Abstract
Binding of the α-factor pheromone to its G-protein-coupled receptor (encoded by STE2) activates the mating pathway in MATa yeast cells. To investigate whether specific interactions between the receptor and the G protein occur prior to ligand binding, we analyzed dominant-negative mutant receptors that compete with wild-type receptors for G proteins, and we analyzed the ability of receptors to suppress the constitutive signaling activity of mutant Gα subunits in an α-factor-independent manner. Although the amino acid substitution L236H in the third intracellular loop of the receptor impairs G-protein activation, this substitution had no influence on the ability of the dominant-negative receptors to sequester G proteins or on the ability of receptors to suppress the GPA1-A345T mutant Gα subunit. In contrast, removal of the cytoplasmic C-terminal domain of the receptor eliminated both of these activities even though the C-terminal domain is unnecessary for G-protein activation. Moreover, the α-factor-independent signaling activity of ste2-P258L mutant receptors was inhibited by the coexpression of wild-type receptors but not by coexpression of truncated receptors lacking the C-terminal domain. Deletion analysis suggested that the distal half of the C-terminal domain is critical for sequestration of G proteins. The C-terminal domain was also found to influence the affinity of the receptor for α-factor in cells lacking G proteins. These results suggest that the C-terminal cytoplasmic domain of the α-factor receptor, in addition to its role in receptor downregulation, promotes the formation of receptor–G-protein preactivation complexes.
In the yeast Saccharomyces cerevisiae, the α-factor pheromone activates a cell-surface receptor on MATa cells, leading to cell division arrest and expression of genes necessary for conjugation (1, 16, 38). The α-factor receptor (encoded by STE2) belongs to the large family of G-protein-coupled receptors (GPCRs), which includes receptors for hormones, neurotransmitters, and sensory stimuli (11, 57). GPCRs transduce their signal by activating a heterotrimeric guanine nucleotide binding protein (G protein) that results in the exchange of GDP for GTP in the Gα subunit (6, 20). In the case of the yeast pheromone pathway, the GTP-bound Gα subunit releases the Gβγ subunits, and the free Gβγ complexes then mediate the subsequent events in the response pathway (1, 16, 38). Although the yeast pheromone receptors and other GPCRs respond to different extracellular signals and share no significant sequence homology, they possess a common structural topology composed of seven transmembrane domains connected by intracellular and extracellular loops. In addition, these receptors exhibit a similar organization of functional domains. For example, as in many GPCRs, the third intracellular loop of the α-factor receptor functions in G-protein coupling (10, 58). Moreover, the cytoplasmic C-terminal domain of both yeast and mammalian receptors mediates ligand-induced endocytosis (46, 49) and plays a role in desensitization (8). The fact that some mammalian GPCRs can activate the pheromone-responsive G protein when they are expressed in yeast further underscores that distant members of this receptor family activate G proteins by similar mechanisms (14, 31, 43, 44).
Ligand binding is thought to drive GPCRs from the inactive (R) state to the active (R*) state (18). The ligand-bound R* forms a ternary complex with a G protein that leads to guanine nucleotide exchange on Gα. Current models also predict that receptors in the R state can associate with G proteins in the absence of ligand (48). We will use the term “preactivation complex” to refer to the complex that forms between unliganded receptor and the G protein (RG). Although this complex is not necessarily a direct intermediate in the formation of the activated ternary complex, it has been proposed that preactivation complexes may play an important role in regulating the specificity and efficiency of G-protein signaling (39, 40, 52). Although receptor–G-protein complexes have been observed in a few cases (9, 35, 36, 41), the analysis of preactivation and activated complexes has been hampered by the technical limitations of the copurification methods used in these experiments. Consequently, most studies have relied on indirect criteria for evaluating the interaction between the R* state and the G protein. These criteria include the ability of mutant receptors to trigger G-protein activation, the ability of G proteins to modulate the affinity of the receptor for its ligand, and the ability of receptor-derived peptides to promote post-receptor signaling events (6, 20, 40, 59). Although these approaches have identified receptor sequences required for G-protein activation, relatively little is known about receptor–G-protein complexes prior to activation (i.e., RG preactivation complexes) (39, 40).
In yeast, several observations suggest the existence of RG preactivation complexes. First, the basal level of signaling through the pheromone pathway is increased in cells lacking receptors (4, 23), consistent with a role for unoccupied receptors in maintaining the G protein in its inactive heterotrimeric state. Second, dominant-negative (DN) mutants of the α-factor receptor inhibit signaling from coexpressed wild-type receptors, apparently by sequestering G proteins (12, 37). Finally, inactive α-factor receptors inhibit the signaling activity of other GPCRs that respond to different ligands or that signal in a ligand-independent fashion (32, 33, 43, 45, 51, 55). In this study, we examine the structural basis for the formation of preactivation complexes between receptors and G proteins in yeast. Our results indicate that sequences within the cytoplasmic C-terminal domain of the receptor are required for the unoccupied receptors to sequester G proteins.
MATERIALS AND METHODS
Yeast strains and plasmids.
Yeast strains used in this study are described in Table 1. Cells were grown in media as described by Sherman (53). Cells were grown in synthetic medium containing adenine and amino acid additives, but lacking uracil to select for plasmid maintenance. High-copy-number YEp vectors for STE2, STE2-Y266C, STE2-F204S, and ste2-T326 and low-copy-number YCp vectors for ste2-T326, ste2-Δ297-360, and ste2-Δ297-391 that were derived from STE2 plasmid pJBK-008 have been described (8, 12). Plasmids with STE2 DN and ste2-T326 alleles under the galactose-inducible promoter were created by subcloning their corresponding AatII-PstI fragments into pJK57. The ste2-L236H point mutant was constructed by using the Quick Change Site-Directed Mutagenesis Kit (Stratagene), and the ste2-T345 and ste2-T360 truncation mutations were generated by PCR and cloned into STE2 plasmid pDB02 (13). To construct the STE2–Δ360-390 deletion mutant, two oligonucleotides were designed with a BspEI site immediately adjacent to the sequences encoding for Ser360 and Gly390 and were used to generate two DNA fragments that, when ligated, resulted in the in-frame deletion of residues 360 to 390. Isolation of the GPA1-A345T mutant will be described elsewhere (K. A. Schandel and D. D. Jenness, unpublished data). The ste2-F423L and ste2-L287S mutations were created by using PCR to amplify codons 195 through 431 of the STE2 gene. The template was STE2 plasmid pDB02 (13), and the primers were 5′GATGTTAGTGCCACCCAAG 3′ and 5′GCATCTGATGAGCACCTGAATC 3′. The intact plasmid was regenerated by using double-strand gap repair (34). Strain DJ926-10-3 was transformed with the PCR product together with plasmid pDB02 that had been digested with ClaI and SalI. Ura+ transformants were screened for fertility and for the inability to correct the 38°C growth defect conveyed by mutation GPA1-A345T. The phenotype was shown to be plasmid dependent by isolating the plasmid and retransforming strain DJ926-10-3. DNA sequencing of the two mutant STE2 alleles identified a single mutation (ste2-F423L) and a double mutation (ste2-L287S,F394S). The SalI-SacI fragment carrying the ste2-F423L mutation was subcloned into pDB02 to eliminate other mutations that may have existed in the unsequenced portion of plasmid. The ClaI-PstI fragment containing the ste2-L287S mutation was subcloned into pDB02, and the resulting plasmid conferred the same phenotype as the original double mutation. When the PstI-SalI fragment containing ste2-F394S was subcloned, the resulting plasmid conferred no detectable phenotype.
TABLE 1.
Yeast strains used in this study
| Strain | Genotypeb |
|---|---|
| DJ211-5-3 | MATa ade2-1 bar1-1 cry1 his4-580a leu2 lys2o trp1a tyr1o ura3 SUP4-3ts |
| DJ925-1-3 | MATa ade2-1 bar1-1 cry1 his4-480 leu2 lys2 ste2-10::LEU2 trp1 ura3 SUP4-3 |
| DJ926-10-3 | Isogenic to DJ925-1-3 except GPA1-A345T |
| JK7441-4-2 | MATa ade2-1o bar1-1 cry1 his4-580a leu2 lys2o trp1a ura3 SUP4-3ts ste2-T326 |
| JKY25 | MATa ade2-1 his4-580a lys2o trp1a tyr1o leu2 ura3 SUP4-3ts bar1-1 mfa2::FUS1-lacZ |
| JKY97 | MATa ade2-1 bar1-1 his4 leu2 lys2 ura3 trp1 tyr1 ste2Δ SUP4-3ts |
| JKY131a | MATa ade2 bar1::hisG far1 his3 leu2 ura3 ste2Δ mfa2::FUS1-lacZ mfα1::LEU2 mfα2::his5+ |
| JKY136a | Isogenic to JKY131 except ste2-P258L |
| MDY2 | Isogenic to JK7441-4-2 except gpal::URA3 and ste4::LEU2 |
| MDY3 | Isogenic to DJ211-5-3 except gpa1::URA3 and ste4::LEU2 |
| YLG123 | Isogenic to JKY25 except ste2-10::LEU2 |
Combined mutations in the α-factor structural genes (MFα1 and MFα2) prevent autocrine stimulation in strains JKY131 and JKY136.
All strains are isogenic to strain 381G (21) except for strains JKY131 and JKY136.
Pheromone-induced responses.
Halo assays for α-factor-induced cell division arrest were performed by spreading ∼3 × 105 cells from an overnight culture onto the appropriate solid media. Sterile filter disks containing the indicated amount of α-factor were placed on the cell lawns and then the plates were incubated at 30°C for 2 days. Spot assays for α-factor-induced cell division arrest were carried out by adjusting cells to 106 cells/ml, and then 5-μl aliquots from a 10-fold dilution series were placed on solid medium plates containing the indicated concentration of α-factor. The growth of the cells was recorded after 2 days for strains incubated at 30°C. Similar results were observed in at least two independent assays for both the spot assays and halo assays for cell division arrest. FUS1-lacZ induction was assayed in cells grown overnight to log phase in selective medium, diluted to 4 × 106 cells/ml, and then incubated with the indicated concentrations of α-factor for 2 h. β-Galactosidase assays were performed in duplicate by using the colorimetric substrate o-nitrophenyl-β-d-galactopyranoside as described previously (33). The averaged value of at least two independent experiments was reported for each assay, and the standard deviation was always less than 10%.
α-Factor receptor analysis.
For Western blot assays, log-phase cells adjusted to 107 per ml were collected directly or treated with α-factor (final concentration, 5 × 10−7 M) for the appropriate time, were poisoned with 10 mM NaN3 and 10 mM KF to halt endocytosis, and were collected. Analysis of protein production was carried out by lysing approximately 2.5 × 108 cells with glass beads in 250 μl of lysis buffer (2% sodium dodecyl sulfate, 100 mM Tris [pH 6.8], 8 M urea). Protein concentration was determined by using a Bio-Rad Protein Assay kit, and equal amounts of extract were separated by sodium dodecyl sulfate–9% polyacrylamide gel electrophoresis, were transferred to nitrocellulose, and then were probed with rabbit anti-Ste2p antibodies (32). For analysis of protein stability, exponentially growing cells were incubated with 20 mg of cycloheximide per ml for 10 min, and α-factor was added to a final concentration of 10−7 M. Samples were withdrawn at various times from 15 to 60 min and were processed for Western blot analysis as described above. 35S-labeled α-factor was prepared and assayed for the ability to bind whole cells as described previously (30, 33, 49). In brief, cells were incubated with radiolabeled α-factor, collected on Whatman GF/C filters, and washed, and then the bound radioactivity was determined by scintillation counting. Analysis of α-factor dissociation was performed with slight modifications to previously described procedures (3). Briefly, membrane fractions from cells expressing full-length or truncated Ste2p were incubated with 15 nM 35S-labeled α-factor (60 Ci/mmol) in a buffer containing 50 mM Tris acetate (pH 8.0), 500 mM potassium acetate, 1 mM magnesium acetate, and 0.1 mM EDTA. After a 30-min incubation at room temperature, the samples were diluted 100-fold in the presence of unlabeled synthetic α-factor with or without 10 mM GTPγS (Boehringer Mannheim). Aliquots were removed, filtered, and washed through polyethyleneimine-presoaked GF/F filters (Whatman), and then the bound radioactivity was quantified by scintillation counting.
RESULTS
The C-terminal domain is essential for DN mutant receptors to interfere with signaling.
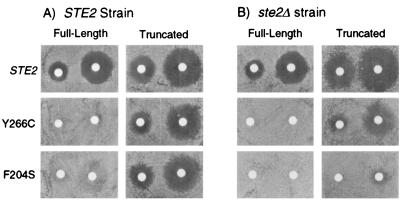
DN receptor mutants were used as a starting point for defining specific interactions between the α-factor receptor and the G protein. DN mutants were previously isolated based on their ability to inhibit the response to mating pheromone in cells that express both the DN mutant and wild-type receptors (12, 37). We identified 16 DN mutations that mapped to the extracellular ends of transmembrane domains (12), defining a region of the α-factor receptor that is critical for ligand binding and signaling. These DN mutants apparently form inactive RG complexes that limit the pool of free G proteins, since the dominant negative phenotype is suppressed by overproducing the three G-protein subunits. DN mutants also inhibit truncated receptors that lack the cytoplasmic C-terminal domain. Receptors truncated at residue 326 (T326), lacking the C-terminal region, exhibit defects in endocytosis and adaptation to pheromone, whereas pheromone binding and G-protein activation are unaffected (32, 45). These T326 receptors do not signal when coexpressed with DN receptors (not shown), suggesting that receptors lacking the C-terminal tail, despite their greater stability and increased signaling activity, are unable to compete effectively with DN receptors for G proteins.
In the present study, we tested whether the C-terminal domain of the receptor plays a role in the formation of inactive RG preactivation complexes. Plasmids that encode truncated and full-length versions of the DN receptors were introduced into a strain that carried a wild-type STE2 gene and into a control strain that carried the ste2Δ allele. Consistent with previous results (12), both the STE2+ (Fig. 1A) and the ste2Δ (Fig. 1B) strains were resistant to pheromone-induced cell division arrest when they contained the plasmids encoding the full-length versions of Y266C or F204S DN receptors. In contrast, STE2+ cells expressing the truncated versions of the DN receptors (Y266C-T326 or F204S-T326) were sensitive to α-factor (Fig. 1A). Although the truncated DN receptors resulted in a moderate-to-slight increase in α-factor responsiveness when expressed in the ste2Δ strain (Fig. 1B), the smaller and more turbid zones of growth inhibition in this strain (Fig. 1B) do not account for the wild-type level of responsiveness observed in the STE2+ cells (Fig. 1A). Western blot analysis confirmed that the Y266C-T326 and F204S-T326 receptors were produced at normal levels (not shown). Together, these results demonstrate that the C-terminal domain is required for the DN receptors to interfere with signaling.
FIG. 1.
Effects of C-terminal truncation on the interfering properties of DN mutant receptors. (A) Assays of pheromone-induced cell division arrest performed with JKY25 cells (STE2) that express wild-type receptors. The cells also contained multicopy plasmids that carried the indicated full-length or T326-truncated version of the following receptor genes: wild-type STE2, DN STE2-Y266C, or DN STE2-F204S. (B) Halo assays performed with YLG123 cells that lack an endogenous receptor gene (ste2Δ) but carried the same multicopy plasmids described in panel A. Assays of α-factor-induced growth arrest (halo assays) were carried out by placing filter disks contained 0.6 or 0.1 nmol of α-factor on agar plates spread with a lawn of the indicated cells derived from strains YLG123 or JKY25 and incubated for 48 h at 30°C to observe the zones of cell division arrest.
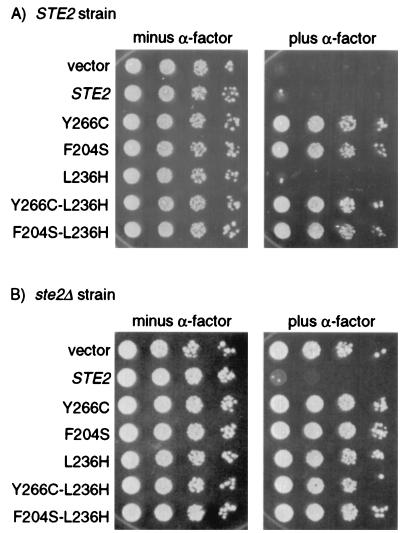
The idea that the C-terminal domain is dispensable for G-protein activation yet important for sequestering G proteins suggests that different domains of the receptor control these two activities. The independence of these receptor functions was explored further by analyzing the effects of the ste2-L236H mutation. This mutation causes an amino acid substitution in the third intracellular loop of the receptor and impairs G-protein activation, but it does not affect cell-surface expression, ligand binding, ligand-induced internalization, or the ability to undergo α-factor-induced changes in conformation (7, 49, 58). These properties suggest that the L236H mutant receptors are similar to rhodopsin mutants that acquire the R* state without catalyzing guanine-nucleotide exchange on Gα (15). The L236H receptors did not result in a DN phenotype when coexpressed with wild-type receptors (Fig. 2A). The L236H receptors differ from the DN receptors in that they bind α-factor and undergo the ligand-induced conformational change. The effect of the L236H amino acid substitution on the DN mutant receptors was tested by transforming wild-type cells with a plasmid containing a ste2 allele with both mutations (L236H and Y266C, or L236H and F204S). Growth of the transformed cells on plates containing α-factor (Fig. 2A) indicated that the defect in the third intracellular loop (L236H) did not impair the dominant inhibitory activity of the DN mutants (Y266C and F204S). Control studies showed that all L236H mutants were defective for signaling when present as the only receptors in the cell (Fig. 2B). Altogether, these results indicate that the structural determinants involved in sequestration of G proteins differ from those involved in G-protein activation.
FIG. 2.
Effect of the L236H mutation in the third intracellular loop on the interfering properties of DN receptors. Growth of (A) JKY25 (STE2) or (B) YLG123 (ste2Δ) cells carrying the indicated STE2 alleles on multicopy vector YEplac195. Serial dilutions of cells were spotted on plates in the presence or absence of 10−7 M α-factor and were incubated for 48 h at 30°C.
Signal inhibition by unoccupied receptors requires the C-terminal domain.
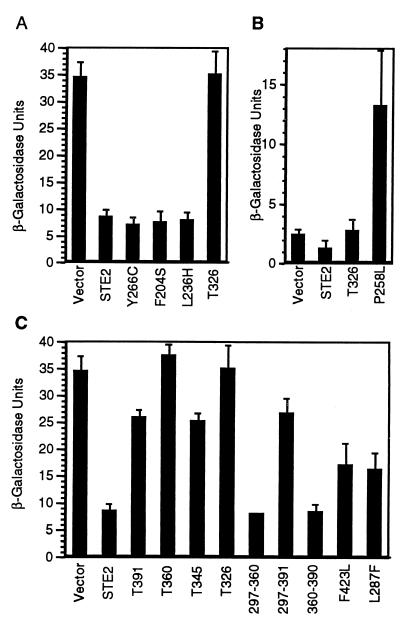
The ability of DN receptors to interfere with signaling does not appear to reflect a novel gain of function, as unoccupied wild-type receptors also inhibit postreceptor signals under certain conditions. For example, wild-type α-factor receptors inhibit signaling in yeast cells that express constitutively active α-factor receptors (33, 55), a-factor receptors (26), or mammalian hormone receptors (43). We tested whether the C-terminal domain is important for wild-type α-factor receptors to inhibit the signal exhibited by the constitutively active ste2-P258L mutant. The test strain for these assays carried a far1 mutation that prevented cell division arrest in the ste2-P258L mutant background and contained a pheromone-responsive FUS1-lacZ reporter gene for monitoring the basal level of postreceptor signal. Interestingly, the high FUS1-lacZ activity exhibited by the ste2-P258L mutant was reversed when wild-type, Y266C, or F204S receptors were coexpressed (Fig. 3A). Thus, in the absence of α-factor, wild-type receptors are similar to DN receptors in that they inhibit the postreceptor signal. The unoccupied L236H mutant receptors also inhibited the constitutive signal of the ste2-P258L mutant, indicating that the inability to activate G proteins does not reflect an inability to sequester G proteins.
FIG. 3.
Effects of coexpressed receptor genes on the basal signaling levels of constitutively active receptors. Strain JKY136 that carries the constitutive receptor gene ste2-P258L in the genome (A and C) and strain JKY131 that lacks the chromosomal receptor gene (ste2Δ) (13) were transformed with a low-copy-number vector carrying the indicated STE2 alleles. The basal levels of signaling of the pheromone-responsive FUS1-lacZ reporter gene in the absence of α-factor were assayed by measuring β-galactosidase activity.
In contrast to the full-length receptors, T326 truncated receptors did not affect the basal signal in the ste2-P258L mutant (Fig. 3A), even when the T326 receptors were overproduced (not shown). The failure of the T326 receptors to reduce the basal signal in the ste2-P258L mutant was not due to the truncated receptors directing an additional constitutive signal. Cells producing T326 receptors in the absence of the ste2-P258L mutant receptors showed a relatively low basal level of signaling that was similar to the ste2Δ control cells (Fig. 3B). Interestingly, the basal signaling levels for both the ste2Δ control cells and the ste2-T326 cells were consistently twofold higher than for cells producing wild-type receptors. Thus, the C-terminal domain also specifies reduced basal signaling, a property previously attributed to a role of the receptor in stabilizing the inactive heterotrimeric form of the G protein (4, 23).
Synthetic lethal interaction between alleles encoding the receptor and the Gα subunit.
As a second method for detecting receptor-G protein preactivation complexes, we exploited synthetic lethal interactions that occur between alleles of the STE2 and GPA1 loci. The advantage of this approach is that the genetic interaction between STE2 and GPA1 is evaluated directly, instead of relying on the ability of G proteins to influence the genetic interaction between two alleles of the STE2 gene. The GPA1-A345T mutation was identified originally based on its ability to suppress the mating defect of the ste2-L236H mutant (K. A. Schandel and D. D. Jenness, unpublished data). The suppressor phenotype was dominant. Halo assays and FUS1-lacZ transcriptional induction assays showed that the GPA1-A345T mutation resulted in an essentially wild-type level of pheromone responsiveness of STE2 control cells. Presumably, the Gpa1-A345T protein is activated more easily than the wild-type Gpa1 protein, thus the weaker signaling activity of the ste2-L236H mutant receptors may be sufficient to cause G-protein activation in the GPA1-A345T mutant.
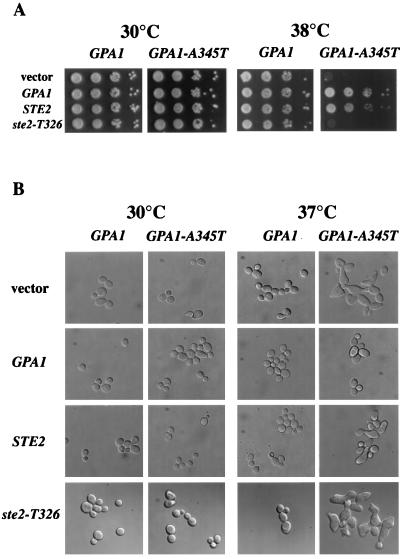
A second phenotype of GPA1-A345T pertains to the physical association of receptor and G protein. At 38°C, the combination of GPA1-A345T and ste2Δ resulted in a synthetic lethal phenotype. As shown in Fig. 4A, GPA1-A345T ste2Δ cells grow normally at 30°C, but fail to form colonies at 38°C. Microscopic images of cells grown at 30°C and then shifted to 37°C (Fig. 4B) revealed that GPA1-A345T ste2Δ cells were larger than wild-type cells. In addition, at 37°C, the double mutant formed cell surface projections similar to the cell surface projections that appear in wild-type MATa cells exposed to α-factor or in gpa1Δ cells without α-factor. The inhibition of growth in the double mutant at 37°C does not appear to be a consequence of Gα degradation since the steady-state level of the mutant Gα protein in an immunoblot assay was slightly higher than that of the wild-type protein at 37°C (not shown). As GPA1 transcription is stimulated by pheromone, the increased amount of Gpa1-A345T protein is consistent with partial activation of the pheromone response pathway.
FIG. 4.
Synthetic phenotypes of the GPA1-A345T and ste2Δ mutations. (A) Growth was assessed at 30 and 38°C for the GPA1+ ste2Δ and GPA1-A345T ste2Δ cells that carried the vector control or carried GPA1 or a STE2 allele on a plasmid. Tenfold serial dilutions of each culture were spotted onto selective medium, and duplicate plates were incubated at 30°C for 36 h or at 38°C for 72 h. (B) Microscopic images were obtained for the same strains grown at 30 and 37°C. The cells were cultured in selective liquid medium for 16 h at 30°C and then for an additional 18 h at either 30 or 37°C. GPA1 and GPA1-A345T shown at the top of each panel indicate the chromosomal allele (strains DJ925-1-3 and DJ926-10-3, respectively). Vector, GPA1, STE2, and ste2-T326 indicate the gene present on the plasmid (pJK67, YCpC3, pDB02, and pJBK023, respectively).
Introduction of a plasmid-borne copy of GPA1 reversed the growth defect of GPA1-A345T ste2Δ cells at 38°C (Fig. 4A). The synthetic lethal phenotype associated with GPA1-A345T was completely recessive to GPA1 as GPA1 restored viability (Fig. 4A) and normal cellular morphology at 37°C (Fig. 4B). Significantly, the introduction of STE2 on a plasmid also suppressed the lethality of the GPA1-A345T ste2Δ mutant cells. The suppression by STE2 was, however, incomplete, as many cells still exhibited morphological defects (Fig. 4B). Thus, the receptor partially corrected a phenotypic defect associated with a mutant Gα protein, providing additional evidence that the receptor forms a complex with the G protein in the absence of pheromone.
To determine whether the C-terminal domain of the receptor is required to stabilize the mutant G protein, we expressed T326 receptors in GPA1-A345T ste2Δ cells and then assayed the cells for their ability to grow at 38°C. The truncated receptors could not suppress the growth defect at elevated temperature (Fig. 4A) or suppress the morphological defects (Fig. 4B), implicating the C-terminal domain in preactivation complex formation. Consistent with the results obtained in the receptor competition assays, ste2-L236H GPA1-A345T cells were able to grow at 38°C (data not shown), indicating that the receptor domain required for G-protein activation is distinct from the receptor domain involved in precoupling to the G protein.
Mutational analysis of the C-terminal domain.
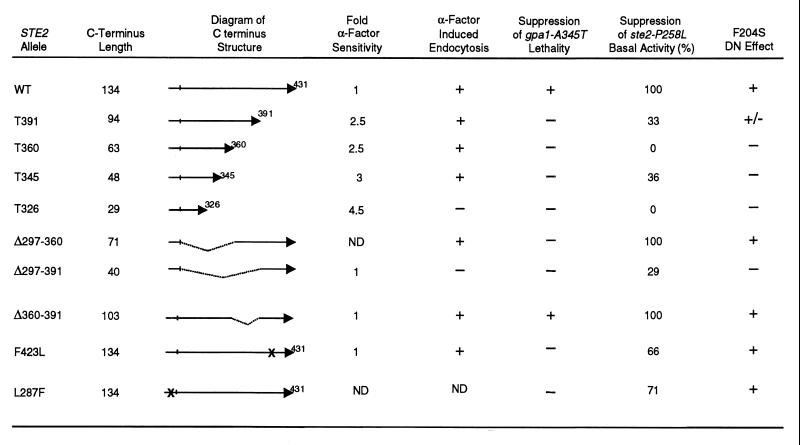
Mutational analyses of the C-terminal domain of the α-factor receptor (residues 297 to 431) have identified sequence elements important for pheromone desensitization and for ligand-induced endocytosis. A well-characterized sequence encompassing amino acids 331 to 339 (SINNDAKSS) undergoes ligand-stimulated ubiquitination (24) and is sufficient to mediate ligand-induced endocytosis and degradation of receptors when added back to truncated receptors that lack the entire C-terminal domain (46). Sequences distal to amino acid 345 have not been tested for their ability to promote internalization (46) but may encode redundant signals for endocytosis (Fig. 5). The sequences that mediate receptor desensitization are not restricted to a single motif. Four phosphorylation sites located within 33 amino acids of the C terminus are partially responsible for regulation of receptor sensitivity (8), and phosphorylation sites in other portions of the C-terminal domain may also contribute to desensitization as well as to ubiquitination (25, 45). The present study focused on deletion mutants that affect the C-terminal domain in order to delineate regulatory elements that control endocytosis, desensitization, and the formation of RG preactivation complexes. Consistent with previous reports, cells containing receptors truncated at positions 391, 360, 345, and 326 (T391, T360, T345, and T326, respectively) were more sensitive to α-factor than wild-type cells (Fig. 5). In contrast, a receptor missing amino acids 297 to 391 (designated Δ297–391) led to nearly normal sensitivity, indicating that residues 392 to 431 are sufficient to mediate some aspects of pheromone sensitivity. Also consistent with previously published studies (8, 46), T345, T360, and T391 receptors were subject to ligand-induced internalization, whereas T326 and Δ297–391 receptors were not.
FIG. 5.
Functional analysis of the C-terminal domain. The length of the C-terminal domain and a diagram of the structure for each mutant receptor are shown on the left. The sensitivity to α-factor and the ability to undergo α-factor-induced endocytosis were analyzed in YLG123 (ste2Δ) cells that expressed the indicated truncation or in-frame deletion receptors. Sensitivity to α-factor was determined from halo assays and normalized to that of wild-type receptors. α-factor-induced endocytosis was determined by analysis of receptor stability after treatment with α-factor. Suppression of GPA1-A345T lethality was assayed as described in the legend to Fig. 4. Suppression of the high basal signaling activity of ste2-P258L cells was assayed as described in the legend to Fig. 3 and was normalized to 100% inhibition for full-length wild-type receptors. Shown in the far right column are the effects of the indicated C-terminal deletions or truncations on the dominant-negative properties of F204S receptors. Receptors containing both the F204S mutation and the indicated deletion or truncation were coexpressed with wild-type receptors in JKY25 cells and were assayed for their ability to interfere with signaling by wild-type receptors in halo assays (+ indicates that receptors are DN, − indicates that receptors are not DN, ± indicates that receptors are DN only when overexpressed).
As judged by the three genetic assays described in this paper, the specific sequences within the C-terminal domain that are important for G protein interaction differ from the sequences that control endocytosis. In the first assay, the T391, T360, T345, and Δ297–360 receptors were unable to suppress the lethality associated with the GPA1-A345T mutation at 38°C, even though they were proficient for endocytosis (Fig. 5). Only full-length and Δ360–390 receptors resulted in suppression, suggesting that stabilization of the mutant Gpa1 protein requires the intact C-terminal domain of the receptor. The second and third assays, based on receptor competition, proved to be less stringent and identified partially functional mutants. Although the T360 receptors were proficient for endocytosis, they failed to diminish the constitutive signal in cells containing P258L mutant receptors (Fig. 3C and 5). In contrast, the Δ297–391 receptors failed to undergo endocytosis, even though they partially inhibited the constitutive signal. The third assay, that tested whether F204S mutant receptors containing defects in the C-terminal domain cause a DN phenotype when expressed in cells containing wild-type receptors, yielded qualitatively similar results. Interestingly, cells expressing Δ297–360 receptors were indistinguishable from wild-type cells in both competition assays, indicating that residues 360 to 431 are sufficient for the formation of preactivation complexes.
Random mutagenesis of the STE2 gene led to the identification of single residues that influence RG preactivation complexes. STE2 mutants were isolated that were unable to support the growth of GPA1-A345T cells at 38°C, and the isolates were screened for pheromone sensitivity and for accumulation of cell-surface receptor protein. Two point mutations were identified, one causing a Leu-to-Phe substitution at position 287 (L287F) and the other causing a Phe-to-Leu substitution at position 423 (F423L). These L287F and F423L receptors were also partially impaired in their ability to inhibit the constitutive signal in ste2-P258L cells (Fig. 3C and 5). The L287F substitution affects a residue within the seventh transmembrane domain and may, therefore, influence the C-terminal domain indirectly. The defects in F423L receptors are consistent with a role for residues 360 to 431 in RG preactivation complexes. Taken together, our results indicate that RG preactivation complexes are largely governed by distal sequences in the receptor C-terminal tail (residues 360 to 431). This region of the receptor plays no essential role in ligand-induced endocytosis. However, it overlaps with sequences that regulate pheromone sensitivity, suggesting that these functions may be interrelated.
The C-terminal domain of the receptor influences affinity for ligand.
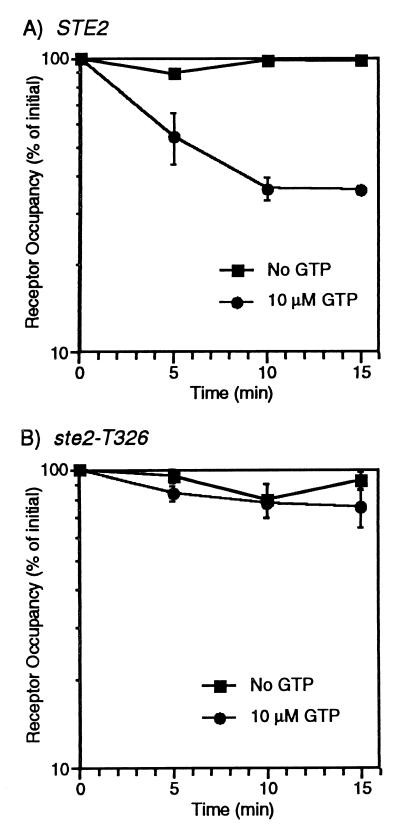
For many GPCRs, including the α-factor receptor, the affinity for the ligand is greater when the receptor is bound to the G protein (3). We wished to determine whether the C-terminal domain plays a role in this aspect of G-protein–receptor coupling in addition to its role in the preactivation complex. Two methods were used for judging the effect of the G protein on α-factor affinity. The first method tested whether GTPγS stimulates release of α-factor from crude membranes containing either wild-type or truncated receptors. Consistent with the original findings of Blumer and Thorner (3), the complexes containing α-factor and full-length receptors dissociated more rapidly when GTPγS was present (Fig. 6A). In contrast, the complexes containing truncated receptors dissociated at a slow rate that was independent of GTPγS (Fig. 6B). Thus, the C-terminal domain apparently leads to a reduction in α-factor affinity when GTPγS is present.
FIG. 6.
Analysis of GTP-promoted dissociation of 35S-α-factor from wild-type and truncated receptors. Membrane preparations from (A) STE2 strain DJ211-5-3 and (B) ste2-T326 strain JK7441-4-2 were incubated with 35S-α-factor and then assayed for the α-factor that remained bound in the presence or absence of 10 μM GTPγS after the indicated periods of time. Values given are the averages with standard deviations of three independent determinations carried out in duplicate.
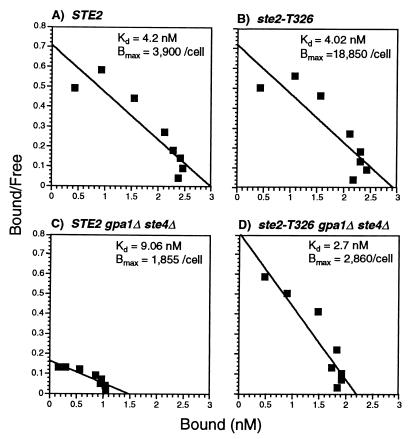
The second method employed equilibrium binding assays to evaluate the effect of G proteins on α-factor affinity (Fig. 7). As previously reported (32, 45), wild-type cells that produce either full-length (STE2) or truncated (ste2-T326) receptors bind α-factor with similar affinity (Kd = 4.2 and 4.0 nM, respectively). The fivefold increase in α-factor binding sites observed for the ste2-T326 mutant is consistent with its endocytosis defect (46, 49). Cells containing wild-type receptors exhibited reduced α-factor affinity (Kd = 9.1 nM) and fewer α-factor binding sites when the Gα and Gβ subunits were absent (STE2 gpa1Δ ste4Δ), consistent with earlier studies on ste4 mutant cells (29). In contrast, cells containing truncated receptors showed no reduction in α-factor affinity (Kd = 2.7 nM) when Gα and Gβ were absent (ste2-T326 gpa1Δ ste4Δ). These results indicate that the C-terminal domain of the receptor promotes a low affinity form of the receptor in the absence of G protein.
FIG. 7.
Equilibrium binding of 35S-α-factor to wild-type or truncated receptors in the presence or absence of G proteins. Scatchard plot analysis of wild-type (A) and truncated (B) T326 receptors in yeast cells that contain G proteins and of wild-type (C) and T326 (D) receptors in strains deleted for both the Gα (gpa1Δ) and Gβ (ste4Δ) subunit genes. The strains for these analyses were DJ211-5-3, JK7441-4-2, MDY3, and MDY2. The concentrations of cells in the assays were 5 × 108/ml (for assays in panels A, C, and D) and 1 × 108/ml (for assays in panel B) in a final volume of 100 μl.
DISCUSSION
Several observations suggest that the yeast pheromone receptors form preactivation complexes with G proteins (4, 12, 23, 33, 37, 55). In the present study, we used three genetic assays that yielded strong support for the ability of unoccupied receptors to interact with G proteins, and we showed for the first time that the C-terminal tail of the receptor facilitates this interaction. First, DN mutant receptors, which are defective in ligand binding, required their C-terminal domain to inhibit signaling from wild-type receptors. Second, the C terminus was required for unoccupied wild-type receptors to interfere with signaling from constitutively active receptors. Lastly, the temperature-sensitive lethal phenotype that resulted from a mutant form of the Gα subunit was alleviated by unoccupied full-length receptors but not by unoccupied truncated receptors.
The three genetic assays employed different criteria in defining preactivation complexes. In the first two assays, mutant and wild-type receptors competed for a common pool of G proteins, providing indirect evidence for receptor–G-protein interactions. Since overexpression of the G proteins overcomes the inhibitory activity of DN receptors (12), these two assays apparently measure the ability of the DN receptors to sequester G proteins. A caveat of the first assay is that the apparent sequestration of G proteins by DN receptors may be a unique property of the mutant receptors. However, in the second assay, unoccupied wild-type receptors interfere with the signaling from constitutively active receptors, thus suggesting that precoupling is a function of normal receptors. The third assay provides a more direct genetic test for receptor-G protein precoupling in that it detects interactions between STE2 and GPA1 alleles, whereas in the first two assays, G-protein sequestration is inferred from interactions between different STE2 alleles. Although it could be argued that all three assays measure the ability of unoccupied receptors to dampen the signaling pathway at a point downstream of the G protein, two results are inconsistent with this alternative hypothesis: G-protein overexpression reverses the DN phenotype, and DN receptors do not overcome signaling in gpa1Δ cells (12). In sum, our results, together with the results of others (23, 37, 55), strongly suggest that unoccupied α-factor receptors precouple with G proteins. It remains to be determined, however, whether the C terminus of the α-factor receptor interacts directly with its cognate G protein. Interestingly, the C terminus of Gpr1p, a putative G-protein-coupled receptor involved in nutritional sensing in yeast, interacts with its Gα subunit (Gpa2p) in the two-hybrid assay (60). In the case of the α-factor receptor, the two-hybrid assay has failed to detect interactions with any of the G-protein subunits (unpublished data); other assays will have to be developed for determining the mechanism by which the C-terminal domain regulates the formation of preactivation complexes.
In interpreting our results, we also considered the possibility that receptor oligomerization plays a role in the genetic interactions that we observed. This phenomenon has been reported for the α-factor receptor (42) and is often invoked to explain interactions observed when different mutant forms of a protein are coexpressed. In particular, oligomerization of wild-type and mutant receptors was previously suggested as a possible explanation for the recessive phenotype of the ste2-T326 allele (32, 45). The ste2-T326 cells are supersensitive to pheromone, but cells that contain both truncated ste2-T326 and full-length STE2+ receptors show normal sensitivity. However, based on the information presented in this paper, the recessive nature of the ste2-T326 allele is also consistent with the C-terminal domain of the wild-type receptors being involved in the sequestration of G proteins in preactivation complexes. It could also be argued that protein oligomerization underlies the dominant nature of the DN mutant alleles of STE2. However, for this hypothesis to be viable, one must also propose that G protein overproduction blocks the ability of the receptors to interact effectively. Moreover, receptor oligomerization does not readily account for the ability of wild-type receptors to reverse the growth defect of the GPA1-A345T mutant cells since these cells express only one form of the receptor. Thus, although oligomerization of receptors may occur, it is unnecessary to invoke this phenomenon to explain the interactions among STE2 alleles, and it fails to explain the interactions between STE2 and GPA1 alleles.
As for other GPCRs, the C-terminal domain of the α-factor receptor functions in signal downregulation by promoting ligand-induced endocytosis (46, 49) and by mediating desensitization of the receptors that remain at the cell surface (8, 32, 45). The relationships between these regulatory functions and preactivation complex formation were, therefore, explored by using deletion mutagenesis. The ability of the receptors to undergo endocytosis was not sufficient for G-protein sequestration. Moreover, the distal C-terminal region of the tail required for sequestration does not coincide with the well-defined endocytosis domain spanning residues 331 to 339, suggesting that endocytosis and sequestration of G proteins are separate and distinct functions. In contrast, the desensitization mechanism that is mediated by phosphorylation requires sequences that are dispersed throughout the C-terminal domain, and these sequences partially overlap the regions of the C-terminal domain that are required for sequestering G proteins. Thus, it is conceivable that desensitization and sequestration are linked in some way. For example, receptor desensitization could be mediated, in part, by α-factor-induced modifications in the C-terminal domain that prevent the receptor from interacting with the G protein.
The third intracellular loop rather than the C-terminal domain of the α-factor receptor is thought to play the major role in G-protein activation (7, 10, 49, 54). Thus, occupied and unoccupied receptors may utilize different structural regions to make the relevant contacts with the G protein. Conformational differences in occupied and unoccupied receptors reflect ligand-mediated changes in both the third intracellular loop and the C-terminal domain. The third intracellular loop is more accessible to trypsin cleavage in the occupied receptors, whereas sites in the C-terminal domain are more accessible in unoccupied receptors (7). Behavior of the L236H mutant receptors is also consistent with a role for the third intracellular loop in G-protein activation. The amino acid substitution in this loop inhibits G-protein activation, yet, as reported here, it has no detectable effect on preactivation complexes. Since trypsin cleavage of the third loop in L236H receptors remains sensitive to α-factor (7), the loss of G-protein activation apparently reflects a defect in the receptor–G-protein contact site rather than a failure of this receptor to undergo the conformational change. However, other changes in the third intracellular loop apparently influence preactivation complexes, since the L236R substitution blocks the ability of wild-type receptors to inhibit constitutive receptor signaling (55) and since the ste2-R233K,G237S gpa1-A345T double mutant exhibits a synthetic lethal phenotype (K. A. Schandel and D. D. Jenness, unpublished data). The differences between these mutant receptors and the L236H mutant may reflect a role for the third loop in preactivation complexes, or it is possible that the L236R mutant receptors may assume a conformational state inconsistent with G-protein binding. Although the third intracellular loop of the yeast α-factor receptor plays a prominent role in G-protein activation, other GPCR proteins use additional regions of the receptor. In some cases, the second intracellular loop or the C-terminal domain has been implicated in G-protein activation in vitro (6, 40, 59).
Pheromone binding studies were used to determine whether the C-terminal domain, in addition to its role in precoupling, also mediates interactions between the G protein and occupied receptors. The α-factor receptor, like many other GPCRs, shows higher affinity for ligand when complexed with G protein (2, 29). Truncated T326 receptors possess the same affinity for α-factor as wild-type receptors in the presence of G proteins (32); however, when expressed in cells that lack G proteins, these truncated receptors did not undergo the distinctive shift in affinity that is observed for wild-type receptors (Fig. 7). Apparently, the cytoplasmic tail modulates the conformation of the ligand-binding pocket. These results suggest that the C-terminal domain of the α-factor receptor plays a role in the transition of the inactive RG complex to the activated state after ligand binding. The C-terminal domains of rhodopsin, adrenergic receptors, and other GPCRs have also been implicated in coupling to the G protein (6, 40, 59). Moreover, truncations affecting the C-terminal tail of the prostaglandin EP3 receptor have been shown to confer ligand-independent activity (22), indicating that, in this case, the C-terminal domain plays a crucial role in constraining the receptor in its inactive conformation. Thus, although the C-terminal regions of many GPCRs are not essential for G-protein activation, these regions appear to play an important role in the normal transition to the activated state upon ligand binding.
Altogether, the results of this study have uncovered novel functions for the C-terminal domain of the α-factor receptor in regulating the ability of receptors to interact with the G protein. These results indicate that the receptor has two opposing roles in governing the intensity of signaling in the pheromone response pathway and that distinct regions of the receptor are required for these functions. On one hand, unoccupied receptors, via their C-terminal domains, form preactivation complexes with G proteins and stabilize the heterotrimeric G proteins to ensure low basal levels of signaling (i.e., a negative role in signaling). On the other hand, occupied receptors, through sequences involving the third intracellular loop, stimulate G-protein signaling to promote signal transduction (i.e., a positive role in signaling). Additionally, the formation of preactivation complexes by unoccupied receptors may contribute to the spatial regulation of signaling that enables yeast cells to locate the position of mating partners. Yeast cells locate potential mating partners by polarizing their growth towards the strongest source of incoming pheromone signal (27, 50). Consistent with this, truncated receptor strains display defects in mating partner selection (28) and in mating under suboptimal conditions (17, 19) that may result from a defect in precoupling in combination with their defect in receptor desensitization. Interestingly, the fact that some mammalian GPCRs appear to sequester G proteins suggests that they form preactivation complexes (5, 39, 47, 56). It has been proposed that these preactivation complexes could be involved in enhancing the rate of G-protein activation, in determining G-protein specificity, and in regulating the balance of signaling between the different types of GPCRs present in mammalian cells (39, 40, 52). In view of the high degree of conservation among GPCRs, it will be interesting to determine whether the C-terminal domains of other receptors are required for the formation of preactivation complexes.
ACKNOWLEDGMENTS
We thank our colleagues for advice and Colleen Davis for technical assistance with the early phases of this project.
This work was supported by NIH grant GM55107, awarded to J.B.K., and by NIH grant GM34719, awarded to D.D.J. K.A.S. was supported, in part, by a Faculty Development Grant from Assumption College.
REFERENCES
- 1.Bardwell L, Cook J G, Inouye C J, Thorner J. Signal propagation and regulation in the mating pheromone response pathway of the yeast Saccharomyces cerevisiae. Dev Biol. 1994;166:363–379. doi: 10.1006/dbio.1994.1323. [DOI] [PubMed] [Google Scholar]
- 2.Blumer K J, Reneke J E, Courchesne W E, Thorner J. The α-factor receptor (STE2 gene product) of yeast Saccharomyces cerevisiae. Cold Spring Harbor Symp Quant Biol. 1988;53:591. doi: 10.1101/sqb.1988.053.01.068. [DOI] [PubMed] [Google Scholar]
- 3.Blumer K J, Thorner J. β and γ subunits of a yeast guanine nucleotide-binding protein are not essential for membrane association of the α subunit but are required for receptor coupling. Proc Natl Acad Sci USA. 1990;87:4363–4367. doi: 10.1073/pnas.87.11.4363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boone C, Davis N G, Sprague G F., Jr Mutations that alter the third cytoplasmic loop of the a-factor receptor lead to a constitutive and hypersensitive phenotype. Proc Natl Acad Sci USA. 1993;90:9921–9925. doi: 10.1073/pnas.90.21.9921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bouaboula M, Perrachon S, Milligan L, Canat X, Rinaldi-Carmona M, Portier M, Barth F, Calandra B, Pecceu F, Lupker J, Maffrand J P, Le Fur G, Casellas P. A selective inverse agonist for central cannabinoid receptor inhibits mitogen-activated protein kinase activation stimulated by insulin or insulin-like growth factor 1. Evidence for a new model of receptor/ligand interactions. J Biol Chem. 1997;272:22330–22339. doi: 10.1074/jbc.272.35.22330. [DOI] [PubMed] [Google Scholar]
- 6.Bourne H R. How receptors talk to trimeric G proteins. Curr Opin Cell Biol. 1997;9:134–142. doi: 10.1016/s0955-0674(97)80054-3. [DOI] [PubMed] [Google Scholar]
- 7.Bukusoglu G, Jenness D D. Agonist-specific conformational changes in the yeast α-factor pheromone receptor. Mol Cell Biol. 1996;16:4818–4823. doi: 10.1128/mcb.16.9.4818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen Q, Konopka J B. Regulation of the G protein-coupled α-factor pheromone receptor by phosphorylation. Mol Cell Biol. 1996;16:247–257. doi: 10.1128/mcb.16.1.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chidiac P. Rethinking receptor-G protein-effector interactions. Biochem Pharmacol. 1998;55:549–556. doi: 10.1016/s0006-2952(97)00361-4. [DOI] [PubMed] [Google Scholar]
- 10.Clark C D, Palzkill T, Botstein D. Systematic mutagenesis of the yeast mating pheromone receptor third intracellular loop. J Biol Chem. 1994;269:8831–8841. [PubMed] [Google Scholar]
- 11.Dohlman H G, Thorner J, Caron M G, Lefkowitz R J. Model systems for the study of 7-transmembrane-segment receptors. Annu Rev Biochem. 1991;60:653–688. doi: 10.1146/annurev.bi.60.070191.003253. [DOI] [PubMed] [Google Scholar]
- 12.Dosil M, Giot L, Davis C, Konopka J B. Dominant-negative mutations in the G protein-coupled α-factor receptor map to the extracellular ends of the transmembrane segments. Mol Cell Biol. 1998;18:5981–5991. doi: 10.1128/mcb.18.10.5981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dube P, Konopka J B. Identification of a polar region in transmembrane domain 6 that regulates the function of the G protein-coupled α-factor receptor. Mol Cell Biol. 1998;18:7205–7215. doi: 10.1128/mcb.18.12.7205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Erickson J R, Wu J J, Goddard J G, Tigyi G, Kawanishi K, Tomei L D, Kiefer M C. Edg-2/Vzg-1 couples to the yeast pheromone response pathway selectively in response to lysophosphatidic acid. J Biol Chem. 1998;273:1506–1510. doi: 10.1074/jbc.273.3.1506. [DOI] [PubMed] [Google Scholar]
- 15.Ernst O P, Hofmann K P, Sakmar T P. Characterization of rhodopsin mutants that bind transducin but fail to induce GTP nucleotide uptake. Classification of mutant pigments by fluorescence, nucleotide release, and flash-induced light-scattering assays. J Biol Chem. 1995;270:10580–10586. doi: 10.1074/jbc.270.18.10580. [DOI] [PubMed] [Google Scholar]
- 16.Ferguson B, Horecka J, Printen J, Schultz J, Stevenson B J, Sprague G F., Jr The yeast pheromone response pathway: new insights into signal transmission. Cell Mol Biol Res. 1994;40:223–228. [PubMed] [Google Scholar]
- 17.Gehrung S, Snyder M. The SPA2 gene of Saccharomyces cerevisiae is important for pheromone-induced morphogenesis and efficient mating. J Cell Biol. 1990;111:1451–1464. doi: 10.1083/jcb.111.4.1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gether U, Kobilka B K. G protein-coupled receptors. II. Mechanism of agonist activation. J Biol Chem. 1998;273:17979–17982. doi: 10.1074/jbc.273.29.17979. [DOI] [PubMed] [Google Scholar]
- 19.Giot L, DeMattei C, Konopka J B. Combining mutations in the incoming and outgoing pheromone signal pathways causes a synergistic mating defect in Saccharomyces cerevisiae. Yeast. 1999;15:765–780. doi: 10.1002/(SICI)1097-0061(19990630)15:9<765::AID-YEA418>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 20.Hamm H E. The many faces of G protein signaling. J Biol Chem. 1998;273:669–672. doi: 10.1074/jbc.273.2.669. [DOI] [PubMed] [Google Scholar]
- 21.Hartwell L H. Mutants of Saccharomyces cerevisiae unresponsive to cell division control by polypeptide mating hormone. J Cell Biol. 1980;85:811–822. doi: 10.1083/jcb.85.3.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hasegawa H, Negishi M, Ichikawa A. Two isoforms of the prostaglandin E receptor EP3 subtype different in agonist-independent constitutive activity. J Biol Chem. 1996;271:1857–1860. doi: 10.1074/jbc.271.4.1857. [DOI] [PubMed] [Google Scholar]
- 23.Hasson M S, Blinder D, Thorner J, Jenness D D. Mutational activation of the STE5 gene product bypasses the requirement for G protein β and γ in the yeast pheromone response pathway. Mol Cell Biol. 1994;14:1054–1065. doi: 10.1128/mcb.14.2.1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hicke L, Riezman H. Ubiquitination of a yeast plasma membrane receptor signals its ligand-stimulated endocytosis. Cell. 1996;84:277–287. doi: 10.1016/s0092-8674(00)80982-4. [DOI] [PubMed] [Google Scholar]
- 25.Hicke L, Zanolari B, Riezman H. Cytoplasmic tail phosphorylation of the α-factor receptor is required for its ubiquitination and internalization. J Cell Biol. 1998;141:349–358. doi: 10.1083/jcb.141.2.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hirsch J P, Cross F P. The pheromone receptors inhibit the pheromone response pathway in Saccharomyces cerevisiae by a process that is independent of their associated Gα protein. Genetics. 1993;135:943–953. doi: 10.1093/genetics/135.4.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jackson C L, Hartwell L H. Courtship in Saccharomyces cerevisiae: both cell types choose mating partners by responding to the strongest pheromone signal. Cell. 1990;63:1039–1051. doi: 10.1016/0092-8674(90)90507-b. [DOI] [PubMed] [Google Scholar]
- 28.Jackson C L, Konopka J B, Hartwell L H. S. cerevisiae α-pheromone receptors activate a novel signal transduction pathway for mating partner discrimination. Cell. 1991;67:389–402. doi: 10.1016/0092-8674(91)90190-a. [DOI] [PubMed] [Google Scholar]
- 29.Jenness D D, Goldman B S, Hartwell L H. Saccharomyces cerevisiae mutants unresponsive to α-factor pheromone: α-factor binding and extragenic suppression. Mol Cell Biol. 1987;7:1311–1319. doi: 10.1128/mcb.7.4.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jenness D D, Spatrick P. Down regulation of the α-factor pheromone receptor in Saccharomyces cerevisiae. Cell. 1986;46:345–353. doi: 10.1016/0092-8674(86)90655-0. [DOI] [PubMed] [Google Scholar]
- 31.Klein C, Paul J I, Sauve K, Schmidt M M, Arcangeli L, Ransom J, Trueheart J, Manfredi J P, Broach J R, Murphy A J. Identification of surrogate agonists for the human FPRL-1 receptor by autocrine selection in yeast. Nat Biotechnol. 1998;16:1334–1337. doi: 10.1038/4310. [DOI] [PubMed] [Google Scholar]
- 32.Konopka J B, Jenness D D, Hartwell L H. The C terminus of the Saccharomyces cerevisiae α-pheromone receptor mediates an adaptive response to pheromone. Cell. 1988;54:609–620. doi: 10.1016/s0092-8674(88)80005-9. [DOI] [PubMed] [Google Scholar]
- 33.Konopka J B, Margarit M, Dube P. Mutation of pro-258 in transmembrane domain 6 constitutively activates the G protein-coupled α-factor receptor. Proc Natl Acad Sci USA. 1996;93:6764–6769. doi: 10.1073/pnas.93.13.6764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kunes S, Ma H, Overbye K, Fox M S, Botstein D. Fine structure recombinational analysis of cloned genes using yeast transformation. Genetics. 1987;115:73–81. doi: 10.1093/genetics/115.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Law S F, Reisine T. Changes in the association of G protein subunits with the cloned mouse delta opioid receptor on agonist stimulation. J Pharmacol Exp Ther. 1997;281:1476–1486. [PubMed] [Google Scholar]
- 36.Law S F, Yasuda K, Bell G I, Reisine T. G1α3 and Goα selectively associate with the cloned somatostatin receptor subtype SSTR2. J Biol Chem. 1993;268:10721–10727. [PubMed] [Google Scholar]
- 37.Leavitt L M, Macaluso C R, Kim K S, Martin N P, Dumont M E. Dominant negative mutations in the α-factor receptor, a G protein-coupled receptor encoded by the STE2 gene of the yeast Saccharomyces cerevisiae. Mol Gen Genet. 1999;261:917–932. doi: 10.1007/s004380051039. [DOI] [PubMed] [Google Scholar]
- 38.Leberer E, Thomas D Y, Whiteway M. Pheromone signalling and polarized morphogenesis in yeast. Curr Opin Genet Dev. 1997;7:59–66. doi: 10.1016/s0959-437x(97)80110-4. [DOI] [PubMed] [Google Scholar]
- 39.Neubig R R. Membrane organization in G-protein mechanisms. FASEB J. 1994;8:939–946. doi: 10.1096/fasebj.8.12.8088459. [DOI] [PubMed] [Google Scholar]
- 40.Neubig R R. Specificity of receptor-G protein coupling: protein structure and cellular determinants. Semin Neurosci. 1998;9:189–197. [Google Scholar]
- 41.Osawa S, Weiss E R. The effect of carboxyl-terminal mutagenesis of Gtα on rhodopsin and guanine nucleotide binding. J Biol Chem. 1995;270:31052–31058. doi: 10.1074/jbc.270.52.31052. [DOI] [PubMed] [Google Scholar]
- 42.Overton M C, Blumer K J. G-protein-coupled receptors function as oligomers in vivo. Curr Biol. 2000;10:341–344. doi: 10.1016/s0960-9822(00)00386-9. [DOI] [PubMed] [Google Scholar]
- 43.Pausch M H. G protein-coupled receptors in Saccharomyces cerevisiae: high-throughput screening assays for drug discovery. Trends Biotechnol. 1997;15:487–494. doi: 10.1016/S0167-7799(97)01119-0. [DOI] [PubMed] [Google Scholar]
- 44.Price L A, Strnad J, Pausch M, Hadcock J R. Pharmacological characterization of the rat A2A-adenosine receptor functionally coupled to the yeast pheromone response pathway. Mol Pharmacol. 1996;50:829–837. [PubMed] [Google Scholar]
- 45.Reneke J E, Blumer K J, Courchesne W E, Thorner J. The carboxyl terminal domain of the α-factor receptor is a regulatory domain. Cell. 1988;55:221–234. doi: 10.1016/0092-8674(88)90045-1. [DOI] [PubMed] [Google Scholar]
- 46.Rohrer J, Benedetti H, Zanolari B, Riezman H. Identification of a novel sequence mediating regulated endocytosis of the G protein-coupled α-pheromone receptor in yeast. Mol Biol Cell. 1993;4:511–521. doi: 10.1091/mbc.4.5.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Roka F, Brydon L, Waldhoer M, Strosberg A D, Freissmuth M, Jockers R, Nanoff C. Tight association of the human Mel1a-melatonin receptor and Gi: precoupling and constitutive activity. Mol Pharmacol. 1999;56:1014–1024. doi: 10.1124/mol.56.5.1014. [DOI] [PubMed] [Google Scholar]
- 48.Samama P, Cotecchia S, Costa T, Lefkowitz R J. A mutation-induced activated state of the β2-adrenergic receptor. Extending the ternary complex model. J Biol Chem. 1993;268:4625–4636. [PubMed] [Google Scholar]
- 49.Schandel K A, Jenness D D. Direct evidence for ligand-induced internalization of the yeast α-factor pheromone receptor. Mol Cell Biol. 1994;14:7245–7255. doi: 10.1128/mcb.14.11.7245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Segall J E. Polarization of yeast cells in spatial gradients of α-mating factor. Proc Natl Acad Sci USA. 1993;90:8332–8336. doi: 10.1073/pnas.90.18.8332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shah A, Marsh L. Role of SST2 in modulating G protein-coupled receptor signaling. Biochem Biophys Res Commun. 1996;226:242–246. doi: 10.1006/bbrc.1996.1340. [DOI] [PubMed] [Google Scholar]
- 52.Shea L, Linderman J J. Mechanistic model of G-protein signal transduction. Determinants of efficacy and effect of precoupled receptors. Biochem Pharmacol. 1997;53:519–530. doi: 10.1016/s0006-2952(96)00768-x. [DOI] [PubMed] [Google Scholar]
- 53.Sherman F. Getting started with yeast. Methods Enzymol. 1991;194:3–21. doi: 10.1016/0076-6879(91)94004-v. [DOI] [PubMed] [Google Scholar]
- 54.Stefan C J, Blumer K J. The third cytoplasmic loop of a yeast G-protein-coupled receptor controls pathway activation, ligand discrimination, and receptor internalization. Mol Cell Biol. 1994;14:3339–3349. doi: 10.1128/mcb.14.5.3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stefan C J, Overton M C, Blumer K J. Mechanisms governing the activation and trafficking of yeast G protein-coupled receptors. Mol Biol Cell. 1998;9:885–899. doi: 10.1091/mbc.9.4.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vasquez C, Lewis D L. The CB1 cannabinoid receptor can sequester G-proteins, making them unavailable to couple to other receptors. J Neurosci. 1999;19:9271–9280. doi: 10.1523/JNEUROSCI.19-21-09271.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Watson S, Arkinstall S. The G-protein linked receptor FactsBook. London, England: Academic Press Limited; 1994. [Google Scholar]
- 58.Weiner J L, Guttierez-Steil S, Blumer K J. Disruption of receptor-G protein coupling in yeast promotes the function of as SST2-dependent adaptation pathway. J Biol Chem. 1993;268:8070–8077. [PubMed] [Google Scholar]
- 59.Wess J. G-protein-coupled receptors: molecular mechanisms involved in receptor activation and selectivity of G-protein recognition. FASEB J. 1997;11:346–354. [PubMed] [Google Scholar]
- 60.Xue Y, Batlle M, Hirsch J P. GPR1 encodes a putative G protein-coupled receptor that associates with the Gpa2p Gα subunit and functions in a Ras-independent pathway. EMBO J. 1998;17:1996–2007. doi: 10.1093/emboj/17.7.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]