Abstract
Background
Patients with COVID-19 pneumonia can have increased inflammation and elevated cytokines, including interleukin (IL)-6, which might be deleterious. Thus, sarilumab, a high-affinity anti-IL-6 receptor antibody, might improve the outcome of patients with moderate-to-severe COVID-19 pneumonia.
Methods
We did a multicentric, open-label, Bayesian randomised, adaptive, phase 2/3 clinical trial, nested within the CORIMUNO-19 cohort, to test a superiority hypothesis. Patients 18 years or older hospitalised with COVID-19 in six French centres, requiring at least 3L/min of oxygen but without ventilation assistance and a WHO Clinical Progression Scale [CPS] score of 5 were enrolled. Patients were randomly assigned (1:1) via a web-based system, according to a randomisation list stratified on centre and with blocks randomly selected among 2 and 4, to receive usual care plus 400 mg of sarilumab intravenously on day 1 and on day 3 if clinically indicated (sarilumab group) or usual care alone (usual care group). Primary outcomes were the proportion of patients with WHO-CPS scores greater than 5 on the 10-point scale on day 4 and survival without invasive or non-invasive ventilation at day 14. This completed trial is closed to new participants and is registered with ClinicalTrials.gov, NCT04324073.
Findings
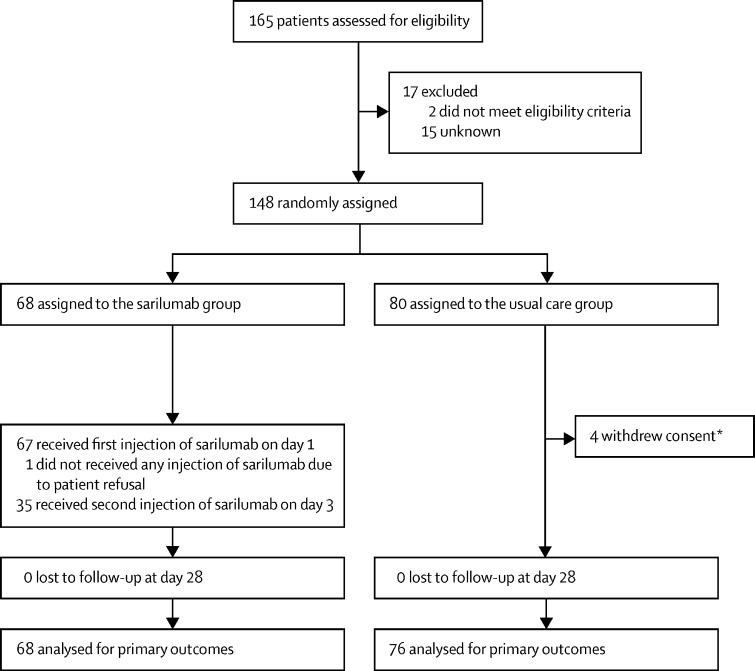
165 patients were recruited from March 27 to April 6, 2020, and 148 patients were randomised (68 patients to the sarilumab group and 80 to the usual care group) and followed up for 90 days. Median age was 61·7 years [IQR 53·0–71·1] in the sarilumab group and 62·8 years [56·0–71·7] in the usual care group. In the sarilumab group 49 (72%) of 68 were men and in the usual care group 59 (78%) of 76 were men. Four patients in the usual care group withdrew consent and were not analysed. 18 (26%) of 68 patients in the sarilumab group had a WHO-CPS score greater than 5 at day 4 versus 20 (26%) of 76 in the usual care group (median posterior absolute risk difference 0·2%; 90% credible interval [CrI] −11·7 to 12·2), with a posterior probability of absolute risk difference greater than 0 of 48·9%. At day 14, 25 (37%) patients in the sarilumab and 26 (34%) patients in the usual care group needed ventilation or died, (median posterior hazard ratio [HR] 1·10; 90% CrI 0·69–1·74) with a posterior probability HR greater than 1 of 37·4%. Serious adverse events occurred in 27 (40%) patients in the sarilumab group and 28 (37%) patients in the usual care group (p=0·73).
Interpretation
Sarilumab treatment did not improve early outcomes in patients with moderate-to-severe COVID-19 pneumonia. Further studies are warranted to evaluate the effect of sarilumab on long-term survival.
Funding
Assistance publique—Hôpitaux de Paris
Introduction
COVID-19 is a respiratory disease caused by the coronavirus SARS-CoV-2, that has already caused more than 4 million deaths globally.1, 2, 3, 4 Most people with COVID-19 have only mild or uncomplicated symptoms. Still, approximately 10% to 15% have moderate or severe disease that requires hospitalisation and oxygen support, and 3% to 5% require admission to an intensive care unit (ICU) mainly for ventilation assistance.4, 5 In severe cases, COVID-19 can be complicated by acute respiratory distress syndrome. Older age, male sex, and co-morbid diseases are risk factors for death.6, 7, 8
Patients with COVID-19 pneumonia present with non-specific inflammatory responses, including oedema and inflammatory cell infiltration in the lungs. Besides the specific pathogenic effect of SARS-CoV-2, this deleterious excessive and non-effective host immune response plays an important role during the disease course. It is, at least in part, related to a hyperinflammatory status associated with the production of a number of proinflammatory cytokines and chemokines, including interleukin (IL)-6.9 Supporting evidence of the deleterious role of hyperinflammation in moderate-to-severe pneumonia, the RECOVERY collaborative group showed that dexamethasone 6 mg per day for 10 days decreased the 28-day mortality of patients receiving mechanical ventilation or oxygen.10 This treatment has been considered the standard of care in these subsets of patients since July, 2020.
At the beginning of the epidemic in France in March, 2020, when no standard of care was yet defined, we set up the publicly supported CORIMUNO-19 platform dedicated to doing cohort embedded, open-label, randomised clinical trials of immune-modulatory drugs in patients hospitalised with moderate or severe COVID-19. Sarilumab is an anti-IL-6 receptor antibody that has an affinity to the IL-6 receptor α chain that is 20 times greater than that of tocilizumab. We aimed to assess the ability of sarilumab to improve the outcome of patients hospitalised with moderate-to-severe COVID-19 pneumonia.
Research in context.
Evidence before the study
Since the beginning of the COVID-19 pandemic, no definitive standard of care for mild-to-moderate pneumonia as a result of COVID-19 (WHO clinical progression scale score of 5) has clearly emerged. Patients with COVID-19 pneumonia have an excess of inflammation and elevated cytokines, including interleukin (IL)-6. We searched PubMed from inception to June 30, 2021, for clinical trials published in English evaluating the effect of sarilumab, a monoclonal anti-IL-6 receptor antibody in patients with laboratory-confirmed COVID-19 using the search terms (“COVID-19”[All Fields] OR “2019-nCoV”[All Fields]) OR “SARS-CoV-2”[All Fields]) AND (“sarilumab”[All Fields] (filters: Clinical Trial, Randomized Controlled Trial). We identified only open studies and one randomised clinical trial that compared sarilumab with usual care in patients with COVID-19. We designed one of the first randomised clinical trials to assess whether sarilumab could improve the outcome of patients hospitalised with moderate-to-severe COVID-19 pneumonia.
Added value of this study
In this open label randomised clinical trial that included 148 patients with moderate-to-severe COVID-19 pneumonia, there was no significant difference in the proportion of patients that required non-invasive or mechanical ventilation that died at day 14 between the patients receiving sarilumab (37%) and those receiving usual care (34%). Survival up to day 90 was not improved by sarilumab: 85% with sarilumab versus 79% with usual care (adjusted HR 0·70, 95% CI 0·31–1·58), possibly due to lack of power. These results on long-term survival are in line with a recent meta-analysis of all randomised controlled trials of IL-6 receptor nhibitors done in patients with moderate-to-severe COVID-19 pneumonia.
Implications of all the available evidence
The primary endpoint of our study was not reached; however, the non-significant effect in favour of better survival with sarilumab at day 90 supports the need for further studies for assessing the efficacy of sarilumab in patients with COVID-19 and moderate-to-severe pneumonia.
Methods
Study design
In this multicentre, adaptive, open-label study we enrolled patients with COVID-19 from six French hospitals to a series of randomised controlled trials testing different therapeutic regimens (CORIMUNO-19 cohort). Patients with moderate-to-severe pneumonia (non-ICU [CORIMUNO-SARI-1]) and patients with critical pneumonia (ICU [CORIMUNO-SARI-2]) were enrolled in independent clinical trials. An institutional review board-approved amendment to the protocol on April 6, 2020 (appendix p 135), clarified the definition of these two populations. Here we report the results ofCORIMUNO-SARI-1, on patients with moderate-to-severe COVID-19 pneumonia; CORIMUNO-SARI-2 will be reported separately.
The CORIMUNO-19 cohort and all embedded trials (ie, trials using data collected in the CORIMUNO-19 cohort) were approved by the Comité de Protection des Personnes Ile-de-France VI ethics committee and the French national agency of medicine and health products (ANSM). Legal issues and trial procedures are presented in detail in the appendix (p 12). Written informed consent was obtained from all patients to enter the CORIMUNO-19 cohort, and longitudinal data (including clinical status, biological data, and outcomes) were recorded as part of their participation in the cohort. Patients who consented were made aware that several trials might occur via the cohort and that they would probably be offered to participate in some of them. A specific additional written consent was obtained from eligible patients who were randomly selected to be offered sarilumab and agreed to receive this treatment. Eligible patients assigned to receive usual care were not notified about the trial, but their CORIMUNO-19 cohort data were available for analysis. The trial is registered with clinicaltrials.gov (NCT04324073) and the full trial protocol is in the appendix (p 22). This trial was reported according to CONSORT guidelines for adaptive randomised trials.11
Participants
Hospitalised patients 18 years or older were eligible for the CORIMUNO-19 cohort if they had confirmed SARS CoV-2 infection (positive on RT-PCR or typical chest CT scan) with mild-to-moderate, severe, or critical pneumonia (receiving > 3L/min of oxygen and having a WHO Clinical Progression Scale [CPS] score > 5; WHO-CPS is a 10-point ordinal scale described in the appendix [p 12–13]). Patients from the CORIMUNO-19 cohort were eligible for the CORIMUNO-SARI-1 trial if they had moderate-to-severe pneumonia with a WHO CPS score of 5, receiving at least 3L/min of oxygen, but without ventilation assistance that included high-flow oxygen, non-invasive ventilation, or mechanical ventilation. Pregnant women were excluded from the study. Other exclusion criteria are detailed in the appendix (p 12).
Randomisation and masking
Participants were randomly assigned in a 1:1 ratio to receive usual care plus sarilumab (sarilumab group) or usual care alone (usual care group) via a web-based secure centralised system. An independent statistician provided a computer-generated randomisation list stratified by centre and blocked with random block size (randomly selected among 2 and 4); the block size was unknown to the investigators and statisticians analysing the data.
Procedures
Sarilumab was administered intravenously at a fixed dose of 400 mg on day 1. Administration of an additional fixed dose of 400 mg intravenously on day 3 was recommended if oxygen requirement had not decreased by more than 50%, but the decision was left to the treating physician. Usual care (antibiotic agents, antiviral agents, corticosteroids, vasopressor support, anticoagulants) was provided at the discretion of the clinicians. Because of the emergency nature of the trial and feasibility issues, no placebo of sarilumab was prepared. Data quality monitoring included both remote and on-site monitoring by dedicated staff independent of the site investigators, with source data verification done for all patients recruited at every site for all crucial data points. Analyses were done by the study statisticians who were blinded to the actual randomisation groups, and investigators were not made aware of results presented to the data and safety monitoring board (DSMB).
Outcomes
The two primary outcomes of the CORIMUNO-SARI-1 trial were the proportion of patients dead or needing non-invasive ventilation or mechanical ventilation on day 4 (patients with a WHO-CPS score of >5) to be analysed as a binary outcome and survival with no need for non-invasive ventilation (including high-flow oxygen) or mechanical ventilation at day 14, to be analysed as a time-to-event outcome. Both outcomes were consistent with the core outcome set proposed by WHO.12 Prespecified secondary outcomes were clinical status assessed with the WHO-CPS at day 7 and day 14, overall survival, time to discharge, time to oxygen supply independence, and safety. We also measured biological factors such as C-reactive protein concentration.
Statistical analysis
The sample size was initially set at 120 patients (60 per group), with Bayesian interim analyses after every 60 patients (30 per group), and a provision to increase the sample size to 180 patients (90 patients, or 30 additional patients, per group) in case of promising but not conclusive results on the first 120 patients. The definition of promising was left to the discretion of the DSMB. For logistical reasons, analyses for all CORIMUNO-19 trials running in parallel were presented weekly to the DSMB. The study statisticians were in charge of the interim and final analyses, because given the short time to plan and launch the trial, the complexity of methods, the tight timelines (interim analyses were carried out every week on the latest data available), there was no possibility for us to have independent statisticians. However, trial statisticians were blinded to the true groups (that were labelled A and B in the data, blinding being done by a data manager of the clinical trials unit, a different unit) when doing the interim analyses. Blinded reports were not shared with the trial investigators, who remained blinded to all results during the trial conduct.
Events rate in the usual care group was estimated to be 0·50, based on opinions of physicians in the scientific committee as there was no available data at that time. The treatment effect was expressed in terms of absolute risk difference for the day 4 outcome and hazard ratio (HR) for the day 14 outcome. According to the protocol, a posterior probability greater than 0·99 at the interim analysis or greater than 0·95 at the final analysis indicated efficacy. We also computed the posterior probability of absolute risk difference as less than −5·5% and HR as less than 0·85 (denoting moderate or greater effect). A futility boundary was set at a posterior probability of moderate or greater effect of less than 20% at the interim analysis. The protocol made explicit that those decision rules were not binding. We computed that the trial would have frequentist power of 97·2% to detect a decrease in event rate from 0·50 to 0·20, and 73·9% to detect a decrease in event rate from 0·50 to 0·30.
We used Bayesian monitoring and analysis of the trial based on the co-primary outcomes. A first interim analysis was done after enrolment of 83 patients (43 had reached 4 days of follow-up; appendix p 16), leading to the recommendation to continue the trial, and then data were presented again to the DSMB after all patients had been randomised (98 analysed for the day 4 outcome), leading to a recommendation to stop accrual without increasing the sample size further. Posterior probabilities of absolute risk difference less than 0 and HR less than 1 were computed at each analysis, analytically for the absolute risk difference and using Markov chain Monte Carlo methods for the HR. For the day 4 outcome, we used a β prior distribution with parameters 1 and 1 for the proportion in each group. For the day 14 outcome, we used a Gaussian prior distribution with mean 0 and variance 106 for the log HR. Sensitivity analyses using a range of prior distribution were then done (appendix p 14–15). The treatment effect was summarised by the posterior median and equal tail credible intervals. A subgroup analysis according to antiviral drug use at baseline was prespecified in the protocol. Analyses according to the use of corticosteroids and particularly dexamethasone were added post-hoc considering recent publications.12, 13 Secondary outcomes were analysed in a frequentist framework, except the analysis of the WHO-CPS scores as an ordinal variable. Details of the statistical analyses are in the appendix (p 14–15).
Analyses were done on an intention-to-treat basis with no correction for multiplicity for prespecified secondary outcomes. Thus, these results are exploratory and reported as point estimates and 95% CIs. Statistical analyses were done using SAS version 9.4 (SAS Institute) and R version 3.6.1.
Role of the funding source
The funder had no role in the study design, data collection, data analysis, data interpretation, or writing of the report.
Results
From March 27 to April 6, 2020, 165 patients were assessed for eligibility in six French university hospitals and 148 patients were randomly assigned (68 patients to the sarilumab group and 80 patients to the usual care group), and the DSMB did not advise further increasing the sample size. Of the 80 patients assigned to the usual care group, four (5%) withdrew consent and were not analysed. Of the 68 patients assigned to the sarilumab group, one (1%) refused the treatment and 35 (51%) received a second injection on day 3 (figure 1 ). The median age was 61·7 years (IQR 53·0–71·1) in the sarilumab group and 62·8 years (56·0–71·7) in the usual care group. In the sarilumab group, 49 (72%) of 68 were men and in the usual care group 59 (78%) of 76 were men (table 1 ). During the trial, including anytime before or after randomisation, remdesivir was administered to no patients in the sarilumab group and one (7%) of 76 patients in the usual care group, glucocorticoids were administered to 10 (15%) of 68 patients in the sarilumab group and 19 (25%) in the usual care group, and antibiotics (61 [90%] vs 59 [78%]) and anticoagulants (63 [93%] vs 67 [88%]) were also administered. Additional immunomodulators were administered to three (4%) patients in the usual care group (tocilizumab [n=2] and sarilumab [n=1]). The details of the treatments received at the time of, and after, randomisation until day 14 are summarised in the appendix (p 17).
Figure 1.
Trial profile
*According to French and European regulations, it is possible to analyse data from patients who withdraw consent until the date of consent withdrawal, except if they ask their personal data to be erased, which was the case here.
Table 1.
Baseline characteristics
| Sarilumab (N=68) | Usual care (N=76) | ||
|---|---|---|---|
| Age, years | 61·7 (53·0–71·1) | 62·8 (56·0–71·7) | |
| Sex | |||
| Male | 49 (72%) | 59 (78%) | |
| Female | 19 (28%) | 17 (22%) | |
| Weight, kg | 81·5 (72·0–96·0) | 85·0 (74·0–93·0; n=66) | |
| BMI, kg/m2 | 27·7 (25·1–29·8; n=50) | 28·7 (24·1–31·2; n=55) | |
| BMI ≥30 kg/m2* | 14/65 (22%) | 17/71 (24%) | |
| WHO-CPS score of 5 | 68 (100%) | 80 (100%) | |
| Temperature (°C) | 37·9 (37·1–38·7) | 37·7 (37·1–38·5) | |
| Respiratory rate, breaths per min | 28·0 (24·0–32·0; n=65) | 26·0 (22·0–33·0; n=74) | |
| Oxygen flow, L/min | 5·0 (4·0–7·0) | 6·0 (4·0–9·0) | |
| SpO2, % | 94·0 (92·0–96·0) | 95·0 (93·0–96·0) | |
| Time from symptoms onset to randomisation, days | 10·0 (7·0–13·0; n=66) | 10·0 (8·0–13·0; n=75) | |
| Coexisting conditions | |||
| Chronic cardiac disease | 17/67 (25%) | 19 (25%) | |
| Diabetes | 22 (32%) | 22 (29%) | |
| Chronic kidney disease (stage 1 to 3) or dialysis | 10/67 (15%) | 7 (9%) | |
| Asthma | 3/67 (4%) | 8 (11%) | |
| Chronic pulmonary disease (not asthma) | 6/67 (9%) | 3 (4%) | |
| Active malignant neoplasm | 3/67 (4%) | 1 (1%) | |
| Smoking status | |||
| Never smoker | 46/61 (75%) | 55/71 (77%) | |
| Current smoker | 6/61 (10%) | 1/71 (1%) | |
| Former smoker | 9/61 (15%) | 15/71 (21%) | |
| Laboratory values | |||
| CRP, mg/L | 160 (98–207; n=67) | 155 (94–216; n=76) | |
| D-Dimer, μg/L | 1265 (765–2030; n=52) | 1125 (740–1660; n=58) | |
| Neutrophil count, G/L | 5·5 (4·2–7·0; n=64) | 5·8 (4·7–7·9; n=73) | |
| Lymphocyte count, G/L | 0·9 (0·7–1·2; n=65) | 0·8 (0·6–1·2; n=73) | |
| Lymphocytes to neutrophils ratio | 0·2 (0·1–0·3; n=64) | 0·1 (0·1–0·2; n=73) | |
| Haemoglobin, g/dL | 12·9 (11·7–13·6; n=67) | 13·0 (11·9–14·3) | |
| Platelet count, g/L | 236 (182–291; n=67) | 226 (182–295) | |
| ALT or SGPT, IU/L | 40·0 (28·0–60·0; n=67) | 37·0 (25·0–59·0; n=71) | |
| AST or SGOT, IU/L | 59·0 (40·0–79·0; n=66) | 51·0 (39·0–77·0; n=70) | |
| Albumin, g/L | 32·0 (28·0–35·0; n=42) | 33·4 (29·8–36·0; n=42) | |
| Creatinine, μmol/L | 82·0 (65·5–117·0; n=68) | 73·0 (59·0–93·5] | |
| Ferritin, mg/L | 937 (517–2237; n=41) | 1138 (561–2064; n=44) | |
| LDH, IU/L | 469 (344–640; n=50) | 444 (369–571; n=54) | |
| CPK (IU/L) | 173 (81–300; n=57) | 172 (77–300; n=49) | |
Data are median (IQR), n (%), or n/N (%). All patients had either positive RT-PCR or typical chest CT scanALT=alanine aminotransferase. AST=aspartate aminotransferase. BMI=body mass index. CPK=creatine phosphokinase. CPS=clinical progression scale. CRP=C-reactive protein. LDH=lactate dehydrogenase. SGBT=serum glutamic-pyruvic transaminase. SGOT=serum glutamic-oxaloacetic transaminase.
This variable was also recorded as a binary condition at screening, which explains the lower number of missing values than when not using a binary condition.
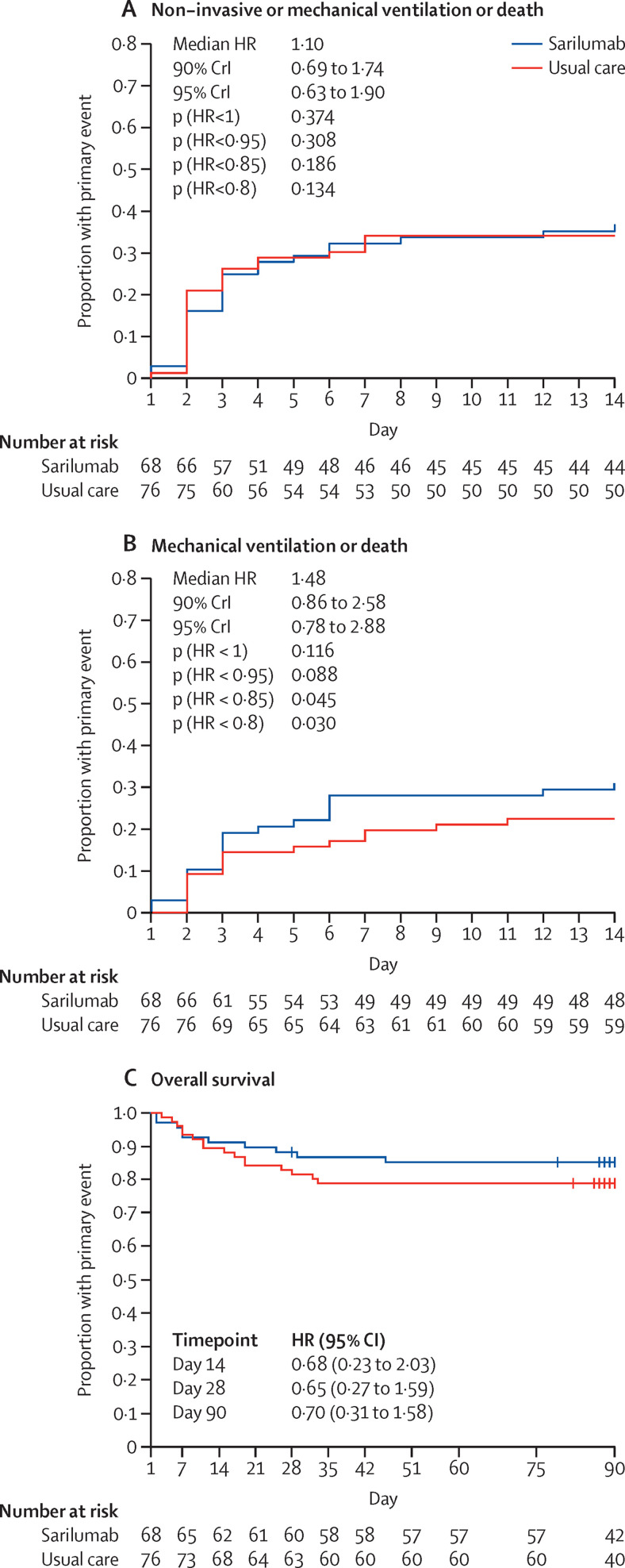
On day 4, 18 (26%) of 68 patients in the sarilumab group had a WHO-CPS score greater than 5 versus 20 (26%) of the 76 patients in the usual care group (median posterior absolute risk difference 0·2%; 90% credible interval [CrI] −11·7 to 12·2; table 2 ; appendix p 17). The posterior probability of absolute risk difference less than 0 (sarilumab better than usual care) was 48·9% (appendix p 19). On day 14, at least one event (non-invasive ventilation includinghigh-flow oxygen, mechanical ventilation, or death) had occurred in 25 (37%) patients in the sarilumab group (cumulative incidence of event 37%; 95% CI 24–47) and 26 (34%) patients in the usual care group (cumulative incidence 34%; 95% CI 23–44; figure 2A ; table 2; appendix p 17). The posterior probability of any efficacy of sarilumab (HR <1) was 37·4% (median posterior HR 1·10; 90% CrI 0·69–1·74), and of moderate or greater efficacy than usual care (HR < 0·85) was 18·6% (appendix p 18). The number of patients with mechanical ventilation or death at day 14 was 21 (31%) in the sarilumab group and 17 (22%) in the usual care group (figure 2B; appendix p 18).
Table 2.
Primary and secondary efficacy outcomes
| Sarilumab (n=68) | Usual care (n=76) | Treatment effect | ||
|---|---|---|---|---|
| Coprimary outcomes | ||||
| WHO-CPS score > 5 at day 4 | 18 (26%) | 20 (26%) | .. | |
| Median posterior absolute risk difference | .. | .. | 0·2% (90% CrI −11·7 to 12·2) | |
| Median posterior odds ratio adjusted for age and centre | .. | .. | 1·02 (90% CrI 0·54 to 1·94) | |
| Posterior probability of any benefit | .. | .. | 48·9% | |
| Posterior probability of moderate or greater benefit than usual care* | .. | .. | 21·6% | |
| Non-invasive ventilation, mechanical ventilation, or death up to day 14 | 25 (37%) | 26 (34%) | .. | |
| Median posterior hazard ratio adjusted for age and centre. | .. | .. | 1·10 (90% CrI 0·69–1·74) | |
| Posterior probability of any benefit | .. | .. | 37·4% | |
| Posterior probability of moderate or greater benefit than usual care* | .. | .. | 18·6% | |
| Secondary outcomes | ||||
| Overall survival | ||||
| Mortality at day 14 | 6 (9%) | 8 (11%) | 0·68 (95% CI 0·23–2·03)† | |
| Mortality at day 28 | 8 (12%) | 14 (18%) | 0·65 (95% CI 0·27–1·59)† | |
| Mortality at day 90 | 10 (15%) | 16 (21%) | 0·70 (95% CI 0·31–1·58)† | |
| WHO-CPS score | ||||
| Day 4 | 5 (5–6) | 5 (5–6) | 1·11 (95% CrI 0·53–2·34)‡ | |
| Day 7 | 5 (5–7; n=52) | 5 (5–6; n=66) | 1·02 (95% CrI 0·49–2·08)‡ | |
| Day 14 | 4 (2–5; n=63) | 5 (2–6; n=67) | 0·79 (95% CrI 0·42–1·47)‡ | |
| Day 2 to 14 (longitudinal analysis) | .. | .. | 0·93 (95% CrI 0·26–3·38)‡ | |
| Discharged at day 28 | 51 (75%) | 53 (70%) | 1·19 (95% CI 0·81 to 1·75)† | |
| Independent from oxygen at day 28 | 50 (74%) | 54 (71%) | 1·06 (95% CI 0·72 to 1·57)† | |
Data are n (%), n/N (%), or median (IQR) unless otherwise stated. CrI=credible interval (Bayesian analysis). CPS=Clinical Progression Scale.
Moderate or greater benefit was defined as an absolute risk difference of less than −5·5% for the day 4 outcome and a hazard ratio of less than 0·85 for the day 14 outcome.
Hazard ratio adjusted for age and centre.
Median posterior odds ratio in a proportional odds model adjusted for age and centre.
Figure 2.
Occurrence of events during follow-up
Kaplan-Meier cumulative estimates of probability of the primary outcome, death, or ventilation support (mechanical ventilation, high-flow oxygen, or non-invasive ventilation; A) and death or mechanical ventilation (B), and overall survival (C) in the sarilumab group as compared with the usual care group. For the primary outcome (death or ventilation support) and death or mechanical ventilation, data are analysed in a Bayesian framework, and median posterior HRs and 90% Crls are presented, together with posterior probabilities of achieving specified effects. Overall survival was analysed in a frequentist framework, so these probabilities are not relevant. Crl=credible interval. HR=hazard ratio.
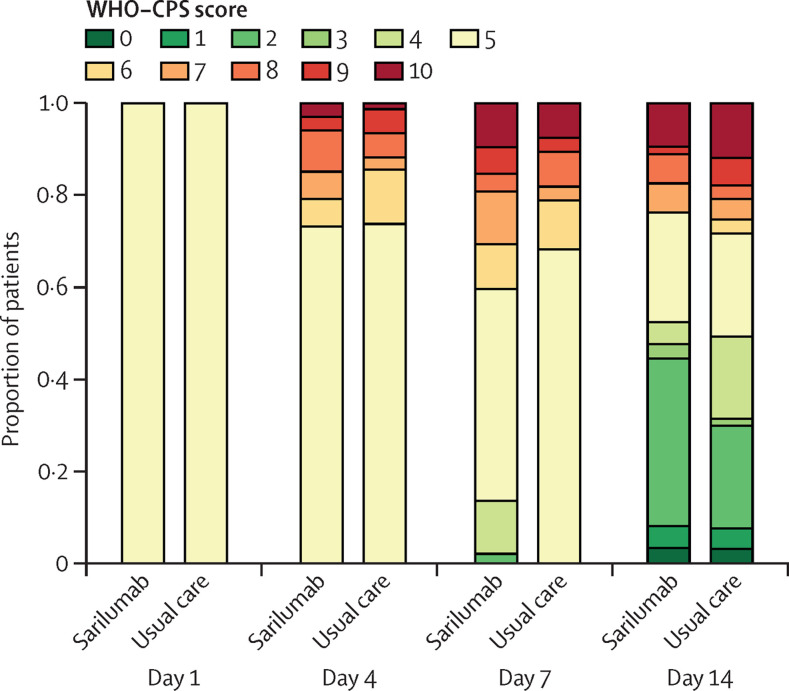
The evolution of WHO-CPS scores during the 14-day follow-up is shown in figure 3 . The groups were similar in their WHO-CPS score outcomes on days 4, 7, and 14 (table 2). At day 90, ten patients had died in the sarilumab group (overall survival 85%; 95% CI 77–94) and 16 patients had died in the usual care group (overall survival 79%; 95% CI 70–89; adjusted HR 0·70; 95% CI 0·31–1·58; figure 2C; table 2; appendix p 18). The cumulative incidence of patients who had been weaned from oxygen at day 28 was 74% (95% CI 61–83) in the sarilumab group and 71% (95% CI 59–80) in the usual care group (HR 1·06; 95% CI 0·72–1·57; table 2). The cumulative incidence of discharge by day 28 was 75% (95% CI 63–84) in the sarilumab group and 70% (95% CI 58–79) in the usual care group (HR 1·19; 95% CI 0·81–1·75; table 2). The decrease in C-reactive protein level and neutrophil count was rapid in the sarilumab arm (appendix p 21). Post-hoc analysis did not show any benefit of sarilumab in subgroups of patients defined by C-reactive protein level of more than 150 mg/L (appendix p 18).
Figure 3.
WHO clinical progression scale score during follow-up
WHO-CPS=WHO clinical progression scale.
A total of 37 (54%) patients in the sarilumab and 33 (43%) patients in the usual care group had adverse events (table 3 ). The number of adverse events in the sarilumab group was 77 and in the usual care group was 58 (p=0·02 for the average number of events per patient). Serious adverse events occurred in 27 (40%) patients in the sarilumab group and 28 (37%) in the usual care group (p=0·73). Bacterial sepsis was the most commonly occurring serious adverse event, occurring in 12 patients in the sarilumab group and seven patients in the usual care group. Ten (15%) patients in the sarilumab died and 16 (21%) patients in the usual care group died, and causes of death are indicated in table 3.
Table 3.
Safety analysis. Averse events, serious adverse events and causes of deaths
| Sarilumab (n=68) | Usual care (n=76) | p value | |||
|---|---|---|---|---|---|
| Adverse events | |||||
| Patients with at least one adverse event* | 37 (54%) | 33 (43%) | 0·24* | ||
| Patients with multiple adverse events | 17 (25%) | 11 (14%) | .. | ||
| Number of adverse events† | 77 | 58 | .. | ||
| Incidence rate per 1000 patient-day (95% CI) | 14·6 (11·7–18·2) | 10·3 (8·0–13·4) | .. | ||
| IRR (95% CI) | 1·41 (1·00–1·98) | ref | 0·048† | ||
| Serious adverse events | |||||
| Patients with at least one serious adverse event | 27 (40%) | 28 (37%) | 0·73* | ||
| Patients with multiple serious adverse events | 10 (15%) | 9 (12%) | .. | ||
| Number of serious adverse events | 44 | 40 | .. | ||
| Incidence rate per 1000 patient-day (95% CI) | 8·3 (6·2–11·2) | 7·1 (5·2–9·7) | .. | ||
| IRR (95% CI) | 1·16 (0·76–1·79) | ref | 0·47† | ||
| ARDS | 7 | 11 | .. | ||
| Bacterial sepsis | 12 | 7 | .. | ||
| Hepatic cytolysis | 6 | 3 | .. | ||
| Neutropenia | 5 | 0 | .. | ||
| Lymphopenia | 2 | 2 | .. | ||
| Anaemia | 0 | 2 | .. | ||
| Thrombopenia | 1 | 0 | .. | ||
| Pulmonary embolism | 1 | 2 | .. | ||
| Other ischemic events | 3 | 5 | .. | ||
| Haemorrhagic events | 3 | 2 | .. | ||
| Sudden death | 0 | 1 | .. | ||
| Multiple organ failure | 0 | 2 | .. | ||
| Acute renal failure | 1 | 1 | .. | ||
| Abdominal pain | 1 | 0 | .. | ||
| Encephalitis | 1 | 0 | .. | ||
| Pneumothorax | 1 | 0 | .. | ||
| Asthenia | 0 | 1 | .. | ||
| Hypernatremia | 0 | 1 | .. | ||
| Deaths | 10 (15%) | 16 (21%) | .. | ||
| ARDS | 7 | 9 | .. | ||
| Bacterial sepsis | 1 | 2 | .. | ||
| Haemorrhagic stroke | 1 | 1 | .. | ||
| Gastrointestinal bleeding | 0 | 1 | .. | ||
| Sudden death | 0 | 1 | .. | ||
| Vasculitis of the limbs | 1 | 0 | .. | ||
| Multiple organ failure | 0 | 1 | .. | ||
| Pulmonary embolism | 0 | 1 | .. | ||
IRR=incidence rate ratio. ARDS=acute respiratory distress syndrome.
Fisher's exact test.
Poisson model.
Discussion
In this trial, we did not find any effect of sarilumab (400 mg administered intravenously on day 1, possibly repeated on day 3) in patients with COVID-19 and moderate-to-severe pneumonia for decreasing the proportion of patients with a WHO-CPS score greater than >5 at day 4, or for decreasing the proportion of patinets with non-invasive ventilation including high-flow oxygen, mechanical ventilation, or death on day 14. Likewise, most of the secondary endpoints did not differ between the sarilumab and the usual care groups. Survival up to 90 days was not different between groups (HR 0·70; 95% CI 0·31–1·58).
To date, only two published randomised controlled trials of sarilumab in patients with COVID-19 have been published. The first trial, from the REMAP-CAP collaborative group, included a different subset of patients: critical patients on non-invasive ventilation or mechanical ventilation and having been in intensive care for less than 24 hours.14 The REMAP-CAP study, which included 48 patients treated with 400 mg of intravenously administered sarilumab, showed a median adjusted odds ratios for in-hospital survival of 2·01 (95% CrI 1·18–4·71) for sarilumab as compared with usual care over 90 days. Conversely, a second randomised controlled trial sponsored by Regeneron, in which 304 (73%) of 416 patients were on oxygen but not in ICU (WHO CPS score of 5), did not show efficacy of sarilumab.15 Nonetheless, in this latter trial, as in our study, at day 29, there were numerical, non-significant survival differences between patients with critical disease receiving 400 mg of sarilumab (88%) and patients receiving placebo (79%; difference of 8·9 [95% CI −7·7 to 25·5]; p=0·25). Lastly, an open-label cohort study that included 28 patients on non-invasive ventilation treated with 400 mg of intravenously administered sarilumab and 28 contemporary patients treated with usual care in Italy showed improved survival with sarilumab at 28 days, but this effect was non-significant.16
Our CORIMUNO-TOCI-1 trial, which evaluated tocilizumab in the same population and with the same methodology as the present study, met its primary endpoint.13 In the CORIMUNO-TOCI-1 study, the probability that tocilizumab reduced the risk of non-invasive ventilation, mechanical ventilation, or death at day 14 was 95% (HR 0·58; 90% CrI 0·33–1·00). The survival at day 28 was not improved but, similar to the present trial, there was a non-statistical difference in favour of better survival up to day 90 in patients treated with tocilizumab (HR 0·65; 95% CI 0·25–1·67).
In July, 2021, a WHO meta-analysis of 27 randomised clinical trials of anti-IL-6 receptor agents showed a significant reduction of 28-day mortality (HR=0·86 [0·79–0·95]) and mechanical ventilation or death (0·77 [0·70–0·85]) in patients with COVID-19.17 Interestingly, in this meta-analysis, day-28 mortality was significantly reduced with tocilizumab (HR=0·83 [0·74–0·92]) but not with sarilumab (HR=1·08 [0·86–1·36]). Of note, conversely to tocilizumab trials, most of the sarilumab trials have been done without associated corticosteroids as part of usual care. Thus, a synergistic effect between corticosteroids and anti-IL-6 receptor antibodies, shown in the WHO meta-analysis, might explain such discrepancy. However, we cannot eliminate a subtle difference of mechanism of action between both drugs.
The mechanism of action of sarilumab and tocilizumab is identical with regard to neutralisation of soluble and membrane IL-6 receptor and thus inhibition of the IL-6 pathway. However, the affinity of sarilumab to the IL-6 receptor alpha chain is 20-times higher than that of tocilizumab.18, 19 Likewise, the dissociation constant of sarilumab for the target receptor is 12·8 pmol/L, which is about 55 times lower than that of tocilizumab, consistent with the higher binding affinity.20 Meanwhile, the dose of both drugs was roughly the same in the different trials. Consequently, we can hypothesise that some trapping of sarilumab in the blood might have occurred in some patients (due to large increases in blood IL-6 receptor concentrations in patients with COVID-19), thus potentially leading to decreased penetration of sarilumab in the lungs; the site of hyperinflammation. Although the conclusion of this study, in contrast to our study with tocilizumab, suggests a lack of efficacy of sarilumab, the difference in effect of both IL-6 receptor inhibitors on patients with COVID-19 remains questionable and was not shown in the WHO meta-analysis if results were analysed separately with or without corticosteroids.
Safety was the same in both groups. The number of serious adverse events and the number of patients with serious adverse events was identical. Of note, five cases of neutropenia (a known side effect of anti-IL-6 receptor antibodies) were observed with sarilumab. There was a numerically non-statistically significant increased number of bacterial infections in the sarilumab group (12) compared to the usual care group (seven).
Strengths of this trial include the multi-centre design, thorough monitoring to ensure data quality, a homogeneous target population of patients with moderate pneumonia requiring at least 3L/min oxygen support, and long-term survival analysis. The groups were well balanced regarding baseline characteristics and cotreatments administered during the study. However, this trial has several limitations. The sample size is small and was planned to detect a very large effect of the drug. The trial was not blinded since it was logistically impossible at the time of the onset of the pandemic to set up a double-blind academic study quickly. There was an imbalance in numbers between groups due to the fact that we did two sarilumab trials (non-ICU [SARI-1], which is presented here, and ICU [SARI-2], which will be reported separately) that were operationally considered as two strata of the same trial on the randomisation platform. Since at the beginning of the pandemic patients on high-flow oxygen (WHO-CPS 6) were very rapidly intubated, there was an amendment on April 6, 2020, as indicated in the appendix (p 135, stating that patients in WHO-CPS 6 be moved from stratum 1 (non-ICU) to stratum 2 (ICU). Another limitation is that usual care might have differed across centres and over time, especially regarding corticosteroid use. However, the short period of accrual and the stratification of randomisation might have limited the effect of non-standardisation. The unblinded nature of the study might have also led to performance bias, disadvantaging the patients in the sarilumab group: three (4%) patients in the usual care group received an anti-IL-6 receptor drug and 19 (25%) patients assigned to the usual care group received corticosteroids, versus only 10 (15%) patients in the sarilumab group. However, the number and percentage of patients receiving corticosteroids was low and dexamethasone was rarely used as the trial accrual stopped before the results of the RECOVERY study were known. The very infrequent use of dexamethasone in our study is another limitation regarding the recent results from RECOVERY that show the benefit of tocilizumab was restricted to the patients taking it concomitantly with dexamethasone.21 Lastly, this trial targeted a specific segment of the COVID-19 patient population (patients with a WHO-CPS score of 5 exactly and requiring at least 3L/min oxygen without any ventilation support, regardless of inflammatory status), and thus our results are not generalisable to the whole COVID-19 population.
In summary, this randomised clinical trial suggests that sarilumab was not effective in reducing the need for non-invasive ventilation, mechanical ventilation, or death in patients with COVID-19 and moderate-to-severe pneumonia. Larger randomised controlled trials with longer follow-up, as well as meta-analyses, are needed for determining the exact effects of sarilumab and other IL-6 receptor antagonists in different subsets of patients, and whether combined therapy with dexamethasone might further improve outcomes.
Data sharing
The protocol, consent form, statistical analysis plan, regulatory documents, and other relevant study materials are available in the appendix (p 22). As described in the protocol, the trial steering committee will facilitate use of the study data and approval will not be unreasonably withheld. Deidentified participant data collected during the CORIMUNO-SARI-1 trial (and the data dictionary) will be made available to bona fide researchers registered with an appropriate institution within 3 months of publication, and for 10 years. Proposals should be addressed via email to raphael.porcher@aphp.fr and will be reviewed by the CORIMUNO-19 scientific committee. The steering committee will need to be satisfied that any proposed publication is of high quality, honours the commitments made to the study participants in the consent documentation and ethical approvals, and is compliant with relevant legal and regulatory requirements (eg, relating to data protection and privacy). To gain access, data requesters will need to sign a data access agreement and to confirm that data will only be used for the agreed purpose for which access was granted. The steering committee will have the right to review and comment on any draft manuscripts before publication.
Declaration of interests
We declare no competing interests.
Acknowledgments
Acknowledgments
This trial was publicly funded by the Ministry of Health, Programme Hospitalier de Recherche Clinique (PHRC COVID-19–20–0151, PHRC COVID-19–20–0029), and Assistance Publique − Hôpitaux de Paris Foundation and Foundation for Medical Research. We are grateful to all patients who participated in the CORIMUNO-19 study, and their families. The authors also thank Prof Maxime Dougados who was in charge of the logistics, as well as the investigators who collaborated in this study and Universities of Paris, Paris-Saclay, Paris-Sorbonne, Paris-Nord Sorbonne, Paris-Est Créteil, Versailles-Saint Quentin and Strasbourg (medical students support), INSERM, and Reacting. Sanofi donated sarilumab as an unrestricted grant and had no role in the study design, no role in the collection, analysis, or interpretation of the data, and no role in the writing of the report.
Contributors
A steering committee was responsible for the design, conduct, and reporting of the trial, and an independent DSMB oversees all CORIMUNO-19 trials (appendix p 1). The manuscript was initially drafted by XM, OH, P-LT, MR-R, RP and PR, and approved by all members of the trial steering committee. MR-R organised collection of the data. RP and PR did the statistical analysis. All authors from the writing committee contributed to design of the trial and of study protocol, data interpretation, and critical review and revision of the manuscript. RP and MR-R had access to the raw data and verified the data and analyses and the fidelity of this report to the study protocol and data analysis plan. All members of the writing committee had full access to all of the data and the final responsibility to submit for publication.
The CORIMUNO-19 Collaborative group
Writing committee: France X Mariette (Université Paris-Saclay, AP-HP, Hôpital Bicêtre, INSERM, Le Kremlin Bicêtre), O Hermine (Université de Paris, PARCC, AP-HP, Hôpital Necker, INSERM, Imagine Institute, Paris), P-L Tharaux (PARCC, Université de Paris, INSERM, Paris), M Resche-Rigon (Université de Paris, INSERM, Hôpital Saint Louis, Paris), R Porcher, P Ravaud (Université de Paris, INSERM, INRAE, AP-HP, Hôpital Hôtel-Dieu, Paris). The six writing committee authors contributed equally. Steering committee: P Ravaud (chair of the CORIMUNO-19 platform), S Bureau, M Dougados, O Hermine, X Mariette, M Resche-Rigon, P-L Tharaux, A Tibi.
Contributor Information
The CORIMUNO-19 Collaborative group:
Xavier Mariette, Olivier Hermine, Pierre-Louis Tharaux, Matthieu Resche-Rigon, Raphael Porcher, Philippe Ravaud, Serge Bureau, Maxime Dougados, Annick Tibi, Elie Azoulay, Jacques Cadranel, Joseph Emmerich, Muriel Fartoukh, Bertrand Guidet, Marc Humbert, Karine Lacombe, Matthieu Mahevas, Frédéric Pene, Valérie Pourchet-Martinez, Frédéric Schlemmer, Yazdan Yazdanpanah, Gabriel Baron, Elodie Perrodeau, Damien Vanhoye, Cécile Kedzia, Lauren Demerville, Anne Gysembergh-Houal, Alexandre Bourgoin, Sarah Dalibey, Nabil Raked, Lakhdar Mameri, Stéphanie Alary, Samir Hamiria, Thinhinane Bariz, Hala Semri, Dhiaa Meriem Hai, Moustafa Benafla, Mohamed Belloul, Pernelle Vauboin, Saskia Flamand, Claire Pacheco, Anouk Walter-Petrich, Emilia Stan, Souad Benarab, Corine Nyanou, Claire Montlahuc, Lucie Biard, Robin Charreteur, Celine Dupré, Kévin Cardet, Blandine Lehmann, Kamyl Baghli, Claire Madelaine, Eric D'Ortenzio, Oriane Puéchal, Caroline Semaille, Laurent Savale, Anatole Harrois, Samy Figueiredo, Jacques Duranteau, Nadia Anguel, Arthur Pavot, Xavier Monnet, Christian Richard, Jean-Louis Teboul, Philippe Durand, Pierre Tissieres, Mitja Jevnikar, David Montani, Sophie Bulifon, Xavier Jaïs, Olivier Sitbon, Stéphan Pavy, Nicolas Noel, Olivier Lambotte, Lelia Escaut, Stéphane Jauréguiberry, Elodie Baudry, Christiane Verny, Mathilde Noaillon, Edouard Lefèvre, Mohamad Zaidan, Clotilde Le Tiec Le Tiec, Céline Verstuyft, Anne-Marie Roques, Lamiae Grimaldi, Domitille Molinari, Gaël Leprun, Alain Fourreau, Laurent Cylly, Myriam Virlouvet, Ramdane Meftali, Solène Fabre, Marion Licois, Asmaa Mamoune, Yacine Boudali, Sophie Georgin-Lavialle, Patricia Senet, Gilles Pialoux, Angèle Soria, Antoine Parrot, Hélène François, Nathalie Rozensztajn, Emmanuelle Blin, Pascaline Choinier, Juliette Camuset, Jean-Simon Rech, Antony Canellas, Camille Rolland-Debord, Nadège Lemarié, Nicolas Belaube, Marine Nadal, Martin Siguier, Camille Petit-Hoang, Julie Chas, Elodie Drouet, Matthieu Lemoine, Audrey Phibel, Lucie Aunay, Eliane Bertrand, Sylviane Ravato, Marie Vayssettes, Anne Adda, Celine Wilpotte, Pélagie Thibaut, Julie Fillon, Isabelle Debrix, Soraya Fellahi, Jean-Philippe Bastard, Guillaume Lefèvre, Vincent Fallet, Jacques-Eric Gottenberg, Yves Hansmann, Emmanuel Andres, Sophie Bayer, Guillaume Becker, Frédéric Blanc, Stéphane Brin, Vincent Castelain, Emmanuel Chatelus, Eva Chatron, Olivier Collange, François Danion, Frédéric De Blay, Eric Demonsant, Pierre Diemunsch, Sophie Diemunsch, Renaud Felten, Bernard Goichot, Valentin Greigert, Aurélien Guffroy, Bob Heger, Anne Hutt, Charlotte Kaeuffer, Loic Kassegne, Anne Sophie Korganow, Pierrick Le Borgne, Nicolas Lefebvre, Tristan Martin, Paul Michel Mertes, Catherine Metzger, Nicolas Meyer, Gabriel Nisand, Eric Noll, Mathieu Oberlin, Sophie Ohlmann-Caillard, Vincent Poindron, Julien Pottecher, Yvon Ruch, Cédric Sublon, Hakim Tayebi, François Weill, Arsène Mekinian, Noémie Abisror, Vincent Jachiet, Dorothée Chopin, Olivier Fain, Marc Garnier, Jessica Krause le Garrec, Marjolaine Morgand, Jerome Pacanowski, Tomas Urbina, Chloe McAvoy, Maria Pereira, Gladys Aratus, Laurence Berard, Tabassome Simon, Anne Daguenel-Nguyen, Marie Antignac, Céline Leplay, Jean-Benoit Arlet, Jean-Luc Diehl, Florence Bellenfant, Anne Blanchard, Alexandre Buffet, Bernard Cholley, Antoine Fayol, Edouard Flamarion, Anne Godier, Thomas Gorget, Sophie-Rym Hamada, Caroline Hauw-Berlemont, Jean-Sébastien Hulot, David Lebeaux, Marine Livrozet, Adrien Michon, Arthur Neuschwander, Marie-Aude Penet, Benjamin Planquette, Brigitte Ranque, Olivier Sanchez, Geoffroy Volle, Sandrine Briois, Mathias Cornic, Virginie Elisee, Denis Jesuthasan, Juliette Djadi-Prat, Pauline Jouany, Ramon Junquera, Mickael Henriques, Amina Kebir, Isabelle Lehir, Jeanne Meunier, Florence Patin, Valérie Paquet, Anne Tréhan, Véronique Vigna, Brigitte Sabatier, Damien Bergerot, Charléne Jouve, Camille Knosp, Olivia Lenoir, Nassim Mahtal, Léa Resmini, F-Xavier Lescure, Jade Ghosn, Antoine BACHELARD, Timothee BIRONNE, Raphael BORIE, Agathe BOUNHIOL, Catherine BOUSSARD, Jeanne CHAUFFiER, Solaya CHALAL, Lynda CHALAL, Malikhone CHANSOMBAT, Paul CRESPIN, Bruno CRESTANI, Olivia DACONCEICAO, Laurene DECONINCK, Philippe DIEUDE, Antoine DOSSIER, Marie DUBERT, Greggory DUCROCQ, Axelle FUENTES, Anne GERVAIS, Marie GILBERT, Valentina ISERNIA, Sophie ISMAEL, Veronique JOLY, Zelie JULIA, Sylvie LARIVEN, Sylvie LE GAC, Diane LE PLUART, Francoise LOUNI, Awa NDIAYE, Thomas PAPO, Marion PARISEY, Bao PHUNG, Annabelle POURBAIX, Anne RACHLINE, Christophe RIOUX, Aurelie SAUTEREAU, Gabriel STEG, Hassan TARHINI, Simon VALAYER, Dorothee VALLOIS, Paul VERMES, Thomas VOLPE, Yann Nguyen, Vasco Honsel, Emmanuel Weiss, Anaïs Codorniu, Virginie Zarrouk, Victoire De Lastours, Matthieu Uzzan, Olivier Olivier, Geoffrey Rossi, Naura Gamany, Roza Rahli, Zeina Louis, David Boutboul, Lionel Galicier, Yaël Amara, Gabrielle Archer, Amira Benattia, Anne Bergeron, Louise Bondeelle, Nathalie De Castro, Melissa Clément, Michaël Darmont, Blandine Denis, Clairelyne Dupin, Elsa Feredj, Delphine Feyeux, Adrien Joseph, Etienne Lengliné, Pierre Le Guen, Geoffroy Liégeon, Gwenaël Lorillon, Asmaa Mabrouki, Eric Mariotte, Grégoire Martin de Frémont, Adrien Mirouse, Jean-Michel Molina, Régis Peffault de Latour, Eric Oksenhendler, Julien Saussereau, Abdellatif Tazi, Jean-Jacques Tudesq, Lara Zafrani, Isabelle Brindele, Emmanuelle Bugnet, Karine Celli Lebras, Julien Chabert, Lalia Djaghout, Catherine Fauvaux, Anne Lise Jegu, Ewa Kozaliewicz, Martine Meunier, Marie-Thérèse Tremorin, Claire Davoine, Isabelle Madeleine, Sophie Caillat-Zucman, Constance Delaugerre, Florence Morin, Damien SENE, Ruxandra BURLACU, Benjamin CHOUSTERMAN, Bruno MEGARBANE, Pascal RICHETTE, Jean-Pierre RIVELINE, Aline FRAZIER, Eric VICAUT, Laure BERTON, Tassadit HADJAM, Miguel Alejandro VASQUEZ-IBARRA, Clément JOURDAINE, Aude JACOB, Julie SMATI, Stéphane RENAUD, Philippe MANIVET, Claire PERNIN, Lydia SUAREZ, Luca Semerano, Sebastien ABAD, Ruben Benainous, Coralie Bloch Queyrat, Nicolas Bonnet, Sabrina Brahmi, johann Cailhol, Yves Cohen, Celine Comparon, Hugues Cordel, Robin Dhote, Nathalie Dournon, Boris Duchemann, Nathan Ebstein, Benedicte Giroux-Leprieur, Jeanne Goupil de Bouille, Anne Jacolot, Hilario Nunes, Johanna Oziel, Vanessa Rathouin, Marthe Rigal, Dominique Roulot, Claire Tantet, Yurdagul Uzunhan, Nathalie COSTEDOAT-CHALUMEAU, Zakaria Ait Hamou, Sarah Benghanem, Philippe BLANCHE, Etienne CANOUI, Nicolas CARLIER, Benjamin CHAIGNE, Adrien CONTEJEAN, Bertrand DUNOGUE, Pierre DUPLAND, Aurélie DUREL - MAURISSE, Remy GAUZIT, Paul JAUBERT, Hassan Joumaa, Mathieu Jozwiak, Solen KERNEIS, Marie LACHATRE, Hélène Lafoeste, Paul LEGENDRE, Liem Binh LUONG NGUYEN, Jonathan MAREY, Caroline MORBIEU, Luc MOUTHON, Lee NGUYEN, Lola-Jade Palmieri, Alexis REGENT, Tali-Anne SZWEBEL, Benjamin TERRIER, Corinne GUERIN, Jérémie ZERBIT, Kahina CHEREF, Kamil CHITOUR, Mamadou Salif CISSE, Ada CLARKE, Gaelle CLAVERE, Isabelle DUSANTER, Caroline GAUDEFROY, Moez JALLOULI, Sami KOLTA, Catherine LE BOURLOUT, Nathalie MARIN, Nathalie MENAGE, Alexandre MOORES, Isabelle PEIGNEY, Cédric PIERRON, Samira SALEH-MGHIR, Mathilde VALLET, Marc MICHEL, Giovanna MELICA, Jean-Daniel LELIEVRE, Elena FOIS, Pascal LIM, Marie MATIGNON, Constance GUILLAUD, Alaki THIEMELE, David SCHMITZ, Marion BOUHRIS, Syllia BELAZOUZ, Laetitia LANGUILLE, Armand MEKONTSO-DESSAPS, Thiziri SADAOUI, Julien Mayaux, Patrice Cacoub, Jean-Christophe Corvol, Céline Louapre, Sara Sambin, Louise-Laure Mariani, Carine Karachi, Florence Tubach, Candice Estellat, Linda Gimeno, Karine Martin, Aïcha Bah, Vixra Keo, Sabrine Ouamri, Yasmine Messaoudi, Nessima Yelles, Pierre Faye, Sébastien Cavelot, Cecile Larcheveque, Laurence Annonay, Jaouad Benhida, Aida Zahrate-Ghoul, Soumeya Hammal, Ridha Belilita, Marie Lecronier, Alexandra Beurton, Luc Haudebourg, Robin Deleris, Julien Le Marec, Sara Virolle, Safaa Nemlaghi, Côme Bureau, Pierre Mora, Martin De Sarcus, Olivier Clovet, Baptiste Duceau, Paul Henri Grisot, Marie hélène Pari, Jérémy Arzoine, Ulrich Clarac, Morgane Faure, Julie Delemazure, Maxence Decavele, Elise Morawiec, Alexandre Demoule, Martin Dres, Mathieu Vautier, Yves Allenbach, Olivier Benveniste, Gaelle Leroux, Aude Rigolet, Perrine Guillaume-Jugnot, Fanny Domont, Anne Claire Desbois, Cloé Comarmond, Nicolas Champtiaux, Segolene Toquet, Amine Ghembaza, Matheus Vieira, Georgina Maalouf, Gonçalo Boleto, Yasmina Ferfar, Fanny Charbonnier, Claire AGUILAR, Fanny ALBY-LAURENT, Marie-Alexandra ALYANAKIAN, Prissile BAKOUBOULA, Christine BROISSAND, Carole BURGER, Clara CAMPOS-VEGA, Nathalie CHAVAROT, Laure CHOUPEAUX, Benjamin FOURNIER, Sophie GRANVILLE, Elodie ISSORAT, Claire ROUZAUD, Damien VIMPERE, Guillaume Geri, Nawal Derridj, Naima Sguiouar, Hakim Meddah, Mourad Djadel, Helene Chambrin-Lauvray, Jean-Charles Duclos-Vallée, Faouzi Saliba, Sophie-Caroline Sacleux, Ilias Koumis, Jean-Marie Michot, Annabelle Stoclin, Emeline Colomba, Fanny Pommeret, Chistophe Willekens, Madona Sakkal, Rosa Da Silva, Valérie Dejean, Yasmina Mekid, Ines Ben-Mabrouk, Caroline Pradon, Laurence Drouard, Valérie Camara-Clayette, Alexandre Morel, Gilles Garcia, Abolfazl Mohebbi, Férial Berbour, Mélanie Dehais, Anne-Lise Pouliquen, Alison Klasen, Loren Soyez-Herkert, Jonathan London, Younes Keroumi, Emmanuelle Guillot, Guillaume Grailles, Younes El Amine, Fanny Defrancq, Hanane Fodil, Chaouki Bouras, Dominique Dautel, Nicolas Gambier, Thierno Dieye, Anaïs Razurel, Boris Bienvenu, Victor Lancon, Laurence Lecomte, Kristina Beziriganyan, Belkacem Asselate, Laure Allanic, Elena Kiouris, Marie-Hélène Legros, Christine Lemagner, Pascal Martel, Vincent Provitolo, Félix Ackermann, Mathilde Le Marchand, Aurélie Clan Hew Wai, Dimitri Fremont, Elisabeth Coupez, Mireille Adda, Frédéric Duée, Lise Bernard, Antoine Gros, Estelle Henry, Claire Courtin, Anne Pattyn, Pierre-Grégoire Guinot, Marc Bardou, Agnes Maurer, Julie Jambon, Amélie Cransac, Corinne Pernot, Bruno Mourvillier, Amélie Servettaz, Gaetan Deslée, Alain Wynckel, Philippe Benoit, Eric Marquis, Damien Roux, Coralie Gernez, Cécile Yelnik, Julien Poissy, Mandy Nizard, Fanette Denies, Hélène Gros, Jean-Jacques Mourad, Emmanuelle Sacco, and Sophie Renet
Supplementary Material
References
- 1.Wu J, Liu J, Zhao X, et al. Clinical characteristics of imported cases of coronavirus disease 2019 (COVID-19) in Jiangsu province: a multicenter descriptive study. Clin Infect Dis. 2020;71:706–712. doi: 10.1093/cid/ciaa199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8:475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gao YD, Ding M, Dong X, et al. Risk factors for severe and critically ill COVID-19 patients: a review. Allergy. 2021;72:428–455. doi: 10.1111/all.14657. [DOI] [PubMed] [Google Scholar]
- 9.Del Valle DM, Kim-Schulze S, Huang HH, et al. An inflammatory cytokine signature predicts COVID-19 severity and survival. Nat Med. 2020;26:1636–1643. doi: 10.1038/s41591-020-1051-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Horby P, Lim WS, Emberson JR, et al. Dexamethasone in hospitalized patients with COVID-19. N Engl J Med. 2021;384:693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dimairo M, Pallmann P, Wason J, et al. The adaptive designs CONSORT extension (ACE) statement: a checklist with explanation and elaboration guideline for reporting randomised trials that use an adaptive design. BMJ. 2020;369:m115. doi: 10.1136/bmj.m115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marshall JC, Murthy S, Diaz J, et al. A minimal common outcome measure set for COVID-19 clinical research. Lancet Infect Dis. 2020;20:e192–e197. doi: 10.1016/S1473-3099(20)30483-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hermine O, Mariette X, Tharaux PL, et al. Effect of tocilizumab vs usual care in adults hospitalized with COVID-19 and moderate or severe pneumonia: a randomized clinical trial. JAMA Intern Med. 2021;181:32–40. doi: 10.1001/jamainternmed.2020.6820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.REMAP-CAP Investigators Interleukin-6 receptor antagonists in critically ill patients with COVID-19. N Engl J Med. 2021;384:1491–1502. doi: 10.1056/NEJMoa2100433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lescure FX, Honda H, Fowler RA, et al. Sarilumab in patients admitted to hospital with severe or critical COVID-19: a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Respir Med. 2021;9:522–532. doi: 10.1016/S2213-2600(21)00099-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Della-Torre E, Campochiaro C, Cavalli G, et al. Interleukin-6 blockade with sarilumab in severe COVID-19 pneumonia with systemic hyperinflammation: an open-label cohort study. Ann Rheum Dis. 2020;79:1277–1285. doi: 10.1136/annrheumdis-2020-218122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group. Shankar-Hari M, Vale CL, et al. Association between administration of IL-6 antagonists and mortality among patients hospitalized for COVID-19: a meta-analysis. JAMA. 2021;326:499–518. doi: 10.1001/jama.2021.11330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rafique A, Martin J, Blome M, Huang T, Ouyang A, Papadopoulos N. AB0037 evaluation of the binding kinetics and functional bioassay activity of sarilumab and tocilizumab to the human IL-6 receptor (IL-6r) alpha. Ann Rheum Dis. 2013;72(suppl 3):A797. A797. [Google Scholar]
- 19.Xu C, Rafique A, Potocky T, et al. Differential binding of sarilumab and tocilizumab to IL-6 Rα and effects of receptor occupancy on clinical parameters. J Clin Pharmacol. 2020;61:714–724. doi: 10.1002/jcph.1795. [DOI] [PubMed] [Google Scholar]
- 20.Kim GW, Lee NR, Pi RH, et al. IL-6 inhibitors for treatment of rheumatoid arthritis: past, present, and future. Arch Pharm Res. 2015;38:575–584. doi: 10.1007/s12272-015-0569-8. [DOI] [PubMed] [Google Scholar]
- 21.RECOVERY Collaborative Group Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet. 2021;397:1637–1645. doi: 10.1016/S0140-6736(21)00676-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The protocol, consent form, statistical analysis plan, regulatory documents, and other relevant study materials are available in the appendix (p 22). As described in the protocol, the trial steering committee will facilitate use of the study data and approval will not be unreasonably withheld. Deidentified participant data collected during the CORIMUNO-SARI-1 trial (and the data dictionary) will be made available to bona fide researchers registered with an appropriate institution within 3 months of publication, and for 10 years. Proposals should be addressed via email to raphael.porcher@aphp.fr and will be reviewed by the CORIMUNO-19 scientific committee. The steering committee will need to be satisfied that any proposed publication is of high quality, honours the commitments made to the study participants in the consent documentation and ethical approvals, and is compliant with relevant legal and regulatory requirements (eg, relating to data protection and privacy). To gain access, data requesters will need to sign a data access agreement and to confirm that data will only be used for the agreed purpose for which access was granted. The steering committee will have the right to review and comment on any draft manuscripts before publication.