Abstract
Objective
To establish a model to predict gestational diabetes mellitus (GDM) based on the clinical characteristics, early pregnancy (10-12 weeks gestation) peripheral blood routine, and biochemical indicators, and to explore its predictive efficiencies.
Methods
Data from 607 pregnant women with GDM were compared to the data from 833 pregnant women without GDM admitted to the Obstetrics Department of Fujian Maternity and Child Health Hospital (affiliated to Fujian Medical University) from May 2018 to December 2018 were retrospectively included. The ages of the pregnant women, paternal ages, number of pregnancies, number of deliveries, pre-pregnancy heights/weights, and the calculated body mass indexes (BMI) were recorded. In all participants, 10-12 weeks of pregnancy, afamin concentration, routine blood work, prenatal aneuploidy screening, and biochemical testing were performed. At weeks 24-28 of gestation, patients underwent oral glucose tolerance test (OGTT) for GDM screening.
Results
Multivariate logistic regression analysis showed that maternal age, early pregnancy afamin level, triglycerides, and platelet/lymphocyte ratio (PLR) were independent risk factors for gestational diabetes. The formula for predicting GDM probability was as follows: P = 1/1 + exp( − 6.054 + 0.774 × triglycerides + 0.002 × afamin + 0.155 × age − 0.012 × PLR)]. From the established ROC curve, the area under the curve (AUC) was 0.748, indicating that the model has a good degree of discrimination. When the predictive probability cut-off value was set on 0.358, sensitivity, specificity, positive predictive value, and negative predictive value were 69.2%, 68.3%, 42.5%, and 86.2%, respectively, and the accuracy rate was 70.2%. The Hosmer-Lemeshow test results showed that the goodness of the model fit has a good calibration ability (χ2 = 12.269, df=8, P=0.140).
Conclusions
Maternal age, early pregnancy afamin level, triglycerides, and PLR are independent risk factors for gestational diabetes. When combined, the above indicators are helpful for prediction, early diagnosis, and intervention of gestational diabetes.
Keywords: gestational diabetes, early prediction, afamin, age, PLR, triglycerides
Introduction
Gestational diabetes mellitus (GDM) refers to diabetes that often starts in the middle or late stages of pregnancy and is considered one of the most common diseases during pregnancy (1). According to the diagnostic criteria from the International Association of Diabetes and Pregnancy Study Group (IADPSG), the incidence rate of GDM is about 13.9% (2). Recent years, with the prevalence of obesity, delayed childbirth, multiple pregnancies, and these factors, there is a gradual increase in GDM occurrence, with a greater impacts to the wellbeing of both mother and baby (3). Gestational diabetes mellitus can increase the incidence of miscarriage, premature delivery, dystocia, infection, pregnancy complications, fetal macrosomia, structural birth defects, and small for gestational age babies (4). Gestational diabetes mellitus also significantly increases predisposition to non-GDM diabetes after pregnancy (5). Since blood glucose screening is usually performed in the middle and late stages of pregnancy, once confirmed, there is little time for patients to receive treatment and prevent the disease from negatively impacting the fetus. Therefore, there is a need to identify some appropriate biochemical markers for the early diagnosis of GDM. Afamin, a vitamin E–binding protein, seems to play a role in anti-apoptotic cellular response to oxidative stress (6). Studies indicate that afamin plasma levels increase linearly (around 2-fold) during an uncomplicated pregnancy (7), and strongly correlate with clinical and laboratory parameters of the metabolic syndrome, such as elevations in BMI and plasma glucose concentrations (8). Recent large population-based study in more than 20,000 participants showed strong association of afamin concentrations with the prevalence and incidence of type 2 diabetes mellitus (9), making it a potential new biomarker for pathological glucose metabolism in pregnancy. The main goal of this retrospective study is to conduct in-depth research of GDM to find new accurate and reliable predictive methods of diagnostics.
Materials and Methods
Research Participants and Groupings
Medical data from pregnant women who gave birth in our hospital from May 2018 to December 2018 were retrieved from the Medical record of our hospital and retrospectively grouped according to glucose tolerance test results of the patients at 24-28 weeks of gestation.
Based on the recorded results of the glucose tolerance tests, 607 cases were included in the GDM group, and 833 pregnant women with normal glucose tolerance, matched according to the similar blood sampling time in the first trimester, were included in the non-GDM group. For all the participants, age of the pregnant woman, age of the father, number of pregnancies, parity, height/weight before pregnancy, and the calculated BMI before pregnancy were recorded.
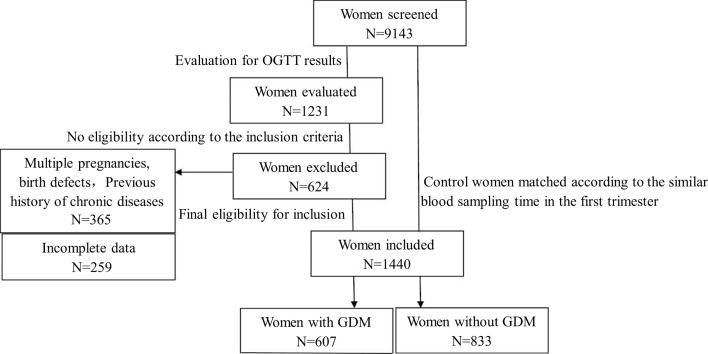
Gestational diabetes was diagnosed based on the diagnostic criteria established by the American Diabetes Association (ADA) in 2012 (10). Inclusion criteria were as follows (1): Singleton pregnancy (2); afamin blood test and routine blood test, prenatal aneuploidy screening (a combination of nuchal translucency thickness measurement, and levels of serum-free human chorionic gonadotropin and the pregnancy-associated plasma protein A), and biochemical tests performed and recorded at 10-12 weeks of gestation, combined with OGTT tests at 24-28 weeks of gestation (3); The data is complete and available. Exclusion criteria included: (1) Previous history of chronic diseases such as hypertension, diabetes, chronic liver or kidney disease, malignant tumor, autoimmune disease, blood system disease or certain infectious diseases; (2) Multiple pregnancy, birth defects, or miscarriage; (3) Incomplete or unavailable data. All participants in this study signed a consent form, and the research protocol was approved by the Ethics Committee of Fujian Maternity and Child Health Hospital (No: 2018-140) from June 12 2018. Figure 1 summarizes a study flowchart.
Figure 1.
Schematic study workflow.
Afamin Level Tests
Two milliliters of fasting venous blood were drawn from all the included study participants in the first trimester (10-12 weeks of gestation). The samples were centrifuged at 4°C, 3,000 rpm for 30 minutes. The supernatant was extracted and stored at -80°C. Afamin concentration in the serum was measured by ELISA, using specific mono- and polyclonal antibodies recognizing human afamin (Shanghai, Huiying, China) according to manufacturer’s instructions.
Routine blood tests, screening for the Downs syndrome, and biochemical analysis were performed and recorded in the laboratory of our hospital. The parameters included afamin, white blood cell count (WBC), neutrophil count (NEU), platelet count (PLT), mean platelet volume (MPV), platelet count test (PCT), platelet distribution width (PDW), platelet large cell ratio (PLCR), lymphocyte count (LYM), hemoglobin (HGB), pregnancy-associated plasma protein A, triglyceride (TG), total cholesterol (CHOL), high density lipoprotein (HDLC), low density lipoprotein (LDL), serum ferritin (Ferr), Calcium (CA), and magnesium (MG). In addition, the neutrophil/lymphocyte ratio (NLR) and platelet/lymphocyte ratio (PLR) were calculated and recorded. NLR is defined as the ratio between the absolute value of neutrophil count and the absolute value of lymphocyte count, and PLR is defined as the ratio between the absolute value of platelet count and the absolute value of lymphocyte count.
Statistical Analysis
SPSS 24.0 software was used for statistical analysis. Data were expressed as mean ± standard deviation, and independent sample t-test was used for comparison between groups. Variables with P < 0.05 in univariate analysis were further analyzed using multivariate logistic regression to identify independent risk factors for GDM. SPSS’s histogram chart was used to investigate the normal distribution of the data. When evaluating the specificity and sensitivity of certain parameter as a potential diagnostic prediction factor, receiver operating characteristic curve (ROC) and area under the curve (AUC) methods were used, and Youden index was employed to select the most appropriate cut-off value. We consider P<0.05 as a statistically significant value.
Results
Clinical and Laboratory Characteristics of Pregnant Women
There was no statistically significant difference between the two groups of pregnant women in pre-pregnancy BMI, number of pregnancies, and parity (P>0.05). There was a statistically significant difference between the study and the GDM groups in terms of maternal age (N (SD) (30.39 (3.30) and 28.42 (3.30) respectively; P<0.001). Similarly, the paternal age was significantly higher in the non-GDM group as compared to the GDM group (P<0.001). The results are summarized in Table 1 .
Table 1.
Comparison of clinical characteristics and laboratory indicators between the two groups of pregnant women.
| Project | GDM group (n = 607 cases) | Non-GDM Group (n = 833 cases) | P value |
|---|---|---|---|
| Maternal age (years) | 30.39 ± 3.66 | 28.42 ± 3.30 | <0.001 |
| Husband age (years) | 31.80 ± 4.55 | 30.23 ± 4.03 | <0.001 |
| Number of pregnancies | 2.05 ± 1.19 | 1.87 ± 1.04 | 0.004 |
| Number of births (Times) | 1.45 ± 0.57 | 1.41 ± 0.65 | 0.202 |
| Pre-pregnancy BMI index(Kg/m2) | 22.26 ± 7.31 | 21.67 ± 2.72 | 0.075 |
| Afamin (ng/ml) | 663.42 ± 226.13 | 594.26 ± 143.80 | <0.001 |
| PAPPA (mg/L) | 2205.50 ± 1880.49 | 2563.45 ± 2066.32 | 0.001 |
| MPV (fL) | 10.00 ± 0.80 | 10.09 ± 0.91 | 0.063 |
| PLT (×103/L) | 244.63 ± 47.95 | 237.47 ± 51.50 | 0.008 |
| PCT (%) | 0.24 ± 0.04 | 0.24 ± 0.05 | 0.024 |
| PDW (%) | 11.18 ± 1.68 | 11.31 ± 1.93 | 0.194 |
| PLCR (%) | 24.71 ± 6.54 | 25.34 ± 7.37 | 0.102 |
| WBC (/uL) | 8.98 ± 1.96 | 8.33 ± 1.86 | <0.001 |
| NEU (/uL) | 7.01 ± 6.94 | 5.92 ± 3.52 | 0.001 |
| LYM (/uL) | 2.16 ± 2.02 | 1.96 ± 0.83 | 0.036 |
| HGB (g/L) | 127.76 ± 9.42 | 126.40 ± 9.86 | 0.009 |
| NLR | 3.45 ± 2.37 | 3.19 ± 1.72 | 0.011 |
| PLR | 113.98 ± 34.53 | 128.63 ± 37.51 | <0.001 |
| TG (mmol/L) | 1.56 ± 0.73 | 1.31 ± 0.47 | <0.001 |
| CHOL (mmol/L) | 4.65 ± 0.76 | 4.49 ± 0.69 | <0.001 |
| HDLC (mmol/L) | 1.72 ± 0.32 | 1.71 ± 0.32 | 0.698 |
| LDL (mmol/L) | 2.09 ± 0.58 | 1.97 ± 0.52 | <0.001 |
| CA (mmol/L) | 2.33 ± 0.81 | 2.33 ± 0.81 | 0.954 |
| MG (mmol/L) | 0.88 ± 0.70 | 0.89 ± 0.73 | 0.088 |
| Ferr (μg/L) | 84.61 ± 58.83 | 87.79 ± 65.18 | 0.404 |
GDM, Gestational Diabetes Mellitus; BMI, body mass index; WBC, white blood cells; NEU, neutrophils; PLT, platelets; MPV, mean platelet volume; PCT, platelet count test; PDW, platelet distribution width; PLCR, platelet large cell ratio; LYM, lymphocytes; HGB, hemoglobin; PAPPA, pregnancy-associated plasma protein A; TG, triglycerides; CHOL, total cholesterol; HDLC, high density lipoprotein; LDL, low density lipoprotein; Ferr, serum ferritin; CA, Calcium; MG, magnesium, (MG); NLR, neutrophil/lymphocyte ratio; PLR, platelet/lymphocyte ratio.
Serum afamin levels were significantly elevated in the women, diagnosed with GDM, compared to GDM group (P<0.001), Table 1 . Similarly, parameters, such as WBC, PLT, NEU, LYM, HGB, NLR, TG, CHOL and LDL, were significantly elevated, while levels of PAPPA and PLR were markedly lower in non-GDM group as compared to GDM group (P<0.05). There was no statistically significant difference in MPV, PDW, PLCR, HDLC, CA, MG and serum ferritin between the two groups of pregnant women (P>0.05), Table 1 .
Multivariate Logistic Regression Analysis and Predictive Model Construction
We next carried out multivariant logistic regression analysis to identify possible risk factors of GDM. As summarized in Table 2 , age, early pregnancy maternal blood platelet/lymphocyte ratio, triglycerides and afamin are found to be independent risk factors for GDM (P<0.001). The formula for predicting GDM probability was as follows:
Table 2.
Risk factors associated with gestational diabetes mellitus: multi-factor logistic regression analysis.
| Index | OR value | (95% confidence interval) | P value |
|---|---|---|---|
| Age (years) | 1.167 | 1.123,1.213 | <0.001 |
| Afamin(ng/ml) | 1.002 | 1.001,1003 | <0.001 |
| TG (mmol/L) | 2.168 | 1.674,2.809 | <0.001 |
| PLR | 0.988 | 0.984,0.992 | <0.001 |
TG, triglycerides; PLR, platelet/lymphocyte ratio;OR, odds ratio.
Evaluation of the Prediction Model
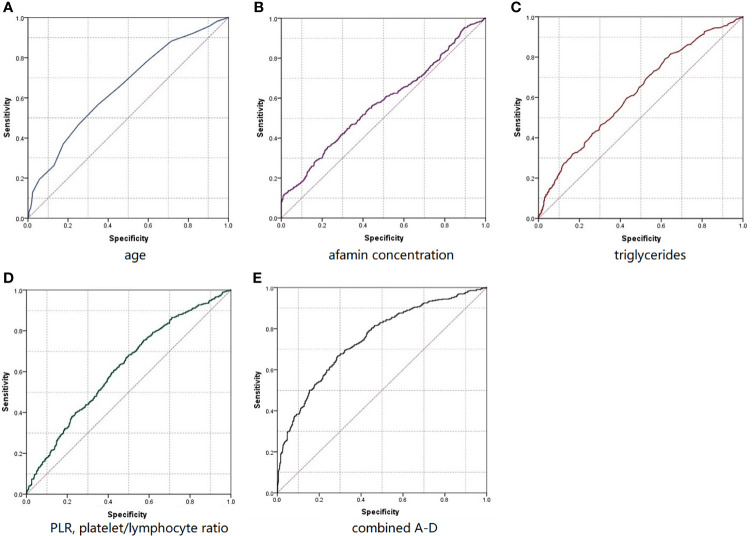
We next evaluated the performance of a classification model using ROC and the area under the curve (AUC) metrics. As summarized in Figure 2 and Table 3 , AUC for age, afamin levels, TG, and PLR were 0.653, 0.574, 0.622, and 0.618, respectively. The AUC of the ROC curve established for the prediction model was 0.748, greater than the AUC of any single factor. The discrimination of the prediction model was, therefore, satisfactory. When the prediction probability cut-off value was 0.358, the sensitivity, specificity, positive predictive value, and negative predictive value for age, afamin levels, TG, and PLR were 69.2%, 68.3%, 42.5%, and 86.2%, respectively, with the accuracy rate of 70.2%. The Hosmer-Leme show test results showed that the model has a good discrimination ability (χ2 = 12.269, df=8, P=0.140), Table 3 and Figure 2 .
Figure 2.
RoC curves predicted individually (A–D) or combined (E) using age (A), afamin concentration (B), triglyceride (C), and platelet lymphocyte ratio (D) levels in the early stages of pregnancy.
Table 3.
Risk factors associated with gestational diabetes mellitus: predictive capabilities.
| Index | Truncation value | Sensitivity | Specificity | AUC value (95% confidence interval) | P Value |
|---|---|---|---|---|---|
| Age (years) | 29.50 | 56.6 | 65.3 | 0.653 (0.624,0.682) | <0.001 |
| Afamin (ng/ml) | 720.10 | 35.6 | 77.3 | 0.574 (0.543,0.605) | <0.001 |
| TG (mmol/L) | 1.095 | 79.1 | 38.4 | 0.622 (0.591,0.653) | <0.001 |
| PLR | 124.56 | 68.0 | 50.2 | 0.618 (0.588, 0.648) | <0.001 |
| The predictive model | 0.358 | 69.2 | 68.3 | 0.748 (0.719, 0.777) | <0.001 |
TG, triglycerides; PLR, platelet/lymphocyte ratio; AUC, area under curve.
Discussion
Most patients with GDM will return to normal blood glucose levels shortly after delivery. However, GDM diagnosis carries a up to a 60% lifetime risk of progression to type 2 diabetes mellitus (T2DM) (11). GDM and T2DM share many common risk factors and pathological pathways. Studies showed that GDM women with severe form of blood glucose disorders during pregnancy generally progress to T2DM within 6 months to 5 years postpartum. Over time, the cumulative incidence of postpartum diabetes gradually increases (12, 13), indicating that GDM may be the early stage of T2DM (14). International Diabetes Foundation predicts that by 2040, the total number of diabetic patients in the world will reach 642 million (15). Therefore, early diagnosis and treatment of GDM can greatly reduce the incidence of maternal and infant complications, improve the prognosis of the disease, and also reduce the burden on healthcare system. Previous studies have shown that appropriate physical activity before and during pregnancy can reduce the risk of GDM (16). Another study confirmed that GDM screening in early pregnancy can effectively reduce the risk of GDM and fetal macrosomia. This indicates that control of blood sugar through diet and exercise in response to GDM screening can yield positive outcomes (17). With the liberalization of the second-child policy in China, more and more families choose to have second baby that leads to more advanced age pregnancies, contributing to the significant increase in the incidence of GDM (18). Early screening for the occurrence of GDM can, therefore, not only reduce the incidence of GDM and its negative outcomes, but also help to conform to the national advocacy of “eugenics” in China (19, 20). From both individual family’s welfare and national policy point of view, finding indicators to screen for early GDM, provide early prevention measures, give early diagnostic approaches, and implement early treatment methods have profound clinical application values.
The results of this study confirms previous reports that age is an independent risk factor for the onset of GDM (21). At present, there is relatively little controversy over the fact that age is an independent risk factor for the onset of GDM. Aging induces the overall physiological function declination of women, and insulin affinity also decreases with aging (22). The metabolism of various system organs increases during pregnancy to meet the needs of fetal growth and development. As result, compensatory insulin secretion increases, which in turn aggravates insulin resistance, potentially leading to development of GDM (23, 24).
Afamin, secreted mainly by liver, is also known as vitamin E binding protein, it a glycoprotein with multiple biological effects, including immune and inflammatory response (25). Recent studies showed that afamin can bind to thioredoxin, leading to oxidative stress and, consequently, to insulin resistance. Animal studies also showed that the deletion of afamin gene led to increased insulin sensitivity in rats (25). Koninger et al. found that serum afamin levels in patients with polycystic ovary syndrome was significantly higher than that of the normal control group, showing strong correlation with insulin sensitivity, and directly proportional to BMI (26). Potential mechanisms of the effect of afamin on insulin sensitivity may involve activation of the NLRP3 inflammasome and conversion of inactive caspase-1 precursors into active caspase-1, which mediates the release of IL-β. This model if further supported by the observation that afamin levels are directly proportional to the levels of hs-CRP (high-sensitivity C-reactive protein), an acute inflammatory protein (27). Studies show that a number of factors associated with diabetes, such as high glucose, adipokines, modified lipoproteins and free fatty acids may trigger CRP production by endothelial and smooth muscle cells and monocytes/macrophages. CRP is regulated by cytokines, and plays a critical role in T2DM by its action on pancreatic β-cells (28). Moreover, the serum level of high-sensitivity CRP are associated with β-cell dysfunction and insulin resistance (29).
A small-sample preliminary study also found that patients, requiring insulin to control blood sugar in late pregnancy, had higher concentrations of afamin compared to patients, whose blood glucose was managed with dietary modifications (30). A meta-analysis of the results of 8 prospective cohort studies involving 20136 samples, carried out by Kollerits B et al. (9), reported that afamin levels positively correlated with the HOMA-IR score of insulin resistance (β= 0.110, 95% CI 0.089–0.132, P = 1.37 × 1023), and were an independent prediction factor for 585 cases of diabetes. Adding serum afamin levels to the prediction model significantly improved its prediction accuracy. Every 10mg/L increase in Afamin level was associated with an increase in the incidence of type 2 diabetes (OR=1.19, 95% CI 1.12–1.26, P= 5.96×10−8). In our study, afamin levels of GDM patients were higher by an average of 69.16 mg/L, as compared to a non-GDM group. Furthermore, the results of multivariant logistic analysis indicated that afamin is a strong independent risk factor of GDM (P<0.001). Together with previous evidence, our results suggest that afamin can be used as an early biomarker for detection for abnormal glucose metabolism during pregnancy. We speculate that increased serum afamin levels may contribute to oxidative stress, insulin resistance and inflammation, ultimately leading to the development of GDM. This hypothesis is further strengthened by the observed evidence of chronic inflammation in GDM group, as indicated by the PLR and NLR levels. Platelet to lymphocyte and neutrophile to lymphocyte ratio (PLR and NLR respectively) are considered indicators of subclinical inflammation and useful predictive markers in prediabetes and diabetes mellitus. Studies show that PLR significantly decreases in prediabetes and early stages of diabetes, while NLR is significantly increased (31). Platelet functions can be evaluated by measuring platelet parameters such as platelet count, mean platelet volume (MPV), and platelet distribution width (PDW) (32). Gestational diabetes is a systemic disease, and the activation of platelets in diabetic patients often precedes the appearance of vascular pathological changes (33). The interaction between endothelial cells and platelets can be accompanied by the release of a variety of inflammatory factors, leading to the adhesion and migration of leukocytes (34–36). Platelet count and platelet-related parameters such as MPV have been studied in GDM, and it was found that MPV can effectively reflect blood glucose level (37). Our results indicated that the PLR of GDM patients in the first trimester was significantly lower than that of the non-GDM group (P<0.001). This decrease coincided with the marked increase in NLR (P=0.011). However, as indicated by multivariant logistic regression analysis, only PLR may be considered a predictor and independent GDM risk factor in our study. Since the occurrence of GDM may be due to the activation of the immune system, lymphocyte-mediated immune system disorders may be the key pathophysiological cause of GDM, and platelets may play a particularly important role in the regulation of GDM immunity and inflammation. Therefore, the PLR value is more effective than other platelet-related parameters in predicting GDM. Using PLR as an early marker of GDM also has the advantage of high efficiency, quick turn-around, and low price.
During pregnancy, blood lipid levels are physiologically elevated and the intestinal absorption of fat is increased to meet the needs of placental and fetal growth and development. This physiological increase generally does not cause adverse consequences and will gradually return to normal after the end of pregnancy (38). However, abnormally elevated blood lipids during pregnancy may lead to certain metabolic diseases like gestational diabetes and acute pancreatitis (39). At the same time, dyslipidemia can also induce oxidative stress in the body, aggravate vascular endothelial damage, and cause a series of pregnancy complications such as hypertension and cardiovascular disease (40). Recent studies have shown that elevated triglyceride (TG) levels may aggravate insulin resistance (40), lead to vascular endothelial cell damage (41) and correlate with the occurrence of GDM (42). In our study, hypertriglyceridemia in the first trimester was an independent risk factor of GDM, and demonstrated predictive significance. Early triglyceride screening may, thus, potentially help reduce the incidence of maternal and child complications.
In this study, we combined maternal age, early pregnancy parameters such as afamin level, TG, and PLR to investigate and build a predictive model for GDM. We concluded that the prediction accuracy of age combined with afamin level, TG, and PLR (AUC=0.748) is higher than any single factor, with AUC 0.653, 0.574, 0.622, 0.618 respectively, P<0.001. The prediction sensitivity, specificity, positive predictive value, and negative predictive value were 69.2%, 68.3%, 42.5%, and 86.2% respectively, with an accuracy rate of 70.2%. Overall, our results clearly indicate a good predictive value of the proposed model.
Additional potential risk factors, analyzed in this study, merit further investigation. BMI is an important indicator of human body fitness. Previous studies have shown that the probability of GDM in overweight pregnant women is significantly higher than that of normal pregnant women (43). However, there was no statistically significant difference in the pre-pregnancy body mass index between the two groups of pregnant women in this study (P>0.05), which needs to be further explored.
PAPP-A is a zinc-binding metalloproteinase, which is secreted by the placental trophoblast tissue in pregnant women. PAPP-A in maternal serum can be detected after 28 days of fertilization, and then it grows rapidly, but the growth rate is slower in the second trimester. At present, the biological function of the PAPP-A molecule during pregnancy is still unclear, but a number of studies have shown that low levels of PAPP-A are related to GDM (44, 45). In this study, the concentration of PAPP-A in the GDM group was lower than that in the control group. Although further multivariate analysis was not statistically significant, it still suggests that PAPP-A may be closely related to glucose metabolism, which merits further investigation.
In summary, the establishment of a simple, low-cost and effective predictive model for early prediction of GDM has clinical practical values and can provide a reliable basis for clinical decision-making. We also acknowledge that this study is a single-center study. Therefore, the proposed GDM prediction model needs further improvement. Additional in-depth prospective, larger-sample size-, and multi-center studies are needed to provide more powerful support for clinical diagnosis and treatment of GDM.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by ethics committee of Fujian Maternity and Child Health Hospital, Affiliated Hospital of Fujian Medical University. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
XW conceived and designed the study. XZ, MX, and LZ were involved in literature search and data collection. LX and ZL analyzed the data. XW wrote the paper. JY and RX reviewed and edited the manuscript. All authors read and approved the final manuscript.
Funding
This study is supported by Science and Technology Innovation Research Funding of Fujian Maternity and Child Health Hospital (YCXZ 18-20).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- 1. Cho NH, Shaw JE, Karuranga S, Huang Y, da Rocha Fernandes JD, Ohlrogge AW, et al. IDF Diabetes Atlas: Global Estimates of Diabetes Prevalence for 2017 and Projections for 2045. Diabetes Res Clin Pract (2018) 138:271–81. doi: 10.1016/j.diabres.2018.02.023 [DOI] [PubMed] [Google Scholar]
- 2. International Association of Diabetes and Pregnancy Study Groups Consensus Panel. Metzger BE, Gabbe SG, Persson B, Buchanan TA, Catalano PA, et al. International Association of Diabetes and Pregnancy Study Groups Recommendations on the Diagnosis and Classification of Hyperglycemia in Pregnancy. Diabetes Care (2010) 33:676–82. doi: 10.2337/dc09-1848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ferrara A, Kahn HS, Quesenberry CP, Riley C, Hedderson MM. An Increase in the Incidence of Gestational Diabetes Mellitus: Northern California, 1991-2000. Obstet Gynecol (2004) 103:526–33. doi: 10.1097/01.AOG.0000113623.18286.20 [DOI] [PubMed] [Google Scholar]
- 4. O’Sullivan EP, Avalos G, O’Reilly M, Dennedy MC, Gaffney G, Dunne F. Atlantic DIP Collaborators. Atlantic Diabetes in Pregnancy (DIP): The Prevalence and Outcomes of Gestational Diabetes Mellitus Using New Diagnostic Criteria. Diabetologia (2011) 54:1670–5. doi: 10.1007/s00125-011-2150-4 [DOI] [PubMed] [Google Scholar]
- 5. Bellamy L, Casas J-P, Hingorani AD, Williams D. Type 2 Diabetes Mellitus After Gestational Diabetes: A Systematic Review and Meta-Analysis. Lancet (2009) 373:1773–9. doi: 10.1016/S0140-6736(09)60731-5 [DOI] [PubMed] [Google Scholar]
- 6. Heiser M, Hutter-Paier B, Jerkovic L, Pfragner R, Windisch M, Becker-André M, et al. Vitamin E Binding Protein Afamin Protects Neuronal Cells In Vitro. J Neural Transm Suppl (2002) 62:337–45. doi: 10.1007/978-3-7091-6139-5_32 [DOI] [PubMed] [Google Scholar]
- 7. Hubalek M, Buchner H, Mörtl MG, Schlembach D, Huppertz B, Firulovic B, et al. The Vitamin E-Binding Protein Afamin Increases in Maternal Serum During Pregnancy. Clin Chim Acta (2014) 434:41–7. doi: 10.1016/j.cca.2014.03.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kronenberg F, Kollerits B, Kiechl S, Lamina C, Kedenko L, Meisinger C, et al. Plasma Concentrations of Afamin are Associated With the Prevalence and Development of Metabolic Syndrome. Circ Cardiovasc Genet (2014) 7:822–9. doi: 10.1161/CIRCGENETICS.113.000654 [DOI] [PubMed] [Google Scholar]
- 9. Kollerits B, Lamina C, Huth C, Marques-Vidal P, Kiechl S, Seppälä I, et al. Plasma Concentrations of Afamin Are Associated With Prevalent and Incident Type 2 Diabetes: A Pooled Analysis in More Than 20,000 Individuals. Diabetes Care (2017) 40:1386–93. doi: 10.2337/dc17-0201 [DOI] [PubMed] [Google Scholar]
- 10. American Diabetes Association . Standards of Medical Care in Diabetes–2012. Diabetes Care (2012) 35 Suppl 1:S11–63. doi: 10.2337/dc12-s011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Noctor E, Dunne FP. Type 2 Diabetes After Gestational Diabetes: The Influence of Changing Diagnostic Criteria. World J Diabetes (2015) 6:234–44. doi: 10.4239/wjd.v6.i2.234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Metzger BE, Cho NH, Roston SM, Radvany R. Prepregnancy Weight and Antepartum Insulin Secretion Predict Glucose Tolerance Five Years After Gestational Diabetes Mellitus. Diabetes Care (1993) 16:1598–605. doi: 10.2337/diacare.16.12.1598 [DOI] [PubMed] [Google Scholar]
- 13. Kim C, Newton KM, Knopp RH. Gestational Diabetes and the Incidence of Type 2 Diabetes: A Systematic Review. Diabetes Care (2002) 25:1862–8. doi: 10.2337/diacare.25.10.1862 [DOI] [PubMed] [Google Scholar]
- 14. Cho YM, Kim TH, Lim S, Choi SH, Shin HD, Lee HK, et al. Type 2 Diabetes-Associated Genetic Variants Discovered in the Recent Genome-Wide Association Studies are Related to Gestational Diabetes Mellitus in the Korean Population. Diabetologia (2009) 52:253–61. doi: 10.1007/s00125-008-1196-4 [DOI] [PubMed] [Google Scholar]
- 15. International Diabetes Federation . IDF Diabetes Atlas. Brussels: International Diabetes Federation; (2015). [Google Scholar]
- 16. Dempsey JC, Butler CL, Sorensen TK, Lee I-M, Thompson ML, Miller RS, et al. A Case-Control Study of Maternal Recreational Physical Activity and Risk of Gestational Diabetes Mellitus. Diabetes Res Clin Pract (2004) 66:203–15. doi: 10.1016/j.diabres.2004.03.010 [DOI] [PubMed] [Google Scholar]
- 17. Seshiah V, Cynthia A, Balaji V, Balaji MS, Ashalata S, Sheela R, et al. Detection and Care of Women With Gestational Diabetes Mellitus From Early Weeks of Pregnancy Results in Birth Weight of Newborn Babies Appropriate for Gestational Age. Diabetes Res Clin Pract (2008) 80:199–202. doi: 10.1016/j.diabres.2007.12.008 [DOI] [PubMed] [Google Scholar]
- 18. Wei Y, Yang H, Zhu W, Yang H, Li H, Yan J, et al. International Association of Diabetes and Pregnancy Study Group Criteria Is Suitable for Gestational Diabetes Mellitus Diagnosis: Further Evidence From China. Chin Med J (Engl) (2014) 127:3553–6. [PubMed] [Google Scholar]
- 19. Lachmann EH, Fox RA, Dennison RA, Usher-Smith JA, Meek CL, Aiken CE. Barriers to Completing Oral Glucose Tolerance Testing in Women at Risk of Gestational Diabetes. Diabetes Med (2020) 37:1482–9. doi: 10.1111/dme.14292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Association AD. 12. Management of Diabetes in Pregnancy. Diabetes Care (2015) 38:S77–9. doi: 10.2337/dc15-S015 [DOI] [PubMed] [Google Scholar]
- 21. Kintiraki E, Goulis DG, Mameletzi S, Kasmas S, Athanasiadis A, Assimakopoulos E, et al. Large- and Small-for-Gestational-Age Neonates Born by Women With Gestational Diabetes Mellitus Diagnosed by the New IADPSG Criteria: A Case-Control Study of 289 Patients and 1 108 Controls. Exp Clin Endocrinol Diabetes (2013) 121:262–5. doi: 10.1055/s-0033-1334907 [DOI] [PubMed] [Google Scholar]
- 22. Marozio L, Picardo E, Filippini C, Mainolfi E, Berchialla P, Cavallo F, et al. Maternal Age Over 40 Years and Pregnancy Outcome: A Hospital-Based Survey. J Matern Fetal Neonatal Med (2019) 32:1602–8. doi: 10.1080/14767058.2017.1410793 [DOI] [PubMed] [Google Scholar]
- 23. Shapiro MR, Atkinson MA, Brusko TM. Pleiotropic Roles of the Insulin-Like Growth Factor Axis in Type 1 Diabetes. Curr Opin Endocrinol Diabetes Obes (2019) 26:188–94. doi: 10.1097/MED.0000000000000484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Karaçam Z, Çelİk D. The Prevalence and Risk Factors of Gestational Diabetes Mellitus in Turkey: A Systematic Review and Meta-Analysis. J Matern Fetal Neonatal Med (2021) 34:1331–41. doi: 10.1080/14767058.2019.1635109 [DOI] [PubMed] [Google Scholar]
- 25. Dieplinger H, Dieplinger B. Afamin–A Pleiotropic Glycoprotein Involved in Various Disease States. Clin Chim Acta (2015) 446:105–10. doi: 10.1016/j.cca.2015.04.010 [DOI] [PubMed] [Google Scholar]
- 26. Köninger A, Edimiris P, Koch L, Enekwe A, Lamina C, Kasimir-Bauer S, et al. Serum Concentrations of Afamin Are Elevated in Patients With Polycystic Ovary Syndrome. Endocr Connect (2014) 3:120–6. doi: 10.1530/EC-14-0053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Seeber B, Morandell E, Lunger F, Wildt L, Dieplinger H. Afamin Serum Concentrations are Associated With Insulin Resistance and Metabolic Syndrome in Polycystic Ovary Syndrome. Reprod Biol Endocrinol (2014) 12:88. doi: 10.1186/1477-7827-12-88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Arora P, Garcia-Bailo B, Dastani Z, Brenner D, Villegas A, Malik S, et al. Genetic Polymorphisms of Innate Immunity-Related Inflammatory Pathways and Their Association With Factors Related to Type 2 Diabetes. BMC Med Genet (2011) 12:95. doi: 10.1186/1471-2350-12-95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Huang Y-C, Chen C-C, Wang T-Y, Nguyen HTT, Chen Y-H, Wu C-M, et al. C-Reactive Protein Gene Variants and Their Serum Levels in Early Adult-Onset Type 2 Diabetes Mellitus. In Vivo (2019) 33:1685–90. doi: 10.21873/invivo.11656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Köninger A, Mathan A, Mach P, Frank M, Schmidt B, Schleussner E, et al. Is Afamin a Novel Biomarker for Gestational Diabetes Mellitus? A Pilot Study. Reprod Biol Endocrinol (2018) 16:30. doi: 10.1186/s12958-018-0338-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mertoglu C, Gunay M. Neutrophil-Lymphocyte Ratio and Platelet-Lymphocyte Ratio as Useful Predictive Markers of Prediabetes and Diabetes Mellitus. Diabetes Metab Syndr (2017) 11 Suppl 1:S127–31. doi: 10.1016/j.dsx.2016.12.021 [DOI] [PubMed] [Google Scholar]
- 32. Thachil J. Platelets in Inflammatory Disorders: A Pathophysiological and Clinical Perspective. Semin Thromb Hemost (2015) 41:572–81. doi: 10.1055/s-0035-1556589 [DOI] [PubMed] [Google Scholar]
- 33. Tavil Y, Sen N, Yazici H, Turfan M, Hizal F, Cengel A, et al. Coronary Heart Disease is Associated With Mean Platelet Volume in Type 2 Diabetic Patients. Platelets (2010) 21:368–72. doi: 10.3109/09537101003628421 [DOI] [PubMed] [Google Scholar]
- 34. Gawaz M, Langer H, May AE. Platelets in Inflammation and Atherogenesis. J Clin Invest (2005) 115:3378–84. doi: 10.1172/JCI27196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Silasi M, Cohen B, Karumanchi SA, Rana S. Abnormal Placentation, Angiogenic Factors, and the Pathogenesis of Preeclampsia. Obstet Gynecol Clin North Am (2010) 37:239–53. doi: 10.1016/j.ogc.2010.02.013 [DOI] [PubMed] [Google Scholar]
- 36. Steegers EAP, von Dadelszen P, Duvekot JJ, Pijnenborg R. Pre-Eclampsia. Lancet (2010) 376:631–44. doi: 10.1016/S0140-6736(10)60279-6 [DOI] [PubMed] [Google Scholar]
- 37. Goncalves SC, Labinaz M, Le May M, Glover C, Froeschl M, Marquis J-F, et al. Usefulness of Mean Platelet Volume as a Biomarker for Long-Term Outcomes After Percutaneous Coronary Intervention. Am J Cardiol (2011) 107:204–9. doi: 10.1016/j.amjcard.2010.08.068 [DOI] [PubMed] [Google Scholar]
- 38. Butte NF. Carbohydrate and Lipid Metabolism in Pregnancy: Normal Compared With Gestational Diabetes Mellitus. Am J Clin Nutr (2000) 71:1256S–61S. doi: 10.1093/ajcn/71.5.1256s [DOI] [PubMed] [Google Scholar]
- 39. Herrera E, Ortega-Senovilla H. Disturbances in Lipid Metabolism in Diabetic Pregnancy - Are These the Cause of the Problem? Best Pract Res Clin Endocrinol Metab (2010) 24:515–25. doi: 10.1016/j.beem.2010.05.006 [DOI] [PubMed] [Google Scholar]
- 40. Ryckman KK, Spracklen CN, Smith CJ, Robinson JG, Saftlas AF. Maternal Lipid Levels During Pregnancy and Gestational Diabetes: A Systematic Review and Meta-Analysis. BJOG (2015) 122:643–51. doi: 10.1111/1471-0528.13261 [DOI] [PubMed] [Google Scholar]
- 41. Miller M, Cannon CP, Murphy SA, Qin J, Ray KK, Braunwald E. PROVE IT-TIMI 22 Investigators. Impact of Triglyceride Levels Beyond Low-Density Lipoprotein Cholesterol After Acute Coronary Syndrome in the PROVE IT-TIMI 22 Trial. J Am Coll Cardiol (2008) 51:724–30. doi: 10.1016/j.jacc.2007.10.038 [DOI] [PubMed] [Google Scholar]
- 42. Lippi G, Albiero A, Montagnana M, Salvagno GL, Scevarolli S, Franchi M, et al. Lipid and Lipoprotein Profile in Physiological Pregnancy. Clin Lab (2007) 53:173–7. [PubMed] [Google Scholar]
- 43. National Institutes of Health Consensus Development Conference Statement: Diagnosing Gestational Diabetes Mellitus, March 4-6, 2013. Obstet Gynecol (2013) 122:358–69. doi: 10.1097/AOG.0b013e31829c3e64 [DOI] [PubMed] [Google Scholar]
- 44. Beneventi F, Simonetta M, Lovati E, Albonico G, Tinelli C, Locatelli E, et al. First Trimester Pregnancy-Associated Plasma Protein-A in Pregnancies Complicated by Subsequent Gestational Diabetes. Prenat Diagn (2011) 31:523–8. doi: 10.1002/pd.2733 [DOI] [PubMed] [Google Scholar]
- 45. Wells G, Bleicher K, Han X, McShane M, Chan YF, Bartlett A, et al. Maternal Diabetes, Large-For-Gestational-Age Births, and First Trimester Pregnancy-Associated Plasma Protein-A. J Clin Endocrinol Metab (2015) 100:2372–9. doi: 10.1210/jc.2014-4103 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.