Abstract
Despite the development of safe and effective vaccines, effective treatments for COVID-19 disease are still urgently needed. Several antiviral drugs have shown to be effective in reducing progression of COVID-19 disease. In the present work, we use an agent-based mathematical model to assess the potential population impact of the use of antiviral treatments in four countries with different demographic structure and current levels of vaccination coverage: Kenya, Mexico, United States (US) and Belgium. We analyzed antiviral effects on reducing hospitalization and death, and potential antiviral effects on reducing transmission. For each country, we varied daily treatment initiation rate (DTIR) and antiviral effect in reducing transmission (AVT). Irrespective of location and AVT, widespread antiviral treatment of symptomatic adult infections (≥20% DTIR) prevented the majority of COVID-19 deaths, and recruiting 6% of all adult symptomatic infections daily reduced mortality by a third in all countries. Furthermore, our model projected that targeting antiviral treatment to the oldest age group (65 years old and older, DTIR of 20%) can prevent over 47% of deaths. Our results suggest that early antiviral treatment (as soon as possible after inception of infection) is needed to mitigate transmission, preventing 50% more infections compared to late treatment (started 3 to 5 days after symptoms onset). Our results highlight the synergistic effect of vaccination and antiviral treatment: as the vaccination rate increases, antivirals have a larger relative impact on population transmission. These results suggest that antiviral treatments can become a strategic tool that, in combination with vaccination, can significantly reduce COVID-19 hospitalizations and deaths and can help control SARS-CoV-2 transmission.
Keywords: antiviral, treatment, COVID-19, SARS-CoV-2, mathematical model
Introduction
With over 5 million deaths worldwide [1], the COVID-19 pandemic has proven difficult to contain. Despite the development, advent, licensure, and rollout of many safe and effective vaccines [2], controlling SARS-CoV-2 transmission has shown to be elusive for several reasons, including vaccine supply shortages in low- and middle-income countries [3], vaccine hesitancy [4], and the emergence of new variants [5]. Indeed, the Delta and Omicron variants, that emerged in summer and fall of 2021, quickly became the predominant strains and have caused large epidemic outbreaks, even in highly vaccinated regions [1]. Rapidly producing such COVID-19 vaccines has been an amazing scientific endeavor, but effective tools to treat COVID-19 disease are still urgently needed. Monoclonal antibodies, antibody cocktails and antiretroviral treatments have been, and continue to be studied to treat SARS-CoV-2 infection and to prevent progression to severe disease [6]. Several treatments have been found to reduce hospitalizations by 30% to 89% [7–10] when taken within the first five days after developing symptoms. Some of them are approved for early treatment of patients with mild-to moderate COVID-19 who are at high-risk of progression to severe disease while others are approved for hospitalized patients, with one approved as a pre-exposure prophylaxis [11]. Furthermore, most studies have shown that these antiviral treatments significantly reduced the amount of infectious virus in the nasal mucosa of treated individuals [12, 13]. Hence, the advent of effective antiviral drugs raises the possibility that in treating infected individuals we may reduce onward transmission (indirect population benefit) while also protecting the treated person from severe disease (direct benefit). The use of antiviral treatments as an effective means of prevention and epidemic control is not new. During the 2009 influenza A H1N1 pandemic, just a few weeks after the first case of influenza A H1N1 was identified in the US, the US government released 11 million courses of antiviral drugs for influenza (25% of the antiviral supply) from the National Stockpile as a potential tool to control transmission and mitigate disease [14]. Treatment as Prevention is considered a primary method of epidemic control for HIV, as research has demonstrated that earliest detection and treatment suppressing HIV replication stops secondary transmission while having the the greatest effect at the individual level [15–17].
Over the past several months, the availability of antigen tests has expanded considerably, facilitating the early diagnosis of SARS-CoV-2 infection and possible early treatment [18, 19]. The US government has purchased 20 million courses of the antiviral pill paxlovid; these are expected to be delivered in early 2022. Furthermore, a “Test to Treat” initiative was recently announced as part of a new phase in the US government pandemic response [20]. The Medicines Patent Pool and the manufacturers of molnupiravir and paxlovid (Merck and Pfizer respectively) have announced license agreements to facilitate global access for these drugs [21, 22], and Pfizer will donate 4 million courses of paxlovid to UNICEF for use in lower-income countries in the following months [23]. Hence, it is possible that in the next few months antiviral treatments will become widely available globally.
In this work, we use an agent-based mathematical model to evaluate the potential population impact of widespread use of antiviral treatments in reducing hospitalization risk and population-level transmission. We explored the use of antiviral treatments in four different countries (Kenya, Mexico, US and Belgium) with very different demographic composition and vastly different proportions of vaccinated individuals. We showed that the synergistic use of vaccine and antiviral treatments can significantly reduce the burden of COVID-19. Further, our model suggested that targeted use of antiviral treatments can be used to prevent the majority of deaths.
Results
Briefly, we used COVASIM, a previously developed agent-based model of SARS-CoV-2 spread calibrated to Seattle, WA [24, 25]. Our model simulates a population of 500,000 people interacting through a network over the course of 6 months, where each individual in the population is an agent. Every day, individuals contact others in four possible locations: home, school, work or community. At a given point in time, individuals can be susceptible, infected asymptomatic, pre-symptomatic or symptomatic, and recovered. Symptomatic infected individuals can develop a mild, severe or critical infection after symptoms onset. A fixed age-dependent proportion of infections is assumed to remain asymptomatic. Within each age stratum, asymptomatic infections are assumed 30% less transmissible than symptomatic infections (Table S1). Infectivity is time dependent, being highest around symptom onset and decreasing afterwards. We assumed that 40% of the population has been infected in previous epidemic waves and protected from re-infection over the study duration, and compared these results to scenarios assuming 20 and 60% pre-existing immunity. We simulated populations of equal size in four different countries: Kenya, Mexico, US and Belgium. For each country, the model uses country-specific demographics to inform population structure and household sizes. Because we are interested in investigating population effects of antivirals in different epidemic contexts that may modulate their effects, we explored two main scenarios: first, we assumed deployment would take place under an Omicron-like wave, representing a high transmissible variant for which vaccines might not be very effective at preventing infections. In this scenario, we assumed vaccination coverages in each country as of January 3rd 2022, separating the proportion of the population that received full doses or boosters in US and Belgium. Then, we repeated the analysis assuming antiviral deployment under a Delta-like wave, a less transmissible variant for which vaccines remained moderately effective in preventing infections. Here, we assumed vaccination coverage as of October 12th, 2021. For each scenario, we used different parameters for viral transmission, and vaccine effectiveness (Methods for full details).
We assumed that the antiviral treatment would have two primary effects. First, in line with results from the EPIC-HR (paxlovid), PINETREE (remdesivir), COMET-ICE (sotrovimab), and MOVe-OUT (molnupiravir) clinical trials, that showed a very high reduction in hospitalization or deaths, we assumed an antiviral effect on hospitalization (denoted by AVH) by which a course of antiviral treatment would reduce the rate of hospitalization of symptomatic infected individuals by 88% (main results) or 30% (sensitivity analysis) [7–10]. Second, because clinical trials have reported an antiviral effect on viral load, [7, 12, 13], we assumed that the antiviral treatment would have an effect in overall transmission (denoted by AVT). Given uncertainty in how a reduction in viral load translates into a reduction in transmission, we explored reductions in secondary transmission 25, 50, 75, or 100% due to antiviral treatment.
We assumed that antiviral treatment would be given to symptomatically infected adults (18 years or older) within five days from symptoms onset. We explored scenarios with treatment initiated within five days, within two days or between days 3 and 5 from symptoms onset, to study the impact of treatment timing on the results. To reflect real-world constraints on resources and infrastructure, we also varied the rate at which symptomatic adults could be identified and recruited for treatment, considering scenarios with a daily treatment initiation rate (DTIR) of 2, 4, 6, 8, 10, 20, … 100% of symptomatic adults. For example, a 6% DTIR resulted on treating roughly 26% of the overall adult symptomatic infections (total symptomatic infections over the duration of the study), while a DTIR of 40% resulted in treating over 90% of the overall adult symptomatic infections. To capture variability, 100 simulations were run for each scenario. Throughout the text, we present the median percentage deaths and infections averted compared to base-case scenario assuming that existing non-pharmaceutical measures remain in place for the next 6 months without additional vaccination. Cumulative deaths and infections (and respective confidence bounds) are presented in the Supplemental Material.
Omicron-like wave.
Targeted use of antiviral treatment can avert large numbers of deaths.
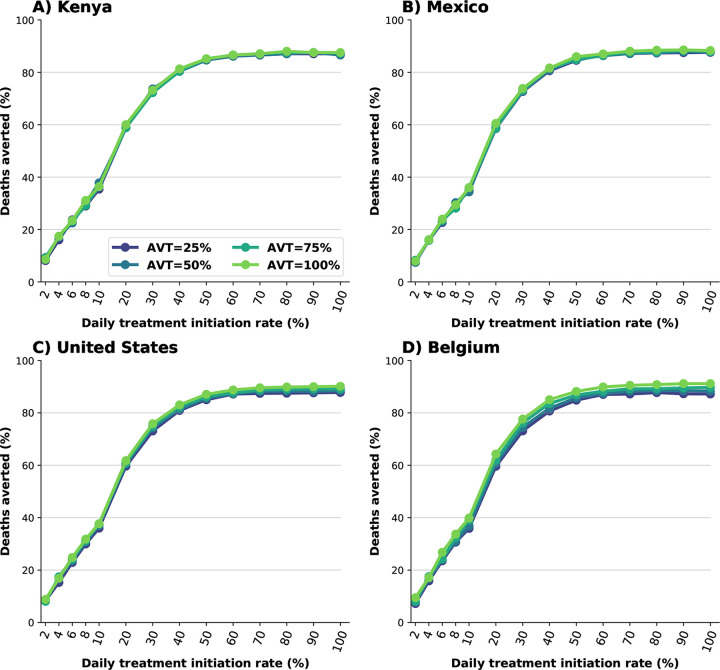
We first examine the use of antiviral treatment in all adults. For all the countries considered, irrespective of the antiviral effect on viral transmission, large numbers of deaths were averted for all scenarios, even if the DTIR was low. A 6% DTIR among all adult symptomatic infections averted over 20% of deaths when compared to no antiviral use in all countries (24, 23, 23 and 24% deaths averted for Kenya, Mexico, US and Belgium respectively assuming AVT=25%). Over 58% of deaths would be averted if 20% of all adult symptomatic infections were identified and recruited for treatment daily (58, 58, 59 and 59% of deaths averted for Kenya, Mexico, US and Belgium respectively assuming AVT=25%). If the DTIR increased to 50% or more, over 85% of deaths would be averted in all countries. We observed minimal differences between countries and assumed levels of antiviral reduction in transmission, pointing to the fact that these reductions are a result of direct rather than indirect antiviral treatment protection (Figs. 1 and S1).
Figure 1:
Percentage of deaths averted (compared to a baseline of no antiviral treatment) for A) Kenya, B) Mexico, C) United States and D) Belgium. Here, we assumed an epidemic wave with parameters similar to those of the Omicron epidemic wave (transmissibility, vaccine effectiveness, and vaccination coverage). For each country, the colors represent four possible values of AVT (25, 50, 75 or 100% reduction in viral transmission in treated symptomatic individuals) and a daily treatment initiation rate (DTIR) of 2–100% of adult symptomatic individuals within the first 5 days of symptoms.
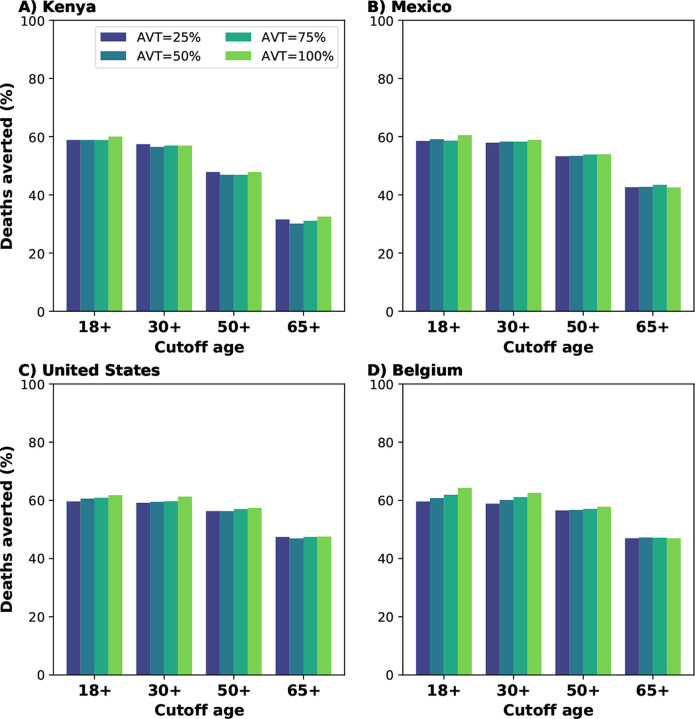
Because identifying, recruiting and treating large numbers of all the eligible symptomatic adults (individuals aged 18 or older) might be difficult to implement in practice, especially in countries where testing is not widely available or where antiviral treatment is in short supply, we then evaluated additional targeted strategies where we restricted antiviral treatment to adults over 30, 50 or 65 years of age and considered a 20% DTIR among these populations. Concentrating antiviral treatment in the older age groups can achieve high population impact: despite the fact that the older age groups are the smallest and have the highest vaccination rates (table S4), identifying and treating 20% of the daily symptomatic infections among older adults (aged 65 years old and older) averted 32, 43, 47 and 47% of all deaths in Kenya, Mexico, US and Belgium respectively (assuming 25% reduction in viral transmission). Adults aged 50 and older represent 26, 40 and 44% of the adult populations in Mexico, US and Belgium respectively, but recruiting 20% of the daily symptomatic infections in this age group averted the majority of deaths in these countries (53, 54 and 56% deaths averted in Mexico, US and Belgium respectively). More impressively, while this age group represents only 13% of the adult population in Kenya, initiating antiviral treatment in 20% of the symptomatic infections in this age group daily resulted in 47% of deaths averted. Extending the use of antiviral treatment to younger adults resulted in a marginal gain in Mexico, US and Belgium (a maximum 7, 4 and 6% more deaths averted respectively) when compared with targeted treatment in adults older than 50 (Fig. 2).
Figure 2:
Percentage of deaths averted (compared to a baseline of no antiviral treatment) for A) Kenya, B) Mexico, C) United States and D) Belgium. Here, we assumed an epidemic wave with parameters similar to those of the Omicron epidemic wave (transmissibility, vaccine effectiveness, and vaccination coverage). For each country, the colors represent four possible values of AVT (25, 50, 75 or 100% reduction in viral transmission in treated symptomatic individuals) and targeting the antiviral treatment to symptomatic adults older than 18, 30, 50 or 65 years of age with a daily treatment initiation rate of 20%.
Synergistic effect of antiviral treatment and vaccine can mitigate SARS-CoV-2 transmission.
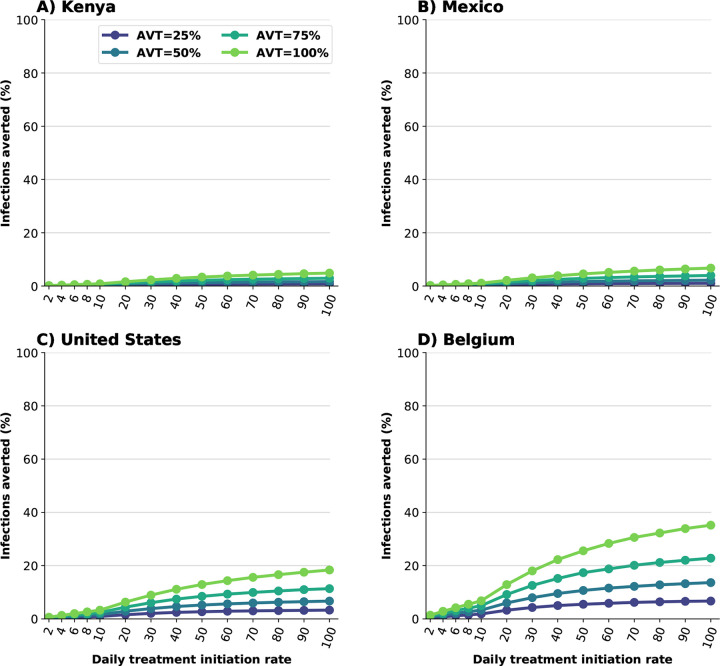
We evaluated the potential effect of antiviral treatment on population incidence of SARS-CoV-2, mediated through reduced transmission. Our model projected that antiviral treatment will have a very limited impact in mitigating transmission in the presence of a variant as transmissible as Omicron in countries like Kenya or Mexico that have low vaccination and boosting rates. In highly vaccinated countries like the US and Belgium, our results showed that antiviral treatment had some impact provided that a large proportion of symptomatic infections are identified and recruited for treatment and the antiviral effect on transmission is high (Figs. 3 and S2). This is because the Omicron variant was very transmissible and vaccination with two doses had a minimal effect against preventing infection, but adding a third dose of vaccine provided some protection against acquisition. Further, we found a clear synergy of combining antiviral treatment and vaccination. While Belgium and the US have similar population structure, vaccination coverage in Belgium has been much higher than in the US (both with full doses and with boosters, tables S5 and S6). When combined with antiviral treatment, this difference had a big effect in mitigating transmission: for example, in the most optimistic scenario, assuming that the antiviral treatment completely blocked transmission, a 30% DTIR of adult symptomatic infections averted 9% of overall infections in the US, and twice as many infections were averted in Belgium (18%). In contrast, for this scenario, only 7% of overall infections were averted in Mexico, which had a high vaccination coverage but had not been boosted the population at that time, and 5% of the overall infections were averted in Kenya. Indeed with very limited vaccine supply, the effective reproductive number in Kenya is much larger than in the other countries, resulting in epidemics that are more difficult to control (Figs. 3 and 4). As expected, the antiviral effect on viral transmission played a bigger role in controlling SARS-CoV-2 transmission. With a low AVT (25%), a very limited number of infections were averted, irrespective of treatment initiation rate or country, with a maximum of 6% of infections averted, but a maximum of 35% of infections were averted with high AVT (Belgium, Fig. 3D). Note that, for AVT= 25, 50 or 75%, the number of infections averted plateaued for medium and high antiviral treatment coverage. For example, in the US, a DTIR of 50% of adult symptomatic infections with an antiviral treatment with AVT=50% averted 5% of infections, and treating an additional 20% of symptomatic adults per day (70% DTIR) resulted in only 2% more infections averted (Fig. 3).
Figure 3:
Percentage of infections averted (compared to a baseline of no antiviral treatment) for A) Kenya, B) Mexico, C) United States and D) Belgium. Here, we assumed an epidemic wave with parameters similar to those of the Omicron epidemic wave (transmissibility, vaccine effectiveness, and vaccination coverage). For each country, the colors represent four possible values of AVT (25, 50, 75 or 100% reduction in viral transmission in treated symptomatic individuals) and a daily treatment initiation rate (DTIR) of 2–100% of adult symptomatic individuals within the first 5 days of symptoms.
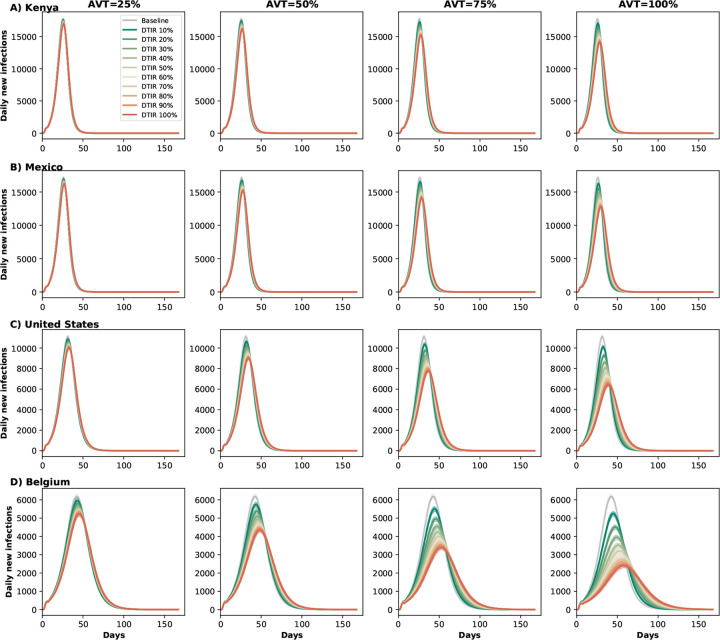
Figure 4:
Daily new infections assuming no antiviral treatment (Baseline) or assuming coverage of 10–100% of eligible symptomatic individuals in A) Kenya, B) Mexico, C) United States and D) Belgium. Here, we assumed an epidemic wave with parameters similar to those of the Omicron epidemic wave (transmissibility, vaccine effectiveness, and vaccination coverage). For each location, each column represents a different value of AVT (25, 50, 75 or 100% reduction in viral load).
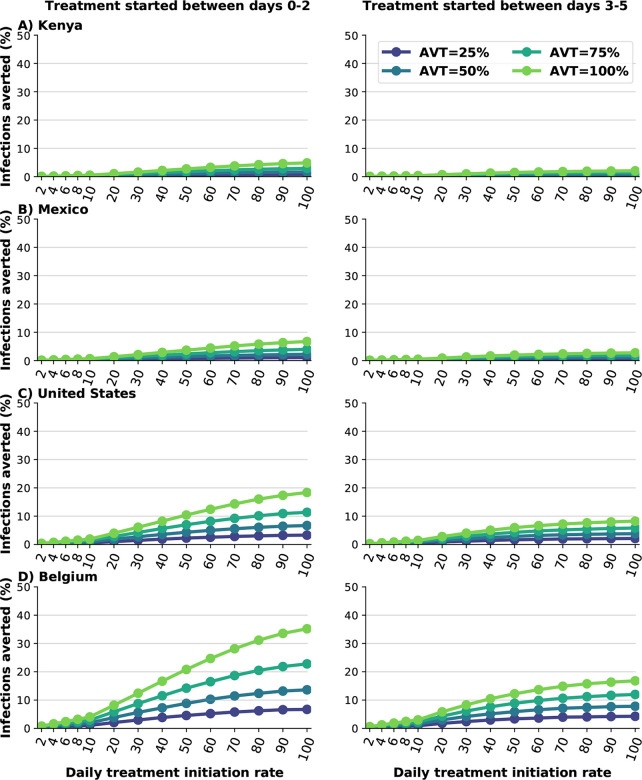
Early treatment is needed to mitigate transmission.
We analyzed the difference between early and late treatment by considering treating eligible symptomatic infections within the first two days of symptoms or alternatively between days 3 and 5 after symptom onset. For Kenya and Mexico, late treatment had no impact on transmission (Fig. 5A and B). This is because viral transmission is extremely high and antiviral treatment is happening after most of the secondary infections among symptomatic individuals have occurred. For the US or Belgium, starting antiviral treatment during the first two days of symptom onset prevented roughly twice as many subsequent infections compared to late treatment (Fig. 5C and D). For example, with a DTIR of 50% of symptomatic infections, and AVT = 75%, 14% and 8% of overall infections were averted in Belgium with early and late treatment respectively (Fig. 5D).
Figure 5:
Percentage of deaths averted (compared to a baseline of no antiviral treatment) for A) Kenya, B) Mexico, C) United States and D) Belgium for early (within the first two days of symptoms, left column) or late (between days 2 and 5 of symptoms, right column) treatment. Here, we assumed an epidemic wave with parameters similar to those of the Omicron epidemic wave (transmissibility, vaccine effectiveness, and vaccination coverage). For each country, the colors represent four possible values of AVT (25, 50, 75 or 100% reduction in viral transmission in treated symptomatic individuals) and covering 10–100% of eligible symptomatic individuals.
Delta-like epidemic wave.
In this section we present the results assuming antiviral treatment was given under similar conditions as those experienced during the Delta wave: we considered vaccination coverages as of October 12th, 2021, higher vaccine efficacy (table S2), and a virus as transmissible as the Delta variant (full details in Methods). Under this scenario, the number of deaths averted was slightly higher than for the Omicron wave with an average 2% more deaths averted in all countries (Figs. 1 and S3). Additionally, there were some differences in the number of deaths averted depending on the assumed antiviral effect on transmission, with a maximum of 3, 7, 9 and 12% more deaths averted in Kenya, Mexico, US and Belgium respectively for an antiviral treatment fully blocking transmission compared with one reducing transmission by 25%, suggesting a small indirect effect on the number of deaths averted as a result of reduced transmission. More importantly, under this scenario, we observed that antiviral treatment markedly cut overall viral transmission, and the synergy with vaccination was more apparent. This is because the reduced transmissibility of a Delta-like variant combined with higher vaccine efficacy and higher proportions of the population vaccinated result in a lower effective reproductive number. For example, assuming a DTIR of 30% of symptomatic adults and AVT=50%, 5, 12, 16 and 32% of infections were averted in Kenya, Mexico, US and Belgium respectively (compared to 1, 1, 4 and 8% under an Omicron wave respectively), Figs. 3 and S4.
Sensitivity analysis
In this section we analyze the sensitivity of our results to key model parameters. All the results presented here assumed an Omicron-type epidemic.
Increase or decreased infection-acquired pre-existing immunity.
Different places, even within a country, have experienced the COVID-19 pandemic in different ways, depending on local non-pharmaceutical interventions, culture (e.g. vaccine hesitancy, adherence to mask use) and population demographics [26, 27]. Hence, we considered the effect of pre-existing infection-acquired immunity on our results, assuming a lower (20%) or higher (60%) fraction of the population previously infected and currently immune. We observed minimal differences in the number of deaths averted for lower or higher assumed infection-acquired pre-existing immunity. This is expected, as the majority of deaths averted in our model are the result of direct effect of the antiviral treatment. However, the model projected differences in transmission assuming lower or higher infection-acquired pre-existing immunity. Lower infection-acquired immunity resulted in bigger epidemic waves and lower proportions of infections averted with antiviral treatment compared to the main results for all countries, all coverages and all AVEs (maximum 4, 6, 12 and 21% infections averted in Kenya, Mexico, US and Belgium respectively), Figs. S5 left column, S6. In contrast, higher infection-acquired immunity resulted in lower epidemic curves and more infections averted (maximum 13, 18, 41 and 82% infections averted in Kenya, Mexico, US and Belgium respectively), Figs. S5 right column and S7.
Reduced antiviral effectiveness against hospitalization, AVH = 30%.
One antiviral treatment (molnupiravir) was shown to have a 30% reduction in hospitalizations and deaths [7], so we repeated our analysis assuming this lower value for AVH. As expected, an antiviral treatment which reduces the hospitalization risk of symptomatically infected by 30% averted considerably less deaths than our main scenario (with AVH = 88%). For example, a DTIR of 20% among adult symptomatic infections resulted in over 20% of the deaths averted for all four countries, a ~40% reduction compared to the main scenario. Under this scenario, there was a stronger synergistic effect of vaccination and with the antiviral effect both on deaths averted and on transmission that was compounded with the antiviral effect on transmission: for example, for a DTIR of 20% and AVT=25% there were 19% and 21% deaths averted in Kenya and Belgium respectively (2% more deaths averted in Belgium), but if AVT=100% there was a 10% difference between the number of deaths averted in Belgium and Kenya (21% and 31% deaths averted in Kenya and Belgium respectively) difference Fig. S8.
Decrease in asymptomatic infectiousness
The relative infectivity of asymptomatic individuals remains uncertain [28]. We explored scenarios in which asymptomatic individuals are 50% less infectious than symptomatic individuals. The overall antiviral effect on mortality was not sensitive to this parameter: our model projected nearly identical deaths averted for each scenario considered, Fig. S9. However, the number of infections averted assuming a lower infectivity of asymptomatic infections was higher, with higher differences observed in countries with higher vaccination coverages. For example, under this scenario, 20% of the infections were averted in the US assuming a DTIR of 50% and an antiviral treatment fully reducing transmission, compared with 12% infections averted in the main scenario, Fig. S10.
Discussion
Despite the existence of effective vaccines, the number of deaths due to COVID-19 globally is still above 7,000 per day, with over 1,000 deaths per day in the US [1], due to issues with supply, hesitancy and logistics. This highlights the need of effective and affordable treatment options. Several antiviral treatments have shown to be highly efficacious against COVID-19 hospitalization and death, provided that treatment is started early, within 5 days of symptoms onset [7–10]. In the present work we analyzed the potential population impact of antiviral treatments for reducing SARS-CoV-2 transmission and COVID-19 related hospitalizations and deaths. We considered four equal-sized populations from four different countries (Kenya, Mexico, US and Belgium), representing different population structures and vaccination coverage. We further explored the impact of this intervention under epidemic waves with parameters similar to the Delta and Omicron waves. Our results suggest that irrespective of country, AVT and variant, widespread use of antiviral treatment (daily treatment initiation rate of 20% of the daily adult symptomatic infections) could prevent the majority of deaths. We projected that under an Omicron-like epidemic wave, with extremely high viral transmissibility and low vaccine effectiveness against infection acquisition, antiviral treatment of symptomatic infections will have very limited impact on transmission. This is expected as a large proportion of the infection chain is occurring in asymptomatic infections, and treating symptomatic infections under high transmissibility is not sufficient to curb overall transmission. Under this scenario, expanding antiviral treatment access to all detected infections might increase the effectiveness of antiviral treatments in this regard. However, if newer variants behaved more like the Delta variant, then our model showed that antiviral treatment in symptomatic adults can have a larger impact on curbing overall infections. Considerable practical challenges would be faced in identifying, testing, and treating symptomatic infections among all adults, especially among the younger adults, who are at much lower risk to progress to severe disease and death.
A clinical outcome of considerable interest is long COVID, to which all age groups appear to be susceptible [29, 30]. Long COVID consists of persistent symptoms including abnormal breathing, headache, fatigue, muscle weakness, anxiety or depression, headache, myalgia, and cognitive dysfunction [31]. To date, it is not known if, how and which (if any) antiviral treatments are effective against any of these post-COVID conditions. If antiviral treatments (either monoclonal antibodies or antiviral chemotherapy) were shown to be effective against long COVID, this could justify widespread use of antiviral treatments among all strata of the population. Of course, continuing promoting and financing wide scale testing would be critical for achieving widespread antiviral use.
Moreover, our model suggests that there is a synergistic effect of combining antiviral treatment and vaccination: countries with larger proportion of their populations vaccinated are expected to benefit more from adding antiviral treatment to their pandemic control toolbox. This emphasizes the need of continuous effort to improve vaccination coverage especially in settings where it is extremely low. Finally, our simulations showed that early treatment, when viral transmission is highest [32, 33], is important for mitigating transmission and could be used as a prevention tool. This is in agreement with previous work [34–38] that has shown the potential use of antiviral therapies to reduce COVID-19 related mortality.
Our model, like all mathematical models, is subject to several limitations. We did not consider the development of antiviral resistance, yet it is possible that if antiviral treatments are widely used, resistance could rapidly develop. In fact, monoclonal antibody treatments that were highly effective against older variants have become ineffective against newer ones [39, 40]. However, small molecule oral antiviral treatments have been shown to remain efficacious against new variants [41]. We analyzed the use of antiviral treatment in symptomatic individuals, and did not explore its use in asymptomatic infections or as a prophylaxis. Further, we assumed that an antiviral effect reducing transmission was independent of when treatment was started (as long as it started within the first five days of treatment). In reality, it is possible that antiviral treatments might have different effects in reducing overall transmission depending on when during the course of an infection they are taken, e.g. reducing overall viral load and hence transmission if taken early on but having only a modest effect as time goes by. Because studies have shown contradictory results regarding the effect of vaccines in reducing infectiousness [42–45], we conservatively assumed that the vaccine had no effect in reducing infectiousness. If vaccination does reduce infectiousness then the synergy between antiviral and vaccine might be less than reported here. We assumed no further vaccination during the period of our analysis, which corresponds to the current situation in most middle- and high-vaccinated countries, where the adult population who are willing to be vaccinated and boosted has mostly been immunized. Nevertheless, vaccines for children younger than 5 years old are currently in clinical trials, and are expected to be available during summer 2022. Vaccinating this age group will increase the overall proportion of the population vaccinated in high-income countries, hence reducing further on-going transmission. In addition, WHO recently released an ambitious plan to vaccinate 70% of the global population by mid 2022 [46]. Because we modeled four different countries with different non-pharmaceutical interventions and different cultures, we did not model any additional interventions, such as masking, or behavioral changes among diagnosed people. For simplicity, we did not include waning immunity, but explored scenarios where a higher or lower proportions of the population are currently immune. While it is extremely hard to predict when and how different populations will become vaccinated and the percentage of pre-existing immunity in each population, we believe that the scenarios that we have considered here, with different combinations of vaccination coverage and proportions of the population with pre-existing infection-induced immunity, are sufficient to capture the potential population-level effect of the potential use of antiviral treatment on transmission and COVID-19-related severe outcomes.
Taken together, our results suggest that antiviral treatment can be an extremely useful tool to reduce COVID-19 related deaths and to alleviate the COVID-19 burden on overwhelmed and exhausted healthcare systems. In particular, antiviral treatments, especially oral antiviral drugs that can be easily distributed, can have a huge impact in countries which have had less access to vaccines and boosters. Further, our model shows that the population level impact of antiviral treatments are enhanced by their synergistic use in combination with vaccination, particularly in the presence of less transmissible variants. Our results suggest that in the face of highly transmissible variants, unless antiviral treatments are shown to protect against or diminish long COVID conditions, antiviral treatment is best used by targeting it to groups being at high risk for disease progression. As more data emerges, mathematical modeling can be extremely useful to determine the optimal use of antiviral treatments.
Methods
Main model
We adapted a previously developed agent-based transmission model, COVASIM [24]. Namely, we extended the model to include the use of antiviral treatments in the population. We briefly describe here the main features of this model (as were used in the present work) and we describe in full detail the adaptations we made. We refer to the original article by Kerr et al. [24] for the full details of the model implementation. This is an agent-based model that simulates SARS-CoV-2 transmission and interventions that was originally calibrated to data for Seattle, WA, US. Each individual in the population is modeled as an agent in a network, with 500,000 agents. The model has four possible contact layers: home, school, work or community. For each location, the model uses country-specific demographics and household sizes. The model simulates infections, interventions antiviral treatment and vaccination. We did 100 runs for each scenario. We report the median of these runs and the 10th and 90th percentiles for the lower and upper bounds.
Disease dynamics
Individuals in the network can be susceptible, exposed (infected but not infectious), asymptomatic, presymptomatic or symptomatic. Symptomatic individuals have one of three fates: they develop a mild, severe, or critical disease. All mild infected individuals recover, and infected individuals reaching a critical state can recover or die. The latent period is sampled from a log-normal distributions with a mean of 4.6 days. The length of time between becoming infectious and developing symptoms is sampled from a log-normal distribution with mean 1.1 days. The times to develop severe symptoms, to progress to become critically ill and to death are sampled from lognormal distributions with means 6.6, 1.5 and 10.7 days respectively. Asymptomatic and mild cases recover on average on 8 days (sampled from log-normal distributions), while severe and critically ill cases recover on average on 18.1 days. Infectiousness was assumed to be linearly correlated to viral load [47]. As with the original model [24], we modeled viral load having two modes: first, a high mode where viral load is highest, around or before symptom onset, and a low mode having a longer duration but a lower viral load (50% lower than during the high mode). We assumed that children are less likely to develop symptoms than adults but equally likely to become infected [48, 49], Table S3. Further, asymptomatic individuals are assumed to be 30% less infectious than symptomatic individuals [50].
Viral transmission
We assumed that antiviral treatment would be deployed under epidemic waves with characteristics similar to the Delta or the Omicron waves. For the Delta wave, we assumed an increased transmissibility of 97% with respect to the ancestral variant [5]. For the Omicron wave, we assumed an increased transmissibility of 66% with respect to the Delta variant [51]. These assumptions resulted in basic reproduction number R0 for a Delta-like variant of approximately 4.95–5.68 and of 7.75–8.63 for an Omicron-like variant. We assumed that 0.5% of the population is infected at the beginning of the simulation and that 40% of the population (selected at random) in each country was previously infected and is immune for the duration of the study (20 and 60% were also explored in Sensitivity analysis). Table S1 summarizes all the parameters used in the model.
Antiviral treatment
As described above, we assumed that antiviral treatment had two main effects. First, antiviral treatment reduced the probability of symptomatic infected individuals becoming hospitalized. Second, we reduced viral load in treated individuals by 25, 50, 75 or 100%. Because in our model, viral load linearly correlates with infectiousness, this assumption resulted in equal reductions in the overall transmissibility of treated infected individuals. Each day of the simulation, the model identified eligible individuals (symptomatic adults over 18, 30, 50 or 65 years of age) whose symptoms onset was within the time frame studied (5 days, 2 days or within 3–5 days of symptoms onset). It then randomly selected a percentage of these individuals (excluding those who are already in treatment) for antiviral treatment initiation. Once an infected symptomatic individual initiated treatment, we assumed that antiviral effects would last for the subsequent days of his or her infection.
Vaccination
We considered a leaky vaccine (that is, a vaccine that confers partial protection to all vaccinated individuals) [52] having three effects on vaccinated individuals: to reduce the probability of acquiring a SARS-CoV-2 infection (denoted byVESUS), to reduce the probability of developing COVID-19 symptoms after infection (denoted by VESYMP), and to reduce the probability of hospitalization conditioned on symptomatic infection (denoted by VEH). Then it follows that
where VEDIS and VEHOSP are the unconditional vaccine effectiveness on symptomatic infection and hospitalization respectively. Importantly, under this model vaccination does not influence onward transmission of infection except through a reduction in SARS-CoV-2 infection. Vaccine effectiveness estimates for each modeled wave can be found in table S2. For Mexico, the US and Belgium, we used country- and age-specific vaccination rates (vaccination rates as of October 12th 2021 and January 3rd, 2022 for the Delta and Omicron waves respectively), tables S5–S7 [53–56]. For the US and Belgium, vaccinated individuals were further split between those having high (boosted individuals) or low protection for the Omicron wave. Since Kenya had fully vaccinated only a very small proportion of its population −1.5% and 7.7% of its population for each of these dates– and targeted front-line health workers, teachers, police and military, we distributed its vaccine randomly among all adults [3, 56, 57].
Supplementary Material
Acknowledgements
Funding:
Scientific Computing Infrastructure at Fred Hutch was funded by ORIP grant S10OD028685. HJ was supported by R56AI143418 and R01CA152089 from the National Institutes of Health. LM, DD and HJ were supported by UM1 AI068635 from the National Institutes of Health. ERB was supported by the Infectious Diseases Clinical Research Consortium through the National Institute of Allergy and Infectious Diseases, part of the National Institutes of Health, under award number UM1AI148684. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health
Footnotes
Competing interests: The authors declare no competing interests.
Code availability: Code will be available at: https://github.com/lulelita
References
- [1].Johns Hopkins Coronavirus Resource Center. Home - Johns Hopkins Coronavirus Resource Center. https://coronavirus.jhu.edu/, 2021. last accessed September 28th, 2021.
- [2].Basta Nicole and Moodie Erika, on behalf of the McGill University COVID19 Vaccine Tracker Team. COVID-19 Vaccine Development and Approvals Tracker. https://covid19.trackvaccines.org/, 2020. last accessed September 28th, 2021.
- [3].Mathieu Edouard, Ritchie Hannah, Ortiz-Ospina Esteban, Roser Max, Hasell Joe, Appel Cameron, Giattino Charlie, and Rodés-Guirao Lucas. A global database of covid-19 vaccinations. Nature Human Behaviour, 5(7):947–953, 2021. doi: 10.1038/s41562-021-01122-8. URL 10.1038/s41562-021-01122-8. [DOI] [PubMed] [Google Scholar]
- [4].Lazarus Jeffrey V., Ratzan Scott C., Palayew Adam, Gostin Lawrence O., Larson Heidi J., Rabin Kenneth, Kimball Spencer, and El-Mohandes Ayman. A global survey of potential acceptance of a covid-19 vaccine. Nature Medicine, 27(2): 225–228, 2021. doi: 10.1038/s41591-020-1124-9. URL 10.1038/s41591-020-1124-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Campbell Finlay, Archer Brett, Laurenson-Schafer Henry, Jinnai Yuka, Konings Franck, Batra Neale, Pavlin Boris, Vandemaele Katelijn, Van Kerkhove Maria D, Jombart Thibaut, Morgan Oliver, and le Polain de Waroux Olivier. Increased transmissibility and global spread of sars-cov-2 variants of concern as at june 2021. Eurosurveillance, 26(24):2100509, 2021. doi: 10.2807/1560-7917.ES.2021.26.24.2100509. URL https://www.eurosurveillance.org/content/10.2807/1560-7917.ES.2021.26.24.2100509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Westendorf Kathryn, Wang Lingshu, Žentelis Stefanie, Foster Denisa, Vaillancourt Peter, Wiggin Matthew, Lovett Erica, van der Lee Robin, Hendle Jörg, Pustilnik Anna, Sauder J. Michael, Kraft Lucas, Hwang Yuri, Siegel Robert W., Chen Jinbiao, Heinz Beverly A., Higgs Richard E., Kallewaard Nicole, Jepson Kevin, Goya Rodrigo, Smith Maia A., Collins David W., Pellacani Davide, Xiang Ping, de Puyraimond Valentine, Ricicova Marketa, Devorkin Lindsay, Pritchard Caitlin, O’Neill Aoise, Dalal Kush, Panwar Pankaj, Dhupar Harveer, Garces Fabian A., Cohen Courtney, Dye John, Huie Kathleen E., Badger Catherine V., Kobasa Darwyn, Audet Jonathan, Freitas Joshua J., Hassanali Saleema, Hughes Ina, Munoz Luis, Palma Holly C., Ramamurthy Bharathi, Cross Robert W., Geisbert Thomas W., Menacherry Vineet, Lokugamage Kumari, Borisevich Viktoriya, Lanz Iliana, Anderson Lisa, Sipahimalani Payal, Corbett Kizzmekia S., Yang Eun Sung, Zhang Yi, Shi Wei, Zhou Tongqing, Choe Misook, Misasi John, Kwong Peter D., Sullivan Nancy J., Graham Barney S., Fernandez Tara L., Hansen Carl L., Falconer Ester, Mascola John R., Jones Bryan E., and Barnhart Bryan C.. Ly-cov1404 (bebtelovimab) potently neutralizes sars-cov-2 variants. bioRxiv, 2022. doi: 10.1101/2021.04.30.442182. URL https://www.biorxiv.org/content/early/2022/01/07/2021.04.30.442182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Bernal Angélica Jayk, Gomes da Silva Monica M., Musungaie Dany B., Kovalchuk Evgeniy, Gonzalez Antonio, Reyes Virginia Delos, Martín-Quirós Alejandro, Caraco Yoseph, Williams-Diaz Angela, Brown Michelle L., Du Jiejun, Pedley Alison, Assaid Christopher, Strizki Julie, Grobler Jay A., Shamsuddin Hala H., Tipping Robert, Wan Hong, Paschke Amanda, Butterton Joan R., Johnson Matthew G., and Anda Carisa De. Molnupiravir for oral treatment of covid-19 in nonhospitalized patients. New England Journal of Medicine, 386(6):509–520, 2022/February/15 2021. doi: 10.1056/NEJMoa2116044. URL 10.1056/NEJMoa2116044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Gottlieb Robert L., Vaca Carlos E., Paredes Roger, Mera Jorge, Webb Brandon J., Perez Gilberto, Oguchi Godson, Ryan Pablo, Nielsen Bibi U., Brown Michael, Hidalgo Ausberto, Sachdeva Yessica, Mittal Shilpi, Osiyemi Olayemi, Skarbinski Jacek, Juneja Kavita, Hyland Robert H., Osinusi Anu, Chen Shuguang, Camus Gregory, Abdelghany Mazin, Davies Santosh, Behenna-Renton Nicole, Duff Frank, Marty Francisco M., Katz Morgan J., Ginde Adit A., Brown Samuel M., Schiffer Joshua T., and Hill Joshua A.. Early remdesivir to prevent progression to severe covid-19 in outpatients. New England Journal of Medicine, 386(4):305–315, 2022/February/15 2021. doi: 10.1056/NEJMoa2116846. URL 10.1056/NEJMoa2116846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Gupta Anil, Gonzalez-Rojas Yaneicy, Juarez Erick, Casal Manuel Crespo, Moya Jaynier, Falci Diego R., Sarkis Elias, Solis Joel, Zheng Hanzhe, Scott Nicola, Cathcart Andrea L., Hebner Christy M., Sager Jennifer, Mogalian Erik, Tipple Craig, Peppercorn Amanda, Alexander Elizabeth, Pang Phillip S., Free Almena, Brinson Cynthia, Aldinger Melissa, and Shapiro Adrienne E.. Early treatment for covid-19 with sars-cov-2 neutralizing antibody sotrovimab. New England Journal of Medicine, 385(21):1941–1950, 2022/February/15 2021. doi: 10.1056/NEJMoa2107934. URL 10.1056/NEJMoa2107934. [DOI] [PubMed] [Google Scholar]
- [10].Pfizer. Pfizer’s Novel COVID-19 Oral Antiviral Treatment Candidate Reduced Risk of Hospitalization or Death by 89EPIC-HR Study | Pfizer. https://www.pfizer.com/news/press-release/press-release-detail/pfizers-novel-covid-19-oral-antiviral-treatment-candidate, Nov 2021. last accessed Nov 5th, 2021. [Google Scholar]
- [11].FDA. Coronavirus Disease 2019 (COVID-19) EUA Information. https://www.fda.gov/emergency-preparedness-and-response/mcm-legal-regulatory-and-policy-framework/emergency-use-authorization#coviddrugs, February 2022. last accessed: February 28th, 2022.
- [12].Fischer William, Eron Joseph J, Holman Wayne, Cohen Myron S, Fang Lei, Szewczyk Laura J, Sheahan Timothy P, Baric Ralph, Mollan Katie R, Wolfe Cameron R, Duke Elizabeth R, Azizad Masoud M, Borroto-Esoda Katyna, Wohl David A, Loftis Amy James, Alabanza Paul, Lipansky Felicia, and Painter Wendy P. Molnupiravir, an Oral Antiviral Treatment for COVID-19., Jun 2021.
- [13].Pfizer. Pfizer Announces Additional Phase 2/3 Study Results Confirming Robust Efficacy of Novel COVID-19 Oral Antiviral Treatment Candidate in Reducing Risk of Hospitalization or Death. https://www.pfizer.com/news/press-release/press-release-detail/pfizer-announces-additional-phase-23-study-results, Dec 2021. last accessed March 18th, 2022.
- [14].Centers for Disease Control and Prevention. The 2009 H1N1 Pandemic: Summary Highlights, April 2009-April 2010. https://www.cdc.gov/h1n1flu/cdcresponse.htm, June 2010. last accessed March 18th, 2022.
- [15].Centers for Disease Control and Prevention. How to Optimize HIV Treatment | Treatment, Care, and Prevention for People with HIV | Clinicians | HIV | CDC. https://www.cdc.gov/hiv/clinicians/treatment/partner-prevention.html. last accessed October 12th, 2021. [Google Scholar]
- [16].Cohen Myron S., Chen Ying Q., McCauley Marybeth, Gamble Theresa, Hosseinipour Mina C., Kumarasamy Nagalingeswaran, Hakim James G., Kumwenda Johnstone, Grinsztejn Beatriz, Pilotto Jose H.S., Godbole Sheela V., Chariyalertsak Suwat, Santos Breno R., Mayer Kenneth H., Hoffman Irving F., Eshleman Susan H., Piwowar-Manning Estelle, Cottle Leslie, Zhang Xinyi C., Makhema Joseph, Mills Lisa A., Panchia Ravindre, Faesen Sharlaa, Eron Joseph, Gallant Joel, Havlir Diane, Swindells Susan, Elharrar Vanessa, Burns David, Taha Taha E., Nielsen-Saines Karin, Celentano David D., Essex Max, Hudelson Sarah E., Redd Andrew D., and Fleming Thomas R.. Antiretroviral therapy for the prevention of hiv-1 transmission. New England Journal of Medicine, 375(9):830–839, 2016. doi: 10.1056/NEJMoa1600693. URL 10.1056/NEJMoa1600693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Donnell Deborah, Baeten Jared M, Kiarie James, Thomas Katherine K, Stevens Wendy, Cohen Craig R, McIntyre James, Lingappa Jairam R, and Celum Connie. Heterosexual hiv-1 transmission after initiation of antiretroviral therapy: a prospective cohort analysis. The Lancet, 375(9731):2092–2098, 2010. ISSN 0140–6736. doi: 10.1016/S0140-6736(10)60705-2. URL https://www.sciencedirect.com/science/article/pii/S0140673610607052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].FDA. Coronavirus (COVID-19) Update: FDA Authorizes Additional OTC Home Test to Increase Access to Rapid Testing for Consumers. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-additional-otc-home-test-increase-access-rapid-testing October 2021. last accessed March 18th, 2022.
- [19].UNICEF. Most affordable COVID-19 rapid diagnostic test now available. https://www.unicef.org/supply/stories/most-affordable-covid-19-rapid-diagnostic-test-now-available, April 2021. last accessed March 18th, 2022.
- [20].The White House. Remarks of President Joe Biden – State of the Union Address As Prepared for Delivery. https://www.whitehouse.gov/briefing-room/speeches-remarks/2022/03/01/remarks-of-president-joe-biden-state-of-the-union-address-as-delivered/, March 2022. last accessed March 11th, 2022.
- [21].Medicines Patent Pool, UN. MPP and MSD announce new licence for investigational COVID-19 treatment. https://medicinespatentpool.org/news-publications-post/mpp-msd-new-licence-announcement-molnupiravir/, October 2021. URL https://medicinespatentpool.org/news-publications-post/mpp-msd-new-licence-announcement-molnupiravir/. last accessed October 27th, 2021.
- [22].Pfizer. Pfizer and The Medicines Patent Pool (MPP) Sign Licensing Agreement for COVID-19 Oral Antiviral Treatment Candidate to Expand Access in Low- and Middle-Income Countries. https://www.pfizer.com/news/press-release/press-release-detail/pfizer-and-medicines-patent-pool-mpp-sign-licensing, Nov 2021. last accessed February 24, 2022.
- [23].The New York Times. Pfizer will send 4 million courses of its Covid pill treatment to poorer countries. https://www.nytimes.com/live/2022/03/22/world/covid-19-mandates-cases-vaccine/pfizer-4-million-covid-pill-poorer-countries, March 2022. last accessed March 22, 2022.
- [24].Kerr Cliff C., Stuart Robyn M., Mistry Dina, Abeysuriya Romesh G., Rosenfeld Katherine, Hart Gregory R., Núñez Rafael C., Cohen Jamie A., Selvaraj Prashanth, Hagedorn Brittany, George Lauren, Jastrzębski Michał, Izzo Amanda S., Fowler Greer, Palmer Anna, Delport Dominic, Scott Nick, Kelly Sherrie L., Bennette Caroline S., Wagner Bradley G., Chang Stewart T., Oron Assaf P., Wenger Edward A., Panovska-Griffiths Jasmina, Famulare Michael, and Klein Daniel J.. Covasim: An agent-based model of covid-19 dynamics and interventions. PLOS Computational Biology, 17 (7):1–32, July 2021. doi: 10.1371/journal.pcbi.1009149. URL 10.1371/journal.pcbi.1009149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Kerr Cliff C., Mistry Dina, Stuart Robyn M., Rosenfeld Katherine, Hart Gregory R., Núñez Rafael C., Cohen Jamie A., Selvaraj Prashanth, Abeysuriya Romesh G., Jastrzębski Michał, George Lauren, Hagedorn Brittany, Panovska-Griffiths Jasmina, Fagalde Meaghan, Duchin Jeffrey, Famulare Michael, and Klein Daniel J.. Controlling covid-19 via test-trace-quarantine. Nature Communications, 12(1):2993, 2021. doi: 10.1038/s41467-021-23276-9. URL 10.1038/s41467-021-23276-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].COVIDESTIM. covidestim: COVID-19 nowcasting. https://covidestim.org/, 2020. URL https://covidestim.org/. last accessed October 12th, 2021.
- [27].Washington State Department of Health. COVID-19 Data Dashboard, 2021. URL https://www.doh.wa.gov/Emergencies/COVID19/DataDashboard{\#}dashboard. last accessed October 12th, 2021.
- [28].Buitrago-Garcia Diana, Egli-Gany Dianne, Counotte Michel J., Hossmann Stefanie, Imeri Hira, Ipekci Aziz Mert, Salanti Georgia, and Low Nicola. Occurrence and transmission potential of asymptomatic and presymptomatic sars-cov-2 infections: A living systematic review and meta-analysis. PLOS Medicine, 17(9):e1003346–, September 2020. URL 10.1371/journal.pmed.1003346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Davis Hannah E., Assaf Gina S., McCorkell Lisa, Wei Hannah, Low Ryan J., Re’em Yochai, Redfield Signe, Austin Jared P., and Akrami Athena. Characterizing long covid in an international cohort: 7 months of symptoms and their impact. eClinicalMedicine, 38, 2022/March/22 2021. doi: 10.1016/j.eclinm.2021.101019. URL 10.1016/j.eclinm.2021.101019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Taquet Maxime, Dercon Quentin, Luciano Sierra, Geddes John R., Husain Masud, and Harrison Paul J.. Incidence, co-occurrence, and evolution of long-covid features: A 6-month retrospective cohort study of 273,618 survivors of covid-19. PLOS Medicine, 18(9):1–22, September 2021. doi: 10.1371/journal.pmed.1003773. URL 10.1371/journal.pmed.1003773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Centers for Disease Control and Prevention. Post-COVID Conditions. https://www.cdc.gov/coronavirus/2019-ncov/long-term-effects/index.html, September 2021. last accessed March 18th, 2022.
- [32].He Xi, Lau Eric H.Y., Wu Peng, Deng Xilong, Wang Jian, Hao Xinxin, Lau Yiu Chung, Wong Jessica Y., Guan Yujuan, Tan Xinghua, Mo Xiaoneng, Chen Yanqing, Liao Baolin, Chen Weilie, Hu Fengyu, Zhang Qing, Zhong Mingqiu, Wu Yanrong, Zhao Lingzhai, Zhang Fuchun, Cowling Benjamin J., Li Fang, and Leung Gabriel M.. Temporal dynamics in viral shedding and transmissibility of COVID-19. Nature Medicine, 26(May), 2020. ISSN 1546170X. doi: 10.1038/s41591-020-0869-5. [DOI] [PubMed] [Google Scholar]
- [33].Killingley Ben, Mann Alex, Kalinova Mariya, Boyers Alison, Goonawardane Niluka, Zhou Jie, Lindsell Kate, Hare Samanjit S., Brown Jonathan, Frise Rebecca, Smith Emma, Hopkins Claire, Noulin Nicolas, Londt Brandon, Wilkinson Tom, Harden Stephen, Helen McShane Mark Baillet, Gilbert Anthony, Jacobs Michael, Charman Christine, Mande Priya, Nguyen-Van-Tam Jonathan S., Semple Malcolm G., Read Robert C., Ferguson Neil M., Open-shaw Peter J., Rapeport Garth, Barclay Wendy S., Catchpole Andrew P., and Chiu Christopher. Safety, tolerability and viral kinetics during SARS- CoV-2 human challenge. Nature Portfolio, 2022. doi: 10.21203/rs.3.rs-1121993/v1. URL 10.21203/rs.3.rs-1121993/v1. [DOI] [PubMed] [Google Scholar]
- [34].Jo Youngji, Jamieson Lise, Edoka Ijeoma, Long Lawrence, Silal Sheetal, Pulliam Juliet R.C., Moultrie Harry, Sanne Ian, Meyer-Rath Gesine, and Nichols Brooke E. Cost-effectiveness of remdesivir and dexamethasone for covid-19 treatment in south africa. medRxiv, 2020. doi: 10.1101/2020.09.24.20200196. URL https://www.medrxiv.org/content/early/2020/09/27/2020.09.24.20200196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Goyal Ashish, Cardozo-Ojeda E. Fabian, and Schiffer Joshua T.. Potency and timing of antiviral therapy as determinants of duration of sars-cov-2 shedding and intensity of inflammatory response. Science Advances, 6(47):eabc7112, 2021/November/08 2020. doi: 10.1126/sciadv.abc7112. URL 10.1126/sciadv.abc7112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Leung Kathy, Jit Mark, Leung Gabriel M, and Wu Joseph T. Comparative effectiveness of allocation strategies of covid-19 vaccines and antivirals against emerging sars-cov-2 variants of concern in east asia and pacific region. medRxiv, 2021. doi: 10.1101/2021.10.20.21265245. URL https://www.medrxiv.org/content/early/2021/10/20/2021.10.20.21265245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Torneri Andrea, Libin Pieter, Vanderlocht Joris, Vandamme Anne-Mieke, Neyts Johan, and Hens Niel. A prospect on the use of antiviral drugs to control local outbreaks of covid-19. medRxiv, 2020. doi: 10.1101/2020.03.19.20038182. URL https://www.medrxiv.org/content/early/2020/03/30/2020.03.19.20038182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Whittaker Charles, Watson Oliver J., Alvarez-Moreno Carlos, Angkasekwinai Nasikarn, Boonyasiri Adhiratha, Triana Luis Carlos, Chanda Duncan, Charoenpong Lantharita, Chayakulkeeree Methee, Cooke Graham S., Croda Julio, Cucunubá Zulma M, Djaafara Bimandra A., Estofolete Cassia F., Grillet Maria Eugenia, Faria Nuno R., Costa Silvia Figueiredo, Forero-Peña David A., Gibb Diana M., Gordon Anthony C, Hamers Raph L., Hamlet Arran, Irawany Vera, Jitmuang Anupop, Keurueangkul Nukool, Kimani Teresia Njoki, Lampo Margarita, Levin Anna S., Lopardo Gustavo, Mustafa Rima, Nayagam Shevanthi, Ngamprasertchai Thundon, Njeri Ng’ang’a Irene Hannah, Nogueira Mauricio L., Ortiz-Prado Esteban, Perroud Mauricio W., Phillips Andrew N., Promsin Panuwat, Qavi Ambar, Rodger Alison J., Sabino Ester C., Sangkaew Sorawat, Sari Djayanti, Sirijatuphat Rujipas, Sposito Andrei C., Srisangthong Pratthana, Thompson Hayley A., Udwadia Zarir, Valderrama-Beltrán Sandra, Winskill Peter, Ghani Azra C., Walker Patrick G.T., and Hallett Timothy B.. Understanding the potential impact of different drug properties on sars-cov-2 transmission and disease burden: A modelling analysis. medRxiv, 2021. doi: 10.1101/2021.06.17.21259078. URL https://www.medrxiv.org/content/early/2021/06/20/2021.06.17.21259078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Aggarwal Anupriya, Stella Alberto Ospina, Walker Gregory, Akerman Anouschka, Milogiannakis Vanessa, Brilot Fabienne, Amatayakul-Chantler Supavadee, Roth Nathan, Coppola Germano, Schofield Peter, Jackson Jennifer, Henry Jake Y., Mazigi Ohan, Langley David, Lu Yonghui, Forster Charles, McAllery Samantha, Mathivanan Vennila, Fichter Christina, Hoppe Alexandra Carey, Munier Mee Ling, Jack Hans-Martin, Cromer Deborah, Darley David, Matthews Gail, Christ Daniel, Khoury David, Davenport Miles, Rawlinson William, Kelleher Anthony D., and Turville Stuart. Sars-cov-2 omicron: evasion of potent humoral responses and resistance to clinical immunotherapeutics relative to viral variants of concern. medRxiv, 2021. doi: 10.1101/2021.12.14.21267772. URL https://www.medrxiv.org/content/early/2021/12/15/2021.12.14.21267772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Zhou Hao, Tada Takuya, Dcosta Belinda M., and Landau Nathaniel R.. Sars-cov-2 omicron ba.2 variant evades neutralization by therapeutic monoclonal antibodies. bioRxiv, 2022. doi: 10.1101/2022.02.15.480166. URL https://www.biorxiv.org/content/early/2022/02/16/2022.02.15.480166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Takashita Emi, Kinoshita Noriko, Yamayoshi Seiya, Sakai-Tagawa Yuko, Fujisaki Seiichiro, Ito Mutsumi, Iwatsuki-Horimoto Kiyoko, Halfmann Peter, Watanabe Shinji, Maeda Kenji, Imai Masaki, Mitsuya Hiroaki, Ohmagari Norio, Takeda Makoto, Hasegawa Hideki, and Kawaoka Yoshihiro. Efficacy of Antiviral Agents against the SARS-CoV-2 Omicron Subvariant BA.2. 10.1056/NEJMc2201933, March 2022. URL 10.1056/NEJMc2201933. last accessed March 18th, 2022. [DOI] [PMC free article] [PubMed]
- [42].Brown CM, Vostok J, Johnson H, and et al. Outbreak of SARS-CoV-2 Infections, Including COVID-19 Vaccine Break-through Infections, Associated with Large Public Gatherings — Barnstable County, Massachusetts, July 2021. MMWR Morb Mortal Wkly Rep, 70:1059–1062, August 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Hay James A., Kissler Stephen M., Fauver Joseph R., Mack Christina, Tai Caroline G., Samant Radhika M., Connelly Sarah, Anderson Deverick J., Khullar Gaurav, MacKay Matthew, Patel Miral, Kelly Shannan, Manhertz April, Eiter Isaac, Salgado Daisy, Baker Tim, Howard Ben, Dudley Joel T., Mason Christopher E., Ho David D., Grubaugh Nathan D., and Grad Yonatan H.. Viral dynamics and duration of pcr positivity of the sars-cov-2 omicron variant. medRxiv, 2022. doi: 10.1101/2022.01.13.22269257. URL https://www.medrxiv.org/content/early/2022/01/14/2022.01.13.22269257. [DOI] [Google Scholar]
- [44].Layan Maylis, Gilboa Mayan, Gonen Tal, Goldenfeld Miki, Meltzer Lilac, Andronico Alessio, Hozé Nathanaël, Cauchemez Simon, and Regev-Yochay Gili. Impact of BNT162b2 vaccination and isolation on SARS-CoV-2 transmission in Israeli households: an observational study. American Journal of Epidemiology, March 2022. ISSN 0002–9262. doi: 10.1093/aje/kwac042. URL 10.1093/aje/kwac042. kwac042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Prunas Ottavia, Warren Joshua L., Crawford Forrest W., Gazit Sivan, Patalon Tal, Weinberger Daniel M., and Pitzer Virginia E.. Vaccination with bnt162b2 reduces transmission of sars-cov-2 to household contacts in israel. Science, 375 (6585):1151–1154, 2022. doi: 10.1126/science.abl4292. URL https://www.science.org/doi/abs/10.1126/science.abl4292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].WHO. WHO, UN set out steps to meet world COVID vaccination targets. https://www.who.int/news/item/07-10-2021-who-un-set-out-steps-to-meet-world-covid-vaccination-targets, 2021. URL https://www.who.int/news/item/07-10-2021-who-un-set-out-steps-to-meet-world-covid-vaccination-targets
- [47].Despres Hannah W, Mills Margaret G, Shirley David J, Schmidt Madaline M, Huang Meei-Li, Jerome Keith R, Greninger Alexander L, and Bruce Emily A. Quantitative measurement of infectious virus in sars-cov-2 alpha, delta and epsilon variants reveals higher infectivity (viral titer:rna ratio) in clinical samples containing the delta and epsilon variants. medRxiv, Sep 2021. doi: 10.1101/2021.09.07.21263229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Bi Q, Lessler J, Eckerle I, Lauer S A, Kaiser L, Vuilleumier N, Cummings D A T, Flahault A, Petrovic D, Guessous I, Stringhini S, Azman A S, and SeroCoV-POP Study Group. Household transmission of SARS-CoV-2: Insights from a population-based serological survey. medRxiv, 2021. URL https://www.medrxiv.org/content/10.1101/2020.11.04.20225573v2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Meuris Christelle, Kremer Cécile, Geerinck Anton, Locquet Medea, Bruyère Olivier, Defêche Justine, Meex Cécile, Hayette Marie-Pierre, Duchene Loic, Dellot Patricia, Azarzar Samira, Maréchal Nicole, Sauvage Anne-Sophie, Frippiat Frederic, Giot Jean-Baptiste, Léonard Philippe, Fombellida Karine, Moutschen Michel, Durkin Keith, Artesi Maria, Bours Vincent, Faes Christel, Hens Niel, and Darcis Gilles. Transmission of SARS-CoV-2 After COVID-19 Screening and Mitigation Measures for Primary School Children Attending School in Liège, Belgium. JAMA Network Open, 4 (10):e2128757–e2128757, October 2021. ISSN 2574–3805. doi: 10.1001/jamanetworkopen.2021.28757. URL 10.1001/jamanetworkopen.2021.28757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].CDC. COVID-19 Pandemic Planning Scenarios. URL https://www.cdc.gov/coronavirus/2019-ncov/hcp/planning-scenarios.html.
- [51].Pearson Carl A. B., Silal Sheetal P., Li Michael W.Z., Dushoff Jonathan, Bolker Benjamin M., Abbott Sam, van Schalkwyk Cari, Davies Nicholas G., Barnard Rosanna C., Edmunds W. John, Bingham Jeremy, Meyer-Rath Gesine, Jamieson Lise, Glass Allison, Wolter Nicole, Govender Nevashan, Stevens Wendy S., Scott Lesley, Mlisana Koleka, Moultrie Harry, and Pulliam Juliet R. C.. Bounding the levels of transmissibility & immune evasion of the omicron variant in south africa. medRxiv, 2021. doi: 10.1101/2021.12.19.21268038. URL https://www.medrxiv.org/content/early/2021/12/21/2021.12.19.21268038. [DOI] [Google Scholar]
- [52].Halloran M Elizabeth, Struchiner Claudio J, and Longini Ira M. Study designs for evaluating different efficacy and effectiveness aspects of vaccines. American Journal of Epidemiology, 146(10):789–803, 1997. ISSN 0002–9262 (Print). [DOI] [PubMed] [Google Scholar]
- [53].de Salud Secretaria, Mexico. Vacuna COVID. http://vacunacovid.gob.mx/wordpress/, 2021. last accessed October 12th, 2021. [Google Scholar]
- [54].Centers for Disease Control and Prevention. COVID-19 Vaccination and Case Trends by Age Group, United States Vaccinations, 2021. URL https://data.cdc.gov/Vaccinations/COVID-19-Vaccination-and-Case-Trends-by-Age-Group-/gxj9-t96f. last accessed October 12th, 2021.
- [55].Sciensano. Belgium COVID-19 Dashboard - Sciensano › Vaccination, 2021. URL https://datastudio.google.com/u/0/reporting/c14a5cfc-cab7-4812-848c-0369173148ab/page/hOMwB. last accessed October 12th, 2021.
- [56].OurWorldInData.org. COVID-19 vaccine doses, people with at least one dose, people fully vaccinated, and boosters per 100 people. https://ourworldindata.org/explorers/coronavirus-data-explorer?zoomToSelection=true&time=2020-03-01..latest&uniformYAxis=0&pickerSort=asc&pickerMetric=location&Metric=Vaccine+doses%2C+people+vaccinated%2C+and+booster+doses&Interval=7-day+rolling+average&Relative+to+Population=false&Color+by+test+positivity=false&country=USA~GBR~CAN~DEU~ITA~IND, 2021. last accessed February 24, 2022.
- [57].GAVI. Kenya completes its first round of COVID-19 vaccinations | Gavi, the Vaccine Alliance. https://www.gavi.org/vaccineswork/kenya-completes-its-first-round-covid-19-vaccinations, 2021. Last accessed October 12th, 2021.
- [58].Lauer Stephen A, Grantz Kyra H, Bi Qifang, Jones Forrest K, Zheng Qulu, Meredith Hannah R, Azman Andrew S, Reich Nicholas G, and Lessler Justin. The incubation period of coronavirus disease 2019 (COVID-19) from publicly reported confirmed cases: Estimation and application. Annals of Internal Medicine, 2020. ISSN 1539–3704. doi: 10.7326/M20-0504. URL http://www.ncbi.nlm.nih.gov/pubmed/32150748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Roman Wölfel Victor M. Corman, Guggemos Wolfgang, Seilmaier Michael, Zange Sabine, Müller Marcel A., Niemeyer Daniela, Jones Terry C., Vollmar Patrick, Rothe Camilla, Hoelscher Michael, Bleicker Tobias, Brünink Sebastian, Schneider Julia, Ehmann Rosina, Zwirglmaier Katrin, Drosten Christian, and Wendtner Clemens. Virological assessment of hospitalized patients with covid-2019. Nature, 581(7809):465–469, 2020. doi: 10.1038/s41586-020-2196-x. URL 10.1038/s41586-020-2196-x. [DOI] [PubMed] [Google Scholar]
- [60].Linton Natalie M, Kobayashi Tetsuro, Yang Yichi, Hayashi Katsuma, Akhmetzhanov Andrei R, Jung Sung-Mok, Yuan Baoyin, Kinoshita Ryo, and Nishiura Hiroshi. Incubation period and other epidemiological characteristics of 2019 novel coronavirus infections with right truncation: A statistical analysis of publicly available case data. Journal of clinical medicine, 9(2):538, February 2020. doi: 10.3390/jcm9020538. URL https://pubmed.ncbi.nlm.nih.gov/32079150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Wang Dawei, Hu Bo, Hu Chang, Zhu Fangfang, Liu Xing, Zhang Jing, Wang Binbin, Xiang Hui, Cheng Zhenshun, Xiong Yong, Zhao Yan, Li Yirong, Wang Xinghuan, and Peng Zhiyong. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus–Infected Pneumonia in Wuhan, China. JAMA, 323(11):1061–1069, March 2020. ISSN 0098–7484. doi: 10.1001/jama.2020.1585. URL 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Chen Jun, Qi Tangkai, Liu Li, Ling Yun, Qian Zhiping, Li Tao, Li Feng, Xu Qingnian, Zhang Yuyi, Xu Shuibao, Song Zhigang, Zeng Yigang, Shen Yinzhong, Shi Yuxin, Zhu Tongyu, and Lu Hongzhou. Clinical progression of patients with covid-19 in shanghai, china. Journal of Infection, 80(5):e1–e6, 2020. ISSN 0163–4453. doi: 10.1016/j.jinf.2020.03.004. URL https://www.sciencedirect.com/science/article/pii/S0163445320301195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Verity Robert, Okell Lucy C, Dorigatti Ilaria, Winskill Peter, Whittaker Charles, Imai Natsuko, Cuomo-Dannenburg Gina, Thompson Hayley, Walker Patrick G T, Fu Han, Dighe Amy, Griffin Jamie T, Baguelin Marc, Bhatia Sangeeta, Boonyasiri Adhiratha, Cori Anne, Cucunubá Zulma, FitzJohn Rich, Gaythorpe Katy, Green Will, Hamlet Arran, Hinsley Wes, Laydon Daniel, Nedjati-Gilani Gemma, Riley Steven, van Elsland Sabine, Volz Erik, Wang Haowei, Wang Yuanrong, Xi Xiaoyue, Donnelly Christl A, Ghani Azra C, and Ferguson Neil M. Estimates of the severity of coronavirus disease 2019: a model-based analysis. The Lancet Infectious Diseases, 20(6):669–677, 2021/November/01 2020. doi: 10.1016/S1473-3099(20)30243-7. URL 10.1016/S1473-3099(20)30243-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].O’Driscoll Megan, Santos Gabriel Ribeiro Dos, Wang Lin, Cummings Derek A. T., Azman Andrew S., Paireau Juliette, Fontanet Arnaud, Cauchemez Simon, and Salje Henrik. Age-specific mortality and immunity patterns of sars-cov-2. Nature, 590(7844):140–145, 2021. doi: 10.1038/s41586-020-2918-0. URL 10.1038/s41586-020-2918-0. [DOI] [PubMed] [Google Scholar]
- [65].Brazeau Nick F, Verity Robert, Jenks Sara, Fu Han, Whittaker Charles, Winskill Pete, Dorigatti Ilaria, Walker Patrick G T, Riley Steven, Schnekenbert Ricardo P, Hoeltgebaum Henrique, Mellan Thomas A, Mishra Swapnil, Unwin H Juliette T, Watson Oliver J, Cucunuba Zulma, Baguelin Marc, Whittles Lilith K, Bhatt Samir, Ghani Azra C., Ferguson Neil M., and Okell Lucy C. Report 34 - COVID-19 Infection Fatality Ratio Estimates from Seroprevalence. https://www.imperial.ac.uk/mrc-global-infectious-disease-analysis/covid-19/report-34-ifr/, 2020.
- [66].Ferguson Neil M, Laydon Daniel, Nedjati-Gilani Gemma, Imai Natsuko, Ainslie Kylie, Baguelin Marc, Bhatia Sangeeta, Boonyasiri Adhiratha, Cucunubá Zulma, Cuomo-Dannenburg Gina, Dighe Amy, Dorigatti Ilaria, Fu Han, Gaythorpe Katy, Green Will, Hamlet Arran, Hinsley Wes, Okell Lucy C, van Elsland Sabine, Thompson Hayley, Verity Robert, Volz Erik, Wang Haowei, Wang Yuanrong, Walker Patrick G T, Walters Caroline, Winskill Peter, Whittaker Charles, Donnelly Christl A, Riley Steven, and Ghani Azra C. Impact of non-pharmaceutical interventions (NPIs) to reduce COVID-19 mortality and healthcare demand. 2020. URL 10.25561/77482. [DOI] [PMC free article] [PubMed]
- [67].Viner Russell M., Mytton Oliver T., Bonell Chris, Melendez-Torres G. J., Ward Joseph, Hudson Lee, Waddington Claire, Thomas James, Russell Simon, Van Der Klis Fiona, Koirala Archana, Ladhani Shamez, Panovska-Griffiths Jasmina, Davies Nicholas G., Booy Robert, and Eggo Rosalind M.. Susceptibility to SARS-CoV-2 infection among children and adolescents compared with adults: A systematic review and meta-analysis. JAMA Pediatrics, 175(2):143–156, 2021. ISSN 21686211. doi: 10.1001/jamapediatrics.2020.4573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Lee Yong-Hoon, Hong Chae Moon, Kim Dae Hyun, Lee Taek Hoo, and Lee Jaetae. Clinical course of asymptomatic and mildly symptomatic patients with coronavirus disease admitted to community treatment centers, South Korea. Emerging Infectious Diseases, Oct, 2020. doi: 10.3201/eid2610.201620. URL https://wwwnc.cdc.gov/eid/article/26/10/20-1620{\_}article. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Mizumoto Kenji, Kagaya Katsushi, Zarebski Alexander, and Chowell Gerardo. Estimating the asymptomatic proportion of coronavirus disease 2019 (COVID-19) cases on board the Diamond Princess cruise ship, Yokohama, Japan, 2020. Euro Surveillance, 25(10), 2020. URL [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Gilead. Veklury® (Remdesivir) Significantly Reduced Risk of Hospitalization in High-Risk Patients with COVID-19. https://www.gilead.com/news-and-press/press-room/press-releases/2021/9/veklury-remdesivir-significantly-reduced-risk-of-hospitalization-in-highrisk-patients-with-covid19 September 2021. last accessed October 19th, 2021.
- [71].Merck. Merck and Ridgeback’s Investigational Oral Antiviral Molnupiravir Reduced the Risk of Hospitalization or Death by Approximately 50 Percent Compared to Placebo for Patients with Mild or Moderate COVID-19 in Positive Interim Analysis of Phase 3 Study - Merc. https://www.merck.com/news/merck-and-ridgebacks-investigational-oral-antiviral-molnupiravir-reduced-the-risk-of-hospitalization-or-death-by-approximately-50-percent-compared-to-placebo-for-patients-with-mild-or-moderat October 2021. last accessed October 19th, 2021.
- [72].Centers for Disease Control and Prevention. CDC COVID Data Tracker. https://covid.cdc.gov/covid-data-tracker/\#vaccine-effectiveness, 2020. last accessed October 19th, 2021.
- [73].Ferguson Neil, Ghani Azra, Cori Anne, Hogan Alexandra, Hinsley Wes, Volz Erik, and on behalf of the Imperial College COVID-19 response Team. Report 49 - Growth, population distribution and immune escape of Omicron in England | Faculty of Medicine | Imperial College London, 2021. URL https://www.imperial.ac.uk/mrc-global-infectious-disease-analysis/covid-19/report-49-Omicron/.
- [74].UK Health Security Agency. SARS-CoV-2 variants of concern and variants under investigation in England, Technical Briefing 31. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment\_data/file/1042367/technical\_briefing-31-10-december-2021.pdf, Dec 2021. last accessed February 24, 2022.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.