ABSTRACT
Therapeutic strategies against systemic mycoses can involve antifungal resistance and significant toxicity. Thus, novel therapeutic approaches to fight fungal infections are urgent. Monoclonal antibodies (MAbs) are promising tools to fight systemic mycoses. In this study, MAbs of the IgM isotype were developed against chitin oligomers. Chitooligomers derive from chitin, an essential component of the fungal cell wall and a promising therapeutic target, as it is not synthesized by humans or animals. Surface plasmon resonance (SPR) assays and cell-binding tests showed that the MAbs recognizing chitooligomers have high affinity and specificity for the chitin derivatives. In vitro tests showed that the chitooligomer MAbs increased the fungicidal capacity of amphotericin B against Cryptococcus neoformans. The chitooligomer-binding MAbs interfered with two essential properties related to cryptococcal pathogenesis: biofilm formation and melanin production. In a murine model of C. neoformans infection, the combined administration of the chitooligomer-binding MAb and subinhibitory doses of amphotericin B promoted disease control. The data obtained in this study support the hypothesis that chitooligomer antibodies have great potential as accessory tools in the control of cryptococcosis.
KEYWORDS: Cryptococcus, chitin, monoclonal antibodies
INTRODUCTION
Fungal infections affect more than one billion people, resulting in approximately 13.5 million life-threatening infections and more than 1.7 million deaths annually (1). Approximately 30% of patients diagnosed with histoplasmosis living with HIV/AIDS die in Latin America (2). Each year, 220,000 new cases of cryptococcal meningitis occur worldwide, resulting in approximately 180,000 deaths (3). More than 400,000 cases of Pneumocystis pneumonia occur in Africa each year, with an estimated fatality rate of 100% if the disease is not treated (3). Although these numbers are alarming, there are only a few options for the treatment of fungal diseases. Immunotherapeutic strategies have been proposed as tools to fight fungal diseases. Antibodies with therapeutic potential were developed against Histoplasma capsulatum histone 2B (4), melanin (5), and heat shock proteins (6); Candida albicans and Aspergillus fumigatus β-glucans (7); and Cryptococcus neoformans glycosylceramide (8), melanin (9), and glucuronoxylomannan (10), among others.
Chitin is essential for the integrity of fungal cell walls (11). Since this polysaccharide is not synthesized by humans or animals, chitin is a promising candidate for the antifungal therapy (11). The inhibition of chitin synthesis in fungi is not trivial, due to the general redundancy of genes regulating chitin formation in fungal cells (12). Chitin oligomers or chitooligomers are formed by the partial enzymatic hydrolysis of chitin in fungal cells (13). In C. neoformans, the blocking of chitooligomers with a lectin prior to injection of the fungus in mice reduced brain colonization (14), suggesting that these structures could be targeted by antifungal approaches. Monoclonal antibodies (MAbs) to chitooligomers are still not available, which limits the evaluation of these molecules as therapeutic tools.
In this study, MAbs against chitooligomers were developed using the hybridoma technique. These MAbs recognized chitooligomers on the surface of C. neoformans and Candida albicans. Surface plasmon resonance (SPR) assays demonstrated that the MAbs are avid and specific for the chitooligomers. In vitro, these MAbs inhibited fungal growth when combined with amphotericin B. Moreover, one of the chitooligomer-binding MAbs was highly effective in controlling cryptococcosis in a mouse model of infection, in the presence of subinhibitory amphotericin B. Our findings suggest that chitooligomer-binding MAbs are promising as accessory tools to fight cryptococcosis.
RESULTS
Generation of MAbs against chitooligomers.
For immunization of mice, we first .challenged the animals with Cryptococcus deuterogattii followed by 2 intraperitoneal injections at 15-day intervals with the β-1,4-linked N-acetylglucosamine trimer, chitotriose, in aluminum hydroxide. Mice were then challenged intravenously with chitotriose in phosphate-buffered saline (PBS) 3 days after the last intraperitoneal injection. Fifty-eight hybridomas interacting with chitotriose were selected, and finally, the 10 hybridomas that showed the highest response were selected for enzyme-linked immunosorbent assay (ELISA) using chitotriose coupled to bovine serum albumin (chitotriose-BSA) as the primary antigen. The most reactive antibodies, accordingly to the ELISA results, were purified and sequenced (Table 1), and 2 MAbs (namely, DD11 and CC5) were identified with different complementarity-determining regions (CDR), which were characterized as of the IgM subtype (Fig. 1). The light and heavy regions (VL and VH) were characterized with bands of approximately 23 and 70 kDa, respectively (Fig. 1).
TABLE 1.
CDR sequence comparisons of the chitooligomer MAbs
| Chain and MAb | Sequence in: |
||
|---|---|---|---|
| CDR1 | CDR2 | CDR3 | |
| Variable light chain (VL) | |||
| CC5 | SASSSISYMH | DTSKLAS | QRSSYPCT |
| DD11 | SASSSVSYMH | STSNLAS | QQRSSYPLT |
| Variable heavy chain (VH) | |||
| CC5 | GFTFSDAWMD | IRSKANNHA | HRYDGFDY |
| DD11 | GFTFSDYGMA | ISNLAYSIY | DYYGSSYWYFDV |
FIG 1.
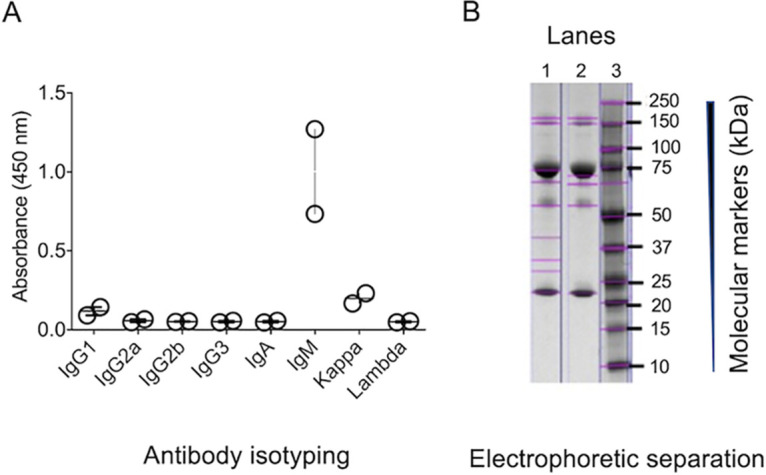
Characterization of MAbs to chitooligomers. (A) Isotyping of MAbs DD11 and CC5 was performed using culture supernatants of the hybridomas producing each antibody. Results from each hybridoma were compiled; each circle represents the signal obtained from each antibody. The highest signals were obtained for IgM, with MAb DD11 producing the highest reactivity. (B) Denaturing gel of purified MAbs denoting the predominant bands corresponding to the heavy (∼70-kDa) and light (∼23- to 24-kDa) chains of IgM. Lane 1, MAb CC5; lane 2, MAb DD11; lane 3, molecular mass markers.
Antigen recognition assays.
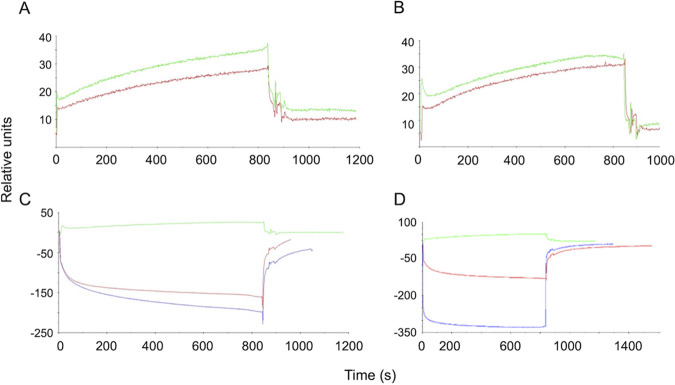
The MAbs were immobilized by amine coupling chemistry, and chitotriose was tested at two concentrations (0.1 and 0.06 mM) to analyze the association constant (Ka) and the dissociation constant (Kd). SPR was adjusted to a 1:1 interaction model, and, finally, Ka and Kd were determined for each antibody. Regardless of the concentration of the antigen, MAb DD11 demonstrated higher affinity than CC5 (Table 2). Both MAbs were tested against other molecules (glycine and BSA), and they showed no affinity or specificity for these molecules (Fig. 2). We also included cell-binding assays in our MAb characterization. In these assays, C. neoformans and C. albicans were tested first by immunofluorescence and then by an adaptation of conventional ELISA to allow the use of intact cells. Immunofluorescence analysis with each of the antibodies revealed that, in both pathogens, chitooligomers localized to the cell surface. While the antibody-binding sites were more uniformly distributed on the cell surface of C. neoformans (Fig. 3A), the antibodies reacted with surface structures that localized to cell division sites in C. albicans (Fig. 3B).
TABLE 2.
Kinetics of the binding of chitooligomer MAbs to chitotriose
| MAb | Chitotriose concn (M) |
Ka (1/M) |
Kd (M) |
||
|---|---|---|---|---|---|
| Value | Mean ± SD | Value | Mean ± SD | ||
| DD11 | 6.8 × 10−5 | 4.5 × 104 | (4.16 ± 0.47) × 104 | 2.24 × 10−5 | (2.8 ± 0.76) × 10−5 |
| 1 × 10−4 | 3.83 × 104 | 3.32 × 10−5 | |||
| CC5 | 6.8 × 10−5 | 1.61 × 103 | (1.69 ± 0.11) × 103 | 6.21 × 10−4 | (5.9 ± 0.38) × 10−4 |
| 1 × 10−4 | 1.77 × 103 | 5.67 × 10−4 | |||
FIG 2.
SPR sonogram representative of the interaction of the chitooligomer MAbs with chitotriose. The ligands tested were the MAbs DD11 (A) and CC5 (B). (C and D) Negative controls for MAbs DD11 and CC5, respectively. The surfaces of the flow cells were activated and the ligands immobilized at 100 μg/ml in 10 mM sodium acetate, pH 5.0. In panels A and B, red and green lines correspond to the interaction of the MAbs with of 0.06 nM and 0.1 nM chitotriose, respectively. In panels C and D, the green lines represent chitotriose (0.06 nM), while red and blue lines represent BSA and glycine, respectively, at the same concentration. The analyte injection corresponds to time zero. The rise of the curves represents analyte-substrate binding. Approximately 800 s after injection, disassociation starts, resulting in the abrupt drop of the curves. Response units were generated by the equipment's software.
FIG 3.
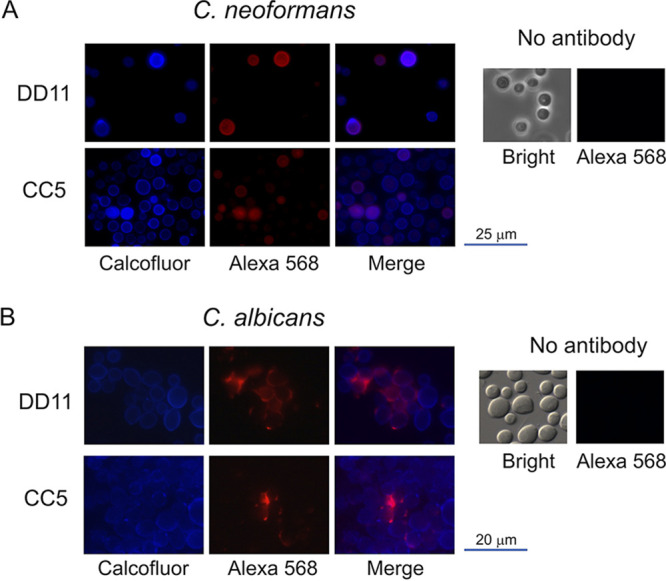
Reactivity of chitooligomer MAbs with the cell surface of C. neoformans (A) and C. albicans (B). Cell wall chitin was stained with calcofluor white (blue fluorescence), and chitooligomers were stained with an Alexa Fluor 568 secondary antibody (red fluorescence) after incubation with MAb DD11 or CC5. Merge panels illustrate the surface localization of the chitooligomers in more detail. Control systems (no antibody) were not incubated with the primary antibodies. In these systems, fungal cells were observed in bright-field and red fluorescence (Alexa 568) modes.
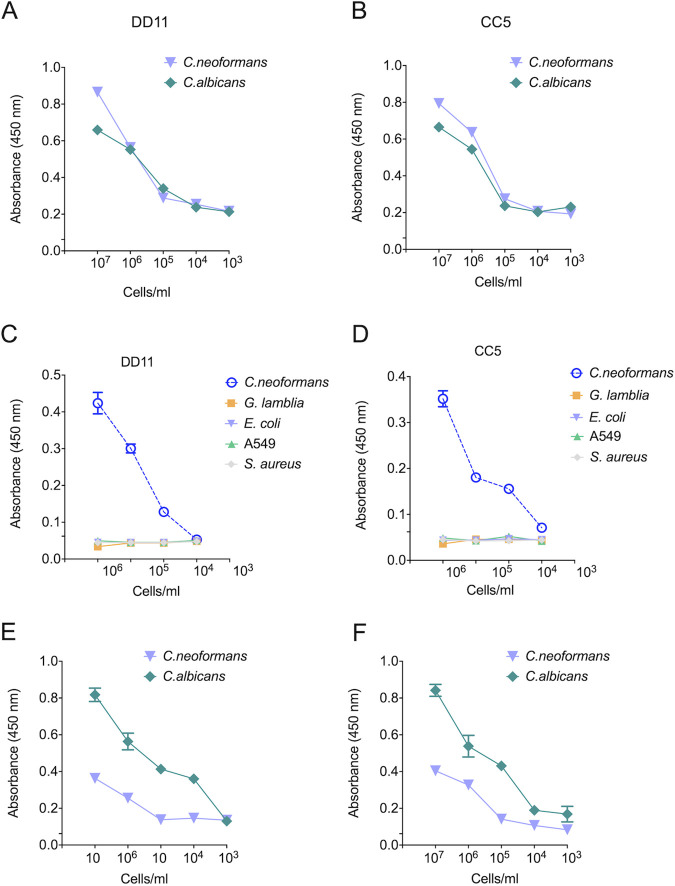
In the ELISA-like tests, the MAbs gave positive reactions above a cutoff of 103 cells/ml for both fungi (Fig. 4A and B). The reactions were considered positive when they produced spectrophotometric values 3-fold greater than the negative control. Although the chitooligomer MAbs bound to the surface of C. neoformans and C. albicans, they did not show any agglutinating activity when tested at concentrations of up to 25 μg/ml (data not shown). The MAbs were also tested for their ability to recognize other cell types (Fig. 4C and D), including human pulmonary cells (A549), Gram-positive bacteria (Staphylococcus aureus), Gram-negative bacilli (Escherichia coli), and parasites (Giardia lamblia). The MAbs did not interact with G. lamblia, A549 cells, E. coli, or S. aureus at 104 cells/ml. We also used dot blot assays to test the MAb-fungus interactions. In these tests, the MAbs required at least 106 cells/ml to recognize C. neoformans. However, the DD11 MAb required only 104 cells/ml to recognize C. albicans, in contrast to CC5 (Fig. 4E and F). Once again, reactions were considered positive when values were 3-fold greater than the control cutoff.
FIG 4.
Binding of chitooligomer MAbs to whole cells of C. neoformans and C. albicans. The wells of ELISA plates were coated with cells of C. albicans (green lines) or C. neoformans (purple lines) at cell densities ranging from 102 to 107/ml. The reactivities of MAbs DD11 (A) and CC5 (B) at 12.5 μg/ml are shown. Similar tests were performed with MAb DD11 (C) and MAb CC5 (D) with G. lamblia (green lines), A549 human cells (orange lines), Gram-negative E. coli (purple lines), and Gram-positive S. aureus (gray lines). Dot blotting also shows the binding of chitooligomer MAbs to whole cells of C. neoformans and C. albicans. MAbs DD11 (E) and CC5 (F) were tested against whole cells of C. albicans (green lines) and C. neoformans (purple lines) at various cell densities. The MAbs were used at 12.5 μg/ml. The results illustrate a representative experiment, with three independent replicates producing similar results.
Effects of the chitooligomer MAbs on the formation of cryptococcal biofilms.
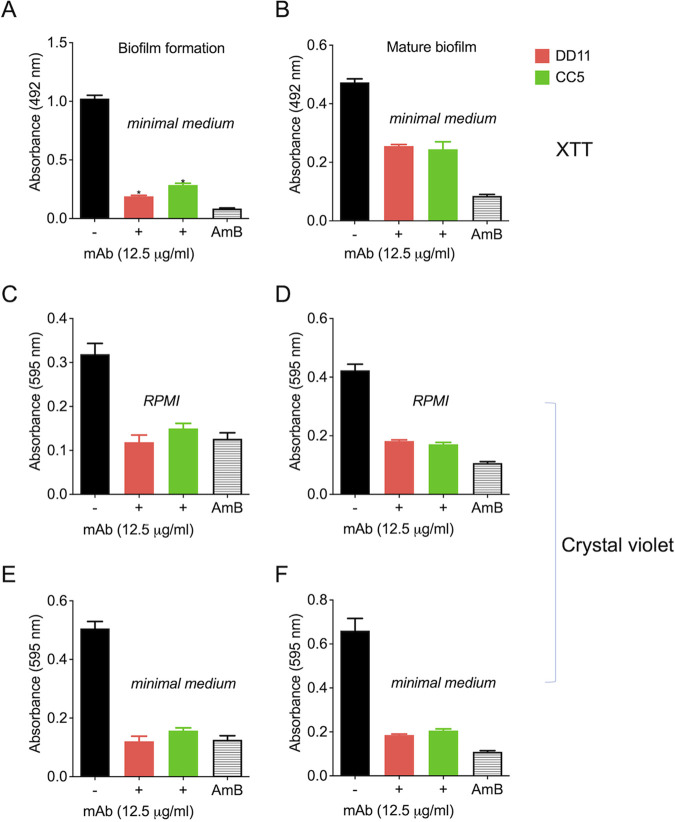
Due to the roles of fungal biofilms in pathogenesis (15), we tested the effect of the chitooligomer MAbs on the formation of C. neoformans biofilms through two different approaches, including XTT metabolization and assimilation of crystal violet. At 12.5 μg/ml, the MAbs significantly inhibited the formation of biofilms and also affected mature biofilms (P < 0.01) (Fig. 5). Similar results were observed for the two different approaches to biofilm analyses. As a control of biofilm inhibition, amphotericin B was used at 1 μg/ml. Lower MAb concentrations were also tested; however, no effects were observed. Similar results were obtained for C. albicans biofilms (data not shown).
FIG 5.
Effect of MAbs to chitooligomers on cryptococcal biofilms. Metabolic activity of mature biofilm and biofilm formation was measured indirectly by the XTT reduction assay (A and B) or crystal violet assimilation (C to F). MAb DD11 (red), MAb CC5 (green), and amphotericin B (AmB; 1 μg/ml) were tested for their ability to affect biofilms. (A, C, and E) Treatment of mature biofilms with MAbs; (B, D, and F) incubation of C. neoformans with the MAbs before biofilm formation. Data illustrate a representative experiment, with three independent replicates producing similar results. In all cases, statistically significant differences (P < 0.01) in comparison to control systems were observed when the biofilms were exposed to AmB or the MAbs.
Chitooligomer MAbs affect the melanization of C. neoformans.
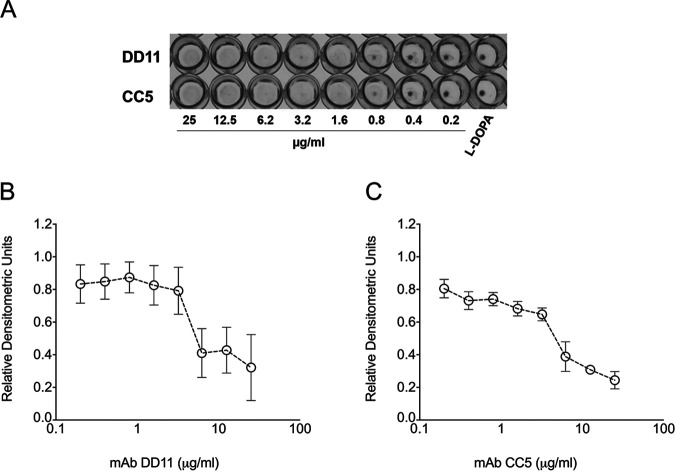
The capacity of C. neoformans to produce melanin represents an important virulence factor (16). Considering that the MAbs tested here target components of the cell wall, where melanin is deposited, we evaluated the ability of these antibodies to affect pigmentation. MAbs DD11 and CC5 were tested at concentrations ranging from 0.2 to 25 μg/ml, and l-3,4-dihydroxyphenylalanine (l-DOPA) was used as a substrate for melanization. The DD11 MAb partially inhibited cryptococcal melanization at concentrations higher than or equal to 6.2 μg/ml (P < 0.05), while at lower concentrations, no inhibition was detected (P > 0.05). At concentrations greater than 6.2 μg/ml (P < 0.001), the CC5 MAb also prevented melanization completely, while at 3.2 μg/ml, a partial inhibition occurred. Lower concentrations had no effect on the fungal pigmentation (P > 0.05) (Fig. 6).
FIG 6.
Effect of the chitooligomer MAbs on the melanization of C. neoformans. (A) Pigmentation of C. neoformans in the presence of MAbs DD11 and CC5. Pigmentation in C. neoformans was visually assessed by sedimentation of brown to black color at the bottoms of wells in 96-well plates. (B and C) Densitometric quantification of pigments treated with MAbs DD11 and CC5, respectively. The results illustrate a representative experiment, with three independent replicates producing similar results.
Antifungal activity.
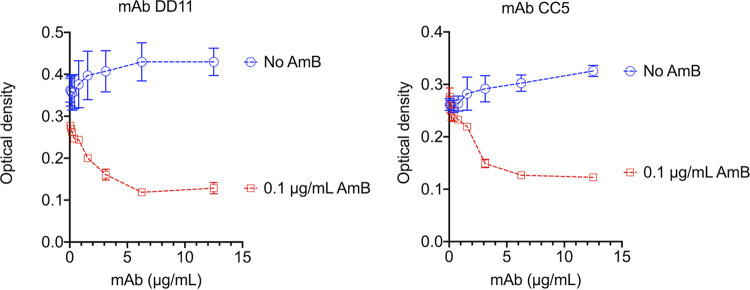
The potential antifungal activity of the MAbs against C. neoformans was tested using standardized methods proposed by the European Committee on Antimicrobial Susceptibility Testing (EUCAST) (17). When tested alone, the MAbs did not show any effect on the fungal growth (Fig. 7). We then tested the MAbs in the presence of subinhibitory (0.1 μg/ml) amphotericin B. In the presence of the antifungal, a dose-dependent inhibition of fungal growth was observed for both antibodies. The effects of the MAbs and amphotericin B were synergistic (Table 3). We also observed an additive effect of amphotericin B with MAb DD11 at 1.6 μg/ml. These calculations were based on the fractional inhibitory index (FII).
FIG 7.
Analysis of cryptococcal growth in the presence of subinhibitory amphotericin B and MAbs DD11 or CC5. C. neoformans cells were incubated in the absence or presence of MAbs, both at concentrations varying from 0 to 12.5 μg/ml. Alternatively, fungal cells were cultivated in the presence of a subinhibitory concentration of amphotericin B (AmB; 0.1 μg/ml) alone (no MAb [0 μg/ml]) or in combination with the MAbs. The combination of the MAbs with subinhibitory amphotericin B resulted in inhibition of fungal growth. The results illustrate a representative experiment, with three independent replicates producing similar results.
TABLE 3.
Antifungal effects of chitooligomer MAbs in combination with 0.1 μg/ml amphotericin Ba
| MAb | Concn (μg/ml) | FII |
|---|---|---|
| DD11 | 12.5 | 0.8 |
| 6.2 | 0.7 | |
| CC5 | 12.5 | 0.8 |
| 6.2 | 0.9 |
Additive or synergistic effects were determined on the basis of the calculation of the fractional inhibitory index (FII). Values of 1.0 represent an additive effect, while values of <1.0 denote a synergistic effect (48).
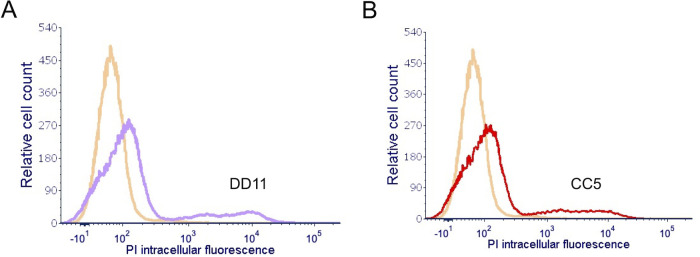
We asked why the chitooligomer MAbs would increase the antifungal capacity of amphotericin B. Ergosterol, the cellular target of amphotericin B, is a plasma membrane component (18), implying that the antifungal has to cross the cell wall to exert its inhibitory effect. We then speculated that the chitooligomer MAbs altered cell wall permeability after binding to C. neoformans. To address this hypothesis, we stained C. neoformans with propidium iodide, which binds to the nucleus, after treatment of the fungus with the chitooligomer MAbs. The treatment of fungal cells with MAb DD11 or CC5 for 2 h at 37°C resulted in a increased staining of C. neoformans with propidium iodide (Fig. 8). These results supported the supposition that the chitooligomers MAbs rendered C. neoformans more permeable to externally added molecules of low molecular mass, including amphotericin B and propidium iodide.
FIG 8.
Treatment of C. neoformans with chitooligomer MAbs results in increased intracellular staining of the fungus with propidium iodide. C. neoformans was treated with MAb DD11 (A, purple histogram) or CC5 (B, red histogram) at 12.5 μg/ml for 2 h at 37°C, stained with 0.5 μg/ml propidium iodide, and immediately analyzed by flow cytometry. Control systems (no antibody; orange histograms) were incubated in PBS. These results represent two independent replicates that produced similar results.
The association of a chitooligomer MAb and subinhibitory amphotericin B results in the control of animal cryptococcosis.
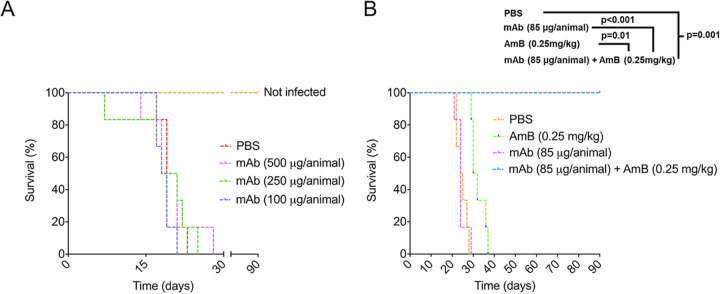
On the basis of the antifungal effects of the association between the chitooligomer MAbs and subinhibitory amphotericin B, we tested the ability of this association to control cryptococcosis in mice (Fig. 9). MAb DD11 was selected for these assays due to its broader activity (synergistic and additive effects) in the presence of amphotericin B. The mortality rate of untreated animals infected with C. neoformans (control system) was 100% on the 28th day after infection. Similarly, the treatment of lethally infected mice with the MAb alone at different concentrations resulted in 100% death at day 29 postinfection. All animals placed in the group treated only with amphotericin B (0.25 mg/kg of body weight) died at day 37 postinfection, while all animals placed in the group treated with a combination of MAb DD11 (85 μg/animal) with amphotericin B (0.25 mg/kg) survived until the experiment was interrupted at day 90 postinfection. In the control group treated with the standard dose of amphotericin B (2.5 mg/kg), no animals died (data not shown).
FIG 9.
An association of MAb DD11 with subinhibitory amphotericin B (AmB) improves the survival of mice infected with lethal dose of C. neoformans. (A) BALB/c mice (n = 7) receiving PBS or MAb DD11 died 22 to 29 days after infection with C. neoformans. (B) Animals receiving a subinhibitory concentration of AmB (0.25 mg/kg) postinfection died at day 37 postinfection. All mice receiving the combination of a subinhibitory concentration of AmB and the MAb survived until the experiment was interrupted (P = 0.001).
DISCUSSION
Several studies have shown that monoclonal antibodies can be protective against fungal infections (7). Proteins, polysaccharides, pigments, and even fungal glycolipids were characterized as the targets of protective antibodies (6–9, 19, 20). Our laboratory previously demonstrated that blocking cell wall chitooligomers is beneficial for the control of animal cryptococcosis (21), but MAbs to chitin and its derivatives are still not available. Thus, we chose chitotriose, a water-soluble chitin oligomer composed of three units of β1,4-linked N-acetyglucosamine, as a target for developing MAbs.
The immunization strategy using C. deuterogattii whole cells was adopted based on our previous study showing that chitooligomers are exposed on the outer layers of the cryptococcal surface (22). This immunization strategy, which certainly stimulated immune responses to multiple antigens, was followed by sequential boosts of the humoral response to the chitooligomers using chitotriose combined with AlOH3 (23, 24). In contrast to IgM, IgG was not detected, suggesting a greater proliferation of memory cells of the IgM type (25, 26). We assumed that their rapid expansion followed by the generation of plasma cells independently of T cells led to a greater accumulation of IgM-producing cells, probably due to the stimulation caused by chitotriose (27).
Amphotericin B, the main drug used to fight cryptococcosis and other systemic mycoses, is toxic and expensive (28). Therefore, therapeutic protocols using smaller doses of amphotericin B have the potential to be more affordable and produce less accentuated collateral effects. The chitooligomer MAbs had no effects on the fungal growth alone. However, cryptococci were more susceptible in vitro to amphotericin B in the presence of the chitooligomer MAbs. We still do not know why the antibodies render C. neoformans more susceptible to amphotericin B, but it is likely that the antibodies interfere with the cell wall, as suggested by their ability to affect melanization. In fact, the cell wall is the cellular site where melanin is finally deposited in C. neoformans (16), leading to the supposition that binding of the MAbs to the cryptococcal surface can induce disorganization of the wall matrix and, consequently, altered melanization. Considering that amphotericin B acts on the plasma membrane (29), we speculate that changes in the cell wall could also make the C. neoformans cells more permeable to the antifungal, increasing its efficacy. This supposition was supported by the observation that the MAbs rendered C. neoformans more susceptible to intracellular staining with propidium iodide. Interference with the cell wall could explain the effects on biofilm formation, considering the essential role of the fungal surface in this process (30).
The development of MAbs against fungal targets has been explored over the past 2 decades (9, 31, 32). Passively administered IgMs raised to a polysaccharide antigen protected mice against cryptococcosis (33), supporting the notion that this class of antibody can be therapeutic against Cryptococcus. The obvious differences between mouse and human cryptococcosis raise questions about the applicability of the murine model to selection of antibodies with therapeutic potential. In this regard, an IgG1 MAb to cryptococcal glucuronoxylomannan (GXM; MAb 18B7) was selected for human clinical trials based on its effects in mice (34), supporting the use of this animal model for preclinical tests.
MAbs with therapeutic potential vary according to their mechanisms of protection. Two examples are the MAbs 18B7 against Cryptococcus GXM and 2G8 against Candida β1,3-glucan (35, 36). MAb 18B7 is an immunomodulator with no antifungal effects, while MAb 2G8 interferes with fungal growth (35, 36). Other MAbs raised against fungal components can promote growth inhibition (37), inhibition of biofilm formation (38), reduced adherence and germination (39), and inhibition of melanin formation (9). Our results and these reports suggest that the chitooligomer antibodies could facilitate infection control in mice without affecting the host’s physiology, as inferred from their affinity and specificity for fungal cells.
Considering the ability of the chitooligomer MAbs to participate in antifungal activity, biofilm formation and pigmentation, we asked whether these antibodies would be effective in vivo. In fact, MAb DD11 promoted the control of animal cryptococcosis in the presence of a subinhibitory concentration of amphotericin B. This combination resulted in 100% survival of animals infected with lethal inocula of C. neoformans. The mechanisms explaining these effects are still unclear, but they could be related to the effects of the antibody in vitro, in addition to the modulation of the immune response in favor of the host (40), and interference with the immunological recognition of chitooligomers and chitin. We demonstrated previously that blocking cryptococcal chitooligosaccharides by other approaches resulted in attenuated pathogenesis (14). We cannot rule out the possibility that blocking the chitooligomers will also affect the immune response during cryptococcosis. For instance, host chitinase is regularly expressed in the lungs of rats, but not the brain (21). We demonstrated in an earlier study that surface chitooligomers supposedly deriving from host chitinase activity are more abundant in C. neoformans cells infecting the lungs of rats (21). We also demonstrated that chitinase induces the formation of chitooligomers that associate with capsular GXM to induce unique immunological responses (41). If antibody binding to the surface of C. neoformans affects its sensitivity to host chitinase, all these events would likely have different outcomes, including the modulation of the host’s immune response.
Our results support the notion that MAbs against chitooligomers might be useful as biopharmaceuticals for treating fungal diseases along with lower doses of amphotericin B. Thus, the MAbs developed in this study could be used as auxiliary tools in the therapy against fungal infections. Future studies with humanized antibodies on optimal doses and immunomodulation in vivo could be the basis for the formulation of more efficient protocols to fight cryptococcosis.
MATERIALS AND METHODS
Animal use and ethics statement.
Female BALB/c mice (6 to 8 weeks old) were obtained from the animal facility of Fiocruz (CECAL). All experimental procedures were approved by the Oswaldo Cruz Foundation's Ethical Committee on the Use of Animals (CEUA; protocol LW-13/16).
Eukaryotic and prokaryotic cells.
Fungal strains used in this study were C. neoformans H99, C. deuterogattii R265, and C. albicans ATCC 90028. The microorganisms were maintained in Sabouraud broth (C. neoformans and C. deuterogattii) or liquid brain heart infusion (BHI; C. albicans). For in vitro assays, the cells were cultured in a minimal medium (15 mM glucose, 10 mM MgSO4, 29.4 mM KH2PO4, 13 mM glycine, 3 μM thiamine-HCl; pH 5.5) and kept under rotation for 24 h at 37°C. The cells were obtained by centrifugation, washed in PBS, and counted in a Neubauer hematocytometer. Antibody specificity tests included Giardia lamblia (ATCC 30957), A549 human cells (ATCC CCL-185), Escherichia coli (ATCC 9637), and Staphylococcus aureus (ATCC 25923).
Mouse immunization.
Here, we describe the methods that resulted in the production of the MAbs used in all the subsequent assays. However, we need to mention that several other immunization protocols were tested for optimization of antibody production, with poorer results. These other protocols can be made available to any colleague upon request.
Mice (female BALB/c, 6 weeks old) were immunized intraperitoneally (i.p.) every 14 days as described by Guimarães et al., with some adaptations (6). The animals were immunized i.p. with C. deuterogattii (strain R265; 1 × 106 cells/ml, 100 μl/animal) previously fixed with 4% paraformaldehyde (PFA) and washed in 10 mM phosphate-buffered saline (PBS), pH 7.4. This procedure was followed by two immunizations via i.p. with an interval of 14 days with 200 μg of chitotriose combined with aluminum hydroxide (1.5 mg, 100 μl/animal). Finally, the animals were immunized intravenously (i.v.) with 50 μg of free chitotriose 3 days after the last immunization, using PBS as a vehicle (50 μl/animal). Bleeding was performed at the end of the immunization to check the serum antibody titer by indirect ELISA. The preimmune sera from all animals were used as controls and to determine reaction cutoffs for the screenings.
After the four immunizations, the animals were subjected to splenectomy and splenocyte processing to perform cell fusion with murine myeloid cells (SP2/0) (42). Splenocytes and SP2/0 cells in a proportion of 1:2 were fused in the presence of 50% (vol/vol) polyethylene glycol (PEG) 3000 to 3700, preheated to 37°C. Subsequently, the cell homogenate was suspended in Dulbecco’s modified Eagle medium (DMEM) containing glutamine (6.4 mM), 100 U/ml penicillin, 0.1 mg/ml streptomycin, 0.25 μg/ml amphotericin B, and 20% fetal bovine serum (FBS). The cells were transferred to 96-well plates (100 μl/well). Three wells were reserved as controls containing the selection medium (DMEM with hypoxanthine, aminopterin, and thymidine [HAT]) to which 6 × 104 SP2/0 cells were added. The plates were incubated at 37°C, in an atmosphere of 5% CO2 for 24 h. After the initial incubation, 100 μl of the selection medium (DMEM with 6.4 mM glutamine, 1× ATB, 20% SFB, and 2× HAT) was added to the wells to select viable hybrid cells. After 14 days, the culture supernatants were used in indirect ELISAs using chitotriose as the primary antigen.
ELISA for the determination of serum titers.
ELISA (96-well) plates were coated with chitotriose complexed with serum bovine albumin (chitotriose-BSA) (Vector Laboratories catalog no. G-5000) at 0.5 μg/ml in PBS and incubated overnight at 4°C. Subsequently, the plate was incubated with PBS containing 1% BSA for 1 h at 37°C followed by washing with PBS containing 0.05% Tween. The sera of the animals were added at different dilutions and incubated for 2 h at 37°C. The plate was washed three times with PBS-Tween with subsequent addition of anti-murine IgG and anti-IgM antibodies conjugated to peroxidase, followed by incubation for 2 h at 37°C. The plates were then washed as described above and incubated with tetramethylbenzidine (TMB) for 30 min at 37°C. The reaction was stopped with 1 M HCl, and the spectrophotometric readings were obtained at 450 nm. The reactions considered positive were those with absorbance values corresponding to 3 times the cutoff, without the addition of primary antibodies. For determining polyclonal and monoclonal antibody reactivity, the same procedure was performed, with the difference that the primary antibody of the reaction came from the hybridoma culture supernatants.
Cloning of polyclonal hybridomas.
The cloning of positive polyclonal hybridomas in the ELISA was performed by limiting dilution (20). The cells were incubated at 37°C in a 5% CO2 atmosphere for 14 days, and clonality (monoclonal or polyclonal) was observed from the 5th day. The cultures that remained viable had their supernatants tested by ELISA using chitotriose-BSA as the primary antigen. Once again, the reactions were considered positive when the absorbance values corresponded to 3 times the cutoff, without the addition of primary antibodies. The isotyping of the selected clones was performed using the commercial rapid ELISA mouse MAb isotyping kit (Thermo Fisher). The kit determines the presence of the murine isotypes IgG1, IgG2a, IgG2b, IgG3, IgA, and IgM.
Purification of MAbs.
The purification of the MAbs took place in three phases: precipitation with PEG, molecular exclusion chromatography, and high-performance liquid exclusion and ion exchange liquid chromatography on an AKTA purifier 10 (GE Healthcare). Precipitation with PEG was carried out by supplementing the culture supernatant with PEG 6000 to a final concentration of 4%. The suspension was maintained with stirring for 3 h at room temperature and then subjected to centrifugation (1,600 × g; 30 min at 4°C). The supernatant obtained after centrifugation was subjected to a second precipitation step with 6% PEG 6000, followed by centrifugation under the same conditions. The precipitate was dissolved in 15 ml of 50 mM Tris-HCl buffer solution, pH 8.0. The sample was fractionated by molecular exclusion chromatography (SEC) using the Superdex 200 high-load column (26 by 600 mm; 320 ml) with a flow rate of 3.0 ml/min, using 50 mM Tris-HCl, pH 8.0, as the eluent. The collection volume was equal to 10 ml per tube.
After selecting and pooling samples from the SEC, anion-exchange chromatography was performed on a Poros HQ 10- by 100-mm column. Fractions were eluted at a flow rate of 5.0 ml/min with 50 mM Tris-HCl buffer solution, pH 8.0, in a gradient from 20% to 50% with saline. The fractions were collected with a volume of 4.0 ml per tube. The homogeneity of the samples obtained at each stage of the purification process was evaluated by denaturing electrophoresis on 12% polyacrylamide gels (SDS-PAGE) at a constant voltage of 200 V for 45 min. To estimate the molecular weight, the commercial standard dual-color Precision Plus protein (Bio-Rad) was used. The proteins were developed with Coomassie blue R350 dye solution and the result analyzed using Image Lab software, after image processing in the Gel Doc XR+ system (Bio-Rad).
Antibody sequencing.
The cells of each positive clone were collected by centrifugation (400 × g for 10 min at room temperature) and the RNA extracted with the commercial RNeasy minikit (Qiagen), following the protocol established by the manufacturer. For reverse transcriptase PCR (RT-PCR), cDNA synthesis was performed from RNA using the commercial kit SuperScript III first-strand synthesis system (Invitrogen). Subsequently, PCR was performed with universal primers for murine VH and VL (43). RT-PCR was performed under the following conditions (43): initial denaturation 94°C for 5 min, denaturation 94°C for 2 min, annealing 48°C for 1 min, and extension 72°C for 1 min 30 s. The cycles were repeated 35 times, and the final extension was 72°C for 10 min for the VH chain; the same conditions were used for VL but with annealing at 55°C for 1 min. The bands were visualized using 1.5% agarose gels (data not shown). The sequencing of the selected MAbs was performed according with the protocol described in the commercial kit BigDye Terminator v3.1 (Life Technologies), and for that purpose the primers described for PCR were used. The sequences were analyzed using the SeqMan program (DNAStar), and to identify CDR1, −2, and −3 gene sequences, we used the IgBlast tool (NCBI, NIH; https://www.ncbi.nlm.nih.gov/igblast/) through the Kabat database (http://www.bioinf.org.uk/abs/).
Determination of affinity and dissociation constant by SPR.
The SPR experiments were performed using the Biacore X system (GE Healthcare) equipped with a CM5 sensor chip. The ligands tested were the CC5 and DD11 MAbs, which were immobilized using amine coupling chemistry (Biacore X 202AD). The surfaces of the two flow cells were activated for 7 min with a 1:1 mixture of 0.1 M NHS (N-hydroxysuccinimide) and 0.1 M EDC [3- (N,N-dimethylamino)propyl-N-ethylcarbodiimide] at a flow rate of 10 μl/min. The ligands were immobilized at 100 μg/ml in 10 mM sodium acetate, pH 5.0. Ester residues were deactivated with a 7 min injection of 1 M ethanolamine, pH 8.0. To collect kinetic binding data, the BSA-(GlcNAc)3 analyte was injected over the two flow cells at concentrations of 0.1 and 0.06 nM at a flow rate of 5 μl/min and at 25°C using HBS-EP (10 mM HEPES, 150 mM NaCl, 3 mM EDTA and 0.005% P20), pH 7.4. The data were adjusted by concentration in a simple model of interaction (1:1) of the ligand and analyte using the option of global data analysis, which makes it possible to adjust all the graphs obtained simultaneously. All results were analyzed using the BiaEvaluation 4.1 software.
Immunofluorescence.
C. neoformans (107 cells) or C. albicans (106 cells) were fixed in 4% paraformaldehyde for 30 min at room temperature. The cell suspensions were washed with PBS and then blocked with PBS supplemented with 1% BSA for 1 h at room temperature. The cells were washed again with PBS and incubated with each of the chitooligomer MAbs under conditions that differed for each pathogen. C. albicans cells were incubated with MAb DD11 or CC5 (25 μg/ml) for 1 h at 37°C. For staining of C. neoformans, longer periods were required, since the capsule impairs the access of antibodies to the cell wall, as we demonstrated in early studies (44). Therefore, C. neoformans cells were initially incubated with each MAb (25 μg/ml) for 1 h at 37°C, followed by transfer of the antibody-binding systems to 4°C for incubation for 24 h. Shorter periods resulted in the detection of fluorescence at background levels (data not shown). The cells were washed with PBS and then incubated with an anti-IgM Alexa Fluor 568 antibody (2 μg/ml) under the conditions described for the primary antibodies. The fungal cells were washed with PBS and stained with calcofluor white (25 μM) for 30 min at room temperature. After washing with PBS, the cells were analyzed by fluorescence microscopy. C. albicans was observed on an Olympus AX70 fluorescence microscope coupled to a QImaging Retiga 1300 camera system. The images were captured and processed with the QCapture software (V2.46). C. neoformans was observed with a Leica AF6000 modular system (DMI6000B microscope). Images were recorded with the LasAF software.
ELISA for determination of antibody binding to intact cells.
For this test, C. neoformans, C. albicans, Giardia lamblia, the human lung cell line A549, E. coli, and Staphylococcus aureus were used. The fungal cells were washed in PBS three times, suspended at a density of 107 cells/ml in poly-l-lysine (5 μg/ml in PBS), and placed in 96-well polystyrene microplates for overnight incubation at 4°C (45). On the next day the plates were blocked with 5% PBS–BSA for 1 h at 37°C, followed by incubation for 2 h at 37°C with the anti-chitooligomer MAbs at concentration ranges of 5 to 50 μg/ml. Subsequently, the systems were washed 3 times with PBS-Tween, and murine anti-IgM conjugated to peroxidase (1:5,000 dilution) was added to the system, followed by incubation for 2 h at 37°C. After washing, TMB was added, and the plates were incubated for 30 min at 37°C. The reaction was stopped with 1 M HCl, and readings were obtained at 450 nm (45). Subsequently, the experiment was repeated, using serial dilutions (1:10) of the cell suspensions, which ranged from 102 to 107 cells/ml for fungi, and 104 to 107 cells/ml for the other cell types. The antichitooligomer MAb was tested at 25 μg/ml.
Dot blotting for determination of antibody binding to intact cells.
Suspensions of C. neoformans and C. albicans were prepared at densities ranging from 102 to 107 cells/ml in 5 μg/ml poly-l-lysine in PBS. These suspensions (10 μl) were loaded onto nitrocellulose membranes, and blocking and antibody binding were performed as in the ELISA. For reactivity determination, the membrane was cut into small pieces and deposited into the wells of 96-well plates, to which 50 μl of TMB was added. The systems were incubated for 30 min at 37°C. The solution (50 μl) was removed and transferred to a new plate, and development was carried out as described above (46).
Anticryptococcal activity.
Antimicrobial tests followed the EUCAST protocol adapted for yeast cells (17). C. neoformans cells were inoculated in RPMI 1640 buffered with 3-(n-morpholino)-propanesulfonic acid (MOPS), pH 7, at 105 cells/well in 96-well plates, in a final volume of 200 μl. The systems were supplemented with the MAbs (12.5, 6.25, 3.2, 1.6, 0.8, 0.4, 0.2, and 0.1 μg/ml) and amphotericin B (AmB; 1 and 0.1 μg/ml). All substances were used alone or in combination with the MAbs. After 48 h of incubation at 37°C with shaking, the cells were homogenized with the aid of a pipette and subjected to reading at 592 nm (47). Synergistic activity between AmB alone and associated with the MAbs was determined on the basis of the calculation of the fractional inhibitory index (FII), and the synergism was categorized as follows: synergistic effect, FII < 1; additive effect, FII = 1 (48).
Propidium iodide staining.
C. neoformans (1 × 107 cells/ml) was treated with MAb DD11 or CC5 for 2 h at 37°C (12.5 μg/ml). Control systems were incubated in PBS under the same conditions. The cells were washed with PBS by centrifugation, suspended in a 0.5-μg/ml solution of propidium iodide, and immediately analyzed by flow cytometry for detection of red fluorescence on a SR Fortessa BD cytometer. The results were acquired and analyzed with the use of FlowJo software. Intracellular staining of C. neoformans with propidium iodide was interpreted as an increased cellular permeability after antibody treatment.
Biofilm formation.
The cell suspensions (C. neoformans H99) were prepared in minimal medium at 1 × 106 cells/ml and added (100 μl) to the wells of 96-well plates in the presence of the MAbs DD11 and CC5, all at 12.5 μg/ml. As a control for biofilm inhibition, amphotericin B was used at 1 μg/ml (47). Two independent systems were prepared; one was incubated for 48 h at 37°C and then washed to remove nonadherent cells, and the fungal cells that remained attached to the wells were considered mature biofilms. The other was not subjected to washes, and the fungal cells were classified as biofilm formation. The first system, after the washes, was incubated at 37°C for 24 h with antifungal drugs and MAbs; the second system was incubated with antifungal drugs and MAbs at 37°C for 48 h. The metabolic activity of viable cells in the two assays was evaluated by the reduction of 2,3-bis-(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide (XTT), as determined spectrophotometrically at 492 nm. Alternatively, C. neoformans grown in minimal medium or RPMI was prepared for biofilm analysis as described above but stained with a 0.4% solution of crystal violet for 45 min at room temperature (49). The biofilms were washed 4 times with ultrapure water and destained with 95% ethanol for 45 min at room temperature, and the optical density was determined at 595 nm.
Melanization.
C. neoformans (1 × 106 cells/ml) was cultured for 72 h in minimal medium supplemented with 1 mM l-DOPA in a 96-well plate (U bottom) in the presence of MAb DD11 or CC5 at concentrations ranging from 0.2 to 25 μg/ml. Melanin production was determined densitometrically after digitalization of the images with an iBright FL1000 imager (Invitrogen) (50).
Murine infection.
The influence of anti-chitooligomer antibodies on the survival of animals infected with lethal doses of C. neoformans was assessed using two different protocols. Mice (female BALB/c, 8 weeks old) (n = 7 mice per group) were challenged i.p. with 105 cells of C. neoformans H99 in PBS (100 μl/animal). After 2 h, the animals were treated i.p. with 100 μl of solutions of MAb DD11 at concentrations of 100, 250, and 500 μg/ml. As a control, the animals were treated with PBS only. The survival curve was monitored up to 90 days after infection. Alternatively, the mice were similarly infected but treated after 2 h with 100 μl solutions of amphotericin B (2.5 mg/kg or 0.25 mg/kg) (51), MAb DD11 (85 μg/ml), or MAb DD11 (85 μg/ml) containing amphotericin B (0.25 mg/kg) (52). In all systems, the treatments were repeated twice at 10-day intervals. As controls, the animals were injected with 100 μl of PBS, AmB (2.5 mg/kg or 0.25 mg/kg), or MAb (85 μg/ml) (53). The survival curve was monitored up to 90 days after infection.
Statistical analysis.
All statistical analyses were performed using GraphPad Prism software, version 5.00. Two-way analysis of variance (ANOVA) with Bonferroni’s posttest was used for individual comparison between groups, with a 95% confidence interval for all experiments. For the survival curves, differences between groups were analyzed using the Gehan-Breslow-Wilcoxon test.
ACKNOWLEDGMENTS
M.L.R. is currently on leave from the position of associate professor at the Microbiology Institute of the Federal University of Rio de Janeiro, Brazil.
M.L.R. is supported by grants from the Brazilian Ministry of Health (grant 440015/2018-9), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq; grants 405520/2018-2 and 301304/2017-3) and Fiocruz (grants PROEP-ICC 442186/2019-3, VPPCB-007-FIO-18, and VPPIS-001-FIO18). This study was financed in part by scholarships from the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES, Brazil, Finance Code 001). M.L.R. also acknowledges support from the Instituto Nacional de Ciência e Tecnologia de Inovação em Doenças de Populações Negligenciadas (INCT-IDPN). The funders had no role in the decision to publish or preparation of the manuscript.
We are grateful to the Program for Technological Development in Tools for Health-RPT-FIOCRUZ for using the microscopy facility, RPT07C, Carlos Chagas Institute, Fiocruz-Paraná. We also thank Haroldo Oliveira and Beatriz Borges for help with the preparation of immunofluorescence images.
Part of the data presented here is the subject of a pending patent application under the numbers BR 10 2020 002165 6 (Brazil), WO2021/151180 A2 (World Intellectual Property Organization), and PCT/BR2021/050007 (Patent Cooperation Treaty) (54).
REFERENCES
- 1.Kainz K, Bauer MA, Madeo F, Carmona-Gutierrez D. 2020. Fungal infections in humans: the silent crisis. Microb Cell 7:143–145. 10.15698/mic2020.06.718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bongomin F, Gago S, Oladele RO, Denning DW. 2017. Global and multi-national prevalence of fungal diseases—estimate precision. J Fungi (Basel) 3:57. 10.3390/jof3040057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.GAFFI. 2017. Fungal disease frequency. Global Action Fund for Fungal Infections.
- 4.Nosanchuk JD, Steenbergen JN, Shi L, Deepe GS, Casadevall A. 2003. Antibodies to a cell surface histone-like protein protect against Histoplasma capsulatum. J Clin Invest 112:1164–1175. 10.1172/JCI19361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guimarães AJ, de Cerqueira MD, Nosanchuk JD. 2011. Surface architecture of Histoplasma capsulatum. Front Microbiol 2:225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guimarães AJ, Frases S, Gomez FJ, Zancope-Oliveira RM, Nosanchuk JD. 2009. Monoclonal antibodies to heat shock protein 60 alter the pathogenesis of histoplasma capsulatum. Infect Immun 77:1357–1367. 10.1128/IAI.01443-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Di Mambro T, Guerriero I, Aurisicchio L, Magnani M, Marra E. 2019. The yin and yang of current antifungal therapeutic strategies: how can we harness our natural defenses? Front Pharmacol 10:80. 10.3389/fphar.2019.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rodrigues ML, Shi L, Barreto-Bergter E, Nimrichter L, Farias SE, Rodrigues EG, Travassos LR, Nosanchuk JD. 2007. Monoclonal antibody to fungal glucosylceramide protects mice against lethal Cryptococcus neoformans infection. Clin Vaccine Immunol 14:1372–1376. 10.1128/CVI.00202-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rosas ÁL, Nosanchuk JD, Casadevall A. 2001. Passive immunization with melanin-binding monoclonal antibodies prolongs survival of mice with lethal Cryptococcus neoformans infection. Infect Immun 69:3410–3412. 10.1128/IAI.69.5.3410-3412.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martinez LR, Moussai D, Casadevall A. 2004. Antibody to Cryptococcus neoformans glucuronoxylomannan inhibits the release of capsular antigen. Infect Immun 72:3674–3679. 10.1128/IAI.72.6.3674-3679.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garcia-Rubio R, de Oliveira HC, Rivera J, Trevijano-Contador N. 2020. The fungal cell wall: Candida, Cryptococcus, and Aspergillus species. Front Microbiol 10:2993. 10.3389/fmicb.2019.02993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sherrington SL, Sorsby E, Mahtey N, Kumwenda P, Lenardon MD, Brown I, Ballou ER, MacCallum DM, Hall RA. 2017. Adaptation of Candida albicans to environmental pH induces cell wall remodelling and enhances innate immune recognition. PLoS Pathog 13:e1006403. 10.1371/journal.ppat.1006403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fonseca FL, Frases S, Casadevall A, Fischman-Gompertz O, Nimrichter L, Rodrigues ML. 2009. Structural and functional properties of the Trichosporon asahii glucuronoxylomannan. Fungal Genet Biol 46:496–505. 10.1016/j.fgb.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fonseca FL, Guimarães AJ, Kmetzsch L, Dutra FF, Silva FD, Taborda CP, Araujo G de S, Frases S, Staats CC, Bozza MT, Schrank A, Vainstein MH, Nimrichter L, Casadevall A, Rodrigues ML. 2013. Binding of the wheat germ lectin to Cryptococcus neoformans chitooligomers affects multiple mechanisms required for fungal pathogenesis. Fungal Genet Biol 60:64–73. 10.1016/j.fgb.2013.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aslanyan L, Sanchez DA, Valdebenito S, Eugenin EA, Ramos RL, Martinez LR. 2017. The crucial role of biofilms in Cryptococcus neoformans survival within macrophages and colonization of the central nervous system. J Fungi 3:10. 10.3390/jof3010010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nosanchuk JD, Stark RE, Casadevall A. 2015. Fungal melanin: what do we know about structure? Front Microbiol 6:1463–1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arendrup MC, Meletiadis J, Mouton JW, Guinea J, Cuenca-Estrella M, Lagrou K, Howard SJ, Arendrup MC, Howard SJ, Mouton J, Guinea J, Lagrou K, Arikan-Akdagli S, Barchiesi F, Hamal P, Järv H, Lass-Flörl C, Mares M, Matos T, Muehlethaler K, Rogers TR, Torp Andersen C, Verweij P, Subcommittee on Antifungal Susceptibility Testing (AFST) of the ESCMID European Committee for Antimicrobial Susceptibility Testing (EUCAST). 2016. EUCAST technical note on isavuconazole breakpoints for Aspergillus, itraconazole breakpoints for Candida and updates for the antifungal susceptibility testing method documents. Clin Microbiol Infect 22:571.E1–571.E4. 10.1016/j.cmi.2016.01.017. [DOI] [PubMed] [Google Scholar]
- 18.Rodrigues ML. 2021. The multifunctional fungal ergosterol. mBio 9:e01755-18. 10.1128/mBio.01755-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guimarães AJ, Frases S, Pontes B, De Cerqueira MD, Rodrigues ML, Viana NB, Nimrichter L, Nosanchuk JD. 2011. Agglutination of Histoplasma capsulatum by IgG monoclonal antibodies against Hsp60 impacts macrophage effector functions. Infect Immun 79:918–927. 10.1128/IAI.00673-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lopes LCL, Rollin-Pinheiro R, Guimarães AJ, Bittencourt VCB, Martinez LR, Koba W, Farias SE, Nosanchuk JD, Barreto-Bergter E. 2010. Monoclonal antibodies against peptidorhamnomannans of Scedosporium apiospermum enhance the pathogenicity of the fungus. PLoS Negl Trop Dis 4:e853. 10.1371/journal.pntd.0000853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fonseca FL, Nimrichter L, Cordero RJB, Frases S, Rodrigues J, Goldman DL, Andruszkiewicz R, Milewski S, Travassos LR, Casadevall A, Rodrigues ML. 2009. Role for chitin and chitooligomers in the capsular architecture of Cryptococcus neoformans. Eukaryot Cell 8:1543–1553. 10.1128/EC.00142-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rodrigues ML, Alvarez M, Fonseca FL, Casadevall A. 2008. Binding of the wheat germ lectin to Cryptococcus neoformans suggests an association of chitinlike structures with yeast budding and capsular glucuronoxylomannan. Eukaryot Cell 7:602–609. 10.1128/EC.00307-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Petrovsky N, Aguilar JC. 2004. Vaccine adjuvants: current state and future trends. Immunol Cell Biol 82:488–496. 10.1111/j.0818-9641.2004.01272.x. [DOI] [PubMed] [Google Scholar]
- 24.Haji-Ghassemi O, Blackler RJ, Young NM, Evans SV. 2015. Antibody recognition of carbohydrate epitopes. Glycobiology 25:920–952. 10.1093/glycob/cwv037. [DOI] [PubMed] [Google Scholar]
- 25.Racine R, Winslow GM. 2009. IgM in microbial infections: taken for granted? Immunol Lett 125:79–85. 10.1016/j.imlet.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Capolunghi F, Rosado MM, Sinibaldi M, Aranburu A, Carsetti R. 2013. Why do we need IgM memory B cells? Immunol Lett 152:114–120. 10.1016/j.imlet.2013.04.007. [DOI] [PubMed] [Google Scholar]
- 27.Phan TG, Tangye SG. 2017. Memory B cells: total recall. Curr Opin Immunol 45:132–140. 10.1016/j.coi.2017.03.005. [DOI] [PubMed] [Google Scholar]
- 28.Rodrigues ML, Nosanchuk JD. 2020. Fungal diseases as neglected pathogens: a wake-up call to public health officials. PLoS Negl Trop Dis 14:e0007964. 10.1371/journal.pntd.0007964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lemke A, Kiderlen AF, Kayser O. 2005. Amphotericin B. Appl Microbiol Biotechnol 68:151–162. 10.1007/s00253-005-1955-9. [DOI] [PubMed] [Google Scholar]
- 30.Martinez LR, Casadevall A. 2015. Biofilm formation by Cryptococcus neoformans. Microbiol Spectr 3:MB-0006-2014. 10.1128/microbiolspec.MB-0006-2014. [DOI] [PubMed] [Google Scholar]
- 31.Casadevall A, Scharff MD. 1991. The mouse antibody response to infection with Cryptococcus neoformans: VH and VL usage in polysaccharide binding antibodies. J Exp Med 174:151–160. 10.1084/jem.174.1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dromer F, Charreire J. 1991. Improved amphotericin B activity by a monoclonal anti-Cryptococcus neoformans antibody: study during murine cryptococcosis and mechanisms of action. J Infect Dis 163:1114–1120. 10.1093/infdis/163.5.1114. [DOI] [PubMed] [Google Scholar]
- 33.Mr W, Kausik D, Qing C, Lr X, Bradley W, Krishanthi S, Liise-Anne P. 2004. Protective and nonprotective human immunoglobulin M monoclonal antibodies to Cryptococcus neoformans glucuronoxylomannan manifest different specificities and gene use profiles. Infect Immun 72:4810–4818. 10.1128/IAI.72.8.4810-4818.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Larsen RA, Pappas PG, Perfect J, Aberg JA, Casadevall A, Cloud GA, James R, Filler S, Dismukes WE. 2005. Phase I evaluation of the safety and pharmacokinetics of murine-derived anticryptococcal antibody 18B7 in subjects with treated cryptococcal meningitis. Antimicrob Agents Chemother 49:952–958. 10.1128/AAC.49.3.952-958.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Casadevall A, Cleare W, Feldmesser M, Glatman-Freedman A, Goldman DL, Kozel TR, Lendvai N, Mukherjee J, Pirofski LA, Rivera J, Rosas AL, Scharff MD, Valadon P, Westin K, Zhong Z. 1998. Characterization of a murine monoclonal antibody to Cryptococcus neoformans polysaccharide that is a candidate for human therapeutic studies. Antimicrob Agents Chemother 42:1437–1446. 10.1128/AAC.42.6.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rachini A, Pietrella D, Lupo P, Torosantucci A, Chiani P, Bromuro C, Proietti C, Bistoni F, Cassone A, Vecchiarelli A. 2007. An anti-β-glucan monoclonal antibody inhibits growth and capsule formation of Cryptococcus neoformans in vitro and exerts therapeutic, anticryptococcal activity in vivo. Infect Immun 75:5085–5094. 10.1128/IAI.00278-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Torosantucci A, Bromuro C, Chiani P, De Bernardis F, Berti F, Galli C, Norelli F, Bellucci C, Polonelli L, Costantino P, Rappuoli R, Cassone A. 2005. A novel glyco-conjugate vaccine against fungal pathogens. J Exp Med 202:597–606. 10.1084/jem.20050749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Martinez LR, Casadevall A. 2005. Specific antibody can prevent fungal biofilm formation and this effect correlates with protective efficacy. Infect Immun 73:6350–6362. 10.1128/IAI.73.10.6350-6362.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moragues MD, Omaetxebarria MJ, Elguezabal N, Sevilla MJ, Conti S, Polonelli L, Pontón J. 2003. A monoclonal antibody directed against a Candida albicans cell wall mannoprotein exerts three anti-C. albicans activities. Infect Immun 71:5273–5279. 10.1128/IAI.71.9.5273-5279.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Boniche C, Rossi SA, Kischkel B, Barbalho FV, Moura ÁND, Nosanchuk JD, Travassos LR, Taborda CP. 2020. Immunotherapy against systemic fungal infections based on monoclonal antibodies. J Fungi (Basel) 6:31. 10.3390/jof6010031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ramos CL, Fonseca FL, Jessica R, Guimaraes AJ, Cinelli LP, Miranda KM, Nimrichter L, Casadevall A, Travassos LR, Rodrigues ML. 2012. Chitin-like molecules associate with Cryptococcus neoformans glucuronoxylomannan to form a glycan complex with previously unknown properties. Eukaryot Cell 11:1086–1094. 10.1128/EC.00001-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Köhler G, Milstein C. 2005. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature, 1975, 256 (5517):495–497. J Immunol 174:2453–2455. [DOI] [PubMed] [Google Scholar]
- 43.Wang Z, Raifu M, Howard M, Smith L, Hansen D, Goldsby R, Ratner D. 2000. Universal PCR amplification of mouse immunoglobulin gene variable regions: the design of degenerate primers and an assessment of the effect of DNA polymerase 3’ to 5’ exonuclease activity. J Immunol Methods 233:167–177. 10.1016/S0022-1759(99)00184-2. [DOI] [PubMed] [Google Scholar]
- 44.Rodrigues ML, Travassos LR, Miranda KR, Franzen AJ, Rozental S, de Souza W, Alviano CS, Barreto-Bergter E. 2000. Human antibodies against a purified glucosylceramide from Cryptococcus neoformans inhibit cell budding and fungal growth. Infect Immun 68:7049–7060. 10.1128/IAI.68.12.7049-7060.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stearns NA, Zhou S, Petri M, Binder SR, Pisetsky DS. 2016. The use of poly-L-lysine as a capture agent to enhance the detection of antinuclear antibodies by ELISA. PLoS One 11:e0161818. 10.1371/journal.pone.0161818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nimrichter L, Frases S, Cinelli LP, Viana NB, Nakouzi A, Travassos LR, Casadevall A, Rodrigues ML. 2007. Self-aggregation of Cryptococcus neoformans capsular glucuronoxylomannan is dependent on divalent cations. Eukaryot Cell 6:1400–1410. 10.1128/EC.00122-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Joffe LS, Schneider R, Lopes W, Azevedo R, Staats CC, Kmetzsch L, Schrank A, Del Poeta M, Vainstein MH, Rodrigues ML. 2017. The anti-helminthic compound mebendazole has multiple antifungal effects against Cryptococcus neoformans. Front Microbiol 8:535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mor V, Rella A, Farnoud AM, Singh A, Munshi M, Bryan A, Naseem S, Konopka JB, Ojima I, Bullesbach E, Ashbaugh A, Linke MJ, Cushion M, Collins M, Ananthula HK, Sallans L, Desai PB, Wiederhold NP, Fothergill AW, Kirkpatrick WR, Patterson T, Wong LH, Sinha S, Giaever G, Nislow C, Flaherty P, Pan X, Cesar GV, de Melo Tavares P, Frases S, Miranda K, Rodrigues ML, Luberto C, Nimrichter L, Del Poeta M. 2015. Identification of a new class of antifungals targeting the synthesis of fungal sphingolipids. mBio 6:e00647-15. 10.1128/mBio.00647-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Khan SN, Khan S, Iqbal J, Khan R, Khan AU. 2017. Enhanced killing and antibiofilm activity of encapsulated cinnamaldehyde against Candida albicans. Front Microbiol 8:1641. 10.3389/fmicb.2017.01641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Walker CA, Gómez BL, Mora-Montes HM, Mackenzie KS, Munro CA, Brown AJP, Gow NAR, Kibbler CC, Odds FC. 2010. Melanin externalization in candida albicans depends on cell wall chitin structures. Eukaryot Cell 9:1329–1342. 10.1128/EC.00051-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schwarz P, Dromer F, Lortholary O, Dannaoui E. 2006. Efficacy of amphotericin B in combination with flucytosine against flucytosine-susceptible or flucytosine-resistant isolates of Cryptococcus neoformans during disseminated murine cryptococcosis. Antimicrob Agents Chemother 50:113–120. 10.1128/AAC.50.1.113-120.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Han Y. 2010. Efficacy of combination immunotherapy of IgM MAb B6.1 and amphotericin B against disseminated candidiasis. Int Immunopharmacol 10:1526–1531. 10.1016/j.intimp.2010.08.027. [DOI] [PubMed] [Google Scholar]
- 53.Liedke SCSC, Miranda DZDZ, Gomes KXKX, Gonçalves J, Frases S, Nosanchuk JDJD, Rodrigues MLML, Nimrichter L, Peralta JMJM, Guimarães AJAJ. 2017. Characterization of the antifungal functions of a WGA-Fc (IgG2a) fusion protein binding to cell wall chitin oligomers. Sci Rep 7:12187. 10.1038/s41598-017-12540-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Figueiredo ABC, Rodrigues ML, Conte FP, Fonseca FL, Arissawa M. 5 August 2021. Antibody, related use, pharmaceutical composition including method for diagnosing fungal infections, fungal infection diagnosis kit and method for treating fungal infections. Brazil patent application. [Google Scholar]