Abstract
Study Objectives
Binge alcohol consumption is associated with increased cardiovascular risk. The effects of evening binge alcohol consumption (i.e. 4–5 beverages within 2 h) on the vagal components of HRV and cardiovagal baroreflex sensitivity (cvBRS) during sleep remain largely equivocal. The present study examined the effects of evening binge alcohol consumption on nocturnal cardiac vagal tone and baroreflex sensitivity during stage N2, slow wave (SWS), and rapid eye movement (REM) sleep. We hypothesized that evening binge drinking would reduce HRV and cvBRS in each sleep stage.
Methods
Following a familiarization night within the laboratory, twenty-three participants were examined following a night of binge alcohol consumption and a fluid control (randomized, crossover design). A quality nocturnal beat-to-beat blood pressure signal was obtained in both conditions in 16 participants (seven men, nine women; 25 ± 1 years).
Results
Binge drinking reduced both the high frequency (HF) and time-domain components (i.e. pNN50 and RMSSD) of HRV in stage N2 sleep, SWS, and REM. In addition, cvBRS up-up (vagal activation) was reduced following binge alcohol consumption in stage N2 (21 ± 3 vs. 15 ± 3 ms/mmHg, p = 0.035) and REM (15[11–28] vs. 11[9–18] ms/mmHg, p = 0.009). Binge alcohol consumption reduced cvBRS down-down (vagal withdrawal) in stage N2 (23 ± 2 vs. 14 ± 2 ms/mmHg, p < 0.001), SWS (20[14–30] vs. 14[9–17] ms/mmHg, p = 0.022), and REM (14[11–24] vs. 10[7–15] ms/mmHg, p = 0.006).
Conclusions
Evening binge alcohol consumption disrupts cardiac vagal tone and baroreflex function during nearly all sleep stages. These findings provide mechanistic insight into the potential role of binge drinking and alcohol abuse on cardiovascular risk.
Clinical Trials Details
Alcohol and Neural Cardiovascular Control in Binge Drinkers, www.clinicaltrials.gov/ct2/show/NCT03567434, NCT03567434.
Keywords: ethanol, autonomic control, heart rate variability, baroreflex sensitivity, hypertension
Statement of Significance.
Attenuation of cardiac vagal tone and cardiovagal baroreflex sensitivity after evening binge drinking offers a contributing mechanism to the pathogenesis of cardiovascular disease and morning cardiovascular events (i.e. morning surge) in individuals who struggle with alcohol abuse. Evening binge alcohol consumption reduced nocturnal heart rate variability and cardiovagal baroreflex sensitivity across polysomnographic (PSG) stage N2, slow wave, and rapid eye movement sleep. Our findings offer the first PSG-specific comparisons of cardiovagal function after an evening of binge alcohol consumption, and provide new insights relevant to clinical populations of alcohol abuse where repeated binge drinking behavior may chronically depress cardiovagal activity during sleep.
Introduction
Millions of American adults struggle with nightly sleep disturbances including trouble falling asleep and/or staying asleep, with many experiencing symptom severity which may approach a diagnosis similar to clinical insomnia [1]. Nearly 20% of American adults attempt to cope with sleep issues by using alcohol [2]. With repeated use, this can lead to issues of dependance [3], alcoholism and alcohol abuse [4, 5], and contribute to cardiovascular disease progression [6, 7]. Our laboratory has previously investigated both the acute and next-morning impacts of binge alcohol consumption on the sympathetic nervous system (SNS) and blood pressure regulation [8, 9].
The impact of alcohol consumption on sleep is complex. Low to moderate alcohol consumption prior to sleep do not follow the same pattern of sleep disturbance [10] as seen in high dose or binge alcohol consumption [11, 12]. Regardless, a shortened sleep latency [13] often creates the perception that sleep quality is improved, and yet detriments in sleep are still apparent especially as the alcohol dose is increased. At present, there remains a paucity of work related to the nocturnal cardiovascular implications of evening binge alcohol consumption.
Numerous studies have reported the detriments in cardiac vagal control and sensitivity associated with alcohol consumption. Acute alcohol consumption is known to decrease heart rate variability (HRV) in both the time and frequency domain across a variety of alcohol doses [14–20], but little is known if these detriments persist into sleep. One nocturnal HRV study following alcohol consumption in 10 male participants reported no significant interaction of high frequency (HF) component of HRV between alcohol dose and time of night [21], while a large observational study of Finnish employees demonstrated a dose dependent reduction in the time domain of HRV [22]. More recently, de Zambotti et al. [23] demonstrated reductions in nocturnal HF HRV and cardiovagal baroreflex sensitivity (cvBRS) after low and high doses of alcohol. However, a limitation of each of these studies [21–23] was that cardiovascular indices were only compared across hours of sleep rather than in specific sleep stages. Arguably, two of the most important stages of sleep with respect to cardiovascular health are slow wave (SWS) and rapid eye movement (REM) sleep. Both have characteristic cardiovascular profiles, where blood pressure (BP) and heart rate (HR) reach a daily nadir in SWS, and are subsequently increased in REM [24]; often a contributor to the morning blood pressure surge associated with morning cardiovascular events [25]. Thus, examining alterations in vagal indices of HRV and cvBRS during each polysomnographic sleep stage following evening binge alcohol consumption are warranted.
The purpose of the present study was to examine the sleep stage specific changes in nocturnal cardiac vagal tone and cvBRS following evening binge alcohol consumption. Our randomized, crossover, fluid-control trial utilized a pragmatic model of evening binge alcohol consumption in adults with gold-standard assessment of sleep stages and nocturnal blood pressure (i.e. polysomnography and finger plethysmography). We hypothesized that evening binge drinking would attenuate cardiac vagal tone and cvBRS function, and that these detriments would be most evident in SWS and REM sleep.
Methods
Participants
Young, healthy men and women were recruited from Michigan Technological University and surrounding communities. Inclusion criteria for each participant were ages of 21–40 years, body mass index (BMI) 18.5–35 kg/m2, and self-report of at least one binge drinking episode (i.e. 4–5 drink equivalent within two hours) in the past 6 months. Participants were excluded if they reported a history of smoking, had a diagnosis of diabetes, were currently prescribed blood pressure or autonomic medications, or were diagnosed or being treated for obstructive sleep apnea (OSA). In addition, an 11-item alcohol use disorder (AUD) questionnaire disqualified anyone who self-reported moderate to severe AUD. All eligible women could not be pregnant or breastfeeding, were not prescribed oral or intrauterine contraceptive devices in the past 6 months, and reported a normal menstrual cycle (i.e. 25–32 day average). Eligible women were randomized for testing in either the early follicular (i.e. 2–5 days after menstrual period; EF) or mid-luteal (i.e. 8–10 days after ovulation; ML) ovarian phase.
Seventy-nine individuals completed an initial phone screen and 57 were eligible for enrollment in the present study, where they were invited to Michigan Technological University’s Sleep Research Laboratory for an initial orientation session. After detailed study description and consent process, 36 participants confirmed enrollment by confirming aldehyde dehydrogenase 2 (ALDH-2) function [26] and completing an at-home sleep apnea screen. Twenty-three participants gave written informed consent and completed the study; initial morning microneurographic data reported previously [9]. Of the cohort of completed participants, concurrent and stable PSG and beat-to-beat blood pressure were obtained during both alcohol and fluid-control conditions in a total of 16 participants (seven men, nine women; five EF, four ML). All testing procedures were approved by the Michigan Technological University and Montana State University Institutional Review Boards, and are in accordance with the Declaration of Helsinki.
Experimental design
The experimental design for the present study is part of an ongoing randomized control trial (NCT03567434), where details of the alcohol consumption and fluid control protocols are described previously [9]. Briefly, participants reported to the sleep research laboratory for three scheduled visits. The first visit was to familiarize participants for a routine polysomnography (PSG) study with dual-finger beat-to-beat finger plethysmography, in part to confirm the absence of any sleep disorders including, but not limited to, obstructive sleep apnea and periodic limb movements. Using a randomized, cross-over design, participants returned for two other visits that were separated by one month to ensure all women were tested within the same phase of their menstrual cycle. For each of these visits, participants reported to the laboratory at 4:00 pm to begin experiment with baseline testing of breath alcohol content (Intoximeters Inc., St. Louis, MO) and hydration levels (Schmidt Haensch, Berlin, Germany).
At 8:00 and 9:00 pm, two alcohol doses were administered for the alcohol trial to simulate a night of binge drinking. The alcohol doses were diluted in a 1:3 ratio of 95% ethanol to desired fruit juice (i.e. orange or cranberry juice). Three aliquots were provided to the participant at the top of the hour, and again five and ten minutes past the hour. Each participant was given five minutes to consume the provided aliquot. The total alcohol dose was a 1 g/kg dose for men and 0.85 g/kg dose for women. The fluid control condition included the exact volume, but only fruit juice with 1 mL of ethanol to help mask smell. At 9:15 pm, PSG and finger plethysmography instrumentation occurred. Two additional breathalyzer recordings were taken at 10:00 pm and 11:00 pm to monitor breath alcohol content at presumed peak intoxication and prior to lights out, respectively. Upon confirming stable PSG and blood pressure signals, participant entered bed at 10:45pm, with lights out at 11:00 pm. An 8-h sleep opportunity was allotted, with lights turned on promptly at 7:00 am. An overnight sleep technician ensured quality of various sleep signals and beat-to-beat blood pressure throughout the night. Three supine blood pressures were taken ~10 min after conclusion of overnight PSG.
Measurements
Polysomnography
A standard polysomnographic sleep study (Natus Medical; Middleton, WI) was conducted on each participant in the Sleep Research Laboratory. Sleep electroencephalography (EEG) was recorded and scored via 10–20 electrode placement with two frontal, central, and occipital leads referenced to contralateral electrodes placed at the mastoid process on the opposite side of the head. In accordance with established standards of the American Association of Sleep Medicine (AASM), electrooculography (EOG) and electromyography (EMG) were recorded via two electrodes near the eyes and three electrodes placed on the chin, respectively. Thorax and abdomen respiratory inductance plethysmography (RIP) was used to monitor respiratory effort, and airflow flow was measured using a thermistor and nasal pressure sensor. Heart rate (HR) and rhythm was monitored via two-lead electrocardiogram (ECG). Blood oxygen saturation was recorded via pulse oximetry to track desaturations associated with apneic events. Leg movements were measured via EMG with surface electrodes placed on the tibialis anterior of each leg. All physiologic signals were sampled at 250 Hz. Sleep staging, apneic/respiratory events, limb movements, and arousals were defined and scored according to the AASM by a board-certified sleep physician (C.A. Smoot).
Blood pressure and heart rate
Continuous beat-to-beat blood pressure (NOVA, Finapres Medical Systems, Amsterdam, Netherlands) and heart rate were measured throughout the 8-hour PSG recording. Finger cuffs were applied to the middle and ring fingers, where cuff changes occurred every 30 min to minimize discomfort during sleep. Prior to lights out, supine brachial blood pressures (HEM-907XL, Omron, Kyoto, Japan) were used to calibrate finger plethysmography. The arterial blood pressure and heart rate (via two-lead electrocardiogram) were used to quantify nocturnal cardiac vagal tone and cardiovagal baroreflex sensitivity.
Data analysis
Sleep Stage Identification
As previously outlined, all polysomnography recordings were scored in accordance with the AASM guidelines. Further macroarchitecture analysis was performed by finding 10 min of stable sleep in stage N2, SWS, and REM sleep in each participant where there was not a finger plethysmography cuff change and absent of arousals, apneic events, and limb movements per AASM scoring standards. Cardiovagal baroreflex and cardiac vagal tone (i.e. HRV) were quantified within each full 10-min period for each participant.
Baroreflex function: spontaneous methodologies
Spontaneous cardiovagal BRS was assessed via the sequence method during stable 10-min periods of stage N2, SWS, and REM sleep [27, 28]. These periods of sleep were absent of any arousal, respiratory event, leg movement, etc. The continuous beat-to-beat blood pressure and electrocardiogram (ECG) signal was imported into a specialized software program for analysis (WinCPRS, Absolute Aliens, Turku, Finland). In brief, three or more progressive changes of SAP and corresponding R−R interval (RRI; lag 1) were identified as baroreflex sequences. Both up-up (i.e. three or more increases in SAP in tandem with lengthening RRI; vagal activation) and down-down (i.e. progressive decreases in SAP in tandem with shortening RRI; vagal withdrawal) were quantified. Criteria for an acceptable sequence included 1 mmHg change for SAP and 4 ms for RRI. A linear regression analysis of each sequence of RRI and SAP determined the reported slope. A minimum r-value of 0.7 was used as the criteria for accepting sequences. Up-up and down-down sequences within the 10-minute sleep period were averaged for each subject.
Heart rate variability
Indices of cardiac vagal tone were taken from nocturnal heart rate variability (HRV), quantified in the frequency and time domain. Briefly, the nocturnal ECG was imported in specialized software (WinCPRS, Absolute Aliens, Turku, Finland) where individual R-waves were marked and confirmed by the research team for quantification of R−R interval (RRI). Frequency domain analysis of HRV during each 10-min period of stable PSG sleep was performed via fast Fourier power spectral analysis. R-R intervals were made equidistant by spline interpolating and resampling at 5 Hz. Data was passed through a low pass filter with a cut off frequency of 0.5 Hz. Each 10-min period were transformed via fast Fourier with a Hanning window to obtain power spectra. The power spectra were expressed as the integrated area within the high-frequency (HF; 0.15–0.4 Hz) range. Total power of high frequency component was reported for each participant [29]. Time domain analysis of HRV included the root mean squared of successive differences of R−R interval (RMSSD) and the percentage of adjacent R−R intervals that varied by 50 ms or more (pNN50) [30]. Respiratory rate was determined for each period of analysis.
Statistical analysis
All data were analyzed statistically using commercial software (SPSS 26.0; IBM SPSS, Armonk, NY). Assumption of normality tests were conducted on main outcome variables via z-score normalization of variable skewness. If z-score exceeded three standard deviations from the mean, the data were considered non-normally distributed. We utilized paired samples t-tests to compare differences between conditions (i.e. alcohol vs. fluid control) when assumptions of normality were met. The Wilcoxon Signed Rank test was conducted on non-normally distributed variables which were limited to stage N2 HF HRV, SWS SAP-RRI down-down, and REM SAP-RRI up-up, down-down, and HF HRV. Data are presented as mean ± standard error of mean for data that met assumptions of normality, and as median (25th−75th percentile) if assumptions of normality were violated. One-tailed statistical tests were utilized for directional hypotheses of decreased nocturnal cvBRS and HF HRV following evening binge alcohol consumption. All other analyses utilize two-tailed distribution. Significance level was set at α ≤ 0.05.
Results
Participants were 25 ± 1 years with a BMI of 28 ± 1 kg/m2. Table 1 highlights binge alcohol consumption did not alter morning-after supine blood pressure, but did elicit an elevated morning HR. Evening alcohol consumption elicited a decrease in total sleep time (TST) and sleep efficiency (SE) when compared of fluid control. There was no change in the apnea hypopnea index (AHI) between conditions. The estimated peak breath alcohol content at 10 pm (0.000 ± 0.000% vs. 0.090 ± 0.005%, p < 0.001) was increased compared to baseline, and breath alcohol content at 11:00 pm (i.e. lights out) was significantly decreased from 10:00 pm values (0.084 ± 0.004%, p = 0.015).
Table 1.
Participant characteristics: fluid control vs. alcohol
| Variable | Fluid control (n = 16) | Alcohol (n = 16) | p value |
|---|---|---|---|
| Age (years) | 25 ± 1 | − | |
| BMI (kg/m 2 ) | 28 ± 1 | − | |
| SAP (mmHg) | 111 ± 2 | 110 ± 2 | 0.753 |
| DAP (mmHg) | 63 ± 2 | 64 ± 3 | 0.681 |
| HR (beats/min) | 57 ± 2 | 63 ± 3** | 0.004 |
| TST (min) | 439 ± 6 | 410 ± 10** | 0.004 |
| SE (%) | 92 ± 1 | 86 ± 2** | 0.003 |
| AHI (events/min) | 1.57 ± 0.8 | 1.93 ± 0.9 | 0.573 |
Results are means ± SEM; BMI, body mass index; SAP, systolic arterial pressure; DAP, diastolic arterial pressure; HR, heart rate; TST, total sleep time; SE, sleep efficiency; AHI, apnea hypopnea index. **p < 0.01 compared to corresponding fluid control condition.
Table 2 depicts nocturnal HRV reported in the frequency and time domain. RRI was significantly decreased after evening binge alcohol consumption across each sleep stage (p < 0.001 for all). Alcohol-induced respiratory rate was significantly higher in stage N2 sleep (p = 0.003) and SWS (p = 0.002), but not REM sleep (p = 0.453). HF HRV was decreased during stage N2 sleep (p = 0.013), SWS (p < 0.001), and REM sleep (p = 0.004) after evening alcohol consumption. In addition, pNN50 and RMSSD were both reduced after evening alcohol consumption in stage N2 sleep (p = 0.001 for both), SWS (p < 0.001 for both) and REM sleep (p = 0.004 for both).
Table 2.
Time and frequency domain heart rate variability by sleep stage
| Variable | Fluid Control (n = 16) | Alcohol (n = 16) | p value | |
|---|---|---|---|---|
| N2 | RRI (ms) | 1111 ± 59 | 943 ± 46* | <0.001 |
| Resp. rate (breaths/min) | 14 ± 1 | 15 ± 1* | 0.003 | |
| HF (ms 2 ) | 1270 (461−2024) | 357 (124−2155)* | 0.013 | |
| pNN50 (%) | 46 ± 7 | 25 ± 7* | 0.001 | |
| RMSSD (ms) | 82 ± 11 | 52 ± 10* | 0.001 | |
| SWS | RRI (ms) | 1100 ± 51 | 972 ± 41* | <0.001 |
| Resp. rate (breaths/min) | 15 ± 1 | 16 ± 1* | 0.002 | |
| HF (ms 2 ) | 1429 ± 279 | 698 ± 146* | <0.001 | |
| pNN50 (%) | 47 ± 6 | 29 ± 6* | <0.001 | |
| RMSSD (ms) | 81 ± 9 | 48 ± 6* | <0.001 | |
| REM | RRI (ms) | 1046 ± 57 | 936 ± 50* | <0.001 |
| Resp. rate (breaths/min) | 15 ± 1 | 15 ± 1 | 0.453 | |
| HF (ms 2 ) | 978 (340−1864) | 335 (122−1162)* | 0.004 | |
| pNN50 (%) | 33 ± 7 | 20 ± 6* | 0.004 | |
| RMSSD (ms) | 76 ± 11 | 49 ± 8* | 0.004 |
Results are means ± SEM (normal distribution) or median (25th−75th percentile, non-normal distribution); HF, high frequency; pNN50, proportion of R−R intervals that exceed 50 ms; RMSSD, root mean square of successive differences. *p < 0.05, compared with corresponding fluid control condition.
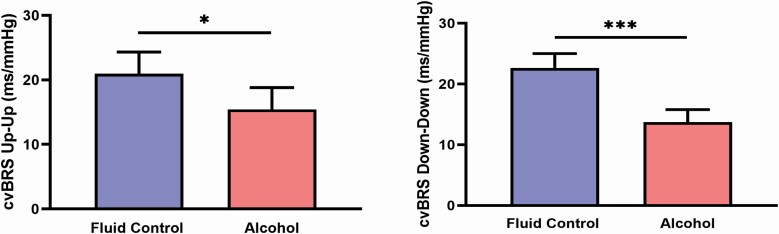
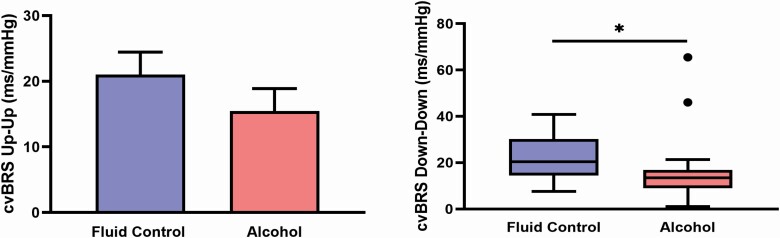
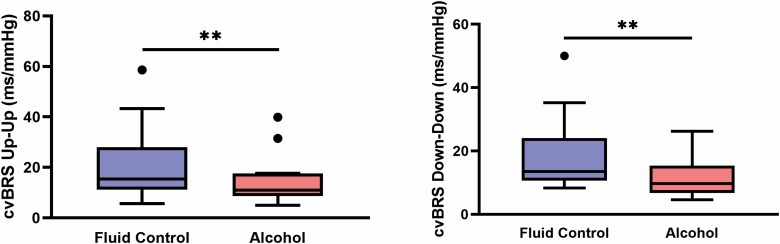
Figure 1 depicts cvBRS up-up and down-down sequencing during stage N2 sleep, where both up-up (21 ± 3 vs. 15 ± 3 ms/mmHg, p = 0.035) and down-down (23 ± 2 vs. 14 ± 2 ms/mmHg, p < 0.001) cvBRS were reduced after evening binge alcohol consumption. Figure 2 demonstrates similar patterns in SWS, where down-down cvBRS was significantly reduced after binge alcohol consumption (20 [14–30] vs. 14 [9–17] ms/mmHg, P = 0.022), but not up-up cvBRS (21 ± 3 vs. 15 ± 3 ms/mmHg, p = 0.112). In similar fashion to N2 sleep, Figure 3 depicts a significant reduction in both up-up (15 [11–28] vs. 11 [9–18] ms/mmHg, p = 0.009) and down-down (14 [11–24] vs. 10 [7–15] ms/mmHg, p = 0.006) cvBRS after evening binge alcohol consumption during REM sleep (n = 14).
Figure 1.
Nocturnal cardiovagal baroreflex (cvBRS) sensitivity during a stable 10-minute period of stage N2 sleep. cvBRS up-up (vagal activation) and down-down (vagal withdrawal) was reduced following evening binge alcohol consumption. *p < 0.05, ***p < 0.001 between conditions.
Figure 2.
Nocturnal cardiovagal baroreflex (cvBRS) sensitivity during a stable 10-minute period of stage slow wave sleep (SWS). cvBRS down-down (vagal withdrawal) was reduced following evening binge alcohol consumption. *p < 0.05, ***p < 0.001 between conditions.
Figure 3.
Nocturnal cardiovagal baroreflex (cvBRS) sensitivity during a stable 10-min period of rapid eye movement (REM) sleep (n = 14). cvBRS up-up (vagal activation) and down-down (vagal withdrawal) was reduced following evening binge alcohol consumption. **p < 0.01 between conditions.
Discussion
The present study examined the impact of evening binge alcohol consumption on cardiac vagal control and baroreflex sensitivity. In line with prior work, indices of HRV and cvBRS were reduced during sleep following alcohol consumption [23]. However, our findings advance these prior studies in several important ways, including an experimental approach of simulated binge drinking, as well an account of the cardiovagal responses to evening binge alcohol consumption by sleep stage using gold-standard PSG. Our primary findings are 4-fold. First, evening binge drinking consistently reduced the HF (i.e. predominantly vagal) component of HRV across all sleep stages, including N2, SWS, and REM. Second, HRV quantified in the time domain (i.e. RMSSD and pNN50) was also decreased consistently in all sleep stages after binge alcohol consumption when compared to fluid control. Third, we demonstrate that down-down cvBRS (vagal withdraw) was attenuated in stage N2, SWS, and REM sleep following alcohol consumption. Finally, up-up cvBRS (vagal activation) was also reduced after evening alcohol consumption during stage N2 and REM sleep. In summary, evening binge alcohol consumption contributes to blunted vagal control of heart rate and baroreflex function across key PSG sleep stages. These findings advance current knowledge regarding nocturnal cardiovascular control following evening binge alcohol consumption, elucidate the PSG sleep stage specific nocturnal cardiovascular regulation impairment, and offer clinical implications for alcohol abuse mediated cardiovascular disease.
Acute consumption of alcohol is known to alter vagal outflow at the level of the heart [16, 17]. Additionally, recent work has revealed vagal withdrawal during undefined sleep following alcohol consumption across hours of the night [23]. The present study addresses a key gap by utilizing PSG to examine cardiovagal function during each sleep stage after evening binge alcohol consumption. Our findings demonstrate that the HF component and time domain indices of HRV are reduced in light non-REM (NREM) sleep (i.e. N2 sleep), SWS, and REM. Vagal indices of the HF component of HRV and time-domain HRV progressively increase from sleep onset and peak during SWS. Vagal HRV is reduced to near wake levels during REM sleep [31, 32]. This typical pattern is disrupted following evening binge alcohol consumption, indicative of poor vagal activation. This is relevant due to the cardiovascular implications for each sleep stage, where increased vagal activation at the heart is an accepted mechanism which contributes to blood pressure dipping in NREM sleep [33]. Chronic use of alcohol, especially at binge consumption levels, can create added stress via blunted blood pressure dipping on the vasculature and contribute to cardiovascular disease pathogenesis. Importantly, the indices of HRV highlighted in the present study all demonstrate high intraindividual reliability and repeatability (i.e. HF component and time domain analyses of HRV) [34]. Several studies have provided evidence against the use of the low frequency (LF) component of HRV as a measure of sympathetic activation due to the poor reliability across participants [35–37]. Even so, recent publications continue to utilize the approach to directly relate LF and LF/HF ratio to sympathetic activation [38], despite clear evidence that these HRV assessments quantify sympathetic and parasympathetic innervation of the heart [39]. As such, we do not report any LF components of HRV for these methodological reasons. However, we reasonably infer that binge alcohol consumption likely augments sympathetic activity during sleep due to the robust activation of the SNS quantified by muscle sympathetic nerve activity (MSNA) following acute alcohol consumption [8], and more importantly recent evidence of an augmented sympathoexcitation to stimuli the morning-after an evening of binge alcohol consumption [9].
The cardiovagal baroreflex is essential for homeostatic beat-to-beat blood pressure regulation at the level of the heart via either vagal activation or withdrawal following baroreflex loading or unloading, respectively. Impaired cvBRS function has multiple implications in diseased populations including heart disease and renal failure [40, 41]. During sleep in healthy adults, cardiovagal baroreflex control is remarkably consistent throughout the night [42], even increasing slightly throughout the progression of sleep stages of light NREM sleep, SWS, and REM sleep [43, 44]. A recent assessment of cardiovagal control throughout the night following low-to-moderate alcohol consumption observed a dose dependent reduction in cvBRS across hours of the night when compared to placebo conditions [23]. The present findings advance these results by documenting similar reduced cvBRS function during PSG sleep specific stages after evening binge alcohol consumption when compared to fluid control. When taken in combination with the findings of de Zambotti et al. [23], our results objectively demonstrate the detrimental effects of evening binge alcohol consumption on cardiovascular regulatory processes, offering mechanistic insights for the observed long-term impact of alcohol use and cardiovascular morbidities [6, 7].
Interestingly, we observed a significantly reduced cvBRS during REM sleep, a critical sleep stage when blood pressure oscillations and absolute nocturnal blood pressure levels increase, and when sympathetic activity is at its highest nocturnal levels [24, 43]. Importantly, the increased cvBRS previously observed in REM (without alcohol consumption) only appears to occur in response to baroreflex loading (i.e. up−up sequence) when blood pressure levels increase [43, 44]. This indicates that enhanced cvBRS function in REM sleep acts primarily to offset any aberrant positive fluctuations in blood pressure at night. Our present findings indicate that up-up cvBRS function during REM sleep is reduced after evening binge alcohol consumption, limiting its buffering effectiveness during this time. These results are similarly reflected in recent work by de Zambotti et al. who observed a significantly reduced up-up cvBRS during the second half of the night that is often dominated by REM [23]. These parallel findings hold significant importance regarding regulation of the circadian blood pressure surge which occurs early in the morning, beginning in primarily REM dominant sleep. Without adequate cvBRS buffering in response to baroreflex loading, the morning blood pressure surge may go partly unregulated, subsequently elevating susceptibility to early morning cardiac events [25].
Our sample consisted of healthy adults that had participated in at least one binge drinking episode in the prior 6 months, and our HRV and cvBRS sampling occurred during stable 10-minute periods of uninterrupted sleep (i.e. absent arousals, apneas, limb movements, etc.). While this adds rigor to our analyses and eliminates the confounding influence of artifact, it limits the generalizability of our findings in diseased populations, such as those with OSA in whom frequent arousals and apneas occur, particularly in REM sleep. However, we have previously reported that overnight alcohol consumption augments blood pressure “overshoot” during phase IV of the Valsalva’s maneuver the morning after binge alcohol consumption [9]. Similar to the current study, cvBRS during Valsalva’s maneuver was diminished in parallel with the heightened blood pressure response, indicating at least a partial role for vagal dysfunction in the exaggerated pressor activity the morning-after an evening of binge alcohol consumption [9]. The detriments of cvBRS during REM and during the early morning hours after wake offer potential mechanisms contributing to increased cardiovascular risk during early morning hours (i.e. 6 am and 12 pm) [25]. The phase IV blood pressure “overshoot” increase during the Valsalva’s maneuver directly parallels the blood pressure response in recovery following an apnea during sleep. Individuals with OSA have been reported to have an exacerbated blood pressure responsiveness to voluntary wake apneas [45], as well as substantial pressor responses to nocturnal apneas, particularly during REM sleep [46]. Indeed, a recent meta-analysis observed a significant impact of alcohol consumption on increased OSA symptom severity [47, 48]. The current findings indicate that in addition to increased OSA severity [47, 48], binge alcohol consumption prior to sleep in OSA patients may be especially harmful through facilitation of further exaggerated cardiovascular responses to apneas due to dampened cvBRS buffering of blood pressure increases. It is important to note that our results are taken from stable portions of sleep. Given the detriments of HF HRV and cvBRS were present during “quiet” periods of sleep, it can be reasonably inferred that the cardiovascular response to arousal stimuli and apneic events following evening binge alcohol consumption are likely further exacerbated.
The consumption of alcohol as a sleep aid and/or as an attempt for self-medication paves a dangerous path for further health complications. As previously highlighted, alcohol consumption prior to bedtime can indeed decrease sleep latency [13], and the individual may subjectively report a paradoxical improvement in sleep quality. However, there is growing support for the concept of this action being a detrimental feed forward mechanism to contribute to future AUD or alcohol abuse [4, 5]. Roehrs and Roth [4] experimentally determined this relationship by administration of an alcohol dose prior to bedtime (i.e. 0.6 g/kg), which increased total sleep time and SWS by the second day in a group of insomniacs, but these effects were abolished by the sixth day of the protocol. This speaks to the vicious cycle of excessive alcohol consumption, where a tolerance can develop to the same dose of alcohol, and the dose needs to be increased to achieve a desired effect. This feed-forward mechanism is a hallmark indicator of AUD progression outlined in the DSM-5 screening criteria [49]. In addition, it is known that evening alcohol consumption, especially at binge levels, contributes to altered sleep architecture in the form of delayed and reduced REM sleep [9, 11, 12]. While we acknowledge the cardiovascular surges during REM sleep, there are a variety of positive psychological processes related to emotional memory processing that also occur during this stage of sleep. Principally, the amygdala appears to play a crucial role in emotional memory processing and encoding during REM, along with coactivation of the hippocampus [50–52]. Experimental studies assessing the impact of REM on amygdala reactivity have observed that consolidated REM sleep reduces amygdala reactivity to emotional paradigms [51, 53], indicating a key role for REM in emotional adaption and habituation. It can be inferred that a similar mechanism may be involved where REM is both shortened and fragmented after evening alcohol consumption. A condition that is often comorbid with both sleep disturbances and alcohol abuse is post-traumatic stress disorder (PTSD) [54]. Studies on veterans of the U.S. Armed Forces report that a significant portion cope with PTSD symptomology via alcohol use [55], which can further exacerbate issues relating to depression and anxiety from disturbed REM sleep [56]. This, again, is part of a vicious feed forward cycle with alcohol abuse further contributing to sleep issues and progression of disorders such as anxiety, depression, and PTSD.
Our laboratory has taken a keen interest in autonomic and neural control sex differences for well over a decade [57–63], and while we report a near equal distribution of men to women, more work is warranted. At this point, we are not powered to examine sex differences, but ongoing data collection from the parent randomized, clinical trial (RCT03567434) will allow future studies to not only address the potential role of sex, but also compare across menstrual phase (i.e. early follicular vs. mid-luteal) in young, eumenorrheic women. This is relevant given the recently reported differences between men and women where nocturnal cvBRS was blunted and the morning blood pressure surge was augmented in women compared to men after evening alcohol consumption [23].
In summary, alcohol is a frequently abused substance which many individuals use to self-medicate sleep problems or disturbances. This behavior can contribute to alcohol abuse and alcoholism progression, increasing likelihood of co-morbid cardiovascular disease. Mechanisms for alcohol abuse mediated cardiovascular risk include our previously reported acute and next morning sympathoexcitation [8, 9], and the present study advances the field by demonstrating blunted nocturnal cardiovagal function as another contributing mechanism across specific PSG sleep stages. We observed a significant decrease in both frequency- and time-domain analyses of cardiovagal indices of HRV within N2, SWS, and REM. Additionally, up-up cvBRS was decreased in stage N2 and REM, while down-down cvBRS was decreased in N2, SWS, and REM after evening binge alcohol consumption. These findings offer new mechanistic insight to the relationship between binge drinking, alcohol abuse, and cardiovascular disease risk.
Acknowledgments
The authors would like to thank our previous master’s student, Hannah Cunningham, and our team of undergraduate research assistants including Maggie Blevins, Abigail Botz, Morgan Colling, Alexa Destrampe, Sarah Dix, Bella Nutini, Grant Thivierge, Alex Rondorf, and Jonathon Worden. We would also like to thank Terry Anderson for administrative support, our sleep technician Jennifer Nicevski, and Kelsae Ruppe for dietary consultation.
Funding
This work was supported by the National Institute on Alcohol Abuse and Alcoholism (AA-024892).
Disclosure Statement
None declared.
References
- 1. Centers for Disease Control and Prevention (CDC). Perceived insufficient rest or sleep among adults - United States, 2008. MMWR Morb Mortal Wkly Rep. 2009;58(42):1175–1179. [PubMed] [Google Scholar]
- 2. Brower KJ. Alcohol’s effects on sleep in alcoholics. Alcohol Res Health. 2001;25(2):110–125. [PMC free article] [PubMed] [Google Scholar]
- 3. Chakravorty S, et al. . Alcohol dependence and its relationship with insomnia and other sleep disorders. Alcohol Clin Exp Res. 2016;40(11):2271–2282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Roehrs T, et al. . Insomnia as a path to alcoholism: tolerance development and dose escalation. Sleep. Aug 1 2018;41(8)1–6. doi: 10.1093/sleep/zsy091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Koob GF, et al. . Alcohol use disorder and sleep disturbances: a feed-forward allostatic framework. Neuropsychopharmacology. 2020;45(1):141–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. O’Keefe JH, et al. . Alcohol and cardiovascular health: the razor-sharp double-edged sword. J Am Coll Cardiol. 2007;50(11):1009–1014. [DOI] [PubMed] [Google Scholar]
- 7. Sundell L, et al. . Increased stroke risk is related to a binge-drinking habit. Stroke. 2008;39(12):3179–3184. [DOI] [PubMed] [Google Scholar]
- 8. Carter JR, et al. . Influence of acute alcohol ingestion on sympathetic neural responses to orthostatic stress in humans. Am J Physiol Endocrinol Metab. 2011;300(5):E771–E778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Greenlund IM, et al. . Morning sympathetic activity after evening binge alcohol consumption. Am J Physiol Heart Circ Physiol. 2021;320(1):H305–H315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Roehrs T, et al. . Sleep, sleepiness, and alcohol use. Alcohol Res Health. 2001;25(2):101–109. [PMC free article] [PubMed] [Google Scholar]
- 11. Chan JK, et al. . The acute effects of alcohol on sleep architecture in late adolescence. Alcohol Clin Exp Res. 2013;37(10):1720–1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Feige B, et al. . Effects of alcohol on polysomnographically recorded sleep in healthy subjects. Alcohol Clin Exp Res. 2006;30(9):1527–1537. [DOI] [PubMed] [Google Scholar]
- 13. Ebrahim IO, et al. . Alcohol and sleep I: effects on normal sleep. Alcohol Clin Exp Res. 2013;37(4):539–549. [DOI] [PubMed] [Google Scholar]
- 14. Weise F, et al. . Acute alcohol ingestion reduces heart rate variability. Drug Alcohol Depend. May 1986;17(1):89–91. [DOI] [PubMed] [Google Scholar]
- 15. Spaak J, et al. . Dose-related effects of red wine and alcohol on heart rate variability. Am J Physiol Heart Circ Physiol. 2010;298(6):H2226–H2231. [DOI] [PubMed] [Google Scholar]
- 16. Koskinen P, et al. . Acute alcohol intake decreases short-term heart rate variability in healthy subjects. Clin Sci (Lond). 1994;87(2):225–230. [DOI] [PubMed] [Google Scholar]
- 17. Fazio M, et al. . Mechanics of the carotid artery wall and baroreflex sensitivity after acute ethanol administration in young healthy volunteers. Clin Sci (Lond). 2001;101(3):253–260. [PubMed] [Google Scholar]
- 18. Romanowicz M, et al. . Changes in heart rate variability associated with acute alcohol consumption: current knowledge and implications for practice and research. Alcohol Clin Exp Res. 2011;35(6):1092–1105. [DOI] [PubMed] [Google Scholar]
- 19. Bau PF, et al. . Acute ingestion of alcohol and cardiac autonomic modulation in healthy volunteers. Alcohol. 2011;45(2):123–129. [DOI] [PubMed] [Google Scholar]
- 20. Brunelle C, et al. . Relationship between the cardiac response to acute intoxication and alcohol-induced subjective effects throughout the blood alcohol concentration curve. Hum Psychopharmacol. 2007;22(7):437–443. [DOI] [PubMed] [Google Scholar]
- 21. Sagawa Y, et al. . Alcohol has a dose-related effect on parasympathetic nerve activity during sleep. Alcohol Clin Exp Res. 2011;35(11):2093–2100. [DOI] [PubMed] [Google Scholar]
- 22. Pietilä J, et al. . Acute effect of alcohol intake on cardiovascular autonomic regulation during the first hours of sleep in a large Real-World sample of finnish employees: observational study. JMIR Ment Health. 2018;5(1):e23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. de Zambotti M, et al. . Impact of evening alcohol consumption on nocturnal autonomic and cardiovascular function in adult men and women: a dose-response laboratory investigation. Sleep. 2021;44(1):1–12. doi:10.1093/sleep/zsaa135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Somers VK, et al. . Sympathetic-nerve activity during sleep in normal subjects. N Engl J Med. 1993;328(5):303–307. [DOI] [PubMed] [Google Scholar]
- 25. Kario K. Morning surge in blood pressure and cardiovascular risk: evidence and perspectives. Hypertension. 2010;56(5):765–773. [DOI] [PubMed] [Google Scholar]
- 26. Yokoyama A, et al. . Reliability of a flushing questionnaire and the ethanol patch test in screening for inactive aldehyde dehydrogenase-2 and alcohol-related cancer risk. Cancer Epidemiol Biomarkers Prev. 1997;6(12):1105–1107. [PubMed] [Google Scholar]
- 27. Blaber AP, et al. . Change in phase relationship between SBP and R-R interval during lower body negative pressure. Am J Physiol. 1995;268(4 Pt 2):H1688–H1693. [DOI] [PubMed] [Google Scholar]
- 28. Bertinieri G, et al. . Evaluation of baroreceptor reflex by blood pressure monitoring in unanesthetized cats. Am J Physiol. 1988;254(2 Pt 2):H377–H383. [DOI] [PubMed] [Google Scholar]
- 29. Cooke WH, et al. . Human cerebrovascular and autonomic rhythms during vestibular activation. Am J Physiol Regul Integr Comp Physiol. 2004;286(5):R838–R843. [DOI] [PubMed] [Google Scholar]
- 30. Cooke WH, et al. . Heart rate variability and its association with mortality in prehospital trauma patients. J Trauma. 2006;60(2):363–370; discussion 370. [DOI] [PubMed] [Google Scholar]
- 31. Brandenberger G, et al. . Is slow wave sleep an appropriate recording condition for heart rate variability analysis? Auton Neurosci. 2005;121(1–2):81–6. [DOI] [PubMed] [Google Scholar]
- 32. Trinder J, et al. . Autonomic activity during human sleep as a function of time and sleep stage. J Sleep Res. 2001;10(4):253–264. [DOI] [PubMed] [Google Scholar]
- 33. Kuo TB, et al. . The role of autonomic and baroreceptor reflex control in blood pressure dipping and nondipping in rats. J Hypertens. 2014;32(4):806–816. [DOI] [PubMed] [Google Scholar]
- 34. Dodds KL, et al. . Heart rate variability in insomnia patients: A critical review of the literature. Sleep Med Rev. 2017;33:88–100. [DOI] [PubMed] [Google Scholar]
- 35. Billman GE. Heart rate variability - a historical perspective. Front Physiol. 2011;2:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Eckberg DL. Sympathovagal balance: a critical appraisal. Circulation. Nov 4 1997;96(9):3224–32. [DOI] [PubMed] [Google Scholar]
- 37. Lord SW, et al. . Low-frequency heart rate variability: reproducibility in cardiac transplant recipients and normal subjects. Clin Sci (Lond). 2001;100(1):43–46. [PubMed] [Google Scholar]
- 38. Qin H, et al. . Heart Rate Variability during Wakefulness as a Marker of Obstructive Sleep Apnea Severity. Sleep. 2021; 44(5). doi: 10.1093/sleep/zsab018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Koh J, et al. . Human autonomic rhythms: vagal cardiac mechanisms in tetraplegic subjects. J Physiol. 1994;474(3):483–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Eckberg DL, et al. . Defective cardiac parasympathetic control in patients with heart disease. N Engl J Med. 1971;285(16):877–883. [DOI] [PubMed] [Google Scholar]
- 41. Johansson M, et al. . Baroreflex effectiveness index and baroreflex sensitivity predict all-cause mortality and sudden death in hypertensive patients with chronic renal failure. J Hypertens. 2007;25(1):163–168. [DOI] [PubMed] [Google Scholar]
- 42. Nakazato T, et al. . Nocturnal variation in human sympathetic baroreflex sensitivity. J Auton Nerv Syst. 1998;70(1–2):32–7. [DOI] [PubMed] [Google Scholar]
- 43. Iellamo F, et al. . Baroreflex buffering of sympathetic activation during sleep: evidence from autonomic assessment of sleep macroarchitecture and microarchitecture. Hypertension. 2004;43(4):814–819. [DOI] [PubMed] [Google Scholar]
- 44. Legramante JM, et al. . Sleep-related changes in baroreflex sensitivity and cardiovascular autonomic modulation. J Hypertens. 2003;21(8):1555–1561. [DOI] [PubMed] [Google Scholar]
- 45. Jouett NP, et al. . Systolic pressure response to voluntary apnea predicts sympathetic tone in obstructive sleep apnea as a clinically useful index. Auton Neurosci. 2016;194:38–45. [DOI] [PubMed] [Google Scholar]
- 46. Somers VK, et al. . Sympathetic neural mechanisms in obstructive sleep apnea. J Clin Invest. 1995;96(4):1897–1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Burgos-Sanchez C, et al. . Impact of Alcohol Consumption on Snoring and Sleep Apnea: A Systematic Review and Meta-analysis. Otolaryngol Head Neck Surg. 2020;163(6):1078–1086. [DOI] [PubMed] [Google Scholar]
- 48. Scanlan MF, et al. . Effect of moderate alcohol upon obstructive sleep apnoea. Eur Respir J. 2000;16(5):909–913. [DOI] [PubMed] [Google Scholar]
- 49. Grant BF, et al. . Epidemiology of DSM-5 Alcohol Use Disorder: Results From the National Epidemiologic Survey on Alcohol and Related Conditions III. JAMA Psychiatry. 2015;72(8):757–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. McGaugh JL. The amygdala modulates the consolidation of memories of emotionally arousing experiences. Annu Rev Neurosci. 2004;27:1–28. [DOI] [PubMed] [Google Scholar]
- 51. van der Helm E, et al. . REM sleep depotentiates amygdala activity to previous emotional experiences. Curr Biol. 2011;21(23):2029–2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Walker MP, et al. . Overnight therapy? The role of sleep in emotional brain processing. Psychol Bull. 2009;135(5):731–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Wassing R, et al. . Restless REM sleep impedes overnight amygdala adaptation. Curr Biol. 2019;29(14):2351–2358.e4. [DOI] [PubMed] [Google Scholar]
- 54. Lande RG. Troublesome triad: trauma, insomnia, and alcohol. J Addict Dis. 2012;31(4):376–381. [DOI] [PubMed] [Google Scholar]
- 55. Norman SB, et al. . The burden of co-occurring alcohol use disorder and PTSD in U.S. Military veterans: comorbidities, functioning, and suicidality. Psychol Addict Behav. 2018;32(2):224–229. [DOI] [PubMed] [Google Scholar]
- 56. Wassing R, et al. . Slow dissolving of emotional distress contributes to hyperarousal. Proc Natl Acad Sci U S A. 2016;113(9):2538–2543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Carter JR, et al. . Sympathetic neural responsiveness to sleep deprivation in older adults: sex differences. Am J Physiol Heart Circ Physiol. 2019;317(2):H315–H322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Carter JR, et al. . Ovarian cycle and sympathoexcitation in premenopausal women. Hypertension. Feb 2013;61(2):395–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Carter JR, et al. . Effects of the menstrual cycle on sympathetic neural responses to mental stress in humans. J Physiol. 2007;585(Pt 2):635–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Carter JR, et al. . Sympathetic neural responses to mental stress: responders, nonresponders and sex differences. Am J Physiol Heart Circ Physiol. 2009;296(3):H847–H853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Daugherty SL, et al. . Cardiovascular disease in women across the lifespan: the importance of sleep. J Womens Health (Larchmt). 2020;29(3):452–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Greenlund IM, et al. . Sex differences in blood pressure responsiveness to spontaneous K-complexes during stage II sleep. J Appl Physiol (1985). Feb 1 2021;130(2):491–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Carter JR, et al. . Sympathetic neural responses to 24-hour sleep deprivation in humans: sex differences. Am J Physiol Heart Circ Physiol. 2012;302(10):H1991–H1997. [DOI] [PMC free article] [PubMed] [Google Scholar]