Alcohol-induced mGluR2 deficits are restored by psilocybin, resulting in a rescue of pathological behaviors in alcoholism.
Abstract
Alcohol-dependent patients commonly show impairments in executive functions that facilitate craving and can lead to relapse. However, the molecular mechanisms leading to executive dysfunction in alcoholism are poorly understood, and new effective pharmacological treatments are desired. Here, using a bidirectional neuromodulation approach, we demonstrate a causal link between reduced prefrontal mGluR2 function and both impaired executive control and alcohol craving. A neuron-specific prefrontal mGluR2 knockdown in rats generated a phenotype of reduced cognitive flexibility and excessive alcohol seeking. Conversely, virally restoring prefrontal mGluR2 levels in alcohol-dependent rats rescued these pathological behaviors. In the search for a pharmacological intervention with high translational potential, psilocybin was capable of restoring mGluR2 expression and reducing relapse behavior. Last, we propose a FDG-PET biomarker strategy to identify mGluR2 treatment-responsive individuals. In conclusion, we identified a common molecular pathological mechanism for both executive dysfunction and alcohol craving and provided a personalized mGluR2 mechanism-based intervention strategy for medication development for alcoholism.
INTRODUCTION
The consumption of alcoholic beverages is popular in many cultures but is also an important cause of morbidity and mortality worldwide, accounting for 3 million deaths (5.3% of all deaths) annually (1). While most people can control their drinking behavior, others become excessive drinkers and can eventually develop an alcohol use disorder (AUD). The defining features of AUD are a pattern of compulsive heavy alcohol use and a loss of control over alcohol intake. Clinical presentation of the disorder is highly heterogeneous but is often accompanied by deficits in executive functions, i.e., impairments in higher cognitive abilities involved in self-control, regulation of emotions, motivation, working memory, decision-making, attention, and cognitive flexibility (2). Alcohol-induced deficits in executive functions usually persist long into abstinence and may therefore contribute to craving and subsequent relapse in abstinent patients (2). While there are now four approved pharmacological treatments for AUD, they all have limited effectiveness, and their prescription rates are low (3, 4). This warrants innovation in terms of drug development, searching for drug targets, and developing new methodologies in treatment (5).
“Psychedelics move from agents of rebellion towards therapeutics” was the title of a recent news feature in Nature Medicine (6). After a long hiatus and a recent revival, psychedelic drugs, including the tryptamine psilocybin {3-[2-(dimethylamino) ethyl]-1H-indol-4-yl dihydrogen phosphate} have emerged as a new possible source of that much needed innovation (7–10). Despite the initiation of several clinical trials for psychiatric disorders, unexpectedly little preclinical research has been conducted so far (11–15). Psychedelics have been shown to promote effects on G protein–coupled signal transduction as well as downstream effectors, second messengers, and gene expression (16–19). A key mechanism that might explain sustained effects in humans (20–22) can be explained by serotonin (5-hydroxytryptamine) 2A (5-HT2A) receptor (5-HT2AR) desensitization (17), which persistently leads to reduced signaling in downstream pathways linked to the 5-HT2AR. Altered or reduced signaling in downstream pathways linked to the 5-HT2AR could, in turn, increase transmission via group II metabotropic glutamate receptors (mGluR2/3) due to the reciprocally inhibitory relationship between 5-HT2AR and mGluR2/3 (17).
mGlu2/3 receptors have received growing attention in addiction research because of their abundance in the pathway from the medial prefrontal cortex (mPFC) to the nucleus accumbens (NAc), which mediates drug craving and relapse (23–25), as well as cognitive flexibility (26). The metabotropic glutamate receptor subtype 2 (mGluR2) is thought to be an especially important target of drug-induced neuroadaptations, as it has been reported that long-term exposure to drugs of abuse results in its down-regulation and reduced function (27, 28). Moreover, we have demonstrated that alcohol dependence, in both humans and rats, leads to a long-lasting reduction of mGluR2 expression, specifically in the infralimbic subregion of the mPFC, which is associated with a loss of control over alcohol-seeking behavior in rats (29). In addition, it has also been shown that mGluR2 can modulate cognitive flexibility, and its stimulation enhances cognitive flexibility in rats (30). Therefore, we hypothesized that an mGluR2 dysfunction in the mPFC may comprise a common molecular mechanism for deficits in several behavioral domains and can be restored by psychedelic treatment.
We tested this hypothesis in established rat models of AUD using an interdisciplinary multimodal approach. After identifying a deficiency in mGluR2 function in the corticoaccumbal pathway as a common pathological mechanism that is necessary and sufficient both for increased alcohol-seeking behavior and impaired cognitive flexibility, we then demonstrate the feasibility and efficiency of a psilocybin-based medication development strategy for restoring mGluR2 expression and reducing relapse-like drinking. Last, to address the large heterogeneity in treatment response to anticraving medications (31), we present a putative biomarker approach that uses [18F]-fluorodeoxyglucose (FDG) positron emission tomography (PET) to identify responders to interventions targeting this mechanism.
RESULTS
Reduced cognitive flexibility after chronic intermittent alcohol exposure
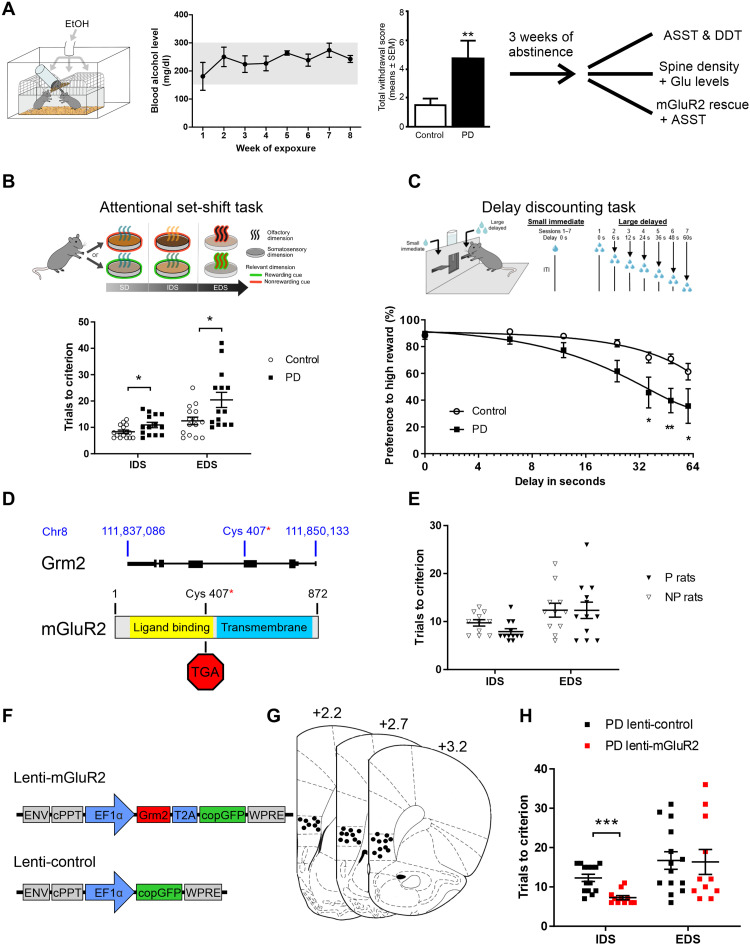
We used an established model of alcohol dependence, in which rats receive chronic intermittent exposure (CIE) to alcohol vapor. This leads to intoxication levels similar to those seen in clinical alcohol addiction and induces long-lasting behavioral and pronounced molecular changes in the brain. Alcohol-dependent rats in this model show persistent escalation of alcohol self-administration, increased motivation to obtain alcohol, and increased relapse-like behavior (32–34). Rats were made alcohol-dependent by CIE for 7 weeks. From weeks 2 to 7, blood alcohol concentrations were 250 to 300 mg/dl. This exposure resulted in pronounced withdrawal signs compared to air-exposed animals (Fig. 1A). After a prolonged abstinence period of 3 weeks, the rats were used to assess cognitive flexibility and craving using the attentional set shifting (35, 36) and delay discounting tests (37). Sixteen alcohol-exposed and 16 air-exposed Wistar rats were tested in an attentional set shifting test (ASST) (Fig. 1B), a rodent version of the Wisconsin card sorting task (35, 38), a widely used neuropsychological test in humans for assessing cognitive flexibility, with this deficit observed in alcohol-dependent patients (39–41). Two alcohol-dependent and one control animal were excluded from the ASST because they failed to learn the initial simple discrimination (SD) task. Repeated measures analysis of variance (ANOVA) revealed a main effect of alcohol exposure on alcohol-dependent and control animals’ ASST performance (F[1,27] = 11.34, P < 0.002) as well as a significant group × alcohol exposure interaction (F [6,162] = 2.62, P < 0.018). While there were no significant differences between the groups in SD, compound discrimination (CD), CD reversal (CDrev), and CD repetition (CDrep) tasks [one-way ANOVAs, SD: F[1,26] = 0.33, P = not significant (n.s.); CD: F[1,26] = 0.36, P = n.s.; CDrev: F[1,26] = 2.72, P = n.s.; and CDrep: F[1,26] = 0.001, P = n.s., respectively], we observed pronounced differences at higher cognitive demands. Thus, alcohol-dependent rats needed significantly more trials to reach criterion than nondependent rats in the subtasks involving an intradimensional shift (IDS) or extradimensional shift (EDS) (one-way ANOVAs, IDS: F[1,26] = 5.99, P < 0.05; EDS: F[1,26] = 7.4, P < 0.05, respectively; Fig. 1B). Thus, ASST performance was not generally impaired after CIE exposure, but significant impairments were observed when the rules of the task were changed (IDS and EDS). This inability to adapt their strategies across testing conditions indicates a reduced cognitive flexibility in alcohol-dependent rats.
Fig. 1. Alcohol-induced infralimbic mGluR2 deficit leads to impaired ASST performance.
(A) Scheme of chronic alcohol-vapor exposure leading to stable blood alcohol concentrations and significantly increased somatic withdrawal scores. After 3 weeks of abstinence, batches of animals were used either for spine density analysis, attentional set-shifting test (ASST), or lentiviral infralimbic mGluR2 rescue. (B) Scheme of ASST, where rats have to make serial discriminative choices based on olfactory or tactile cues/dimensions. ASST performance of alcohol-dependent [postdependent (PD), n = 14, black squares] rats need significantly more trials to criterion in the IDS and EDS subtasks compared to control rats (n = 15, white circles). (C) Scheme of the delay discounting task (DDT). The graph below represents the percentage preference for the high reward against the delay of the high reward in time. Alcohol-dependent rats have a steeper curve (means ± SEM) compared to controls. (D) Schematic representation of genomic location of premature stop codon in P rats (50). (E) Alcohol-naïve Indiana P rats (n = 12, black triangles) do not differ in IDS/EDS tasks compared to Indiana NP rats (n = 12, white triangles). (F) Schematic representation of lentiviral constructs. Lenti-mGluR2 expresses Grm2 and cop green fluorescent protein (copGFP) under control of elongation factor 1-alpha (EF1α) promoter. Lenti-control only expresses copGFP under EF1α control. ENV; Epstein-Barr virus derived vector, cPPT; central polypurine tract, WPRE; Woodchuck posttranscriptional regulatory element. (G) Injection placements are represented by black circles. Injection sites were verified within the infralimbic cortex from +3.2 to +2.2 mm anterior to bregma (85). (H) ASST performance of PD rats injected with either lenti-mGluR2 (n = 16, red squares) or lenti-control (n = 16, black squares) into the infralimbic cortex. In the EDS component of the task, no difference was observed between control and virally infected PD rats. However, in the IDS component, lenti-mGluR2–injected animals overall needed significantly fewer trials to criterion. IDS, intradimensional shift; EDS, extradimensional shift; *P < 0.05, **P < 0.01, and ***P < 0.001.
Cognitive impairments were also seen in a reward delay discounting test. Alcohol-dependent individuals display greater discounting of future rewards than controls, and this is accompanied by decreased activation of the executive system (42–45). Paralleling clinical observations, the alcohol-dependent rats (n = 8 per group) displayed a steeper discounting curve, suggesting a faster switch in preference toward the lower over the delayed higher reward (repeated measures ANOVA, group effect: F[1,12] = 7.78, P < 0.05; delay effect: F[2.09, 25.17] = 29.72, P < 0.001; and treatment × delay interaction: F[2.09, 25.17] = 3.84, P < 0.03). The results of the ASST and delay discounting tests are consistent with a previous study in mice where CIE exposure led to reduced cognitive flexibility and the inability to alter behavioral responses under changing environmental demands (46).
Reduced cognitive flexibility is accompanied by structural and functional changes in the corticostriatal system
Given that CIE exposure induced structural neuronal changes within the PFC in mice (46), we next asked whether reduced cognitive flexibility is accompanied by structural and functional changes within the mPFC. We analyzed spine density in the mPFC of alcohol-dependent and air-exposed control rats and found a significantly higher spine density in the mPFC of alcohol-dependent rats compared to control rats (two-tailed t test, t[1,29] = 2.71, P < 0.011; fig. S1). Projections from the mPFC to the NAc mediate different aspects of the ASST (26). In the NAc of alcohol-dependent rats, we found a significantly lower spine density compared to control rats (t[1,8] = 2.63, P < 0.03; fig. S1). In summary, we found a unique pattern of persisting neuroadaptations in the mPFC-accumbens circuitry after CIE, i.e., increased spine density in the mPFC and reduced in NAc. This pattern is distinct from structural neuroadaptations seen with other drugs of abuse. For example, repeated noncontingent injections or self-administration of psychostimulants induced persistent increases in spine density in NAc and mPFC. In contrast, morphine self-administration had the opposite effect; it caused long-lasting decreases in spine density in both sites (47).
Next, we asked whether these structural changes in the mPFC-NAc pathway were accompanied by functional alterations. It is known that glutamatergic neuroadaptations arise in corticostriatal projections after exposure to drugs of abuse including alcohol (48). In particular, CIE exposure in mice leads to persistent changes in glutamatergic projection neurons from the mPFC (29, 46). Given that infralimbic mPFC to NAc shell projections are, in particular, altered in alcohol-dependent rats and alcohol-dependent patients (29), we measured baseline (BL) and alcohol-induced extracellular glutamate levels in the mPFC and NAc shell by microdialysis in freely moving rats, comparing CIE rats 3 weeks in abstinence versus control rats (fig. S2, A to F). In the mPFC, we found strongly reduced basal levels of glutamate (t[1,169] = 10.65, P < 0.0001), indicative of a hypoglutamatergic state. This suggests that presynaptic glutamate terminals are, in some way, compromised, and it has been shown that alcohol-dependent rats that are derived from the CIE procedure have a significant down-regulation of presynaptic markers, such as synapsin or Syt1 in the mPFC (29, 49, 50). Systemic administration of increasing doses of alcohol [0, 0.5, 1.0, and 2.0 g/kg intraperitoneally (i.p.)] led to robust and dose-dependent increases in extracellular glutamate levels only in alcohol-dependent rats, with no changes in nondependent rats (repeated measures ANOVA time × treatment interaction: F[18, 180] = 2.02, P < 0.01) (fig. S2, A to C). This augmented release of glutamate is most likely due to reduced presynaptic mGluR2 expression within the mPFC (29). In the NAc shell, systemic administration of increasing doses of alcohol did not affect extracellular glutamate levels in alcohol-dependent or control rats (repeated ANOVA time × treatment interaction: F[19,209] = 1.08, n.s.) (fig. S2, D to F).
In summary, we found that alcohol-dependent rats exhibited persistent structural and functional alterations within the corticostriatal system. Whether these alterations, especially in the glutamatergic system, contribute to reduced cognitive flexibility in alcohol-dependent rats was studied in the next set of experiments.
Improved ASST performance in alcohol-dependent rats after mGluR2 rescue in infralimbic cortex
Glutamatergic activity and homeostasis is regulated by mGluR2/3. In particular, these receptors typically provide negative feedback on glutamate actions: Preferentially presynaptic mGluR2 inhibit glutamate release, while in glia, mostly mGluR3 increase glutamate uptake from the synapse by increasing the expression of glial glutamate transporters (49). In our previous work, we demonstrated a significant reduction of mGluR2 within the mPFC, especially in the infralimbic cortex in alcohol-dependent rats and alcohol-dependent patients (29). This deficit in mGluR2 expression may explain the observed heightened sensitivity of glutamate release in response to alcohol in alcohol-dependent rats (fig. S2, A and B). Here, we studied the contribution of an mGluR2 deficit to reduced cognitive flexibility in the ASST. We first used a rat line that carries a spontaneously arising stop codon, introducing mutation in the Grm2 gene locus Fig. 1D) (50) to study whether this global mGluR2 deficit would lead to similar impairments in ASST performance as chronic intermittent alcohol exposure. For this purpose, we used 12 alcohol-naïve male Indiana alcohol-preferring (P) rats and 14 male Indiana alcohol-nonpreferring (NP) rats. Two NP rats were excluded from the experiment because they failed to learn the early stages of ASST. There was a significant difference on the first testing day during simple discrimination (F[1,21] = 8.9, P < 0.007). However, there were no significant differences in the other subtasks of the ASST (CD: F[1,21] = 1.49, n.s.; CDrev: F[1,21] = 0.07, n.s.; CDrep: F[1,21] = 0.74, n.s.; IDS: F[1,21] = 4.15, P = n.s.; and EDS: F[1,21] = 0.0002, n.s.; Fig. 1E). There was also no overall significant difference as confirmed by repeated measures ANOVA analysis (F[1,21] = 1.55; n.s.). Thus, a global functional mGluR2 knockout does not appear to be associated with deficits in ASST performance likely because of compensatory mechanisms during development.
Next, we asked whether a local rescue of mGluR2 expression and function within the infralimbic cortex in alcohol-dependent rats would lead to a normalization of cognitive flexibility. We applied a lentiviral overexpression (Fig. 1, F and G) approach in 32 alcohol-dependent Wistar rats. After CIE exposure, the animals received bilateral injections into the infralimbic mPFC with the respective lentivirus vectors (lenti-mGluR2, n = 16; lenti-control, n = 16; seven rats were excluded because of mistargeting or minimal virus expression, resulting in lenti-mGluR2, n = 12, and lenti-control, n = 13). The animals were allowed to recover for 2 weeks before the start of the ASST. As shown in Fig. 1H, the impairments in alcohol-dependent rats in the IDS was reversed by mGluR2 rescue within the infralimbic cortex. Hence, there was a significant reduction in the IDS in the lenti-mGluR2 group, compared to lenti-control (F[1,23] = 19.45, P < 0.0002). There were no significant differences in the other ASST subtasks (SD: F[1,23] = n.s.; CD: F[1,23] = 0.14, n.s.; CDrev: F[1,23] = 1.35, n.s.; CDrep: F[1,23] = 0.06, n.s.; IDS: F[1,23] = 0.03, n.s.; and EDS: F[1,23] = 0.008, n.s.). In summary, rescue of mGluR2 expression within the infralimbic cortex led to a rescue of cognitive flexibility in the IDS task.
Reduced cognitive flexibility in alcohol-naïve rats after mGluR2 knockdown in the infralimbic cortex
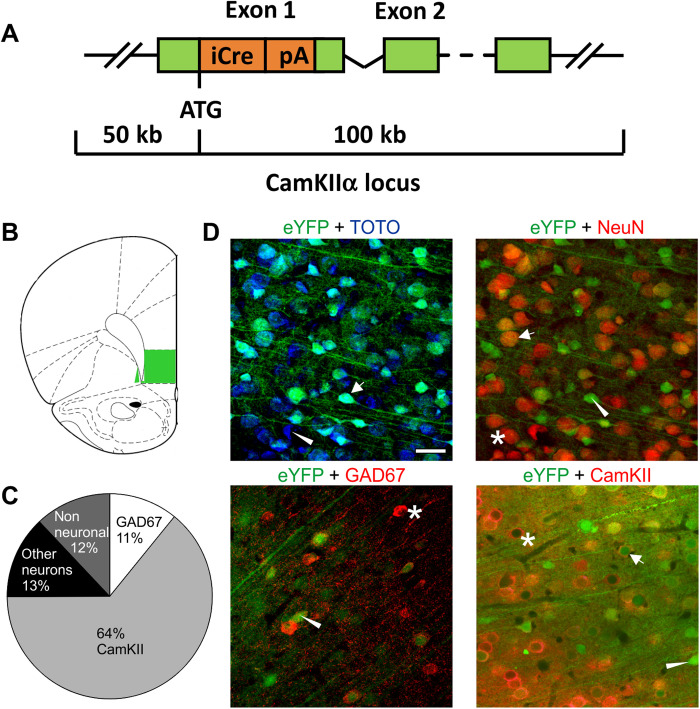
The lentiviral overexpression approach shows that a rescue of ASST performance in alcohol-dependent rats is dependent on a rescue of mGluR2 expression and function within the infralimbic cortex. Our previous findings indicate that corticostriatal projection neurons to the NAc shell are particularly affected by chronic alcohol exposure and are characterized by an alcohol-induced mGluR2 deficit (29). In the next set of experiments, we asked whether a deficit in mGluR2 function in infralimbic cortex projection neurons in alcohol-naïve animals is sufficient to mimic reduced flexibility as seen in alcohol-dependent rats. Very recently, transgenic rat models were introduced with neuron-specific genome modification in the adult rat brain (51). We followed such an approach to specifically down-regulate mGluR2 expression in projection neurons from the infralimbic cortex. For this purpose, we developed a novel calcium/calmodulin-dependent protein kinase (CamKII)–Cre transgenic rat line, expressing cre-recombinase under control of CamKIIα promoter (Fig. 2A), which was demonstrated to drive expression predominantly in excitatory forebrain neurons (52, 53) in combination with a virally delivered short hairpin–mediated RNA (shRNA) targeted against mGluR2 mRNA (Fig. 3B).
Fig. 2. Characterization of a new CamKIIα-iCre rat line.
(A) Bacterial artificial chromosome construct for specific Cre expression in transgenic rats under the CamKII promoter. (B) Targeted region (infralimbic cortex) for immunohistochemical characterization (C) proportion of CamKII and GAD67-positive cells. (D) Representative images of enhanced yellow fluorescent protein (eYFP) colocalization with TOTO-3, NeuN, GAD67, and CamKII. Scale bar, 20 μm. Asterisks indicate single-positive cells for the respective cellular marker, triangles indicate single-positive cells for viral eYFP expression, and arrows indicate double-positive cells for the respective cellular marker and the viral eYFP marker.
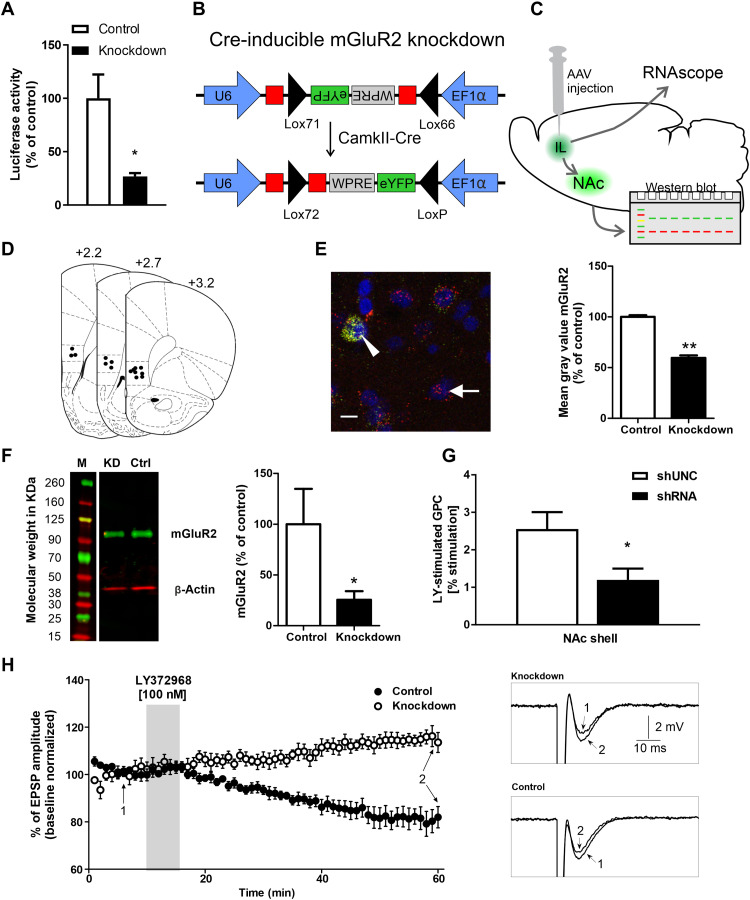
Fig. 3. Characterization of an infralimbic mGluR2 knockdown in CamKII-iCre rats.
(A) Dual luciferase assay of control AAV and mGluR2 knockdown AAV reveals a significant down-regulation of luciferase activity. (B) Schematic representation of the AAV construct. The short hairpin–mediated RNA (shRNA) coding sequence (in red) was split in the middle and inserted in opposite directions into the lox71 and lox66 flanked cassette. The reporter gene eYFP was also inverted and inserted opposite of the EF1α promoter. After Cre recombination, shRNA expression is driven by U6 promoter and eYFP by EF1α. (C) Schematic representation of AAV injection into the infralimbic and projection to the NAc. mGluR2 mRNA was detected via RNAscope, protein levels by Western blot using NAc shell tissue punches (see Fig. 3F). (D) Injection placements of Cre-inducible knockdown AAV and control AAV are represented by black circles. (E) Left: Representative image of eYFP and mGluR2 fluorescent in situ hybridization of CamKII-Cre mGluR2 knockdown AAV rats. Arrow indicates a single cell for mGluR2. Triangle indicate double-positive cells expressing eYFP and mGluR2. Scale bar, 10 μm. Right: Quantification of knockdown efficiency of mGluR2 on mRNA level using fluorescent in situ hybridization in CamkII-Cre rats. (F) Left: Representative Western blot for mGluR2/ß-actin from NAc shell tissue of CamKII-Cre knockdown AAV (KD) or control AAV (Ctrl) injection. M, marker. Right: Signal intensity of mGluR2 protein in NAc shell of CamKII-Cre knockdown AAV (black bar) or control (white bar). *P < 0.05 and **P < 0.01. (G) LY379268-stimulated G protein coupling (GPC) was decreased in the NAc shell following infralimbic mGluR2 knockdown (n = 4 per group; shUNC BL: 291 ± 20 nCi/g; shRNA BL: 279 ± 9 nCi/g; means ± SEM). (H) Normalized EPSP amplitude (means ± standard deviation) before (BL), during a 5-min application of 100-nM mGluR2 agonist LY379268, and during prolonged washout. Representative voltage traces of control and knockdown are shown (“1” labels BL traces; “2” labels traces after 45 min of LY379268 washout).
First, we characterized the Cre expression pattern of CamKII-Cre rats in the infralimbic cortex by bilateral injections of a Cre-inducible adeno-associated virus (AAV) expressing the fluorescent marker enhanced yellow fluorescent protein (eYFP). Four weeks after the AAV injection, rats were euthanized, and brain sections were stained with TOTO-3 for nuclear counterstaining. The cellular location of the fluorescence marker was analyzed by immunolabeling for the neuronal marker hexaribonucleotide binding protein-3 (NeuN), the interneuron marker glutamic acid decarboxylase 67 (GAD67), and CamKII as a marker for prefrontal projection neurons. We found that ~43% of all TOTO-3 cells colocalized with eYFP expression, whereas 52% of all neurons (NeuN) colocalized with eYFP. Of all eYFP positive cells, ~88% were NeuN positive, ~64% were CamKII positive, and ~11% were GAD67 positive (Fig. 2, B to D). Thus, there is no exclusive colocalization of Cre with CamKII neurons in the infralimbic cortex of CamKII-Cre rats. However, most Cre-expressing neurons are CamKII positive, which indicates that this transgenic rat line provides a strong preference for manipulations of projection neurons from the infralimbic mPFC.
We then tested the knockdown efficiency of a newly designed conditional mGluR2 shRNA expression system in vitro and in vivo on the mRNA and protein levels, as well as by guanosine 5′-O-(3′-thiotriphosphate) (GTP-γ-S) autoradiography and local field potential recordings (Fig. 3, A to H). For initial verification of the knockdown strategy, a dual luciferase assay was performed with the in vitro–recombined Cre-dependent shRNA AAV expression vector, together with a firefly luciferase reporter construct, harboring the mGluR2 shRNA target sequences. The mGluR2 knockdown AAV construct reduced firefly luciferase activity to ~27% compared to control AAV (t[1,4] = 3.255, P < 0.03; Fig. 3A). We then tested the knockdown efficiency of the Cre-inducible knockdown AAV in vivo; CamKII-Cre rats were injected with the mGluR2 shRNA-AAV or control AAV (Fig. 3, C to G). After 4 weeks of virus expression, mRNA levels of mGluR2 were analyzed using fluorescent in situ hybridization. Compared to the control AAV, the shRNA-expressing AAV significantly reduced mGluR2 mRNA levels by ~40% (t[1,2] = 16.8, P < 0.004; Fig. 3E). Knockdown efficiency of the Cre-dependent knockdown AAV on the protein levels was tested by Western blots of NAc shell tissue samples (n = 8 per group). Compared to control AAV, the knockdown AAV significantly reduced mGluR2 protein levels by ~75% (t[1,14] = 1.84, P < 0.04; Fig. 3F). We also tested the effect of the mGluR2 knockdown on downstream signaling. G protein activation by the mGluR2 agonist LY379268 was measured by [35S]GTP-γ-S autoradiography (Fig. 3G). Compared to the control shRNA, infralimbic cortex injections of mGluR2 knockdown shRNA significantly reduced mGluR2 receptor binding in the NAc shell (one-way ANOVA: F[1,6] = 6.28, P < 0.05). Last, we tested the mGluR2 knockdown at a functional level by recording field excitatory postsynaptic potentials (EPSPs) in slice preparations, containing NAc shell and infralimbic cortex. Presynaptically located mGluR2 act as autoreceptors (54), and their activation induces long-term depression of glutamatergic transmission (50, 55). Here, we activated mGluR2 pharmacologically using 100 nM of the mGluR2/3 agonist LY379268 (Fig. 3H). EPSPs were recorded in NAc shell by stimulating glutamatergic neurons in the infralimbic cortex. Bath perfusion of LY379268 (5 min) reduced the amplitude of EPSPs in control rats by ~20% but increased the amplitude of EPSPs in knockdown rats by ~10%, suggesting an uncompensated loss of mGluR2 function (Fig. 3H). In summary, our site-specific mGluR2 receptor knockdown could be successfully demonstrated on various levels—in vitro, in vivo, as well as pharmacologically and functionally. We conclude that, following our targeting strategy, infralimbic corticostriatal projection neurons showed the pronounced deficit in mGluR2 function similar to that observed in alcohol-dependent rats.
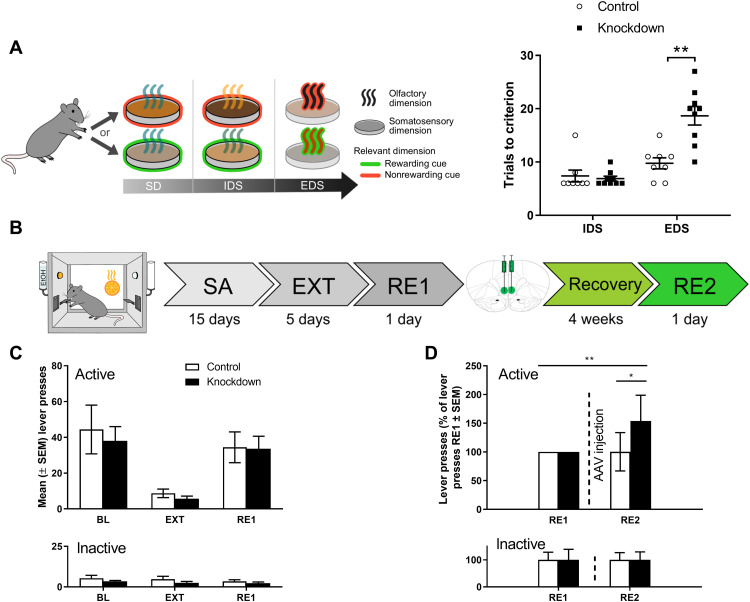
To test whether a deficit in mGluR2 function in infralimbic cortex and its corticostriatal projection neurons affects ASST performance and would mimic an alcohol-dependent phenotype, we injected CamKII-Cre rats (n = 18) with the knockdown AAV or the control AAV. An overall significant difference between the control and knockdown group was seen as confirmed by a repeated measures ANOVA (F[1,15] = 9.02, P < 0.009) (Fig. 4A). There were no significant differences in ASST subtasks SD-IDS as confirmed by one-way ANOVA (SD: F[1,15] = 2.85, P = n.s.; CD: F[1,15] = 0.16, n.s.; CDrev: F[1,15] = 0.16, n.s.; CDrep: F[1,15] = 1.18, n.s.; and IDS: F[1,15] = 2.78, n.s.. However, there was a significant difference between the groups in EDS performance (F[1,15] = 18.07, P < 0.001; Fig. 4A). Thus, as in alcohol-dependent rats (Fig. 1C), there was a pronounced increase in EDS performance trial numbers indicative of reduced cognitive flexibility. In a refined analysis of the three ASST experiments, we considered the probabilities of making correct responses and of reversals in the IDS and EDS trials (fig. S3, A to L). Alcohol-dependent rats show a trend toward lower probability of making correct choices in the IDS (pcorr = 0.108, panel A) and EDS (pcorr = 0.088, panel C) but do not differ in their probability to shift sides after a trial. Furthermore, a latency analysis demonstrates faster response time during incorrect trails by alcohol-dependent rats compared to controls, in particular at higher cognitive demands (IDS: 19.9 s for controls versus 11.1 s for CIE rats, pcorr = 0.808; EDS: 32.5 s for controls versus 14.8 s for CIE rats, pcorr = 0.03). This behavioral pattern reflects acting without prior consideration or, in other words, impulsivity. Impulsive behavior is strongly linked to increased dopaminergic neurotransmission. We have previously described a hyperdopaminergic state for CIE rats after prolonged periods of abstinence (56). Nevertheless, after restoring mGluR2 levels in the infralimbic cortex (IL) of alcohol-dependent rats, we found improvements of the above-mentioned behavioral features for the IDS (fig. S3, E to H). In contrast, knockdown of infralimbic mGluR2 in normal rats results in a significantly lower probability of shifting response sides (reversals) at high cognitive demands at the EDS (pcorr = 0.036; fig. S3L) that animals with a specifically induced mGluR2 deficit show perseverative behavior, a phenotype typically seen in AUD (39). The results of this analysis are consistent with the notion that chronic alcohol causes multiple molecular pathologies and that mGluR2 in infralimbic corticostriatal projection neurons is an important mechanism for cognitive flexibility.
Fig. 4. Effect of mGluR2 knockdown in infralimbic and corticostriatal projection neurons on impairments of executive functions and cue-induced reinstatement of alcohol-seeking behavior.
(A) Scheme of ASST, where rats have to make serial discriminative choices based on olfactory or tactile cues/dimensions. ASST performance of CamKII-Cre rats injected with either control AAV (n = 9, white bars) or Cre-dependent knockdown AAV (n = 9, black bars) into the infralimbic mPFC. The knockdown animals needed significantly more trials to criterion in the EDS subtask and overall needed more trials to criterion as compared to control animals. **P < 0.01. (B) Experimental timeline of alcohol-seeking behavior: All animals underwent alcohol self-administration (SA) and extinction (EXT) training, followed by a cue-induced reinstatement session of alcohol seeking (RE1). Following stereotaxic AAV injection (control AAV or mGluR2 knockdown AAV, n = 9 per group) and a recovery period of 4 weeks, the animals were tested during a second cue-induced reinstatement session (RE2). (C) Active and inactive operant responses of CamKII-Cre rats before control (white bars, n = 7) and knockdown (black bars, n = 7). AAV injection (two control and two knockdown animals had to be excluded from analysis because they failed to reach the criterion for successful cue-induced reinstatement of alcohol seeking, which was >10 active lever presses). There was no significant difference between the groups during BL, EXT, and cue-induced RE1 responding. (D) Performance of CamKII-Cre rats injected with control or Cre-induced mGluR2 knockdown AAV. Data were normalized to RE1 performance (before AAV injection). There was a significant difference between the groups in RE2 and a significant difference in the knockdown group between RE1 (before AAV injection) and RE2. *P < 0.05, **P < 0.01 indicate significant differences between control, knockdown/alcohol-dependent (PD) rats, and between RE2 and RE1, respectively.
Cre-dependent mGluR2 knockdown in the infralimbic cortex is sufficient to induce excessive alcohol seeking in nondependent rats
In our previous study, we found that excessive alcohol-seeking behavior in rats with a history of alcohol dependence could be normalized by reexpressing mGluR2 in the infralimbic cortex (29), demonstrating that reduced mGluR2 levels within the infralimbic cortex are necessary to induce this phenotype. To test sufficiency, we here used the above-described viral mGluR2 knockdown strategy to reduce infralimbic cortex mGluR2 levels in nondependent animals. We trained 19 CamKII-Cre transgenic rats to self-administer a 10% alcohol solution. After reaching a stable self-administration BL, all animals underwent extinction training (EXT) and one cue-induced reinstatement session before AAV injection (RE1) Fig. 4B). Animals were ranked on the basis of their RE1 performance and divided into two groups with matched performance. There were no significant differences between the prospective experimental groups during BL, EXT, and RE1 (Fig. 4C and table S2).
Next, rats were bilaterally injected with control or Cre-dependent mGluR2 knockdown AAV (n = 9 per group; four rats had to be excluded post hoc because of incorrect placement or minimal virus expression). Four weeks after AAV injection, the animals were again tested for their operant alcohol-seeking performance. There was a significant difference within the knockdown group between RE1 and RE2, i.e., before and after AAV injection, respectively (t[1,11] = 3.2, P < 0.01; Fig. 4D). For comparison of the effect size, we reanalyzed our previous experiment, which was done in a cohort of alcohol-dependent and control rats (n = 15 per group) injected with an mGluR2 overexpression or control lentivector, which were tested for cue-induced reinstatement several weeks after cessation of CIE exposure (29). As shown in Fig. 5D, relative changes between alcohol-dependent and control rats were of similar magnitude as that for the infralimbic mPFC mGluR2 knockdown rats used here, and this effect was fully reversed by reexpression of mGluR2 in the infralimbic cortex of those alcohol-dependent rats. Thus, we demonstrate that a bidirectional modulation of drug-seeking behavior–infralimbic cortex mGluR2 knockdown increased alcohol seeking, while overexpression in mGluR2-deficient alcohol-dependent rats reduces it. From this direct comparison, we conclude that a down-regulation of mGluR2 in infralimbic corticostriatal projection neurons is necessary and sufficient to induce an alcohol-dependent phenotype with respect to excessive alcohol-seeking behavior.
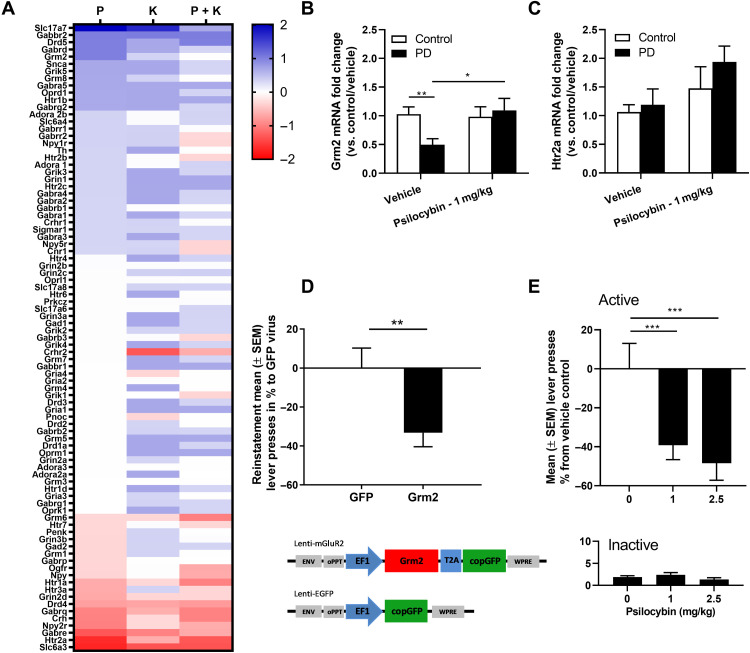
Fig. 5. Psilocybin restores the alcohol dependence–induced mGluR2 down-regulation and reduces alcohol-seeking behavior.
(A) Heatmap representing the fold change of increased (blue) or decreased (red) expression of genes in psilocybin (P)–, ketanserin (K)–, or psilocybin + ketanserin (P + K)–treated rats. (B) Accumbal gene expression changes of Grm2 and (C) Htr2a represented as fold change in relation to vehicle nonexposed control (white bar). Grm2 was significantly reduced in vehicle-treated PD rats and could be restored by psilocybin treatment. (D) Top: Lenti-mGluR2 significantly attenuated drug-seeking behavior only in alcohol-dependent rats (PD). Bottom: Schematic representation of the lentiviral expression plasmids used for the production of lenti-mGluR2 and lenti–enhanced GFP (EGFP). Presentation of the conditioned stimulus (CS+) elicited significant reinstatement in both control and alcohol-dependent rats with lenti-EGFP; data are adapted from (29). (E) Relapse to alcohol was evaluated with a regular fixed ratio 1 (FR1)–1-hour session of self-administration and was evaluated in PD rats (black bar) with either 0, 1, or 2.5 mg/kg psilocybin. *P < 0.05, **P < 0.01, and ***P < 0.001 indicate significant differences between control and alcohol-dependent (PD) rats and between psilocybin treatment and vehicle, respectively.
Together, we have identified a common pathological mechanism for two co-occurring phenotypes in addiction: excessive alcohol seeking and impaired cognitive flexibility. These results identify mGluR2 function in the corticostriatal pathway as a target for medication development for alcoholism. Given that, during withdrawal and protracted abstinence, reduced executive function and craving can result in relapse, we next searched for pharmacological interventions targeting the described mGluR2 deficit.
mGluR2 mechanism-based interventions to treat alcohol relapse
In the search of an mGluR2 mechanism-based intervention with high translational potential, psychedelics such as psilocybin have been shown to promote effects on G protein–coupled signal transduction as well as downstream effectors, second messengers, and gene expression (16–19). Altered or reduced signaling in downstream pathways linked to the primary target (5-HT2AR) could, in turn, increase transmission via mGluR2 because of the reciprocally inhibitory relationship between 5-HT2AR and mGluR2 (17).
Accordingly, we first investigated whether psilocybin is capable of modulating gene expression levels in naïve Wistar rats. In this experiment, we analyzed gene expression in the NAc of rats 4 hours after receiving either psilocybin (1 mg/kg, i.p.), a 5-HT2AR antagonist ketanserin (0.12 μg, intracerebroventricularly), or the combination psilocybin/ketanserin with a custom RT2 Profiler PCR arrays, containing sets of rat neurotransmission-specific genes. The results of this experiment are summarized in Fig. 5A. Screening of neurotransmission genes demonstrated alteration in the expression of several candidate genes following psilocybin intraperitoneal injection in naive rats. Among the genes, most strongly up-regulated in the psilocybin group was Grm2 (fold change, 2.35). Moreover, this up-regulation seems 5-HT2AR dependent, as ketanserin blocks this change in gene expression in the psilocybin/ketanserin group. We next evaluated whether psilocybin could also increase Grm2 levels in alcohol-dependent rats. As expected by our previous results (29), alcohol-dependent rats showed a significant twofold decrease of accumbal Grm2 expression (F[1,16] = 10.416, P < 0.005). Psilocybin intraperitoneal treatment restored Grm2 expression (F[1,16] = 8.399, P < 0.01) to vehicle control levels (Fig. 5B). No change of Htr2a gene expression was observed (Fig. 5C).
Last, we wanted to know whether this molecular mGluR2 rescue with psilocybin could also reduce relapse to alcohol. Psilocybin (1 and 2.5 mg/kg) or vehicle (0 mg/kg) were administered 4 hours before the relapse session. We observed a highly significant dose but no dose-response effect with similar reduction in relapse for alcohol with both doses of psilocybin as compared to the vehicle (repeated measures ANOVA, dose effect: F[2,28] = 10.08, P < 0.001; lever effect: F[1, 28] = 77.69, P < 0.001; and dose × lever interaction: F[2,28] = 10.40, P < 0.001). The post hoc test indicated a significant difference between the vehicle and both doses (P < 0.001) but not between the doses of 1 and 2.5 mg/kg (P = 0.49) (Fig. 5E). The effects of reduced relapse were comparable with our viral mGluR2 rescue (Fig. 5D). Inactive lever responding was unaffected by psilocybin treatment, indicating that no sedative effects of the drug were present at time of the operant relapse session.
A candidate biomarker strategy for personalized treatment of individuals with an mGluR2 deficit
A challenge for medication development in alcoholism is the heterogeneity of the clinical population. The heterogeneity makes it unlikely that targeting a single pathophysiological mechanism among the multitude of those known to be involved in alcohol addiction will be uniformly effective (57) or will be beneficial for all patients. A predictive biomarker able to identify patients responsive to interventions targeting mGluR2 would be a major advance and improve the prospects of successful clinical development. In the next set of experiments, we propose an FDG-PET approach that may provide a proof of concept for a possible biomarker strategy (Fig. 6A).
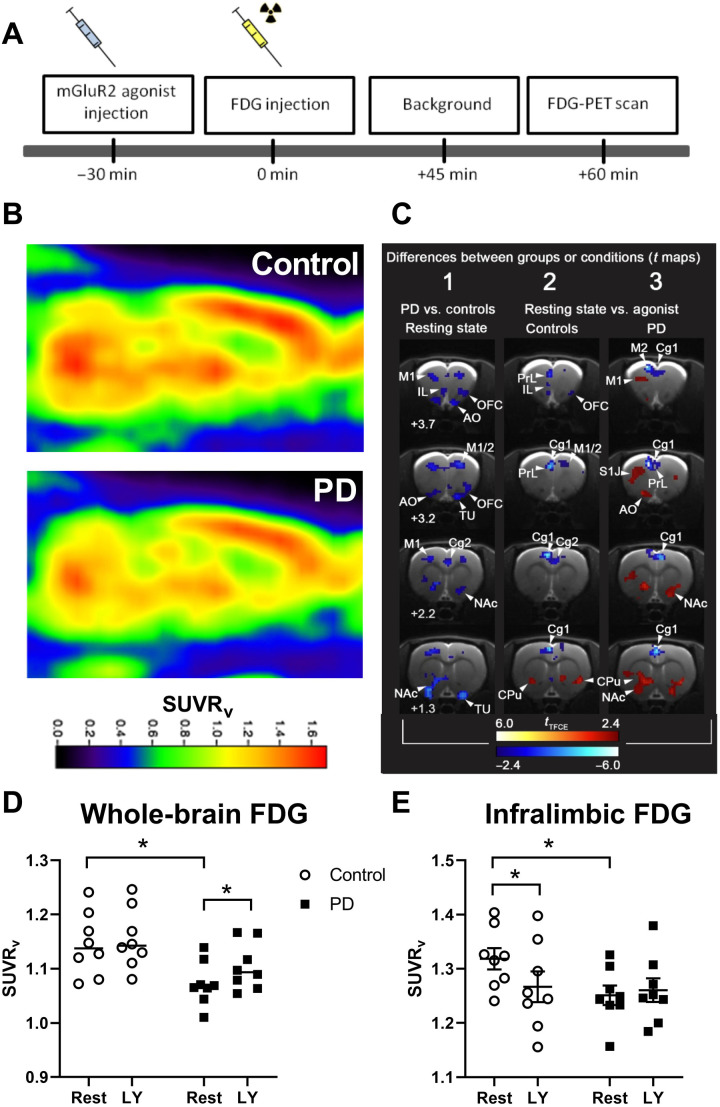
Fig. 6. [18F]-FDG-PET in alcohol-dependent and control rats after application of the mGluR2/3 agonist LY379268.
(A) Experimental timeline: Animals received either vehicle or the mGluR2/3 agonist LY379268 (n = 8 per group) 30 min before [18F]-FDG injection. After the injection of [18F]-FDG, rats were placed in their home cage for 45 min followed by a PET scan. (B) Representative normalized PET images of control and alcohol-dependent (PD) rats in a sagittal section. (C) Differences between groups (PD versus control) or conditions (resting condition or mGluR2/3 agonist treatment) represented in t maps. White numbers indicate bregma levels. tTFCE bars are ranging from deep red to light red, indicating significant increases in [18F]-FDG uptake ratio, whereas deep blue to light blue indicates significant decreases. (D) Whole-brain analysis between PD and controls shows a significant decrease in [18F]-FDG uptake [given as standardized uptake value ratio (SUVRv)] in PD rats. (E) mGluR2/3 agonist LY379268 (LY) challenge led to a decrease in [18F]-FDG uptake ratio (LY versus vehicle) under resting state (rest) conditions in the infralimbic cortex of control rats, while no effect of the drug was found in the alcohol-dependent group. Cg1, cingulate cortex area 1; M1, primary motor cortex; M2, secondary motor cortex; PrL, prelimbic cortex; IL, infralimbic cortex; NAc, nucleus accumbens; OFC, orbitofrontal cortex; AO, accessory olfactory bulb; CPU, caudate putamen; TU, olfactory tubercle; S1J, primary somatosensory cortex. Data are presented as means ± SEM. *P < 0.05 indicates significant differences from the vehicle group.
The PET radiotracer [18F]-FDG not only is widely used in clinical diagnostics of central nervous system tumors but also offers a valuable tool with which to identify impaired brain glucose metabolism in psychiatric conditions such as addiction (58). Several [18F]-FDG-PET studies in alcohol-dependent patients have consistently reported decreases in overall brain glucose metabolism, and FDG-PET has been proposed for biomarker development (31). We have used FDG-PET to compare brain glucose uptake in alcohol-dependent rats, which have an mGluR2 deficit in the infralimbic cortex, versus control nondependent rats. As described in AUD patients, we found a reduction in glucose utilization in the whole brain of alcohol-dependent rats compared to controls (factor group: F[1,14] = 6.4, P < 0.05; Fig. 6, B to D). This effect could be reversed by activation of the mGluR2 on a whole-brain level (factor treatment: F[1,14] = 21.8, P < 0.001).
In a refined region of interest (ROI) analysis, we focused on the mGluR2-impaired infralimbic cortex. In control rats, the regional FDG signal was decreased by mGluR2 activation. In contrast, no such effect of mGluR2 activation was found in the infralimbic of alcohol-dependent rats (Fig. 6E). A two-way ANOVA revealed a significant treatment effect (F[1,14] = 2.56, P < 0.01) and significant treatment × group interaction (F[1,14] = 5.4, P < 0.05). Post hoc analysis demonstrated a significant difference between control and alcohol-dependent rats under BL condition (P < 0.05), confirming the results observed for whole-brain analysis, as well as a significant difference between BL and agonist treatment only in controls (P < 0.01). We conclude that alcohol-dependent rats with reduced numbers of mGluR2 fail to modulate glucose utilization in the infralimbic cortex upon pharmacological receptor stimulation.
DISCUSSION
In this work, we use a translational multilevel approach to characterize the role of mGluR2 receptors in alcohol addiction. Using advanced genetic tools and pharmacologically validated rat models of AUD, we first provide evidence that a corticoaccumbal mGluR2 deficit is both necessary and sufficient for diminished cognitive flexibility and increased drug craving. These findings establish a common molecular-pathological mechanism for two, mostly co-occurring intermediate phenotypes in addicted patients. Prefrontal mGluR2 deficits can be caused by excessive alcohol and cocaine exposure (28, 29, 59). We then demonstrate that pharmacological targeting of mGluR2 dysfunction via psilocybin may provide an effective strategy for relapse prevention in AUD. Given the large heterogeneity of AUD patient populations, successful pharmacotherapies have to be based on personalized medicine approaches. To identify potential treatment responders, i.e., those patients that have an mGluR2 deficit, we propose a PET-based biomarker strategy and demonstrate its feasibility.
In the first set of experiments, we examined executive functions, in particular, cognitive flexibility of alcohol-dependent rats. In the ASST and in the delay discounting test, we showed cognitive impairments in alcohol-dependent rats. This is in line with findings in alcohol-dependent patients who discount temporally distant rewards more than controls, a cognitive deficit that is accompanied by a decreased activation of the executive system (44, 45). In summary, we show cognitive impairments in alcohol-dependent rats—a finding that is consistent with studies in alcohol-dependent mice (46) and patients.
By using genetic manipulations, including a rat model with an infralimbic cortex–specific knockdown of mGluR2, we were able to modulate cognitive flexibility bidirectionally. These findings are consistent with improved ASST performance in rats treated with the mGluR2-positive allosteric modulator LY487379 (30). In addition, our novel transgenic rat model expressing Cre-recombinase constitutively under CamKII promoter control provided a valuable addition to the recently published tool box of transgenic rats supporting neuron-specific genome modification in the adult rat (51). In conclusion, an mGluR2 deficit is necessary and sufficient to mediate reduced cognitive flexibility in alcohol-dependent rats.
Using the same genetic manipulations, we further demonstrated that an mGluR2 deficit is also necessary and sufficient for enhanced cue-induced alcohol-seeking in rats. Alcohol seeking is a critical component of alcohol craving (60), and thus, we suggest that an mGluR2 deficit may also be involved in cue-elicited craving responses in humans. Reduced cognitive flexibility and cue-induced craving can result in a lapse or relapse by abstinent alcohol-dependent patients. Building on our findings, a mechanism-based intervention targeting the mGluR2 deficit in alcohol-dependent individuals is suggested. It has been previously demonstrated that 5-HT2AR and mGluR2 can assemble into a functional complex and modulate each other’s function (61–64). This heteromeric complex has been implicated in the mechanism of action of psychedelics, and its role has been demonstrated in several in vivo systems. In rats and mice, the psychedelic behavioral response measured by the head-twitch response was shown to be altered in the presence of mGluR2/3 agonists, antagonists, and mGlu2R-selective–positive allosteric modulators (65–67). The necessity of mGluR2 in evoking the head-twitch response was shown in mGluR2 knockout mice in two independent studies (68, 69). Together, these studies suggest a strong interaction between the 5-HT2AR and the mGluR2 and indicate that mGluR2 modulation can counteract and enhance the behavioral effects of hallucinogenic drugs, respectively. We here demonstrate that psilocybin administration was capable of restoring mGluR2 deficit in alcohol-dependent rats and thus could decrease relapse behavior. In addition, psilocybin might also have a positive impact on cognitive flexibility, as the human literature could also show beneficial effects of psilocybin use on cognitive flexibility (70). Therefore, we conclude that psilocybin should be considered for clinical trials in alcohol-dependent patients, such as in Bogenschutz and Johnson (20). In addition, future animal studies need to assess the long-lasting effects of psilocybin to be able to compare the treatment to the clinic, where effects are observed over days and even months after the drug administration.
Last, we provide a proof-of-concept biomarker approach. [18F]-FDG PET, an index of regional glucose metabolism widely used in clinical diagnostics, can also be applied to biomarker development (31). Several FDG-PET studies in alcohol-dependent patients have consistently reported decreases in overall brain glucose metabolism at rest and withdrawal (71). This is also seen in alcohol-dependent rats that had long-term intermittent access to alcohol (72). In the present study, alcohol-dependent rats also showed a decrease in whole-brain glucose utilization, an effect that was also observed with the infralimbic cortex, in a more specific ROI analysis. Therefore, we conclude that FDG-PET studies in rat models of alcoholism may have translational utility and can specifically be used for biomarker development. Following treatment that activates mGluR2/3, no alterations in glucose levels were detected in alcohol-dependent rats with the infralimbic cortex, whereas in control rats, reduced whole-brain uptake was detected in this region. A blunted response to mGluR2 agonist treatment can be explained by the pronounced mGluR2 deficit in alcohol-dependent rats, especially in the corticostriatal system (29). This FDG-PET finding most likely translates to humans, as we have also reported strong prefrontal mGluR2 expression deficits in deceased alcohol-dependent patients (29). We conclude that alcohol-dependent individuals with an mGluR2 deficit do not respond in a detectable range to glucose update but show a beneficial treatment effect in respect to craving and relapse. A likely interpretation of these findings is that the FDG response reveals a decreased sensitivity of mGlu2 activation due to decreased receptor expression.
Our multidisciplinary series of experiments has at least two limitations. First, we show that a general loss of mGluR2 function did not affect ASST performance, an unexpected finding given that we conclude here that mGluR2 is critically involved in cognitive flexibility. For a general loss of mGluR2 function model, we used Indiana P rats, which carry the mGluR2 cys407* point mutation, preventing expression of functional mGluR2 receptors in the entire brain (50, 73). Indiana P rats did not show any impairment in ASST performance compared to their respective control rat line, the Indiana NP rats. This finding suggested that a general loss of mGluR2 function does not affect ASST performance. One possible explanation could be that mGluR3 can compensate for the loss of mGluR2 function in this rat line as both receptors can act as presynaptic autoinhibitors in neurons (74, 75). Second, we used a neuron-targeted viral mGluR2 knockdown in the infralimbic mPFC of CamKII-Cre rats to prevent general compensatory molecular mechanisms. However, we did not achieve an exclusive colocalization of Cre with CamKII neurons in the infralimbic cortex of CamKII-Cre rats. There was therefore not a complete neuronal restriction of the Cre-dependent AAV expression, which may explain why we observed only a partial rescue in the ASST.
Combined with other reports (76–79), our preclinical results provide support for mGluR2 as a molecular target for treating reduced cognitive flexibility, craving, and relapse responses in alcohol-dependent patients. In terms of further clinical translation, we propose the following key steps. First, we suggest performing an experimental medicine trial in alcohol-dependent patients to demonstrate improved cognitive flexibility in response to a single administration of psilocybin. Such a trial would benefit from an enrichment strategy based on the FDG-PET biomarker described here. Second, we suggest performing a cue-elicited craving study in alcohol-dependent patients in the magnetic resonance imaging (MRI) scanner to demonstrate normalized functional connectivity in brain areas known to be involved in neuronal cue reactivity following a single application of psilocybin (80–82). In the case that both proposed human experimental studies yield positive results, a randomized controlled trial (RCT) for testing the antirelapse properties of psilocybin is indicated.
MATERIALS AND METHODS
All experiments were done in accordance with the European Union guidelines for the care and use of laboratory animals and were approved by the local animal care committee (Regierungspraesidium Karlsruhe, Karlsruhe, Germany) and (Comité Régional d’Ethique en Matière d’Expérimentation Animale de Picardie, no. 96).
Animals
All animals were housed in groups of four under a 12-hour light/12-hour dark cycle with food and water available ad libitum in home cages. The generation of CamKII-Cre transgenic rat, Grm2 *407 genotyping, and generation of a Cre-inducible mGluR2 knockdown virus are described in the Supplementary Materials.
General experimental design
Six cohorts were used for behavioral studies. First, 18 CamKII-Cre transgenic rats were trained for operant alcohol self-administration to test Cre-inducible shRNA-AAV mGluR2 knockdown in the infralimbic cortex. After reinstatement tests, rats underwent the ASST.
Then, four Wistar batches (n = 32 each) were randomly assigned into two groups, which either underwent 8 weeks of CIE or air exposure. The first batch was used for the ASST, the second received bilateral lentivirus injections before the ASST, the third batch underwent the delay discounting task, and the fourth batch was used for operant self-administration following psilocybin treatment. Last, Indiana P (n = 12) and NP (n = 14) rats were directly tested on their ASST performance.
All other procedures, such as behavioral procedures [alcohol vapor exposure, ASST, delay discounting test, operant alcohol self-administration, cue-induced reinstatement testing, neuroanatomical experiments [immunohistochemistry procedures, RNAscope fluorescent in situ hybridization, and spine density analysis], biochemical assays (dual-luciferase assay, Western blot of mGluR2, and [35S] GTP-γ-S autoradiography), electrophysiology, MRI, and PET are described in the Supplementary Materials.
Statistics
All data are expressed as means ± SEM. Alpha level for significant effects was set to 0.05. Operant alcohol seeking between-group data were analyzed using repeated measures ANOVA, followed by Newman-Keuls post hoc test. Data of ASST subtasks and probability analysis were analyzed using one-way ANOVA, followed by Bonferroni adjustments for multiple comparisons indicated in Results as “pcorr.” Overall group differences across all ASST subtasks were analyzed using repeated measures ANOVA. Spine density data were analyzed using two-tailed t tests. Luciferase assay and RNAscope in situ hybridization data were analyzed using two-tailed t tests. Western blot data were analyzed using one-tailed t tests because of the expected reduction in protein levels in the mGluR2 knockdown group. For GTP-γ-S binding assays, values were calculated as percent of BL value in the same region and animal and expressed as percent stimulation (± SEM) and were statistically analyzed by one-way ANOVA. For FDG-PET experiments, two-way ANOVAs were performed. To correct for multiple testing with 19,536 brain voxels, a threshold-free cluster enhancement (TFCE) procedure with subsequent permutation testing, thresholded at P < 0.05, was used (83) for all t maps. In general, data for ANOVAs were not transformed, and repeated measures ANOVA were tested for sphericity using Mauchly’s test. When appropriate, corrections in using Greenhouse-Geisser for ε < 0.75 and Huynh-Feldt for ε > 0.75 were performed.
Acknowledgments
We would like to thank M. Schneider and A. Göpfrich for sharing ASST protocol and help implementing it. Special thanks to S. Küppers for schematic drawings for all figures. Furthermore, we would like to thank E. Röbel, S. Koch, A. Gallego-Roman, N. Hirth, B. Alharastani, and T. Kuner’s laboratory for technical assistance.
Funding: Financial support for this work was provided by the Bundesministerium für Bildung und Forschung (BMBF)–funded ERA-NET program: Psi-Alc (FKZ: 01EW1908), the BMBF-funded SysMedSUDs consortium (FKZ: 01ZX1909A), and the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation), project IDs: ME 5279/3-1 and 402170461–TRR 265 (84), and the European Union’s Horizon 2020 program (668863-SyBil-AA).
Author contributions: M.W.M., S.P., G.F., M.N., W.H.S., and R.S. designed the research. M.W.M., S.P., G.F., C.R., J.J., J.B.-F., R.H., M.L.M., E.P., A.C.H., G.K., K.W., O.v.B.u.H., and N.M. performed the research. M.W.M., S.P., G.F., C.R., M.L.M., and W.H.S. analyzed the data. R.L.B., H.E., B.N., K.S., and D.B. provided critical infrastructure or specific (transgenic) rat lines. M.W.M., S.P., M.N., R.S., and W.H.S. wrote the paper.
Competing interests: The authors declare that they have no competing interests.
Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials.
Supplementary Materials
This PDF file includes:
Supplementary Methods
Figs. S1 to S3
Tables S1 and S2
REFERENCES AND NOTES
- 1.UNODC, World Drug Report (2018), pp. 35–42. [Google Scholar]
- 2.Le Berre A. P., Fama R., Sullivan E. V., Executive functions, memory, and social cognitive deficits and recovery in chronic alcoholism: A critical review to inform future research. Alcohol. Clin. Exp. Res. 41, 1432–1443 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jonas D. E., Amick H. R., Feltner C., Bobashev G., Thomas K., Wines R., Kim M. M., Shanahan E., Gass C. E., Rowe C. J., Garbutt J. C., Pharmacotherapy for adults with alcohol use disorders in outpatient settings: A systematic review and meta-analysis. JAMA 311, 1889–1900 (2014). [DOI] [PubMed] [Google Scholar]
- 4.Mark T. L., Kassed C. A., Vandivort-Warren R., Levit K. R., Kranzler H. R., Alcohol and opioid dependence medications: Prescription trends, overall and by physician specialty. Drug Alcohol Depend. 99, 345–349 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heilig M., Augier E., Pfarr S., Sommer W. H., Developing neuroscience-based treatments for alcohol addiction: A matter of choice? Transl. Psychiatry 9, 255 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McCall B., Psychedelics move from agents of rebellion towards therapeutics. Nat. Med. 1–4 (2020). [DOI] [PubMed] [Google Scholar]
- 7.Carhart-Harris R. L., Goodwin G. M., The therapeutic potential of psychedelic drugs: Past, present, and future. Neuropsychopharmacology 42, 2105–2113 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bogenschutz M. P., Studying the effects of classic hallucinogens in the treatment of alcoholism: Rationale, methodology, and current research with psilocybin. Curr. Drug Abuse Rev. 6, 17–29 (2013). [DOI] [PubMed] [Google Scholar]
- 9.Rucker J. J. H., Iliff J., Nutt D. J., Psychiatry & the psychedelic drugs. Past, present & future. Neuropharmacology 142, 200–218 (2018). [DOI] [PubMed] [Google Scholar]
- 10.Tullis P., The rise of psychedelic psychiatry. Nature 508, 506–509 (2021). [Google Scholar]
- 11.Cameron L. P., Benson C. J., Defelice B. C., Fiehn O., Olson D. E., Chronic, intermittent microdoses of the psychedelic N, N-Dimethyltryptamine (DMT) produce positive effects on mood and anxiety in rodents. ACS Chem. Nerosci. 10, 3261–3270 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cameron L. P., Tombari R. J., Lu J., Pell A. J., Hurley Z. Q., Ehinger Y., Vargas M. V., McCarroll M. N., Taylor J. C., Myers-Turnbull D., Liu T., Yaghoobi B., Laskowski L. J., Anderson E. I., Zhang G., Viswanathan J., Brown B. M., Tjia M., Dunlap L. E., Rabow Z. T., Fiehn O., Wulff H., McCorvy J. D., Lein P. J., Kokel D., Ron D., Peters J., Zuo Y., Olson D. E., A non-hallucinogenic psychedelic analogue with therapeutic potential. Nature 589, 474–479 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jefsen O., Højgaard K., Christiansen S. L., Elfving B., Nutt D. J., Wegener G., Müller H. K., Psilocybin lacks antidepressant-like effect in the Flinders Sensitive Line rat. Acta Neuropsychiatr. 31, 213–219 (2019). [DOI] [PubMed] [Google Scholar]
- 14.Ly C., Greb A. C., Cameron L. P., Wong J. M., Barragan E. V., Wilson P. C., Burbach K. F., Soltanzadeh Zarandi S., Sood A., Paddy M. R., Duim W. C., Dennis M. Y., McAllister A. K., Ori-McKenney K. M., Gray J. A., Olson D. E., Psychedelics promote structural and functional neural plasticity. Cell Rep. 23, 3170–3182 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meinhardt M. W., Güngör C., Skorodumov I., Mertens L. J., Spanagel R., Psilocybin and LSD have no long-lasting effects in an animal model of alcohol relapse. Neuropsychopharmacology 45, 1316–1322 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Halberstadt A. L., Recent advances in the neuropsychopharmacology of serotonergic hallucinogens. Behav. Brain Res. 277, 99–120 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nichols D. E., Psychedelics. Pharmacol. Rev. 68, 264–355 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.López-Giménez J. F., González-Maeso J., Hallucinogens and serotonin 5-HT2A receptor-mediated signaling pathways. Curr. Top. Behav. Neurosci. 36, 45–73 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.D. A. Martin, C. D. Nichols, in Current Topics in Behavioral Neurosciences (2018), vol. 36, pp. 137–158. [DOI] [PubMed] [Google Scholar]
- 20.Bogenschutz M. P., Johnson M. W., Classic hallucinogens in the treatment of addictions. Prog. Neuro-Psychopharmacology Biol. Psychiatry. 64, 250–258 (2016). [DOI] [PubMed] [Google Scholar]
- 21.Vollenweider F. X., Kometer M., The neurobiology of psychedelic drugs: Implications for the treatment of mood disorders. Nat. Rev. Neurosci. 11, 642–651 (2010). [DOI] [PubMed] [Google Scholar]
- 22.Ross S., Bossis A., Guss J., Agin-Liebes G., Malone T., Cohen B., Mennenga S. E., Belser A., Kalliontzi K., Babb J., Su Z., Corby P., Schmidt B. L., Rapid and sustained symptom reduction following psilocybin treatment for anxiety and depression in patients with life-threatening cancer: A randomized controlled trial. J. Psychopharmacol. 30, 1165–1180 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Javitt D. C., Schoepp D., Kalivas P. W., Volkow N. D., Zarate C., Merchant K., Bear M. F., Umbricht D., Hajos M., Potter W. Z., Lee C.-M., Translating glutamate: From pathophysiology to treatment. Sci. Transl. Med. 3, 102mr2 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bell R. L., Hauser S. R., McClintick J., Rahman S., Edenberg H. J., Szumlinski K. K., McBride W. J., Ethanol-associated changes in glutamate reward neurocircuitry: A minireview of clinical and preclinical genetic findings. Prog. Mol. Biol. Transl. Sci. 137, 41–85 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bell R. L., Sable H. J. K., Colombo G., Hyytia P., Rodd Z. A., Lumeng L., Animal models for medications development targeting alcohol abuse using selectively bred rat lines: Neurobiological and pharmacological validity. Pharmacol. Biochem. Behav. 103, 119–155 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stefani M. R., Moghaddam B., Rule learning and reward contingency are associated with dissociable patterns of dopamine activation in the rat prefrontal cortex, nucleus accumbens, and dorsal striatum. J. Neurosci. 26, 8810–8818 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moussawi K., Kalivas P. W., Group II metabotropic glutamate receptors (mGlu2/3) in drug addiction. Eur. J. Pharmacol. 639, 115–122 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kasanetz F., Lafourcade M., Deroche-Gamonet V., Revest J. M., Berson N., Balado E., Fiancette J. F., Renault P., Piazza P. V., Manzoni O. J., Prefrontal synaptic markers of cocaine addiction-like behavior in rats. Mol. Psychiatry 18, 729–737 (2013). [DOI] [PubMed] [Google Scholar]
- 29.Meinhardt M. W., Hansson A. C., Perreau-Lenz S., Bauder-Wenz C., Stählin O., Heilig M., Harper C., Drescher K. U., Spanagel R., Sommer W. H., Rescue of infralimbic mGluR2 deficit restores control over drug-seeking behavior in alcohol dependence. J. Neurosci. 33, 2794–2806 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nikiforuk A., Popik P., Drescher K. U., van Gaalen M., Relo A. L., Mezler M., Marek G., Schoemaker H., Gross G., Bespalov A., Effects of a positive allosteric modulator of group II metabotropic glutamate receptors, LY487379, on cognitive flexibility and impulsive-like responding in rats. J. Pharmacol. Exp. Ther. 335, 665–673 (2010). [DOI] [PubMed] [Google Scholar]
- 31.M. Heilig, W. H. Sommer, R. Spanagel, in Current Topics in Behavioral Neurosciences (Springer Verlag, 2016), vol. 28, pp. 151–171; www.ncbi.nlm.nih.gov/pubmed/27240677. [DOI] [PubMed]
- 32.Rimondini R., Arlinde C., Sommer W., Heilig M., Long-lasting increase in voluntary ethanol consumption and transcriptional regulation in the rat brain after intermittent exposure to alcohol. FASEB J. 16, 27–35 (2002). [DOI] [PubMed] [Google Scholar]
- 33.Sommer W. H., Rimondini R., Hansson A. C., Hipskind P. A., Gehlert D. R., Barr C. S., Heilig M. A., Upregulation of voluntary alcohol intake, behavioral sensitivity to stress, and amygdala Crhr1 expression following a history of dependence. Biol. Psychiatry 63, 139–145 (2008). [DOI] [PubMed] [Google Scholar]
- 34.Meinhardt M. W., Sommer W. H., Postdependent state in rats as a model for medication development in alcoholism. Addict. Biol. 20, 1–21 (2015). [DOI] [PubMed] [Google Scholar]
- 35.Birrell J. M., Brown V. J., Medial frontal cortex mediates perceptual attentional set shifting in the rat. J. Neurosci. 20, 4320–4324 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Klugmann M., Goepfrich A., Friemel C. M., Schneider M., AAV-mediated overexpression of the CB1 receptor in the mPFC of adult rats alters cognitive flexibility, social behavior, and emotional reactivity. Front. Behav. Neurosci. 5, 37 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Floresco S. B., Tse M. T. L., Ghods-Sharifi S., Dopaminergic and glutamatergic regulation of effort- and delay-based decision making. Neuropsychopharmacology 33, 1966–1979 (2008). [DOI] [PubMed] [Google Scholar]
- 38.Berg E. A., A simple objective technique for measuring flexibility in thinking. J. Gen. Psychol. 39, 15–22 (1948). [DOI] [PubMed] [Google Scholar]
- 39.Wicks S., Hammar J., Heilig M., Wisén O., Factors affecting the short-term prognosis of alcohol dependent patients undergoing inpatient detoxification. Subst. Abus. 22, 235–245 (2001). [DOI] [PubMed] [Google Scholar]
- 40.Zhou Z., Zhu H., Li C., Wang J., Internet addictive individuals share impulsivity and executive dysfunction with alcohol-dependent patients. Front. Behav. Neurosci. 8, 288 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stephan R. A., Alhassoon O. M., Allen K. E., Wollman S. C., Hall M., Thomas W. J., Gamboa J. M., Kimmel C., Stern M., Sari C., Dalenberg C. J., Sorg S. F., Grant I., Meta-analyses of clinical neuropsychological tests of executive dysfunction and impulsivity in alcohol use disorder. Am. J. Drug Alcohol Abuse 43, 24–43 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bickel W. K., Yi R., Temporal discounting as a measure of executive function: Insights from the competing neuro-behavioral decision system hypothesis of addiction. Adv. Health Econ. Health Serv. Res. 20, 289–309 (2008). [PubMed] [Google Scholar]
- 43.Dennis L. E., Kohno M., McCready H. D., Schwartz D. L., Schwartz B., Lahna D., Nagel B. J., Mitchell S. H., Hoffman W. F., Neural correlates of reward magnitude and delay during a probabilistic delay discounting task in alcohol use disorder. Psychopharmacology (Berl) 237, 263–278 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Petry N. M., Delay discounting of money and alcohol in actively using alcoholics, currently abstinent alcoholics, and controls. Psychopharmacology (Berl) 154, 243–250 (2001). [DOI] [PubMed] [Google Scholar]
- 45.MacKillop J., Amlung M. T., Few L. R., Ray L. A., Sweet L. H., Munafò M. R., Delayed reward discounting and addictive behavior: A meta-analysis. Psychopharmacology (Berl) 216, 305–321 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kroener S., Mulholland P. J., New N. N., Gass J. T., Becker H. C., Chandler L. J., Chronic alcohol exposure alters behavioral and synaptic plasticity of the rodent prefrontal cortex. PLOS ONE 7, e37541 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Badiani A., Belin D., Epstein D., Calu D., Shaham Y., Opiate versus psychostimulant addiction: The differences do matter. Nat. Rev. Neurosci. 12, 685–700 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Knackstedt L. A., Kalivas P. W., Glutamate and reinstatement. Curr. Opin. Pharmacol. 9, 59–64 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Anwyl R., Metabotropic glutamate receptors: Electrophysiological properties and role in plasticity. Brain Res. Rev. 29, 83–120 (1999). [DOI] [PubMed] [Google Scholar]
- 50.Zhou Z., Karlsson C., Liang T., Xiong W., Kimura M., Tapocik J. D., Yuan Q., Barbier E., Feng A., Flanigan M., Augier E., Enoch M.-A., Hodgkinson C. A., Shen P.-H., Lovinger D. M., Edenberg H. J., Heilig M., Goldman D., Loss of metabotropic glutamate receptor 2 escalates alcohol consumption. Proc. Natl. Acad. Sci. U.S.A. 110, 16963–16968 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bäck S., Necarsulmer J., Whitaker L. R., Coke L. M., Koivula P., Heathward E. J., Fortuno L. V., Zhang Y., Yeh C. G., Baldwin H. A., Spencer M. D., Mejias-Aponte C. A., Pickel J., Hoffman A. F., Spivak C. E., Lupica C. R., Underhill S. M., Amara S. G., Domanskyi A., Anttila J. E., Airavaara M., Hope B. T., Hamra F. K., Richie C. T., Harvey B. K., Neuron-specific genome modification in the adult rat brain using CRISPR-Cas9 transgenic rats. Neuron 102, 105–119.e8 (2019). [DOI] [PubMed] [Google Scholar]
- 52.Mayford M., Bach M. E., Huang Y. Y., Wang L., Hawkins R. D., Kandel E. R., Control of memory formation through regulated expression of a CaMKII transgene. Science 274, 1678–1683 (1996). [DOI] [PubMed] [Google Scholar]
- 53.Schönig K., Weber T., Frömmig A., Wendler L., Pesold B., Djandji D., Bujard H., Bartsch D., Schönig K., Weber T., Frömmig A., Wendler L., Pesold B., Djandji D., Bujard H., Bartsch D., Conditional Gene Expression Systems in the Transgenic Rat Brain. BMC Biol. 10, 77 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Galici R., Jones C. K., Hemstapat K., Nong Y., Echemendia N. G., Williams L. C., De Paulis T., Conn P. J., Biphenyl-indanone A, a positive allosteric modulator of the metabotropic glutamate receptor subtype 2, has antipsychotic- and anxiolytic-like effects in mice. J. Pharmacol. Exp. Ther. 318, 173–185 (2006). [DOI] [PubMed] [Google Scholar]
- 55.Walker A. G., Conn P. J., Group i and group II metabotropic glutamate receptor allosteric modulators as novel potential antipsychotics. Curr. Opin. Pharmacol. 20, 40–45 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hirth N., Meinhardt M. W., Noori H. R., Salgado H., Torres-Ramirez O., Uhrig S., Broccoli L., Vengeliene V., Roßmanith M., Perreau-Lenz S., Köhr G., Sommer W. H., Spanagel R., Hansson A. C., Convergent evidence from alcohol-dependent humans and rats for a hyperdopaminergic state in protracted abstinence. Proc. Natl. Acad. Sci. 113, 3024–3029 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Spanagel R., Alcoholism: A systems approach from molecular physiology to addictive behavior. Physiol. Rev. 89, 649–705 (2009). [DOI] [PubMed] [Google Scholar]
- 58.Volkow N. D., Wang G.-J., Kojori E. S., Fowler J. S., Benveniste H., Tomasi D., Alcohol decreases baseline brain glucose metabolism more in heavy drinkers than controls but has no effect on stimulation-induced metabolic increases. J. Neurosci. 35, 3248–3255 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cannella N., Halbout B., Uhrig S., Evrard L., Corsi M., Corti C., Deroche-Gamonet V., Hansson A. C., Spanagel R., The mGluR2/3 agonist LY379268 induced anti-reinstatement effects in rats exhibiting addiction-like behavior. Neuropsychopharmacology 38, 2048–2056 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Spanagel R., Animal models of addiction. Dialogues Clin. Neurosci. 19, 247–258 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Toneatti R., Shin J. M., Shah U. H., Mayer C. R., Saunders J. M., Fribourg M., Arsenovic P. T., Janssen W. G., Sealfon S. C., López-Giménez J. F., Benson D. L., Conway D. E., González-Maeso J., Interclass GPCR heteromerization affects localization and trafficking. Sci. Signal. 13, eaaw3122 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Baki L., Fribourg M., Younkin J., Eltit J. M., Moreno J. L., Park G., Vysotskaya Z., Narahari A., Sealfon S. C., Gonzalez-Maeso J., Logothetis D. E., Cross-signaling in metabotropic glutamate 2 and serotonin 2A receptor heteromers in mammalian cells. Pflugers Arch. Eur. J. Physiol. 468, 775–793 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fribourg M., Moreno J. L., Holloway T., Provasi D., Baki L., Mahajan R., Park G., Adney S. K., Hatcher C., Eltit J. M., Ruta J. D., Albizu L., Li Z., Umali A., Shim J., Fabiato A., MacKerell A. D. Jr., Brezina V., Sealfon S. C., Filizola M., González-Maeso J., Logothetis D. E., Decoding the signaling of a GPCR heteromeric complex reveals a unifying mechanism of action of antipsychotic drugs. Cell 147, 1011–1023 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.González-Maeso J., Ang R. L., Yuen T., Chan P., Weisstaub N. V., López-Giménez J. F., Zhou M., Okawa Y., Callado L. F., Milligan G., Gingrich J. A., Filizola M., Meana J. J., Sealfon S. C., Identification of a serotonin/glutamate receptor complex implicated in psychosis. Nature 452, 93–97 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gewirtz J. C., Marek G. J., Behavioral evidence for interactions between a hallucinogenic drug and group II metabotropic glutamate receptors. Neuropsychopharmacology 23, 569–576 (2000). [DOI] [PubMed] [Google Scholar]
- 66.Klodzinska A., Bijak M., Tokarski K., Pilc A., Group II mGlu receptor agonists inhibit behavioural and electrophysiological effects of DOI in mice. Pharmacol. Biochem. Behav. 73, 327–332 (2002). [DOI] [PubMed] [Google Scholar]
- 67.Benneyworth M. A., Xiang Z., Smith R. L., Garcia E. E., Conn P. J., Sanders-Bush E., A selective positive allosteric modulator of metabotropic glutamate receptor subtype 2 blocks a hallucinogenic drug model of psychosis. Mol. Pharmacol. 72, 477–484 (2007). [DOI] [PubMed] [Google Scholar]
- 68.Moreno J. L., Holloway T., Albizu L., Sealfon S. C., González-Maeso J., Metabotropic glutamate mGlu2 receptor is necessary for the pharmacological and behavioral effects induced by hallucinogenic 5-HT2A receptor agonists. Neurosci. Lett. 493, 76–79 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Benvenga M. J., Chaney S. F., Baez M., Britton T. C., Hornback W. J., Monn J. A., Marek G. J., Metabotropic Glutamate2 receptors play a key role in modulating head twitches induced by a serotonergic hallucinogen in mice. Front. Pharmacol. 9, 208 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Prochazkova L., Lippelt D. P., Colzato L. S., Kuchar M., Sjoerds Z., Hommel B., Exploring the effect of microdosing psychedelics on creativity in an open-label natural setting. Psychopharmacology (Berl) 235, 3401–3413 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wong D. F., Maini A., Rousset O. G., Brasić J. R., Positron emission tomography--a tool for identifying the effects of alcohol dependence on the brain. Alcohol Res. Health 27, 161–173 (2003). [PMC free article] [PubMed] [Google Scholar]
- 72.Gispert J. D., Figueiras F. P., Vengeliene V., Herance J. R., Rojas S., Spanagel R., Changes in cerebral [18F]-FDG uptake induced by acute alcohol administration in a rat model of alcoholism. Behav. Brain Res. 327, 29–33 (2017). [DOI] [PubMed] [Google Scholar]
- 73.L. Lumeng, T. Hawkins, T. Li, Alcohol and Aldehyde Metabolizing Systems (Academic Press, 1977), pp. 537–544. [Google Scholar]
- 74.Niswender C. M., Conn P. J., Metabotropic glutamate receptors: Physiology, pharmacology, and disease. Annu. Rev. Pharmacol. Toxicol. 50, 295–322 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.De Filippis B., Lyon L., Taylor A., Lane T., Burnet P. W. J., Harrison P. J., Bannerman D. M., The role of group II metabotropic glutamate receptors in cognition and anxiety: Comparative studies in GRM2−/−, GRM3−/− and GRM2/3−/− knockout mice. Neuropharmacology 89, 19–32 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kufahl P. R., Martin-Fardon R., Weiss F., Enhanced sensitivity to attenuation of conditioned reinstatement by the mGluR2/3 Agonist LY379268 and increased functional activity of mGluR2/3 in Rats with a history of ethanol dependence. Neuropsychopharmacology 36, 2762–2773 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Holmes A., Spanagel R., Krystal J. H., Glutamatergic targets for new alcohol medications. Psychopharmacology (Berl) 229, 539–554 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Augier E., Dulman R. S., Rauffenbart C., Augier G., Cross A. J., Heilig M., The mGluR2 positive allosteric modulator, AZD8529, and cue-induced relapse to alcohol seeking in rats. Neuropsychopharmacology 41, 2932–2940 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Caprioli D., Justinova Z., Venniro M., Shaham Y., Effect of novel allosteric modulators of metabotropic glutamate receptors on drug self-administration and relapse: A review of preclinical studies and their clinical implications. Biol. Psychiatry 84, 180–192 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Noori H. R., Cosa Linan A., Spanagel R., Largely overlapping neuronal substrates of reactivity to drug, gambling, food and sexual cues: A comprehensive meta-analysis. Eur. Neuropsychopharmacol. 26, 1419–1430 (2016). [DOI] [PubMed] [Google Scholar]
- 81.Strosche A., Zhang X., Kirsch M., Hermann D., Ende G., Kiefer F., Vollstädt-Klein S., Investigation of brain functional connectivity to assess cognitive control over cue-processing in Alcohol Use Disorder. Addict. Biol. 26, e12863 (2021). [DOI] [PubMed] [Google Scholar]
- 82.Bach P., Weil G., Pompili E., Hoffmann S., Hermann D., Vollstädt-Klein S., Mann K., Perez-Ramirez U., Moratal D., Canals S., Dursun S. M., Greenshaw A. J., Kirsch P., Kiefer F., Sommer W. H., Incubation of neural alcohol cue reactivity after withdrawal and its blockade by naltrexone. Addict. Biol. 25, e12717 (2020). [DOI] [PubMed] [Google Scholar]
- 83.Rohleder C., Wiedermann D., Neumaier B., Drzezga A., Timmermann L., Graf R., Leweke F. M., Endepols H., The functional networks of prepulse inhibition: Neuronal connectivity analysis based on fdg-pet in awake and unrestrained rats. Front. Behav. Neurosci. 10, 148 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Heinz A., Kiefer F., Smolka M. N., Endrass T., Beste C., Beck A., Liu S., Genauck A., Romund L., Banaschewski T., Bermpohl F., Deserno L., Dolan R. J., Durstewitz D., Ebner-Priemer U., Flor H., Hansson A. C., Heim C., Hermann D., Kiebel S., Kirsch P., Kirschbaum C., Koppe G., Marxen M., Meyer-Lindenberg A., Nagel W. E., Noori H. R., Pilhatsch M., Priller J., Rietschel M., Romanczuk-Seiferth N., Schlagenhauf F., Sommer W. H., Stallkamp J., Ströhle A., Stock A. K., Winterer G., Winter C., Walter H., Witt S., Vollstädt-Klein S., Rapp M. A., Tost H., Spanagel R., Addiction Research Consortium: Losing and regaining control over drug intake (ReCoDe)—From trajectories to mechanisms and interventions. Addict. Biol. 25, e12866 (2020). [DOI] [PubMed] [Google Scholar]
- 85.G. Paxinos, C. Watson, The Rat Brain in Stereotaxic Coordinates (Elsevier, ed. 4, 1998). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Methods
Figs. S1 to S3
Tables S1 and S2