Abstract
Background
The detailed causes of death in non–ST-segment–elevation myocardial infarction (NSTEMI) have not been adequately evaluated compared to those in ST-segment elevation myocardial infarction (STEMI).
Methods
The study population was 6,228 AMI patients who underwent percutaneous coronary intervention (STEMI: 4,625 patients and NSTEMI: 1,603 patients). The primary outcome was all-cause death.
Results
Within 6 months after AMI, the adjusted mortality risk was not significantly different between NSTEMI patients and STEMI patients (HR: 0.83, 95%CI: 0.67–1.03, P = 0.09). Regarding the causes of death within 6 months after AMI, mechanical complications more frequently occurred in STEMI patients than in NSTEMI patients, while proportions of post resuscitation status on arrival and heart failure were higher in in NSTEMI patients than in STEMI patients. Beyond 6 months after AMI, the adjusted mortality risk of NSTEMI relative to STEMI was not significantly different. (HR: 1.04, 95%CI: 0.90–1.20, P = 0.59). Regarding causes of death beyond 6 months after AMI, almost half of deaths were cardiovascular causes in both groups, and breakdown of causes of death was similar between NSTEMI and STEMI.
Conclusion
The mortality risk within and beyond 6 months after AMI were not significantly different between STEMI patients and NSTEMI patients after adjusting confounders. Deaths due to post resuscitation status and heart failure were more frequent in NSTEMI within 6 months after AMI.
Introduction
Primary percutaneous coronary intervention (PCI) in ST-segment elevation myocardial infarction (STEMI) has been widely performed worldwide and mortality of STEMI patients has been improved [1–5] Non ST-segment elevation myocardial infarction (NSTEMI) is different from STEMI in many aspects including pathophysiology and treatment [4, 6, 7]. Clinical outcomes of NSTEMI patients may also be different from those of STEMI patients. In-hospital mortality risk in NSTEMI patients was reported to be better or equivalent as compared with that in STEMI patients, while long-term mortality risk in NSTEMI patients was reported to be higher than in STEMI patients in real world practice [8–12]. However, the reasons for the higher mortality risk and the causes of death in NSTEMI patients have not been fully evaluated yet. We, therefore, sought to compare short- and long-term clinical outcomes and the causes of death between NSTEMI and STEMI in a large-scale Japanese cohort study.
Materials and methods
Study population
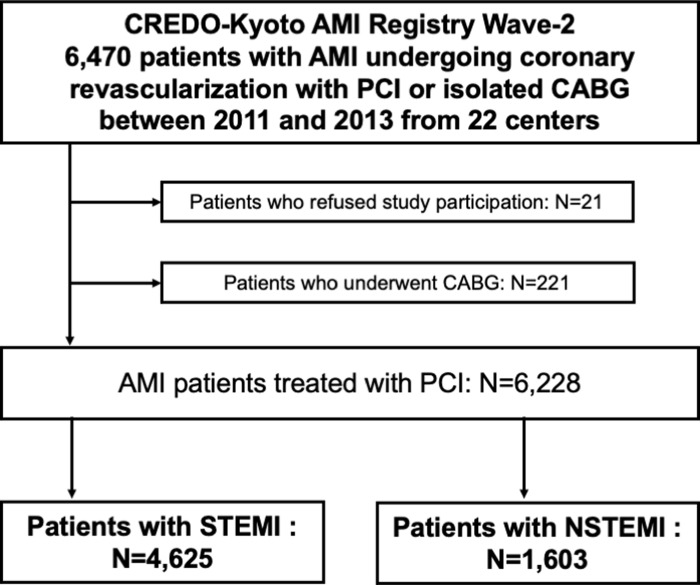
The Coronary REvascularization Demonstrating Outcome Study in Kyoto (CREDO-kyoto) acute myocardial infarction (AMI) Registry Wave-2 is a physician-initiated, non-company sponsored, multi-center registry enrolling 6,470 consecutive AMI patients who underwent coronary revascularization within 7 days of the onset of symptoms between January 2011 and December 2013 among 22 participating centers in Japan (S1 Text). In this study, the study population consisted of 6,228 AMI patients who underwent PCI after excluding those patients who refused study participation (N = 21) and those who underwent coroanry artery bypass grafting (CABG) (N = 221) (Fig 1). In the present study, we compared the baseline characteristics, clinical outcomes, and causes of death between those patients with STEMI and NSTEMI.
Fig 1. Study flowchart.
CREDO-Kyoto = Coronary REvascularization Demonstrating Outcome study in Kyoto; AMI = acute myocardial infarction; PCI = percutaneous coronary intervention, CABG = coronary artery bypass grafting; NSTEMI = non-ST-segment elevation myocardial infarction; STEMI = ST-segment elevation myocardial infarction.
The relevant institutional review boards at all participating centers approved the study protocol, and we performed the study in accordance with the Declaration of Helsinki. Written informed consent for this study was waived because of the retrospective nature of the study; however, we excluded those patients who refused participation in the study when contacted at follow-up. This strategy is concordant with the guidelines of the Japanese Ministry of Health, Labor and Welfare.
Definitions and clinical outcome measures
STEMI patients were defined as patients with electrocardiographic signs of ≥0.1 mV of ST-segment elevation in ≥2 limb leads or ≥0.2 mV in ≥2 contiguous precordial leads, accompanied by chest pain lasting at least 30 minutes or increased serum levels of cardiac biomarkers [13]. NSTEMI patients were defined as AMI other than STEMI, with elevating cardiac biomarkers, consisting of at least a value exceeding the upper reference limit for troponin, or >3× of the upper reference limit for creatine kinase MB (CK-MB). Experienced clinical research coordinators from the independent clinical research organization (Research Institute for Production Development, Kyoto, Japan; S2 Text) collected baseline clinical, angiographic and procedural characteristics from the hospital charts or hospital databases according to the pre-specified definitions.
The primary outcome measure of this study was all-cause death. The secondary outcome measures included cardiovascular death, non-cardiovascular death, myocardial infarction, stroke, hospitalization for heart failure (HF), major bleeding, target vessel revascularization, and any coronary revascularization. Death of unknown cause and any death during the index hospitalization for coronary revascularization were regarded as cardiac death. Cardiovascular death included cardiac death and other vascular death related to stroke, renal disease, and vascular disease. The definition of baseline characteristics and other endpoints were described at S3 Text. Death, myocardial infarction, stroke, and major bleeding events were adjudicated by the independent clinical event committee (S4 Text).
Classification of causes of death
The detailed causes of cardiovascular death were classified into the followings; death related to the index AMI, recurrent AMI, sudden cardiac death or ventricular tachycardia (VT)/ventricular fibrillation (VF), heart failure, stroke, and other cardiovascular death. Furthermore, death related to the index AMI was sub-classified into cardiogenic shock, mechanical complication, anoxic brain damage, cardiopulmonary arrest (CPA) on arrival, and other causes during index hospitalization for AMI. The detailed causes of non-cardiovascular death were classified into malignancies, pulmonary disease, infection, gastrointestinal diseases, accident/trauma, and other non-cardiovascular death. To assess detailed causes of deaths, two physicians reviewed the patients’ records, and ascertained the causes of death independently. In case of disagreement, the third physician reviewed the patients’ records and discussed to reach a consensus about the causes of death.
Statistical analyses
Continuous variables were expressed as mean ± standard deviation (SD) or median with interquartile range (IQR). Continuous variables were compared using the Student’s t-test or Wilcoxon rank sum test based on their distributions. Categorical variables are expressed as numbers and percentages and were compared using χ2 test. Cumulative incidence was estimated by the Kaplan-Meier method and differences were assessed with the log-rank test. To estimate the adjusted hazard ratios (HRs) and their 95% confidence intervals (CIs) of NSTEMI relative to STEMI for the outcome measures, we used the multivariable Cox proportional hazard models by incorporating the 28 clinically relevant factors listed in Table 1 in consistent with our previous report [13]. Continuous variables were dichotomized by clinically meaningful reference values to make proportional hazard assumptions robust and to be consistent with our previous reports [14]. Proportional hazard assumptions for the risk-adjusting variables were assessed on the plots of log (time) versus log [-log (survival)] stratified by the variable. The assumptions were verified to be acceptable for all the variables except for the primary variable (NSTEMI versus STEMI). A prior study also demonstrated that the primary variable did not meet the proportional hazard assumption.6 Therefore we conducted landmark analyses and estimated the adjusted HRs and their 95% CIs within and beyond 6 months after the index AMI. The missing values at baseline characteristics were described at S5 Text. All analyses were performed using R version 3.6.1 (R Foundation for Statistical Computing, Vienna, Austria). All reported P values were two-tailed, and P values less than 0.05 were considered statistically significant.
Table 1. Baseline characteristics comparing between NSTEMI and STEMI.
| NSTEMI | STEMI | P value | |
|---|---|---|---|
| (N = 1603) | (N = 4625) | ||
| (A) Clinical characteristics | |||
| Age (years) | 70.5±11.5 | 68.8±12.6 | <0.001 |
| Age ≥75 years* | 657 (41%) | 1652 (36%) | <0.001 |
| Men* | 1214 (76%) | 3459 (75%) | 0.47 |
| Body mass index (kg/m2) | 23.8±3.6 | 23.6±3.6 | 0.30 |
| Body mass index <25.0 kg/m2* | 1077 (67%) | 3207 (69%) | 0.12 |
| Hypertension* | 1350 (84%) | 3691 (80%) | <0.001 |
| Diabetes mellitus* | 636 (40%) | 1614 (35%) | 0.001 |
| on insulin therapy | 128 (8.0%) | 259 (5.6%) | 0.001 |
| Current smoking* | 450 (28%) | 1676 (36%) | <0.001 |
| Heart failure (current and/or prior)* | 509 (32%) | 1508 (33%) | 0.55 |
| LVEF (%) | 56.3±13.4 | 53.8±12.3 | <0.001 |
| LVEF ≤40% | 170 (12%) | 580 (14%) | 0.17 |
| Prior PCI | 348 (22%) | 514 (11%) | <0.001 |
| Prior CABG | 64 (4.0%) | 59 (1.3%) | <0.001 |
| Prior myocardial infarction* | 242 (15%) | 417 (9.0%) | <0.001 |
| Prior stroke (symptomatic)* | 235 (15%) | 505 (11%) | <0.001 |
| Peripheral vascular disease* | 105 (6.6%) | 202 (4.4%) | 0.001 |
| eGFR<30mL/min/1.73m2, without hemodialysis* | 122 (7.6%) | 279 (6.0%) | 0.03 |
| Hemodialysis* | 104 (6.5%) | 119 (2.6%) | <0.001 |
| eGFR <30 ml/min/1.73m2 or hemodialysis | 226 (14%) | 398 (8.6%) | <0.001 |
| Atrial fibrillation* | 179 (11%) | 410 (8.9%) | 0.008 |
| Anemia (Hemoglobin <11.0g/dl) * | 261 (16%) | 505 (11%) | <0.001 |
| Thrombocytopenia (Platelet <100×109 /L) * | 43 (2.7%) | 93 (2.0%) | 0.14 |
| Chronic obstructive pulmonary disease* | 68 (4.2%) | 169 (3.7%) | 0.33 |
| Liver cirrhosis* | 35 (2.2%) | 99 (2.1%) | 0.99 |
| Malignancy* | 189 (12%) | 501 (11%) | 0.31 |
| (B) Presentation | |||
| Transport pattern | <0.001 | ||
| Direct admission | 984 (61%) | 2580 (56%) | |
| Inter-facility transfer | 542 (34%) | 1937 (42%) | |
| Others | 77 (4.8%) | 108 (2.3%) | |
| Systolic blood pressure | 134±40 | 128±40 | <0.001 |
| Killip class III/IV | 262 (16%) | 882 (19%) | 0.02 |
| Cardiogenic shock* | 180 (11%) | 741 (16%) | <0.001 |
| Cardiopulmonary arrest | 60 (3.7%) | 190 (4.1%) | 0.57 |
| Maximum creatine kinase | 442 (163–1198) | 1865 (788–3690) | <0.001 |
| (C) Angiographic characteristics | |||
| Multivessel disease* | 991 (62%) | 2560 (55%) | <0.001 |
| Target of proximal LAD* | 799 (50%) | 2597 (56%) | <0.001 |
| Target of unprotected left main coronary artery* | 111 (6.9%) | 213 (4.6%) | <0.001 |
| Anterior wall infarction* | 701 (44%) | 2283 (49%) | <0.001 |
| Infarct related artery location: | |||
| Left anterior descending coronary artery | 636 (40%) | 2156 (47%) | <0.001 |
| Left circumflex coronary artery | 455 (28%) | 470 (10%) | <0.001 |
| Right coronary artery | 450 (28%) | 1875 (41%) | <0.001 |
| Left main coronary artery | 71 (4.4%) | 137 (3.0%) | 0.006 |
| Coronary artery bypass graft | 14 (0.9%) | 23 (0.5%) | 0.13 |
| (D) Procedural characteristics | |||
| Intra-aortic balloon pump use | 209 (13%) | 915 (20%) | <0.001 |
| Percutaneous cardiopulmonary support use | 46 (2.9%) | 146 (3.2%) | 0.63 |
| Transradial approach | 430 (27%) | 733 (16%) | <0.001 |
| Transfemoral approach | 1055 (66%) | 3640 (79%) | 0.02 |
| Intravascular ultrasound use for the culprit lesion | 973 (61%) | 2653 (57%) | 0.24 |
| Stent use for the culprit lesion | 1454 (91%) | 4241 (92%) | 0.22 |
| Stent type for the culprit lesion | <0.001 | ||
| Bare metal stent | 400 (28%) | 1735 (41%) | |
| Drug-eluting stent | 1054 (72%) | 2506 (59%) | |
| Staged PCI | 368 (23%) | 1018 (22%) | 0.45 |
| (E) Baseline Medications | |||
| Antiplatelet therapy | |||
| Thienopyridine | 1562 (97%) | 4485 (97%) | 0.38 |
| Ticlopidine | 79 (5.1%) | 123 (2.7%) | <0.001 |
| Clopidogrel | 1454 (93%) | 4304 (96%) | <0.001 |
| Aspirin | 1577 (98%) | 4544 (98%) | 0.82 |
| Cilostazol | 61 (3.8%) | 114 (2.5%) | 0.007 |
| Statins* | 1278 (80%) | 3849 (83%) | 0.06 |
| Beta-blockers* | 732 (46%) | 2507 (54%) | <0.001 |
| ACE inhibitors/ARB* | 1117 (70%) | 3525 (76%) | <0.001 |
| Nitrates | 356 (22%) | 819 (18%) | <0.001 |
| Calcium channel blockers* | 587 (37%) | 941 (20%) | <0.001 |
| Nicorandil | 324 (20%) | 939 (20%) | 0.97 |
| Oral anticoagulant | 174 (11%) | 613 (13%) | 0.01 |
| Warfarin | 154 (9.6%) | 553 (12%) | 0.01 |
| Direct oral anticoagulant | 21 (1.3%) | 60 (1.3%) | 1.00 |
| Proton pump inhibitors or Histamine type-2 receptor blockers * | 1304 (81%) | 3961 (86%) | <0.001 |
| Proton pump inhibitors | 1089 (68%) | 3431 (74%) | <0.001 |
| Histamine type-2 receptor blockers | 224 (14%) | 544 (12%) | 0.02 |
*Risk-adjusting variables for the Cox proportional hazard models.
STEMI = ST-segment elevation myocardial infarction; NSTEMI = Non ST-segment elevation myocardial infarction; LVEF = left ventricular ejection fraction; PCI = percutaneous coronary intervention; CABG = coronary artery bypass grafting; eGFR = estimated glomerular filtration rate; LAD = left anterior descending artery; PCI = percutaneous coronary intervention; ACE inhibitor/ARB = angiotensin-converting enzyme inhibitor/angiotensin receptor blocker.
Results
Study population and baseline characteristics in NSTEMI and STEMI
Among the study population of 6,228 AMI patients who underwent PCI, there were 4,625 patients with STEMI, and 1603 patients with NSTEMI. As compared with STEMI patients, NSTEMI patients were older, and more often had comorbidities such as hypertension, diabetes, severe renal dysfunction, peripheral vascular disease, and atrial fibrillation. The NSTEMI group also included more patients with prior myocardial infarction, prior coronary revascularization and prior stroke than the STEMI group (Table 1). However, the proportion of patients complicated by cardiogenic shock was significantly lower in the NSTEMI group than in the STEMI group, and infarct size estimated by maximum creatinine kinase (CK) was significantly smaller in the NSTEMI group than in the STEMI group (S1 Fig).
NSTEMI patients more often had their culprit lesions in left main coronary artery and left circumflex coronary artery, and more often had multivessel disease than STEMI patients. Regarding procedural characteristics, transradial approach and drug-eluting stents (DES) were more often used in the NSTEMI group than in the STEMI group.
In terms of medications at hospital discharge, STEMI patients more often received guideline-recommended medical therapy such as statins, beta-blockers, angiotensin-converting enzyme inhibitors/angiotensin receptor blockers than NSTEMI patients (Table 1).
Long-term clinical outcomes in NSTEMI and STEMI
Median follow-up duration was 5.5 years (IQR, 3.6–6.6 years). Complete 1-, 3-, and 5-year follow-up information was obtained in 96.5%, 93.6%, and 83.1% of patients.
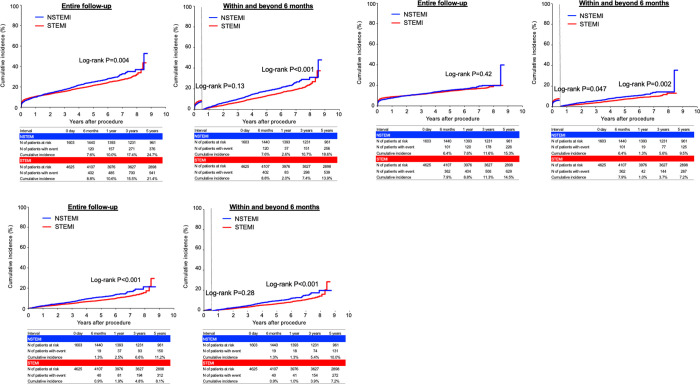
The cumulative 5-year incidence of all-cause death was significantly higher in the NSTEMI group than in the STEMI group (24.7% versus 21.4%, P = 0.004) (Fig 2). The cumulative 5-year incidence of cardiovascular death was not significantly different between the 2 groups (15.3% versus 14.5%, P = 0.42), while the cumulative 5-year incidence of non-cardiovascular death was significantly higher in the NSTEMI group than in the STEMI group (11.2% versus 8.1%, P<0.001) (Fig 2). The cumulative 5-year incidences of myocardial infarction, hospitalization for heart failure, target vessel revascularization, and any coronary revascularization were significantly higher in the NSTEMI group than in the STEMI group (9.1% versus 6.7%, P = 0.002, 13.0% versus 9.6%, P<0.001, 24.5% versus 21.4%, P = 0.04, and 33.3% versus 29.4%, P = 0.02, respectively) (S2 Fig). The cumulative 5-year incidences of stroke and major bleeding were not significantly different between the 2 groups (7.5% versus 7.5%, P = 0.93, and 23.5% versus 21.5%, P = 0.35, respectively) (S2 Fig).
Fig 2. Kaplan-Meier curves for mortality outcomes comparing between NSTEMI and STEMI.
(A) all-cause death, (B) cardiovascular death, and (C) non-cardiovascular death. NSTEMI = non-ST-segment elevation myocardial infarction; STEMI = ST-segment elevation myocardial infarction.
Difference of mortality and causes of deaths between NSTEMI and STEMI in acute phase (within 6 months after AMI)
The cumulative incidence of all-cause death within 6 months after AMI was not significantly different between the NSTEMI and STEMI groups (7.6% versus 8.8%, log-rank P = 0.13) (Fig 2, and Table 2). The adjusted risk for all-cause death was not significantly different between NSTEMI group and STEMI group (adjusted HR: 0.83, 95%CI: 0.67–1.03, P = 0.09) (Table 2). The cumulative incidence of cardiovascular death within 6 months were significantly lower in the NSTEMI group than in the STEMI group, while the adjusted risk for cardiovascular death within 6 months was not significantly different between the NSTEMI group and the STEMI group (6.4% versus 7.9%, log-rank P = 0.047, and adjusted HR: 0.82, 95%CI: 0.65–1.03, P = 0.09) (Fig 2 and Table 2). The adjusted risks of NSTEMI relative to STEMI for stroke, hospitalization for HF tended to be lower but statistically not significant (Table 2). The adjusted risks for major bleeding was significantly lower in the NSTEMI group than in the STEMI group (adjusted HR: 0.81, 95%CI: 0.68–0.96, P = 0.01) (Table 2).
Table 2. Clinical outcomes comparing between NSTEMI and STEMI within and beyond 6 months.
| Endpoints | NSTEMI | STEMI | Crude HR | P value | Adjusted HR | P value | ||
|---|---|---|---|---|---|---|---|---|
| N of patients with event | (95%CI) | (95%CI) | ||||||
| (Cumulative incidence) | ||||||||
| All-cause death | ||||||||
| Within 6 months | 120 | (7.6%) | 402 | (8.8%) | 0.86(0.70–1.05) | 0.13 | 0.83(0.67–1.03) | 0.09 |
| Beyond 6 months | 321 | (18.6%) | 694 | (13.9%) | 1.37(1.20–1.56) | <0.001 | 1.04(0.90–1.20) | 0.59 |
| Cardiovascular death | ||||||||
| Within 6 months | 101 | (6.4%) | 362 | (7.9%) | 0.80(0.64–1.00) | 0.047 | 0.82(0.65–1.03) | 0.09 |
| Beyond 6 months | 151 | (9.5%) | 329 | (7.2%) | 1.35(1.12–1.64) | 0.002 | 0.94(0.77–1.15) | 0.55 |
| Non-cardiovascular death | ||||||||
| Within 6 months | 19 | (1.3%) | 40 | (0.9%) | 1.35(0.78–2.34) | 0.28 | - | - |
| Beyond 6 months | 170 | (10.0%) | 365 | (7.2%) | 1.38(1.15–1.66) | 0.001 | 1.13(0.93–1.37) | 0.21 |
| Myocardial infarction | ||||||||
| Within 6 months | 41 | (2.7%) | 132 | (3.0%) | 0.89(0.62–1.26) | 0.501 | 0.75(0.52–1.08) | 0.12 |
| Beyond 6 months | 102 | (6.5%) | 174 | (3.8%) | 1.76(1.38–2.25) | <0.001 | 1.38(1.06–1.78) | 0.02 |
| Stroke | ||||||||
| Within 6 months | 37 | (2.4%) | 120 | (2.7%) | 0.88(0.61–1.28) | 0.51 | 0.71(0.48–1.04) | 0.08 |
| Beyond 6 months | 80 | (5.3%) | 224 | (4.9%) | 1.05(0.81–1.35) | 0.71 | 0.83(0.63–1.08) | 0.16 |
| Hospitalization for heart failure | ||||||||
| Within 6 months | 54 | (3.6%) | 145 | (3.4%) | 1.06(0.77–1.45) | 0.72 | 0.75(0.54–1.04) | 0.08 |
| Beyond 6 months | 156 | (9.7%) | 291 | (6.4%) | 1.58(1.30–1.92) | <0.001 | 1.15(0.94–1.42) | 0.19 |
| Major bleeding | ||||||||
| Within 6 months | 191 | (12.1%) | 638 | (14.1%) | 0.85(0.72–1.00) | 0.052 | 0.81(0.68–0.96) | 0.01 |
| Beyond 6 months | 177 | (12.9%) | 359 | (8.6%) | 1.43(1.20–1.71) | <0.001 | 1.16(0.96–1.40) | 0.13 |
| Target vessel revascularization | ||||||||
| Within 6 months | 107 | (7.1%) | 305 | (7.1%) | 1.00(0.80–1.25) | 0.99 | 0.84(0.67–1.06) | 0.14 |
| Beyond 6 months | 252 | (18.7%) | 610 | (15.4%) | 1.21(1.05–1.4) | 0.01 | 1.16(0.99–1.35) | 0.07 |
| Any coronary revascularization | ||||||||
| Within 6 months | 139 | (9.3%) | 404 | (9.4%) | 0.98(0.81–1.19) | 0.84 | 0.84(0.69–1.03) | 0.09 |
| Beyond 6 months | 350 | (26.5%) | 853 | (22.2%) | 1.21(1.07–1.37) | 0.003 | 1.14(1.00–1.30) | 0.053 |
Number of patients with event beyond 6 months was counted until the end of follow-up.
Cumulative incidence was presented at 6-month for within 6 months, and at 5-year for beyond 6 months.
The risk of NSTEMI relative to STEMI was expressed as HR with 95%CI. The risk-adjusting variables in the multivariate Cox proportional hazard models were indicated in Table 1.
STEMI = ST-segment elevation myocardial infarction; NSTEMI = Non ST-segment elevation myocardial infarction; HR = hazard ratio; CI = confidence interval.
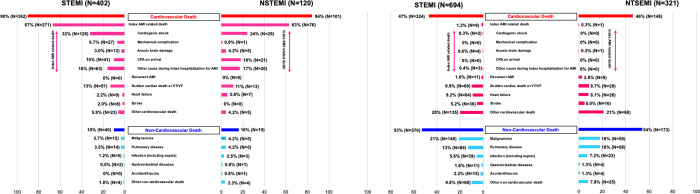
Regarding the detailed causes of cardiovascular death, approximately two thirds of deaths were related to the index AMI event in both NSTEMI and STEMI (Fig 3). Among the index AMI related death, mechanical complications (including ventricular septal perforation and myocardial rupture) occurred more frequently in the STEMI group than in the NSTEMI group (6.7% [N = 27], and 0.8% [N = 1], respectively), and the proportion of death due to cardiogenic shock was numerically higher in the STEMI group than in the NSTEMI group (32% [N = 128] and 24% [N = 29], respectively). However, the proportion of death due to post resuscitation status after CPA on arrival was greater in the NSTEMI group than in the STEMI group (18% [N = 21], and 10% [N = 41], respectively). Among 21 deaths due to post resuscitation status after CPA on arrival in the NSTEMI group, 15 patients had totally occluded culprit lesions on angiography. The proportion of death due to HF within 6 months after AMI was also higher in the NSTEMI group than in the STEMI group (5.8% [N = 7], and 2.2% [N = 9], respectively) (Fig 3).
Fig 3.
The detailed causes of deaths after NSTEMI and STEMI (A) in the early phase (within 6 months), and (B) in the late phase (beyond 6 months). STEMI = ST-segment elevation myocardial infarction; NSTEMI = Non ST-segment elevation myocardial infarction; VT = ventricular tachycardia; VF = ventricular fibrillation; CPA = cardiopulmonary arrest; AMI = acute myocardial infarction.
Difference of mortality and causes of deaths between NSTEMI and STEMI in late phase (beyond 6 months after AMI)
Beyond 6 months after AMI, the cumulative incidences of all-cause death, cardiovascular death, and non-cardiovascular death were significantly higher in the NSTEMI group than in the STEMI group (18.6% versus 13.9%, P<0.001, and 9.5% versus 7.2%, P = 0.002, and 10% versus 7.2%, P = 0.001, respectively). However, after adjusting for confounders, the risk of NSTEMI relative to STEMI was no longer significant for all-cause death, cardiovascular death, and non-cardiovascular death (adjusted HR: 1.04, 95%CI: 0.90–1.20, P = 0.59, and adjusted HR: 0.94, 95%CI: 0.77–1.15, P = 0.55, and adjusted HR: 1.13, 95%CI: 0.93–1.37, P = 0.21, respectively) (Table 2). The risk of NSTEMI relative to STEMI was significantly higher for myocardial infarction beyond 6 months (Table 2).
In both the NSTEMI and STEMI groups, approximately half of deaths were accounted for cardiovascular death (N = 148/321 [46%], and N = 324/694 [47%], respectively) (Fig 3B). Detailed causes of cardiovascular and non-cardiovascular deaths beyond 6 months after AMI were similar between the NSTEMI and STEMI groups (Fig 3).
Discussion
The main findings of this study were as follows; 1) The mortality risk within 6 months after AMI was not significantly different between STEMI patients and NSTEMI patients; 2) Within 6 months after AMI, deaths due to post resuscitation status and HF were more frequent in NSTEMI patients than in STEMI patients, while deaths due to mechanical complication and cardiogenic shock were more frequent in STEMI patients than in NSTEMI patients; 3) The mortality risk of NSTEMI relative to STEMI beyond 6 months after AMI was no longer significant after adjusting confounders; 4) Approximately half of deaths beyond 6 months were cardiovascular death in both STEMI and NSTEMI patients, and detailed causes of deaths were similar in NSTEMI and STEMI patients.
Several previous studies reported that long-term prognosis in NSTEMI patients was worse than that in STEMI patients despite of smaller infarct size [8–12]. In this study, adjusted risk of NSTEMI relative to STEMI for long-term mortality was neutral. Discrepancy among studies including our study might be largely explained the heterogenous NSTEMI population and different follow-up duration among studies. After widespread use of troponin, especially high-sensitive troponin, the paradigm shift occurred in the concept of NSTEMI, which included many low risk patients who were formerly diagnosed as unstable angina.
Consistent with our study results, most previous studies reported that mortality risk of NSTEMI in acute phase of AMI was equivalent or slightly lower than that of STEMI, but the mortality risk of STEMI patients relative to NSTEMI patients attenuated overtime, because long-term mortality risk of NSTEMI beyond acute phase of AMI was higher than that of STEMI due to high risk patients’ backgrounds in NSTEMI patients such as older age and multiple comorbidities [4, 6, 7].
Even within 6 months after AMI, the absolute difference of mortality rate was only 1.2% (7.6% versus 8.8%), indicating that NSTEMI patients did not mean low risk population, but included high-risk patients in the heterogenous population. As mentioned above, NSTEMI patients were generally older and had more comorbidity, and more complex anatomy such as left main disease and multivessel disease than STEMI patients. The proportion of left main coronary artery and left circumflex coronary artery as a culprit lesion was significantly higher in NSTEMI patients than in STEMI patients in this study, suggesting that NSETMI population included high-risk subset of patients who presented “non-ST elevation” electrocardiographic findings, but had a “STEMI like” hemodynamic consequence during evolving myocardial infarction. The recent study reported the favorable clinical outcome of shorter door to balloon time even in NSTEMI population, suggesting the importance of appropriate risk stratification and timely reperfusion therapy in NSTEMI population [15].
According to the findings of this study results, the detailed causes of deaths in acute phase of AMI were somewhat different between STEMI and NSTEMI. Among those differences, it was worth noting that the proportion of deaths of post resuscitation status after CPA on arrival was higher in NSTEMI patients than in STEMI patients. Previous studies reported that ECG findings at the time of recovery of spontaneous circulation often did not show typical changes including ST segment elevation, and, therefore, clinical guidelines recommend coronary angiography unless other causes of cardiac arrest are identified and patients are comatose [16–18]. Actually, in this study, more than 70% of NSTEMI patients with death due to post resuscitation status after CPA on arrival had totally occluded culprit lesions on angiography. On the other hand, the proportion of mechanical complication such as ventricular septal perforation and cardiac rupture in this study was higher in STEMI patients than in NSTEMI patients. This could be explained by the angiographic characteristics. A prior study suggested that left anterior descending (LAD) artery and right coronary artery (RCA) were the vast majority of infarct related lesion for patients with ventricular septal perforation, and another study demonstrated single vessel disease was the risk factor for cardiac rupture [19, 20]. In this study, more patients with STEMI had LAD and RCA as infarct related artery than those with NSTEMI, and more patients with STEMI had single vessel disease compared with those with NSTEMI.
Beyond 6 months after AMI in this study, the mortality risk was not different between NSTEMI and STEMI. Among deaths during long-term follow-up, approximately half of deaths were cardiovascular death, and detailed caused of deaths were similar between NSTEMI and STEMI, indicating that secondary prevention and appropriate medical treatment is important in not only STEMI patients but also in NSTEMI patients. Considering high risk feature of NSTEMI patients with elderly and many comorbidities, further studies investigating appropriate management of NSTEMI patients might be needed.
Limitations
There are several limitations of this study. First, the current study was an observational study with its limitations inherent to observational study design, such as selection bias and unmeasured confounders. Second, the specific landmark point of 6 months was not prespecified, but visually assessed by viewing the entire follow-up results in consistent with our previous report. Finally, we included only NSTEMI patients who underwent coronary revascularization. NSTEMI patients who were selected conservative therapy and who were contraindication for intervention were excluded, therefore the prognosis of NSTEMI patients in this study might be different from that of the entire NSTEMI population in daily clinical practice.
Conclusions
The mortality risk within and beyond 6 months after AMI were not significantly different between STEMI patients and NSTEMI patients after adjusting confounders. Deaths due to post resuscitation status and heart failure were more frequent in NSTEMI within 6 months after AMI.
Supporting information
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Acknowledgments
We deeply appreciate the following investigators and co-investigators of the participating centers: Kyoto University Hospital; Takeshi Kimura, Kenji Minatoya, Kazuhiro Yamazaki, Hiroki Shiomi, Kishiwada City Hospital; Mitsuo Matsuda, Tatsuya Ogawa, Takashi Uegaito, Tenri Hospital; Toshihiro Tamura, Atsushi Iwakura, Hyogo Prefectural Amagasaki General Medical Center; Yukihito Sato, Nobuhisa Ohno, Ryoji Taniguchi, Kitano Hospital; Moriaki Inoko, Michiya Hanyu, Koto Memorial Hospital; Tomoyuki Murakami, Teruki Takeda, Kokura Memorial Hospital; Kenji Ando, Takenori Domei, Yoshiharu Soga, Akira Marui, Kindai University Nara Hospital; Manabu Shirotani, Nobushige Tamura, Kobe City Medical Center General Hospital; Yutaka Furukawa, Tadaaki Koyama, Natsuhiko Ehara, Kobe City Nishi-Kobe Medical Center; Hiroshi Eizawa, Kansai Denryoku Hospital: Katsuhisa Ishii, Eiji Tada, Osaka Red Cross Hospital; Masaru Tanaka, Tsukasa Inada, Shogo Nakayama, Shizuoka City Shizuoka Hospital; Tomoya Onodera, Ryuzo Nawada, Fumio Yamazaki, Yasuhiko Terai, Hamamatsu Rosai Hospital; Eiji Shinoda, Miho Yamada, Junichiro Nishizawa, Shiga University of Medical Science Hospital; Takashi Yamamoto, Hiroshi Sakai, Japanese Red Cross Wakayama Medical Center; Takashi Tamura, Mamoru Toyofuku, Naoki Kanemitsu, Hiroyuki Hara, Shimabara Hospital; Mamoru Takahashi, Shizuoka General Hospital; Hiroki Sakamoto, Tomohisa Tada, Hiroshi Tsuneyoshi, Kurashiki Central Hospital; Kazushige Kadota, Tatsuhiko Komiya, Takeshi Tada, Mitsubishi Kyoto Hospital; Shinji Miki, Kazuhisa Kaneda, Jiro Esaki, Shimada Municipal Hospital; Takeshi Aoyama, Juntendo University Shizuoka Hospital; Satoru Suwa, Keiichi Tambara.
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
This study was supported by an educational grant from the Research Institute for Production Development (Kyoto, Japan). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Fox KA, Steg PG, Eagle KA, Goodman SG, Anderson FA Jr., Granger CB, et al. Decline in rates of death and heart failure in acute coronary syndromes, 1999–2006. JAMA. 2007;297:1892–900. doi: 10.1001/jama.297.17.1892 [DOI] [PubMed] [Google Scholar]
- 2.Gale CP, Allan V, Cattle BA, Hall AS, West RM, Timmis A, et al. Trends in hospital treatments, including revascularisation, following acute myocardial infarction, 2003–2010: a multilevel and relative survival analysis for the National Institute for Cardiovascular Outcomes Research (NICOR). Heart. 2014;100:582–9. doi: 10.1136/heartjnl-2013-304517 [DOI] [PubMed] [Google Scholar]
- 3.Jernberg T, Johanson P, Held C, Svennblad B, Lindback J and Wallentin L. Association between adoption of evidence-based treatment and survival for patients with ST-elevation myocardial infarction. JAMA. 2011;305:1677–84. doi: 10.1001/jama.2011.522 [DOI] [PubMed] [Google Scholar]
- 4.Puymirat E, Simon T, Cayla G, Cottin Y, Elbaz M, Coste P, et al. Acute Myocardial Infarction: Changes in Patient Characteristics, Management, and 6-Month Outcomes Over a Period of 20 Years in the FAST-MI Program (French Registry of Acute ST-Elevation or Non-ST-Elevation Myocardial Infarction) 1995 to 2015. Circulation. 2017;136:1908–1919. doi: 10.1161/CIRCULATIONAHA.117.030798 [DOI] [PubMed] [Google Scholar]
- 5.Rogers WJ, Frederick PD, Stoehr E, Canto JG, Ornato JP, Gibson CM, et al. Trends in presenting characteristics and hospital mortality among patients with ST elevation and non-ST elevation myocardial infarction in the National Registry of Myocardial Infarction from 1990 to 2006. Am Heart J. 2008;156:1026–34. doi: 10.1016/j.ahj.2008.07.030 [DOI] [PubMed] [Google Scholar]
- 6.Chan MY, Sun JL, Newby LK, Shaw LK, Lin M, Peterson ED, et al. Long-term mortality of patients undergoing cardiac catheterization for ST-elevation and non-ST-elevation myocardial infarction. Circulation. 2009;119:3110–7. doi: 10.1161/CIRCULATIONAHA.108.799981 [DOI] [PubMed] [Google Scholar]
- 7.Rosengren A, Wallentin L, Simoons M, Gitt AK, Behar S, Battler A, et al. Age, clinical presentation, and outcome of acute coronary syndromes in the Euroheart acute coronary syndrome survey. Eur Heart J. 2006;27:789–95. doi: 10.1093/eurheartj/ehi774 [DOI] [PubMed] [Google Scholar]
- 8.Allen LA, O’Donnell CJ, Camargo CA Jr., Giugliano RP and Lloyd-Jones DM. Comparison of long-term mortality across the spectrum of acute coronary syndromes. Am Heart J. 2006;151:1065–71. doi: 10.1016/j.ahj.2005.05.019 [DOI] [PubMed] [Google Scholar]
- 9.Darling CE, Fisher KA, McManus DD, Coles AH, Spencer FA, Gore JM, et al. Survival after hospital discharge for ST-segment elevation and non-ST-segment elevation acute myocardial infarction: a population-based study. Clin Epidemiol. 2013;5:229–36. doi: 10.2147/CLEP.S45646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.García-García C, Subirana I, Sala J, Bruguera J, Sanz G, Valle V, et al. Long-term prognosis of first myocardial infarction according to the electrocardiographic pattern (ST elevation myocardial infarction, non-ST elevation myocardial infarction and non-classified myocardial infarction) and revascularization procedures. Am J Cardiol. 2011;108:1061–7. doi: 10.1016/j.amjcard.2011.06.003 [DOI] [PubMed] [Google Scholar]
- 11.Ishihara M, Nakao K, Ozaki Y, Kimura K, Ako J, Noguchi T, et al. Long-Term Outcomes of Non-ST-Elevation Myocardial Infarction Without Creatine Kinase Elevation—The J-MINUET Study. Circ J. 2017;81:958–965. doi: 10.1253/circj.CJ-17-0033 [DOI] [PubMed] [Google Scholar]
- 12.Terkelsen CJ, Lassen JF, Nørgaard BL, Gerdes JC, Jensen T, Gøtzsche LB, et al. Mortality rates in patients with ST-elevation vs. non-ST-elevation acute myocardial infarction: observations from an unselected cohort. Eur Heart J. 2005;26:18–26. doi: 10.1093/eurheartj/ehi002 [DOI] [PubMed] [Google Scholar]
- 13.Yamashita Y, Shiomi H, Morimoto T, Yaku H, Furukawa Y, Nakagawa Y, et al. Cardiac and Noncardiac Causes of Long-Term Mortality in ST-Segment-Elevation Acute Myocardial Infarction Patients Who Underwent Primary Percutaneous Coronary Intervention. Circ Cardiovasc Qual Outcomes. 2017;10. doi: 10.1161/CIRCOUTCOMES.116.002790 [DOI] [PubMed] [Google Scholar]
- 14.Kimura T, Morimoto T, Furukawa Y, Nakagawa Y, Shizuta S, Ehara N, et al. Long-term outcomes of coronary-artery bypass graft surgery versus percutaneous coronary intervention for multivessel coronary artery disease in the bare-metal stent era. Circulation. 2008;118:S199–209. doi: 10.1161/CIRCULATIONAHA.107.735902 [DOI] [PubMed] [Google Scholar]
- 15.Case BC, Yerasi C, Wang Y, Forrestal BJ, Hahm J, Dolman S, et al. Admissions Rate and Timing of Revascularization in the United States in Patients With Non-ST-Elevation Myocardial Infarction. Am J Cardiol. 2020;134:24–31. doi: 10.1016/j.amjcard.2020.08.010 [DOI] [PubMed] [Google Scholar]
- 16.Monsieurs KG, Nolan JP, Bossaert LL, Greif R, Maconochie IK, Nikolaou NI, et al. European Resuscitation Council Guidelines for Resuscitation 2015: Section 1. Executive summary. Resuscitation. 2015;95:1–80. doi: 10.1016/j.resuscitation.2015.07.038 [DOI] [PubMed] [Google Scholar]
- 17.Noc M, Fajadet J, Lassen JF, Kala P, MacCarthy P, Olivecrona GK, et al. Invasive coronary treatment strategies for out-of-hospital cardiac arrest: a consensus statement from the European association for percutaneous cardiovascular interventions (EAPCI)/stent for life (SFL) groups. EuroIntervention. 2014;10:31–7. doi: 10.4244/EIJV10I1A7 [DOI] [PubMed] [Google Scholar]
- 18.Roffi M, Patrono C, Collet JP, Mueller C, Valgimigli M, Andreotti F, et al. 2015 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: Task Force for the Management of Acute Coronary Syndromes in Patients Presenting without Persistent ST-Segment Elevation of the European Society of Cardiology (ESC). Eur Heart J. 2016;37:267–315. doi: 10.1093/eurheartj/ehv320 [DOI] [PubMed] [Google Scholar]
- 19.Nozoe M, Sakamoto T, Taguchi E, Miyamoto S, Fukunaga T and Nakao K. Clinical manifestation of early phase left ventricular rupture complicating acute myocardial infarction in the primary PCI era. J Cardiol. 2014;63:14–8. doi: 10.1016/j.jjcc.2013.06.012 [DOI] [PubMed] [Google Scholar]
- 20.Poulsen SH, Praestholm M, Munk K, Wierup P, Egeblad H and Nielsen-Kudsk JE. Ventricular septal rupture complicating acute myocardial infarction: clinical characteristics and contemporary outcome. Ann Thorac Surg. 2008;85:1591–6. doi: 10.1016/j.athoracsur.2008.01.010 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.