ABSTRACT
Infections with enteropathogenic Escherichia coli (EPEC) cause severe diarrhea in children. The noninvasive bacteria adhere to enterocytes of the small intestine and use a type III secretion system (T3SS) to inject effector proteins into host cells to modify and exploit cellular processes in favor of bacterial survival and replication. Several studies have shown that the T3SSs of bacterial pathogens are essential for virulence. Furthermore, the loss of T3SS-mediated effector translocation results in increased immune recognition and clearance of the bacteria. The T3SS is, therefore, considered a promising target for antivirulence strategies and novel therapeutics development. Here, we report the results of a high-throughput screening assay based on the translocation of the EPEC effector protein Tir (translocated intimin receptor). Using this assay, we screened more than 13,000 small molecular compounds of six different compound libraries and identified three substances which showed a significant dose-dependent effect on translocation without adverse effects on bacterial or eukaryotic cell viability. In addition, these substances reduced bacterial binding to host cells, effector-dependent cell detachment, and abolished attaching and effacing lesion formation without affecting the expression of components of the T3SS or associated effector proteins. Moreover, no effects of the inhibitors on bacterial motility or Shiga-toxin expression were observed. In summary, we have identified three new compounds that strongly inhibit T3SS-mediated translocation of effectors into mammalian cells, which could be valuable as lead substances for treating EPEC and enterohemorrhagic E. coli infections.
KEYWORDS: EHEC, EPEC, T3SS, virulence inhibitors, antivirulence strategy, small-molecule inhibitors
INTRODUCTION
Infection with enteropathogenic Escherichia coli (EPEC) is a major cause of infantile diarrhea in children in developing countries (1). EPEC is a member of the family of attaching and effacing (A/E) pathogens, which also includes enterohemorrhagic E. coli (EHEC) and the mouse pathogen Citrobacter rodentium. The bacteria in this family intimately adhere to the surface of enterocytes by inducing the formation of characteristic actin-rich pedestals (attachment) and the loss of microvilli (effacement) (2). Subversion of the host actin signaling is mediated by the injection of bacterial effector proteins via a type III secretion system (T3SS). Both the components of the T3SS, as well as effectors involved in A/E lesion formation, are encoded on the locus of enterocyte effacement (LEE) pathogenicity island located in the bacterial genome (3, 4).
The T3SS of EPEC consists of the basal body, needle, filament and translocon pore (5). The needle is formed by multiple copies of EscF, which form a hollow tube (6) essential for T3S and thus for virulence (7). It is associated on one end with the basal body that powers the translocation and on the other end with a filament made of EspA multimers (6). Together, needle and filament form a channel that upon host cell contact connects the bacterial cytosol with the host cell membrane and initiates the formation of the translocation pore. The translocon pore is formed by heterooligomers of EspB and EspD subunits that are translocated through the channel to the top of which they bind. This complex then inserts into the host cell membrane to form the pore for effector translocation (8). Between 25 and 50 effector proteins are translocated directly into the host cell cytosol by this T3SS where they manipulate a set of host cell processes important for the establishment of the infection (9, 10). Most translocated effector proteins that are essential for intimate attachment and pathogenesis are located on the LEE. The translocated intimin receptor (Tir) is the most important and best-studied effector protein of EPEC. After translocation, it inserts into the host cell membrane and acts as a receptor for the bacterial outer membrane protein intimin (11, 12). Other LEE-encoded effectors (EspH, EspF, and EspG) and non-LEE-encoded effectors (including EspM, EspT-EspW) manipulate actin, inhibit phagocytosis and protein secretion, and induce cell death (10). EspZ restricts translocation of other effector proteins through the T3SS, and its loss results in aberrant protein translocation and host cell detachment (13). Many of the effectors encoded on minor pathogenicity islands (NleA-NleH) are essential for bacterial survival during infection, as they subvert host innate immune signaling and cell death pathways (9, 10). Importantly, EPEC strains with a nonfunctional secretion system, such as ΔescN and ΔespA mutants, are unable to intimately adhere to enterocytes and were shown to be avirulent in infection models (7).
T3SSs are highly conserved and shared by more than 25 human, plant and zoonotic Gram-negative pathogens (14). Loss of a functional T3SS was also linked with avirulence in the human enteric pathogens Salmonella, Yersinia and Shigella sp. (14). High conservation among the structural components of the T3SSs (14) makes it likely that inhibitors may be found that target the T3SS of more than one bacterial pathogen. In addition, off-target effects of inhibitors against the T3SS, such as destruction of protective members of the microbiota should be rare, given as T3SSs are only found in pathogenic bacteria and symbionts. Moreover, the development of resistance mutations to cope with the stresses produced by an inhibitor of the T3SS is less likely as such a substance does not affect bacterial viability or structural integrity (15).
A growing number of studies have identified promising inhibitory compounds that target the T3SS of pathogenic bacteria (16–20). While several, such as Aurodox (21), were shown to be selective in inhibiting the T3SS of only one genus, other compounds, including the salicylidene acylhydrazides, showed a broader specificity (22–25). Furthermore, some compounds targeted not only the T3SSs but also bacterial motility (26, 27), suggesting that they affect a conserved target in the basal structure shared between virulence-associated T3SSs and the flagellar export system (28).
Here, we used a high throughput screen to identify substances that inhibit T3S-mediated Tir-effector translocation from EPEC into eukaryotic cells. We identified three promising substances that were able to inhibit Tir translocation into host cells without adverse effects on bacterial or eukaryotic cell viability. We were able to show that the inhibitors interfered with intimate bacterial attachment as well as T3SS-dependent cell detachment in response to infection with an EPEC ΔespZ mutant exhibiting an uncontrolled high effector translocation. The inhibitors did not affect the amount of LEE-encoded protein expression or motility.
RESULTS
Screening of natural and chemical compound libraries identified substances that interfere with effector translocation.
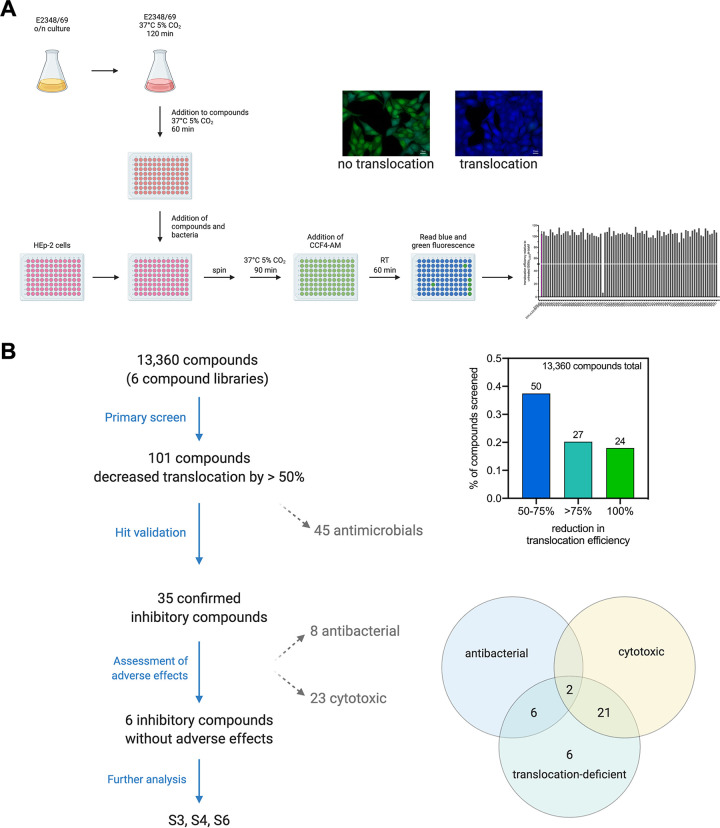
Translocation of the EPEC effector protein Tir into the host cell was monitored using an EPEC E2348/69 strain expressing a Tir-β-lactamase (TEM) fusion protein encoded under the native promoter in the EPEC genome (29) and the β-lactamase fluorescence resonance energy transfer substrate CCF4-AM, which contains the coumarin- and fluorescein-conjugated β-lactam cephalosporin and is green fluorescent. Using a 96-well plate setup, cells were seeded and subsequently infected with EPEC E2348/69 wild type or E2346/69 PLEE5tir-blaM. The bacteria were first grown in T3SS-inducing conditions for 2 h and then preincubated in the presence of the screening substances (see Table 1 for concentrations) for 1 h before infection. If translocated into the host cells, the β-lactamase cleaves the CCF4-AM substrate, changing its fluorescence signal from green to blue, which can be quantified in a microtiter plate reader (Fig. 1A).
TABLE 1.
Screened natural and chemical compound libraries
| Compound library | Source | No. of compounds | Stock solution concn [mM] | Remarks |
|---|---|---|---|---|
| NCh + ExNCl | HIPS, Univ. Tübingen via DZIF | 264 + 352 | 1 and 10 | Microbial secondary metabolites |
| SPECS | Specs, The Netherlands | 2171 | 10 | Chemical synthesis |
| LOPAC | Sigma | 1408 | 10 | Known bioactive compounds |
| Var | Various research groups | 2933 | 5 or 10 | Chemical synthesis |
| EMC | EMC microcollections GmbH, Tübingen | 6232 | 1 | Chemical synthesis |
FIG 1.
Illustration of inhibitor screen setup and screen summary. For the translocation assay, bacteria were grown overnight in Luria Bertani (LB) broth and diluted 1:50 into Dulbecco’s modified Eagle medium (DMEM). The infection cultures were grown under type III secretion system-inducing conditions (37°C, 5% CO2) for 2 h and subsequently added to 96-well plates containing one μl of the screen substances per well. The bacteria were incubated for an additional hour in the presence of substances before being added to a 96-well plate containing HEp-2 cells. Infection was synchronized by centrifugation. Cells were infected in the presence of inhibitors for 1.5 h. Bacteria were removed and cells stained with the fluorescence resonance energy transfer substrate CCF4-AM for 1 h. Translocation efficiency was assessed by measuring blue and green fluorescence (A). Of all the libraries screened, compounds that reduced the translocation by more than 50% in the initial screen were further assessed for cytotoxic and antibacterial side effects. Three inhibitory compounds without adverse effects were used for further analysis (B). Figures were created with BioRender.com.
Using this assay, we screened two natural and four chemical compound libraries with a total of 13,360 substances (Table 1; Table S1 in the supplemental material). The z'-values ranged between 0.5 and 0.94 with an average z'-value of 0.76 and an average standard to noise ratio (S/N) of 31.8. Of the tested substances, 50 reduced translocation efficiency by 50 to 75%, while 27 were able to reduce the translocation efficiency of Tir-TEM by more than 75% compared with the control (translocation by untreated E2346/69 PLEE5tir-blaM). Twenty-four compounds showed a reduction of 100% (Fig. 1B; Fig. S1 and Table S1 in the supplemental material). The latter commonly corresponded to known or published antimicrobial substances, including chloramphenicol and rifampin, thuggacin (30–32), tartrolon (33, 34), as well as derivatives of myxovirescin (35), myxovalargin (36, 37), and sorangicin (38). In total, 45 of the primary hit compounds were identified as antimicrobials or compounds with structural homology to known antibiotics and were thus excluded from further analyses.
Effects of primary hit substances on bacterial growth and cell viability.
The remaining 56 inhibitory substances that were neither published antibiotics nor showed structural homology to antibiotics were then tested for their dose-dependent effect on bacterial growth behavior. For this, overnight cultures of E2348/69 wild type were diluted to an OD600 of 0.02 and grown in the presence of increasing concentrations of each substance (2.5–50 μM) at 37°C without shaking for 8 h. At regular intervals (every 2 h), the optical density of each sample was measured. Of the 56 compounds retested in dose-response assays, only 35 were confirmed to inhibit translocation. Eight of these substances showed an antibacterial effect, reducing the growth of EPEC E2348/69 by >75% at the highest tested concentration (50 μM) (Fig. 1B).
To further determine whether the inhibitory substances themselves had any effect on the health of the eukaryotic cells (HEp-2), these were seeded in the presence of the substances and incubated at 37°C with 5% CO2 for 3 days. Subsequently, the cell viability was determined by XTT assay. Here, 23 of the tested substances showed marked effects on cellular viability of which two were also shown to have antibiotic activity (Fig. 1B). In summary, of all tested substances that were neither antimicrobial nor cytotoxic to eukaryotic cells, only six were able to inhibit translocation repeatedly.
Dose-response assays confirmed three substances that inhibit effector translocation without cytotoxicity to eukaryotic cells or EPEC.
The six substances, which were able to inhibit T3SS-mediated translocation of the Tir-TEM fusion repeatedly, were purchased or acquired from their respective sources to allow for further analyses. Repetition of the growth, cell viability and translocation assays with the freshly prepared substances demonstrated that three substances (S3 and S4 from SPECS and S6 from the Var library) robustly inhibited Tir-TEM translocation in a dose-dependent manner (Fig. 2A) with no adverse effects on bacterial or eukaryotic cell viability (Fig. S2 in the supplemental material), with their ID50 values given in Table 2. The inhibitory effect of the other three compounds (S1, S2, and S5) could not be reproduced with a new batch of the substances in the same manner (data not shown).
FIG 2.
Three substances of the initial high-throughput screen inhibit translocated intimin receptor (Tir) translocation in a dose-dependent manner. Translocation assays were carried out as described for the high throughput screen. The dose-dependent translocation of Tir-β-lactamase in the presence of the three inhibitory substances identified in the high throughput screen was determined with purchased substances. The ratio of blue/green fluorescence was determined, and values are given as mean (± SEM) of two independent experiments. Curves are analyzed in GraphPad Prism using a nonlinear regression fit, variable slope (four parameters) with 95% confidence interval (A). Chemical structures of the three substances (drawn using ChemDraw) (B).
TABLE 2.
IC50 values for translocation efficiency of translocated intimin receptor (Tir)-β-lactamase as determined with GraphPad Prism using a non-linear regression fit, variable slope (four parameters) with 95% confidence interval
| Compound | IC50 [μM] |
|---|---|
| S3 | ± 33.77 |
| S4 | ± 8.207 |
| S6 | ± 9.347 |
To ensure that the observed inhibition of translocation is not due to an effect on the host cells, preventing host-pathogen interaction, HEp-2 cells were pretreated with the compounds prior to infection. As shown in Fig. S3 in the supplemental material, no inhibition of Tir-TEM translocation was observed.
The chemical structures of the three identified substances are shown in Fig. 2B. The substances can be categorized as follows: S3 is a 1,2,4-triazine-5-one, S4 is a p-methoxy-hydrocinnamamide, and substance S6 is a 3-chloroquinoxalin-2(1H)-one 4-oxide.
Intimate attachment of EPEC to eukaryotic cells was impaired in the presence of compounds.
T3SS-mediated translocation of Tir is followed by its integration into the host cell membrane and interaction with the bacterial outer membrane protein intimin. This induces a phosphorylation cascade that results in the accumulation of F-actin underneath the adherent bacteria and the formation of actin-rich pedestals upon which the bacteria reside (2). HEp-2 cells seeded 1 day before infection were infected with fluorescently-labeled EPEC strain E2348/69 (E2348/69 pPgapdhamCyan) as described for the translocation assay. Cells were fixed after 1.5 h of infection, and actin pedestals were visualized by fluorescent actin staining (FAS) using phalloidin. While cells infected with wild-type EPEC in the presence of dimethyl sulfoxide (DMSO) (negative control) showed the characteristic actin aggregation where bacteria were attached to the cells, no microcolonies or pedestals could be observed in cells treated with either of the compounds (Fig. 3; Fig. S4 in the supplemental material). For these and following assays, the concentrations of the compounds (50 μM S3 or S4, or 25 μM S6) which showed maximal inhibition of T3SS effector translocation without adverse effects (see Fig. 2A; Fig. S2) was used. In the presence of the compounds, the majority of the bacteria still visible on the cells were not intimately attached to the surface of the cells, no pedestals were formed (Fig. 3). No obvious differences were observed between the different compounds. This confirmed previous results showing that all three substances blocked Tir translocation, a prerequisite for pedestal formation.
FIG 3.

Intimate attachment of enteropathogenic Escherichia coli (EPEC) is abolished in the presence of inhibitory substances. HEp-2 cells were infected with EPEC strain E2348/69 pPgapdhamCyan (green) in the presence of either inhibitory substances (50 μM S3 or S4, 25 μM S6) or dimethyl sulfoxide (DMSO) (control) for 1.5 h. After rinsing with Dulbecco’s phosphate-buffered saline (DPBS), cells were fixed, permeabilized and stained with tetramethyl rhodamine isocyanate-Phalloidin to visualize F-actin (red). Coverslips were mounted with ProLong Diamond mounting medium containing DAPI (4′,6-diamidino-2-phenylindole) for staining of DNA (blue). Cells were visualized using a Keyence Biorevo BZ-9000 microscope and an overlay of representative fluorescence microscopy images of infected and uninfected cells are shown. White arrowheads indicate areas with bacteria forming pedestals. Scale bars depict 10 μm.
Substances inhibited translocation-dependent host cell detachment.
In a previous study, Berger et al. identified EspZ as essential for regulating T3SS-dependent protein translocation (13). They showed that a ΔespZ mutant induced host cell detachment and cell death due to uncontrolled high effector translocation into host cells (13). Here, we used infection of HeLa cells with an EPEC E2348/69 ΔespZ mutant in the presence of inhibitors or DMSO to assess whether the inhibitors could block uncontrolled translocation of effector proteins into the host cell and thus inhibit the described cell detachment phenotype. Although infection of HeLa cells with wild-type EPEC or the T3SS mutant ΔescN had little effect on cell loss, only approximately 15% of cells remained attached after infection with the ΔespZ mutant in the presence of DMSO (Fig. 4A and B). Interestingly, although cell detachment was inhibited in the presence of inhibitors 50 μM S4 or 25 μM S6, which had an average of ∼98% and ∼108% of adherent cells compared with uninfected, 50 μM S3 had a less significant effect on cell loss compared with DMSO-treated cells, with ∼75% of cells remaining after infection (Fig. 4A and B; Fig. S5 in the supplemental material).
FIG 4.
Substances inhibit effector-mediated cell loss. Cells were infected with an enteropathogenic Escherichia coli (EPEC) E2348/69 ΔespZ mutant in the presence of either 50 μM S3 or S4 and 25 μM S6 or an equivalent amount of dimethyl sulfoxide (DMSO) for 3 h. Cells were thoroughly washed with Dulbecco’s phosphate-buffered saline (DPBS) five times every hour. The number of adherent cells after infection was determined by trypsinizing remaining cells at the end of the experiment and counting them in a hemocytometer. The number of cells in uninfected, untreated samples was set to 100%. Shown are the mean values (±SEM) of adherent cells determined in three independent experiments performed at least in triplicate. Statistical analysis was performed using the Kruskal-Wallis test followed by Dunn's multiple-comparison test (*P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001), with all treated groups compared to DMSO-treated, wild-type EPEC E2348/69-infected control cells (A). To visualize the amount of cells remaining after 3 h, cells were fixed and stained with Hematoxylin Eosin staining solution. Cells were visualized using a Keyence Biorevo BZ-9000 (B). Pictures are representative of three independent experiments conducted in triplicate.
Expression of LEE-encoded proteins remained unaffected in the presence of the inhibitory compounds.
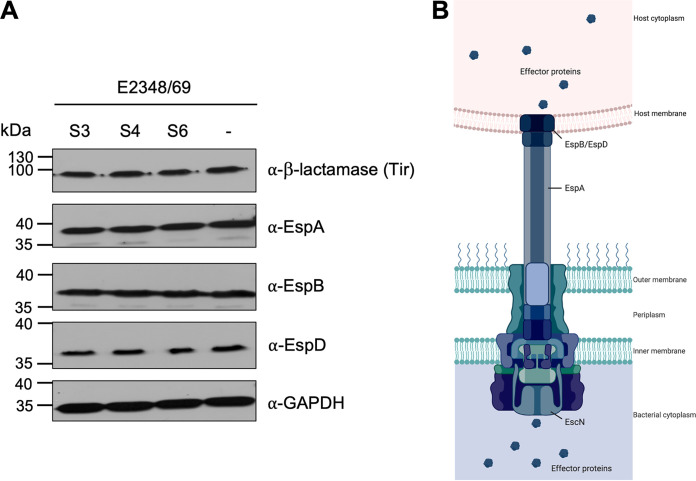
The observed effect of the inhibitors on T3SS-mediated translocation of effector proteins such as Tir may be due to a direct effect on the function of the T3S machinery or an indirect inhibition of the process. An indirect effect could reduce the amount of LEE-proteins (either structural components of the T3SS system or translocated effector proteins) expressed in the presence of the substances. To determine whether such an effect on protein expression could be detected, bacteria were grown under T3S-inducing conditions in the presence of Tir translocation-inhibiting concentrations of the inhibitors (50 μM S3 or S4, and 25 μM S6; Fig. 2A), and bacterial cell lysates were assessed by immunoblotting. As can be seen in Fig. 5, the expression levels of the structural components EspA, EspB and EspD as well as the effector protein Tir (fused to β-lactamase as described above) were comparable in inhibitor-treated bacteria and the DMSO-treated negative control (-) (Fig. 5). We further tested whether type III-mediated secretion of the effectors is affected in the presence of the inhibitors, but the amount of effectors secreted into the supernatant remained unchanged (Fig. S6 in the supplemental material). This indicated that either the assembly or the translocation function of the effectors of the T3S machinery is inhibited by the compounds.
FIG 5.
The expression levels of locus of enterocyte effacement (LEE) pathogenicity island-encoded type III secretion system (T3SS) proteins are unaffected by the translocation inhibitors. Cell lysates of bacteria grown under T3SS-inducing conditions (37°C, 5% CO2) for 3 h in the presence of 50 μM S3 or S4, or 25 μM S6. Equalized for cell numbers were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis. The protein expression levels of the proteins EspA, EspB and EspD, which make up the T3SS filament and translocon pore, as well as the level of the translocated intimin receptor (Tir)-β-lactamase fusion protein were evaluated by immunoblotting. Depicted blots are representative of at least three independent experiments (A). The schematic of the T3SS shows the localization of the analyzed components in the T3SS complex. The figure was created with BioRender.com (B).
The hemolytic activity of EPEC is affected differently by the different inhibitors.
T3SS-dependent hemolysis of sheep red blood cells (RBCs) was described for EPEC (39) and has been attributed to the formation of the T3SS translocon pore in the erythrocyte membrane (8). In a previous study, Kimura et al. used this phenotype in their high-throughput screen, which identified Aurodox (21). Here, RBCs were infected with EPEC wild type in the presence or absence of inhibitors and hemolysis was determined after 2 h. A ΔescN mutant was used as a control for the T3SS-mediated effect, uninfected RBCs were used as a negative, and saponin-treated cells as a positive control. S3 had no effect on hemolysis, while S4 and S6 significantly inhibited hemolysis by ∼20% and ∼10%, respectively, compared with DMSO-treated cells (Fig. 6). This indicated that S3 does not seem to interfere with the assembly and translocon pore formation by the T3SS system.
FIG 6.
Hemolysis of sheep red blood cells (RBC) was reduced in the presence of S4 and S6 but not by S3. A 3% RBC suspension was incubated with 1 × 108 bacteria in the presence of decreasing concentrations of the inhibitors or dimethyl sulfoxide (DMSO) for 2 h. Cells were pelleted, supernatants transferred to 96-well plates, and the amount of hemoglobin released from the cells was determined by measuring absorbance at 543 nm in a plate reader. Shown are the mean values (±SEM) of the percentage of hemolysis inhibition determined in three independent experiments performed in triplicate with all treated groups compared to DMSO-treated, wild-type-infected control cells. Statistical analysis was performed via one-way ANOVA with Geisser-Greenhouse correction followed by Dunnett’s multiple-comparison test (*P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001).
Compounds showed no effect on bacterial motility.
Due to the high homology between the basal bodies of the T3SS and flagella (28), a possible effect of the identified compounds on bacterial motility was investigated. Bacterial motility assays were conducted using Luria Bertani (LB; Carl Roth, Germany) and Dulbecco’s modified Eagle medium (DMEM) soft agar plates supplemented with 50 μM each substance. As the motility diameter indicates (Fig. 7), bacteria were more motile on LB plates (∼15 mm) compared with on DMEM plates (∼4 mm). Nevertheless, none of the investigated compounds affected bacterial motility when compared to the negative control (DMSO; Fig. 7). Bacterial growth in both LB and DMEM was confirmed to be unaffected under the respective conditions (Fig. S7 in the supplemental material).
FIG 7.

Type III secretion system (T3SS) inhibiting compounds S3, S4 and S6 do not affect the flagellar T3SS. Bacterial motility was assessed by stab-inoculating bacteria into Luria Bertani (LB)- or Dulbecco’s modified Eagle medium (DMEM)-motility agar containing inhibitors (50 μM S3 or S4, 25 μM S6) or dimethyl sulfoxide (DMSO). After 6 h (LB; A), 24 h and 48 h (DMEM; B) the diameters of the motility halo were measured. All assays were carried out three times in triplicate. Differences in diameter in the presence of substances compared to DMSO were not significant. Statistical analysis was performed using the Kruskal-Wallis test followed by Dunn's multiple-comparison test.
Inhibitory substances did not induce Shiga toxin production in reporter-gene assays.
Previous results of this study suggest that S3, S4, and S6 are effective compounds suppressing the translocation of crucial virulence factors by the T3SS expressed by EPEC but also by EHEC. This could make them promising candidates for the treatment or the prevention of bloody diarrhea and the development of hemolytic-uremic syndrome in infected patients (40, 41), which is associated with the expression of the Shiga toxin (Stx) by EHEC. As many antimicrobial compounds, that induce DNA damage also induce the expression of the Shiga toxin genes (stx) which are encoded on a lambdoid prophage, we wanted to ensure that the induction of toxin expression in response to the identified inhibitors can be excluded. To test this, Shiga toxin expression was assessed using Gaussia luciferase reporter gene assays in both, E. coli K12 (C600) and Citrobacter rodentium (DBS100) strains encoding the Gaussia luciferase gene (Gluc) under the control of the respective stx2 promoters (42). Ciprofloxacin, a potent inducer of Shiga toxin expression, was used as a positive control. As expected, reporter strains treated with ciprofloxacin showed a strong and significant induction of luciferase expression (Fig. 8A and B). In contrast, no or only a very small, negligible activation of the luciferase reporter was detectable in E. coli K-12 ϕstx2a::Gluc (Fig. 8A) and C. rodentium ϕstx2dact::Gluc (Fig. 8B) when treated with tested maximum concentrations of the inhibitors (50 μM for S3 and S4, 25 μM for S6) compared with DMSO-treated control samples. Therefore, it can be concluded that neither of the inhibitors affects Shiga toxin expression, making them safe not only for the treatment of EPEC infections but also as potential drugs against infections with EHEC.
FIG 8.

The expression of Shiga toxin is unaffected by the inhibitory compounds. E. coli (A) or C. rodentium (B) expressing the Gaussia luciferase gene (Gluc) under the control of the Shiga toxin promoter were grown overnight in the presence of either compounds (50 μM S3 or S4, 25 μM S6), dimethyl sulfoxide (DMSO), or Ciprofloxacin (positive control), equalized and the amount of luciferase released into the supernatant was determined by Gaussia luciferase assay. Given are the mean RLU values (±SEM) of three independent experiments performed in triplicate. Statistical analysis was performed using the Kruskal-Wallis test followed by Dunn's multiple-comparison test (*P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001), with all treated groups compared to DMSO-treated control.
DISCUSSION
The dramatic increase in antibiotic resistance in recent years has led to a shift in research from antibacterial strategies toward more focused antivirulence-targeted approaches. Interference with virulence mechanisms instead of bacterial survival is promising to reduce the necessity for resistance development as bacteria will only be impaired in their colonization or pathogenicity but able to survive. Anti-virulence strategies have targeted different virulence factors and virulence-associated processes including motility, adhesion and invasion, effector secretion, and inter-bacterial signaling (quorum sensing) (15, 19, 20, 25, 43, 44).
Bacterial secretion systems, especially type III and IV secretion systems, are strongly associated with bacterial virulence, as they are used to inject bacterial effectors into the host cells to modulate disease. The loss of functional secretion systems has, therefore, been associated with a loss of pathogenicity of the respective bacteria, rendering pathogens virtually harmless (14). The phenotypes that are associated with functional T3SSs are diverse. Pathogenic E. coli translocate several effector proteins involved (i) in the intimate host cell attachment of the bacteria, (ii) in cell-cell junction integrity to invade lower tissues, and (iii) in the inhibition of innate immune signaling pathways which interfere with the recruitment of and clearance by immune cells (9, 10).
Here, we monitored effector translocation assays in a primary screen to identify small molecule inhibitors that interfere with this process. A Tir-β-lactamase (TEM)-expressing EPEC strain has been used before to study the dynamics of effector translocation in this pathogen (29). Furthermore, this TEM-based assay is commonly used to either screen or confirm identified inhibitory compounds not only in enteropathogenic E. coli but also in other T3SS or T4SS-containing pathogens. It has been used to identify or characterize secretion system inhibitors of Salmonella (SipB) (26), Pseudomonas aeruginosa (ExoS) (45), and Yersinia (YopE and YopB, (46, 47) but has also been employed for the T4SSs of Helicobacter pylori (48) and Coxiella burnetii (49). On the other hand, T3SS-mediated hemolysis was employed as a primary screen in the study, which initially identified the LEE-inhibitor Aurodox (21), while subversion of the host NF-κB inflammatory signaling pathway has been assessed in a study which aimed to identify inhibitors of the Yersinia T3SS and identified the inhibitory substance Piericidin A (50).
In our study setup, bacteria were first grown in T3SS-inducing conditions for 2 h before exposing them to the inhibitory substances. Therefore, the inhibitors that were identified in this screen are unlikely to affect the induction of T3SS-gene expression and injectisome formation as the T3SSs have already formed. This hypothesis is supported by the fact that the expression of components of the T3SS as well the Tir-TEM fusion protein is unaffected in the presence of the inhibitors (Fig. 5). All identified T3SS inhibitors S3, S4 and S6 reduced Tir-TEM translocation, effector-dependent cell detachment and A/E lesion formation to a similar extent/in a comparable manner. Most likely, Tir integration into the host cell membrane is impaired as reduced translocation into the host cell cytosol could be observed for the Tir-TEM fusion protein (Fig. 2, Fig. S2 in the supplemental material). Furthermore, the absence of pedestals in the presence of the inhibitors conclusively indicated that no intimate interaction occurred between the bacterial outer membrane protein intimin and its receptor, Tir (Fig. 3; Fig. S4) (11, 12). In contrast, T3SS-mediated hemolysis of erythrocytes was only inhibited by S4 and S6 but not by S3. The inhibitory effect of S3 appears to be at the translocation/effector level and does not seem to interfere with T3SS assembly as this inhibitor was unable to inhibit hemolysis of erythrocytes (Fig. 6). As hemolysis is a consequence of T3 translocon pore formation in the erythrocyte membrane, which is dependent on the translocon pore components EspB and EspD (8, 39), it is possible that S4 and S6 affect translocation of all effectors (including the translocation of EspB and EspD) or that they affect translocon pore-formation per se (Fig. 6). Interestingly, pre-exposure of HEp-2 cells to these inhibitors did not alter the translocation of Tir-TEM by EPEC (Fig. S3), suggesting that S3, S4, and S6 do not act by influencing host cells. It is likely, that they interfere with the assembly or the function of the T3S apparatus, and it will be interesting to determine the exact mechanism of action of these compounds in future studies.
All three identified inhibitors do not belong to the previously identified classes of T3SS inhibitors. Earlier screens commonly identified inhibitors that affect either gene expression—Aurodox (51), cytosporone B (52), benzimidazoles (53), sulfonyl amino benzanilides (54), quinolines (55), and salicylidene anilides (27)—or bacterial metabolism—salicylidene acylhydrazides (19), omeprazole ATPase inhibitors (56–58). An effect on the bacterial respiratory chain, which would decrease the formation of the T3SS and thus translocation of effectors was ruled out for the compounds S3, S4, and S6 identified in this study, as a short-term treatment of bacteria with these inhibitors did not result in significant ATP release (Fig. S8 in the supplemental material).
Considering that the identified inhibitors did not affect the expression of the proteins that make up the T3SS or the translocated effector Tir (Fig. 5), we tested the effect of the inhibitors on the translocation of other effector proteins into the host cell by making use of known effector deletion mutants and their associated phenotypes. Deletion of the gene for the LEE-encoded effector protein EspZ results in aberrant effector translocation into host cells, resulting in host cell detachment due to cell death (13). Preincubation of bacteria in the presence of the inhibitors efficiently reduced cell detachment compared to the positive control (ΔespZ + DMSO), suggesting that effector translocation into the host cells was indeed impaired (Fig. 4; Fig. S5 in the supplemental material).
Despite the homologies between the T3SS and the bacterial flagellar basal body (28), no inhibitory effect on bacterial motility was observed with the new T3SS inhibitors (Fig. 7). This, too, supports the hypothesis that the inhibitors do not interfere with conserved basal body structures of the T3SS, but rather with the assembly of the injectisome or the translocation of the effector proteins. Also, other compounds have been identified that interfere with T3SS function; for example, (-)-hopeaphenol, which seem to localize on the outer membrane and interfere with the closely related T3S apparatus of Yersinia pseudotuberculosis, Pseudomonas aeruginosa, and Chlamydia trachomatis (59), and thiazolidinone derivatives that inhibit T3SSs in S. enterica serovar Typhimurium and Pseudomonas syringae, most likely by targeting the outer membrane secretin component of the T3SS (60). In addition, a picolinic acid derivative and a symmetric dipropionate were found to be active against effector secretion of Y. pestis without cytotoxicity against mammalian cells, and notably, they were also active against the translocated intimin receptor Tir in EPEC (46). As their molecular targets are also still unknown, it would be interesting to investigate whether they inhibit identical T3SS functions as the identified compounds S3, S4 or S6.
The effective blockage of T3SS expressed by EPEC as well as by frequent EHEC strains makes the identified inhibitors promising candidates for therapies against hemorrhagic uremic syndrome, which is not curable by common antibiotics such as ciprofloxacin which induce the Shiga toxin genes encoded on lysogenic phages within the bacterial genome (61, 62). As all three inhibitory substances showed no inducing effect on Shiga toxin reporter-gene expression (Fig. 8), they most likely do not induce/enhance disease progression to hemolytic-uremic syndrome. Furthermore, we discovered that even 5-fold excess concentrations (250 μM) of the inhibitors did not affect bacterial growth in MIC studies (Fig. S9 in the supplemental material), providing further promise that resistance development against these inhibitory substances may be slow as little selective pressure is induced.
Taken together, our study revealed three potent small molecule inhibitors blocking T3SS-mediated translocation of crucial virulence factors of EPEC/EHEC. As all three compounds interfere with the infection at later stages (e.g., after the T3SS has formed), they constitute promising drugs for therapeutic treatment of the infection, as T3SS-formation will have already occurred once treatment commences.
MATERIALS and METHODS
Bacterial strains and cell lines.
Bacterial strains (listed in Table S2 in the supplemental material) were grown in LB (Carl Roth, Germany) or Müller-Hinton (Sigma-Aldrich, Germany) broth or DMEM GlutaMAX (Gibco) as indicated. LB overnight cultures were supplemented with antibiotics tetracycline (30 μg/ml), carbenicillin (100 μg/ml) or kanamycin (50 μg/ml) for selection where required.
Cell lines and their respective growth media are given in Table S3. Eukaryotic cells were cultured at 37°C, 5% CO2.
Construction of strain E2348/69 pPgapdhamCyan.
The egfp gene encoded on a SalI/NotI fragment on plasmid pFU31 (63) was exchanged by SalI/NotI fragment encoding the amCyan gene of pFU78 (63). Subsequently, a fragment carrying the promoter of the E. coli gapdh gene was amplified with primer (GCA CGG GAT CCG CCA TTT GCT CAC ATC TC and GCA CGG TCG ACA TAT TCC ACC AGC TAT TTG TTA) and cloned into the BamHI/SalI sites generating plasmid pPgapdhamCyan which was transformed into E2348/69.
Infection of eukaryotic cells.
One day before infection, bacteria were inoculated into LB broth and grown at 37°C and 180 rpm overnight. On the day of infection, overnight cultures were diluted 1:75 in DMEM GlutaMAX (Gibco) and grown statically for 3 h at 37°C with 5% CO2.
HEp-2 cells were washed twice with Dulbecco's phosphate-buffered saline (DPBS; Sigma) and infected with EPEC grown to an OD600 nm of 0.03 for 3 h.
Small molecule compound libraries.
All used two natural and four chemical libraries are given in Table 1 with concentration of stock solutions and sources.
Effector translocation assay.
For translocation assays, 2 × 105 HEp-2 (ATCC CCL-23) cells/ml were seeded into black 96-well plates with transparent bottom (Costar, Germany). Bacterial overnight cultures were diluted 1:50 into an appropriate amount of DMEM with GlutaMAX (Gibco, Germany) and incubated for 2 h at 37°C 5% CO2. 2.5 mM probenecid were added to the cultures which were diluted 1:2 in DMEM with GlutaMAX (Gibco, Germany). 50 μl of the bacterial suspension were subsequently added to the screening plates which contained 1 μl of compound per well (concentrations are given in Table 1). These plates were incubated for an additional hour. HEp-2 cells were washed once with DPBS and 50 μl DMEM with GlutaMAX was added to each well. The bacteria-inhibitor suspension (50 μl per well) was then added to the cells, the plates were centrifuged at 1,000 × g for 1 min (Eppendorf Centrifuge 5810R with a A-2-DWP-AT plate rotor) and incubated for 1.5 h (37°C, 5% CO2). Media was then removed, and infected cells were washed twice with DPBS. DMEM with GlutaMAX supplemented with 1 mM HEPES (Biochrom, Germany) and 1x gentamicin (Sigma) was added to the cells and mixed with LifeBLAzer CCF4-AM staining solution (Invitrogen). The plates were then incubated for 1 h at room temperature. Subsequently, the fluorescence was determined in a VarioSkan (Fisher Scientific, Germany) or ClarioStar Plus (BMG Labtech, Germany) plate reader using an excitation wavelength of 405 nm (10 nm bandwidth). Emission was detected with 460 nm (20 nm bandwidth, blue fluorescence) and 530 nm (15 nm bandwidth, green fluorescence) filters. Effector translocation was determined by calculating the ratio of blue to green fluorescence (Em520 nm/Em460 nm). The translocation efficiency of untreated, EPEC-infected cells was set to 100% while that of untreated EPEC-infected cells was used as a negative control.
Analysis of inhibitors on in vitro growth and cell viability.
Wild-type EPEC E2348/69 was grown overnight in LB at 37°C and resuspended to an optical density (OD600nm) of 0.02. 100 μl were added to each well of a 96-well plate containing appropriate amounts of substance or control. Plates were incubated at 37°C without shaking and the OD600nm was determined every 2 h for 8 h in a VarioSkan (Fisher Scientific, Germany) or ClarioStar Plus (BMG Labtech, Germany) plate reader.
To determine the effect of inhibitory substances on the viability of eukaryotic cells, 100 μl of a 2 × 104 cells/ml solution was used to resuspend the inhibitors of interest, and the resulting solution was transferred to a tissue culture treated 96-well plate. Plates were incubated at 37°C, 5% CO2 for 3 days. Subsequently, media was aspirated, and a solution of XTT/PMS (Cell Proliferation Kit II; Merck, Germany) and culture medium was added to the wells. Cells were then incubated at 37°C 5% CO2 for an additional 2 h after which cell viability was determined at 475 nm in a VarioSkan (Fisher Scientific, Germany) or ClarioStar Plus (BMG Labtech, Germany) plate reader.
Fluorescent actin stain (FAS).
Cells seeded on coverslips at 2 × 105 cells/ml were infected as described above. After infection, cells were washed twice with DPBS, fixed with 4% paraformaldehyde (Sigma, Germany) for 20 min and permeabilized with 0.1% Triton X-100 (OMNI Life Science, Germany) for 5 min at RT. After washing, cells were stained with tetramethyl rhodamine isocyanate-Phalloidin (Sigma-Aldrich, Germany), washed again and mounted using Prolong Diamond mounting medium containing DAPI (4′,6-diamidino-2-phenylindole) (Thermo Fisher Scientific, Germany). Actin pedestals on cells were visualized using a Keyence Biorevo BZ-9000 (Keyence, Germany).
Cell detachment assay.
Cell detachment assays were carried out, as described in (13). In short, HeLa cells were seeded 48 h prior to infection with EPEC primed in the presence or absence of inhibitors. After 1 h of infection, cells were washed five times with DPBS, incubated in the presence of inhibitors for an additional hour and washed again five times with DPBS. Cells were trypsinized and counted in a hemocytometer to determine the percentage of remaining cells. For visualization, HeLa cells grown in 8-well removable cell culture chambers (Sarstedt, Germany) or on coverslips were infected and treated as described above. Following the second washing step, cells were fixed with 4% PFA and subsequently stained using the Hematoxylin-Eosin fast staining kit (Carl Roth, Germany) according to Ref. 13.
Motility assay.
LB and DMEM motility agar plates (0.3% agar) containing inhibitory substances at a concentration of 50 μM/ml were stab-inoculated using 2 μl of an EPEC E2348/69 overnight culture and incubated at 37°C overnight. The diameter of the motility halos was documented after 6 (LB) or 24 (DMEM) h. Triplicate stabs were done per assay for each inhibitor.
Protein expression and extraction.
For analysis of effector protein expression, LB overnight cultures of bacteria were diluted 1:50 into DMEM GlutaMAX in the presence or absence of inhibitors. After stationary incubation of the cultures at 37°C, 5% CO2 for 3 h, equalized amounts of cells were harvested by centrifugation at 8,000 × g for 10 min. The supernatants were discarded, and the bacterial pellets were resuspended in BugBuster protein extraction reagent (Merck, Germany). The cell suspensions were incubated on a rotary shaker for 20 min. Sodium dodecyl sulfate (SDS) sample buffer was added before the samples were boiled at 95°C for 10 min before further analysis.
SDS-PAGE and immunoblotting.
Twelve percent SDS-polyacrylamide gels were used to separate proteins with the Bio-Rad MiniPROTEAN Electrophoresis System (Bio-Rad, Germany) followed by transfer of the proteins onto Immobilon FL PVDF membrane (Millipore, Germany). Antibodies against the T3SS components EspA (1:500), EspB (1:10,000) and EspD (1:10,000) (64) and anti-β-lactamase antibody (Abcam; 1:5,000) against the Tir-β-lactamase fusion protein were used to detect protein by immunoblotting. GAPDH (MA5-15738; 1:5,000; ThermoFisherScientific, Germany) was used as a loading control. Using anti-mouse IgG HRP-linked secondary antibody (Cell Signaling Technology, Germany), proteins were detected with WesternLightning ECL Reagent (Perkin Elmer, Germany) and exposure of the membrane to CL-XPosure film (ThermoFisher Scientific, Germany).
Hemolysis assay.
EPEC overnight cultures were diluted 1:25 in DMEM high glucose without Phenol Red (Sigma, Germany) and grown for 3 h at 37°C, 5% CO2 in the presence or absence of decreasing concentrations of the inhibitors. Sheep RBCs in Alsever (Fiebig Nährstofftechnik, Germany) were washed three times in PBS and resuspended to 5% (vol/vol) in DMEM high glucose without Phenol Red (Sigma, Germany). Bacterial cultures were equalized to 1 × 108 in 500 μl and added to 500 μl sheep RBCs (5% vol/vol) in 2 ml Eppendorf tubes. Uninfected RBCs in DMEM were used as a negative control. Total lysis was achieved by the addition of 0.2% saponin to the culture medium. To synchronize infection and mediate bacterial-cell contact, tubes were centrifuged 1 min at 2,500 × g (Eppendorf MiniSpin) before incubation at 37°C, 5% CO2. After 2 h, cells were gently resuspended, followed by centrifugation at 2,500 × g for 1 min. 100 μl of each supernatant was transferred to a 96-well plate, and the amount of hemoglobin released was assessed at 543 nm in a ClarioStar Plus plate reader (BMG Labtech, Germany). Hemolysis was calculated as the percentage of hemoglobin released by the DMSO-treated wild-type-infected RBCs.
Reporter-gene assays: C600 (stx2::Gluc) and DBS100 (stx2dact::Gluc).
Bacteria were inoculated into LB broth and grown at 37°C, 200 rpm for 16 h in the presence of either the inhibitors at their highest noncytotoxic concentration, DMSO (negative control; AppliChem) or 40 ng/ml ciprofloxacin (positive control; Sigma). The optical density of each culture was determined, and a volume corresponding to an OD600nm of one (109 cells) was pelleted at 14,000 × g for 1 min (Eppendorf MiniSpin). 20 μl of supernatant was mixed with 50 μl BioLux Gaussia Luciferase Assay substrate (New England Biolabs, Germany) and the luciferase activity was determined in a ClarioStar Plus plate reader (BMG Labtech, Germany) according to the manufacturer's recommendations. Values were compared to untreated culture supernatant, and LB medium was used as blank.
ACKNOWLEDGMENTS
We thank Ilan Rosenshine for kindly providing the E2348/69 PLEE5tir-blaM strain and Martin Koeppel and Bärbel Stecher for the use of C600 ϕstx2a::Gluc and DBS770 ϕstx2dact::Gluc. We also thank Sandra Stengel and Amrei Rolof for technical assistance with the HTS. Petra Dersch, Ursula Bilitewski and Sabrina Mühlen are supported by the Deutsche Zentrum für Infektionsforschung (DZIF TTU-GI, TTU 06.801-2, TTU_06_819_00).
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.Nataro JP, Kaper JB. 1998. Diarrheagenic Escherichia coli. Clin Microbiol Rev 11:142–201. 10.1128/CMR.11.1.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Frankel G, Phillips AD, Rosenshine I, Dougan G, Kaper JB, Knutton S. 1998. Enteropathogenic and enterohaemorrhagic Escherichia coli: more subversive elements. Mol Microbiol 30:911–921. 10.1046/j.1365-2958.1998.01144.x. [DOI] [PubMed] [Google Scholar]
- 3.Iguchi A, Thomson NR, Ogura Y, Saunders D, Ooka T, Henderson IR, Harris D, Asadulghani M, Kurokawa K, Dean P, Kenny B, Quail MA, Thurston S, Dougan G, Hayashi T, Parkhill J, Frankel G. 2009. Complete genome sequence and comparative genome analysis of enteropathogenic Escherichia coli O127:H6 strain E2348/69. J Bacteriol 191:347–354. 10.1128/JB.01238-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McDaniel TK, Jarvis KG, Donnenberg MS, Kaper JB. 1995. A genetic locus of enterocyte effacement conserved among diverse enterobacterial pathogens. Proc Natl Acad Sci USA 92:1664–1668. 10.1073/pnas.92.5.1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gaytan MO, Martinez-Santos VI, Soto E, Gonzalez-Pedrajo B. 2016. Type Three Secretion System in Attaching and Effacing Pathogens. Front Cell Infect Microbiol 6:129. 10.3389/fcimb.2016.00129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wilson RK, Shaw RK, Daniell S, Knutton S, Frankel G. 2001. Role of EscF, a putative needle complex protein, in the type III protein translocation system of enteropathogenic Escherichia coli. Cell Microbiol 3:753–762. 10.1046/j.1462-5822.2001.00159.x. [DOI] [PubMed] [Google Scholar]
- 7.Deng W, Puente JL, Gruenheid S, Li Y, Vallance BA, Vazquez A, Barba J, Ibarra JA, O'Donnell P, Metalnikov P, Ashman K, Lee S, Goode D, Pawson T, Finlay BB. 2004. Dissecting virulence: systematic and functional analyses of a pathogenicity island. Proc Natl Acad Sci USA 101:3597–3602. 10.1073/pnas.0400326101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ide T, Laarmann S, Greune L, Schillers H, Oberleithner H, Schmidt MA. 2001. Characterization of translocation pores inserted into plasma membranes by type III-secreted Esp proteins of enteropathogenic Escherichia coli. Cell Microbiol 3:669–679. 10.1046/j.1462-5822.2001.00146.x. [DOI] [PubMed] [Google Scholar]
- 9.Riebisch AK, Muhlen S. 2020. Attaching and effacing pathogens: the effector ABC of immune subversion. Future Microbiol 15:945–958. 10.2217/fmb-2019-0274. [DOI] [PubMed] [Google Scholar]
- 10.Shenoy AR, Furniss RCD, Goddard PJ, Clements A. 2018. Modulation of Host Cell Processes by T3SS Effectors. Curr Top Microbiol Immunol 416:73–115. 10.1007/82_2018_106. [DOI] [PubMed] [Google Scholar]
- 11.Kenny B, DeVinney R, Stein M, Reinscheid DJ, Frey EA, Finlay BB. 1997. Enteropathogenic E. coli (EPEC) transfers its receptor for intimate adherence into mammalian cells. Cell 91:511–520. 10.1016/s0092-8674(00)80437-7. [DOI] [PubMed] [Google Scholar]
- 12.Hartland EL, Batchelor M, Delahay RM, Hale C, Matthews S, Dougan G, Knutton S, Connerton I, Frankel G. 1999. Binding of intimin from enteropathogenic Escherichia coli to Tir and to host cells. Mol Microbiol 32:151–158. 10.1046/j.1365-2958.1999.01338.x. [DOI] [PubMed] [Google Scholar]
- 13.Berger CN, Crepin VF, Baruch K, Mousnier A, Rosenshine I, Frankel G. 2012. EspZ of enteropathogenic and enterohemorrhagic Escherichia coli regulates type III secretion system protein translocation. mBio 3 10.1128/mBio.00317-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cornelis GR. 2006. The type III secretion injectisome. Nat Rev Microbiol 4:811–825. 10.1038/nrmicro1526. [DOI] [PubMed] [Google Scholar]
- 15.Muhlen S, Dersch P. 2016. Anti-virulence Strategies to Target Bacterial Infections. Curr Top Microbiol Immunol 398:147–183. 10.1007/82_2015_490. [DOI] [PubMed] [Google Scholar]
- 16.Charro N, Mota LJ. 2015. Approaches targeting the type III secretion system to treat or prevent bacterial infections. Expert Opin Drug Discov 10:373–387. 10.1517/17460441.2015.1019860. [DOI] [PubMed] [Google Scholar]
- 17.Duncan MC, Linington RG, Auerbuch V. 2012. Chemical inhibitors of the type three secretion system: disarming bacterial pathogens. Antimicrob Agents Chemother 56:5433–5441. 10.1128/AAC.00975-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pendergrass HA, May AE. 2019. Natural Product Type III Secretion System Inhibitors. Antibiotics (Basel) 8 10.3390/antibiotics8040162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zambelloni R, Marquez R, Roe AJ. 2015. Development of antivirulence compounds: a biochemical review. Chem Biol Drug Des 85:43–55. 10.1111/cbdd.12430. [DOI] [PubMed] [Google Scholar]
- 20.McShan AC, De Guzman RN. 2015. The bacterial type III secretion system as a target for developing new antibiotics. Chem Biol Drug Des 85:30–42. 10.1111/cbdd.12422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kimura K, Iwatsuki M, Nagai T, Matsumoto A, Takahashi Y, Shiomi K, Omura S, Abe A. 2011. A small-molecule inhibitor of the bacterial type III secretion system protects against in vivo infection with Citrobacter rodentium. J Antibiot (Tokyo) 64:197–203. 10.1038/ja.2010.155. [DOI] [PubMed] [Google Scholar]
- 22.Veenendaal AK, Sundin C, Blocker AJ. 2009. Small-molecule type III secretion system inhibitors block assembly of the Shigella type III secreton. J Bacteriol 191:563–570. 10.1128/JB.01004-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wolf K, Betts HJ, Chellas-Gery B, Hower S, Linton CN, Fields KA. 2006. Treatment of Chlamydia trachomatis with a small molecule inhibitor of the Yersinia type III secretion system disrupts progression of the chlamydial developmental cycle. Mol Microbiol 61:1543–1555. 10.1111/j.1365-2958.2006.05347.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Izore T, Job V, Dessen A. 2011. Biogenesis, regulation, and targeting of the type III secretion system. Structure 19:603–612. 10.1016/j.str.2011.03.015. [DOI] [PubMed] [Google Scholar]
- 25.Beckham KS, Roe AJ. 2014. From screen to target: insights and approaches for the development of anti-virulence compounds. Front Cell Infect Microbiol 4:139. 10.3389/fcimb.2014.00139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Negrea A, Bjur E, Ygberg SE, Elofsson M, Wolf-Watz H, Rhen M. 2007. Salicylidene acylhydrazides that affect type III protein secretion in Salmonella enterica serovar typhimurium. Antimicrob Agents Chemother 51:2867–2876. 10.1128/AAC.00223-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kauppi AM, Nordfelth R, Uvell H, Wolf-Watz H, Elofsson M. 2003. Targeting bacterial virulence: inhibitors of type III secretion in Yersinia. Chem Biol 10:241–249. 10.1016/s1074-5521(03)00046-2. [DOI] [PubMed] [Google Scholar]
- 28.Diepold A, Armitage JP. 2015. Type III secretion systems: the bacterial flagellum and the injectisome. Philos Trans R Soc Lond B Biol Sci 370 10.1098/rstb.2015.0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mills E, Baruch K, Charpentier X, Kobi S, Rosenshine I. 2008. Real-time analysis of effector translocation by the type III secretion system of enteropathogenic Escherichia coli. Cell Host Microbe 3:104–113. 10.1016/j.chom.2007.11.007. [DOI] [PubMed] [Google Scholar]
- 30.Irschik H, Reichenbach H, Hofle G, Jansen R. 2007. The thuggacins, novel antibacterial macrolides from Sorangium cellulosum acting against selected Gram-positive bacteria. J Antibiot (Tokyo) 60:733–738. 10.1038/ja.2007.95. [DOI] [PubMed] [Google Scholar]
- 31.Buntin K, Irschik H, Weissman KJ, Luxenburger E, Blocker H, Muller R. 2010. Biosynthesis of thuggacins in myxobacteria: comparative cluster analysis reveals basis for natural product structural diversity. Chem Biol 17:342–356. 10.1016/j.chembiol.2010.02.013. [DOI] [PubMed] [Google Scholar]
- 32.Bock M, Dehn R, Kirschning A. 2008. Total synthesis of thuggacin B. Angew Chem Int Ed Engl 47:9134–9137. 10.1002/anie.200803271. [DOI] [PubMed] [Google Scholar]
- 33.Irschik H, Schummer D, Gerth K, Hofle G, Reichenbach H. 1995. The tartrolons, new boron-containing antibiotics from a myxobacterium, Sorangium cellulosum. J Antibiot (Tokyo) 48:26–30. 10.7164/antibiotics.48.26. [DOI] [PubMed] [Google Scholar]
- 34.Elshahawi SI, Trindade-Silva AE, Hanora A, Han AW, Flores MS, Vizzoni V, Schrago CG, Soares CA, Concepcion GP, Distel DL, Schmidt EW, Haygood MG. 2013. Boronated tartrolon antibiotic produced by symbiotic cellulose-degrading bacteria in shipworm gills. Proc Natl Acad Sci USA 110:E295–304. 10.1073/pnas.1213892110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gerth K, Irschik H, Reichenbach H, Trowitzsch W. 1982. The myxovirescins, a family of antibiotics from Myxococcus virescens (Myxobacterales). J Antibiot (Tokyo) 35:1454–1459. 10.7164/antibiotics.35.1454. [DOI] [PubMed] [Google Scholar]
- 36.Irschik H, Gerth K, Kemmer T, Steinmetz H, Reichenbach H. 1983. The myxovalargins, new peptide antibiotics from Myxococcus fulvus (Myxobacterales). I. Cultivation, isolation, and some chemical and biological properties. J Antibiot 36:6–12. 10.7164/antibiotics.36.6. [DOI] [PubMed] [Google Scholar]
- 37.Irschik H, Reichenbach H. 1985. The mechanism of action of myxovalargin A, a peptide antibiotic from Myxococcus fulvus. J Antibiot (Tokyo) 38:1237–1245. 10.7164/antibiotics.38.1237. [DOI] [PubMed] [Google Scholar]
- 38.Irschik H, Jansen R, Gerth K, Hofle G, Reichenbach H. 1987. The sorangicins, novel and powerful inhibitors of eubacterial RNA polymerase isolated from myxobacteria. J Antibiot (Tokyo) 40:7–13. 10.7164/antibiotics.40.7. [DOI] [PubMed] [Google Scholar]
- 39.Warawa J, Finlay BB, Kenny B. 1999. Type III secretion-dependent hemolytic activity of enteropathogenic Escherichia coli. Infect Immun 67:5538–5540. 10.1128/IAI.67.10.5538-5540.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Karmali MA, Steele BT, Petric M, Lim C. 1983. Sporadic cases of haemolytic-uraemic syndrome associated with faecal cytotoxin and cytotoxin-producing Escherichia coli in stools. Lancet 1:619–620. 10.1016/S0140-6736(83)91795-6. [DOI] [PubMed] [Google Scholar]
- 41.O'Brien AD, Lively TA, Chang TW, Gorbach SL. 1983. Purification of Shigella dysenteriae 1 (Shiga)-like toxin from Escherichia coli O157:H7 strain associated with haemorrhagic colitis. Lancet 2:573. 10.1016/S0140-6736(83)90601-3. [DOI] [PubMed] [Google Scholar]
- 42.Muhlen S, Ramming I, Pils MC, Koeppel M, Glaser J, Leong J, Flieger A, Stecher B, Dersch P. 2020. Identification of antibiotics that diminish disease in a murine model of enterohemorrhagic Escherichia coli infection. Antimicrob Agents Chemother doi:10.1128/AAC.02159–19. 10.1128/AAC.02159-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Escaich S. 2008. Antivirulence as a new antibacterial approach for chemotherapy. Curr Opin Chem Biol 12:400–408. 10.1016/j.cbpa.2008.06.022. [DOI] [PubMed] [Google Scholar]
- 44.Dickey SW, Cheung GYC, Otto M. 2017. Different drugs for bad bugs: antivirulence strategies in the age of antibiotic resistance. Nat Rev Drug Discov 16:457–471. 10.1038/nrd.2017.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Aiello D, Williams JD, Majgier-Baranowska H, Patel I, Peet NP, Huang J, Lory S, Bowlin TL, Moir DT. 2010. Discovery and characterization of inhibitors of Pseudomonas aeruginosa type III secretion. Antimicrob Agents Chemother 54:1988–1999. 10.1128/AAC.01598-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pan NJ, Brady MJ, Leong JM, Goguen JD. 2009. Targeting type III secretion in Yersinia pestis. Antimicrob Agents Chemother 53:385–392. 10.1128/AAC.00670-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Harmon DE, Davis AJ, Castillo C, Mecsas J. 2010. Identification and characterization of small-molecule inhibitors of Yop translocation in Yersinia pseudotuberculosis. Antimicrob Agents Chemother 54:3241–3254. 10.1128/AAC.00364-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schindele F, Weiss E, Haas R, Fischer W. 2016. Quantitative analysis of CagA type IV secretion by Helicobacter pylori reveals substrate recognition and translocation requirements. Mol Microbiol 100:188–203. 10.1111/mmi.13309. [DOI] [PubMed] [Google Scholar]
- 49.Newton P, Thomas DR, Reed SCO, Lau N, Xu B, Ong SY, Pasricha S, Madhamshettiwar PB, Edgington-Mitchell LE, Simpson KJ, Roy CR, Newton HJ. 2020. Lysosomal degradation products induce Coxiella burnetii virulence. Proc Natl Acad Sci USA 117:6801–6810. 10.1073/pnas.1921344117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Duncan MC, Wong WR, Dupzyk AJ, Bray WM, Linington RG, Auerbuch V. 2014. An NF-kappaB-based high-throughput screen identifies piericidins as inhibitors of the Yersinia pseudotuberculosis type III secretion system. Antimicrob Agents Chemother 58:1118–1126. 10.1128/AAC.02025-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.McHugh RE, O'Boyle N, Connolly JPR, Hoskisson PA, Roe AJ. 2019. Characterization of the Mode of Action of Aurodox, a Type III Secretion System Inhibitor from Streptomyces goldiniensis. Infect Immun 87 10.1128/IAI.00595-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li J, Lv C, Sun W, Li Z, Han X, Li Y, Shen Y. 2013. Cytosporone B, an inhibitor of the type III secretion system of Salmonella enterica serovar Typhimurium. Antimicrob Agents Chemother 57:2191–2198. 10.1128/AAC.02421-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Marsden AE, King JM, Spies MA, Kim OK, Yahr TL. 2016. Inhibition of Pseudomonas aeruginosa ExsA DNA-Binding Activity by N-Hydroxybenzimidazoles. Antimicrob Agents Chemother 60:766–776. 10.1128/AAC.02242-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gauthier A, Robertson ML, Lowden M, Ibarra JA, Puente JL, Finlay BB. 2005. Transcriptional inhibitor of virulence factors in enteropathogenic Escherichia coli. Antimicrob Agents Chemother 49:4101–4109. 10.1128/AAC.49.10.4101-4109.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Koppolu V, Osaka I, Skredenske JM, Kettle B, Hefty PS, Li J, Egan SM. 2013. Small-molecule inhibitor of the Shigella flexneri master virulence regulator VirF. Infect Immun 81:4220–4231. 10.1128/IAI.00919-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Case HB, Mattock DS, Dickenson NE. 2018. Shutting Down Shigella Secretion: Characterizing Small Molecule Type Three Secretion System ATPase Inhibitors. Biochemistry 57:6906–6916. 10.1021/acs.biochem.8b01077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Puiac S, Sem X, Negrea A, Rhen M. 2011. Small-molecular virulence inhibitors show divergent and immunomodulatory effects in infection models of Salmonella enterica serovar Typhimurium. Int J Antimicrob Agents 38:409–416. 10.1016/j.ijantimicag.2011.06.009. [DOI] [PubMed] [Google Scholar]
- 58.Layton AN, Hudson DL, Thompson A, Hinton JC, Stevens JM, Galyov EE, Stevens MP. 2010. Salicylidene acylhydrazide-mediated inhibition of type III secretion system-1 in Salmonella enterica serovar Typhimurium is associated with iron restriction and can be reversed by free iron. FEMS Microbiol Lett 302:114–122. 10.1111/j.1574-6968.2009.01847.x. [DOI] [PubMed] [Google Scholar]
- 59.Zetterstrom CE, Hasselgren J, Salin O, Davis RA, Quinn RJ, Sundin C, Elofsson M. 2013. The resveratrol tetramer (-)-hopeaphenol inhibits type III secretion in the gram-negative pathogens Yersinia pseudotuberculosis and Pseudomonas aeruginosa. PLoS One 8:e81969. 10.1371/journal.pone.0081969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Felise HB, Nguyen HV, Pfuetzner RA, Barry KC, Jackson SR, Blanc MP, Bronstein PA, Kline T, Miller SI. 2008. An inhibitor of gram-negative bacterial virulence protein secretion. Cell Host Microbe 4:325–336. 10.1016/j.chom.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kakoullis L, Papachristodoulou E, Chra P, Panos G. 2019. Shiga toxin-induced haemolytic uraemic syndrome and the role of antibiotics: a global overview. J Infect 79:75–94. 10.1016/j.jinf.2019.05.018. [DOI] [PubMed] [Google Scholar]
- 62.Zimmerhackl LB. 2000. E. coli, antibiotics, and the hemolytic-uremic syndrome. N Engl J Med 342:1990–1991. 10.1056/NEJM200006293422611. [DOI] [PubMed] [Google Scholar]
- 63.Uliczka F, Pisano F, Kochut A, Opitz W, Herbst K, Stolz T, Dersch P. 2011. Monitoring of gene expression in bacteria during infections using an adaptable set of bioluminescent, fluorescent and colorigenic fusion vectors. PLoS One 6:e20425. 10.1371/journal.pone.0020425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Beinke C, Laarmann S, Wachter C, Karch H, Greune L, Schmidt MA. 1998. Diffusely adhering Escherichia coli strains induce attaching and effacing phenotypes and secrete homologs of Esp proteins. Infect Immun 66:528–539. 10.1128/IAI.66.2.528-539.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material. Download aac.00958-21-s0001.pdf, PDF file, 7.6 MB (7.6MB, pdf)