Abstract
Although the CRISPR/Cas9 system has been successfully used for crop breeding, its application remains limited in forest trees. Here, we describe an efficient gene editing strategy for hybrid poplar, (Populus tremula × alba INRA clone 717-1B4) based on the Golden Gate MoClo cloning. To test the system efficiency for generating single gene mutants, two single guide RNAs (sgRNAs) were designed and incorporated into the MoClo Tool Kit level 2 binary vector with the Cas9 expression cassette to mutate the SHORT ROOT (SHR) gene. Moreover, we also tested its efficiency for introducing mutations in two genes simultaneously by expressing one sgRNA targeting a single site of the YUC4 gene and the other sgRNA targeting the PLT1 gene. For a robust evaluation of the approach, we repeated the strategy to target the LBD12 and LBD4 genes simultaneously, using an independent construct. We generated hairy roots by Agrobacterium rhizogenes-mediated leaf transformation. Sequencing results confirmed the CRISPR/Cas9-mediated mutation in the targeted sites of PtaSHR. Biallelic and homozygous knockout mutations were detected. A deletion spanning both target sites and small insertions/deletions were the most common mutations. Out of the 22 SHR alleles sequenced, 21 were mutated. The phenotype’s characterization showed that transgenic roots with biallelic mutations for the SHR gene lacked a defined endodermal single cell layer, suggesting a conserved gene function similar to its homolog in Arabidopsis Arabidopsis thaliana (L.) Heynh. Sequencing results also revealed the high efficiency of the system for generating double mutants. Biallelic mutations for both genes in the yuc4/plt1 and lbd12/lbd4 roots were detected in three (yuc4/plt1) and two (lbd12/lbd4) out of four transgenic roots evaluated. A small deletion or a single nucleotide insertion at the single target site was the most common mutations. This CRISPR/Cas9 strategy arises as a rapid, simple and standardized gene-editing tool to evaluate the gene role in essential developmental programs such as radial cell differentiation of poplar roots.
Keywords: CRISPR/Cas9, gene editing, Golden Gate, MoClo Plant Parts Kit, MoClo Toolkit, Populus tremula × alba
Introduction
The CRISPR/Cas9 system is an efficient genome editing technology that has been applied successfully to a wide range of plant species (Bortesi and Fischer 2015, Gao 2018). This system requires two essential components, the Cas9 nuclease and a single guide RNA (sgRNA), which directs the nuclease to make double-strand breaks (DSBs) at specific genomic sites (Jinek et al. 2012). The intrinsic cellular DNA repair of CRISPR/Cas9-mediated DSBs frequently results in modifications to the sequence targeted by the sgRNA (Bortesi and Fischer 2015). By targeting genes of interest, this system has enabled plant breeders to develop new crop varieties with desired characteristics, including increased grain yield in rice (Liu et al. 2017), low-gluten wheat (Sánchez-León et al. 2018) or tomato varieties resistant to powdery mildew (Nekrasov et al. 2017).
In contrast, the application of the CRISPR/Cas9 system to genetically modify woody, perennial tree species has been limited. A commonly targeted genotype is the INRA 717-1B4, a female Populus tremula × Populus alba interspecific hybrid, widely used as a woody plant model species for gene functional characterization due to its high susceptibility to Agrobacterium-mediated transformation and organogenesis (Leple et al. 1992). A system that allows the expression of a single sgRNA was first used to mutate 4CL1 and 4CL2 lignin and flavonoid biosynthesis genes in INRA 717-1B4 (Zhou et al. 2015). The same strategy that targets a single site was applied to mutate EVE, a novel regulator of vessel element size (Ribeiro et al. 2020), and LHY2, a circadian gene involved in the photoperiodic control of shoot growth (Ramos-Sánchez et al. 2019). For multiple site editing, the strategy described by Fan et al. (2015) has been applied to assemble up to four gRNAs to mutate a single gene (reviewed in Bewg et al. 2018).
The Agrobacterium-mediated transformation incorporating the T-DNA containing expression cassettes with Cas9 and numerous sgRNAs showed high efficiency for multiple sites genome editing (Ma et al. 2015, Xie et al. 2015). Several multi-site CRISPR/Cas9 systems have been reported for plants (Xing et al. 2014, Zhang et al. 2016). These systems usually combine PCR amplification steps with Golden Gate, Gibson, Gateway or similar cloning methods to assemble the required DNA fragments in the final vector. Several subcloning steps are usually necessary to build the plant expression vector containing the sgRNAs and Cas9 expression cassettes (Fan et al. 2015, Lowder et al. 2015, Xie et al. 2015, Vazquez-Vilar et al. 2016, Hu et al. 2019), increasing cost and time.
Here, we propose a CRISPR/Cas9 genome editing strategy for poplar, based on the plasmids contained in the ‘MoClo Toolkit’ and ‘MoClo Plant Parts Kit’ (Weber et al. 2011, Engler et al. 2014). Altogether, these two kits have become versatile, precise and efficient tools for plant biologists to assemble multiple gene constructs, based in the Golden Gate cloning system. Golden Gate cloning has been successfully applied in human cells to mutate multiple genes simultaneously (Sakuma et al. 2015). The MoClo Toolkit contains a collection of cloning vectors required for the assembly of DNA parts during the subcloning steps. The MoClo Plant Parts Kit contains promoters, terminators, reporter genes, selectable markers, signal peptides, subcellular localization signals and other parts suitable for plant transformation. These include the 4-nt fusion sites that predefine their position based on a standardized genetic grammar, simplifying the assembly rules and the exchange of DNA constructs among laboratories. Both complete kits and individual parts are available at the non-profit plasmid repository Addgene (http://www.addgene.org/). By combining parts from these two kits, it is possible to design many standardized T-DNA fragments for different purposes: gene overexpression, tissue-specific gene expression, promoter–reporter fusion, protein–reporter fusion, protein-subcellular localization fusion and multiple genes expression combinations.
The Golden Gate MoClo cloning-based toolkit has been used for efficient single gene editing by expressing one or several gRNAs in yeast (Akhmetov et al. 2018), diatoms (Hopes et al. 2016, 2017), Arabidopsis Arabidopsis thaliana (L.) Heynh. (Castel et al. 2019) and tomato (Brooks et al. 2014, Nekrasov et al. 2017). Here, we show a MoClo kits-based strategy to express two sgRNAs to direct Cas9-mediated mutations in hybrid poplar target genes. Following this strategy, two Golden Gate ligation steps are required, with no PCR sub-steps. We demonstrate that this rapid, cloning-friendly and open-source CRISPR/Cas9 strategy is a powerful tool for gene function research in hybrid poplar.
Materials and methods
General strategy
Golden Gate is a DNA assembly method based on Type IIS restriction enzymes. It is an efficient cloning system with the potential of avoiding the need for primer design, PCR amplification and gel purification. Golden Gate only requires four base-pair junctions or overhangs (‘fusion sites’) to join adjacent parts. A limitation of Golden Gate cloning is that the recognition sequences for the type IIS enzymes used for assembly have to be removed from all the components to be assembled, in a process known as ‘domestication’. The MoClo Plant Parts Kit is a repository of standard domesticated parts that can be readily used in plants. The MoClo Tool Kit contains all the vector backbones required for assembly of the desired parts in single or multigene constructions.
The Golden Gate MoClo system uses a standardized grammar for the fusion sites for promoter, coding and terminator sequences. These are referred to as ‘level 0’. Selected level 0 parts can be assembled into ‘level 1’ acceptor vectors in a single cloning step, resulting in level 1 constructs containing assembled transcriptional units (TUs). Multigene constructs are then assembled from level 1 constructs in acceptor backbones referred to as ‘level 2’, M or P acceptors. All level 1, 2, M and P backbones have left and right borders and an origin of replication that functions in Agrobacterium.
Our genome editing strategy uses the Golden Gate MoClo system and plasmids available in the MoClo Toolkit and MoClo Plant Parts Kit. Following the procedure described below, up to six TUs can be assembled in the level 2 acceptor with no additional steps. This feature makes it possible to express up to four sgRNAs, together with the Cas9 and a selectable marker to detect transformation events. To test the efficiency of this strategy, we first targeted two genomic regions in a gene of interest, the poplar homolog of the Arabidopsis SHORT ROOT (SHR) gene (Potri.007G063300), by expressing two sgRNAs. The target sites were in the same exon, at a distance of 100 base pairs (bp) from each other. We also evaluated the potential of this strategy for generating mutations in two genes simultaneously. For this, we expressed two sgRNAs, one targeting a poplar homolog of the Arabidopsis YUCCA 4 (YUC4; Potri.006G248200) and the other a poplar homolog of the Arabidopsis PLETHORA 1 (PLT1; Potri.003G205700). We repeated this strategy to target poplar homologs of the Arabidopsis LATERAL ORGAN BOUNDARIES-DOMAIN 4 (LBD4; Potri.005G097800) and 12 (LBD12; Potri.001G281600) for a robust evaluation of the system’s efficiency for generating poplar double mutants.
To identify the sgRNAs used in the present study, predicted to have the highest efficiency, we used the CRISPR2 tool (http://cbi.hzau.edu.cn/CRISPR2/).
Generation of the toolkit components
The level 0 components, not contained in the MoClo Toolkit, consisted of the following essential elements: the Cas9 gene sequence, the promoters that drive the expression of sgRNAs and the sgRNAs. Level 0 of the promoters and Cas9 were generated by PCR. The pAtU6–26 and pAtU6–29 promoter sequences were amplified from the pHSE401 and pCBC-DT1T2 plasmids, respectively (Xing et al. 2014, Addgene). The Cas9 gene sequence from Streptococcus pyogenes was amplified from the pRGEB32 plasmid (Xie et al. 2015, Addgene). During the PCR step, the BsaI target site and the 4-nt fusion sites were added in the 5′ strand at both borders (Figures 1 and 2), by incorporating these sequences to the primers (see Table S1 available as Supplementary data at Tree Physiology Online). The PCR products were cloned into pGEM-T Easy (Promega Corp., Madison, Wis., USA). The resulting plasmids constitute the basic elements of this toolkit. These three plasmids, containing the Cas9 gene sequence, and the pAtU6–26 and pAt-U6–29 promoter sequences were deposited and are available in Addgene (www.addgene.org) under the following reference identifiers: pGEMT-Easy-Cas9-nls-CDS1, pGEMT-Easy-AtpU6–26 and pGEMT-Easy-AtpU6–29. The level 0 components of the sgRNAs double-stranded DNA containing the target sequence GN19 spacer of the sgRNA, the scaffold and the T7 terminator were synthesized by Synbio Technologies (Monmouth Junction, NJ, USA) (Figure 1). The BsaI target site was also incorporated in the 5′ strand at both borders, as well as the 4-nt fusion sites (Figures 1 and 2).
Figure 1.

Basic toolkit pieces prepared for the CRISPR/Cas9 strategy. (a, b) PCR amplified pU6–26 and pU6–29 DNA sequence (yellow). Primers contained the elements needed to make these pieces compatible with the Golden Gate cloning system: BsaI site (red), a prefix to assemble the promoter in the MoClo toolkit level 1 acceptor (gray). The suffix is the first bp of the sgRNA (blue). (c) sgRNA double DNA sequence (blue) is synthesized together with the scaffold (green) and T7 terminator (purple) and BsaI sites (red). The last three bp of the pU6–26 or pU6–29 promoters were added as prefix (yellow), and the standard suffix to assemble the piece into the level 1 acceptor was also added (gray). Hence, the last three nucleotides of the promoters and the first nucleotide of the sgRNA form the 4-nt fusion site needed to assemble the promoters and the sgRNA after the level 1 reaction. (d) Finally, Cas9 DNA sequence was amplified. This piece was compatible with the Golden Gate cloning by adding BsaI sites at 5′ strand of both border (red) and the MoClo standard prefix and suffix (gray) to make the piece compatible with the Golden Gate system (position CDS1).
Figure 2.

Assembly strategy of level 0 pieces of sgRNAs and promoters into the level 1 acceptor. GN19 sequence of the sgRNA, synthesized together with the sgRNA scaffold and T7 terminator (blue), is assembled with the promoters using the Golden Gate level 1 restriction-ligation reaction. As 4 nucleotides overhangs, the last 3 nucleotides of the promoters (yellow) and the first nucleotide of the sgRNA (blue) were used.
Assembly of TUs of the toolkit
For assembly of the TU from level 0 parts, the sgRNA-scaffold-T7 fragment and the promoter were first assembled into level 1 acceptor vectors using the Golden Gate BsaI/T4 restriction-ligation reaction (New England Biolabs, Ipswich, MA, USA) (level 1 reaction) (Figure 2). In our system, the 4-nt fusion site required was generated using the promoters’ last three nucleotides and the first guanine of the sgRNA-scaffold-T7 fragment (Figure 2). Using this overhang, we avoid introducing additional nucleotides between the promoter and the sgRNA that could potentially reduce the genome editing’s efficiency. It is worth mentioning that the source of the sgRNA is a crucial factor to be considered for setting the level 1 reaction. In our case, we synthesized a double-stranded DNA containing the target sequence GN19 spacer of the sgRNA, the scaffold and the T7 terminator. This sequence is cloned in the pUC57 plasmid by the company before it is received in the lab. As our sgRNA is contained in a plasmid, we used the standard level 1 reaction, which consists of adding 100 ng of each level 0 pieces: pU6–26 or pU6–29 (cloned in pGEM-T Easy plasmid), sgRNA1-scaffold-T7 or sgRNA2-scaffold-T7 (cloned in pUC57) and the level 1 acceptor (MoClo toolkit). An alternative approach to synthesizing the sgRNAs is to generate them by PCR by amplifying the scaffold from the plasmid pICH86966::AtU6p::sgRNA_PDS (Addgene, catalog number: 46966) using primers containing the target sequences (GN19) as previously described (Hopes et al. 2017). In this case, for the level 1 reaction, the sgRNA level 0 piece consists of a short DNA PCR product (about 100 bp) compared with the level 1 acceptor plasmid (4968 bp). Because of this difference in size, it is recommended to apply the following formula to maximize the reaction efficiency:
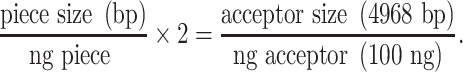 |
Accordingly, the level 1 reaction is performed as follows:
-Level 1 acceptor (MoClo toolkit): 100 ng
-pU6–26 or pU6–29 (cloned in pGEM-T Easy): 100 ng
-sgRNA1-scaffold-T7 or sgRNA2-scaffold-T7: about 4 ng (PCR product) or 100 ng (plasmid)
-10x T4 buffer: 1.5 μl
-100x BSA: 0.15 μl
-BsaI: 1 μl
-T4 DNA ligase: 1 μl
-dH20: to 15 μl
Following the reactions described above, sgRNA1-scaffold-T7 and pU6–26 were assembled in the level 1 acceptor, position 3-reverse (MoClo toolkit-pICH47822). Similarly, sgRNA2-scaffold-T7 and pU6–29 were assembled in the level 1 acceptor, position 4-reverse (MoClo Toolkit—pICH47831) (Figure 3). Following the same strategy, we generated the TU for the Cas9 endonuclease from S. pyogenes. pAtact2 promoter (pAtact2-pro) and tSlATPase terminator (pS1ATPase-term) containing plasmids were obtained from the MoClo Plant Parts Kit (pICH87644 and pICH71431, respectively); 100 ng of the level 1 acceptor, position 2-reverse vector (MoClo toolkit-pICH47811) and plasmids containing Cas9 level 0 (pGEMT-Easy-Cas9-nls-CDS1), pAtact2-pro and tSlATPase-term were used for the level 1 reaction described above. As a marker to select transformation events, we used the tdTomato-ER fluorescent protein; 35S promoter (MoClo plant part-pICH51288), tdTomato-ER and NOS terminator (MoClo plant part-pICH41421) were assembled in the level 1 acceptor, position 1-reverse (MoClo toolkit-pICH47802). These Golden Gate Level 1 plasmids containing the TUs of the Cas9 and tdTomato-ER were also deposited at Addgene under the following reference identifiers: pICH47811-pAtact2:Cas9-nls::tATPase and pICH47802-p35S::tdTomato-ER::tNOS.
Figure 3.
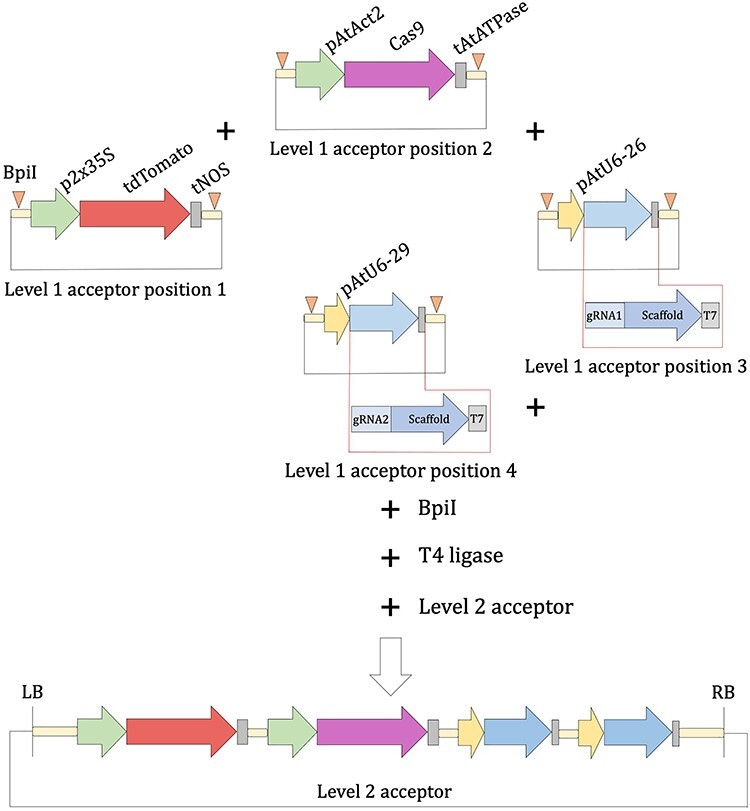
Golden Gate-based assembly of the four TUs into the final Golden Gate level 2 acceptor binary vector.
Final constructs were obtained by level 2 reactions, which are similar to level 1 reactions described above with the exception that the enzyme BsaI was replaced with BpiI, and the DNA components were replaced by level 1 plasmids and a level 2 acceptor. To assemble the four independent TUs into the final expression vector, 100 ng of level 1 plasmids containing TUs, 100 ng of level 2 acceptor (MoClo toolkit-pAGM4673) and 100 ng of end-link 4 (MoClo toolkit-pICH41780) were used to assemble the four expression cassettes using the BpiI/T4 Ligase restriction-ligation reactions (level 2 reaction) (Figure 3). This final binary plasmid was used for Agrobacterium rhizogenes ARqua1-mediated plant transformation.
Evaluation of the system efficiency in hybrid poplar
To evaluate the system’s efficiency in the genome editing of hybrid poplar, we generated hairy roots by transforming hybrid poplar (P. tremula × alba INRA 717–1B4) leaves with A. rhizogenes ARqua 1, as described previously (Yoshida et al. 2015).
Two sgRNAs were targeted against the hybrid poplar homolog of the Arabidopsis SHR gene, PtaSHR (Helariutta et al. 2000, Wang et al. 2011). Two sgRNAs were targeted against the hybrid poplar homologs of Arabidopsis YUC4 and PLT1, respectively. Finally, two sgRNAs were targeted against the hybrid poplar homologs of Arabidopsis LBD4 and LBD12, respectively.
After 3 weeks, hairy roots arose from the transformed leaves. The efficiency of the transformation, measured as the percentage of transgenic roots, was calculated by observing the tdTomato-ER fluorescent presence in 200 hairy roots.
Characterization of mutation events
Sixteen, six and six transgenic (tdTomato-ER fluorescent) hairy roots were harvested to characterize the mutation at PtaSHR, PtaYUC4/PtaPLT1 and PtaLBD4/PtaLBD12, respectively. Genomic DNA was extracted from each root (1–3 cm long), as described in Causevic et al. (2005), with modifications. Briefly, roots were frozen in liquid nitrogen and ground in 2-ml tubes containing 2 grinding beads using a homogenizer (MiniG 1600, Spex, Metuchen, NJ, USA); 200 μl of CTAB buffer containing 0.2% β-mercaptoethanol, 4% PVP and 0.1 mg ml−1 Proteinase K (Invitrogen) was added to the ground tissue. The suspension was digested for 1 h at 60 °C. After adding 1 volume of chloroform/isoamyl alcohol (24:1, v/v), samples were mixed and then centrifuged at 14 000 r.p.m. for 10 min. The aqueous phase was transferred to a new tube and incubated with 1 μl of RNase (Macherey-Nagel, 12-mg ml−1 stock) for 30 min at 37°C. The chloroform/isoamyl alcohol cleaning step was repeated. The aqueous phase was mixed with 1 volume of isopropanol and incubated for 30 min at −20°C. The sample was centrifuged at 14,000 r.p.m. for 15 min, and the pellet was washed with 70 and 95% ethanol by centrifugation for 5 min. The pellet was air-dried and resuspended in 50 μl of low TE.
DNA fragments spanning both target sites were amplified by PCR using primers reported in Table S1 available as Supplementary data at Tree Physiology Online. The PCR products were separated in a 1% agarose gel, using 0.5 × TBE during the electrophoresis, and purified using the Monarch DNA Gel Extraction Kit (New England Biolabs). Purified PCR products were cloned into a pGEM-T Easy vector (Promega) and transformed in DH5α Escherichia coli cells (New England Biolabs). Ten colonies per transgenic root were sequenced using the T7 primer to identify biallelic mutations.
Phenotyping CRISPR/Cas9 shr mutant
A 1-cm portion of roots showing biallelic mutations for shr was fixed in 4% formaldehyde as previously described (Conde et al. 2013). Roots were then embedded in Kulzer Technovit 7100 resin (Emgrid Australia) following the manufacturers’ instructions. Semi-thin sections (7–8 μm) of resin embedded samples were prepared using a Leica RM2045 microtome and stained with 0.1% Ruthenium Red (Sigma-Aldrich) in PBS. Cross-sections were analyzed using a confocal microscope (Zeiss). Images were obtained using a Leica TCS SP5 confocal microscope under the laser excitation line of 405 and 488 nm.
Results
To evaluate the efficiency of this CRISPR/Cas9 strategy, we targeted the hybrid poplar homolog of the Arabidopsis SHR gene, PtaSHR (Helariutta et al. 2000, Wang et al. 2011), by generating two sgRNAs against two DNA sites separated by 100 bp (Figure 4). SHR is a transcription factor expressed in the stele that moves into the adjacent cell layer to control SCARECROW transcription and endodermis specification (Helariutta et al. 2000). Endodermis and cortex in the root are derived from the same initial cells through asymmetric cell divisions (Scheres and Benfey 1999). AtSHR is essential for the endodermal cell layer specification in both roots and shoots (Helariutta et al. 2000, Cui et al. 2007). Notably, although the number of cortex cell layers varies considerably, nearly all plant species have only one layer of endodermis. Populus SHR shares high similarity with AtSHR over the entire length of the coding sequence. Populus genome contains two other SHR-like genes, SHR2A and SHR2B, that are significantly shorter at their 5′ ends than AtSHR (Miguel et al. 2016). Based on sequence similarity and functional studies with the Populus SHR coding sequence, SHR is considered the putative ortholog of AtSHR (Wang et al. 2011). SHR and SHR2B expression patterns markedly differ, and SHR2B has been associated with the bark development in Populus (Miguel et al. 2016). In hybrid poplar, the phenotypic alterations produced by shr mutation in roots have not yet been characterized.
Figure 4.
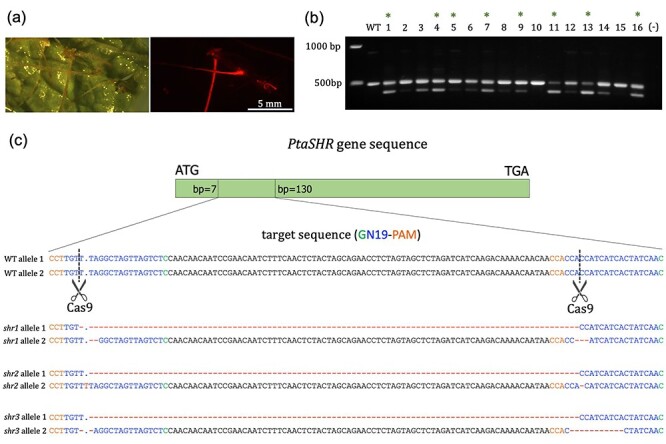
(a) Transgenic hairy root formation by A. rhizogenes ARQUA 1, transformed with the vector containing the construct to mutate SHR. Three weeks after transformation, transgenic carrying tdTOMATO fluorescence and WT roots are visible. (b) Genomic DNA was extracted from 16 transgenic roots. A fragment of SHR gene spanning both target sites was amplified and run in a 1% agarose gel. Most of the transgenics showed two bands corresponding to the different sizes of both alleles of SHR. Eight lines that showed two bands were selected for purification (indicated with a green asterisk), pGEM-T easy cloning, E. coli transformation and colony sequencing. (c) Three representative biallelic shr mutants are shown. Scale bar: 5 mm.
To evaluate the efficiency of this CRISPR/Cas9 strategy for introducing mutations in several genes simultaneously, we targeted the hybrid poplar homolog of the Arabidopsis YUC4 and PLT1 genes by generating two sgRNAs: one targeting PtaYUC4 and the other targeting PtaPLT1. For a robust evaluation of the strategy for generating poplar double mutants, we used the same approach to target the hybrid poplar homolog of the Arabidopsis LBD4 and LBD12 genes by expressing two sgRNAs, one targeting PtaLBD4 and the other targeting PtaLBD12.
High frequency of PtaSHR Cas9-mediated deletions spanning the sgRNA sites
Transgenic hairy roots were first identified as those emitting the tdTomato fluorescence (Figure 4a). The efficiency of transformation measured as the percentage of transgenic roots was 36% (72 fluorescent roots out of 200 hairy roots). The sgRNA1 and sgRNA2 sequences perfectly matched the SHR sequence in both alleles of the hybrid poplar (P. tremula and P. alba, Figure 4). With the PtaSHORT_testCas9_fwd/PtSHORT_testCas9_rev primer pair (Table S1 available as Supplementary data at Tree Physiology Online), a DNA segment spanning the two target sites was amplified from genomic DNA extracted from 16 independent transgenic roots and wild-type (WT) and separated in 1% agarose gel. Among these, 14 events showed two distinct bands, one with the expected size, and a second, smaller band (Figure 4b), suggesting a deletion. The PCR products from eight of the events that showed two amplified bands of different sizes were then purified and cloned into pGEM-T Easy. Five lines showed biallelic mutations (shr1, shr2, shr3, shr5 and shr6), while one line showed a mutation only in one allele (shr4). In addition, chimeric mutations were identified in two of the mutant hairy roots (shr7, shr8, see Figure S1 available as Supplementary data at Tree Physiology Online). These contained three and seven different mutated shr alleles, respectively. All eight lines had one allele with a deletion spanning the DNA sequence between the two Cas9 target sites (Figure 4c, Figure S1 available as Supplementary data at Tree Physiology Online). In total, out of the 22 SHR alleles sequenced in the transgenic roots, 21 presented a mutation derived from the Cas9 DSBs, and one allele remained not mutated (Figure 4c, Figure S1 available as Supplementary data at Tree Physiology Online).
High frequency of Cas9-mediated double mutants
As described above, transgenic hairy roots were first identified as those emitting the tdTomato fluorescence. Using the PtaYUC4_genotype/PtaPLT1_genotype, or the PtaLBD4_genotype/PtaLBD12_genotype primer pairs (Table S1 available as Supplementary data at Tree Physiology Online), a DNA segment spanning the target site for each gene was amplified from genomic DNA extracted from six independent transgenic roots for each construct and WT and separated in 1% agarose gel. The PCR products from four of the events for each construct were then purified and cloned into pGEM-T Easy.
In the case of yuc4/plt1 double mutants, three lines showed biallelic mutations for PtaYUC4 and PtaPLT1 (yuc4/plt1 #1, yuc4/plt1 #2 and yuc4/plt1 #3) (Figure 5a, Figure S2a available as Supplementary data at Tree Physiology Online). One line (yuc4/plt1 #4) showed biallelic mutations for PtaPLT1, while PtaYUC4 showed a mutation only in one allele (Figure 5a, Figure S2a available as Supplementary data at Tree Physiology Online). In the case of lbd12/lbd4 double mutants, two lines showed biallelic mutations for PtaLBD12 and PtaLBD4 (lbd12/lbd4 #1, lbd12/lbd4 #2) (Figure 5b, Figure S2b available as Supplementary data at Tree Physiology Online). One line (lbd12/lbd4 #3) showed a biallelic mutation for PtaLBD4, while only one allele was mutated in PtaLBD12 (Figure 5b, Figure S2b available as Supplementary data at Tree Physiology Online). One line showed only one allele mutated for PtaLBD12 and PtaLBD4 (Figure 5b, Figure S2b available as Supplementary data at Tree Physiology Online).
Figure 5.
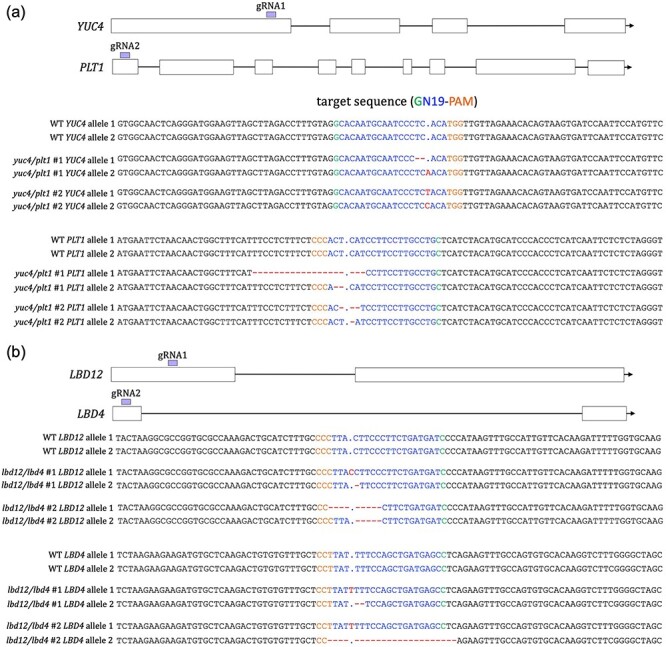
(a) Strategy followed to generate poplar double mutants for PtaYUC4 and PtaPLT1. Two sgRNAs were generated with one targeted to a single site of PtaYUC4 and the other targeted to PtaPLT1. Genomic DNA was extracted from four transgenic roots. A fragment of YUC4 and PLT1 spanning the target site was amplified. Two biallelic yuc4/plt1 double mutants are shown. (b) Strategy followed to generate poplar double mutants for PtaLBD12 and PtaLBD4. Two sgRNAs generated with one targeted to a single site of PtaLBD12 and the other targeted to PtaLBD4. Genomic DNA was extracted from four transgenic roots. A fragment of LBD12 and LBD4 spanning the target site was amplified. Two biallelic lbd12/lbd4 double mutants are shown.
The mutations at the single target site for each gene of interest consisted in a small deletion or a single nucleotide insertion located in the Cas9 cutting site (3–4 nucleotides upstream the protospacer adjacent motif).
Cortex-endodermis development is disrupted in PtaSHR CRISPR/Cas9 transgenic roots
To determine if the Cas9-mediated shr mutations affect the cortex-endodermis development, 3-week-old poplar biallelic mutant hairy roots were fixed in 4% formaldehyde and embedded in Kulzer Technovit 7100 resin. Analysis of 8-μm cross-sections under the scope showed a very defined single endodermis cell layer in WT roots. In contrast, hairy roots showing shr biallelic mutations lacked a defined endodermis layer, as well as a compacted cortex layer (Figure 6), suggesting that this gene, as the Arabidopsis homolog, plays a critical role in radial differentiation patterning during hybrid poplar root development. This observation indicated that our Cas9 strategy, combined with the Agrobacterium-mediated hairy roots generation, is a powerful system to evaluate the gene function of essential developmental programs such as radial cell differentiation of hybrid poplar roots.
Figure 6.

Characterization of the shr mutant phenotype. Hairy roots were fixed in formaldehyde and embedded in Technovit 7100 resin; 8-μm cross-section was obtained. CRISPR/Cas9 shr mutant hairy roots lack a single defined layer of endodermis as well as compact cortex, clearly visible in WT roots. Abbreviations: epidermis (ep), cortex (co), endodermis (ed), mutant layer (m). Scale bars: 50 or 100 μm as indicated in the images.
Discussion
The interspecific hybrid poplar clone INRA 717-1B4 is widely used as a plant model genotype for functional genetic experiments in woody species that require the generation of mutant lines. However, despite its importance as a model species, the number of tested CRISPR/Cas9 strategies remains limited (Fan et al. 2015, Bewg et al. 2018, Elorriaga et al. 2018). Furthermore, the nature of the Populus genome evolution often hampers functional gene characterization using CRISPR/Cas9 transgenic approaches, using a single sgRNA. The complex history of whole-genome duplications, chromosomal rearrangements and tandem duplications that occurred in Populus resulted in a large number of paralogous genes (Tuskan et al. 2006). Paralogous genes can often develop a divergent function due to relaxed selective pressure on one copy of the duplicated gene (Soria et al. 2014). However, a very recent whole-genome duplication occurred in Populus, resulting in nearly 8000 pairs of paralogous genes of similar age (Tuskan et al. 2006). Since the recent whole-genome duplication, most of the paralogs could still share the same function, leading plant breeders and molecular biologists to face functional redundancy. Consequently, the design of efficient CRISPR/Cas9 strategies able to assemble multiple sgRNAs expression cassettes for generation of transgenic knockout lines for multiple genes is of great interest. In the present work, we show that the MoClo Toolkit and MoClo Plant Parts Kit can be easily implemented to express multiple sgRNAs, Cas9 and a selectable marker for transformation, with two Golden Gate restriction-ligation steps. This transgenic implementation strategy is highly versatile. While we expressed the Cas9 under the constitutive pAtact2 promoter, its expression could also be directed under a tissue-specific promoter or an inducible promoter. This approach has recently been described as a powerful strategy to characterize gene function in a tissue or cell-type specific manner (Decaestecker et al. 2019, X. Wang, et al. 2020).
To test the efficiency of the system we described, we targeted two sgRNA to two sites of SHR gene spaced by 100 bp from one another. Our data revealed that 87.5% (14 out of 16) of the transgenic roots presented a deletion in one of the SHR alleles. This facilitated screening and selection of mutant lines by PCR amplification of the DNA fragment spanning both target sites, followed by product separation by agarose gel electrophoresis. In contrast, Cas9 mutations targeted by a single sgRNA generally result in minor sequence changes that are often only distinguishable by DNA sequencing. Furthermore, 62.5% (five out of eight) of the transgenic lines selected for sequencing were biallelic mutants, while 12.5% (one out of eight) presented the mutation only in one allele. Two of eight transgenic roots (25%) resulted in being chimeric, presenting all the detected alleles mutated. A similar percentage of chimeric plants was observed in Populus sp. when generating stable CRISPR/Cas9 knockout transgenic plants after Agrobacterium tumefaciens transformation (Muhr et al. 2018, J. Wang, et al. 2020). It is unclear if the chimeric plants are a consequence of the CRISPR/Cas9 system combined with the cellular DNA repair mechanisms, or due to the Agrobacterium-mediated organogenesis. In the latter case, more than one cell could have been recruited giving rise to independent mutations in each cell during root generation, resulting in chimeric events.
To test the system’s efficiency for generating poplar mutants for several genes simultaneously, we generated two sgRNA, one targeted to a single site of PtaYUC4 and the other targeted to PtaPLT1. Three out of four transgenic roots presented biallelic mutations for both genes, while one line showed biallelic mutation for PtaPLT1 and single allele mutation for PtaYUC4. For a robust evaluation of the system for generating poplar double mutants, we repeated this approach to express two sgRNAs, with one targeting a single site of PtaLBD12 and the other PtaLBD4 simultaneously. Similarly, two out of four transgenic roots presented biallelic mutation for the target genes. These results suggest that it is unnecessary to sequence many transgenic lines to identify biallelic mutations on both target genes when generating poplar double mutants using this strategy.
The MoClo-based cloning strategy described here to express multiple sgRNAs resulted in a potent approach when combined with A. rhizogenes-mediated hairy root formation, to explore a critical developmental program such as radial cell differentiation. Moreover, this system can also be used for A. tumefaciens-mediated plant transformation for the generation of stable transgenic plants to explore gene function related to any biological process. In our shr mutant screening, shr2_1, shr3_1, shr4_2, shr5_1, shr7_1 and shr8_1 alleles showed a 99 bp deletion, resulting in a truncated but still in-frame SHR protein version. The observation that the deletion between sgRNA target sites is frequent suggests that this approach may be useful for functional protein domain characterization in hybrid poplar, whenever a precise excision is necessary. A similar strategy to the one presented in the present work was used to produce a precise deletion in a single gene in diatom and tomato (Brooks et al. 2014, Hopes et al. 2017). In this work, we demonstrate that a precise deletion can be introduced in poplar by targeting two sgRNAs to the gene of interest. Moreover, we also show that the system can be applied to generate double mutants by targeting each sgRNA to a single target site for each gene of interest.
Following the strategy described in the present work, up to four sgRNAs can be expressed using the Golden Gate level 1 and 2 reactions. However, we demonstrated the high effectiveness of AtpU6–26 and Atp-29 to express sgRNAs in hybrid poplar. In Populus, it has been observed that the transgene inactivation is related to repeats at the T-DNA sequence (Kumar and Fladung 2001). Under this scenario, it would be recommended to test more promoters instead of expressing several sgRNAs under the same AtpU6–26 and Atp-29 promoters, which could be potentially harmful to the multiple site mutation efficiency. These scenarios will be evaluated in the future.
Finally, a significant advantage of this strategy relies on the fact that by using the level 0 and level 1 parts prepared for AtpU-26, AtpU-29 and Cas9, MoClo kit parts can be used to generate single and double CRISPR/Cas9 mutants. This approach simplifies the cloning method when designing strategies to explore gene function in the hybrid poplar clone INRA 717-1B4, and potentially other woody tree species.
Supplementary Material
Contributor Information
Paolo M Triozzi, School of Forest, Fisheries and Geomatics Sciences, University of Florida, 136 Newins-Ziegler Hall, Gainesville, FL 32611, USA.
Henry W Schmidt, School of Forest, Fisheries and Geomatics Sciences, University of Florida, 136 Newins-Ziegler Hall, Gainesville, FL 32611, USA.
Christopher Dervinis, School of Forest, Fisheries and Geomatics Sciences, University of Florida, 136 Newins-Ziegler Hall, Gainesville, FL 32611, USA.
Matias Kirst, School of Forest, Fisheries and Geomatics Sciences, University of Florida, 136 Newins-Ziegler Hall, Gainesville, FL 32611, USA; Plant Molecular and Cellular Biology Graduate Program, University of Florida, 2550 Hull Road Fifield Hall, room 1509 Gainesville, FL 32611, USA; Genetics Institute, University of Florida, 2033 Mowry Road, Gainesville, FL 32611, USA.
Daniel Conde, School of Forest, Fisheries and Geomatics Sciences, University of Florida, 136 Newins-Ziegler Hall, Gainesville, FL 32611, USA.
Authors' contributions
D.C. designed the research; P.M.T., H.W.S., C.D. and D.C. performed research; P.M.T., M.K. and D.C. analyzed data. P.M.T., M.K. and D.C. wrote the paper. All authors read and approved the manuscript.
Funding
This work was supported by the Department of Energy Office of Science Biological and Environmental Research (Grant DE-SC0018247) to M.K.
Conflict of interest
None declared.
References
- Akhmetov A, Laurent J, Gollihar J, Gardner E, Garge R, Ellington A, Kachroo A, Marcotte E (2018) Single-step precision genome editing in yeast using CRISPR-Cas9. BIO-Protoc 8. https://bio-protocol.org/e2765 (19 March 2021, date last accessed). [DOI] [PMC free article] [PubMed]
- Bewg WP, Ci D, Tsai C-J (2018) Genome editing in trees: from multiple repair pathways to long-term stability. Front Plant Sci 9:1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bortesi L, Fischer R (2015) The CRISPR/Cas9 system for plant genome editing and beyond. Biotechnol Adv 33:41–52. [DOI] [PubMed] [Google Scholar]
- Brooks C, Nekrasov V, Lippman ZB, Van Eck J (2014) Efficient gene editing in tomato in the first generation using the clustered regularly interspaced short palindromic repeats/CRISPR-Associated9 system. Plant Physiol 166:1292–1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castel B, Tomlinson L, Locci F, Yang Y, Jones JDG (2019) Optimization of T-DNA architecture for Cas9-mediated mutagenesis in Arabidopsis Lai E-M (ed). PLoS One 14:e0204778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Causevic A, Delaunay A, Ounnar S, Righezza M, Delmotte F, Brignolas F, Hagège D, Maury S (2005) DNA methylating and demethylating treatments modify phenotype and cell wall differentiation state in sugarbeet cell lines. Plant Physiol Biochem 43:681–691. [DOI] [PubMed] [Google Scholar]
- Conde D, González-Melendi P, Allona I (2013) Poplar stems show opposite epigenetic patterns during winter dormancy and vegetative growth. Trees 27:311–320. [Google Scholar]
- Cui H, Levesque MP, Vernoux T et al. (2007) An evolutionarily conserved mechanism delimiting SHR movement defines a single layer of endodermis in plants. Science 316:421–425. [DOI] [PubMed] [Google Scholar]
- Decaestecker W, Buono RA, Pfeiffer ML et al. (2019) CRISPR-TSKO: a technique for efficient mutagenesis in specific cell types, tissues, or organs in Arabidopsis. Plant Cell 31:2868–2887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elorriaga E, Klocko AL, Ma C, Strauss SH (2018) Variation in mutation spectra among CRISPR/Cas9 mutagenized poplars. Front Plant Sci 9:594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engler C, Youles M, Gruetzner R, Ehnert T-M, Werner S, Jones JDG, Patron NJ, Marillonnet S (2014) A golden gate modular cloning toolbox for plants. ACS Synth Biol 3:839–843. [DOI] [PubMed] [Google Scholar]
- Fan D, Liu T, Li C, Jiao B, Li S, Hou Y, Luo K (2015) Efficient CRISPR/Cas9-mediated targeted mutagenesis in Populus in the first generation. Sci Rep 5:12217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao C (2018) The future of CRISPR technologies in agriculture. Nat Rev Mol Cell Biol 19:275–276. [DOI] [PubMed] [Google Scholar]
- Helariutta Y, Fukaki H, Wysocka-Diller J, Nakajima K, Jung J, Sena G, Hauser M-T, Benfey PN (2000) The SHORT-ROOT gene controls radial patterning of the Arabidopsis root through radial signaling. Cell 101:555–567. [DOI] [PubMed] [Google Scholar]
- Hopes A, Nekrasov V, Kamoun S, Mock T (2016) Editing of the urease gene by CRISPR-Cas in the diatom Thalassiosira pseudonana. Plant Methods 12:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopes A, Nekrasov V, Belshaw N, Grouneva I, Kamoun S, Mock T (2017) Genome Editing in Diatoms Using CRISPR-Cas to Induce Precise Bi-allelic Deletions. BIO-Protoc 7. https://bio-protocol.org/e2625 (19 March 2021, date last accessed). [DOI] [PMC free article] [PubMed]
- Hu N, Xian Z, Li N, Liu Y, Huang W, Yan F, Su D, Chen J, Li Z (2019) Rapid and user-friendly open-source CRISPR/Cas9 system for single- or multi-site editing of tomato genome. Hortic Res 6:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jinek M, Chylinski K, Fonfara I, Hauer M, Doudna JA, Charpentier E (2012) A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science 337:816–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S, Fladung M (2001) Gene stability in transgenic aspen (Populus). II. Molecular characterization of variable expression of transgene in wild and hybrid aspen. Planta 213:731–740. [DOI] [PubMed] [Google Scholar]
- Leple J, Brasileiro A, Michel M, Delmotte F, Jouanin L (1992) Transgenic poplars: expression of chimeric genes using four different constructs. Plant Cell Rep 11:137–141. [DOI] [PubMed] [Google Scholar]
- Liu J, Chen J, Zheng X et al. (2017) GW5 acts in the brassinosteroid signalling pathway to regulate grain width and weight in rice. Nat Plants 3:17043. [DOI] [PubMed] [Google Scholar]
- Lowder LG, Zhang D, Baltes NJ et al. (2015) A CRISPR/Cas9 toolbox for multiplexed plant genome editing and transcriptional regulation. Plant Physiol 169:971–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma X, Zhang Q, Zhu Q et al. (2015) A robust CRISPR/Cas9 system for convenient, high-efficiency multiplex genome editing in monocot and dicot plants. Mol Plant 8:1274–1284. [DOI] [PubMed] [Google Scholar]
- Miguel A, Milhinhos A, Novák O, Jones B, Miguel CM (2016) The SHORT-ROOT-like gene PtSHR2B is involved in Populus phellogen activity. J Exp Bot 67:1545–1555. [DOI] [PubMed] [Google Scholar]
- Muhr M, Paulat M, Awwanah M, Brinkkötter M, Teichmann T (2018) CRISPR/Cas9-mediated knockout of Populus BRANCHED1 and BRANCHED2 orthologs reveals a major function in bud outgrowth control. Tree Physiol 38:1588–1597. [DOI] [PubMed] [Google Scholar]
- Nekrasov V, Wang C, Win J, Lanz C, Weigel D, Kamoun S (2017) Rapid generation of a transgene-free powdery mildew resistant tomato by genome deletion. Sci Rep 7:482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos-Sánchez JM, Triozzi PM, Alique D, Geng F, Gao M, Jaeger KE, Wigge PA, Allona I, Perales M (2019) LHY2 integrates night-length information to determine timing of poplar photoperiodic growth. Curr Biol 29:2402–2406. [DOI] [PubMed] [Google Scholar]
- Ribeiro CL, Conde D, Balmant KM et al. (2020) The uncharacterized gene EVE contributes to vessel element dimensions in Populus. Proc Natl Acad Sci USA 117:5059–5066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakuma T, Nishikawa A, Kume S, Chayama K, Yamamoto T (2015) Multiplex genome engineering in human cells using all-in-one CRISPR/Cas9 vector system. Sci Rep 4:5400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez-León S, Gil-Humanes J, Ozuna CV, Giménez MJ, Sousa C, Voytas DF, Barro F (2018) Low-gluten, nontransgenic wheat engineered with CRISPR/Cas9. Plant Biotechnol J 16:902–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheres B, Benfey PN (1999) Asymmetric cell division in plants. Annu Rev Plant Physiol Plant Mol Biol 50:505–537. [DOI] [PubMed] [Google Scholar]
- Soria PS, McGary KL, Rokas A (2014) Functional divergence for every paralog. Mol Biol Evol 31:984–992. [DOI] [PubMed] [Google Scholar]
- Tuskan GA, DiFazio S, Jansson S et al. (2006) The genome of black cottonwood, Populus trichocarpa (Torr. & Gray). Science 313:1596–1604. [DOI] [PubMed] [Google Scholar]
- Vazquez-Vilar M, Bernabé-Orts JM, Fernandez-del-Carmen A, Ziarsolo P, Blanca J, Granell A, Orzaez D (2016) A modular toolbox for gRNA–Cas9 genome engineering in plants based on the GoldenBraid standard. Plant Methods 12:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Andersson-Gunnerås S, Gaboreanu I et al. (2011) Reduced expression of the SHORT-ROOT gene increases the rates of growth and development in hybrid poplar and Arabidopsis. PLoS ONE 6:e28878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Wu H, Chen Y, Yin T (2020) Efficient CRISPR/Cas9-mediated gene editing in an interspecific hybrid poplar with a highly heterozygous genome. Front Plant Sci 11:996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Ye L, Lyu M, Ursache R, Löytynoja A, Mähönen AP (2020) An inducible genome editing system for plants. Nat Plants 6:766–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber E, Engler C, Gruetzner R, Werner S, Marillonnet S (2011) A modular cloning system for standardized assembly of multigene constructs. PLoS One 6:e16765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie K, Minkenberg B, Yang Y (2015) Boosting CRISPR/Cas9 multiplex editing capability with the endogenous tRNA-processing system. Proc Natl Acad Sci USA 112:3570–3575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing H-L, Dong L, Wang Z-P, Zhang H-Y, Han C-Y, Liu B, Wang X-C, Chen Q-J (2014) A CRISPR/Cas9 toolkit for multiplex genome editing in plants. BMC Plant Biol 14:327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida K, Ma D, Constabel CP (2015) The MYB182 protein Down-regulates proanthocyanidin and anthocyanin biosynthesis in poplar by repressing both structural and regulatory flavonoid genes. Plant Physiol 167:693–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Mao Y, Ha S, Liu W, Botella JR, Zhu J-K (2016) A multiplex CRISPR/Cas9 platform for fast and efficient editing of multiple genes in Arabidopsis. Plant Cell Rep 35:1519–1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, Jacobs TB, Xue L-J, Harding SA, Tsai C-J (2015) Exploiting SNPs for biallelic CRISPR mutations in the outcrossing woody perennial Populus reveals 4-coumarate:CoA ligase specificity and redundancy. New Phytol 208:298–301. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


