Vexas syndrome (vacuoles, E1 enzyme, X-linked, autoinflammatory, somatic) is a recently identified, treatment-refractory, inflammatory syndrome developing in late adulthood with fevers, cytopenias, characteristic vacuoles in myeloid and erythroid precursor cells, dysplastic bone marrow, neutrophilic cutaneous and pulmonary inflammation, chondritis, vasculitis, and an often fatal outcome.1,2 Effective treatments have not yet been established. Here, we report genetic, morphologic, and clinical remissions in 2 of 3 VEXAS syndrome patients treated with the hypomethylating agent azacytidine.
Following the initial report by Beck et al,1 we identified 6 cases at our institute with a clinical phenotype compatible with VEXAS syndrome. The presence of UBA1 mutations in the majority of blood cells was confirmed in all patients and we detected concomitant mutations in the DNA (cytosine-5)-methyltransferase 3A (DNMT3A) gene in 3/6 patients with relatively high VAFs (30%–59%). UBA1 mutation detection and panel-based sequencing is described in detail in Supplemental Digital Table 1, http://links.lww.com/HS/A211.
Three patients were treated with the hypomethylating agent azacytidine, among which two patients with a concomitant DNMT3A mutation, and these patients are described in detail in this report (Table 1). Azacytidine was administered at a dose of 75 mg/m2 s.c. QD for 7 days in a 4-week schedule, as outlined in the case descriptions. The DNMT3A variants found in 2 patients involving the R882 and R688 residues are well known in the context of age-related clonal hematopoiesis (ARCH),3,4 and DNMT3A R882H and R688C are established as pathogenic variants in hematologic malignancies (respectively, COSV53036153 and COSV99258673). The third patient had a mutation in TET2 in a small subset (VAF 4%) of hematopoietic cells, as can be observed in the context of ARCH.4
Table 1.
Clinical Characteristics of Three Cases of VEXAS Syndrome Treated With Azacytidine.
| Case 1 | Case 2 | Case 3 | |
|---|---|---|---|
| Mutation UBA1a[first presentation] | c.122T>Cb (VAF 86%) p.Met41Thr |
c.121A>Gc (VAF 56%) p.Met41Val |
c.122T>Cc (VAF 86%) p.Met41Thr |
| Co-occurring mutations |
DNMT3Ad c.2062C> T(VAF 59%)f Arg688Cys |
DNMT3Ad c.2645G>A (VAF 30%) Arg882His |
TET2e c.2290dup (VAF4%) p.(Gln764Profs*5) |
| Year of first symptoms | 2009 | 2018 | 2011 |
| Follow-up | Alive | Alive | Alive |
| Age at presentation (year) | 61 | 77 | 67 |
| Fever | + | + | + |
| Sweet syndrome | + | − | + |
| Cutaneous small-vessel vasculitis | + | + | + |
| Chondritis | + | − | + |
| Pulmonary infiltrates | − | + | − |
| Venous thromboembolism | − | − | − |
| Scleritis | + | − | + |
| Periorbital inflammation | − | + | − |
| Abducens paralysis | − | − | − |
| Axonal polyneuropathy | − | + | + |
| Macrocytic anemia | + | + | + |
| Thrombocytopenia | + | − | + |
| Leukopenia | − | − | + |
| C-reactive protein (mg/L)g median (range) | 189 (62–183) |
160 (58–221) |
150 (182–343) |
| ESR (mm/h) median (range) |
119 (98–150) |
115 (49–150) |
109 (50–119) |
| Bone marrow vacuoles | |||
| Erythropoiesis | + | − | + |
| Granulopoiesis | + | + | + |
| Bone marrow dysplasia, range dysplasia score | |||
| Erythropoiesis | <10%−39% | 0%−3% | 10%−26% |
| Granulopoiesis | 2%−14% | 0%−12% | 4%−19% |
| Megakaryopoiesis | 0%−47% | 0%−13% | 22%−23% |
| Bone marrow blast percentage | <2% | <2% | <5% |
| Bone marrow cytogenetics | 46XY | 46XY | 46XY |
| Response to corticosteroids | ± | ± | ± |
| Response to colchicin | − | N/A | N/A |
| Response to DMARDs | − | − | − |
| Response to anti-TNFα | N/A | − | N/A |
| Response to IVIG | − | N/A | N/A |
| Response to ESA | − | N/A | − |
| Response to azacytidine | + | + | − |
aNM_003334.3 (UBA1).
bBone marrow.
cPeripheral blood.
dNM_022552.4 (DNMT3A).
eNM_001127208.2 (TET2).
fSince the DNA for the detection of this DNMT3A variant at diagnosis was of limited quantity and quality no conclusion other than that the majority of cells carry a DNMT3A mutation can be drawn.
gValues at flares of disease to avoid bias by treatment or intercurrent infections.
DMARDs = disease modifying antirheumatic drugs: azathioprine, mycophenolate, methotrexate; ESA = erythroid stimulating agent; ESR = erythrocyte sedimentation rate; IVIG = intravenous immunoglobulins; N/A = not applicable.
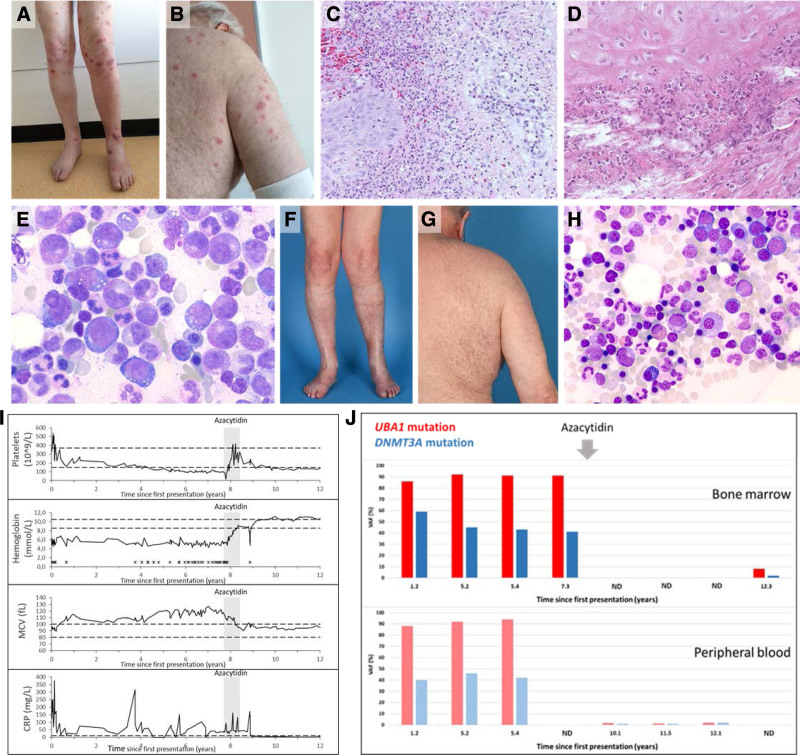
Case 1 is a male patient with a previous unnoticeable medical history who presented with a clinical phenotype that was characterized over time by fever, Sweet syndrome, cutaneous small-vessel vasculitis, relapsing polychondritis located in both ears and the nose, and scleritis (Table 1 and Figure 1A–D). Laboratory examination demonstrated an elevation of inflammatory parameters and a transfusion dependent macrocytic anemia with thrombocytopenia (Figure 1I). Bone marrow examination demonstrated hypercellularity, erythroid macrocytosis and a pronounced vacuolization in erytropoiesis and myelopoiesis in the context of mild dysplasia (Figure 1E). Dysplasia scores varied in subsequent aspirates over time (Table 1), while the pronounced vacuolization with toxic granulation of myeloid cells was a consistent finding in multiple bone marrow examinations. No ringsideroblasts were noted and there was no increase in blast frequency. No cytogenetic abnormalities were detected upon repeated testing.
Figure 1.
Clinical course and response to azacytidine in VEXAS syndrome (case 1). Skin lesions on extremities and trunk (A and B) and histopathological findings of a leg lesion (C) were consistent with a diagnosis of neutrophilic dermatosis or Sweet syndrome. A clinical picture of relapsing polychondritis corresponded with histopathological findings in an auricular biopsy (D). Bone marrow aspirate showed characteristic vacuolisation of erythroid and myeloid precursor cells (E). Skin lesions disappeared (F and G) and bone marrow cytology normalized (H) after azacytidin treatment (8 cycles). Hematoxylin and eosin staining was used in (C) and (D), and May-Grünwald Giemsa staining in (E) and (H) (magnification 10 × 100 and 10 × 63, respectively). (I) Laboratory parameters from start of first symptoms until last follow-up are shown. During and after azacytidine treatment (indicated by shaded area), laboratory parameters (platelet count, hemoglobin, MCV, and CRP levels) normalized. Horizontal dashed black lines in each graph represent lower and upper limits of the normal range for each variable. X: red blood cell transfusion. (J) Variant allele frequencies (VAFs) of UBA1 and DNMT3A mutations in bone marrow (BM) and peripheral blood (PB) from start of first presentation (years) during and after azacytidine treatment demonstrating near complete eradication of the UBA1-DNMT3A mutated clone after azacytidine treatment. ND = no data.
The clinical course was characterized by a remitting relapsing pattern under prednisone maintenance (15 mg/d) with partial, temporary, responses to increasing doses of corticosteroids (10–60 mg) during exacerbation of clinical symptoms, with gradual overall worsening of the clinical condition to a WHO performance status of 4 in 2016 with wheel-chair dependency (due to fatigue) and persisting transfusion-dependency (2–4 units of red blood cells/month) throughout the years. The clinical picture was unresponsive to disease modifying antirheumatic drugs (DMARDS), such as azathioprine (150 mg/d), mycophenolic acid (360 mg twice daily), intravenous immunoglobulins (1 g/kg, every 4 wks for a total of 6 administrations), and erythroid-stimulating agents (darbopoietin 300 µg/wk, by subcutaneous injections).
A variant in the DNA methylase domain of the DNMT3A gene (NM_022552.4(DNMT3A):c.2062C>T; p.(Arg688Cys)) was detected at VAFs corresponding to a heterozygous mutation in virtually all mononuclear cells in the bone marrow that, in retrospect, co-occurred with an UBA1 (NM_003334.3(UBA1):c.122T>C; p.(Met41Thr)) mutation in all available earlier bone marrow and blood samples (Figure 1J). Based on the presence of predominantly clonal hematopoiesis, a provisional diagnosis of “clonal hematopoiesis-associated inflammatory syndrome” was made and the lack of other options in this severely affected, refractory patient, prompted us to start treatment with azacytidine, for which anecdotal responses in MDS with inflammatory symptoms had been reported.5
The patient received azacytidine (75 mg/m2 s.c. QD for 7 d in a 4-week schedule) from August 2016 for a total of 8 courses until April 2017. Administration of azacytidine was associated with rapid and complete disappearance of disease related inflammatory symptoms (polychondritis and Sweet syndrome), loss of transfusion-dependency with normalization of blood values and inflammatory parameters and eradication of the UBA1-DNMT3A mutated clone (VAF 92% (mutant UBA1) and 41% (mutant DNMT3A) to 1% (mutant UBA1) and 1% (mutant DNMT3A)) (Figure 1F–J). Improvement of blood values was already apparent after 2 cycles with disappearance of transfusion dependency. The azacytidine was stopped because of remitting diverticulitis and a diagnosis of local colorectal adenocarcinoma for which a laparoscopic subtotal colectomy was performed in September 2017. The patient has not received azacytidine, or any other disease modulating drug, since, and corticosteroid exposure was weaned.
The clinical course since 2017 is characterized by a persistent complete long-term remission without any VEXAS-related disease symptoms (or any other pathology), an ongoing WHO performance status of 0, normal blood values and continued suppression of the mutant UBA1 clone in blood and bone marrow for now 4.5 years after the last azacytidin administration (Figure 1).
Case 2 is a male patient who presented with a clinical picture of recurrent episodes of fever, leukocytoclastic vasculitis, periorbital inflammation, relapsing polychondritis located in both ears, lung infiltrates, axonal polyneuropathy of the feet, and macrocytic anemia. Laboratory examination demonstrated recurrent elevation of inflammatory parameters during episodes of exacerbation. Bone marrow examination in March 2021 demonstrated normocellularity without signs of dysplasia but pronounced vacuolization of myelopoiesis (Supplemental Digital Figure 1A, http://links.lww.com/HS/A211). No cytogenetic abnormalities were detected upon repeated testing. The diagnosis VEXAS syndrome was confirmed by the demonstration of the UBA1 mutation in the bone marrow (c.121A>G; p.Met41Val; VAF 56%) as well as a mutation in the DNMT3A gene (c.2645G>A, Arg882His; VAF 30%). The similar VAFs strongly suggest the co-existence of the UBA1 and DNMT3A mutations in approximately 60% of all bone marrow cells (of note, the UBA1 gene is X-linked resulting in VAFs twice as high as those of DNMT3A). The patient did not fulfill diagnostic WHO 2016 criteria for MDS.
The disease was refractory to azathioprine (150 mg once daily) and anakinra (100 mg once daily) and high-dose corticosteroids (15–40 mg/d) were required to maintain control of inflammation, which he poorly tolerated. Azacytidine (75 mg/m2 s.c. QD for 7 d in a 4-week schedule) was started on March 22, 2021 (1.3 y after first presentation) for a total of 3 courses until June 2021. Response evaluation after 3 cycles of azacytidine demonstrated near complete eradication of the UBA1-DNMT3A mutated clone (VAF 52% [mutant UBA1] and 28% [mutant DNMT3A] to <1% [mutant UBA1] and 2% [mutant DNMT3A] with normalization of bone marrow abnormalities [Supplemental Digital Figure 1A and B, http://links.lww.com/HS/A211]). The clinical course was characterized by gradual improvement of periocular complaints without new episodes of systemic inflammation under successful tapering of prednisone dose to 7.5 mg once daily currently.
Case 3 is a male with polychondritis, Sweet syndrome, vasculitis, axonal neuropathy, and pancytopenia. The clinical syndrome was unresponsive to mycophenolic acid (360 mg twice daily) and erythroid-stimulating agents and displayed a relapse/remitting pattern under corticosteroid maintenance treatment (7.5–60 mg once daily). Bone marrow examination (February 2021) demonstrated hypercellularity with pronounced vacuolisation of erythropoiesis and myelopoiesis and dysplasia >10% of the erythroid and megakaryocytic lineages. No cytogenetic abnormalities were detected upon repeated testing. The diagnosis VEXAS syndrome was made by the demonstration of the UBA1 mutation in the bone marrow (c.122T>C, p.Met41Thr; VAF 86% (at the first presentation [Table 1]) in the bone marrow. In addition, a mutation in TET2 (c.2290dup, p.(Gln764Profs*5); VAF4%) was detected. This low-level TET2 mutation could either be part of the UBA1 mutant cells or the result of ARCH. There were no variants detected in the DNMT3A gene in this VEXAS patient.
Azacytidine (75 mg/m2 s.c. QD for 7 d in a 4-week schedule) was started in March 2021 for a total of 3 courses until May 2021. Azacytidine was discontinued after 3 cycles because of pulmonary pathology (interstitial pneumonitis, possibly related to azacytidine), which resolved. Response assessment after 3 cycles did not show reduction in disease burden (VAF UBA1 45% pretreatment and 43% post treatment) with persistent, unaltered, bone marrow abnormalities (vacuolization) (data not shown). The clinical course was characterized by persistent anemia and fatigue.
These cases demonstrate common co-occurrence of mutant UBA1 with mutations in DNMT3A, substantiating the finding of a DNMT3A mutation in 2 of 9 tested VEXAS patients in the original description of the syndrome.1
The etiology and pathogenetic significance of DNMT3A mutations in VEXAS syndrome remains to be elucidated. Both DNMT3A and UBA1 occurred at high VAFs in both patients, indicating that the vast majority of hematopoietic cells carried these mutations and that they therefore likely co-occurred in the same cell (in at least a fraction of cells). Loss-of-function mutations in DNMT3A may be hypothesized to contribute to the proinflammatory pathology in VEXAS syndrome since DNMT3A is thought to function as a repressor of inflammation6 and deficiency in DNMT3A has been associated with activation of innate immune inflammatory signaling in myeloid cells.7–9 We could not assess the clonal hierarchy because VAFs for UBA1 and DNMT3A were approximately equally high for both mutations. It is, however, conceivable that mutations in DNMT3A provide a competitive advantage to UBA1 mutated cells because they confer “resistance” to apoptosis caused by inflammatory stress in hematopoietic progenitor cells.10 Acquisition of a DNMT3A mutation may therefore provide UBA1 mutated cells with a competitive survival advantage in the VEXAS inflammatory micromilieu in the bone marrow.
Another interesting aspect of the reported cases is the distinction between VEXAS syndrome and MDS. Formally, the 3 cases described herein would have met the WHO2016 classification criteria for the diagnosis of MDS11 at some, but, importantly, not all moments of evaluation in their disease course. This is illustrated by case 2, in whom pancytopenia and myelodysplasia in 10%–15% of hematopoietic cells in two lineages was observed at a time of severe systemic inflammatory exacerbation, but no longer later in the course of disease when acute inflammatory symptoms had weaned. This illustrates that severe systemic inflammation caused by VEXAS syndrome may cause myelodysplasia and cytopenia which may lead to a presumptive diagnosis of MDS, but this diagnosis requires exclusion of non-malignant causes of dysplasia and, in hindsight, a clinical and genetic diagnosis of VEXAS syndrome could be made in these patients.
Both patients with VEXAS syndrome associated with DNMT3A mutations displayed a genetic and clinical response to azacytidine, while another patient, not carrying concurrent DMNT3A mutations, was unresponsive to the drug. Future clinical trialing should elucidate whether response to azacytidine is indeed associated with the DNMT3A mutational status. Interestingly, DNMT3A mutations in MDS are not uniformly related to favorable responses to hypomethylating agents,12 but recently it was shown that DNMT3AR882 mutations may sensitize hematopoietic cells to azacytidine.13
Our findings bring further the interesting observations in a recent retrospective survey of treatment modalities in VEXAS syndrome in which treatment of azacytidine in four patients was associated with a time-to next line of treatment of 39.7 months in one patient, suggesting responsiveness to the drug in a subset of VEXAS syndrome patients.14
Collectively, the data prompt the consideration of treatment with azacytidine on a trial basis in severe cases of VEXAS syndrome, in particular when associated with DNMT3A mutations. Future clinical trials will be pivotal to investigate the exact value of azacytidine in subsets of patients with VEXAS syndrome.
Acknowledgments
The authors would like to acknowledge Dr Kirsten Gussinklo (Department of Hematology) and Dr King Lam (Department of Pathology) for cytologic and histologic evaluation and providing images presented in this article.
Disclosures
The authors have no conflicts of interest to disclose.
Supplementary Material
Footnotes
Supplemental digital content is available for this article.
References
- 1.Beck DB, Ferrada MA, Sikora KA, et al. Somatic mutations in UBA1 and severe adult-onset autoinflammatory disease. N Engl J Med. 2020;383:2628–2638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grayson PC, Patel BA, Young NS. VEXAS syndrome. Blood. 2021;137:3591–3594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Genovese G, Kähler AK, Handsaker RE, et al. Clonal hematopoiesis and blood-cancer risk inferred from blood DNA sequence. N Engl J Med. 2014;371:2477–2487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jaiswal S, Fontanillas P, Flannick J, et al. Age-related clonal hematopoiesis associated with adverse outcomes. N Engl J Med. 2014;371:2488–2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fraison JB, Mekinian A, Grignano E, et al. Efficacy of Azacitidine in autoimmune and inflammatory disorders associated with myelodysplastic syndromes and chronic myelomonocytic leukemia. Leuk Res. 2016;43:13–17. [DOI] [PubMed] [Google Scholar]
- 6.Leoni C, Montagner S, Rinaldi A, et al. Dnmt3a restrains mast cell inflammatory responses. Proc Natl Acad Sci U S A. 2017;114:E1490–E1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abplanalp WT, Cremer S, John D, et al. Clonal hematopoiesis-driver DNMT3A mutations alter immune cells in heart failure. Circ Res. 2021;128:216–228. [DOI] [PubMed] [Google Scholar]
- 8.Li X, Zhang Q, Ding Y, et al. Methyltransferase Dnmt3a upregulates HDAC9 to deacetylate the kinase TBK1 for activation of antiviral innate immunity. Nat Immunol. 2016;17:806–815. [DOI] [PubMed] [Google Scholar]
- 9.Lim JY, Duttke SH, Baker TS, et al. DNMT3A haploinsufficiency causes dichotomous DNA methylation defects at enhancers in mature human immune cells. J Exp Med. 2021;218:e20202733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hormaechea-Agulla D, Matatall KA, Le DT, et al. Chronic infection drives Dnmt3a-loss-of-function clonal hematopoiesis via IFNγ signaling. Cell Stem Cell. 2021;28:1428–1442.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arber DA, Orazi A, Hasserjian R, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127:2391–2405. [DOI] [PubMed] [Google Scholar]
- 12.Falconi G, Fabiani E, Piciocchi A, et al. Somatic mutations as markers of outcome after azacitidine and allogeneic stem cell transplantation in higher-risk myelodysplastic syndromes. Leukemia. 2019;33:785–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Scheller M, Ludwig AK, Göllner S, et al. Hotspot DNMT3A mutations in clonal hematopoiesis and acute myeloid leukemia sensitize cells to azacytidine via viral mimicry response. Nat Cancer. 2021;2:527–544. [DOI] [PubMed] [Google Scholar]
- 14.Bourbon E, Heiblig M, Gerfaud Valentin M, et al. Therapeutic options in VEXAS syndrome: insights from a retrospective series. Blood. 2021;137:3682–3684. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.