Abstract
The rapid progression of chronic kidney disease and higher incidence of cardiovascular complications are well known in hyperaldosteronism patients. However, detailed renal histopathological characteristics of this disease have remained unknown. Therefore, renal biopsy specimens of 19 cases with unilateral hyperaldosteronism were compared with 22 autopsy renal cases of estimated glomerular filtration rate-matched essential hypertension without nephropathy or endocrine disorders to explore the hyperaldosteronism- specific histopathological renal changes in this study. Global and segmental glomerulosclerosis, interstitial fibrosis, infiltration of inflammatory cells, arteriosclerosis, hyalinization of arterioles and immunoreactivity of mineralocorticoid receptor, 11β-hydroxysteroid dehydrogenase type 1 and 2 and renin were all quantitatively evaluated. The ultrastructural analysis was added in 3 hyperaldosteronism cases. Both mineralocorticoid receptor (p<0.01) and 11β-hydroxysteroid dehydrogenase type 2 (p<0.01) were significantly higher in renal tubules of hyperaldosteronism, which could result in enhancement of in situ aldosterone effects in hyperaldosteronism kidneys. Interstitial fibrosis was significantly more marked in hyperaldosteronism (p< 0.01). The proportion of segmental glomerulosclerosis was also significantly higher in hyperaldosteronism (p<0.01). There were no significant differences of global glomerulosclerosis between two groups (p=0.08). Glomerular size was significantly larger in hyperaldosteronism (p<0.01). In medium size artery, luminal stenosis tended to be more marked (p=0.08) and intima-to-media ratio was significantly lower (p=0.02) in hyperaldosteronism. Arteriolar hyalinization was significantly more pronounced (p<0.01), especially at efferent arterioles (p<0.01) in hyperaldosteronism. Results above demonstrated more pronounced whole renal damages in hyperaldosteronism. Results of our present study also indicated the potential clinical significance of early intervention using mineralocorticoid receptor antagonists or blockers.
Keywords: primary aldosteronism, aldosterone, mineralocorticoid receptor, renal injury, hypertension, histopathology
Introduction
Primary aldosteronism (PA) is a major cause of secondary hypertension, accounting for 5–10% of all hypertensive patients [1–3]. PA patients are also well known to harbor significantly higher incidence of cardiovascular complications than those with essential hypertension (EH), even at the same severity of hypertension [4]. For instance, high plasma aldosterone levels frequently caused left ventricular hypertrophy [5] and vascular smooth muscle cell proliferation and fibrosis of vascular wall [6]. In addition to those cardiovascular events above, renal injury is also one of the critical and life-threatening complications resulting in hemodialysis in some PA patients. Early and more pronounced clinical renal involvement were reported in PA patients compared to EH [7]. For instance, we previously reported that 11.7 % of PA patients had already had chronic kidney disease (CKD) at the time of their initial diagnosis [8]. In addition, higher estimated glomerular filtration rate (eGFR) and more marked albuminuria caused by the changes of intrarenal hemodynamic patterns were reported in early-stage PA than those who had similar blood pressure without aldosterone excess and deterioration of eGFR was reported even under medically or surgically treated patients [9, 10]. Marked albuminuria, lower serum K levels and plasma aldosterone concentration at first visit were also reported to predict subsequent post-treatment decline of eGFR [8, 11, 12].
Several underlying molecular pathways including NF-κB pathways or ROS/Redox signaling, have been reported to be involved in “aldosterone-specific renal injuries” based on results of both in vitro and in vivo studies using animal models [13], indicating the important roles of inflammation or oxidative stress. However, the detailed in situ analysis of renal damages using histopathological and molecular analysis in PA patients have remained virtually unknown. Therefore, in this study, we performed quantitative histopathological analysis using the renal tissue specimens of PA patients and compared the findings with those of eGFR-matched EH cases in order to clarify histological features of PA related renal injuries.
Materials and Methods
The data that support the findings of this study are available from the corresponding author upon reasonable request. We also provided the further information of this section in the Supplement materials.
PA cases
The total of 19 renal tissue specimens were retrospectively and prospectively collected from PA patients in this study. The enrollment of the cases was summarized in Figure 1-A.
Figure 1.
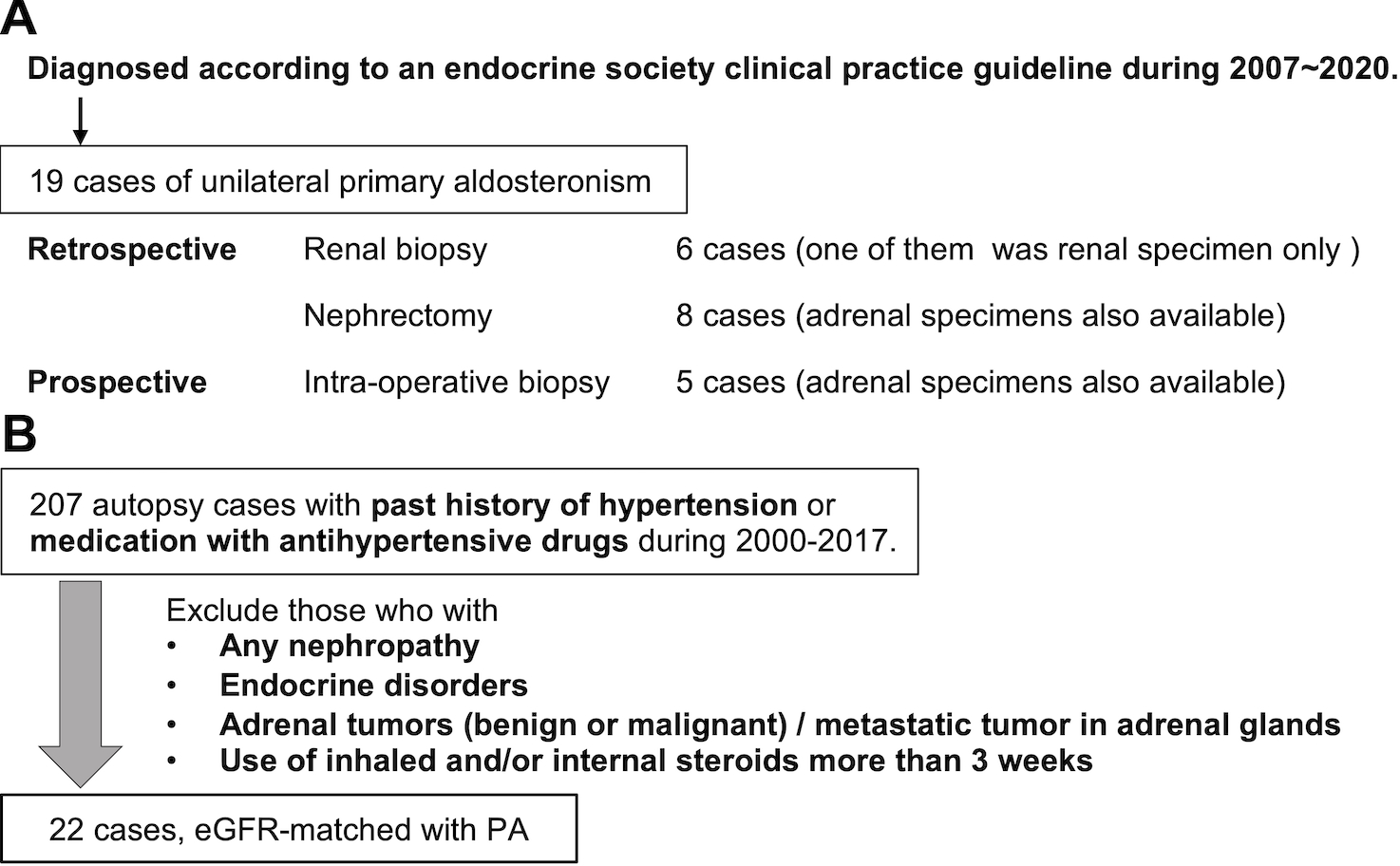
A. Selection of PA cases. We studied 19 APA cases in this study. Fourteen of renal specimens were retrospectively and 5 prospectively obtained. Retrospective cohort contained 6 renal biopsy and 8 nephrectomy cases. Prospective cohort underwent intraoperative renal biopsy. B. Selection of EH cases. We collected 207 EH cases with past history of hypertension or use of antihypertensive agents from autopsy cases performed in Tohoku University Hospital from 2000 to 2017. We excluded those who had any nephropathies or endocrine disorders, had adrenal tumor both benign and malignant, and were given inhaled or/and medication of steroids more than 3 weeks. After that, 22 cases with matched eGFR with PA were retrieved.
Among those 19 cases, 14 cases (8 from nephrectomy for concomitant renal cell carcinoma and 6 from core needle biopsy) were retrospectively retrieved from the following institutions in Japan; from Tohoku University Hospital (6 cases), Chiba University Hospital (1), Sapporo City General Hospital (2), Hamamatsu University School of Medicine (1), Hiroshima University Hospital (3) and Hirosaki University (1). In addition, 5 cases were prospectively collected by intraoperative renal biopsy performed at the same time of unilateral adrenalectomy in Tohoku University Hospital. All of those five patients who underwent intraoperative renal biopsy were voluntarily registered with the sufficient explanation including its potential risks in the events of complications and all of the relevant procedures were performed according to the declaration of Helsinki, after obtaining a written informed consent from the patients and approval of the institutional review board in Tohoku University School of Medicine (No. 2017–2-261–1). In all the cases examined, clinical diagnosis of unilateral PA was made according to an endocrine society clinical practice guideline.
In addition, 18 adrenal specimens were available for our present study among those cases above. Eighteen of them had aldosterone-producing adenomas (APAs), which was confirmed by histopathological studies including immunohistochemistry (IHC) of Cytochrome P450 Family 11 Subfamily B Member 2 (CYP11B2; aldosterone synthase). Adrenalectomy was not performed in one case among those 19 cases.
EH cases
Total of 22 e-GFR matched EH cases were examined as the control group for PA patients in our present study (Figure 1-B). These cases were retrieved from autopsy files at Tohoku University Hospital, Sendai, Japan from 2000 to 2017 and all of them met the following criteria: 1) presence of the past history of hypertension or administration of antihypertensive agents. 2) absence of any primary nephropathy, 3) absence of any endocrine disorders, 4) absence of adrenal neoplasms (either primary or metastatic) determined by individual clinical history and/or detailed histopathological examination at the time of autopsy, 5) absence of inhaled or internal use of steroids more than three weeks in past history. Both renal and adrenal tissues were available for examination.
The whole research protocol of this study was approved by the institutional review board of Tohoku University School of Medicine (No. 2017–2-161–1) and all contributing institutions, respectively.
Clinical features of the patients studied
Clinical features of PA and EH cases examined in this study were summarized in Table 1. Age of the patients corresponded to the time when the renal biopsy or nephrectomy was performed in PA and the autopsy in EH. eGFR was calculated based on age, sex, and serum creatinine level of the patients. The values of eGFR in PA were obtained at two different periods before and after adrenalectomy: pre-operative screening test (pre-operative eGFR) and one month after operation (post-operative eGFR). Δ(Delta) eGFR was calculated by the value subtracting pre-operative from the post-operative eGFR. Durations of hypertension were the periods from the time when the diagnosis of hypertension was firstly made to when the renal biopsy, nephrectomy or autopsy was performed. These cohorts included several patients with diabetes without evident complications. Plasma aldosterone concentration (PAC, ng/dl) and plasma renin activity (PRA, ng/ml/h) were both measured in individual institutions in PA cases and aldosterone-renin ratio (ARR) were calculated when the highest PAC and ARR over 20 was obtained. ARR of two cases was lower than 20 but results of other clinical findings including adrenal vein sampling led to the diagnosis of PA in these two patients. Correlation of histopathological findings with body mass index (BMI), serum potassium level (serum K), potassium replacement (K replacement), serum cortisol on 1mg dexamethasone suppression test (cortisol on DST), urine albumin-to-creatinine ratio (UACR), urinary potassium (U-K), urinary sodium (U-Na) and systolic/diastolic blood pressure (SBP/DBP) could be analyzed only in the cases from Tohoku University Hospital because of availability of the data. Only one from 11 cases was over 1.8μg/dl of cortisol on DST.
Table 1.
Clinical characteristics of PA and EH patients.
| Clinical factors | PA (n=19) | EH (n=22) | P value |
|---|---|---|---|
| Age (y) | 58 (40~75) | 67 (53~93) | <0.01* |
| Sex (male/female) | 14/5 | 16/6 | 0.95 |
| eGFR (mL/min/1.73m2) (pre-operative in PA) |
68.9 (27.9~105.8) | 54.3 (18.3~84.3) | |
| Post-operative eGFR (mL/min/1.73m2) |
50.5 (16.8~83.6) | 0.70 (compared with EH) |
|
| ΔeGFR | −11.7 (−47.1~3.0) | ||
| Duration of hypertension (y) | 15 (2~36) | 14 (2~33) | 0.73 |
| Number of antihypertensive agents (n) | 2 (0~6) | 1 (0~2) | 0.02* |
| Body Mass Index (kg/mm2) | 25.7 (18.5~30.2) | 23.9 (15.8~28.5) | 0.10 |
| Past history of diabetes (+/−) | 3/13 | 5/17 | 0.77 |
| Systolic blood pressure (mmHg) † | 150 (138~183) | 130 (123~206) | 0.01* |
| Diastolic blood pressure (mmHg) † | 88 (60~103) | 80 (59~123) | 0.15 |
| Serum potassium level (mM) † | 3.5 (2.7~4.5) | 4.4 (3.1~5.9) | 0.02* |
| Potassium replacement (mmol/d) † | 36.0 (3.6~156) | NA | |
| Plasma Aldosterone Concentration (ng/dl) | 47.7 (12.2~244.6) | NA | |
| Plasma Renin Activity (ng/ml/h) | 0.48±0.9 (0.1~0.8) | NA | |
| Aldosterone-Renin Ratio | 345.7±1109 (15.3~2446) | NA | |
| Serum cortisol (μg/dl) on 1mg dexamethasone suppression test† |
1.1 (0.6~2.0) | NA | |
| Urine Albumin-to-Creatinine Ratio† | 85.2 (5.6~838.8) | NA | |
| Urinary Potassium (mmol/d) † | 65.2 (20.4~108.5) | NA | |
| Urinary Sodium (mmol/d) † | 88.0 (67.0~325.5) | NA | |
| Urinary Aldosterone (μg/d) † | 25.3 (4.4~374.5) | NA |
Median of each parameters was written in this table. eGFR of EH cases were matched with post-operative eGFR of PA. The age of the patients was lower and the number of antihypertensive agents was higher in PA than in EH. PA patients also had higher systolic blood pressure and lower serum K level.
included only 11 cases from Tohoku University Hospital. PA; primary aldosteronism, EH; essential hypertension, eGFR; estimated glomerular filtration rate. (The Mann-Whitney U test and the chi-squared test for gender difference.)
Median (range)
p<0.05
Histopathological analysis
All of the specimens were submitted for histopathological examination. Hematoxylin and eosin (H&E), Elastica-Masson (EM) and periodic acid-Schiff (PAS) stained tissue sections of all renal specimens were prepared for the quantitative histopathological analysis. Protocols of immunostaining and primary antibodies used for IHC were summarized in major resources table. The histopathological diagnosis of APA was confirmed by CYP11B2 immunolocalization in the tumor area. Histopathological finding of each component in kidney, such as glomeruli, interstitium, arcuate-lobular arteries and arterioles was quantitatively analyzed in individual cases. All immunostained tissue sections were digitally scanned and captured by Image Scope AT2 (Leica) for further image analysis using the digital imaging software, HALO TM (Leica, Buffalo Grove, IL USA).
Glomeruli:
PAS-stained sections were used for the quantitative evaluation of glomerulosclerosis. The percentages of global glomerulosclerosis (GGS) or segmental glomerulosclerosis (SGS) in the total glomeruli were counted and glomerular size was analyzed by measuring the greatest diameter of glomerular capillary. Representative illustrations of glomeruli were demonstrated in Fig. S1.
Interstitium:
Interstitial fibrosis was quantitatively evaluated by collagen type 3 IHC. The percentage of interstitial fibrosis was also calculated by measuring its immunolocalized area within renal cortex analyzed by HALO TM (Area Quantification v1.0). The area of large vessels (arcuate or interlobular artery) and glomerulus was excluded from the renal cortex according to the previous report [14]. The degree of inflammation in renal interstitium was evaluated by counting the number of infiltrating inflammatory cells per 1mm2 of the renal cortical area analyzed by HALO TM (CytoNuclear v1.5) (Fig. S2).
Tubules:
Immunoreactivity of mineralocorticoid receptor (MR), renin, 11β-hydroxysteroid dehydrogenase (11βHSD) type 1 and type 2 was analyzed. Nuclear immunolocalization of MR was specifically evaluated as its activated status in tubular epithelium, and its labeling index (L.I.) was also obtained. Renin immunoreactivity was evaluated by the ratio of the renin-positive area divided by the number of glomeruli according to the method reported in the previous study [15]. Semi-quantified evaluation, H-score (histo-score), was applied for analyzing cytoplasmic immunoreactivity of 11βHSDs [16] (Fig. S3).
Intermediate arteries (Arcuate or interlobular arteries):
The percentage of luminal stenosis and intima-to-media ratio (IMR) in arcuate or interlobular arteries in kidneys were analyzed according to the previous report [17], using EM-stained sections (Fig. S4).
Arterioles (Afferent, efferent and all arterioles):
Arteriole hyalinization was identified as intensely PAS positive area within arteriolar wall. All hyalinized arterioles in the renal cortex were counted and substituted into the following formula: Hy = √(number/cm2), according to the previous report [18]. We assessed 3 patterns of arteriole hyalinization based on their sites of involvement; Hy included all arterioles, Hy-af only afferent and Hy-ef only efferent. Hy-af and Hy-ef were only evaluated in the cases when they could be histologically discernible (Fig. S5).
Adrenals:
CYP11B2 immunoreactivity in APA was analyzed by HALO TM, using H-score (Fig. S6). CYP11B2 H-score was calculated by evaluating tumor volume in order to explore aldosterone-producing ability from the whole tumor as the responsible lesion for PA in individual patients. The detailed formula for calculating three-dimensional CYP11B2 H-score in APA (APA H-score) was provided in the Supplement. In EH, the percentage of CYP11B2 positive area within the whole adrenal cortex of the specimens was measured [19].
Ultrastructural analysis
Renal tissues from 3 PA cases were examined for ultrastructural analysis using transmission electron microscopy. Individual structural components including glomerulus, tubules and vessels were all detected in the specimens from three cases. Representative images of glomerular ultrastructural findings were captured from one glomerulus of each case which was kept from glomerulosclerosis.
Statistical analysis
JMP Pro Version 14 (SAS Institute Inc. Cary, NC, USA) was applied for statistical analysis. The comparison of histopathological findings and clinicopathological factors among 2 cases were analyzed by the Mann-Whitney U test, and gender difference by the chi-squared test. The Spearman’s rank test was used for the analysis of the correlation among the clinicopathological parameters. P-values smaller than 0.05 were considered as statistical significance.
Results
1. Comparison of the renal histopathological findings between PA and eGFR-matched EH cases
Results were summarized in Table 2.
Table 2.
Comparison of histopathological findings between PA and EH.
| Histological factors | PA (n=19) | EH (n=22) | P value |
|---|---|---|---|
| GGS (%) | 11.1 (1.9~64.9) | 6.9 (1.3~35.9) | 0.08 |
| SGS (%) | 5.9 (0~42.9) | 0 (0~2.5) | <0.01** |
| SGS/GGS | 0.2 (0~5.0) | 0 (0~0.3) | <0.01** |
| Glomerular hypertrophy (μm) † | 153.3 (0.5~536.0) | 150.9 (23.6~297.3) | <0.01** |
| Interstitial fibrosis (%) | 4.2 (0.1~28.5) | 1.1 (0.1~7.6) | <0.01** |
| Inflammatory cells (/mm2) | 187.4 (5.5~867.8) | 39.6 (5.5~424.8) | 0.05* |
| Luminal stenosis (%) | 80.0 (21.6~98.1) | 60.0 (43.8~95.0) | 0.08 |
| IMR | 2.8 (0.3~8.2) | 3.6 (1.2~31.3) | 0.02* |
| Hy | 6.2 (0~11.1) | 2.3 (0~5.3) | <0.01* |
| Hy-af | 0 (0~3.5) | 1.3 (0~2.2) | <0.05* |
| Hy-ef | 1.6 (0~5.8) | 0 (0~1.4) | <0.01** |
| 11βHSD1 | 76.8 (33.4~153.8) | 89.1 (31.6~147.0) | 0.03* |
| 11βHSD2 | 58.5 (20.0~122.1) | 33.5 (5.8~80.2) | 0.03* |
| 11βHSD2/11βHSD1 | 0.86 (0.3~2.4) | 0.4 (0.1~0.9) | <0.01** |
| Renin (μm2/glomerulus) | 35.5 (0~197.7) | 208.4 (3.0~1003.8) | <0.01** |
| MR (%) | 11.9 (0~53.7) | 0 (0~3.4) | <0.01** |
There were no significant differences of the proportion of global glomerulosclerosis (GGS) (p=0.08). Segmental glomerulosclerosis (SGS) was significantly more pronounced in PA than EH (p<0.01). SGS was rare in EH. SGS/GGS ratio was also significantly higher in PA than in EH (p<0.01). Glomerular size† was more pronounced in PA than EH (p<0.01). Interstitial fibrosis and the number of infiltrating inflammatory cells were significantly more pronounced in PA than EH kidney (p<0.01, p=0.05, respectively). Luminal stenosis tended to be more marked in PA than in EH but the difference did not reach statistical significance (p=0.08). IMR was significantly lower in PA than EH (p=0.02). Hy including all arterioles was significantly higher in PA than EH (p<0.01). Hy-af was significantly lower (p=0.03) and Hy-ef was higher in PA than EH (p<0.01). 11βHSD1 H-score was significantly higher in EH (p=0.03). 11βHSD2 H-score and 11βHSD2/11βHSD1 ratio were significantly higher in PA than EH (11βHSD2 H-score, p<0.01: 11βHSD2/11βHSD1 ratio, p<0.01). Renin immunoreactivity was significantly higher in EH than PA (p<0.01). MR nuclear immunoreactivity in tubular epithelium was significantly more abundant in PA than EH (p< 0.01). †Glomerular size was demonstrated after counting the greatest dimension of 2683 and 3463 glomeruli in renal tissues of PA and EH, respectively. GGS; global glomerulosclerosis, SGS; segmental glomerulosclerosis, IMR; intima-to-media ratio, Hy; arteriolar hyalinization, 11βHSD; 11-beta hydroxysteroid dehydrogenase, MR; mineralocorticoid receptor. (the Mann-Whitney U test)
Median (range)
p<0.05
p<0.01
1–1. Glomeruli:
The number of glomeruli harboring SGS and the ratio of SGS/GGS were significantly higher in renal tissues of PA than EH (SGS: p < 0.01, SGS/GGS: p <0.01) but there were no significant differences of the number of glomeruli harboring GGS (p=0.08) between PA and EH. Glomerular size was significantly larger in PA than EH (p<0.01) by measuring 2683 glomeruli in PA and 3463 in EH, respectively.
1–2. Interstitium:
Interstitial fibrosis and the degrees of inflammatory cell infiltration were significantly much higher in PA than EH kidney (p<0.01, p=0.05).
1–3. Intermediate arteries:
Luminal stenosis tended to be more marked in PA than EH (p=0.08) but the difference did not reach statistical significance. In contrast, IMR was significantly lower (p=0.02) in PA than EH. Perivascular fibrosis was more pronounced in PA compared with EH, but its quantitation was technically difficult.
1–4. Arterioles:
Arteriole hyalinization in whole arterioles examined was more pronounced in PA than EH (Hy; p<0.01). Hy-ef of PA was significantly higher than that of EH (p<0.01) but Hy-af was significantly lower in PA than in EH (p=0.03), with the limited number of apparently recognizable afferent or efferent arterioles.
1–5. Tubules:
Representative images of IHC were presented in Fig. 2-A. 11βHSD2 and MR were predominantly detected in distal tubules and significantly higher in PA than EH (11βHSD2: p<0.01, MR: p<0.01). On the other hand, 11βHSD1 was higher in EH (p=0.03). The ratio of 11βHSD2/11βHSD1 was also significantly higher in PA than EH (p<0.01). Renin-positive area per glomeruli was significantly lower in PA than EH (p< 0.01). MR immunoreactivity was also detected in pericapillary cells, podocytes and mesangial cells (Fig. S6).
Figure 2.
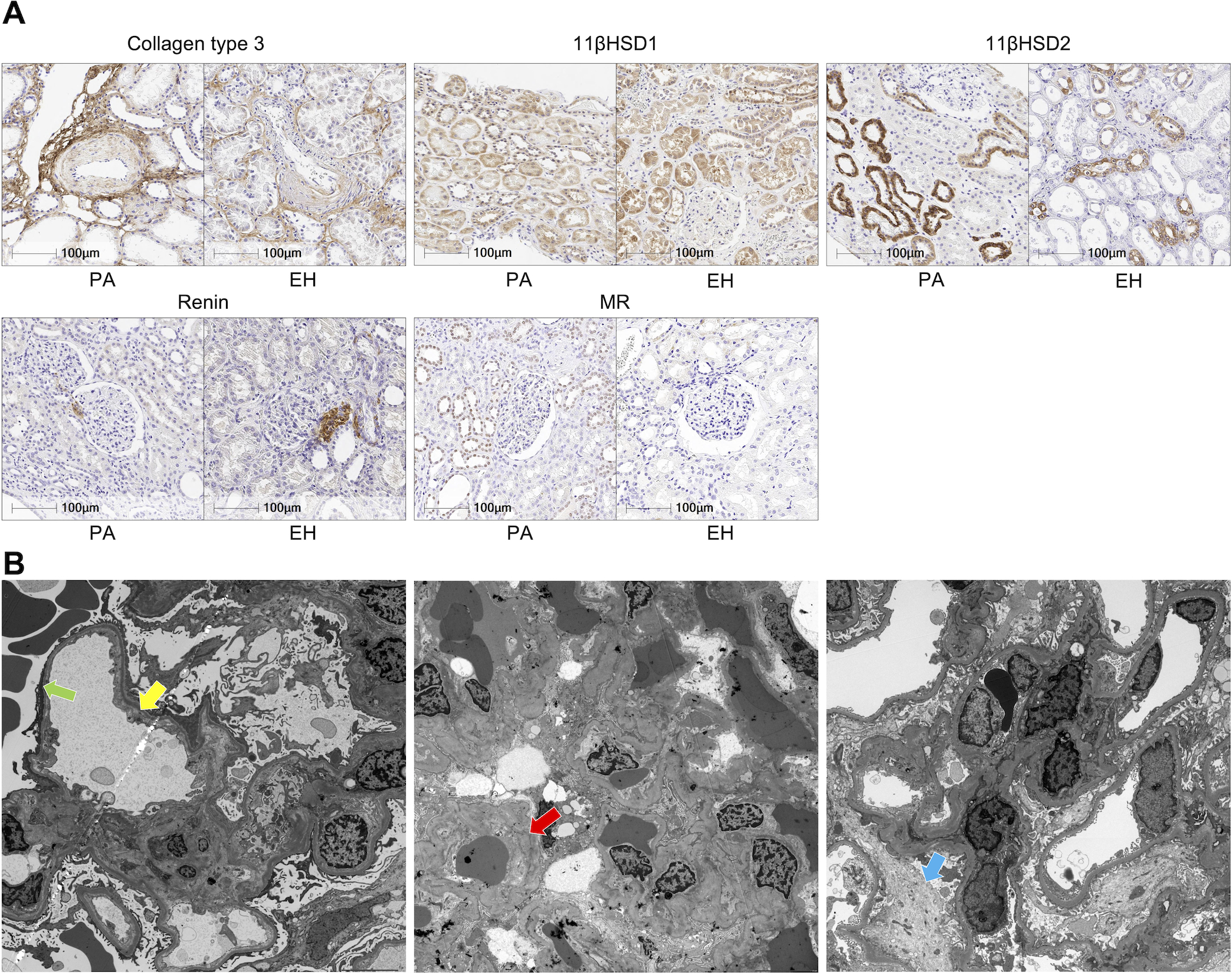
A. Representative illustrations of immunohistochemistry. PA appeared to harbor more dense perivascular fibrosis than EH (collagen type 3). Both had similar immunoreactivity of 11βHSD1. 11βHSD2 was detected mostly in distal tubules and more marked in PA. Juxtaglomerular cells were immunohistochemically positive for renin. MR nuclear immunoreactivity was detected in distal tubules but rarely in EH. B. Ultrastructural findings of 3 cases. Dilatation of subendothelial area, recognized as electron lucent lesions (yellow arrow) and foot process effacement approximately 30% of the observative area were detected (green arrow). Extra-capillary red blood cells were also detected in the left picture. Glomerular basement membrane was almost normal in the left but foci of wrinkling of basement membrane (red arrow) were detected in the middle. The latter and the right also had partial foot process effacement and slight mesangial proliferation (blue arrow). Clinical information of the cases examined was provided in Supplemental material.
In all the histological components examined in this study, there were no significant differences between those with past medical history of DM and those without and no histological findings indicative of diabetic nephropathy such as nodular glomerulosclerosis were detected in the renal specimens with past medical history of DM.
2. Ultrastructural findings of 3 PA cases by transmission electron microscope
We assessed non-sclerosing glomeruli from three PA cases, individually. There were no ruptures of capillary structure, abnormal glomerular basement membrane, nor significant mesangial proliferation detected by ultrastructural examination. However, all of the three cases demonstrated foot process effacement, from 20 to 30 percent within the captured image. One of them had dilatation of subendothelial electron lucent layer, consistent with endothelial injury. Others demonstrated slight increased mesangial matrix and one of them had wrinkling of basement membrane. However, no specific ultrastructural findings were detected (Fig. 2-B).
3. Correlations among clinicopathological parameters in PA cases.
Correlation between histopathological findings and clinical data was summarized in Table 3 and Table S1. PAC was not significantly correlated with any of the histopathological findings above but ARR was significantly correlated with SGS/GGS ratio (ρ= −0.6347, p= 0.04), indicating higher proportion of GGS than SGS in those with higher autonomous aldosterone excess. U-Na were significantly correlated with GGS (ρ= 0.6091, p= 0.05) and MR (ρ= 0.7909, p<0.01). UACR was associated with glomerular size (ρ= 0.7455, p<0.01) and Hy-af (ρ= 0.7862, p<0.01). UACR was also significantly correlated with decreased pre-operative eGFR (ρ= −0.6000, p= 0.05). Post-operative eGFR was inversely correlated with GGS (ρ= −0.7091, p= 0.02), Hy-ef (ρ= −0.6941, p= 0.03), MR L.I. (ρ= −0.7754, p= 0.01).
Table 3.
Correlations of clinicopathological findings in PA.
| Factors | PAC | PRA | ARR | APA H score | Serum K | K replacement | U-K | U-Na | UACR | Post-operative eGFR |
| GGS | ρ= 0.2000 p= 0.56 |
ρ= −0.5001 p= 0.12 |
ρ= 0.3455 p= 0.30 |
ρ= −0.0061 p= 0.99 |
ρ= 0.3562 p= 0.28 |
ρ= −0.7335
p= 0.01 |
ρ= 0.1185 p= 0.73 |
ρ= 0.6091
p= 0.05 |
ρ= 0.1455 p= 0.67 |
ρ= −0.7091
p= 0.02 |
| SGS | ρ= −0.2877 p= 0.39 |
ρ= 0.4652 p= 0.15 |
ρ= −0.5708 p= 0.07 |
ρ= −0.1459 p= 0.69 |
ρ= 0.3280 p= 0.32 |
ρ= 0.4874 p= 0.13 |
ρ= 0.0123 p= 0.97 |
ρ= −0.1324 p= 0.70 |
ρ= −0.2831 p= 0.40 |
ρ= 0.2006 p= 0.58 |
| SGS/GGS | ρ= −0.4795 p= 0.14 |
ρ= 0.5439 p= 0.09 |
ρ= −0.6347
p= 0.04 |
ρ= −0.1033 p= 0.78 |
ρ= 0.1881 p= 0.58 |
ρ= 0.7117
p= 0.01 |
ρ= 0.0510 p= 0.88 |
ρ= −0.2466 p= 0.46 |
ρ= −0.2420 p= 0.47 |
ρ= 0.5410 p= 0.11 |
| Glomerular size | ρ= 0.0364 p= 0.92 |
ρ= 0.5093 p= 0.11 |
ρ= −0.3091 p= 0.36 |
ρ= 0.3455 p= 0.30 |
ρ= −0.1416 p= 0.68 |
ρ= 0.0592 p= 0.86 |
ρ= 0.2627 p= 0.41 |
ρ= −0.0909 p= 0.79 |
ρ= 0.7455
p< 0.01 |
ρ= 0.3091 p= 0.38 |
| Interstitial fibrosis | ρ= 0.0727 p= 0.83 |
ρ= 0.1019 p= 0.77 |
ρ= 0.0000 p= 1.00 |
ρ= −0.1394 p= 0.70 |
ρ= −0.3543 p= 0.09 |
ρ= 0.0228 p= 0.95 |
ρ= 0.2382 p= 0.46 |
ρ= −0.2182 p= 0.52 |
ρ= 0.1727 p= 0.61 |
ρ= 0.5273 p= 0.12 |
| Inflammation | ρ= 0.2182 p= 0.52 |
ρ= −0.0648 p= 0.85 |
ρ= 0.1818 p= 0.59 |
ρ= 0.0909 p= 0.80 |
ρ= 0.3836 p= 0.24 |
ρ= −0.5695 p= 0.07 |
ρ= 0.3554 p= 0.28 |
ρ= 0.0545 p= 0.87 |
ρ= 0.4182 p= 0.20 |
ρ= −0.5030 p= 0.14 |
| Luminal stenosis | ρ= 0.3455 p= 0.33 |
ρ= −0.6648
p= 0.04 |
ρ= 0.5030 p= 0.14 |
ρ= −0.2333 p= 0.55 |
ρ= 0.0732 p= 0.84 |
ρ= −0.7295
p= 0.02 |
ρ= −0.0820 p= 0.81 |
ρ= 0.5515 p= 0.10 |
ρ= −0.0788 p= 0.83 |
ρ= −0.4667 p= 0.21 |
| IMR | ρ= 0.0909 p= 0.80 |
ρ= −0.3293 p= 0.35 |
ρ= 0.1394 p= 0.70 |
ρ= 0.2667 p= 0.49 |
ρ= 0.4146 p= 0.23 |
ρ= −0.3343 p= 0.35 |
ρ= −0.1367 p= 0.69 |
ρ= 0.3333 p= 0.35 |
ρ= 0.2727 p= 0.45 |
ρ= −0.5000 p= 0.17 |
| Hy | ρ= −0.1727 p= 0.61 |
ρ= 0.0556 p= 0.87 |
ρ= 0.0091 p= 0.98 |
ρ= 0.0303 p= 0.93 |
ρ= −0.1050 p= 0.76 |
ρ= −0.2916 p= 0.38 |
ρ= −0.2172 p= 0.50 |
ρ= 0.0545 p= 0.87 |
ρ= 0.3636 p= 0.27 |
ρ= 0.0061 p= 0.99 |
| Hy-af | ρ= −0.0578 p= 0.87 |
ρ= 0.0294 p= 0.93 |
ρ= −0.1387 p= 0.68 |
ρ= 0.2312 p= 0.52 |
ρ= 0.5866 p= 0.06 |
ρ= −0.0348 p= 0.92 |
ρ= −0.0646 p= 0.84 |
ρ= 0.1445 p= 0.67 |
ρ= 0.7862
p< 0.01 |
ρ= −0.4250 p= 0.22 |
| Hy-ef | ρ= −0.3337 p= 0.32 |
ρ= −0.1457 p= 0.67 |
ρ= −0.0477 p= 0.89 |
ρ= 0.3439 p= 0.33 |
ρ= 0.7711
p< 0.01 |
ρ= −0.1003 p= 0.77 |
ρ= 0.3047 p= 0.34 |
ρ= 0.1335 p= 0.70 |
ρ= 0.4672 p= 0.15 |
ρ= −0.6941
p= 0.03 |
| 11βHSD2 | ρ= 0.4000 p= 0.22 |
ρ= 0.2686 p= 0.42 |
ρ= 0.0000 p=1.00 |
ρ= 0.4909 p= 0.15 |
ρ= −0.2922 p= 0.38 |
ρ= 0.0000 p= 1.00 |
ρ= 0.2907 p= 0.36 |
ρ= 0.3909 p= 0.23 |
ρ= 0.2545 p= 0.45 |
ρ= −0.0909 p= 0.80 |
| 11βHSD2/11βHSD1 | ρ= −0.0182 p= 0.96 |
ρ= 0.3890 p= 0.24 |
ρ= −0.2364 p= 0.48 |
ρ= −0.0667 p= 0.85 |
ρ= −0.1918 p= 0.57 |
ρ= −0.1913 p= 0.57 |
ρ= −0.1086 p= 0.74 |
ρ= −0.0636 p= 0.85 |
ρ= 0.3636 p= 0.27 |
ρ= 0.1273 p= 0.73 |
| Renin | ρ= −0.2288 p= 0.50 |
ρ= 0.0194 p= 0.95 |
ρ= −0.1335 p= 0.70 |
ρ= −0.2376 p= 0.51 |
ρ= −0.1676 p= 0.62 |
ρ= 0.3631 p= 0.27 |
ρ= −0.0182 p= 0.96 |
ρ= 0.1430 p= 0.67 |
ρ= −0.6293
p= 0.04 |
ρ= 0.4502 p= 0.19 |
| MR | ρ= 0.2605 p= 0.44 |
ρ= −0.4834 p= 0.13 |
ρ= 0.4280 p= 0.19 |
ρ= 0.1751 p= 0.63 |
ρ= 0.2757 p= 0.4118 |
ρ= −0.7787
p< 0.01 |
ρ= 0.2959 p= 0.35 |
ρ= 0.7909
p< 0.01 |
ρ= 0.3396 p= 0.31 |
ρ= −0.7754
p< 0.01 |
| Pre-operative eGFR | ρ= 0.3818 p= 0.25 |
ρ= −0.3149 p= 0.35 |
ρ= 0.2909 p= 0.39 |
ρ= 0.1030 p= 0.78 |
ρ= −0.4110 p= 0.21 |
ρ= 0.3235 p= 0.33 |
ρ= −0.1686 p= 0.62 |
ρ= −0.1091 p= 0.75 |
ρ= −0.6000 p= 0.05 |
ρ= 0.4061 p= 0.24 |
Comparison among clinicopathological parameters of renal injury and aldosterone-producing ability in PA patients. Relatively higher aldosterone concentration to renin was inversely correlated with SGS/GGS ratio. U-Na was significant correlated with GGS and MR positivity. UACR was correlated with glomerular size and Hy-af. GGS, Hy-ef and MR positivity directly reflected decrease of post-operative eGFR.
PAC; plasma aldosterone concentration, PRA; plasma renin activity, ARR; aldosterone-to-renin ratio, eGFR; estimated glomerular filtration rate, GGS; global glomerular sclerosis, SGS; segmental glomerular sclerosis, IMR; intima-to-media ratio, Hy; arteriolar hyalinization, Hy-af; afferent arteriolar hyalinization, Hy-ef; efferent arteriolar hyalinization, 11βHSD; 11-beta hydroxysteroid dehydrogenase, MR; mineralocorticoid receptor, K; potassium, Na; sodium, U; urinary, UACR; urinary albumin-to-creatinine ratio. (The Spearman’s rank test was used.)
4. Correlations between immunoreactivity CYP11B2 and renal histopathological findings.
Median of APA H-score was 7.56 (range: 0.71~91.50) and APA H-score did not have any significant correlations with renal histopathological findings. In EH, median of the percentage of CYP11B2 positive area was 0.06% (range: 0.01%~0.75%), there was no significant correlations with any histopathological parameters too.
Discussion
Results of our present study did reveal that histologically confirmed renal injuries were significantly more pronounced in PA in all the histological components of the kidneys, especially in vessels, than EH even when matching the status of eGFR. Aldosterone effects on renal injury were summarized in Fig. 3-A. Results of our present study also demonstrated higher 11βHSD2/11βHSD1 ratio and abundant MR nuclear immunolocalization in renal tubules in PA than EH. As tentatively summarized in Fig. 3-B (referring to [20]), 11βHSD2 converts 11β-hydroxyglucocorticoids (cortisol) into their inactive 11-keto forms (cortisone) and 11βHSD1 vice versa. 11βHSD2 has been known to localize in close proximity to MR on the membrane of endoplasmic reticulum, resulting in the most efficient protection of MR from cortisol binding [21]. In our present study, we evaluated nuclear immunoreactivity of MR which was considered to represent its active status, translocated from cytoplasm into the nucleus [22], which also indicated that aldosterone could possibly bind with MR with increased higher affinity due to more in situ predominance of 11βHSD2 in PA. On the other hand, cortisone, one of the metabolites of 11βHSD2, was reported to act like an endogenous MR antagonist and inhibit MR activation and its nuclear translocation [23]. Therefore, the up-regulation of 11βHSD2 is reasonably postulated to compensate for the aldosterone excess in PA by increasing cortisone in situ.
Figure 3. Schematic illustration of the possible pathophysiology of aldosterone induced renal injuries.
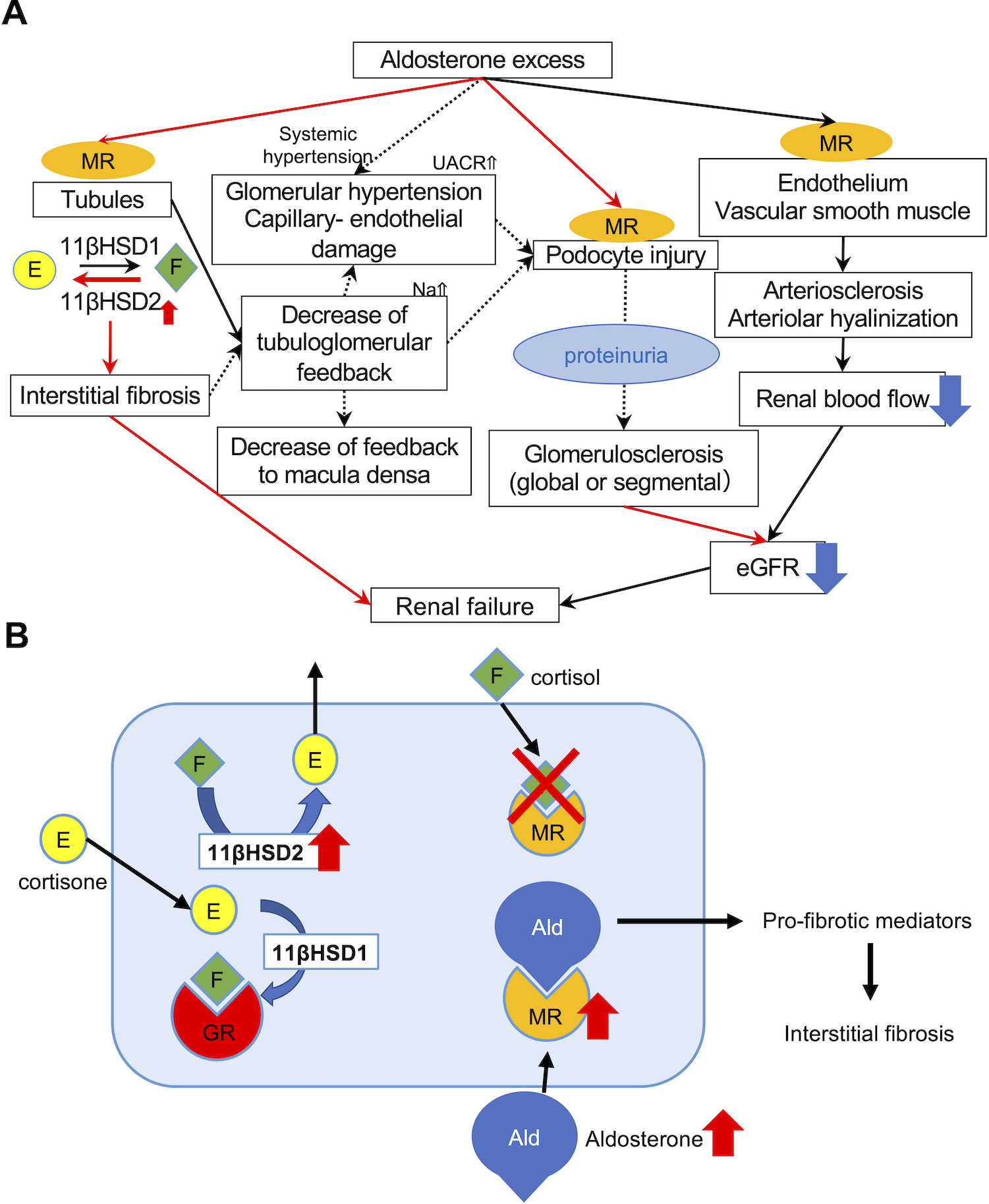
A. MR nuclear immunolocalization in PA was higher than that in EH, demonstrating that MR was more activated and translocated into nucleus as a result of abundant binding with aldosterone. Predominant 11βHSD2 catalytic status detected could compensate for aldosterone excess. Autonomous aldosterone excess (low renin and high aldosterone) could also reduce tubulo-glomerular feedback, resulting in hyperfiltrating status of the remaining glomerulus, histologically recognizable as glomerular hypertrophy. Aldosterone could also directly damage podocytes, and cause foot process effacement. Subsequently induced proteinuria also exacerbated glomerulosclerosis, histologically recognizable as progressive glomerular sclerosis demonstrated by the higher proportion of not only GGS but also SGS. In particular, the prevalence of global glomerulosclerosis accurately reflected the post-operative eGFR after adrenalectomy. Aldosterone could also influence vessels via non-genomic and genomic pathways and cause endothelial swelling and dysfunction, increasing vascular stiffness or peripheral resistance. Therefore, topical renal blood flow could be finally reduced. Therefore, aldosterone-induced renal injury could be systemically induced by multiple pathways. B. 11βHSD2 and MR immunoreactivity was more abundant in PA than EH. 11βHSD2 converted cortisol into cortisone, an inactive form of cortisol. In contrast, 11βHSD1 converted cortisone into cortisol. When 11βHSD2 was not active, MR was occupied by cortisol and aldosterone could not bind with MR. In PA, 11βHSD2-predominant status allowed aldosterone to bind MR and activate pro-fibrotic pathways.
Results of our present study were also consistent with the report that pro-fibrotic mediators are activated via genomic process when aldosterone binds to MR [13,24]. In addition, aldosterone was also known to decrease tubuloglomerular feedback, subsequently resulting in glomerular hypertension and hyperfiltration [25,26]. Larger glomerular size of PA could characterize the status of glomerular hyperfiltration in the residual glomerulus caused by aldosterone excess associated with albuminuria (Fig. S7). Ultrastructural analysis also suggested capillary-endothelial injuries associated with glomerular hypertension. These changes were also reported to be caused by hemodynamic effects and clinically reversible [27]. In addition, foot process effacement was detected and SGS was more pronounced in PA. Foot process effacement occurs when podocytes are damaged and podocytes injury was reported as a common pathogenic mechanism of focal segmental glomerulosclerosis, which also rise in systemic hypertension [28]. Nagase et al had reported that podocytes express MR, as we showed, and that aldosterone-induced podocyte injury occurs through genomic pathway [29]. Damaged podocytes reflected in SGS were also finally detached and lead to GGS [30]. Therefore, even if PA patients had the same levels of eGFR and GGS with EH, their eGFR is reasonably postulated to prospectively decline compared to EH. Furthermore, when aldosterone was corrected, eGFR would drop as a result of reset in tubuloglomerular feedback. In our present study, the patient who had the highest ARR (2446) harbored higher proportion (64.9%) of GGS with the greatest decrease of eGFR after adrenalectomy (ΔeGFR −47.1).
Hypothetical scheme of vascular remodeling associated with PA was illustrated in Fig. S7. MR also localize in vascular smooth muscle cells (VSMC) [30] and endothelial cells (EC) [31] and increased ICAM-1, induced by EC-MR activation by aldosterone, was detected (Fig. S8) [32]. We demonstrated marked luminal stenosis and lower IMR in PA. PA cases also harbored vascular luminal stenosis not only by intimal fibrosis but also by medial thickening due to VSMC proliferation. In particular, the latter finding was considered unique in kidney of PA patients due to the direct effects of aldosterone on vascular smooth muscles [30, 33]. In addition, perivascular fibrosis, illustrated in Fig. 2-A was also more pronounced in PA. These results were all consistent with that aldosterone induced VSMC proliferation and fibrosis via MR after endothelial injury caused by aldosterone itself or hypertension [6]. Nakamura et al reported that MDM2 protein, one of the responsive genes of MR and associated with VSMC proliferation, predominantly expressed in distal small arteries and significantly more abundant in APA patients [30]. Aldosterone may also induce MR-independent arteriolar vasoconstriction [34]. Thus, predominant hyalinization of efferent arterioles was considered to be characteristic of PA due to aldosterone effects contracting or producing vascular hypertrophy of efferent VSMC that ultimately would produce increased hyalinization, although there were no significant correlations between PAC and arteriolar hyalinization.
Limitation of the Study
Several limitations should be noted in the present study. First, this was definitively the largest study using the human renal tissue sections reported in the literature, but the number of the cohorts examined were still relatively small. Second, the limited information of the clinical history or detailed laboratory data among autopsy cases, although inevitable in this nature of investigation. Third, different types of the specimens studied including those from biopsy, surgery and autopsy in this study. This also indicated that the areas of evaluation were different among the types of specimens available for evaluation. It was also true that age, blood pressure and serum K levels were different among the cohorts which could affect renal functions. We also could not investigate detailed associations of recently identified important factors related to PA, including pendrin and IL-6 and they should be clarified by further studies.
Perspectives
We firstly demonstrated that aldosterone itself could harm the whole renal tissue via MR. As previously reported, MR antagonist reduced proteinuria and deterioration of eGFR in PA patients [27, 35, 36]. However, we also demonstrated pronounced histopathological renal damages in PA including those treated with MR antagonists than controlled EH cases. Hundemer et al., reported the higher risks of developing CKD in medically treated PA patients than surgically treated ones [37]. Renal dysfunction in PA patients were considered to be at least partially reversible [27]. Therefore, results of our present study demonstrated early medical intervention using MR antagonists or blockers with dose sufficient to prevent the irreversible renal injuries in PA patients. Further investigations are required to clarify the mechanisms of renal injuries and the novel therapeutic targets for preventing chronic renal failure in PA patients.
Supplementary Material
Novelty and Significance.
What is new
This is the first study to histopathologically characterize the renal injuries and explore in situ aldosterone effects on kidneys of PA patients by simultaneously analyzing both kidneys and adrenals of the same patients. In addition, we also compared the findings with those of eGFR-matched EH cases. We subsequently analyzed the correlation between those histopathological findings above and clinical parameters associated with aldosterone-producing ability and renal function of the patients.
What is relevant
Early medical intervention especially using MR antagonists or blockers with sufficient dose could provide benefits in preventing the progression into irreversible renal injuries in PA patients.
Summary
Histopathological findings of renal injuries in PA were characterized as enlarged glomeruli consistent with clinically reported hyperfiltration, thickening of tunica media due to VSMC proliferation and efferent-predominant arteriolar hyalinization, which could all represent the effects of aldosterone-specific actions.
Acknowledgments;
H.O. is supported by a scholarship from the Takeda Science Foundation. We thank Mr. Katsuhiko Ono for the preparation of the sections of biopsy specimens and Ms. Kiyomi Kisu for the preparation of the sections of electron microscopy.
Sources of Funding;
This work was supported by Japan Society for the Promotion of Science Grants-in-Aid for Scientific Research No. JP15H04711.
Footnotes
Disclosures; None.
References
- [1].Funder JW. The genetics of primary aldosteronism. Science. 2011;331(6018):685–686. doi: 10.1126/science.1202887. [DOI] [PubMed] [Google Scholar]
- [2].Rossi GP, Bernini G, Caliumi C, Desideri G, Fabris B, Ferri C, Ganzaroli C, Giacchetti G, Letizia C, Maccario M, Mallamaci F, Mannelli M, Mattarello MJ, Moretti A, Palumbo G, Parenti G, Porteri E, Semplicini A, Rizzoni D, Rossi E, Boscaro M, Pessina AC, Mantero F; for PAPY Study Investigators. A prospective study of the prevalence of primary aldosteronism in 1125 hypertensive patients. J Am Coll Cardiol. 2006;48(11):2293–2300. doi: 10.1016/j.jacc.2006.07.059. [DOI] [PubMed] [Google Scholar]
- [3].Williams JS, Williams GH, Raji A, Jeunemaitre X, Brown NJ, Hopkins PN, Conlin PR. Prevalence of primary hyperaldosteronism in mild to moderate hypertension without hypokalemia. J Hum Hypertens. 2006; 20: 129–36. [DOI] [PubMed] [Google Scholar]
- [4].Milliez P, Girerd X, Plouin PF, Blacher J, Safar ME, Mourad JJ. Evidence for an increased rate of cardiovascular events in patients with primary aldosteronism. J Am Coll Cardiol. 2005;45(8):1243–1248. doi: 10.1016/j.jacc.2005.01.015. [DOI] [PubMed] [Google Scholar]
- [5].Leopold JA. Aldosterone, mineralocorticoid receptor activation, and cardiovascular remodeling. Circulation. 2011;124(18):e466–468. doi: 10.1161/CIRCULATIONAHA.111.067918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Pruthi D, Mccurley A, Aronovitz M, Galayda C, Karumanchi SA, Jaffe IZ. Aldosterone promotes vascular remodeling by direct effects on smooth muscle cell mineralocorticoid receptors. Arterioscler Thromb Vasc Biol. 2014;34(2):355–364. doi: 10.1161/ATVBAHA.113.302854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Rossi GP, Bernini G, Desideri G, Fabris B, Ferri C, Giacchetti G, Letizia C, Maccario M, Mannelli M, Matterello MJ, Montemurro D, Palumbo G, Rizzonii D, Rossi E, Pessina AC, Mantero F; for PAPY Study Investigators. Renal damage in primary aldosteronism: results of the PAPY Study. Hypertens. 2006;48(2):232–238. doi: 10.1161/01.HYP.0000230444.01215.6a. [DOI] [PubMed] [Google Scholar]
- [8].Iwakura Y, Morimoto R, Kudo M, Ono Y, Takase K, Seiji K, Arai Y, Nakamura Y, Sasano H, Ito S, Satoh F. Predictors of decreasing glomerular filtration rate and prevalence of chronic kidney disease after treatment of primary aldosteronism: Renal outcome of 213 cases. J Clin Endocrinol Metab. 2014;99(5):1593–1598. doi: 10.1210/jc.2013-2180. [DOI] [PubMed] [Google Scholar]
- [9].Monticone S, Sconfienza E, D’Ascenzo F, Cuffolo F, Satoh F, Sechi L, Veglio F, Mulatero P. Renal damage in primary aldosteronism. J Hypertens. 2020;38(1):3–12. doi: 10.1097/HJH.0000000000002216. [DOI] [PubMed] [Google Scholar]
- [10].Sechi LA, Di Fabio A, Bazzocchi M, Uzzau A, Catena C. Intrarenal Hemodynamics in Primary Aldosteronism before and after Treatment. J Clin Endocrinol Metab. 2009;94(4):1191–1197. doi: 10.1210/jc.2008-2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Reincke M, Rump LC, Quinkler M, Hahner S, Diederich S, Lorenz R, Seufert J, Schirpenbach C, Beeuschlein F, Bidlingmaier M, Meisinger C, Holle R, Endres S. Risk Factors Associated with a Low Glomerular Filtration Rate in Primary Aldosteronism. J Clin Endocrinol Metab. 2009;94(3):869–875. doi: 10.1210/jc.2008-1851. [DOI] [PubMed] [Google Scholar]
- [12].Fourkiotis V, Vonend O, Diederich S, Fischer E, Lang K, Endres S, Beuschlein F, Willenberg HS, Rump LS, Allolio B, Reincke M, Quinkler M; for the Mephisto Study Group. Effectiveness of eplerenone or spironolactone treatment in preserving renal function in primary aldosteronism. Eur J Endocrinol. 2013;168(1):75–81. doi: 10.1530/EJE-12-0631 [DOI] [PubMed] [Google Scholar]
- [13].Brem AS, Morris DJ, Gong R. Aldosterone-induced fibrosis in the kidney: questions and controversies. Am J Kidney Dis. 2011;58(3):471–479. doi: 10.1053/j.ajkd.2011.03.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Farris AB, Adams CD, Brousaides N, Pelle PAD, Collins AB, Moradi E, Smith RN, Grimm PC, Colvin RB. Morphometric and visual evaluation of fibrosis in renal biopsies. J Am Soc Nephrol. 2010;22(1):176–186. doi: 10.1681/asn.2009091005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Iijima K, Hamahira K, Kobayashi A, Nakamura H, Yoshikawa N. Immunohistochemical analysis of renin activity in chronic cyclosporine nephropathy in childhood nephrotic syndrome. J Am Soc Nephrol. 2000;11(12):2265–2271. [DOI] [PubMed] [Google Scholar]
- [16].Ueki S, Fujishima F, Kumagai T, Ishida H, Okamoto H, Takaya K, Sato C, Taniyama Y, Kamei T, Sasano H. GR, Sgk1, and NDRG1 in esophageal squamous cell carcinoma: Their correlation with therapeutic outcome of neoadjuvant chemotherapy. BMC Cancer. 2020;20(1):161. doi: 10.1186/s12885-020-6652-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Jain S, Biligi DS. An autopsy study on coronary atherosclerosis with morphological and morphometric analysis. Int J Sci Res. 2015;4(8):1522–1526. [Google Scholar]
- [18].Tracy RE. Age trends of renal arteriolar hyalinization explored with the aid of serial sections. Nephron Clin Pract. 2007;105(4):171–177. doi: 10.1159/000099036. [DOI] [PubMed] [Google Scholar]
- [19].Yamazaki Y, Nakamura Y, Omata K, Ise K, Tezuka Y, Ono Y, Morimoto R, Nozawa Y, Gomez-Sanchez C, Tomlins S, Rainey W, Ito S, Satoh F, Sasano H. Histopathological classification of cross-sectional image-negative hyperaldosteronism. J Clin Endocrinol Metab. 2017;102(4):1182–1192. doi: 10.1210/jc.2016-2986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Luft FC. 11β-hydroxysteroid dehydrogenase-2 and salt-sensitive hypertension. Circulation. 2016;133(14):1335–1337. doi: 10.1161/CIRCULATIONAHA.116.022038. [DOI] [PubMed] [Google Scholar]
- [21].Odermatt A, Arnold P, Frey FJ. The intracellular localization of the mineralocorticoid receptor is regulated by 11β-hydroxysteroid dehydrogenase type 2. J Biol Chem. 2001;276(30):28484–28492. doi: 10.1074/jbc.M100374200. [DOI] [PubMed] [Google Scholar]
- [22].Grossmann C, Ruhs S, Langenbruch L, et al. Nuclear Shuttling Precedes Dimerization in Mineralocorticoid Receptor Signaling. Chem Biol. 2012;19(6):742–751. doi: 10.1016/J.CHEMBIOL.2012.04.014. [DOI] [PubMed] [Google Scholar]
- [23].Brem AS, Morris DJ, Ge Y, et al. Direct fibrogenic effects of aldosterone on normotensive kidney: an effect modified by 11β-HSD activity. Am J Physiol Physiol. 2010;298(5):F1178–F1187. doi: 10.1152/ajprenal.00532.2009. [DOI] [PubMed] [Google Scholar]
- [24].Lavall D, Selzer C, Schuster P, Lenski M, Adam O, Schäfers HJ, Böhm M, Laufs U. The mineralocorticoid receptor promotes fibrotic remodeling in atrial fibrillation. J Biol Chem. 2014;289(10):6656–6668. doi: 10.1074/jbc.M113.519256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Ren Y, D’Ambrosio MA, Garvin JL, Leung P, Kutskill K, Wang H, Peterson EL, Carretero OA. Aldosterone sensitizes connecting tubule glomerular feedback via the aldosterone receptor GPR30. Am J Physiol Ren Physiol. 2014;307(4):427–434. doi: 10.1152/ajprenal.00072.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Iwakura Y, Ito S, Morimoto R,Kudo M, Ono Y, Kezu M, Takase K, Seiji K, Ishidoya S, Arai Y, Funamizu Y, Miki T, Nakamura Y, Sasano H, Satoh F. Renal resistive index predicts postoperative blood pressure outcome in primary aldosteronism. Hypertension. 2016;67(3):654–660. doi: 10.1161/HYPERTENSIONAHA.115.05924. [DOI] [PubMed] [Google Scholar]
- [27].Sechi LA, Novello M, Lapenna R, et al. Long-term Renal Outcomes in Patients With Primary Aldosteronism. JAMA. 2006;295(22):2638–2645.doi: 10.1001/jama.295.22.2638. [DOI] [PubMed] [Google Scholar]
- [28].Kim JS, Han BG, Choi SO, Cha SK. Secondary focal segmental glomerulosclerosis: from podocyte injury to glomerulosclerosis. Biomed Res Int. 2016;2016:1630365. doi: 10.1155/2016/1630365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Nagase M, Yoshida S, Shibata S, Nagase T, Gotoda T, Ando K, Fujita T. Enhanced aldosterone signaling in the early nephropathy of rats with metabolic syndrome: possible contribution of fat-derived factors. J Am Soc Nephrol. 2006;17(12):3438–3446. doi: 10.1681/ASN.2006080944. [DOI] [PubMed] [Google Scholar]
- [30].Nakamura Y, Suzuki S, Suzuki T, Ono K, Miura I, Satoh F, Moriya T, Saito H, Yamada S, Ito S, Sasano H. MDM2: a novel mineralocorticoid-responsive gene involved in aldosterone-induced human vascular structural remodeling. Am J Pathol. 2006;169(2):362–371. doi: 10.2353/ajpath.2006.051351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Jaffe IZ, Jaisser F. Endothelial cell mineralocorticoid receptors turning cardiovascular risk factors into cardiovascular dysfunction. Hypertension. 2014;63(5):915–917. doi: 10.1161/HYPERTENSIONAHA.114.01997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Marzolla V, Armani A, Mammi C, et al. Essential role of ICAM-1 in aldosterone-induced atherosclerosis. Int J Cardiol. 2017;232:233–242. doi: 10.1016/j.ijcard.2017.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Schiffrin EL. Effects of aldosterone on the vasculature. Hypertension. 2006;47(3):312–318. doi: 10.1161/01.HYP.0000201443.63240.a7. [DOI] [PubMed] [Google Scholar]
- [34].Arima S, Kohagura K, Xu H-L, Sugawara A, Abe T, Satoh F, Takeuchi K and Ito S. Nongenomic vascular action of aldosterone in the glomerular microcirculation. J Am Soc Nephrol. 2003;14(9):2255–2263. doi: 10.1097/01.asn.0000083982.74108.54. [DOI] [PubMed] [Google Scholar]
- [35].Bianchi S, Bigazzi R, Campese VM. Long-term effects of spironolactone on proteinuria and kidney function in patients with chronic kidney disease. Kidney Int. 2006;70(12):2116–2123. doi: 10.1038/sj.ki.5001854. [DOI] [PubMed] [Google Scholar]
- [36].Minakuchi H, Wakino S, Urai H, et al. The effect of aldosterone and aldosterone blockade on the progression of chronic kidney disease: a randomized placebo-controlled clinical trial. Sci Rep. 2020;10(1):16626. doi: 10.1038/s41598-020-73638-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Hundemer GL, Curhan GC, Yozamp N, Wang M, Vaidya A. Renal Outcomes in Medically and Surgically Treated Primary Aldosteronism. Hypertens. 2018;72(3):658–666. doi: 10.1161/HYPERTENSIONAHA.118.11568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Wuthrich RP, Jevnikar AM, Takei F, Glimcher LH, Kelley VE. Intercellular adhesion molecule-1 (ICAM-1) expression is upregulated in autoimmune murine lupus nephritis. Am J Pathol. 1990;136(2):441–450. http://www.ncbi.nlm.nih.gov/pubmed/1968316. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


