Abstract
Introduction:
With the emergence of multidrug-resistant gram-negative bacterial infections, there has been a surge in the use of Colistin in recent times. The most important side effect of Colistin use is its nephrotoxicity. The study was designed to assess the effect on kidney function and the risk factors for nephrotoxicity in patients treated with Colistin.
Methods:
The study is a retrospective one, which included patients who received Colistin for more than 48 hours. The estimated glomerular filtration rate (eGFR) was calculated using the Modification of Diet in Renal Disease (MDRD) four-variable equation and acute kidney injury (AKI) was diagnosed as per the Kidney Disease Improving Global Outcome (KDIGO) criteria.
Results:
Of the 150 patients studied, 59 patients (39.2%) developed AKI within a median period of 4 days (Range 2–20 days) of initiation of Colistin. Age, eGFR at the start of therapy and requirement of vasopressor support for treatment of septic shock were the most important risk factors associated with nephrotoxicity. Among patients with AKI, nearly half had only mild worsening of renal functions to KDIGO AKI stage 1. Nearly 75% of patients with AKI had complete or partial recovery of renal functions after stopping Colistin.
Conclusion:
Colistin has significant nephrotoxicity, the risk being higher with older age and baseline renal dysfunction. It is important to monitor renal functions early and at regular intervals after initiating therapy.
Keywords: Colistin, nephrotoxicity, risk factors
Introduction
Colistin became available for clinical use in the 1960s, and was replaced by less toxic antibiotics after almost a decade, due to concerns about its toxicity, especially nephrotoxicity. In the past 10–15 years, the emergence of multidrug-resistant organisms and the drying of the antibiotic development pipeline have led to the increasing use of Colistin worldwide in recent times. Nephrotoxicity is the most worrying adverse effect of this drug. The mechanism of nephrotoxicity is thought to be due to an increase in renal tubular epithelial cell membrane permeability, which results in water and ion influx, leading to cell swelling and cell lysis. Various studies have reported nephrotoxicity ranging from 20 to 50% depending on the dose and the baseline renal function. Despite its toxicity, the emergence of multidrug-resistant organisms forces clinicians to use Colistin even in those with baseline renal dysfunction. This study was done to assess renal functions in patients treated with Colistin.
Methods
The study is a retrospective one, to assess the renal function in patients who received Colistin from April 2017 to June 2019. The data of patients admitted in hospital in both critical and non-critical areas who received Colistin for more than 48 hours was obtained from electronic medical records for the purpose of the study. Patients with chronic liver disease, any structural abnormality of the urinary tract and patients with end-stage renal disease (ESRD) were excluded from the study. Colistin sensitivity was done using Vitek 2 based Automated Antimicrobial Sensitivity testing and the breakpoint Minimum inhibitory Concentration (MIC) for sensitive isolates was <2 microgram/mL.
Patient's demographic variables and serum creatinine at initiation of Colistin therapy were collected. The estimated Glomerular Filtration Rate (eGFR) was calculated using Modification of Diet in Renal Disease (MDRD) four variable equation.[1] All patients received Colistin at a dose adjusted for renal function as per the Stanford guidelines which were based on recommendations by Nation et al.[2] All patients received loading dose calculated based on the body weight and an average reference adult received nine million units as the initial dose and subsequent maintenance dose in two divided doses per day. The dose was adjusted in patients with renal failure based on creatinine clearance calculated as per Cockcroft Gault formula based on patient's body weight. Patients were followed up while they were admitted in hospital. Serum creatinine was measured by Jaffe's kinetic method using COBAS 6000- 501 machine, manufactured by Roche, at Mannhiem city, Germany. MDRD equation was preferred to Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation because of the tendency of the latter to overestimate GFR in Chronic Kidney Disease (CKD) patients.[3] CKD and Acute Kidney Injury (AKI) were defined as per the Kidney Disease Improving Global Outcome (KDIGO) criteria.[4,5] The study was cleared by the hospital ethics committee.
The primary outcome of the study was AKI in patients treated with Colistin. Secondary outcomes were risk factors for AKI, characteristics of AKI including time to onset, severity and reversibility as well as the association between AKI and mortality.
The decision to stop Colistin in patients with AKI was made on a case by case basis, depending on the severity of infections, severity of AKI, relief of symptoms in certain cases like urinary infections and partly based on patient and family concerns regarding rise in creatinine values. Complete recovery of renal functions was defined by improvement of renal function to within 25% of baseline creatinine value and partial recovery as any improvement in creatinine (but not to within 25% of baseline creatinine) after stopping Colistin.
Statistical analysis
The mean and standard deviation were used to describe quantitative variables and percentage distribution for qualitative variables. Independent sample t-test was used to compare quantitative variables and Chi-square test was used to compare discrete variables between groups. Binary logistic regression was performed to ascertain the effects of outcome and predictive variables. A value of P < 0.05 was considered significant.
Results
A total of 150 patients were studied. The characteristics of the patients are described in Table 1 and 2. The main organisms identified were Klebsiella pneumoniae (56%), Pseudomonas aeruginosa (24%), Acinetobacter baumannii (12%) and E. coli (6.8%). Other organisms identified were Elizabethkingia meningoseptica, Achromobacter xylosoxidans, Enterobacter cloacae, Providencia rettgeri and Proteus mirabilis in a small number of patients. Fifty-nine patients (39.2%) had worsening of renal functions after initiation of Colistin. The risk of acute kidney injury was higher in patients with age more than 60 years and baseline glomerular filtration rate less than 60 mL/minute/1.73m2. Patients with eGFR less than 60 mL/minute/1.73m2 had higher risk of worsening of renal functions than patients with higher eGFR {Odds ratio (OR)-9.32, 95%CI (3.08,28.19)}. The lower the GFR, the higher was the risk of worsening of serum creatinine following Colistin therapy. The risk was also higher in patients who required vasopressors for treatment of septic shock {OR- 6.2, 95%CI (1.83,21.01)}. The median time interval from initiation of Colistin to acute kidney injury was 4 days (Range 2-20 days), with around 60% developing renal failure within 7 days. The study found no impact of gender, diabetes, and hypertension on development of AKI. Binary logistic regression analysis was performed to ascertain the effects of age, gender, vasopressor requirement, baseline eGFR, diabetes, hypertension, cumulative dose and duration of Colistin therapy on acute kidney injury. Analysis revealed age {OR-1.074,95%CI (1.024,1.126)}, vasopressor requirement {OR-20.86,95% CI (3.211,135.564)}and baseline GFR {OR- 0.986, 95%CI (0.973,0.998)} as independent risk factors for acute kidney injury. However only age {OR- 1.057, 95%CI (1.010, 1.107)} and vasopressor requirement {OR-3.9, 95% CI (1.035,14.95)} was found to be independent predictors of Stage 3 AKI. Significantly AKI was found to be an independent risk factor for in-hospital mortality {OR 4.2, 95%CI (1.5,11.7)} along with vasopressor requirement {OR- 15.45, 95%CI (3.77,63.19)}.
Table 1.
Characteristics of patients who received Colistin
| Baseline Characteristics | Number - 150 |
|---|---|
| Age in years, mean (SD) | 54.17 (18.2) |
| Gender: Male/Female n (%) | 68 (45.3)/82 (54.6) |
| Diabetes, n (%) | 48 (32%) |
| Hypertension, n (%) | 40 (26.6%) |
| Chronic Kidney disease, n (%)GFR of patients with CKD in ml/minute/173 m2, mean (SD) | 34 (22.6%)40 (16.66) |
| Vasopresor requirement, n (%) | 16 (10.6%) |
| Cumulative dose of Colistine in Million units, Median (range) | 53.5 (18-252) |
| Duration of colistin treatment in days, median (Inter Quartile Range) | 8 (5-10) |
| Time interval from initiation of colistin to AKI in days, Median (Range) | 4 (2-20) |
| Source of sepsis n (%) | |
| Urine | 55 (36.6%) |
| Lung | 43 (28.6%) |
| Skin | 15 (10%) |
| Intraabdominal | 14 (9.3%) |
| Unknown | 23 (15.3%) |
Table 2.
Comparison of characteristics of patients with and without acute kidney injury
| Patient characteristics | Acute Kidney Injury |
P | |
|---|---|---|---|
| Yes (number - 59) | No (number-91) | ||
| Age years, Mean (SD) | 63.65 (11.17) | 48 (19.29) | 0.001 |
| CKD, n (%) | 26 (17%) | 8 (5.3%) | 0.001 |
| Vasopressor requirement, n (%) | 12 (8%) | 4 (2.6%) | 0.01 |
| Gender - Male/Female, n (%) | 27/32 (18%/21.3%) | 41/50 (27.3%/33.3%) | 0.615 |
| Diabetes, n (%) | 23 (15.3%) | 25 (16.66%) | 0.652 |
| Hypertension, n (%) | 16 (10.66%) | 24 (16%) | 0.756 |
| Aminoglycosides, n (%) | 3 (2%) | 5 (3.33%) | 0.236 |
| Intravenous Contrast, n (%) | 4 (2.66%) | 4 (2.66%) | 0.531 |
| ACE Ia/ARBb, n (%) | 2 (1.33%) | 3 (2%) | 0.956 |
| NSAIDsc, n (%) | 3 (2%) | 3 (2%) | 0.898 |
aACEi - Angiotensin Converting Enzyme Inhibitor, bARB - Angiotensin Receptor Blocker, cNSAIDs - Non Steroidal Anti- Inflammatory Drugs
The severity of the AKI was assessed by the KDIGO AKI criteria.[5] Of the fifty-nine patients with AKI, twenty-nine (49.1%) patients had Stage 1 AKI, twelve patients (20.3%) Stage 2 AKI and eighteen patients (30.5%) had Stage 3 of AKI. Dialysis was required in five (8.4%) patients. The planned duration of antibiotic therapy was not completed in twenty six (17.3%) patients due to worsening of renal functions and/or mortality.
Of the 59 patients with worsening of renal functions, 40 patients (67.7%) had complete recovery and five patients had partial renal recovery of kidney functions after stopping the drug. The remaining fourteen patients died in hospital, and there was partial recovery of renal functions in five of these patients after stopping Colistin. The details are further depicted in Figure 1.
Figure 1.
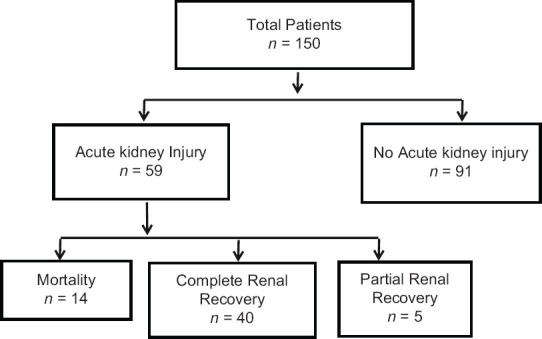
Figure depicting the outcome of patients treated with Colistin
Discussion
The parenteral form of Colistin is sodium colistimethate (CMS), which is converted to Colistin in vivo.[6,7] The bactericidal effect of Colistin is attributed to its binding with lipopolysacharide of bacterial cell wall of Gram-negative bacteria, leading to its increased permeability of bacterial cell wall and cell death. It also binds to endotoxin and neutralizes it.[8,9] The most important side effects of Colistin are nephrotoxicity and neurotoxicity. The mechanism of nephrotoxicity is increase in permeability of renal tubular epithelial cells, causing cell lysis and acute tubular necrosis.[7,8] Apoptosis of the tubular epithelial cells mediated by Caspases have also been thought to be a mechanism of nephrotoxicity.[10,11] Neurotoxicity due to Colistin is dose dependent and reversible, manifesting as paresthesia, peripheral neuropathy, ptosis, ophthalmoplegia or even severe neuromuscular blockade resulting in respiratory paralysis requiring ventilatory support.[7,12]
In our study of 150 patients who received Colistin, 59 patients (39.3%) developed worsening of renal functions after starting Colistin. This was similar to other large studies; Rocco et al.[13] reported 40% nephrotoxicity while Sorli et al. reported 25.5% at day 7 and 49% nephrotoxicity at the end of treatment.[14]
Age was found to be a factor responsible for worsening of renal functions. The mean age of patients who developed AKI was 63.65 ± 11.18 years compared to 48.05 ± 19.29 years for patients whose renal functions remained stable after initiation of Colistin (P < 0.001). Similar findings were observed in other studies as well, with age being an independent risk factor after adjusting for other variables.[15,16] The increased susceptibility of the elderly can be explained by several factors. Age-related decline in kidney function due to increased glomerulosclerosis, interstitial fibrosis and tubular atrophy make the elderly kidneys more prone to further toxic insults. Increased sensitivity to vasoconstrictors and reduced responsiveness to vasodilators impair the normal vascular response in elderly kidneys.[17] Increased oxidative stress due to defective antioxidant mechanisms, increased cell death due to apoptosis, upregulation of inflammation and defective cell repair potential of the cells due to various molecular defects can also explain the increased risk of AKI in elderly due to Colistin.[18]
The risk of worsening was higher if the patients had underlying CKD (eGFR less than 60 mL/minute/1.73m2) at the initiation of Colistin. The incidence of nephrotoxicity varied from 35% in patients with stage 2 CKD to 87.5% in Stage 4 CKD. This risk was much higher compared to the study by Doshi et al.[19] who reported 40% nephrotoxicity in patients with CKD; the mean GFR of patients in that study was 43 mL/minute/1.73 m2 compared to mean GFR of 40 mL/minute/1.73m2 in our study.
Numerous studies have identified concomitant use of aminoglycosides, non-steroidal anti-inflammatory drugs (NSAIDs), vancomycin, calcineurin inhibitors and intravenous contrast agents as risk factors for worsening of renal functions.[15,20,21] However in our study the use of angiotensin-converting enzyme inhibitors/angiotensin receptor Blockers, NSAIDs, aminoglycosides and intravenous contrast did not add to the risk, probably because the numbers were very small and clinicians were cautious to avoid these drugs in this group of patients. The study found no impact of gender, diabetes and hypertension on development of AKI.
Critically ill patients, requiring vasopressor support for treatment of shock, had a 50% risk of worsening of kidney function with use of Colistin {OR 6.21, 95% CI (1.83, 21.0)}. It is likely that the ischemia to the kidneys from septic shock, probably makes the renal tubules more prone to toxic damage from Colistin. Septic shock was found to be a significant risk factor for AKI in the study by Rocco et al.[13]. Deterioration of kidney function with Colistin was also found to be a risk factor for in-hospital mortality. This finding has been consistent even after adjusting for septic shock as a confounder, making it an independent predictor for mortality.
The median time interval between initiation of Colistin therapy and rise in serum creatinine was 4 days (Range 2–20 days). It was observed that among patients with worsening of kidney functions, 60% of patients developed AKI within 7 days of therapy. An early onset of nephrotoxicity is described in the study by Deryke et al.,[22] with all patients developing worsening of kidney function within 5 days.
Among the 59 patients who had worsening of renal functions, 40 patients (67.8%) had complete recovery of renal functions (serum creatinine reduced to 25% of the baseline) within a median period of 10 days (Range 7–15 days) after discontinuing the drug. Lidia et al. reported renal recovery in 64.5% of patients after a median period of 10.5 days which was similar to our findings.[23] A major reason for non-recovery of renal function was early mortality in a majority of patients (73.7%), thus providing the kidneys too short a time to recover.
There was no association of worsening of kidney function with duration or cumulative dose of Colistin. This, contrary to other similar studies, could be attributed to the fact that Colistin was stopped early in patients with nephrotoxicity which led to the data being skewed. The studies by Rattanaumpawan et al.[15] and Pogue et al.[24] reported dose and duration of Colistin therapy to be risk factors for nephrotoxicity.
Our study has many limitations. It was a retrospective, single-center study. The blood levels of Colistin were not assessed. The data of urine output in patients with AKI was not available. While calculating creatinine clearance using Cockroft-Gault formula, bodyweight rather than adjusted body weight was used. The analysis of critically ill patients did not include well-studied scoring systems like Sequential Organ Failure Assessment (SOFA) scores which predict their outcome. Further, the cumulative nephrotoxicity due to other nephrotoxic agents could not be meaningfully assessed due to the small number of patients receiving such agents.
Conclusion
Colistin has significant nephrotoxicity, the risk of AKI is higher with older age, vasopressor requirement and underlying CKD, with the former two factors also associated with Stage 3 AKI. The median time to AKI was 4 days, so it is important to monitor renal functions early and at regular intervals after initiating therapy. The median time to recovery was 10 days. Clinicians should be aware of the risk factors for nephrotoxicity with Colistin for taking an informed decision before starting the drug.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Levey AS, Coresh J, Greene T, Stevens LA, Zhang YL, Hendriksen S, et al. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006;145:247–54. doi: 10.7326/0003-4819-145-4-200608150-00004. [DOI] [PubMed] [Google Scholar]
- 2.Nation RL, Garonzik SM, Thamlikitkul V, Giamarellos-Bourboulis EJ, Forrest A, Paterson DL, et al. Dosing guidance for intravenous colistin in critically ill patients. Clin Infect Dis. 2017;64:565–71. doi: 10.1093/cid/ciw839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Murata K, Baumann NA, Saenger AK, Larson TS, Rule AD, Lieske JC. Relative performance of the MDRD and CKD-EPI equations for estimating glomerular filtration rate among patients with varied clinical presentations. Clin J Am Soc Nephrol CJASN. 2011;6:1963–72. doi: 10.2215/CJN.02300311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Levin A, Stevens PE, Bilous RW, Coresh J, Francisco ALMD, Jong PED, et al. Kidney disease: Improving global outcomes (KDIGO) CKD work group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl. 2013;3:1–150. [Google Scholar]
- 5.Kidney Disease Improving Global Outcomes (KDIGO). Clinical practice guideline for acute kidney injury. Int Suppl. 2012;2:1–38. [Google Scholar]
- 6.Li J, Nation RL, Turnidge JD, Milne RW, Coulthard K, Rayner CR, et al. Colistin: the re-emerging antibiotic for multidrug-resistant Gram-negative bacterial infections. Lancet Infect Dis. 2006;6:589–601. doi: 10.1016/S1473-3099(06)70580-1. [DOI] [PubMed] [Google Scholar]
- 7.Mendes CAC, Burdmann EA. Polymyxins-review with emphasis on nephrotoxicity. Rev Assoc Medica Bras (1992) 2009;55:752–9. doi: 10.1590/s0104-42302009000600023. [DOI] [PubMed] [Google Scholar]
- 8.Nation RL, Li J. Colistin in the 21st century. Curr Opin Infect Dis. 2009;22:535–43. doi: 10.1097/QCO.0b013e328332e672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li J, Nation RL, Milne RW, Turnidge JD, Coulthard K. Evaluation of colistin as an agent against multi-resistant Gram-negative bacteria. Int J Antimicrob Agents. 2005;25:11–25. doi: 10.1016/j.ijantimicag.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 10.Falagas ME, Kasiakou SK. Colistin: The revival of polymyxins for the management of multidrug-resistant gram-negative bacterial infections. Clin Infect Dis Off Publ Infect Dis Soc Am. 2005;40:1333–41. doi: 10.1086/429323. [DOI] [PubMed] [Google Scholar]
- 11.Falagas ME, Kasiakou SK. Toxicity of polymyxins: A systematic review of the evidence from old and recent studies. Crit Care Lond Engl. 2006;10:R27. doi: 10.1186/cc3995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ozkan G, Ulusoy S, Orem A, Alkanat M, Mungan S, Yulug E, et al. How does colistin-induced nephropathy develop and can it be treated? Antimicrob Agents Chemother. 2013;57:3463–9. doi: 10.1128/AAC.00343-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rocco M, Montini L, Alessandri E, Venditti M, Laderchi A, De Gennaro P, et al. Risk factors for acute kidney injury in critically ill patients receiving high intravenous doses of colistin methanesulfonate and/or other nephrotoxic antibiotics: A retrospective cohort study. Crit Care. 2013;17:R174. doi: 10.1186/cc12853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sorli L, Luque S, Grau S, Berenguer N, Segura C, Montero MM, et al. Trough colistin plasma level is an independent risk factor for nephrotoxicity: A prospective observational cohort study. BMC Infect Dis. 2013;13:380. doi: 10.1186/1471-2334-13-380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rattanaumpawan P, Ungprasert P, Thamlikitkul V. Risk factors for colistin-associated nephrotoxicity. J Infect. 2011;62:187–90. doi: 10.1016/j.jinf.2010.11.013. [DOI] [PubMed] [Google Scholar]
- 16.Koksal I, Kaya S, Gencalioglu E, Yilmaz G. Evaluation of risk factors for intravenous colistin use-related nephrotoxicity. Oman Med J. 2016;31:318–21. doi: 10.5001/omj.2016.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jerkić M, Vojvodić S, López-Novoa JM. The mechanism of increased renal susceptibility to toxic substances in the elderly Part I: The role of increased vasoconstriction. Int Urol Nephrol. 2001;32:539–47. doi: 10.1023/a:1014484101427. [DOI] [PubMed] [Google Scholar]
- 18.Wang X, Bonventre JV, Parrish AR. The aging kidney: increased susceptibility to nephrotoxicity. Int J Mol Sci. 2014;15:15358–76. doi: 10.3390/ijms150915358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Doshi NM, Mount KL, Murphy CV. Nephrotoxicity associated with intravenous colistin in critically ill patients. Pharmacotherapy. 2011;31:1257–64. doi: 10.1592/phco.31.12.1257. [DOI] [PubMed] [Google Scholar]
- 20.Kim J, Lee KH, Yoo S, Pai H. Clinical characteristics and risk factors of colistin-induced nephrotoxicity. Int J Antimicrob Agents. 2009;34:434–8. doi: 10.1016/j.ijantimicag.2009.06.028. [DOI] [PubMed] [Google Scholar]
- 21.Kwon JA, Lee JE, Huh W, Peck KR, Kim Y-G, Kim DJ, et al. Predictors of acute kidney injury associated with intravenous colistin treatment. Int J Antimicrob Agents. 2010;35:473–7. doi: 10.1016/j.ijantimicag.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 22.DeRyke CA, Crawford AJ, Uddin N, Wallace MR. Colistin dosing and nephrotoxicity in a large community teaching hospital. Antimicrob Agents Chemother. 2010;54:4503–5. doi: 10.1128/AAC.01707-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dalfino L, Puntillo F, Ondok MJM, Mosca A, Monno R, Coppolecchia S, et al. Colistin-associated acute kidney injury in severely ill patients: A step toward a better renal care? A prospective cohort study. Clin Infect Dis. 2015;61:1771–7. doi: 10.1093/cid/civ717. [DOI] [PubMed] [Google Scholar]
- 24.Pogue JM, Lee J, Marchaim D, Yee V, Zhao JJ, Chopra T, et al. Incidence of and risk factors for colistin-associated nephrotoxicity in a large academic health system. Clin Infect Dis Off Publ Infect Dis Soc Am. 2011;53:879–84. doi: 10.1093/cid/cir611. [DOI] [PubMed] [Google Scholar]


