Abstract
RP215 monoclonal antibody was shown to react with carbohydrate-associated epitope(s) in cancer cell–expressed glycoproteins known as CA215 based on indirect experimental evidences. Efforts have been made to identify glycans in CA215 that may be involved in the epitope recognition. More than 100 tryptic peptides, derived from affinity-purified CA215, consist mainly of immunoglobulin superfamily (IgSF) proteins (~60%), mucins (~7%), and others. Glycoanalysis was performed with affinity-purified CA215 from two cancer cell lines, including (1) N- and O-linked glycan profilings and linked glycoanalysis, (2) glycosylation site mappings, and (3) treatments with selected glycolytic enzymes. High mannose and complex bisecting structures with terminal sialic acid (NeuAc or NeuGc) were detected in N-glycans, whereas as many as 10 O-glycans structurally similar to those of mucins were identified. Through glycosylation site mappings, two N-linked and six out of eight O-linked glycans were detected and matched almost 100% with human immunoglobulin heavy chains. Treatments with several glycolytic enzymes were found to have little effect on the immunoactivity of the RP215-epitope. The same activity was also not affected by the cancer cell culture in human serum instead of bovine serum, indicating that NeuAc and NeuGc are not involved in epitope recognition. The immunoassay results also suggested that the affinity-purified cancer cell–expressed immunoglobulins revealed similar structures and immunoactivities to those of normal human immunoglobulins, except that two additional O-glycans were detected in the former. Supplemental materials are available for this article. Go to the publisher’s online edition of Journal of Carbohydrate Chemistry to view the free supplemental file.
Keywords: CA215, Carbohydrate-associated epitope, Immunoglobulin superfamily proteins, Glycoanalysis and site mapping, RP215 monoclonal antibody
INTRODUCTION
In previous studies, we have identified and characterized an RP215 monoclonal antibody (Mab) that was originally generated against the extract of OC-3-VGH ovarian cancer cells.[1] RP215 was shown to react with a carbohydrate-associated epitope identified in cancer cell–expressed CA215 rich in immunoglobulins or immunoglobulin superfamily (IgSF) proteins.[1] Through extensive biochemical, immunological, and molecular biological studies, we have been able to show that the RP215-specific epitope is mainly present in the IgSF proteins expressed by cancer cells of many human tissue origins.[1,2] CA215 could be detected in cancer cells, both in secreted and membrane-bound forms.[1] RP215 was found to induce apoptosis and complement-dependent cytotoxicity (CDC) of many different cancer cells cultured in vitro and could cause significant inhibition of tumor growth in nude mouse experiments.[3,4] Furthermore, rat Mabs generated against anti-idiotype (aid) of RP215 were shown to induce significant Ab3 (antiaid) response in mice. Ab3 sera were also shown to induce apoptosis in cultured cancer cells, similar to that observed for RP215 (Ab1).[5,6] Therefore, rat aid Mabs, which mimic the internal image of RP215 idiotype, can be good candidates for the development of anticancer vaccines in humans.[5,6] In addition, RP215 was also employed to formulate enzyme immunoassay (EIA) kits to monitor serum levels of CA215 among patients diagnosed with various cancers in humans. It was generally concluded that RP215-based EIA kits are suitable for the monitoring of cancer patients with CA215 as the pan cancer biomarker.[6–8]
In view of the interesting observations of these preclinical studies, it has become essential to elucidate the primary structures of this carbohydrate-associated epitope recognized by RP215. Therefore, through interlab collaborations and contract services, attempts were made to perform comprehensive glycoanalysis as well as glycosylation site mapping of purified CA215 to identify the unique carbohydrate moiety associated with the binding of RP215 in CA215.
RESULTS
Homology Analysis of Tryptic Peptides Derived from CA215
Following tryptic digestions of CA215, a total of 124 tryptic peptides were detected by MALDI-TOF MS. A Protein BLAST Service was conducted to identify proteins with a high degree of peptide sequence homology. The results of such comprehensive analysis are summarized in Table 1 according to the molecular nature of these identified proteins. Among these tryptic peptides detected from CA215, as many as 60% can be matched to IgSF proteins. These include (1) antigen receptors (~47.6%), (2) antigen-presenting MHC molecules (~4.9%), (3) cell adhesion molecules (~8.1%), (4) cytokine and growth factors (~6.5%), (5) receptor tyrosine kinase/phosphatase (~5.7%), and (6) others such as titin (~9.7%).[11] Another major category of these tryptic peptides that are not IgSF protein related is mucins (~7.3%).
Table 1:
Homology analysis of CA215 based on MALDI-TOF MS analysis of tryptic peptides
| Molecule function/Category | Number of peptides matcheda Total = 124 (Percentage) |
|---|---|
| I. Antigen receptors | |
| 1. Antibodies and immunoglobulins | 52 (42.0%) |
| 2. T-cell receptor chains | 7 (5.7%) |
| II. Antigen-presenting molecules (MHC I and MHC II) | 6 (4.9%) |
| III. Adhesion molecules | 10 (8.1%) |
| IV. Cytokine and growth factors | 8 (6.5%) |
| V. Receptor tyrosine kinase/phosphatase | 7 (5.7%) |
| VI. Others | |
| 1. IgSF related (e.g., titin) | 12 (9.7%) |
| Total with homologyb: 75/124 (60.5%) | |
| 2. IgSF unrelated (e.g., mucin) | 9 (7.3%) |
Acid-eluted CA215 (lots CA215A and CA215B) was used for MALDI-TOF MS analysis with MASCOT Program from http://www.matrixscience.com.
Excluding overlapping matched peptides.
Structural Analysis of N-Linked Oligosaccharides
Samples containing human IgG, RP215, and CA215 (acid-eluted form, lots CA215A and CA215B) were analyzed separately by ESI-MS, not by the MALDI-TOF MS method. The profiles of N-linked glycans of each immunoglobulin species were generated and compared. The structures that are unique to CA215, but not in human IgG or RP215, were identified and listed in Table 2, which indicates observed mass, charge state, and proposed structures. As shown in this table, CA215 contains a mixture of high mannose structure as well as N-glycolylneuraminic acid (NeuGc)–terminated complex N-glycans.
Table 2:
Profile of N-linked glycans unique to CA215
| Observed mass (m/z [M+Na]+ + [M+2Na]2+) | Charge state | Proposed structure |
|---|---|---|
| 1169 | Double | GlcNAc5Man3Hex2 |
| 1172 | Single | GlcNAc2Man3 |
| 1330 | Double | GlcNAc4Man3Hex2Fuc1NeuGc1a |
| 1366a | Double | GlcNAc5Man3Hex2NeuGc1 |
| 1438a | Double | GlcNAc4Man3Hex2NeuGc2 |
| 1467a | Double | GlcNAc5Man3Hex3NeuGc1 |
| 1498 | Double | GlcNAc6Man3Hex4 |
| 1525a | Double | GlcNAc4Man3Hex2Fuc1NeuGc2 |
| 1580 | Single | GlcNAc2Man5b,c |
| 1621 | Single | GlcNAc3Man3Hex1 |
| 1785b | Single | GlcNAc2Man6 |
OM with NeuGc as the terminal sialic acid.
OM with high mannose structure.
Lots CA215A and CA215B (acid-eluted) were used for the analysis.
Structural Analysis of O-Linked Oligosaccharides
CA215 samples were subjected to analyses by both NSI-LTQ/MSn and MALDI-TOF MS. A total of three different lots of purified CA215 were employed in triplicate analyses. Two were obtained through purification by acid elution (CA215A and CA215B). However, the CA215 immunoactivity was significantly reduced due to the acid treatment of CA215 during the purification process. Therefore, alternatively, 3M urea was used to elute CA215 (CA215C) from the immunoaffinity column. In the case of O-linked profiling analysis by NSI-LTQ/MSn, CA215A and CA215B, which were obtained from acid purification, were subject to duplicate analyses. Both samples yielded identical results of O-linked glycan profiles in terms of observed mass, charge state, and proposed structure. They are summarized in Table 3 for comparison with parallel analyses of other samples.
Table 3:
Comparative profiles of permethylated O-linked glycans of five different CA215 samples
| Sample ID | Observed mass m/z [M+Na]+ | Proposed structure | Structure |
|---|---|---|---|
| CA215 (lots: A, B, and C)a | 534 | GalNAc1Gal1 |
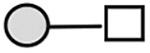 C
C
|
| CA215 (lots: A and B) | 708 | GalNAc1Gal1Fuc1 |
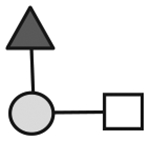
|
| CA215 (lots: A, B, D, C, E, and F)b | 896 | GalNAc1Gal1NeuAc1 |
|
| CA215 (lots: C, E, and F) | 926 | GalNAc1Gal1 NeuGc1 |
|
| CA215C | 940d | GalNAc1GlcNAc1NeuAc1 |
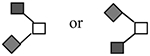
|
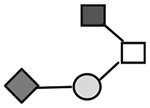
| CA215 (lots: A, B, and C) | 1140 | GalNAc1GlcNAc1Gal1NeuAc1 |
|
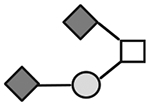
| CA215 (lots: C, D, E, and F) | 1257 | GalNAc1Gal1NeuAc2 |
|
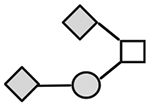
| CA215 (lots: C, E, and F) | 1317 | GalNAc1Gal1NeuGc2 |
|
| CA215 (lots: A, B, C, E, and F) | 1345 | GalNAc1GlcNAc1Gal2NeuAc1 |
|
| CA215 (lots: C, E, and F) | 1375 | GalNAc1GlcNAc1Gal2NeuGc1 |
|
CA215 lots A, B, and C were from OC-3-VGH ovarian cancer cells (CA215-OC-3); lots A and B were obtained through acid elution, whereas lots C, D, E, and F were obtained through elution with 3 M urea.
Lot CA215D was obtained by an additional purification of urea-eluted CA215 (S15K-100425) with goat antihuman IgG affinity column followed by the same analysis. CA215 lots E and F were from C-33A cervical cancer cells (CA215-C33A).
N-acetylgalactosamine (□), N-acetylglucosamine (■), Fucose (▲), Galactose ( ), N-acetylneuraminic acid (
), N-acetylneuraminic acid ( ), and N-glycolylneuraminic acid (
), and N-glycolylneuraminic acid ( ).
).
Detected by MALDI-TOF MS method but not found by NSI-MS method.
From these two acid-eluted lots of CA215 (CA215A and CA215B), five different O-linked structures or fragments were identified by NSI-LTQ/MSn and listed with m/z ranging from 534 to 1346. They were identified as GalNAc1 Gal1, GalNAc1Gal1Fuc1, GalNAc1Gal1NeuAc1, GalNAc1GlcNAc1Gal1NeuAc1, and GalNAcGlcNAcGal2NeuAc1, respectively. NeuAc was the only terminal sialic acid observed among these O-linked fragments. In a separate analysis with urea-eluted CA215 sample (lot CA215C), MALDI-TOF MS was employed. As many as 10 different O-linked glycan structures or fragments were detected and are also listed in Table 3 with m/z ranging from 534 to 1346. Both NeuGc and NeuNAc were found among these O-glycan structures. Four of the detected O-linked fragments were identical to those observed by the ESI-LTQ/MSn method as described above; GalNAc1Gal1Fuc1 was not detected in the latter analysis. The remaining four O-glycans were detected only from the analysis by MALDI-TOF MS. Their primary structures are GalNAc1GluNAc1Gal2NeuAc1, GalNAc1NeuAc1NeuGc1, GalNAc1Gal1NeuAc2, and GalNAc1Gal1NeuGc2, respectively.
In a separate analysis with two urea-eluted lots of CA215 (lots CA215E and CA215F) from the shed medium of C-33A cervical cancer cells, the O-glycan profile was found to be similar to that of CA215 (lot CA215C) from ovarian cancer cells. The typical NSI-MS spectra are presented in Supplementary Figure 1A. In contrast, the O-linked glycan profile was also analyzed with two lots of RP215. Unexpectedly, no significant O-linked glycans were detected. Results of the O-linked glycan profiles are presented in Supplementary Figure 1B.
In a separate experiment, O-linked glycan analysis was also performed with a new lot of CA215 (lot CA215D), which was shown to be cancer cell–expressed human IgG through extensive biochemical and immunological analysis to be described later. CA215D was obtained by affinity purification of urea-eluted CA215 (lot S15K-100425 from OC-3-VGH cancer cells) with goat antihuman IgG column. When subject to analysis by MALDI-TOF MS, only two major O-linked glycans were detected with terminal NeuAc. They are Gal1NAc1Gal1NeuAc1 (m/z 859.6) and GalNAc1GlcNAc1NeuAc2 (m/z 1256.9), respectively. Unexpectedly, other glycans were not detected in CA215D when compared with those of other singly purified CA215 (lot CA215C).
Linked Glycoanalysis by GC-MS
The result of the glycan composition analysis of CA215 (lot S15K-100425) is presented in Supplementary Figure 2. The following monosaccharides were detected in the sample: (1) terminal Gal (time 12.470 min), (2) 3-linked Gal (time 15.692 min), (3) 3-linked GalNAc reduced (time 18.167 min), (4) 3,6-linked GalNAc reduced (time 24.029 min), and (5) 4-linked GlcNAc (time25.571 min). Based on the results of this analysis, the proposed tetra- as well as penta-saccharide structures shown in Table 3 are consistent with the result of the linkage analysis. Glycan analysis was also performed by GC-MS for N-glycans isolated from CA215. The predominant ones are listed as follows: terminal Fuc (8.4%), terminal Gal (20.7%), 2-substituted Man (28.9%), 6-substituted Gal (5.9%), 3-substituted Gal (2.9%), 4-substituted GlcNAc (19.1%), and 1,3,6-substituted Man (6.6%). Details of this analysis are presented in Supplementary Figure 3A.
N- and O-Linked Glycosylation Site Mapping
Affinity-purified CA215 was subjected to glycosylation site mapping analysis. The most notable N-linked and O-linked glycopeptides with potential glycosylation sites were identified and listed in Table 4. A total of two N-linked and eight O-linked glycopeptides were detected through site mapping analysis. These 10 glycopeptides with accession numbers of the originating proteins and potential glycosylation sites are listed in Table 4. Through NCBI Protein BLAST Services, known proteins with high peptide sequence homology are also listed.
Table 4:
N-linked and O-linked glycosylation site mappings of CA215
| Accession number | Peptide detecteda | Peptide sequence homology of proteins (%) |
|---|---|---|
| I. CAC12842.1 | 1. EEQFNSTFR | Immunoglobulin heavy chain (Fc) (100%) |
| II. CAA04843.1 | 2. EEQFNSTYR | Immunoglobulin heavy chain (Fc) (100%) |
| III. AAB60643.2 | 3. LSVPTSEWQR | Cathepsin S (100%) |
| IV AAK68690.1 | 4. FTCLATNDAGDSSK | Hemicentin (100%) |
| Titin (100%) | ||
| Palladin isoform 4 (92%) | ||
| LRN4 (78%) (IgSF proteins) | ||
| V AAD38158.1 | 5. DTLMISR | Immunoglobulin heavy chain (Fc) (100%) |
| VI. AAC39746.2 | 6. GYLPEPVTVTWNSGTLTNGVR | Immunoglobulin heavy chain (Fab) (90%) |
| VII. AAN76042.1 | 7. SVSLTCMINGFYPSDISVEWEK | Immunoglobulin heavy chain (Fc) (90%) |
| VIII. CAJ75462.1 | 8. QSSGLYSLSSVVSVTSSSQPVTCNV | Immunoglobulin heavy chain (Fab and Fc) (100%) |
| IX. ABY48864.2 | 9. VYTMGPPREELSSR | Immunoglobulin heavy chain (Fc) (98%) |
| IgA variable region (89%) | ||
| IgM (98%) | ||
| X. NP_001139647.1 | 10. TFPSVR | Zinc finger protein 414 isoform I (100%) |
| Forkhead box protein C2 (100%) | ||
| Immunoglobulin heavy chain variable region (83%) |
Bold letters indicate glycosylation sites.
Fc, constant region of immunoglobulins; Fab, variable region of immunoglobulins.
Lot CA215C (urea-eluted) was used for this analysis.
Source Protein BLAST Service: http://blast.ncbi.nlm.nih.gov/Blast.cgi.
The two peptides with potential N-glycosylation sites (bold letter), EFQFNSTFR (CAC12842.1) and EFQFNSTYR (CAA04843.1), were shown to match 100% with those of immunoglobulin heavy chains mostly in the constant region. One of the O-linked glycopeptides, LSVPTSEWQR (sites with bold letter), was found to match 100% with that of a lysosomal protease, cathepsin S.[12] The other O-linked glycopeptide, FTCLATNDAGDSSK (sites with bold letter), was found to match 100% with those of IgSF proteins including hemicentin,[13] titin,[11] and several others.[14,15] Unexpectedly, the remaining six O-linked glycopeptides were highly matched mostly to the variable Fab or Fc domains of immunoglobulin heavy chains.
Biochemical and Immunochemical Analysis of CA215
Efforts have been made to study the effects of treatments with selected glycolytic enzymes on RP215-epitope activity. So far, the results are inconclusive. None of the following enzymes in various doses and incubation times showed any significant reductions of RP215-epitope activity in CA215. These enzymes included (1) β-galactosidase (0.1 to 10 units/mL, 24-h incubation), (2) neuraminidase (0.001 to 0.1 unit/mL), (3) O-glycosidase (1 unit/mL, 3- to 18-h incubation), and (4) PNGase F (1 unit/mL, 3- to 24-h incubation).
Efforts were also made to show that the affinity-purified CA215 contains cancer cell–expressed human IgG containing RP215-specific epitope. An affinity column with goat antihuman IgG as the ligand was used to repurify CA215 (lot S15K-100425, from OC-3-VGH cancer cells), which had been affinity purified by RP215-linked affinity column. The doubly purified CA215 designated as CA215D (lot D0425) was shown to be cancer cell–expressed IgG by analysis with SDS-PAGE, EIA, and Western blot.
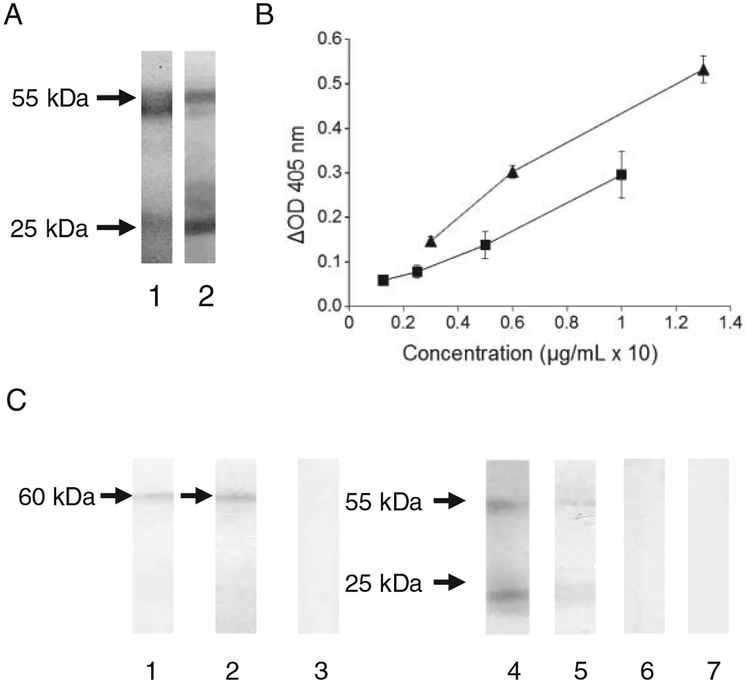
By SDS-PAGE, CA215D was found to consist of mainly heavy and light chains with a molecular weight similar to those of normal human IgG (55 kDa and 25 kDa, respectively) (Fig. 1A). By enzyme immunoassay (EIA) with goat antihuman IgG for both capturing and signal detection, both human IgG and CA215D revealed similar dose-dependent curves as shown in Figure 1B. Furthermore, by Western blot assay, both goat antihuman IgG and RP215 were shown to react with CA215D with a protein band of 60 kDa, which is typical of the heavy chain of human IgG. The results are demonstrated in Figure 1C. However, RP215 does not react with normal human IgG by the same assay due to the absence of RP215-specific epitope.
Figure 1:
Demonstrations of molecular identity of CA215D as human IgG except with RP215-specific epitope. (A) SDS-PAGE of CA215D (1) and human IgG (2) (12% gel). (B) Sandwich enzyme immunoassay to reveal dose-dependent signals with microwells coated with goat antihuman IgG and ALP-labeled goat antihuman IgG in the presence of human IgG (▲) or CA215D (■) in a 2-h assay at 37°C (activity expressed in relative scale). (C) Western blot assay to reveal the differences between CA215D and human IgG; Lane 1: CA215D strip probed with RP215 (1 μg/mL) followed by ALP-labeled goat antimouse IgG; Lane 2: CA215D strip probed directly with ALP-labeled goat antihuman IgG-Fc (0.5 μg/mL); Lane 3: CA215D strip probed with normal mouse IgG (1 μg/mL); Lane 4: CA215D strip probed directly with ALP-labeled goat antihuman IgG (0.5 μg/mL); Lane 5: Human IgG strip probed directly with ALP-labeled goat antihuman IgG (0.5 μg/mL); Lane 6: Human IgG strip probed with RP215 (1 μg/mL) followed by ALP-labeled goat antimouse IgG; Lane 7: Human IgG strip probed with normal mouse IgG (1 μg/mL). Details of experimental conditions have been described in the text.
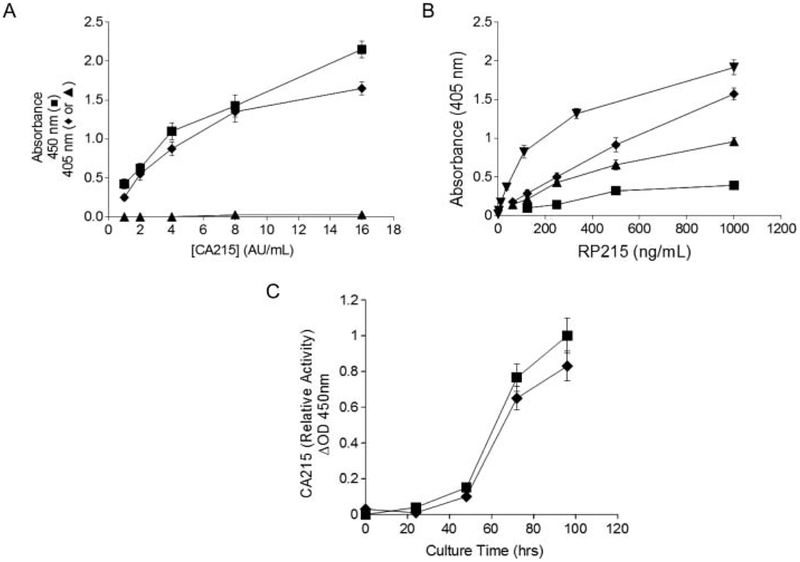
Since the majority of glycopeptides were matched to immunoglobulin heavy chains, sandwich EIAs were performed to determine the relative locations of RP215-specific carbohydrate-associated epitope(s). Three different independent antibody probes were used to sandwich CA215 with well-coated RP215 in the EIA system. Both enzyme-labeled RP215 and goat antihuman IgG Fc gave rise to good dose-dependent signals to sandwiched CA215. In contrast, enzyme-labeled goat antihuman IgG Fab did not pair with well-coated RP215 in the presence of CA215, under the same assay conditions. Results of these comparative sandwich EIAs are presented in Figure 2A. In a separate experiment, ELISA was performed to assess relative binding affinity between RP215 and well-coated CA215 obtained from various sources including those of the cancer cell lines OC-3-VGH, C33A, and ME108, as well as that affinity purified from the shed medium of the OC-3-VGH ovarian cell line. The results of such comparative ELISAs are presented in Figure 2B. Based on this assay, the apparent Kds (dissociation constants) between different binding pairs were estimated to be in the range of 2 to 4 nM.[10] Attempts were made to study if NeuGc is involved in the epitope recognition by RP215 Mab. OC-3-VGH ovarian cancer cells initially in RPMI medium containing 10% bovine serum were subsequently cultured in FS293 serum-free medium (Invitrogen) for 72 h. The RP215-epitope activity for CA215 was lost as judged by RP215-based EIA or Western blot assay.[16] However, the shed medium still contained human IgG by the assay method. In a separate experiment, the cancer cells were cultured instead in RPMI medium containing 10% human serum for 48 to 72 h. The RP215-epitope activity of CA215 was basically unchanged when compared to that found in the shed medium containing bovine serum as judged from the same EIA determinations. The assay results are summarized in Figure 2C.
Figure 2:
(A) Sandwich enzyme immunoassays (EIAs) to reveal the binding signals between different concentrations of CA215 (expressed in AU/mL) and well-coated RP215 in the presence of HRP-labeled RP215 (■), ALP-labeled goat antihuman IgG-Fc (◆), and ALP-labeled goat antihuman IgG-Fab (▲) in a 2-h one-step incubation at 37°C. No binding signals were detected when CA215 was replaced by human IgG (1 μg/mL) under the same assay conditions with any of the detecting probes (data not shown). Data presented are the averages of duplicated experiments with error bars indicated. (B) Enzyme-linked immunosorbent assay (ELISA) to reveal relative dose-dependent bindings between RP215 and well-coated cancer cells of C33A (■), OC-3-VGH (▲), and ME180 (◆), as well as affinity-purified CA215 (▼) from OC-3-VGH cancer cells. Data presented are the averages of the duplicated experiments with error bars indicated. (C) RP215-based EIA to reveal that the RP215-epitope of CA215 activity was not changed significantly in the shed media of cultured OC-3-VGH ovarian cancer cells between that in two different serum conditions for 48 to 96 hours of culture; (■) CA215 activity in RPMI medium containing 10% bovine serum; (◆) CA215 activity in RPMI medium containing 10% human serum.
DISCUSSION
Prior to this communication, several experimental evidences led us to suggest that RP215-epitope is carbohydrate associated in CA215. Briefly, a significant loss of RP215-epitope activity was observed, when CA215 was treated with (1) sodium periodate in solution,[1] (2) extreme heat (e.g., 100°C for 5 min),[8](3) long-term culturing of cancer cells in serum-free medium,[16] or (4) incubations at extreme pHs (≤2.0 or 12.0). In contrast, normal ≥ the immunoactivity of human IgG remains unchanged under the same conditions of treatments. Furthermore, we also observed that the amino acid sequence of cancer cell–expressed IgG1 in the Fc region is almost identical to that of normal human IgG1.[2] In contrast to these observations, treatments of CA215 with several selected glycolytic enzymes gave little or no effect on the immunoactivity of RP215-epitope. The discrepancy in these experimental observations will be resolved by the research in progress. Nevertheless, glycoanalysis of CA215 may still be a logical approach to differentiate between normal human IgGs and the cancer cell–derived ones in terms of glycan structures and to identify RP215-epitope structures.
Extensive analyses were performed with MALDI-TOF MS of tryptic peptides from CA215 and the subsequent peptide sequence homology. It was clearly demonstrated that the majority of tryptic peptides of CA215 were matched with those of IgSF proteins (Table 1) and mucins. The results of these analyses clearly suggested the existence of common domain structures in IgSF proteins that could result in the preferential glycosylation of this group of glycoproteins.[17] Among glycoproteins, mucins might be the most heavily glycosylated glycoproteins in normal or cancer cells. RP215-specific epitope(s) are also generated in mucins expressed preferentially by cancer cells. It remains to be shown how abnormal glycosylation can take place in mucins to generate such new and unique epitope(s) recognized by RP215.
The glycan structures of mucins in the normal and cancerous tissues were analyzed and reported previously.[18–20] It was generally observed that the terminal sialic acid residue might be different between the two types of colonic tissues. The terminal NeuAc of α2–6 linkages was preferentially identified in the normal tissue. In contrast, α2–3 linkage of terminal NeuAc or NeuGc was predominantly found in the cancerous tissue. In addition to differential linkages of the terminal sialic acid residue, the linkages between Gal and GlcNAc (β−1,3 vs. β−1,4) were also different in the normal versus cancerous tissue.[21–24] Whether the differential sialic acid linkages as well as the appearance of NeuGc can result in the creation of new RP215-specific epitope(s) remains to be investigated. Similarly, cancer cell–expressed IgSF proteins might also be abnormally glycosylated through similar pathways or mechanisms in CA215.
The widespread appearance of NeuGc and its antibodies in human cancer cells or tissues has been a subject of interest in cancer research.[9,24–29] Due to the gene mutation, conversion of NeuAc to NeuGc by CMP-NAc hydroxylase in humans cannot take place. It was generally accepted that NeuGc in humans could be derived from the consumption of red meat and could result in the generation of an anti-NeuGc immune response. An alternative hypothesis was also proposed regarding the high appearance of NeuGc in cancer cells through alternative metabolic pathways.[23] The contribution of NeuGc derived from bovine serum–based culture to the glycans attached in CA215 cannot be ruled out. Attempts have been made to culture cancer cells in FS293 serum-free medium. Unexpectedly, RP215-specific epitope activity could no longer be detected in the shed serum-free culture medium or on the cell surface by immunohistochemical staining. In contrast, the cancer cells that express human IgG activity could still be detected by the same assay methods.[6] Basically, in serum-free medium, cancer cells lose the ability to assemble the required carbohydrate moiety to be recognized by RP215. Since NeuGc incorporation may be one of the assembly steps, it is difficult to prove the point with our current experimental observations. The culture of cancer cells in human serum revealed that NeuGc may not be involved in the epitope recognition by RP215, since NeuGc is a very minor component of glycans in human serum (Fig. 2C). However, our experimental observations do not rule out the possibility that cancer cells are able to convert NeuAc to NeuGc and that NeuGc-expressing glycans may still be part of the RP215-specific epitope.
In this study, efforts were made to perform profiling analyses of N-linked as well as O-linked glycans of affinity-purified CA215 samples and to compare with those of other known glycoproteins. This is an important step toward the structural elucidation of the carbohydrate-associated epitope recognized by RP215 Mab. At the same time, we attempt to understand its association with immunoglobulins or IgSF proteins known as CA215, expressed preferentially by most of the cancer cells in humans.[1,2,5]
Our previous studies have indicated that CA215 with RP215-specific carbohydrate-associated epitope was widely expressed by almost all of the cancer tissues in humans.[1,2] Abnormal glycosylation in cancer cells may result in the formation of a new immunogenic carbohydrate moiety that has never been identified previously in immunoglobulins of normal B-cell origins, but can be recognized by RP215 Mab used in this study.[1,2] Previous studies by others also indicated that immunoglobulins expressed by cancer cells and other normal human cells of non–B-cell origins might be important for the growth promotion of cancer cells as well as others in vivo.[21] We also observed that RP215 could induce apoptosis of cancer cells cultured in vitro as well as inhibit significantly the tumor growth in nude mouse experiments.[4] These experimental observations constitute a rational basis for the development of RP215-based anticancer drugs or anticancer vaccines.
From the results of glycan profilings presented in this study, we have been able to identify significant differences in the N-linked and O-linked glycans between CA215 and normal human IgG. In the case of N-glycans, high mannose structure and NeuGc were detected in CA215 but not in normal human IgG (Table 1). Whether the variations in the N-glycan structures of CA215 contribute significantly to the immunogenicity of CA215 is unknown (see Table 1). Previously, we have demonstrated that the incubation of cultured cancer cells with tunicamycin had little effect on the CA215 immunoactivity of treated cancer cells, when assessed by RP215-based EIA (data not shown). Therefore, N-linked glycans may not be the major glycans involved in the binding of RP215 to CA215.[4]
On the other hand, we also showed that the instability of RP215-specific immunoactivity in CA215 was observed at extreme pHs (≤2. and ≥12.0). This observation that suggests that RP215-specific epitope may be an O-glycan, possibly containing terminal NeuGc. Judging from the experimental evidence, it may be possible that the RP215-specific epitope is part of the additional oligosaccharides with terminal NeuAc or NeuGc identified in the O-glycan or N-glycan profiling analysis of CA215. These unusual oligosaccharides could constitute a hapten linked to CA215 and induce an immune response in mice to generate hybridomas secreting RP215 Mab, which eventually led to the discovery of CA215 as a unique pan cancer marker in humans.
Additional glycan analysis was performed by GC-MS to establish the linkages of the detected O-glycans unique to CA215. Based on the results of this comprehensive analysis, the deduced tetra- and penta-oligosaccharide structures with terminal NeuGc were also confirmed by the detected linkages of monosaccharides. On the other hand, our results of N-glycan analysis indicate that CA215 contains complex N-glycans as the major glycan components.
Additional experiments were performed for the N-linked and O-linked glycosylation site mappings. Through Protein BLAST analysis, it was concluded that the detected N-linked glycopeptides were shown to have 100% homology to those of immunoglobulin heavy chain Fc regions. One of the O-linked glycopeptides, FTCLATNDAGDSSK, was found to have 100% homology to IgSF proteins, including hemicentin[13] and titin[11] as well as others yet to be identified. Surprisingly, another O-linked glycopeptide, LSVPTSEWQR, revealed a high degree of homology to cathepsin S, which is a known lysosomal protease.[12] Nevertheless, the remaining O-linked glycopeptides were all matched to immunoglobulin heavy chains (Table 4).
It should be mentioned here that O-linked glycans are not detected in normal human IgG[9] but can be detected in cancer cell–expressed immunoglobulins as reported in the present study. Perhaps significant changes in glycosylation profiles can take place among different cancer cells resulting in a creation of cancer-associated RP215-epitope.
By means of biochemical and immunochemical analysis, we have been able to show that human IgG expressed by cancer cells is one of the main components in CA215 (Fig. 1A–C), all of which are attached with RP215-specific carbohydrate-associated epitope. By SDS-PAGE, EIA, and Western blot assay, one can show that CA215D, which was doubly purified by sequential RP215 and antihuman IgG affinity columns, was identical to human IgG except with additional unique carbohydrate-associated epitope recognized by RP215 Mab (Fig. 1). Furthermore, sandwich EIAs were used to determine the relative locations of RP215-specific carbohydrate-associated epitope(s). While the pairing of RP215-RP215-HRP or RP215-goat antihuman IgG-Fc-ALP gave strong dose-dependent signals from CA215, the combination of RP215 and goat antihuman IgG-Fab failed to form sandwiches in EIA. Results of such assays shown in Figure 2 suggested that RP215 may recognize preferentially the carbohydrate-associated epitope in the Fab regions of immunoglobulin heavy chains rather than the Fc region. Additional experiments with ELISA were performed to show relative bindings between RP215 and CA215 obtained from different cancer cell origins. From the results of this assay in Figure 2B, it can be concluded that apparent affinity between RP215 and CA215 from different sources does not differ significantly with Kd in the range of 2 to 4 nM.[10] The results of this experiment seem to suggest that the RP215-specific epitope is unique in carbohydrate structure and does not vary significantly with the associated glycopeptides derived from different cancer cells or origins.
From the results of glycosylation site mapping, it is interesting to note that both the detected N-linked and O-linked glycopeptides were matched to immunoglobulin heavy chains. Therefore, it seems reasonable to suggest that both N-linked and O-linked glycans are involved in epitope recognition by RP215. In view of this observation, the most likely glycan structure can only be those oligosaccharides with terminal sialic acid (NeuAc or NeuGc), which was found in both types of glycans with mucin-like structures. To verify this assumption, experiments are in progress to re-express the RP215-specific epitope(s) in selected glycopeptides through the transfection of cancer cells in vitro.
MATERIALS AND METHODS
Chemicals
All the chemicals were purchased from Sigma Chemical Company (St. Louis, MO) unless otherwise mentioned.
Cell Lines
The epithelial ovarian cancer cell line of serous origin, OC-3-VGH, was initially established in 1986 by the Department of Obstetrics and Gynecology at Veterans General Hospital, Taipei, Taiwan.[3] The cancer cells were cultured in RPMI medium containing 10% fetal calf serum. The shed medium from the cell culture was collected routinely for CA215 isolation. Cervical cancer cell lines, C-33A and ME108, were obtained from the American Type Culture Collection (ATCC) and cultured for the collection of shed medium according to the supplier’s instructions.
Isolation of CA215 from Spent Media of Cultured Cancer Cell Lines for Glycoanalysis
To carry out repeated glycoanalysis of cancer cell–expressed CA215, which contains RP215-specific epitope, several preparations of this antigen were made from spent media of cultured cancer cell lines. Two different elution methods from the affinity columns were used. Initially, CA215 from spent media from OC-3-VGH ovarian cancer cells was isolated by the RP215 affinity column [1] by elution with 5 mM citric acid and lots CA215A and CA215B assigned. They were initially used for (1) MALDI-TOF MS analysis of CA215-derived tryptic peptides, (2) glyco-composition analysis, and (3) N- and O-linked glycan profiling (see Table 3). In view of the instability of the RP215-epitope in CA215 obtained by acid elution, lots CA215C and S15K-100425 were prepared by elution with 3 M urea instead. For comparative purposes, CA215 was also purified from the shed media of C33A cervical cancer cell lines with urea elution and assigned as CA215E and CA215F (shown in Table 3). In order to show if human IgG is one of the main components of CA215, urea-eluted lot S15K-100425 was further purified with goat antihuman IgG affinity column and eluted with 3 M urea. The new lot of doubly purified CA215 designated as CA215D (Table 3) was used to demonstrate that CA215D is indeed cancer cell–expressed human IgG as described in the Results section. This is in contrast to normal human IgG, which was reported to lack O-glycans in this molecule.[9]
Sandwich Enzyme Immunoassays
Sandwich EIAs were performed for the quantification of CA215 with RP215 Mab-coated microwells in the presence of different enzyme-labeled antibody probes.[1] Three different enzyme-labeled antibody probes, HRP-labeled RP215, ALP-labeled goat antihuman IgG Fc and ALP-labeled goat antihuman Fab, were used separately for CA215 assays with one-step 2-h incubation at 37°C. The remaining steps for wash and subsequent color developments with substrate incubations were described previously.[1–3,7]
Binding between RP215 and CA215 from Different Cancer Cells
Enzyme-linked immunosorbent assays (ELISAs) were performed to assess the relative binding of RP215 to well-coated CA215 obtained from three different cancer cell lines (ovarian or cervical) or in affinity-purified form. Briefly, 1 × 104 cultured cancer cells were coated and dried separately on microtiter wells, followed by blocking and washing. Similarly, CA215 affinity purified from the shed medium of cultured OC-3-VGH cancer cells was also coated on microwells for comparison. Standard ELISAs were performed as described previously.[1] Dose-dependent binding of RP215 and well-coated CA215 were plotted against absorbance at 405 nm. The dissociation constants were estimated for each pair of binding assays.[10]
MALDI-TOF MS Analysis of Tryptic Peptides of CA215
Detailed preparations of tryptic peptides of affinity-purified CA215 have been described previously.[1] Tryptic peptides of CA215 (acid-eluted form) were detected with MALDI-TOF MS analysis (Mascot Program at matrix-science.com). An NCBI Protein BLAST Service was performed to identify those known proteins with peptide sequence homology to the detected tryptic peptides in this study.
All of the glycoanalyses were performed at the Complex Carbohydrate Research Center in Athens, Georgia. Analytical procedures of the submitted samples are briefly described as follows:
I. Structural Analysis of N-Linked Oligosaccharides
Step 1. Release of N-Linked Glycans.
All samples were first dissolved with 1 mL nanopure H2O followed by a freeze-drying step. The dried samples were then dissolved with 100 μL ammonium bicarbonate buffer (50 mM, pH 8.4) and followed immediately by reduction with 25 mM dithiothreitol (45 min at 50°C) and carboxyamidomethylation with 90 mM iodoacetamide (45 min at rt in the dark) prior to trypsin digestion (37°C, overnight). A second enzyme, peptide N-glycosidase F (New England Biolabs), was added to each of the tryptic digests and incubated at 37°C for 18 h to release the N-linked glycans. After enzyme digestions, the samples were passed through a C18 reversed-phase cartridge. The N-linked glycans from each sample were eluted with 5% acetic acid and lyophilized thereafter.
Step 2. Preparations of the Per-O-Methylated Carbohydrates.
The lyophilized N-linked fraction of each sample was dissolved in dimethyl sulfoxide and then methylated for glycan structural analysis by mass spectrometry.
Step 3. Matrix-Assisted Laser Desorption/Ionization Time-of-Flight Mass Spectrometry (MALDI-TOF MS).
Initially, N-linked glycans were analyzed by MALDI-TOF MS using a Voyager V-DE mass spectrometer (Applied Biosystems). However, glycans could not be detected in any of the samples. Hence, the samples were analyzed by electrospray ionization mass spectrometry (ESIMS) using an LCQ-MS (Thermo Finnigan) quadruple ion trap. Each sample (~5 pmol/μL) was infused directly into the instrument at a constant flow rate of 1 μL/min via a syringe pump (Harvard Apparatus) and sprayed at 3.5 kV. A normal collision energy of 35 and an isolation mass window of 2 Da were applied to obtain MSn.
II. Structural Analysis of O-Linked Oligosaccharides
The N-glycans were released and removed prior to β-elimination to avoid being mixed with the O-glycans in the latter’s analysis. Briefly, glycoproteins were denatured and treated with trypsin and PNaseF. The O-linked glycans were released from the glycopeptides by β-elimination. After β-elimination, the samples were desalted and cleaned of borate, permethylated, and analyzed either by nano spray ionization–linear ion trap mass spectrometry (NSILTQ/MSn) or by MALDI-TOF MS.
Step 1. Release of N-Linked Glycans.
The dried samples were dissolved with 0.1 M Tris-HCl buffer (pH 8.2 containing 10 mM CaCl2). The samples were denatured by heating for 5 min at 100°C. After cooling, the samples were digested with trypsin at 37°C overnight. The samples were heated subsequently at 100°C for 5 min to deactivate trypsin. A second enzyme, PNGaseF (New England Biolabs), was added to the samples subsequently to release the N-glycans. After the enzymatic digestions, the samples were passed through a C18 reverse-phase cartridge. The carbohydrate fraction (N-linked glycans) was first eluted with 5% acetic acid and the O-linked glycopeptides and peptides were eluted in series with 20% isopropanol in 5% acetic acid and 40% isopropanol in 5% acetic acid and then 100% isopropanol into separated fractions. The carbohydrate fraction was lyophilized immediately and then stored, whereas the isopropanol fractions were evaporated initially under a stream of nitrogen and then lyophilized.
Step 2. Release of O-Linked Glycans by β-Elimination Procedure.
O-linked carbohydrates were cleaved from the glycopeptides by β-elimination procedure. Briefly, 250 μL of 50 mM NaOH was added to each of the samples and then checked for pH. Upon determination that the pH was basic, an additional 250 μL of 50 mM NaOH containing 19 mg of sodium borohydride was added to the samples, vortexed, and incubated overnight at 45°C. The incubated samples were neutralized subsequently with 10% acetic acid and desalted by passing through a packed column of Dowex resins. O-linked glycans were eluted with 5% acetic acid and lyophilized. The dried samples were cleaned of borate with methanol:acetic acid (9:1) under a stream of nitrogen gas before permethylation.
Step 3. Per-O-Methylation of Carbohydrates and Purification.
The released O-linked glycans from each sample were dissolved with dimethyl sulfoxide and methylated with NaOH and methyl iodide. The reaction was quenched by addition of water and per-O-methylated carbohydrates were extracted with dichloromethane. Per-O-methylated glycans were further cleaned of contaminants by passing them through a C18 Sep-Pak cartridge. The adsorbed permethylated glycans were rinsed with nanopure water and eluted eventually with 85% acetonitrile. Purified glycans were dried under a stream of nitrogen gas and dissolved with methanol prior to analysis by mass spectrometry.
Step 4. Nano Spray Ionization–Linear Ion Trap-Mass Spectrometry (NSILTQ-MSn).
Mass spectrometric analysis was performed by using NSI-LTQMSn following the method developed at the Complex Carbohydrate Research Center. Briefly, permethylated glycans were dissolved with 1 mM NaOH with 50% methanol and infused directly into the instrument (LTQ, Thermo Finnigan) at a constant flow rate of 0.4 μL/min. The capillary temperature was set at 210°C and MS analysis was performed in the positive ion mode. Total ion mapping, which is an automated MS/MS analysis of all possible ions, was performed at 2-mass unit intervals from m/z 500 to 2000. Scanning was accomplished in successive 2.8 mass unit windows with a collision energy of 28.
Profiling by Matrix-Assisted Laser Desorption/Ionization Time-of-Flight Mass Spectrometry (MALDI-TOF MS).
Alternatively, profiling of O-linked glycans can be analyzed following per-O-methylation of carbohydrates in Step3. The permethylated glycans were dissolved with methanol and crystallized with α-dihydroxybenzoic acid (DHBA, 20 mg/mL in 50% methanol:water) matrix. Analysis of glycans present in the samples was performed in the positive ion mode by MALDI-TOF MS using Bruker microflex.
III. Linked Glycosyl Composition Analysis
Experiments were performed to analyze linkage relationships among different O-linked glycans, which are unique to CA215 but not detected in normal human IgG.[9] They are briefly described as follows:
Step 1. Preparation of Partially Methylated Alditol Acetates (PMAAs).
PMAAs were prepared from the remainder of permethylated O-linked glycans of CA215. Briefly, permethylated glycans were hydrolyzed with 2N trifluoroacetic acid at 121°C for 2 h, followed by reduction with NaBD4. After hydrolysis and reduction steps, the free hydroxyls of the partially methylated alditols were acetylated with acetic anhydride:pyridine (1:1 v/v) at 100°C for 1 h to produce PMAAs. PMAAs were extracted with methylene chloride.
Step 2. Gas Chromatography–Mass Spectrometry (GC-MS).
The PMAAs were analyzed on a Hewlett Packard 589° GC interfaced to 5970 MSD (mass selective detector, electron impact ionization mode). The separation was performed on a 30-m EC1 bounded phase fused silica capillary column (Altech). Electron impact mass spectra were obtained under the following conditions: oven temperature, 140°C (2.0°C/min), 220°C (20°C/min), 300°C (7.5 min); detector temperature, 280°C; inlet temperature, 250°C.
N- and O-Linked Glycosylation Site Mappings.
The collaborative work was carried out in the Complex Carbohydrate Research Center in Athens, Georgia, with standard protocols. 18O-labeling of CA215 was performed for N-glycosylation site mapping. β-Elimination followed by Michael addition (BEMAD) was used for O-glycosylation site mapping of the same CA215 sample. The 18O-labeled peptides were subsequently analyzed by LC-MS/MS (liquid chromatography with mass spectrometry/mass spectrometry). The peptide sequences as well as the potential N-glycosylation and O-glycosylation sites were determined. Protein BLAST Service was employed to identify those proteins that have the highest homology as well as the potential O- and N-linked glycosylation sites along the peptide sequences.
Supplementary Material
ACKNOWLEDGEMENTS
This work was supported in parts] by the NSERC and IRAP Program (#743918). Excellent technical support of this work by Suzanne Potzold, Dr. Bixia Ge, and Dr. Frank Zhu and helpful discussions and timely proofreading of this manuscript by Dr. Inka Brockhausen (Dept. Biochemistry, Queens University, Kingston Ontario) are acknowledged. This research was supported in part by the National Institutes of Health (NIH/NCRR)-funded grant entitled “Integrated Technology Resource for Biomedical Glycomics’ (grant no. 1 P41 RR018502-01) to the Complex Carbohydrate Research Center.
ABBREVIATIONS
- Aid
anti-idiotype antibodies
- ALP
alkaline phosphatase
- EIA
enzyme immunoassay
- ELISA
enzyme-linked immunosorbent assay
- ESI-MS
electrospray ionization mass spectrometry
- Gal
galactose
- GalNAc
N-acetylgalactose
- GlcNAc
N-acetylglucosame
- HexAc
N-acetyl hexose (glucose or galactose)
- HRP
horse radish peroxidase
- IgSF
immunoglobulin superfamily
- Kd
dissociation constant
- LCMS
liquid chromatography with mass spectrometry
- LCQ-MS
liquid chromatography with mass spectrometry
- LRN4
leucine-rich repeat and fibronectin type III domain-containing protein 4
- Mab
monoclonal antibody
- MALDI-TOF
MS matrix-assisted laser desorption/ionization time-of-flight mass spectrometry
- NeuAc
N-acetylneuraminic acid
- NeuGc
N-glycolylneuraminic acid
- NSI-LTQ/MS
nano spray ionization–linear ion trap-mass spectrometry
- PMAAs
preparation of partially methylated alditol acetates
Footnotes
CONFLICT OF INTEREST STATEMENT
GL is co-founder of Vancouver Biotech Ltd.
REFERENCES
- 1.Lee G; Laflamme E; Chien CH; Ting HH Molecular identity of a pan cancer marker, CA215. Cancer Biol. Ther 2008, 7, 2007–2014. [DOI] [PubMed] [Google Scholar]
- 2.Lee G; Ge B Cancer cell expressions of immunoglobulin heavy chains with unique carbohydrate-associated biomarker. Cancer Biomark. 2009, 5, 177–188. [DOI] [PubMed] [Google Scholar]
- 3.Lee G; Ge B; Huang TK; Zheng G; Duan J; Wang IHY Positive identification of CA215 pan cancer biomarker from serum specimens of cancer patients. Cancer Biomarkers 2010, 6, 111–117. [DOI] [PubMed] [Google Scholar]
- 4.Lee G; Chu RY; Ting HH Preclinical assessment of anti-cancer drugs by using RP215 monoclonal antibody. Cancer Biol. Ther 2009, 8, 161–166. [DOI] [PubMed] [Google Scholar]
- 5.Lee G; Ge B Growth inhibition of tumor cells in vitro by using monoclonal antibodies against gonadotropin-releasing hormone receptor. Cancer Immunol. Immunother 2010, 59, 1011–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee G; Cheung AP; Ge B; Zhu M; Li PP; Hsu E; Huang TK Monoclonal anti-idiotype antibodies against carbohydrate-associated epitope for anti-cancer vaccine development. J Vaccin. Vaccinat 2010, 1, 106. [Google Scholar]
- 7.Lee G Cancer cell-expressed immunoglobulins: CA215 as a pan cancer marker and its diagnostic applications. Cancer Biomarkers 2009, 5, 137–142. [DOI] [PubMed] [Google Scholar]
- 8.Lee CY; Chen KW; Sheu FS; Tsang A; Chao KC; Ng HT Studies of a tumor-associated antigen, COX-1, recognized by a monoclonal antibody. Cancer Immunol. Immunother 1992, 35, 19–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arnold JN; Wormald MR; Sim RB; Rudd PM; Dwek RA The impact of glycosylation on the biological function and structure of human immunoglobulins. Annu. Rev. Immunol 2007, 25, 21–50. [DOI] [PubMed] [Google Scholar]
- 10.Van Heyningen V; Brook DJH; Van Heyningen S A simple method for ranking the affinity of monoclonal antibodies. J. Immunol. Methods 1983, 62, 149–153. [DOI] [PubMed] [Google Scholar]
- 11.Labeit S; Kolmerer B Titins: giant proteins in charge of muscle ultrastructure and elasticity. Science 1995, 270(5234), 293–296. [DOI] [PubMed] [Google Scholar]
- 12.Shi GP; Munger JS; Meara JP; Rich DH; Chapman HA Molecular cloning and expression of human alveolar macrophage cathepsin S, an elastinolytic cysteine protease. J. Biol. Chem 1992, 267(11), 7258–7262. [PubMed] [Google Scholar]
- 13.Vogel BE; Hedgecock EM Hemicentin, a conserved extracellular member of the immunoglobulin superfamily, organizes epithelial and other cell attachments into oriented line-shaped junctions. Development 2001, 128(6), 883–894. [DOI] [PubMed] [Google Scholar]
- 14.Mykkänen OM; Grönholm M; Rönty M; Lalowski M; Salmikangas P; Suila H; Carpén O Characterization of human palladin, a microfilament-associated protein. Mol. Biol. Cell 2001, 12(10), 3060–3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morimura N; Inoue T; Katayama K; Aruga J Comparative analysis of structure, expression and PSD95-binding capacity of Lrfn, a novel family of neuronal trans-membrane proteins. Gene 2006, 380, 72–83. [DOI] [PubMed] [Google Scholar]
- 16.Lee G; Cheung AP; Ge B; Zhu M; Li P; Hsu E; Huang T Monoclonal anti-idiotype antibodies against carbohydrate-associate epitope for anti-cancer vaccine development. J. Vaccines Vaccinations 2010, 1, 1–7. [Google Scholar]
- 17.Barclay AN Membrane proteins with immunoglobulin-like domains–a master superfamily of interaction molecules. Semin Immunol. 2003, 15, 215–223. [DOI] [PubMed] [Google Scholar]
- 18.Yamashita K; Fukushima K; Sakiyama T; Fusayoshi M; Kuroki M; Matsuoka Y Expression of Siaα→6Galβ1→4GlcNAc residues on sugar chains of glycoproteins including carcinoembryonic antigens in human colon adenocarcinoma: applications of Trichosanthes japonica agglutinin I for early diagnosis. Cancer Res. 1995, 55, 1675–1679. [PubMed] [Google Scholar]
- 19.Podolsky DK Oligosaccharide structures of human colonic mucin. J. Biol. Chem 1985, 260, 8262–8271. [PubMed] [Google Scholar]
- 20.Podolsky DK Oligosaccharide structures of isolated human colonic mucin species. J. Biol. Chem 1985, 260, 15510–15515. [PubMed] [Google Scholar]
- 21.Qiu X; Zhu L; Zhang L; Mao Y; Zhang J; Hao P; Li G; Lv P; Li Z; Sun X; Wu L; Zheng J; Deng Y; Hou C; Tang P; Zhang S; Zhang Y Human epithelial cancers secrete immunoglobulin G with unidentified specificity to promote growth and survival of tumor cells. Cancer Res. 2003, 63, 6488–6495. [PubMed] [Google Scholar]
- 22.Kurosaka A; Nakajima H; Funakoshi I; Matsuyama M; Nagayo T; Yamashina I Structures of the major oligosaccharides from a human rectal adenocarcinoma glycoprotein. J. Biol. Chem 1983, 258, 11594–11598. [PubMed] [Google Scholar]
- 23.Capon C; Laboisse CL; Wierruszeski JM; Maoret JJ; Augeron C; Fournet B Oligosaccharide structures of mucins secreted by the human colonic cancer cell line CL.16E. J. Biol. Chem 1992, 267, 19248–19259. [PubMed] [Google Scholar]
- 24.Malykh YN; Schauer R; Lee S N-Glycolylneuraminic acid in human tumours. Biochimie 2001, 83, 623–634. [DOI] [PubMed] [Google Scholar]
- 25.Hedlund M; Padler-Karavani V; Varki NM; Varki A Evidence for human-specific mechanism for diet and antibody-mediated inflammation in carcinoma progression. PNAS 2008, 105, 18936–18941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Irie A; Koyama S; Kozutsumi Y; Kawaski T; Suzuki A The molecular basis of the absence of N-glycolylneuraminic acid in humans. J. Biol. Chem 1998, 273, 15866–15871. [DOI] [PubMed] [Google Scholar]
- 27.King M-C; Wilson AC Evolution at two levels in humans and chimpanzees. Science 1975, 188, 107–116. [DOI] [PubMed] [Google Scholar]
- 28.Tangvoranuntakul P; Ganeux P; Diaz S; Bardon M; Varki N; Varki A; Much-more E Human uptake and incorporation of an immunogenic nonhuman dietary sialic acid. PNAS 2003, 100, 12045–12050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Varki A Loss of N-glycolylneuraminic acid in humans: mechanisms, consequences, and Implications for hominid evolution. Yearbook Phys. Anthropol 2001, 44, 54–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.