Abstract
Background
Coronavirus disease 2019 (COVID-19) pandemic continues to escalate intensively worldwide. Massive studies on general populations with SARS-CoV-2 infection have revealed that pre-existing comorbidities were a major risk factor for the poor prognosis of COVID-19. Notably, 49–75% of COVID-19 patients had no comorbidities, but this cohort would also progress to severe COVID-19 or even death. However, risk factors contributing to disease progression and death in patients without chronic comorbidities are largely unknown; thus, specific clinical interventions for those patients are challenging.
Methods
A multicenter, retrospective study based on 4806 COVID-19 patients without chronic comorbidities was performed to identify potential risk factors contributing to COVID-19 progression and death using LASSO and a stepwise logistic regression model.
Results
Among 4806 patients without pre-existing comorbidities, the proportions with severe progression and mortality were 34.29% and 2.10%, respectively. The median age was 47.00 years [interquartile range, 36.00–56.00], and 2162 (44.99%) were men. Among 51 clinical parameters on admission, age ≥ 47, oxygen saturation < 95%, increased lactate dehydrogenase, neutrophil count, direct bilirubin, creatine phosphokinase, blood urea nitrogen levels, dyspnea, increased blood glucose and prothrombin time levels were associated with COVID-19 mortality in the entire cohort. Of the 3647 patients diagnosed with non-severe COVID-19 on admission, 489(13.41%) progressed to severe disease. The risk factors associated with COVID-19 progression from non-severe to severe illness were increased procalcitonin levels, SpO2 < 95%, age ≥ 47, increased LDH, activated partial thromboplastin time levels, decreased high-density lipoprotein cholesterol levels, dyspnea and increased D-dimer levels.
Conclusions
COVID-19 patients without pre-existing chronic comorbidities have specific traits and disease patterns. COVID-19 accompanied by severe bacterial infections, as indicated by increased procalcitonin levels, was highly associated with disease progression from non-severe to severe. Aging, impaired respiratory function, coagulation dysfunction, tissue injury, and lipid metabolism dysregulation were also associated with disease progression. Once factors for multi-organ damage were elevated and glucose increased at admission, these findings indicated a higher risk for mortality. This study provides information that helps to predict COVID-19 prognosis specifically in patients without chronic comorbidities.
Abbreviations: COVID-19, coronavirus disease 2019; SARS-COV-2, severe acute respiratory syndrome coronavirus 2; CT, chest tomography; RT-PCR, reverse transcription-polymerase chain reaction; WHO, World Health Organization; PaO2, arterial partial pressure of oxygen; FiO2, inhaled oxygen concentration; ICU, intensive care unit; ARDS, acute respiratory distress syndrome; cTnI, cardiac troponin I; cTnT, cardiac troponin T; hs-cTnI, high sensitivity cardiac troponin I; hs-cTnT, high-sensitivity troponin T; ULN, upper limit of normal; ALT, alanine transaminase; ALP, alkaline phosphatase; LLN, lower limit of normal; IQR, interquartile range; LASSO, least absolute shrinkage and selection operator; OR, odds ratio; CI, confidence intervals; SpO2, oxygen saturation; ECMO, extracorporeal membrane oxygenation; LDH, lactate dehydrogenase; CK, creatine phosphokinase; BUN, blood urea nitrogen; PT, prothrombin time; APTT, activated partial thromboplastin time; HDL-c, high-density lipoprotein cholesterol; IL-6, interleukin-6; HDLs, high-density lipoproteins; SAA, serum amyloid A
Keywords: COVID-19, Risk factors, Severity, Mortality, Without comorbidities
Introduction
Coronavirus disease 2019 (COVID-19) is spreading rapidly worldwide, and severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has caused more than 183 million infections and over 3.9 million deaths by 5 July 2021 [1]. Despite the successful development and application of vaccines [2,3], their supply is not adequate to meet the tremendous demand. Controlling COVID-19 progression and death is still a major challenge. Massive studies from our team and others on general populations with SARS-CoV-2 infection have revealed that pre-existing comorbidities are the major risk factors for the poor prognosis of COVID-19. These comorbidities mainly include hypertension, diabetes, cardiovascular diseases, cancer, etc [[4], [5], [6]]. Patients with well-controlled comorbidities have a lower risk of COVID-19 death than those with poorly controlled conditions [7,8]. Notably, 49–75% of COVID-19 patients do not have comorbidities [[9], [10], [11]]. Nevertheless, this cohort can also progress to severe condition or even death. However, the risk factors of COVID-19 patients without pre-existing comorbidities are largely unknown; thus, specific clinical interventions for those patients are challenging.
Here, to identify potential risk factors contributing to COVID-19 progression and death, we performed a multicenter, retrospective study based on 4806 COVID-19 patients without comorbidities collected from 21 hospitals in Hubei, China. These findings provide guiding information for clinical decision-making that could improve COVID-19 prognosis and reduce disease-related death.
Methods
Study design and participants
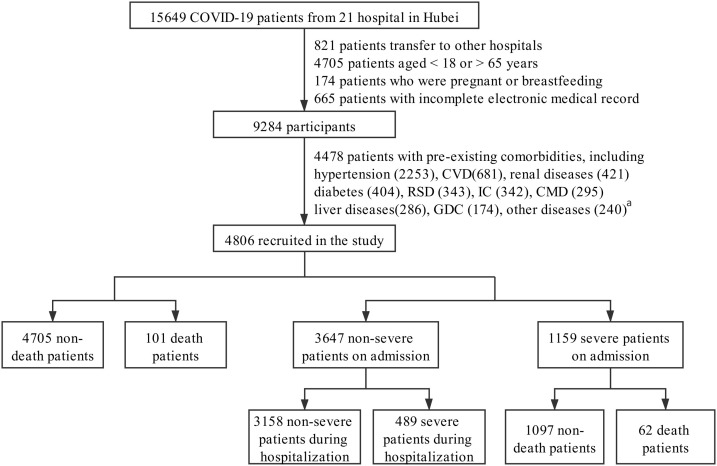
This multicenter, retrospective study was conducted in patients from 21 hospitals in Hubei Province, China. Participants were admitted between December 30, 2019, and April 23, 2020. A total of 15,649 participants diagnosed with COVID-19 were initially enrolled. We excluded 821 patients transferred to other hospitals, 4705 patients younger than 18 or older than 65 years old, 174 patients who were pregnant or breastfeeding, and 665 patients with incomplete electronic medical records. Furthermore, 4478 patients with comorbidities associated with poor outcomes of COVID-19 were excluded based on literature research and clinical experience [[12], [13], [14], [15], [16], [17]]. These comorbidities included hypertension, cardiovascular diseases, renal diseases, respiratory system diseases, immunocompromised conditions, chronic metabolic diseases, liver diseases, etc. were listed in Fig. 1 and eTable 1. Finally, a total of 4806 patients were included in the analysis. The end follow-up date was April 23, and the data for each patient during their hospitalization were collected and reviewed. The study protocol was approved by the Institutional Ethics Committee of Renmin Hospital of Wuhan University and Zhongnan Hospital of Wuhan University and was also individually approved by each collaborating hospital or their institutional ethics boards. Given the urgency of the data collection, written informed consent was waived by each hospital.
Fig. 1.
The flowchart showing the strategy of participants’ enrollment.
Abbreviations: CVD, cardiovascular diseases (except hypertension); RSD, respiratory system disease; IC, immunocompromised conditions; CMD, chronic metabolic diseases (except diabetes); GDC, gastrointestinal and digestive comorbidities.
aOther diseases: rheumatism and autoimmune diseases (55), blood disorders (51), Mental disorders (47), Neurological diseases (41), others that cannot be classified but affect the health of the patients (46, such as history of headache, dizziness, fever of unknown cause, etc.).
Data collection
We collected demographic information, clinical characteristics, initial symptoms, computerized chest tomography (CT) results, and clinical outcomes from electronic medical records by trained clinicians and medical experts. The in-hospital medications and interventions were collected from doctor advice. Regarding the laboratory results, we collected indicators that reflect necrocytosis, inflammatory status, liver function, kidney function, blood glucose level, serum lipid profile, cardiac function, and coagulation function (eTable 2). Before data extraction, personal identifying information (e.g., name and ID) was anonymized, and each participant was given a study ID using an electronic coding system to protect patient privacy.
Definition
In this retrospective observational study, according to the requirements of epidemic prevention and control for residents in Hubei Province, COVID-19 was diagnosed when patients with suspected cases met one or both criteria of reverse transcription-polymerase chain reaction (RT-PCR) and CT manifestations according to the New Coronavirus Pneumonia Prevention and Control Program (5th edition) published by the National Health Commission of China [18]. The severity of COVID-19 was defined according to the COVID-19 Diagnosis and Treatment Protocol (Trial version 5), including mild, moderate, severe, and critical [18]. Mild cases were defined in patients with mild clinical symptoms, and no pneumonia characteristics on CT imaging. Patients in the moderate illness group had fever, respiratory tract symptoms, and CT-reflected pneumonia. Severe cases were defined in patients who met any of the following criteria: (1) respiratory distress with respiratory rate ≥ 30 times/min, (2) oxygen saturation ≤ 93% in the resting state, and (3) arterial partial pressure of oxygen (PaO2)/inhaled oxygen concentration (FiO2) ≤300 mmHg (1 mmHg = .133 kPa). Patients with critical illness met one of the following conditions: (1) respiratory failure requiring mechanical ventilation, (2) shock, and (3) combined with other organ failure and requiring intensive care unit (ICU) monitoring and treatment [18]. Furthermore, according to the WHO severity definitions [19], in which non-severe COVID-19 is defined as the absence of any criteria for severe or critical COVID-19, we grouped patients with mild and moderate illnesses as the non-severe group, while those with severe and critical conditions were classified as the severe group.
The definitions of acute respiratory distress syndrome (ARDS) and septic shock were defined according to the WHO interim guideline “Clinical management of severe acute respiratory infection when novel coronavirus (2019-nCoV) infection is suspected” [20]. Acute kidney injury was defined as an elevation in serum creatinine level equal to or above 26.5 μmmol/L within 48 h [8]. Acute cardiac injury was defined as the serum level of cardiac troponin I (cTnI), cardiac troponin T (cTnT), high-sensitivity troponin I (hs-cTnI), or high-sensitivity troponin T (hs-cTnT) above the upper limit of normal (ULN) [8]. Acute liver injury was defined as the levels of serum alanine transaminase (ALT) or alkaline phosphatase (ALP) above 3-fold of the ULN [21]. The increase or decrease in laboratory indicators was defined as higher than their ULN or lower than the lower limit of normal (LLN), respectively, according to the criteria by the standards in each hospital (eTables 3–5).
Statistical analysis
Categorical variables were expressed as frequencies and percentages. Continuous variables were expressed as median and interquartile range (IQR) values. When comparing the differences between groups, the Mann–Whitney U test was performed for continuous variables, and the χ2 test or Fisher’s exact test was performed for categorical variables. Initially, 51 candidate variables for potential inclusion based on clinical experience and a review of the literature were identified, including clinical characteristics, symptoms, and laboratory tests. Then, 7 variables were excluded because of a missing rate > 40%. To improve clinical utility, all laboratory tests selected were dichotomized according to the reference range in each hospital (eTables 3–5). The rest of the risk factors were identified through three steps. First, all candidate variables for potential inclusion were based on the statistical analysis between groups and clinical experiences. Second, to minimize the potential collinearity of variables and overfitting, the least absolute shrinkage and selection operator (LASSO) regression model was used to further select the risk factors, and the optimal value of the lambda coefficient (λ) with the minimum number of related variables was determined by 10-fold cross-validation [22,23]. Third, a multivariate logistic regression model with a stepwise forward selection process was applied to identify the final risk factors from the previous step [24]. The non-parameter imputation method missForest was conducted to handle missing data, and the estimated imputation error was 8.28%. The odds ratio (OR) and the 95% confidence intervals (CI) were reported. A 2-sided P value < .05 was considered statistically significant. All data were analyzed using R-4.0.2 (R Foundation for Statistical Computing, Vienna, Austria) and SPSS Statistics (version 25.0, IBM, Armonk, NY, USA).
Results
Clinical characteristics and outcomes of all participants stratified by death and non-death
A total of 4806 patients were eventually recruited into the study, with a median age of 47.00 years [IQR, 36.00–56.00], and 2162 (44.99%) were men (Table 1 ). The median duration from symptom onset to hospitalization was 10.00 days [IQR, 6.00–19.00] and from symptom onset to discharge or death was 32.00 days [IQR, 22.00–43.00], respectively (Table 1). On admission, the most common symptom was fever (75.38%) (Table 1). Bilateral lesions (81.48%) and ground-glass opacities (52.70%) were the major radiographic characteristics (eTable 6).
Table 1.
Characteristics, outcomes and laboratory results of the entire cohort without comorbidities.
| Parameters | Total (n = 4806) | Non-death patients (n = 4705) | Death patients (n = 101) | P value |
|---|---|---|---|---|
| Demographics and clinical characteristics | ||||
| Age, median(IQR), years | 47.00 [36.00, 56.00] | 46.00 [36.00, 56.00] | 58.00 [52.00, 62.00] | <.001 |
| 18 ≤ Age ≤ 35, n(%) | 1131 (23.53) | 1126 (23.93) | 5 (4.95) | <.001 |
| 36 ≤ Age ≤ 46, n(%) | 1238 (25.76) | 1230 (26.14) | 8 (7.92) | |
| 47 ≤ Age ≤ 55, n(%) | 1151 (23.95) | 1126 (23.93) | 25 (24.75) | |
| 56 ≤ Age ≤ 65, n(%) | 1286 (26.76) | 1223 (25.99) | 63 (62.38) | |
| Male gender, n(%) | 2162 (44.99) | 2098 (44.59) | 64 (63.37) | <.001 |
| Heart rate, median(IQR), bpm | 84.00 [78.00, 95.00] | 84.00 [78.00, 95.00] | 90.00 [80.00, 100.00] | .002 |
| Respiratory rate, median(IQR), bpm | 20.00 [19.00, 21.00] | 20.00 [19.00, 20.00] | 23.00 [20.00, 27.00] | <.001 |
| SpO2 < 95%, n(%) | 443 (12.64) | 388 (11.31) | 55 (72.37) | <.001 |
| Days from symptom to hospital, median(IQR) | 10.00 [6.00, 19.00] | 10.00 [6.00, 19.00] | 9.00 [6.00, 13.00] | .03 |
| Days from symptom to discharge or death, median(IQR) | 32.00 [22.00, 43.00] | 32.00 [22.00, 43.00] | 23.00 [16.00, 31.00] | <.001 |
| Initial symptoms, n(%) | ||||
| Fever | 3623 (75.38) | 3542 (75.28) | 81 (80.20) | .31 |
| Dyspnea | 737 (15.33) | 692 (14.71) | 45 (44.55) | <.001 |
| Secondary outcome, n(%) | ||||
| ARDS | 399 (8.30) | 305 (6.48) | 94 (93.07) | <.001 |
| Acute liver injury | 406 (8.45) | 369 (7.84) | 37 (36.63) | <.001 |
| Acute cardiac injury | 120 (2.50) | 66 (1.40) | 54 (53.47) | <.001 |
| Septic shock | 57 (1.19) | 24 (.51) | 33 (32.67) | <.001 |
| Acute kidney injury | 51 (1.06) | 15 (.32) | 36 (35.64) | <.001 |
| Laboratory results, n(%) | ||||
| Necrocytosis indicators | ||||
| CK > ULNa | 343 (9.06) | 314 (8.49) | 29 (33.33) | <.001 |
| LDH > ULNa | 1255 (29.97) | 1171 (28.59) | 84 (91.30) | <.001 |
| Inflammatory indicators | ||||
| Leukocyte count > 9.5, 10^9/L | 294 (6.16) | 254 (5.44) | 40 (39.60) | <.001 |
| Neutrophil count > 6.3, 10^9/L | 431 (9.04) | 375 (8.03) | 56 (55.45) | <.001 |
| Lymphocyte count < .8, 10^9/L | 1458 (30.57) | 1374 (29.43) | 84 (83.17) | <.001 |
| CRP > ULNa | 1191 (41.61) | 1123 (40.24) | 68 (95.77) | <.001 |
| Procalcitonin > ULNa | 1024 (27.72) | 961 (26.67) | 63 (69.23) | <.001 |
| Liver function | ||||
| ALT > 40 U/L | 1015 (22.22) | 981 (21.95) | 34 (34.69) | .004 |
| AST > 40 U/L | 762 (16.66) | 706 (15.78) | 56 (57.14) | <.001 |
| Albumin < LLNa | 1935 (41.13) | 1849 (40.16) | 86 (85.15) | <.001 |
| Direct bilirubin > ULNa | 414 (8.89) | 367 (8.05) | 47 (47.47) | <.001 |
| Kidney function | ||||
| UA < LLNa | 449 (9.67) | 413 (9.09) | 36 (36.36) | <.001 |
| BUN > ULNa | 102 (2.19) | 79 (1.74) | 23 (23.23) | <.001 |
| Creatinine > ULNa | 101 (2.18) | 94 (2.07) | 7 (7.22) | .005 |
| Blood glucose | ||||
| Blood glucose > ULNa | 1132 (25.96) | 1059 (24.84) | 73 (76.04) | <.001 |
| Dyslipidemia | ||||
| HDL-c < LLNa | 1359 (42.83%) | 1314 (42.41%) | 45 (60.00) | .003 |
| Coagulation function | ||||
| PT > ULNa | 462 (10.94) | 426 (10.33) | 36 (36.73) | <.001 |
| APTT > ULNa | 390 (9.38) | 368 (9.06) | 22 (23.40) | <.001 |
| D-dimer > ULNa | 1238 (30.09) | 1162 (28.91) | 76 (79.17) | <.001 |
Abbreviations: IQR, interquartile range; SpO2, oxygen saturation; ARDS, acute respiratory distress syndrome; CRP, C-reactive protein; ALT, alanine transaminase; AST, aspartate transaminase; UA, uric acid; BUN, blood urea nitrogen; HDL-c, high-density lipoprotein cholesterol; CK, creatine phosphokinase; LDH, lactate dehydrogenase; PT, prothrombin time; APTT, activated partial thromboplastin time.
Data are median (IQR) and n (%).
P values were calculated by the Mann–Whitney U test for continuous variables and Fisher’s exact test or χ2 test for categorical variables.
Upper limit of normal (ULN) and lower limit of normal (LLN) were defined according to criteria in each hospital.
There were 4705 survivors and 101 non-survivors, with a mortality rate of 2.10% in the entire cohort. Compared with survivors, non-survivors were older, had a higher heart rate and respiratory rate, and had a greater proportion of oxygen saturation (SpO2) reduction on admission. Laboratory results showed that the frequencies of abnormal indicators reflecting tissue and organ necrocytosis, inflammation, liver dysfunction, cardiac dysfunction, kidney dysfunction, and coagulation dysfunction were higher in the death group than in the non-death group (Table 1 and eTable 6). Consequently, patients in the death group required more active treatments, including oxygen inhalation, mechanical ventilation, extracorporeal membrane oxygenation (ECMO), ICU admission and systemic corticosteroid and immunoglobin intervention (eTable 6).
Risk factors associated with COVID-19 mortality in the entire participant cohort
To explore the potential risk factors for death from COVID-19 in all participants, we first selected 30 candidate variables based on differences between groups and clinical significance, put them into the LASSO regression model, and screened out 14 risk factors related to death from COVID-19. Finally, through a stepwise forward logistic regression model, 10 variables were identified as risk factors for COVID-19 mortality in the entire population cohort. The variable screening strategy was shown in eTable 7.
Notably, age was stratified as quartiles and was identified as an independent risk factor for mortality. By treating 18 ≤ age ≤ 35 as a reference, 47 ≤ age ≤ 55 yielded an OR of 4.08 (95% CI, 1.35–12.33; P = .01), and 56 ≤ age ≤ 65 had an OR of 6.36 (95% CI, 2.21–18.30; P = .001). SpO2 < 95% was found to be the second most important risk factor, with an OR of 5.80 (95% CI, 3.55–9.48; P < .001), followed by increased lactate dehydrogenase (LDH), neutrophil count, direct bilirubin, creatine phosphokinase (CK), blood urea nitrogen (BUN) levels, dyspnea, increased blood glucose and prothrombin time (PT) levels (Table 2 ).
Table 2.
Logistic regression model of risk factors associated with mortality in the entire cohort.
| Item | OR (95%CI) | P value |
|---|---|---|
| 18 ≤ Age ≤ 35 | Reference | <.001 |
| 36 ≤ Age ≤ 46 | 1.31 (.37,4.64) | .68 |
| 47 ≤ Age ≤ 55 | 4.08 (1.35,12.33) | .01 |
| 56 ≤ Age ≤ 65 | 6.36 (2.21,18.30) | .001 |
| SpO2 < 95% | 5.80 (3.55,9.48) | <.001 |
| Increased LDHa | 4.93 (2.21,10.97) | <.001 |
| Increased neutrophil countb | 3.43 (2.09,5.65) | <.001 |
| Increased Direct bilirubina | 3.22 (1.95,5.34) | <.001 |
| Increased CKa | 2.76 (1.60,4.77) | <.001 |
| Increased BUNa | 2.21 (1.11,4.40) | .02 |
| Dyspnea | 2.21 (1.35,3.62) | .002 |
| Increased blood glucosea | 2.04 (1.18,3.53) | .01 |
| Increased PTa | 2.00 (1.17,3.42) | .01 |
Abbreviations: SpO2, oxygen saturation; LDH, lactate dehydrogenase; CK, creatine phosphokinase; BUN, blood urea nitrogen; PT, prothrombin time.
P values were calculated by logistic regression.
The increase of laboratory indicators was defined as higher than the upper limit of normal (ULN).
Increased neutrophil count was defined as neutrophil count >6.3, 10^9/L.
Risk factors associated with mortality in the severe group
We then divided all participants into non-severe (N = 3647) and severe groups (N = 1159) on admission to analyze and compare risk factors for patients with different severe stratifications. Patients in the severe group on admission were further separated into non-death (N = 1097) and death (N = 62) groups during hospitalization. Compared to the non-death group, patients who progressed to death showed older age, more severe symptoms, a greater proportion of SpO2 < 95%, and higher percentages of abnormal laboratory parameters at admission (eTable 8).
Similar to our analyses in the entire cohort, 23 variables with significant differences between the death and non-death groups were selected into the LASSO model. Furthermore, 14 variables were screened out (eTable 7). Nine risk factors were finally selected with a high correlation with death risk in patients with severe illness at admission by a subsequent logistic regression model. The Odds of death were the highest in patients over 55 years old (OR, 14.15; 95%CI, 2.47–81.12; P = .003), followed by those 47–55 years old (OR, 8.45; 95%CI, 1.41–50.77; P = .02), and those with an SpO2 < 95% (OR, 7.13; 95%CI, 3.60–14.11; P < .001). Furthermore, increased CK levels, decreased albumin levels, increased neutrophil count, blood glucose, direct bilirubin, BUN levels, and dyspnea were also potential risk factors related to the progression from severity on admission to death during hospitalization (Table 3 ). Interestingly, almost all those selected variables were involved in risk factors for death in the entire cohort, suggesting that those factors might be the core risk factors for COVID-19 deterioration.
Table 3.
Logistic regression model of risk factors associated with mortality in severe cases.
| Item | OR (95%CI) | P value |
|---|---|---|
| 18 ≤ Age ≤ 35 | Reference | .007 |
| 36 ≤ Age ≤ 46 | 4.67 (.67,32.50) | .12 |
| 47 ≤ Age ≤ 55 | 8.45 (1.41,50.77) | .02 |
| 56 ≤ Age ≤ 65 | 14.15 (2.47,81.12) | .003 |
| SpO2 < 95% | 7.13 (3.60,14.11) | <.001 |
| Increased CKa | 5.94 (2.94,12.01) | <.001 |
| Decreased albumina | 2.90 (1.11,7.59) | .03 |
| Increased neutrophil countb | 2.88 (1.48,5.62) | .002 |
| Increased blood glucosea | 2.85 (1.24,6.52) | .01 |
| Increased Direct bilirubina | 2.72 (1.40,5.28) | .003 |
| Increased BUNa | 2.45 (1.02,5.90) | .05 |
| Dyspnea | 2.23 (1.15,4.31) | .02 |
Abbreviations: SpO2, oxygen saturation; CK, creatine phosphokinase; BUN, blood urea nitrogen.
P values were calculated by logistic regression.
The increase or decrease of laboratory indicators was defined as higher than the upper limit of normal (ULN) or lower than the lower limit of normal (LLN).
Increased neutrophil count was defined as neutrophil count >6.3, 10^9/L.
Risk factors associated with progression to severe illness in the non-severe group
Of the 3647 patients diagnosed with non-severe cases on admission, 489 (13.41%) progressed to severe illness during hospitalization, and the remaining 3158 were maintained in a non-severe setting (eTable 9). As expected, patients who progressed to severe conditions had more severe symptoms at admission than those who maintained non-severe conditions (eTable 9). By analyzing 26 variables with significant differences between the non-severe and severe groups via LASSO and logistic regression models, we found that eight risk factors were closely related to the progression from mild or moderate features at admission to severe illness during hospitalization (eTable 7). The risk factors that stood out in the final regression model were increased procalcitonin levels, SpO2, age ≥ 47, increased LDH, activated partial thromboplastin time (APTT) levels, decreased high-density lipoprotein cholesterol (HDL-c) levels, dyspnea and increased D-dimer levels (Table 4 ).
Table 4.
Logistic regression model of risk factors associated with severity in non-severe cases.
| Item | OR (95%CI) | P value |
|---|---|---|
| Increased procalcitonina | 2.56 (2.07,3.17) | <.001 |
| SpO2 < 95% | 2.29 (1.66,3.15) | <.001 |
| 18 ≤ Age ≤ 35 | Reference | .03 |
| 36 ≤ Age ≤ 46 | 1.31 (.96,1.78) | .08 |
| 47 ≤ Age ≤ 55 | 1.37 (1.01,1.86) | .05 |
| 56 ≤ Age ≤ 65 | 1.60 (1.18,2.16) | .002 |
| Increased LDHa | 1.60 (1.27,2.01) | <.001 |
| Increased APTTa | 1.50 (1.05,2.13) | .03 |
| Decreased HDL-ca | 1.34 (1.08,1.66) | .008 |
| Dyspnea | 1.33 (1.02,1.73) | .04 |
| Increased D-dimera | 1.27 (1.02,1.59) | .03 |
Abbreviations: SpO2, oxygen saturation; LDH, lactate dehydrogenase; APTT, activated partial thromboplastin time; HDL-c, high-density lipoprotein cholesterol.
P values were calculated by logistic regression.
The increase or decrease of laboratory indicators was defined as higher than the upper limit of normal (ULN) or lower than the lower limit of normal (LLN).
Risk factors associated with secondary outcomes
Since secondary outcomes directly reflected disease severity and were closely associated with COVID-19 death, we reported the incidence of secondary outcomes, including ARDS, acute liver injury, acute cardiac injury, septic shock, and acute kidney injury, in patients during the duration of follow-up. The proportions of each secondary outcome were all higher in the death and severe groups than in the non-death and non-severe groups (Table 1, eTables 8 and 9). We further analyzed the risk factors associated with each secondary outcome in the severe group. The results were demonstrated in eTables 10–14.
Discussion
In this study, we systematically analyzed the clinical characteristics, disease progression and risk factors of COVID-19 patients without pre-existing comorbidities utilizing a large patient dataset. The total severity rate for COVID-19 patients without chronic comorbidities was up to 34.29%, with a death rate of 2.10%. We found that age ≥ 47, parameters indicating tissue or organ damage (elevated LDH and CK), pulmonary impairment (SpO2 < 95% and dyspnea), organ dysfunction (increased direct bilirubin, BUN and blood glucose), and an inflammatory condition (increased neutrophil count) were potential risk factors for COVID-19 death in patients without chronic comorbidities. Comparatively, the progression from non-severe to severe status was more closely correlated with coagulation disorder (increased APTT and D-dimer) and dyslipidemia (decreased HDL-c). These findings can help us assess the status of the disease in COVID-19 patients during hospitalization and provide direct advice for the strategies of clinical interventions for COVID-19 patients without chronic comorbidities.
Previous studies on risk factors for COVID-19 patients mainly focused on the general population [10,[25], [26], [27], [28], [29], [30]]. These studies did not distinguish the clinical characteristics and disease progression between patients with and without pre-existing disease, whose disease progression and intervention measures may be different. In addition, since patients with pre-existing disease have a higher predisposition to COVID-19, specific studies were interested in addressing clinical traits, treatment strategy and outcomes in COVID-19 patients with pre-existing diseases, such as cardiovascular disease, hypertension and diabetes [7,8,[31], [32], [33], [34]]. However, patients without existing comorbidities have been neglected. Only small-scale studies (less than 200 patients) have reported the severity and mortality progression in populations without pre-existing comorbidities [[35], [36], [37], [38]]. Based on a series of studies on COVID-19 in our group [7,8,[31], [32], [33],[39], [40], [41]], we recognized that these patients without pre-existing disease also have specific traits and disease patterns. In this study, we focused on the COVID-19 population without previous underlying diseases. Using a large patient dataset containing 4806 patients, we described the disease progression characteristics and risk factors of patients without previous comorbidities. In addition, we identified and differentiated sets of risk factors that predicted mortality and progression to severe conditions. We found that indicators suggesting aging, the impairment of respiratory function (SpO2 < 95% and dyspnea), coagulation dysfunction (increased APTT and D-dimer), tissue injury (increased LDH), and lipid metabolism dysregulation (decreased HDL-c level) were closely associated with disease progression from non-severe to severe conditions. In addition, we noticed severe bacterial infections, as indicated by procalcitonin levels, largely increased the risk of developing severe cases in patients without pre-existing diseases. Once indicators suggested that multi-organ damage increased at baseline including increased direct bilirubin, CK and BUN levels, as well as increased blood glucose levels, the rate of mortality increased dramatically in patients without pre-existing disease.
It is interesting that the progression and mortality of COVID-19 are closely associated with multiple organ injuries. The indicators reflecting impaired liver, kidney, and pancreas functions were closely associated with the progression of COVID-19 at admission to death. These organ damages likely involve multiple mechanisms, including direct attacks from SARS-CoV-2, the cytokine storm, hypoxemia, or drug interventions. It has been reported that ACE2, the primary receptor of SARS-CoV-2, is detectable in the heart, vasculature, and kidneys [42]. Recent studies have found new receptors (CD147-spike protein, neuropilin-1) that facilitate the entry of SARS-CoV-2 into human cells, which may increase the direct damage of the virus [43,44]. In addition to direct virus attacks, increased leukocyte counts, especially neutrophil counts, may lead to excessive cytokine production, resulting in a cytokine storm and systematic organ injury [45]. Although there were no explicit causal effects between risk factors and COVID-19 progression, clinical interventions for organ protection and anti-inflammation should be considered.
Furthermore, our data showed that elevated LDH increases the risk of progression to severe illness, and double elevated LDH/CK increases the risk of death. LDH is a cytoplasmic enzyme that exists in all major organ systems. It can be used as an indicator of cell integrity disorders caused by pathological conditions. The elevated LDH levels in the serum indicated cell damage or necrosis [28]. Similarly, the increase in CK in serum, which is mainly distributed in skeletal muscle and myocardium, suggests that the permeability of tissue cells is enhanced or tissue cells are necrotic. CK may act synergistically with LDH in the progression of COVID-19 to accelerate the severity of the disease. The mechanism needs further study.
For patients with a non-severe status at admission but who progressed to severe disease during hospitalization, apart from patients with severe bacterial infections, those with coagulation disorder (increased APTT and D-dimer) and dyslipidemia (decreased HDL-c) had increased odds of disease progression. The increase in APTT and D-dimer may be attributed to the new coronavirus damaging vascular endothelial cells, followed by triggering the formation of microthrombi [46]. High-density lipoproteins (HDLs) have a protective effect on the endothelial layer through their antioxidant, anti-inflammatory, antiapoptotic and antithrombotic functions, normally [47]. However, during COVID-19, HDLs seem to lose their protective effect on endothelial cells under inflammatory conditions [30,47]. Inflammation can cause changes in the structure of HDL particles and cause the acute phase protein serum amyloid A (SAA) to accumulate in the protein portion of HDLs. HDL particles rich in SAA are not only cleared from the circulation faster than normal HDLs and consume cholesterol, resulting in decreased HDL-c, but also lose their anti-inflammatory properties and even promote the proinflammatory activation of macrophages and the excessive production of cytokines, which in turn aggravates inflammation and forms a vicious cycle [30,48]. Therefore, abnormal coagulation and lipid homeostasis should be actively intervened to prevent COVID-19 progression for non-severe patients without comorbidities at admission.
There were several limitations to the current study. First, our study is a retrospective study, and the causal relationship between abnormal laboratory indicators and disease progression cannot be determined. Second, the data analyzed in our study were all collected in China. Geographical limitations should be resolved by further analysis of COVID-19 cases worldwide. Third, the emerging new mutant SARS-CoV-2 might promote disease progression with different clinical features or mechanisms. Thus, further prospective studies based on a large cohort are needed to validate the findings of the present study.
Conclusion
COVID-19 patients without pre-existing chronic comorbidities have specific traits and disease patterns. Patients with COVID-19 accompanied by severe bacterial infections, as indicated by increased procalcitonin levels, was highly associated with disease progression from non-severe to severe conditions. Aging, the impairment of respiratory function, coagulation dysfunction, tissue injury, and lipid metabolism dysregulation were also associated with disease progression. Once factors for multi-organ damage were elevated and glucose increased at admission, these findings indicated a higher risk for mortality. This study provides information that helps to predict COVID-19 prognosis specifically in patients without chronic comorbidities.
Data availability
The data and codes related to the findings of this study will be available from the corresponding author after publication upon reasonable request. The research team will provide an email address for communication once the data are approved to be shared with others. The proposal with detailed aims, statistical plan, and other information/materials may be required to guarantee the rationality of requirement and the security of the data. The patient-level data, but without names and other identifiers, will be shared after review and approval of the submitted proposal and any related requested materials.
Ethical approval
The study protocol was approved by the Institution Ethic Committee of Renmin Hospital of Wuhan University and Zhongnan Hospital of Wuhan University and was also individually approved by each collaborating hospital or their institutional ethics boards. Given the urgency of the data collection, written informed consent was waived by each hospital.
Funding
This work was supported by the National Key R&D Program of China [grant numbers 2016YFF0101504, 2020YFC2004702]; the Special Foundation for Emergency Research on Prevention and Control of COVID-19 of Guangdong Province [grant number 2020B1111330003]; the National Science Foundation of China [grant numbers 81630011, 81970364, 81970070, 81970011, 81770053]; the Hubei Science and Technology Support Project [grant numbers 2019BFC582, 2018BEC473]; the Scientific Research Fund of Hunan Provincial Health Committee [grant number C2019005] and Medical flight plan of Wuhan University [grant number TFJH2018006].
Competing interests
The authors declare no competing interests.
Acknowledgments
W.L., C.Y., Y.-G.L. and F.W. contributed equally, designed the study, collected and analyzed data, and wrote the manuscript. L.L., X.H., B.-H.Z. and Y.Y. collected and reviewed clinical, laboratory, and radiological data. P.Z. performed statistical analysis. X.-J.Z. and Z.-G.S wrote manuscript and provided valuable suggestions for study design and data analysis. L.W. and H.L. contributed equally, designed the project, edited manuscript, and supervised the study. All authors have approved the final version of this paper.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.jiph.2021.11.012.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- 1.World Health Organization. WHO coronavirus (COVID-19) dashboard. https://covid19.who.int/.
- 2.Ramasamy M.N., Minassian A.M., Ewer K.J., Flaxman A.L., Folegatti P.M., Owens D.R., et al. Safety and immunogenicity of ChAdOx1 nCoV-19 vaccine administered in a prime-boost regimen in young and old adults (COV002): a single-blind, randomised, controlled, phase 2/3 trial. Lancet (London, England) 2021;396:1979–1993. doi: 10.1016/S0140-6736(20)32466-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Krammer F. SARS-CoV-2 vaccines in development. Nature. 2020;586:516–527. doi: 10.1038/s41586-020-2798-3. [DOI] [PubMed] [Google Scholar]
- 4.Yan Y., Yang Y., Wang F., Ren H., Zhang S., Shi X., et al. Clinical characteristics and outcomes of patients with severe covid-19 with diabetes. BMJ Open Diabetes Res Care. 2020;8 doi: 10.1136/bmjdrc-2020-001343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu P.P., Blet A., Smyth D., Li H. The science underlying COVID-19: implications for the cardiovascular system. Circulation. 2020;142:68–78. doi: 10.1161/CIRCULATIONAHA.120.047549. [DOI] [PubMed] [Google Scholar]
- 6.Tian J., Yuan X., Xiao J., Zhong Q., Yang C., Liu B., et al. Clinical characteristics and risk factors associated with COVID-19 disease severity in patients with cancer in Wuhan, China: a multicentre, retrospective, cohort study. Lancet Oncol. 2020;21:893–903. doi: 10.1016/S1470-2045(20)30309-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhu L., She Z.-G., Cheng X., Qin J.-J., Zhang X.-J., Cai J., et al. Association of blood glucose control and outcomes in patients with COVID-19 and pre-existing type 2 diabetes. Cell Metab. 2020;31:1068–1077.e3. doi: 10.1016/j.cmet.2020.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang P., Zhu L., Cai J., Lei F., Qin J.-J., Xie J., et al. Association of inpatient use of angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers with mortality among patients with hypertension hospitalized with COVID-19. Circ Res. 2020;126:1671–1681. doi: 10.1161/CIRCRESAHA.120.317134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y., et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet (London, England) 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet (London, England) 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liang W.-H., Guan W.-J., Li C.-C., Li Y.-M., Liang H.-R., Zhao Y., et al. Clinical characteristics and outcomes of hospitalised patients with COVID-19 treated in Hubei (epicentre) and outside Hubei (non-epicentre): a nationwide analysis of China. Eur Respir J. 2020;55 doi: 10.1183/13993003.00562-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fang X., Li S., Yu H., Wang P., Zhang Y., Chen Z., et al. Epidemiological, comorbidity factors with severity and prognosis of COVID-19: a systematic review and meta-analysis. Aging (Albany NY) 2020;12:12493–12503. doi: 10.18632/aging.103579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ellul M.A., Benjamin L., Singh B., Lant S., Michael B.D., Easton A., et al. Neurological associations of COVID-19. Lancet Neurol. 2020;19:767–783. doi: 10.1016/S1474-4422(20)30221-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rogers J.P., Chesney E., Oliver D., Pollak T.A., McGuire P., Fusar-Poli P., et al. Psychiatric and neuropsychiatric presentations associated with severe coronavirus infections: a systematic review and meta-analysis with comparison to the COVID-19 pandemic. Lancet Psychiatry. 2020;7:611–627. doi: 10.1016/S2215-0366(20)30203-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Connors J.M., Levy J.H. COVID-19 and its implications for thrombosis and anticoagulation. Blood. 2020;135:2033–2040. doi: 10.1182/blood.2020006000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Taneri P.E., Gómez-Ochoa S.A., Llanaj E., Raguindin P.F., Rojas L.Z., Roa-Díaz Z.M., et al. Anemia and iron metabolism in COVID-19: a systematic review and meta-analysis. Eur J Epidemiol. 2020;35:763–773. doi: 10.1007/s10654-020-00678-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Disser N.P., De Micheli A.J., Schonk M.M., Konnaris M.A., Piacentini A.N., Edon D.L., et al. Musculoskeletal consequences of COVID-19. J Bone Joint Surg Am. 2020;102:1197–1204. doi: 10.2106/JBJS.20.00847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.National Health Commission of China . 2020. New coronavirus pneumonia prevention and control program. [Google Scholar]
- 19.Rochwerg B., Agarwal A., Siemieniuk R.A.C., Agoritsas T., Lamontagne F., Askie L., et al. A living WHO guideline on drugs for covid-19. BMJ. 2020;370 doi: 10.1136/bmj.m3379. [DOI] [PubMed] [Google Scholar]
- 20.World Health Organization . 2020. Clinical management of severe acute respiratory infection when novel coronavirus (2019-nCoV) infection is suspected. [Google Scholar]
- 21.Marrone G., Vaccaro F.G., Biolato M., Miele L., Liguori A., Araneo C., et al. Drug-induced liver injury 2017: the diagnosis is not easy but always to keep in mind. Eur Rev Med Pharmacol Sci. 2017;21:122–134. [PubMed] [Google Scholar]
- 22.Friedman J., Hastie T., Tibshirani R. Regularization paths for generalized linear models via coordinate descent. J Stat Softw. 2010;33:1–22. [PMC free article] [PubMed] [Google Scholar]
- 23.Sauerbrei W., Royston P., Binder H. Selection of important variables and determination of functional form for continuous predictors in multivariable model building. Stat Med. 2007;26:5512–5528. doi: 10.1002/sim.3148. [DOI] [PubMed] [Google Scholar]
- 24.Schober P., Vetter T.R. Logistic regression in medical research. Anesth Analg. 2021;132:365–366. doi: 10.1213/ANE.0000000000005247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alizadehsani R., Alizadeh Sani Z., Behjati M., Roshanzamir Z., Hussain S., Abedini N., et al. Risk factors prediction, clinical outcomes, and mortality in COVID-19 patients. J Med Virol. 2021;93:2307–2320. doi: 10.1002/jmv.26699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Iftimie S., López-Azcona A.F., Vicente-Miralles M., Descarrega-Reina R., Hernández-Aguilera A., Riu F., et al. Risk factors associated with mortality in hospitalized patients with SARS-CoV-2 infection. A prospective, longitudinal, unicenter study in Reus, Spain. PLoS One. 2020;15 doi: 10.1371/journal.pone.0234452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen R., Liang W., Jiang M., Guan W., Zhan C., Wang T., et al. Risk factors of fatal outcome in hospitalized subjects with coronavirus disease 2019 from a nationwide analysis in China. Chest. 2020;158:97–105. doi: 10.1016/j.chest.2020.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yan L, Zhang H-T, Goncalves J, Xiao Y, Wang M, Guo Y, et al. A machine learning-based model for survival prediction in patients with severe COVID-19 infection. MedRxiv Unpublished results. https://doi.org/10.1101/2020.0 2.27.20028027.
- 29.Li X., Xu S., Yu M., Wang K., Tao Y., Zhou Y., et al. Risk factors for severity and mortality in adult COVID-19 inpatients in Wuhan. J Allergy Clin Immunol. 2020;146:110–118. doi: 10.1016/j.jaci.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hu X., Chen D., Wu L., He G., Ye W. Declined serum high density lipoprotein cholesterol is associated with the severity of COVID-19 infection. Clin Chim Acta. 2020;510:105–110. doi: 10.1016/j.cca.2020.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Qin J.-J., Cheng X., Zhou F., Lei F., Akolkar G., Cai J., et al. Redefining cardiac biomarkers in predicting mortality of inpatients with COVID-19. Hypertens (Dallas, Tex 1979) 2020;76:1104–1112. doi: 10.1161/HYPERTENSIONAHA.120.15528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cheng X., Liu Y.-M., Li H., Zhang X., Lei F., Qin J.-J., et al. Metformin is associated with higher incidence of acidosis, but not mortality, in individuals with COVID-19 and pre-existing type 2 diabetes. Cell Metab. 2020;32:537–547.e3. doi: 10.1016/j.cmet.2020.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang X.-J., Qin J.-J., Cheng X., Shen L., Zhao Y.-C., Yuan Y., et al. In-hospital use of statins is associated with a reduced risk of mortality among individuals with COVID-19. Cell Metab. 2020;32:176–187.e4. doi: 10.1016/j.cmet.2020.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alizadehsani R, Eskandarian R, Behjati M, Zahmatkesh M, Roshanzamir M, Hoseini Izadi N, et al. Factors associated with mortality in hospitalized cardiovascular disease patients infected with COVID-19. SSRN Electron J Unpublished results. https://doi.org/10.2139/ssrn.3857678. [DOI] [PMC free article] [PubMed]
- 35.Wang P., Sha J., Meng M., Wang C., Yao Q., Zhang Z., et al. Risk factors for severe COVID-19 in middle-aged patients without comorbidities: a multicentre retrospective study. J Transl Med. 2020;18:461. doi: 10.1186/s12967-020-02655-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li X., Marmar T., Xu Q., Tu J., Yin Y., Tao Q., et al. Predictive indicators of severe COVID-19 independent of comorbidities and advanced age: a nested case-control study. Epidemiol Infect. 2020;148:e255. doi: 10.1017/S0950268820002502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ronderos Botero D.M., Omar A.M.S., Sun H.K., Mantri N., Fortuzi K., Choi Y., et al. COVID-19 in the healthy patient population: demographic and clinical phenotypic characterization and predictors of in-hospital outcomes. Arterioscler Thromb Vasc Biol. 2020;40:2764–2775. doi: 10.1161/ATVBAHA.120.314845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ozturk S., Kurtulus Ozturk E., Yildiz Kaya S. Clinical and radiological characteristics of COVID-19 patients without comorbidities: a single-center study. Wien Klin Wochenschr. 2021;133:875–881. doi: 10.1007/s00508-021-01880-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cai J., Li H., Zhang C., Chen Z., Liu H., Lei F., et al. The neutrophil-to-lymphocyte ratio determines clinical efficacy of corticosteroid therapy in patients with COVID-19. Cell Metab. 2021;33:258–269.e3. doi: 10.1016/j.cmet.2021.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lei F., Liu Y.-M., Zhou F., Qin J.-J., Zhang P., Zhu L., et al. Longitudinal association between markers of liver injury and mortality in COVID-19 in China. Hepatology. 2020;72:389–398. doi: 10.1002/hep.31301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu Y.-M., Xie J., Chen M.-M., Zhang X., Cheng X., Li H., et al. Kidney function indicators predict adverse outcomes of COVID-19. Med (New York, NY) 2021;2:38–48.e2. doi: 10.1016/j.medj.2020.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li M.-Y., Li L., Zhang Y., Wang X.-S. Expression of the SARS-CoV-2 cell receptor gene ACE2 in a wide variety of human tissues. Infect Dis Poverty. 2020;9:45. doi: 10.1186/s40249-020-00662-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cantuti-Castelvetri L., Ojha R., Pedro L.D., Djannatian M., Franz J., Kuivanen S., et al. Neuropilin-1 facilitates SARS-CoV-2 cell entry and infectivity. Science. 2020;370:856–860. doi: 10.1126/science.abd2985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang K., Chen W., Zhang Z., Deng Y., Lian J.-Q., Du P., et al. CD147-spike protein is a novel route for SARS-CoV-2 infection to host cells. Signal Transduct Target Ther. 2020;5:283. doi: 10.1038/s41392-020-00426-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen X., Yan L., Fei Y., Zhang C. Laboratory abnormalities and risk factors associated with in-hospital death in patients with severe COVID-19. J Clin Lab Anal. 2020;34 doi: 10.1002/jcla.23467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Varga Z., Flammer A.J., Steiger P., Haberecker M., Andermatt R., Zinkernagel A.S., et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet (London, England) 2020;395:1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Begue F., Tanaka S., Mouktadi Z., Rondeau P., Veeren B., Diotel N., et al. Altered high-density lipoprotein composition and functions during severe COVID-19. Sci Rep. 2021;11 doi: 10.1038/s41598-021-81638-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sun J.T., Chen Z., Nie P., Ge H., Shen L., Yang F., et al. Lipid profile features and their associations with disease severity and mortality in patients with COVID-19. Front Cardiovasc Med. 2020;7 doi: 10.3389/fcvm.2020.584987. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data and codes related to the findings of this study will be available from the corresponding author after publication upon reasonable request. The research team will provide an email address for communication once the data are approved to be shared with others. The proposal with detailed aims, statistical plan, and other information/materials may be required to guarantee the rationality of requirement and the security of the data. The patient-level data, but without names and other identifiers, will be shared after review and approval of the submitted proposal and any related requested materials.