ABSTRACT
We evaluated antibiotic activity against the intracellular bacterium Coxiella burnetii using an activated THP-1 cell model of infection. At clinically relevant concentrations, the intracellular bacterial load was reduced 300-fold by levofloxacin and finafloxacin, 40-fold by doxycycline, and 4-fold by ciprofloxacin and was unaffected by azithromycin. Acidification of the culture medium reduced antibiotic activity, with the exceptions of doxycycline (no change) and finafloxacin (slight improvement). This model may be used to select antibiotics to be evaluated in vivo.
KEYWORDS: Coxiella, azithromycin, ciprofloxacin, doxycycline, finafloxacin, intracellular infection, levofloxacin
INTRODUCTION
Coxiella burnetii is the causative agent of Q fever, which can cause infections in humans following inhalation of contaminated aerosols (1). The first-line therapy is doxycycline (1), but azithromycin or the fluoroquinolones (known to accumulate in host cells) may constitute useful alternatives due to the obligate intracellular nature of this bacterium. In this work, we compared the intracellular activity of doxycycline with those of azithromycin and of three fluoroquinolones (ciprofloxacin, levofloxacin, and finafloxacin) in a model of activated THP-1 cells infected by the attenuated variant of C. burnetii Nine Mile, phase II RSA439, clone 4 (2). This strain is a relevant surrogate for fully virulent phase I C. burnetii, with similar growth kinetics in THP-1 cells (3). C. burnetii thrives in acidic vacuoles, sharing similarities with phagolysosomes (4, 5), making relevant the comparison between finafloxacin, which shows lower MICs at acidic pH versus neutral pH against other bacterial species (related to a higher accumulation in bacteria at acidic pH), and conventional fluoroquinolones, which have better activity at neutral pH (6, 7). Moreover, because C. burnetii causes infection of the lungs, where the pH of the extravascular space is acidic (8), or results in endocarditis, which may be accompanied by metabolic acidosis (9), we compared infected cells incubated in medium at neutral pH to those incubated at acidic pH.
MICs were determined by microdilution in ACCM-2 medium according to a published method (10) and are reported in footnote g to Table 1. Phorbol myristate acetate (PMA)-differentiated THP-1 human monocytes were infected following a previously described protocol (11) (see Fig. S1 in the supplemental material for the development of the model), except that C. burnetii and the antibiotic stocks were diluted in RPMI supplemented with 10% fetal calf serum and 2 mM glutamine. Infected cells were exposed for 72 h to each antibiotic over a wide range of concentrations (0.003 to 100× the MIC) and then harvested, and the lysates were used to determine CFU counts following plating on ACCM-2 agar (10). The protein content was also determined. Data (expressed as change in CFU from the postphagocytosis inoculum, normalized to the protein content of the samples) were used to fit a Hill equation (see Fig. 1 for individual data for each antibiotic and Fig. S2 in the supplemental material for antibiotic comparisons) and calculate the pharmacodynamic parameters. The antibiotic efficacy (Emax and E at the maximum concentration of drug in serum [Cmax]) and apparent relative potency (Cs and C−1 log) were determined (Table 1). The parameters Emax, E at Cmax, Cs, and C−1 log are defined and described in more detail in the footnotes to Table 1.
TABLE 1.
Pharmacological parameters and statistical analysis of the concentration-response curves of antibiotics against Coxiella burnetii in activated THP-1 cells incubated in medium at neutral or acidic pHa
| Medium and drugb | Emaxc (Δlog CFU from time 0) | Ed at Cmaxe (Δlog CFU from time 0) |
Cs
f
|
C
−1 log
h
|
||
|---|---|---|---|---|---|---|
| ×MICg | mg/liter | ×MIC | mg/liter | |||
| Medium at pH 7.4 | ||||||
| DOX | −1.64 (−0.70 to −2.58) A | −1.61 (−0.71 to −2.5) A | 0.24 (−0.05 to 0.53) | 0.01 (−0.01 to 0.02) A | 2.47 (0.56 to 4.39) | 0.07 (0.02 to 0.13) A |
| AZI | −1.57 (3.39 to −6.5) A | +0.50 (0.25 to 0.75) B | 0.15 (−0.95 to 1.25) | 19.3 (−121.1 to 160.0) B | 1.31 (−0.74 to 3.35) | 169 (−94.1 to 428.8) B |
| CIP | −2.40 (−2.30 to −2.50) B | −0.59 (−0.48 to −0.70) C | 0.56 (0.31 to 0.81) | 1.12 (0.62 to 1.62) C | 2.42 (2.23 to 2.60) | 4.84 (4.47 to 5.21) C |
| LVX | −2.84 (−1.81 to −3.86) B | −2.54 (−2.32 to −3.39) D | 0.05 (−0.02 to 0.11) | 0.18 (−0.07 to 0.43) D | 0.18 (0.15 to 0.21) | 0.72 (0.61 to 0.84) D |
| FIN | −2.67 (−1.89 to −3.45) B | −2.50 (−1.88 to −3.13) D | 0.59 (−0.02 to 1.20) | 0.07 (−0.01 to 0.14) D | 3.35 (2.31 to 4.38) | 0.42 (0.29 to 0.55) D |
| Medium at pH 5.5 | ||||||
| DOX | −1.6 (−1.25 to −1.97)NS A | −1.56 (−1.23 to −1.89)NS A | 0.47 (−0.10 to 1.05) | 0.01NS (−0.01 to 0.03) A | 3.99NS (3.72 to 4.26) | 0.12NS (0.11 to 0.13) A |
| AZI | −1.55 (−1.01 to −2.09)NS A | +0.61 (0.23 to 0.98)NS B | 2.32* (−0.4 to 5.08) | 296.4* (−57.8 to 650.6) B | >8*** | >1,000*** B |
| CIP | −2.70 (−2.37 to −3.03)* B | +0.14 (−0.17 to 0.44)*** C | 1.78** (0.95 to 2.62) | 3.57** (1.90 to 5.23) C | 6.70*** (6.43 to 6.96) | 13.40*** (12.87 to 13.92) C |
| LVX | −2.96 (−2.32 to −3.60)NS B | −2.12 (−1.75 to −2.49)* D | 0.15** (0.09 to 0.21) | 0.59** (0.37 to 0.81) D | 0.56*** (0.44 to 0.67) | 2.24*** (1.78 to 2.70) D |
| FIN | −2.60 (−2.56 to −2.65)NS B | −2.54 (−2.45 to −2.64)NS E | 0.24 (−0.13 to 1.20) | 0.03NS (−0.02 to 0.08) A | 1.39* (−0.40 to 3.18) | 0.17* (−0.05 to 0.39) A |
Statistical analysis for comparison between antibiotics at a given pH was performed by one-way analysis of variance (ANOVA) with Tukey’s post hoc test. Values with different letters are different from one another (P < 0.05). Comparison of data for each antibiotic between media at pH 7.4 and 5.5 was performed by multiple t test. NS, not significant (P > 0.05); *, P < 0.05; **, P < 0.01; ***, P < 0.001. Statistical analysis for Cs and C−1 log was performed on their log10 values (symmetrical distribution). Data are shown as the mean (with 95% confidence interval in parentheses) from three independent experiments.
Abbreviations: DOX, doxycyline; AZI, azithromycin; CIP, ciprofloxacin; LVX, levofloxacin; FIN, finafloxacin.
Emax, maximal efficacy, shown as CFU reduction in log10 units (with confidence intervals in parentheses) at 72 h from the corresponding initial postphagocytosis bacterial inoculum as extrapolated from the Hill equation of the concentration-effect response for an infinitely large antibiotic concentration.
E, CFU reduction in log10 units (with confidence interval in parentheses) at 72 h from the corresponding initial postphagocytosis bacterial inoculum as calculated from the Hill equation of the concentration-effect response for an antibiotic concentration corresponding to the human Cmax.
Human Cmax values for selected doses (given in parentheses) are as follows: DOX, 2 mg/liter (100 mg twice a day); AZI, 0.4 mg/liter (500 mg once a day); CIP, 2.8 mg/liter (500 mg twice a day); LVX, 8.7 mg/liter (500 mg once a day); FIN, 9.0 mg/liter (800 mg once a day). The selected doses are based on the British National Formulary for inhalation anthrax (CIP and LVX), the recommended dose for Q fever (DOX) (1), and the conventional dose (AZI) or the dose used in clinical trials (FIN). Human Cmax values are based on EUCAST rationale documents (https://www.eucast.org/publications_and_documents/rd/) for CIP, LVX, DOX, and AZI or reference 18 for FIN.
Cs, static concentration, shown as extracellular antibiotic concentration (with confidence intervals in parentheses) resulting in no apparent bacterial growth from the postphagocytosis bacterial inoculum following 72 h of incubation, as calculated from the Hill equation of the concentration-response curve.
MICs are as follows: DOX, 0.03 mg/liter; AZI, >128 mg/liter (with 128 mg/liter taken as the MIC in calculations); CIP, 2 mg/liter; LVX, 4 mg/liter; FIN, 0.125 mg/liter.
C−1 log, extracellular antibiotic concentration (with confidence intervals in parentheses) resulting in a reduction of 90% of CFU (1 log10) from the postphagocytosis bacterial inoculum following 72 h of incubation, as calculated from the Hill equation of the concentration-response curve.
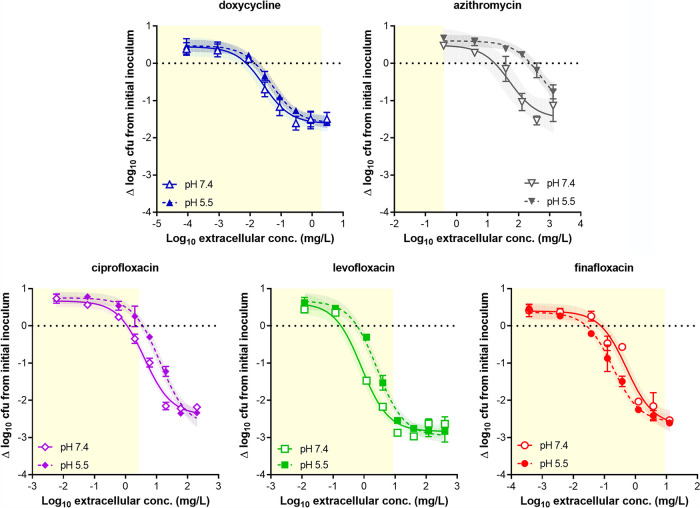
FIG 1.
Concentration-response curves of antibiotics against intracellular C. burnetii in a model of PMA-activated THP-1 human monocytes. The abscissa shows the extracellular concentrations. The ordinate shows the change in the number of CFU from the initial postphagocytosis inoculum following 72 h of incubation. The horizontal dotted line corresponds to the initial inoculum and allows the apparent static effect (static concentration [Cs]) of each antibiotic to be calculated. The data were used to fit Hill curves, represented with their 95% confidence interval. All data are means ± standard error of the mean (SEM) from 3 independent experiments. The yellow-shaded area corresponds to the zone of effects obtained at clinically relevant concentrations (see footnote e to Table 1 for Cmax values). Open symbols and solid lines indicate data from medium at pH 7.4, and closed symbols and dotted lines indicate data from medium at pH 5.5.
The study was approved by the Biosafety Office of the Université catholique de Louvain on 22 May 2019.
Following 72 h of incubation, all antibiotics demonstrated concentration-dependent killing, with azithromycin and doxycycline resulting in a lower (less negative value) Emax (reduction of ∼1.5 log10 CFU) than the fluoroquinolones (reduction of ∼2.5 log10 CFU). Emax was not influenced by the pH of the medium. At an extracellular concentration corresponding to the human Cmax of each antibiotic, levofloxacin and finafloxacin were the most effective in medium at neutral pH, causing a reduction in CFU close to their respective Emax values, followed by doxycycline, ciprofloxacin, and azithromycin, which was ineffective. In acidic medium, the efficacies of finafloxacin and doxycycline remained unaffected, while those of ciprofloxacin and levofloxacin were reduced. Cs was lower than the MIC for all drugs in medium at neutral pH and shifted to significantly higher values at acidic pH for azithromycin, ciprofloxacin, and levofloxacin. The concentration required to result in a 90% reduction in bacterial load (C−1 log) was achieved at clinically relevant concentrations for doxycycline, levofloxacin, and finafloxacin in medium at neutral pH. In acidic medium, the C−1 log values were shifted higher for azithromycin, ciprofloxacin, and levofloxacin, but the value was lower for finafloxacin.
Although C. burnetii is an intracellular pathogen, few studies have examined antibiotic activity intracellularly, and activity has been studied only semiquantitatively or under a few conditions of exposure (11–14). Thanks to the establishment of appropriate axenic cultures in liquid or solid medium (10), we were able to quantify C. burnetii intracellularly under control conditions and following exposure to selected antibiotics.
We found that fluoroquinolones were the most effective drugs among those evaluated, with levofloxacin and finafloxacin (but not ciprofloxacin) able to reduce the intracellular bacterial inoculum 400-fold at clinically relevant concentrations. This is probably related to their known bactericidal activity, as previously suggested (14). The reason why ciprofloxacin was less active in this model remains to be established, but these data are consistent with its recently demonstrated inactivity in vivo, contrasting with the remarkable efficacy of levofloxacin (11). Levofloxacin and finafloxacin also displayed similar relative potencies intracellularly, although we failed to determine a possible advantage of finafloxacin, despite its lower MIC. One possible explanation resides in the lower accumulation of finafloxacin versus levofloxacin in THP-1 cells (2.5-fold versus 10-fold, respectively) (7, 15). This hypothesis is further substantiated by the opposite effect of the pH of the culture medium on the intracellular potency of finafloxacin versus the conventional fluoroquinolones. Finafloxacin is the only fluoroquinolone for which potency was increased in acidic medium (concentration-response curve shifted to the left), most probably because its uptake is increased in THP-1 cells at acidic pH (related to a higher proportion of the zwitterionic form) (see Table S1 in the supplemental material), as opposed to that of other fluoroquinolones (7).
Doxycycline, considered a first line of treatment, was less effective in our model, but remained highly potent, as it was still capable of reducing the intracellular bacterial inoculum 40-fold at clinically relevant concentrations. Previous work showed that the potency of doxycycline could be further increased by neutralizing infected vacuoles (12, 13). However, acidification of the extracellular medium did not affect its intracellular activity, suggesting that its uptake mechanism in THP-1 cells is pH independent in the range of pH values investigated, below the isoelectric point of this molecule (Table S1). Finally, azithromycin was inactive both in axenic culture and intracellularly, despite its high cellular accumulation. The potency of azithromycin is markedly reduced in acidic environments (16), where it is fully protonated (Table S1); this includes the vacuoles where C. burnetii thrives. Its uptake by THP-1 cells is reduced in acidic medium (16), explaining the even lower potency observed under these conditions.
In conclusion, we have demonstrated the utility of a C. burnetii in vitro intracellular infection model that has allowed pharmacological comparisons of antibiotic activity. The data collected for doxycycline, ciprofloxacin, and levofloxacin are consistent with those obtained in vivo with the phase I virulent variant RSA493 (11, 17), suggesting that the attenuated variant, phase II, is an appropriate, easier-to-handle surrogate for larger-scale screening of novel Q fever therapeutics.
Data availability.
Data will be made available upon request via the corresponding author.
ACKNOWLEDGMENTS
We thank Merlion Pharmaceuticals, Berlin, Germany, for the kind gift of finafloxacin powder. Vasileios Yfantis provided excellent technical assistance.
This work was supported by a grant from the Defense Science and Technology Laboratory, UK, and the Belgian Fonds de la Recherche Scientifique (FRS-FNRS [grant T.0189.16]).
F.V.B. is the Research Director of the Belgian Fonds de la Recherche Scientifique (FRS-FNRS).
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.Eldin C, Melenotte C, Mediannikov O, Ghigo E, Million M, Edouard S, Mege JL, Maurin M, Raoult D. 2017. From Q fever to Coxiella burnetii infection: a paradigm change. Clin Microbiol Rev 30:115–190. 10.1128/CMR.00045-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moos A, Hackstadt T. 1987. Comparative virulence of intra- and interstrain lipopolysaccharide variants of Coxiella burnetii in the guinea pig model. Infect Immun 55:1144–1150. 10.1128/iai.55.5.1144-1150.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Howe D, Shannon JG, Winfree S, Dorward DW, Heinzen RA. 2010. Coxiella burnetii phase I and II variants replicate with similar kinetics in degradative phagolysosome-like compartments of human macrophages. Infect Immun 78:3465–3474. 10.1128/IAI.00406-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shaw EI, Voth DE. 2019. Coxiella burnetii: a pathogenic intracellular acidophile. Microbiology (Reading) 165:1–3. 10.1099/mic.0.000707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heinzen RA, Hackstadt T, Samuel JE. 1999. Developmental biology of Coxiella burnetii. Trends Microbiol 7:149–154. 10.1016/S0966-842X(99)01475-4. [DOI] [PubMed] [Google Scholar]
- 6.Chalhoub H, Harding SV, Tulkens PM, Van Bambeke F. 2020. Influence of pH on the activity of finafloxacin against extracellular and intracellular Burkholderia thailandensis, Yersinia pseudotuberculosis and Francisella philomiragia and on its cellular pharmacokinetics in THP-1 monocytes. Clin Microbiol Infect 26:1254.e1–1254.e8. 10.1016/j.cmi.2019.07.028. [DOI] [PubMed] [Google Scholar]
- 7.Lemaire S, Van Bambeke F, Tulkens PM. 2011. Activity of finafloxacin, a novel fluoroquinolone with increased activity at acid pH, towards extracellular and intracellular Staphylococcus aureus, Listeria monocytogenes and Legionella pneumophila. Int J Antimicrob Agents 38:52–59. 10.1016/j.ijantimicag.2011.03.002. [DOI] [PubMed] [Google Scholar]
- 8.Effros RM, Chinard FP. 1969. The in vivo pH of the extravascular space of the lung. J Clin Invest 48:1983–1996. 10.1172/JCI106164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stevenson S, Gowardman J, Tozer S, Woods M. 2015. Life-threatening Q fever infection following exposure to kangaroos and wallabies. BMJ Case Rep 2015:10. 10.1136/bcr-2015-210808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clay KA, Hartley MG, Russell P, Norville IH. 2018. Use of axenic media to determine antibiotic efficacy against Coxiella burnetii. Int J Antimicrob Agents 51:806–808. 10.1016/j.ijantimicag.2017.08.006. [DOI] [PubMed] [Google Scholar]
- 11.Clay KA, Hartley MG, Armstrong S, Bewley KR, Godwin K, Rayner E, Vipond J, Bailey M, Atkins TP, Norville IH. 2021. Evaluation of the efficacy of doxycycline, ciprofloxacin, levofloxacin and cotrimoxazole using in vitro and in vivo models of Q fever. Antimicrob Agents Chemother 10.1128/AAC.00673-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gikas A, Spyridaki I, Scoulica E, Psaroulaki A, Tselentis Y. 2001. In vitro susceptibility of Coxiella burnetii to linezolid in comparison with its susceptibilities to quinolones, doxycycline, and clarithromycin. Antimicrob Agents Chemother 45:3276–3278. 10.1128/AAC.45.11.3276-3278.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maurin M, Benoliel AM, Bongrand P, Raoult D. 1992. Phagolysosomal alkalinization and the bactericidal effect of antibiotics: the Coxiella burnetii paradigm. J Infect Dis 166:1097–1102. 10.1093/infdis/166.5.1097. [DOI] [PubMed] [Google Scholar]
- 14.Yeaman MR, Mitscher LA, Baca OG. 1987. In vitro susceptibility of Coxiella burnetii to antibiotics, including several quinolones. Antimicrob Agents Chemother 31:1079–1084. 10.1128/AAC.31.7.1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Van de Velde S, Nguyen HA, Van Bambeke F, Tulkens PM, Grellet J, Dubois V, Quentin C, Saux MC. 2008. Contrasting effects of human THP-1 cell differentiation on levofloxacin and moxifloxacin intracellular accumulation and activity against Staphylococcus aureus and Listeria monocytogenes. J Antimicrob Chemother 62:518–521. 10.1093/jac/dkn232. [DOI] [PubMed] [Google Scholar]
- 16.Lemaire S, Van Bambeke F, Tulkens PM. 2009. Cellular accumulation and pharmacodynamic evaluation of the intracellular activity of CEM-101, a novel fluoroketolide, against Staphylococcus aureus, Listeria monocytogenes, and Legionella pneumophila in human THP-1 macrophages. Antimicrob Agents Chemother 53:3734–3743. 10.1128/AAC.00203-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Norville IH, Hatch GJ, Bewley KR, Atkinson DJ, Hamblin KA, Blanchard JD, Armstrong SJ, Pitman JK, Rayner E, Hall G, Vipond J, Atkins TP. 2014. Efficacy of liposome-encapsulated ciprofloxacin in a murine model of Q fever. Antimicrob Agents Chemother 58:5510–5518. 10.1128/AAC.03443-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Patel H, Andresen A, Vente A, Heilmann HD, Stubbings W, Seiberling M, Lopez-Lazaro L, Pokorny R, Labischinski H. 2011. Human pharmacokinetics and safety profile of finafloxacin, a new fluoroquinolone antibiotic, in healthy volunteers. Antimicrob Agents Chemother 55:4386–4393. 10.1128/AAC.00832-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental figures and table. Download aac.01061-21-s0001.pdf, PDF file, 0.05 MB (51.8KB, pdf)
Data Availability Statement
Data will be made available upon request via the corresponding author.