Abstract
Background
Observational studies have consistently reported that postmenopausal hormone therapy use is associated with lower colon cancer risk, but epidemiologic studies examining the associations between circulating concentrations of endogenous estrogens and colorectal cancer have reported inconsistent results.
Methods
We investigated the associations between circulating concentrations of estrone, estradiol, free estradiol, testosterone, free testosterone, androstenedione, dehydroepiandrosterone (DHEA), progesterone, and sex hormone–binding globulin (SHBG) with colon cancer risk in a nested case-control study of 1028 postmenopausal European women (512 colon cancer cases, 516 matched controls) who were noncurrent users of exogenous hormones at blood collection. Multivariable conditional logistic regression models were used to compute odds ratios and 95% confidence intervals to evaluate the association between circulating sex hormones and colon cancer risk. We also conducted a dose-response meta-analysis of prospective studies of circulating estrone and estradiol with colorectal, colon, and rectal cancer risk in postmenopausal women. All statistical tests were 2-sided.
Results
In the multivariable model, a nonstatistically significantly positive relationship was found between circulating estrone and colon cancer risk (odds ratio per log2 1-unit increment = 1.17 [95% confidence interval = 1.00 to 1.38]; odds ratioquartile4-quartile1 = 1.33 [95% confidence interval = 0.89 to 1.97], Ptrend = .20). Circulating concentrations of estradiol, free estradiol, testosterone, free testosterone, androstenedione, DHEA, progesterone, and SHBG were not associated with colon cancer risk. In the dose-response meta-analysis, no clear evidence of associations were found between circulating estradiol and estrone concentrations with colorectal, colon, and rectal cancer risk.
Conclusion
Our observational and meta-analysis results do not support an association between circulating concentrations of endogenous sex hormones and colon or rectal cancer in postmenopausal women.
Colorectal cancer is the third-most common cancer globally, with a lower incidence generally found for women than for men (1). It has been hypothesized that the sex disparity in incidence may be explained by higher estrogen concentrations in women, conferring a protective role against colon cancer development (2). Consistent with this hypothesis, multiple observational studies and a clinical trial have found that the use of postmenopausal hormone therapy (HT) was associated with lower colorectal cancer risk in women (3-7).
Epidemiologic data on the association of endogenous estrogens and other sex hormones with colorectal tumorigenesis are relatively limited. Initial analyses of endogenous circulating sex hormone concentrations and colorectal cancer risk in postmenopausal women did not support an antitumorigenic effect of estrogens in the colorectum (8-11), but in a more recent case-control study nested within the Women’s Health Initiative Clinical Trial (WHI-CT), inverse associations were reported between endogenous estrogens and colorectal and colon cancer risk but not rectal cancer, while a positive relationship between sex hormone–binding globulin (SHBG) and colorectal cancer risk was observed (12). Additional studies are needed to provide more clarity on the role of estrogens in colorectal tumorigenesis.
Testosterone is a biologically potent androgen and the main source of estradiol in women after menopause (13). The role of testosterone in relation to colorectal cancer in postmenopausal women is uncertain, but a recent nested case-control study conducted among postmenopausal Japanese women reported a positive association between endogenous testosterone concentrations and colorectal cancer risk (14). Further prospective studies are warranted to examine the role of testosterone and other androgens, dehydroepiandrosterone (DHEA), and androstenedione in colorectal cancer development.
To provide more conclusive evidence of the association between endogenous concentrations of circulating sex hormones and colon cancer, we conducted a nested case-control study within the European Prospective Investigation into Cancer and Nutrition (EPIC) cohort and the Northern Sweden Health and Disease Study (NSHDS) cohort in which circulating concentrations of estradiol, estrone, testosterone, androstenedione, DHEA, progesterone, and SHBG were measured. In addition, we conducted a meta-analysis combining results from the current study with those from previously published prospective studies (8-12,14) to examine the overall evidence linking endogenous estradiol and estrone with colorectal, colon, and rectal cancer risk.
Methods
Study Population and Collection of Blood Samples and Data
EPIC is an ongoing multicenter prospective cohort of 521 330 participants who were recruited between 1992 and 2000, predominantly from the general population of 10 European countries (Denmark, France, Germany, Greece, Italy, the Netherlands, Norway, Spain, Sweden, and the United Kingdom) (15-17). Blood samples were collected at the time of recruitment by standardized procedures (16,17) and stored at the International Agency for Research on Cancer (IARC) (–196°C, liquid nitrogen) except for Denmark (–150°C, nitrogen vapor) and Sweden (–80°C, freezers). All participants completed lifestyle questionnaires at recruitment, and most of the participants had anthropometric measurements and completed a validated food frequency questionnaire. All participants provided written informed consent at recruitment. Ethical approval of the study was obtained from the review boards of IARC and from local participating centers.
NSHDS is an ongoing population-based cohort of 135 000 participants that began in 1985. It consists of 3 subcohorts: the Västerbotten Intervention Programme, the mammography screening cohort, and the Northern Sweden MONItoring of trends and determinants in CArdiovascular disease (MONICA) study (18,19). Approximately 80% of the Västerbotten Intervention Programme and MONICA cohort participants donated their blood after overnight fasting. In the mammography screening cohort, the time since the last meal was recorded. All blood samples were stored in freezers at –80°C. At recruitment, all participants underwent a health examination (including measurement of height and weight) and completed a validated food frequency questionnaire and lifestyle questionnaire. The study was approved by the Research Ethics Committee of Umeå University Hospital and the Regional Ethics Committee in Uppsala (No. 2013-124).
Follow-up for Cancer Incidence
Information about cancer incidence was retrieved from local cancer registries, except for France and Germany, where incident cases were identified through a combination of health insurance records, cancer and pathology registries, and active follow-up of participants (20,21). Greece were excluded from the current analysis because of an ongoing administrative issue. Colon cancer cases were coded according to the International Classification of Diseases for Oncology, Third Edition (C18-C20) (22). We included colon cancer cases within the proximal (C18.0-18.5), distal (C18.6-C18.7), overlapping (C18.8), and unspecified regions (C18.9).
Selection of Cases and Controls
Because of funding constraints and for efficiency, we used a nested case-control design. As our focus was on endogenous circulating sex hormone concentrations, our study was limited to postmenopausal women who were not taking menopausal HT when blood samples were collected. Additionally, women who self-reported diabetes at baseline or those with unknown diabetic status were excluded [because diabetes affects the concentrations of sex hormones (23-25)]. For the selection of controls among EPIC participants, we sampled from all participants who were living, noncurrent users of exogenous hormones; postmenopausal; and free of cancer (except nonmelanoma skin cancer) at the time of diagnosis of the cases, using incidence density sampling and matched by age, study center, follow-up time since blood collection, time of day at blood collection, and fasting status. For NSHDS participants, we matched by study center, follow-up time, age, date of blood collection, and fasting status. We identified 557 incident colon cancer cases and 564 matched controls. Of these, we excluded participants with missing estradiol concentration (n = 1) and those in unmatched case-control sets (n = 92). After these exclusions, the current study included 512 colon cancer cases and 516 matched controls. Of these, 109 participants were also part of the NSHDS cohort but were regarded as EPIC participants in the current study. The 26 cases and 26 matched controls were those participants unique to NSHDS and not the wider EPIC study.
Laboratory Methods and Assessment of Sex Hormone Concentrations and SHBG
Plasma sex hormones and SHBG concentrations were measured at IARC (Lyon, France) using a validated analytical method adapted from previous publications (26,27). Full details are included in the Supplementary Methods (available online). Sex hormones were assayed using in a liquid chromatography–mass spectrometry system consisting of a ultra-high-performance liquid chromatograph (Agilent 1290, Agilent, Santa Clara, CA) and a QTRAP 5500 mass spectrometer (SCIEX, Framingham, MA). Solid-phase “sandwich” enzyme-linked immunoassay (DRG International, Springfield, NJ) was used for the measurement of SHBG concentrations. Lower limits of quantification (LLOQ) for each sex hormone was 7.5 pg/mL for androstenedione, 125 pg/mL for DHEA, 1.25 pg/mL for estradiol, 1.25 pg/mL for estrone, 7.5 pg/mL for progesterone, 7.5 pg/mL for testosterone, and 4 nmol/L for SHBG. Three quality control samples at different concentration levels were measured in duplicate in each batch of analyses. Intrabatch coefficients of variation in sex hormone and SHBG concentrations ranged from 1.4% to 8%. Interbatch coefficients of variations were less than 10% for all analytes.
Plasma concentration of free estradiol was calculated using a validated algorithm (28), taking into consideration measured estradiol and SHBG concentrations and an assumed constant for albumin. Free testosterone was also computed from previously validated mass-action equation using absolute concentrations of testosterone and an assumed albumin constant of 43 g/L (29,30). We also calculated the estradiol-to-testosterone ratio (by dividing the estradiol concentration by the testosterone concentration) because a higher ratio indicates greater production of estradiol from aromatase conversion.
Statistical Analysis
We imputed values to be half the LLOQ for those participants with a lower LLOQ value (4.4% for estradiol and 0.1% for DEHA). Pearson correlation coefficients (adjusted for age at blood collection and batch) between circulating concentrations of log2-transformed sex hormones and SHBG and body mass index (BMI) were calculated for control participants. Participants in the control group were divided into either tertiles or quartiles based on sex hormone concentrations. Statistical tests for trends in the present analyses were performed using the ordinal tertile or quartile entered into the models as continuous variables. We also conducted continuous analyses with each log2-transformed sex hormone. Multivariable conditional regression models, stratified by case–control set, were used to examine the association between circulating sex hormone concentrations and colon cancer risk. The multivariable models were adjusted for BMI, smoking status, physical activity, ever HT use, and alcohol consumption. In further analyses, the estrone, estradiol, and testosterone models were additionally adjusted for SHBG and vice versa. Further adjustment for dietary intakes of total energy, dietary fiber, and red and processed meat resulted in similar results, so these variables were not included in the final multivariable models. False-discovery rate correction was performed using the Benjamini-Hochberg method for the main analysis (31).
We also examined the sex hormone and colon cancer associations according to subgroups of BMI (<25 kg/m2 and ≥25 kg/m2) and follow-up time (below or above median follow-up in years) and computed the P value for interaction with the addition of an interaction term in the model by the aforementioned categories. We used a likelihood ratio test to assess the difference between the models with and without the interaction term.
Analyses for proximal colon cancer and distal colon cancer were also conducted. In further analyses, to assess the potential influence of preclinical disease on the results, cases diagnosed within the first 2 years of follow-up were excluded. In an additional sensitivity analysis, we used a multiple-imputation procedure (SAS command: PROC MI [SAS Institute Inc, Cary, NC]) to impute values below the LLOQ (estradiol, DHEA, estradiol-to-testosterone ratio, and free estradiol models only) (32,33).
All statistical analyses were performed using SAS software, version 9.4. All statistical tests were 2-sided.
Meta-Analysis
We performed a hand search up to July 2020 on PubMed using the keywords (“colorectal” OR “colorectum” OR “colon” OR “rectum”) and “cancer” and “sex hormone.” We limited our search to studies published in English that prospectively evaluated the association between circulating estradiol and estrone with colorectal, colon, or rectal cancer risk. Full details of the meta-analysis methods are described in the Supplementary Methods (available online).
We calculated summary relative risks (RRs) and 95% confidence intervals (CIs) for a 5 pg/mL increment in estradiol and a 10 pg/mL increment in estrone using a random effects model. The average of the natural logarithm of the relative risks was estimated, and the relative risk of each study was weighted using random effects weighting.
The dose-response analysis described by Greenland and Longnecker (34) was used to compute specific slopes (linear trends) and 95% confidence intervals from the natural logs of the reported relative risks and confidence intervals across categories of estradiol and estrone concentrations. Heterogeneity in results across studies was also examined using Cochran Q and I2 statistics. Statistical analyses were performed with Stata software, version 15.1 (StataCorp, College Station, TX).
Results
Nested Case-Control Study
We included 486 cases and 490 controls from the EPIC population and 26 cases and 26 controls from the NSHDS population, with a median follow-up time of 13.9 years. No substantial differences in baseline characteristics were found between cases and controls in both cohorts (Table 1). The baseline characteristics for cases and controls are presented in Supplementary Table 1 (available online). We found strong correlations between log2-transformed concentrations of estrone and estradiol (r = 0.81) and androstenedione and DHEA concentrations (r = 0.79). Relatively strong correlations were observed between concentrations of estrone and androstenedione (r = 0.5), testosterone and androstenedione (r = 0.58), and progesterone and androstenedione (r = 0.54) (Table 2).
Table 1.
Baseline characteristics of colon cancer cases and controls in EPIC and NSHDS participantsa
| Variable | EPIC |
NSHDS |
||
|---|---|---|---|---|
| Cases (n = 486) | Controls (n = 490) | Cases (n = 26) | Controls (n = 26) | |
| Mean (SD) age at blood collection, y | 62.0 (5.4) | 61.9 (5.4) | 60.4 (2.1) | 60.3 (2.0) |
| Mean (SD) body weight, kg | 68.6 (11.8) | 67.5 (10.5) | 75.9 (12.9) | 69.5 (13.4) |
| BMI, No. (%) | 26.6 (4.6) | 26.5 (4.2) | 28.4 (5.5) | 26.6 (4.9) |
| Underweight (>18.5 kg/m2) | 5 (1.0) | 5 (1.0) | 0 (0.0) | 1 (3.9) |
| Normal (18.5-24.9 kg/m2) | 196 (40.3) | 190 (38.8) | 8 (30.8) | 12 (46.2) |
| Overweight (25.0-29.9 kg/m2) | 203 (39.1) | 203 (41.4) | 11 (42.3) | 7 (26.9) |
| Obese (≥ 30 kg/m2) | 92 (19.6) | 92 (18.8) | 7 (26.9) | 6 (23.1) |
| Smoking status, No. (%) | ||||
| Never | 299 (61.5) | 309 (63.1) | 10 (38.5) | 14 (53.9) |
| Former | 111 (22.8) | 104 (21.2) | 12 (46.2) | 7 (26.9) |
| Current | 68 (14.0) | 71 (14.5) | 4 (15.4) | 5 (19.2) |
| Unknown | 8 (1.7) | 6 (1.2) | N/A | N/A |
| Physical activity, No. (%) | ||||
| Inactive | 169 (34.8) | 172 (35.1) | 4 (15.4) | 9 (34.6) |
| Moderately inactive | 151 (31.1) | 150 (30.6) | 6 (23.1) | 6 (23.1) |
| Moderately active | 87 (17.9) | 88 (18.0) | 6 (23.1) | 4 (15.4) |
| Active | 65 (13.4) | 68 (13.9) | 8 (30.8) | 5 (19.2) |
| Missing | 14 (2.9) | 12 (2.5) | 2 (7.7) | 2 (7.7) |
| Ever used menopausal HT, No. (%) | ||||
| No | 387 (79.6) | 392 (80.0) | 26 (100.0) | 26 (100.0) |
| Yes | 70 (14.4) | 72 (14.7) | N/A | N/A |
| Unknown/missing | 29 (6.0) | 26 (5.3) | N/A | N/A |
| Mean (SD) alcohol consumption, g/d | 5.7 (9.8) | 5.9 (10.2) | 3.0 (3.5) | 1.5 (2.1) |
| Serologic variables, median (IQR) | ||||
| Estrone, pg/mL | 18.1 (12.3-25.1) | 17.7 (12.9-23.6) | 25.1 (22.0-40.5) | 23.9 (20.9-33.6) |
| Estradiol, pg/mL | 3.9 (2.6-6.0) | 4.0 (2.6-6.0) | 6.2 (4.3-11.6) | 5.9 (5.1-11.5) |
| Testosterone, pg/mL | 185.9 (129.0-257.2) | 183.5 (127.9-246.2) | 227.4 (166.8-298.8) | 213.1 (162.4-275.8) |
| Androstenedione, ng/mL | 490.1 (346.1-660.4) | 466.9 (348.6-641.7) | 709.0 (517.9-878.4) | 566.7 (497.4-832.9) |
| DHEA, ng/mL | 1.9 (1.2-2.8) | 1.8 (1.2-2.9) | 2.5 (1.5-3.7) | 2.4 (1.8-3.6) |
| Progesterone, pg/mL | 52.2 (37.5-76.1) | 53.1 (39.6-73.5) | 65.3 (51.3-100.8) | 62.3 (43.5-83.0) |
| SHBG, nmol/L | 54.2 (38.4-74.6) | 52.9 (40.4-70.1) | 51.8 (37.5-68.8) | 65.4 (52.1-86.9) |
| Free estradiol, pg/mL | 85.8 (53.7-149.5) | 91.8 (56.8-138.3) | 159.0 (102.0-266.6) | 125.3 (95.7-251.0) |
| Free testosterone, ng/mL | 5.2 (3.2-7.8) | 5.2 (3.3-7.4) | 7.0 (4.7-9.9) | 5.5 (3.4-8.9) |
BMI= body mass index; DHEA = dehydroepiandrosterone; EPIC = European Prospective Investigation into Cancer and Nutrition; HT = hormone therapy; IQR = interquartile range; N/A = not applicable; NSHDS = The Northern Sweden Health and Disease Study; SD = standard deviation; SHBG = sex hormone–binding protein.
Table 2.
Pearson correlation matrix for circulating sex hormone concentrations, SHBG, and BMI adjusted for age and batcha
| Concentrations of sex hormone, SHBG, and BMI | Estrone | Estradiol | Testosterone | Androstenedione | DHEA | Progesterone | SHBG | BMI |
|---|---|---|---|---|---|---|---|---|
| Estrone | – | – | – | – | – | – | – | – |
| Estradiol | 0.81 | – | – | – | – | – | – | – |
| Testosterone | 0.40 | 0.38 | – | – | – | – | – | – |
| Androstenedione | 0.50 | 0.33 | 0.58 | – | – | – | – | – |
| DHEA | 0.35 | 0.20 | 0.42 | 0.79 | – | – | – | – |
| Progesterone | 0.24 | 0.15 | 0.38 | 0.54 | 0.42 | – | – | – |
| SHBG | –0.09 | –0.19 | 0.14 | –0.09 | –0.02 | 0.11 | – | – |
| BMI | 0.28 | 0.39 | 0.08 | 0.07 | –0.02 | –0.08 | –0.37 | – |
Serologic variables were log2 transformed. BMI = body mass index; DHEA = dehydroepiandrosterone; SHBG = sex hormone–binding globulin.
In the multivariable model, a nonstatistically significantly positive relationship was found between circulating estrone levels and colon cancer risk (odds ratio [OR] per log2 1-unit increment = 1.17 [95% CI = 1.00 to 1.38; ORquartile4-quartile1[q4-q1] = 1.33 [95% CI = 0.89 to 1.97], Ptrend = .20) (Table 3). We found no associations between circulating concentrations of estradiol (ORq4-q1 = 1.04 [95% CI = 0.70 to 1.56], Ptrend = .98), free estradiol (ORq4-q1 = 1.05 [95% CI = 0.71 to 1.57], Ptrend = .90), testosterone (ORq4-q1 = 1.17 [95% CI = 0.81 to 1.68], Ptrend = .44), free testosterone (ORq4-q1 = 1.25 [95% CI = 0.87 to 1.79], Ptrend = 0.32), androstenedione (ORq4-q1 = 1.12 [95% CI = 0.77 to 1.62], Ptrend = .42), DHEA (ORq4-q1 = 0.85 [95% CI = 0.58 to 1.23], Ptrend = .78), progesterone (ORq4-q1 = 1.03 [95% CI = 0.72 to 1.48], Ptrend = .94), and SHBG (ORq4-q1 = 1.00 [95% CI = 0.69 to 1.45], Ptrend = .91) and colon cancer risk, with similar relationships also found in the continuous models (Table 3). However, the positive relationship between circulating estrone levels and colon cancer risk was nonstatistically significant after false-discovery rate correction. Similar associations were observed when circulating estrone, estradiol, and testosterone models were additionally adjusted for SHBG concentrations and vice versa (Table 3). In addition, we found no evidence of an association between the circulating estradiol-to-testosterone ratio and colon cancer risk (ORq4-q1=1.07 [95% CI = 0.72 to 1.61], Ptrend = .96). Also, a similar pattern of results was found after the multiple imputation of hormone concentrations with lower LLOQ values (Supplementary Table 2, available online). We found a similar pattern of associations when cases diagnosed within the first 2 years of follow-up were excluded from the analyses (Supplementary Table 3, available online).
Table 3.
Associations between circulating concentrations of sex hormones and SHBG with colon cancer in postmenopausal women (n = 1028)
| Variables | Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | P trend a | Continuous modelb | FDR (Q value) |
|---|---|---|---|---|---|---|---|
| Estrone | |||||||
| Quartile cut points | <13.1 | 13.1-18.1 | 18.1-24.0 | ≥24.0 | – | – | – |
| No. (cases/controls) | 128/129 | 118/129 | 111/129 | 155/129 | – | – | – |
| Unadjusted OR (95% CI)c | 1 [Referent] | 0.92 (0.64 to 1.31) | 0.88 (0.61 to 1.27) | 1.25 (0.87 to 1.81) | .26 | 1.16 (0.99 to 1.35) | – |
| Multivariable-adjusted OR (95% CI)c,d | 1 [Referent] | 0.94 (0.65 to 1.36) | 0.91 (0.62 to 1.32) | 1.32 (0.89 to 1.96) | .23 | 1.17 (1.00 to 1.38) | 0.49 |
| Multivariable-adjusted OR (95% CI)c,e | 1 [Referent] | 0.95 (0.65 to 1.37) | 0.92 (0.63 to 1.35) | 1.33 (0.89 to 1.97) | .20 | 1.17 (1.00 to 1.38) | – |
| Estradiol | |||||||
| Quartile cut points | <2.6 | 2.6-4.1 | 4.1-6.1 | ≥6.1 | – | – | – |
| No. (cases/controls) | 128/129 | 133/129 | 116/129 | 135/129 | – | – | – |
| Unadjusted OR (95% CI)c | 1 [Referent] | 1.03 (0.72 to 1.47) | 0.90 (0.63 to 1.28) | 1.04 (0.73 to 1.50) | .97 | 1.03 (0.92 to 1.15) | – |
| Multivariable-adjusted OR (95% CI)c,d | 1 [Referent] | 1.03 (0.72 to 1.47) | 0.89 (0.61 to 1.29) | 1.04 (0.70 to 1.56) | .98 | 1.04 (0.92 to 1.17) | 0.89 |
| Multivariable-adjusted OR (95% CI)c,e | 1 [Referent] | 1.04 (0.73 to 1.48) | 0.91 (0.62 to 1.32) | 1.08 (0.72 to 1.61) | .86 | 1.04 (0.92 to 1.18) | – |
| Testosterone | |||||||
| Quartile cut points | <129.3 | 129.3-184.5 | 184.5-249.9 | ≥249.9 | – | – | – |
| No. (cases/controls) | 126/129 | 122/129 | 117/129 | 147/129 | – | – | – |
| Unadjusted OR (95% CI)c | 1 [Referent] | 0.98 (0.70 to 1.38) | 0.93 (0.65 to 1.34) | 1.18 (0.83 to 1.68) | .41 | 1.02 (0.86 to 1.22) | – |
| Multivariable-adjusted OR (95% CI)c,d | 1 [Referent] | 0.96 (0.68 to 1.36) | 0.92 (0.64 to 1.33) | 1.17 (0.81 to 1.68) | .44 | 1.02 (0.86 to 1.22) | 0.89 |
| Multivariable-adjusted OR (95% CI)c,e | 1 [Referent] | 0.95 (0.67 to 1.35) | 0.90 (0.63 to 1.31) | 1.14 (0.79 to 1.65) | .51 | 1.01 (0.85 to 1.21) | – |
| Androstenedione | |||||||
| Quartile cut points | <353.8 | 353.8-477.7 | 477.7-645.5 | ≥645.5 | – | – | – |
| No. (cases/controls) | 136/129 | 104/129 | 123/129 | 149/129 | – | – | – |
| Unadjusted OR (95% CI)c | 1 [Referent] | 0.77 (0.53 to 1.11) | 0.91 (0.65 to 1.29) | 1.11 (0.77 to 1.60) | .40 | 1.04 (0.86 to 1.26) | – |
| Multivariable-adjusted OR (95% CI)c,d | 1 [Referent] | 0.78 (0.54 to 1.14) | 0.92 (0.65 to 1.31) | 1.12 (0.77 to 1.62) | .42 | 1.03 (0.85 to 1.25) | 0.89 |
| DHEA | |||||||
| Quartile cut points | <1.2 | 1.2-1.8 | 1.8-2.9 | ≥2.9 | – | – | – |
| No. (cases/controls) | 134/129 | 112/129 | 149/129 | 117/129 | – | – | – |
| Unadjusted OR (95% CI)c | 1 [Referent] | 0.85 (0.60 to 1.19) | 1.13 (0.80 to 1.59) | 0.87 (0.60 to 1.25) | .84 | 0.98 (0.86 to 1.13) | – |
| Multivariable-adjusted OR (95% CI)c,d | 1 [Referent] | 0.82 (0.58 to 1.17) | 1.12 (0.79 to 1.59) | 0.85 (0.58 to 1.23) | .78 | 0.98 (0.85 to 1.12) | 0.89 |
| Progesterone | |||||||
| Quartile cut points | <39.6 | 39.6-53.6 | 53.6-73.6 | ≥73.6 | – | – | – |
| No. (cases/controls) | 139/129 | 124/128 | 106/130 | 143/129 | – | – | – |
| Unadjusted OR (95% CI)c | 1 [Referent] | 0.90 (0.63 to 1.27) | 0.76 (0.53 to 1.08) | 1.03 (0.72 to 1.47) | .90 | 0.95 (0.80 to 1.12) | – |
| Multivariable-adjusted OR (95% CI)c,d | 1 [Referent] | 0.90 (0.63 to 1.28) | 0.76 (0.53 to 1.09) | 1.03 (0.72 to 1.48) | .94 | 0.95 (0.80 to 1.13) | 0.89 |
| SHBG | |||||||
| Quartile cut points | <40.8 | 40.8-53.5 | 53.5-70.7 | ≥70.7 | – | – | – |
| No. (cases/controls) | 146/128 | 108/130 | 111/129 | 147/129 | – | – | – |
| Unadjusted OR (95% CI)c | 1 [Referent] | 0.71 (0.50 to 1.02) | 0.74 (0.52 to 1.06) | 0.99 (0.71 to 1.40) | .93 | 0.99 (0.83 to 1.19) | – |
| Multivariable-adjusted OR (95% CI)c,d | 1 [Referent] | 0.72 (0.50 to 1.04) | 0.75 (0.52 to 1.09) | 1.00 (0.69 to 1.45) | .91 | 0.99 (0.81 to 1.21) | 0.92 |
| Multivariable-adjusted OR (95% CI)c,e | 1 [Referent] | 0.73 (0.50 to 1.05) | 0.77 (0.53 to 1.12) | 0.99 (0.68 to 1.44) | .96 | 0.99 (0.81 to 1.20) | – |
| Free estradiol | |||||||
| Quartile cut points | <57.7 | 57.7-94.7 | 94.7-142.3 | ≥142.3 | – | – | – |
| No. (cases/controls) | 136/129 | 135/129 | 95/129 | 146/129 | – | – | – |
| Unadjusted OR (95% CI)c | 1 [Referent] | 0.98 (0.70 to 1.37) | 0.67 (0.46 to 0.98) | 1.04 (0.73 to 1.46) | .88 | 1.02 (0.92 to 1.13) | – |
| Multivariable-adjusted OR (95% CI)c,d | 1 [Referent] | 0.96 (0.68 to 1.36) | 0.66 (0.45 to 0.99) | 1.05 (0.71 to 1.57) | .90 | 1.03 (0.92 to 1.15) | 0.89 |
| Free testosterone | |||||||
| Quartile cut points | <3.3 | 3.3-5.2 | 5.2-7.4 | ≥7.4 | – | – | – |
| No. (cases/controls) | 126/129 | 123/129 | 108/129 | 155/129 | – | – | – |
| Unadjusted OR (95% CI)c | 1 [Referent] | 0.99 (0.70 to 1.39) | 0.87 (0.61 to 1.24) | 1.25 (0.88 to 1.78) | .31 | 1.02 (0.92 to 1.13) | – |
| Multivariable-adjusted OR (95% CI)c,d | 1 [Referent] | 0.98 (0.69 to 1.39) | 0.86 (0.60 to 1.24) | 1.25 (0.87 to 1.79) | .32 | 1.03 (0.92 to 1.15) | 0.89 |
| Estradiol-to-testosterone ratio | |||||||
| Quartile cut points | <0.19 | 0.19-0.27 | 0.27-0.33 | ≥0.33 | – | – | – |
| No. (cases/controls) | 124/129 | 146/129 | 105/129 | 137/129 | – | – | – |
| Unadjusted OR (95% CI)c | 1 [Referent] | 1.15 (0.82 to 1.60) | 0.83 (0.57 to 1.20) | 1.08 (0.76 to 1.55) | .97 | 1.21 (0.52 to 2.83) | – |
| Multivariable-adjusted OR (95% CI)c,d | 1 [Referent] | 1.13 (0.81 to 1.60) | 0.83 (0.56 to 1.22) | 1.07 (0.72 to 1.61) | .96 | 1.28 (0.51 to 3.24) | 0.89 |
Statistical tests for trend (2-sided) were calculated using ordinal quartile variables entered into the model as a single continuous variable. CI = confidence intervals; DHEA = dehydroepiandrosterone; FDR = false-discovery rate; OR = odds ratio; SHBG = sex hormone–binding globulin.
Serologic variables were log2 transformed in continuous models.
Odds ratios and 95% confidence intervals were estimated by conditional logistic regression models.
Adjusted for body mass index (underweight, normal, overweight, or obese), smoking status (never, former, current, or unknown), physical activity (inactive, moderately inactive, moderately active, active, or unknown), ever used hormone therapy (yes, no, or unknown/missing), and alcohol consumption (continuous).
Additionally adjusted for estrone, estradiol, and testosterone for SHBG and vice versa.
In analyses by colon subsite, there was a nonstatistically significantly positive relationship between circulating concentrations of estrone and proximal colon cancer in the continuous model only (OR per log2 1-unit increment = 1.22 [95% CI = 1.00 to 1.48]), with no association found for distal colon cancer (OR per log2 1-unit increment = 1.00 [95% CI = 0.71 to 1.41]). Other circulating concentrations of sex hormones were not associated with proximal or distal colon cancer risk (Supplementary Table 4, available online).
Table 4 shows the results of subgroup analyses according to BMI categories (<25 kg/m2 and ≥25 kg/m2). None of the associations of sex hormones with colon cancer risk differed according to BMI categories (all Pinteraction ≥ .07), but circulating estrone concentrations were positively associated with colon cancer risk among the overweight/obese group (BMI ≥25 kg/m2) (OR per log2 increment = 1.33 [95% CI = 1.01 to 1.75) but not the normal-weight group (OR per log2 increment = 1.05 [95% CI = 0.66 to 1.68], Pinteraction = .07). We found a similar pattern of associations according to follow-up time group (all Pinteraction ≥ .07) (Supplementary Table 5, available online).
Table 4.
Associations between circulating concentrations of sex hormones and SHBG with colon cancer in postmenopausal women (n = 1028), by strata of BMI
| Variable | Tertile 1 | Tertile 2 | Tertile 3 | Pt rend a | Continuous modelb | P valuec | P interaction d |
|---|---|---|---|---|---|---|---|
| Estrone | |||||||
| Tertile cut points | <14.5 | 14.5-21.7 | ≥21.7 | – | – | – | – |
| No. (cases/controls) | 161/171 | 161/173 | 190/172 | – | – | – | – |
| BMI, multivariable-adjusted OR (95% CI)e | .07 | ||||||
| <25 kg/m2 | 1 [Referent] | 0.83 (0.39 to1.73) | 1.07 (0.36 to 3.16) | .91 | 1.05 (0.66 to 1.68) | .82 | – |
| ≥25 kg/m2 | 1 [Referent] | 0.92 (0.51 to 1.64) | 1.47 (0.83 to 2.62) | .15 | 1.33 (1.01 to 1.75) | .04 | – |
| Estradiol | |||||||
| Tertile cut points | <3.0 | 3.0-5.2 | ≥5.2 | – | – | – | – |
| No. (cases/controls) | 169/171 | 177/173 | 166/172 | – | – | – | – |
| BMI, multivariable-adjusted OR (95% CI)e | .15 | ||||||
| <25 kg/m2 | 1 [Referent] | 1.57 (0.71 to 3.45) | 0.75 (0.27 to 2.02) | .83 | 0.89 (0.64 to 1.25) | .51 | – |
| ≥25 kg/m2 | 1 [Referent] | 1.16 (0.68 to 1.97) | 1.26 (0.68 to 2.33) | .46 | 1.10 (0.87to 1.39) | .41 | – |
| Testosterone | |||||||
| Tertile cut points | <149.5 | 149.5-221.1 | ≥221.1 | – | – | – | – |
| No. (cases/controls) | 170/171 | 147/172 | 195/173 | – | – | – | – |
| BMI, multivariable-adjusted OR (95% CI)e | .51 | ||||||
| <25 kg/m2 | 1 [Referent] | 0.71 (0.32 to 1.57) | 0.97 (0.39 to 2.40) | .91 | 0.91 (0.57 to 1.45) | .69 | – |
| ≥25 kg/m2 | 1 [Referent] | 0.91 (0.54 to 1.52) | 1.21 (0.71 to 2.06) | .48 | 1.08 (0.81 to 1.44) | .59 | – |
| Androstenedione | |||||||
| Tertile cut points | <394.7 | 394.7-589.2 | ≥589.2 | – | – | – | – |
| No. (cases/controls) | 165/171 | 166/171 | 181/173 | – | – | – | – |
| BMI, multivariable-adjusted OR (95% CI)e | .12 | ||||||
| <25 kg/m2 | 1 [Referent] | 0.56 (0.27 to 1.16) | 0.77 (0.36 to 1.66) | .42 | 0.85 (0.54 to 1.35) | .50 | – |
| ≥25 kg/m2 | 1 [Referent] | 1.07 (0.63 to 1.84) | 1.23 (0.73 to 2.07) | .44 | 1.14 (0.84 to 1.55) | .41 | – |
| DHEA | |||||||
| Tertile cut points | <1414.3 | 1414.3-2482.3 | ≥2482.3 | – | – | – | – |
| No. (cases/controls) | 171/171 | 173/172 | 168/173 | – | – | – | – |
| BMI, multivariable-adjusted OR (95% CI)e | .23 | ||||||
| <25 kg/m2 | 1 [Referent] | 0.27 (0.11 to 0.66) | 0.53 (0.23 to 1.21) | .17 | 0.84 (0.62 to 1.16) | .29 | – |
| ≥25 kg/m2 | 1 [Referent] | 1.15 (0.69 to 1.93) | 0.96 (0.56 to 1.63) | .91 | 1.01 (0.81 to 1.26) | .91 | – |
| Progesterone | |||||||
| Tertile cut points | <44.1 | 44.1-65.4 | ≥65.4 | – | – | – | – |
| No. (cases/controls) | 170/172 | 168/172 | 174/172 | – | – | – | – |
| BMI, multivariable-adjusted OR (95% CI)e | .86 | ||||||
| <25 kg/m2 | 1 [Referent] | 0.50 (0.24 to 1.06) | 1.29 (0.56 to 2.95) | .73 | 1.04 (0.68 to 1.59) | .86 | – |
| ≥25 kg/m2 | 1 [Referent] | 1.27 (0.73 to 2.21) | 1.27 (0.74 to 2.16) | .39 | 1.01 (0.75 to 1.36) | .95 | – |
| SHBG | |||||||
| Tertile cut points | <46.0 | 46.0-63.7 | ≥63.7 | – | – | – | – |
| No. (cases/controls) | 195/172 | 131/172 | 186/172 | – | – | – | – |
| BMI, multivariable-adjusted OR (95% CI)e | .73 | ||||||
| <25 kg/m2 | 1 [Referent] | 0.75 (0.26 to 2.17) | 0.95 (0.37 to 2.39) | .91 | 1.31 (0.73 to 2.36) | .36 | – |
| ≥25 kg/m2 | 1 [Referent] | 0.70 (0.41 to 1.19) | 1.04 (0.59 to 1.84) | .94 | 0.94 (0.68 to 1.30) | .69 | – |
| Free estradiol | |||||||
| Tertile cut points | <69.5 | 69.5-123.4 | ≥123.4 | – | – | – | – |
| No. (cases/controls) | 185/171 | 152/172 | 175/173 | – | – | – | – |
| BMI, multivariable-adjusted OR (95% CI)e | .07 | ||||||
| <25 kg/m2 | 1 [Referent] | 1.21 (0.53 to 2.77) | 0.51 (0.19 to 1.39) | .29 | 0.85 (0.62 to 1.16) | .30 | – |
| ≥25 kg/m2 | 1 [Referent] | 0.74 (0.43 to 1.28) | 1.07 (0.60 to 1.91) | .70 | 1.09 (0.88 to 1.35) | .46 | – |
| Free testosterone | |||||||
| Tertile cut points | <3.9 | 3.9-6.6 | ≥6.6 | – | – | – | – |
| No. (cases/controls) | 157/171 | 161/172 | 194/173 | – | – | – | – |
| BMI, multivariable-adjusted OR (95% CI)e | .55 | ||||||
| <25 kg/m2 | 1 [Referent] | 0.99 (0.47 to 2.11) | 1.11 (0.50 to 2.47) | .81 | 0.85 (0.62 to 1.16) | .30 | – |
| ≥25 kg/m2 | 1 [Referent] | 1.07 (0.63 to 1.83) | 1.23 (0.72 to 2.10) | .45 | 1.09 (0.88 to 1.35) | .46 | – |
| Estradiol-to-testosterone ratio | |||||||
| Tertile cut points | <0.22 | 0.22-0.31 | ≥0.31 | – | – | – | – |
| No. (cases/controls) | 168/171 | 175/172 | 169/173 | – | – | – | – |
| BMI, multivariable-adjusted OR (95% CI)e | .20 | ||||||
| <25 kg/m2 | 1 [Referent] | 1.24 (0.60 to 2.54) | 0.67 (0.24 to 1.87) | .69 | 0.42 (0.05 to 3.93) | .45 | – |
| ≥25 kg/m2 | 1 [Referent] | 1.06 (0.61 to 1.82) | 1.43 (0.76 to 2.71) | .26 | 2.10 (0.37 to 11.87) | .40 |
Statistical tests for trend (2-sided) were calculated using ordinal tertile variables entered into the model as a single continuous variable. BMI = body mass index; CI = confidence interval; DHEA = dehydroepiandrosterone; OR = odds ratio; SHBG = sex hormone–binding globulin.
Serologic variables were log2 transformed in a continuous model.
Statistical tests (2-sided) were calculated as a continuous variable.
Heterogeneity by BMI categories were tested using χ2 tests. The test was 2-sided.
Odds ratios and 95% confidence intervals were estimated by conditional logistic regression models. Models were adjusted for BMI (underweight, normal, overweight, or obese), smoking status (never, former, current, or unknown), physical activity (inactive, moderately inactive, moderately active, active, or unknown), ever use hormone therapy (yes, no, or unknown/missing), and alcohol consumption (continuous). Estrone, estradiol, and testosterone were adjusted for SHBG and vice versa.
Meta-analysis
We identified 74 articles, with an additional 6 articles based on screening of titles or abstracts. Of the 80 articles, 7 nested case-control studies (8-12,14), including the current study, were eligible for inclusion in the meta-analysis for colorectal, colon, and rectal cancer. We performed 5 meta-analyses: 1) estradiol and colorectal cancer (including 6 studies) (8-12,14), 2) estradiol and colon cancer (including 2 studies, 1 of which is the current study) (12), 3) estrone and colorectal cancer (including 4 studies), 4) estrone and colon cancer (including 3 studies, 1 of which is the current study) (10,12), and 5) estrone and rectal cancer (including 2 studies) (10,12) (Supplementary Table 6, available online).
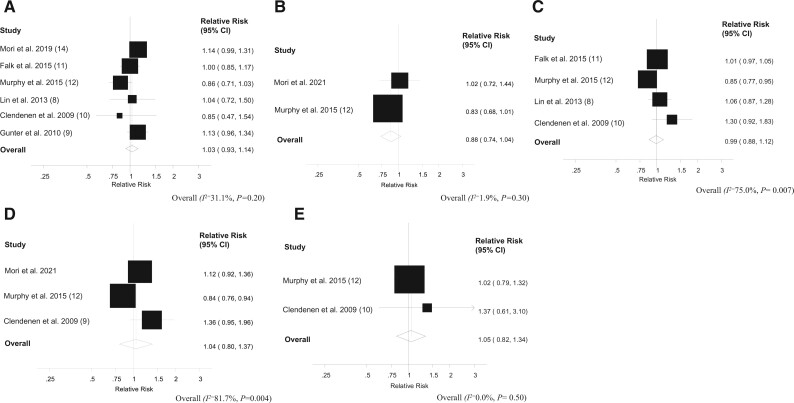
In the dose-response meta-analysis, we found no evidence of an association between circulating estradiol concentrations and colorectal cancer risk (summary RR per 5-pg/mL increase in estradiol concentration = 1.03 [95% CI = 0.93 to 1.14]), with low heterogeneity (I2 = 31.1%, P = .20) (Figure 1). We also found no association between circulating estrone concentrations and colorectal cancer risk (summary RR for a 10-pg/mL increase in estrone concentration = 0.99 [95% CI = 0.88 to 1.12]), with relatively high heterogeneity (I2 = 75.0%, P = .007). For the subsites, a nonstatistically significant inverse association was found between circulating estradiol concentrations and colon cancer risk (summary RR for 5-pg/mL increase in estradiol concentration = 0.88 [95% CI = 0.74 to 1.04]), with low heterogeneity (I2 = 1.9%, P = .30). No evidence of an association between circulating estrone concentrations and colon cancer risk (summary RR for 10-pg/mL increase in estrone concentration = 1.04 [95% CI = 0.80 to 1.37]), with high heterogeneity detected (I2 = 81.7%, P = .004). Little evidence of an association was found between circulating estrone concentrations and rectal cancer risk (summary RR for 10-g/mL increase in estrone concentration = 1.05 [95% CI = 0.82 to 1.34]), with low heterogeneity (I2 = 0.0%, P = .50). (Figure 1). We were unable to conduct a meta-analysis for the association between circulating estradiol concentrations and rectal cancer risk because only 1 study was available (12).
Figure 1.
Dose-response analysis between circulating estradiol, estrone, and colorectal cancer risk. A) Estradiol and colorectal cancer, per 5 pg/mL. B) Estradiol and colon cancer, per 5 pg/mL. C) Estrone and colorectal cancer, per 10 pg/mL. D) Estrone and colon cancer, per 10 pg/mL. E) Estrone and rectal cancer, per 10 pg/mL. The average of the natural logarithm of the relative risks was estimated, and the relative risk from each study was weighted using random effects weighting. A 2-tailed P < .05 was considered statistically significant. Heterogeneity between studies was quantitatively assessed by the Q test and I2. The black squares represent the odds ratios of the individual studies and the error bars their 95% confidence intervals (CIs). The area of the black squares reflects the weight each trial contributes in the meta-analysis.
Discussion
In this prospective study of postmenopausal European women, we found limited evidence for associations between circulating concentrations of sex hormones and SHBG with colon cancer risk. Associations were generally similar when the analyses were stratified according to follow-up time and for distal and proximal colon cancer. Similarly, we found no clear associations between circulating estradiol and estrone with risks of colorectal, colon, and rectal cancer when we conducted dose-response meta-analyses encompassing all available prospective data (including results from the current study).
Consistent with the lower colon cancer risk observed for HT users, several sources of experimental data suggest that estrogens may confer antitumorigenic effects on colon tumorigenesis. It has been shown, for example, that expression of estrogen receptor-β results in the inhibition of proliferation and G1 phase cell-cycle arrest in colon cancer cells; in xenograft mouse studies, estrogen receptor-β inhibits cMyc expression and tumor growth (35). Previously, in the WHI-CT, we observed robust inverse associations between circulating estradiol and estrone with colorectal and colon cancer risks (12). In contrast, the results from the current study suggest a borderline positive association between circulating estrone and cancer of the colon, particularly in the proximal region. This result is somewhat consistent with a study conducted within the Women’s Health Initiative Observational Study, which reported a positive association between estradiol levels and colorectal cancer risk (9). Other published data (8,10-12,14), however, did not observe associations between endogenous estrogens and colon cancer risk. Because of these inconsistent findings and the relatively small size of each prospective study that has examined estrogens and colorectal cancer in postmenopausal women, we conducted a meta-analysis to combine these data. Importantly, results from this meta-analysis found no evidence of an association between prediagnostic estrogen concentrations and colorectal, colon, or rectal cancer risk in postmenopausal women.
In our nested case-control study, we found a positive association between circulating estrone concentrations and colon cancer for overweight/obese women but not for normal-weight women. This heterogeneity according to BMI group, however, did not reach the threshold of statistical significance, and prior studies have found limited evidence that the sex hormone–colorectal cancer association may differ according to body size (10). Given the multiple statistical analyses conducted, it is possible that the positive association we observed between estrone and colon cancer for overweight/obese women was a chance finding. Further studies are needed to examine the role of body size on the sex hormone–colorectal cancer association.
Previously, we reported a positive association between circulating testosterone levels and colorectal cancer risk in a Japanese population (14). It should be noted, however, that this study used a less sensitive assay to measure sex hormone concentrations, and more than 60% of the participants had measured testosterone concentrations below the LLOQ (14). In the current analysis of postmenopausal European women, similar to studies of UK and US women (8,36) and a recent Mendelian randomization study (37), we found no association between circulating testosterone levels and colon cancer risk. We also found no association between circulating concentrations of androstenedione and DHEA with colon cancer risk. Taken together, these results provide little support for androgens having a prominent role in colon cancer development for postmenopausal women.
SHBG is a hepatically derived glycoprotein and principal transport protein of estrogens and androgens and is therefore an important regulator of their bioactivity (38). In addition, SHBG is reported to be correlated with inflammation and insulin sensitivity (39). Previously, in the WHI-CT, we found a more than 2-fold higher colorectal cancer risk when the highest and lowest quartiles of SHBG were compared (12). In contrast, results from our current analysis of postmenopausal European women found no association between SHBG concentrations and colon cancer risk, consistent with results from US, UK, and Japan-based studies (10,14,36) as well as a recent Mendelian randomization study (37). Overall, these results do not support a causal role for SHBG in colorectal cancer development and suggest that the previously reported associations were likely biased (eg, through confounding) or were the result of chance.
The current study was the largest conducted to date to examine circulating sex hormone concentrations and colon cancer risk associations in postmenopausal women. A major strength of the study was our use of a highly sensitive analytical method to measure sex hormone concentrations, with low intra- and interbatch coefficients of variation and the breadth of the sex hormones we were able to study. We were also able to include important confounders, such as fasting status, BMI, physical activity, and whether the patient ever used HT. In addition, we conducted a meta-analysis of circulating estrogens and colorectal cancer and its subsites, including estimates from previous studies as well as our current investigation. A limitation of the study was that sex hormone concentrations were measured in a single plasma sample; therefore, these measurements may not reflect longer-term exposures. Such measurement error may not have been substantial, however, because a prior analysis of postmenopausal women reported within-person correlation coefficients ranging from 0.66 to 0.92 for estrone, free estradiol, SHBG, androstenedione, testosterone, and DHEA measurements over a 2- to 3-year period (40), indicating that single measures of sex hormone concentrations provide good estimates of longer-term exposures.
In this prospective investigation of postmenopausal European women and meta-analysis of all published studies conducted to date, we found limited evidence of an association between circulating concentrations of endogenous sex hormones and colorectal, colon, and rectal cancer risks.
Funding
This work was supported by the French National Cancer Institute (INCa SHSESP17, grant No. 2017-127 to N. Murphy). The coordination of EPIC is financially supported by International Agency for Research on Cancer (IARC) and also by the Department of Epidemiology and Biostatistics, School of Public Health, Imperial College London, which has additional infrastructure support provided by the NIHR Imperial Biomedical Research Centre (BRC). The national cohorts are supported by: Danish Cancer Society (Denmark); Ligue Contre le Cancer, Institut Gustave Roussy, Mutuelle Générale de l’Education Nationale, Institut National de la Santé et de la Recherche Médicale (INSERM) (France); German Cancer Aid, German Cancer Research Center (DKFZ), German Institute of Human Nutrition Potsdam-Rehbruecke (DIfE), Federal Ministry of Education and Research (BMBF) (Germany); Associazione Italiana per la Ricerca sul Cancro-AIRC-Italy, Compagnia di SanPaolo and National Research Council (Italy); Dutch Ministry of Public Health, Welfare and Sports (VWS), Netherlands Cancer Registry (NKR), LK Research Funds, Dutch Prevention Funds, Dutch ZON (Zorg Onderzoek Nederland), World Cancer Research Fund (WCRF), Statistics Netherlands (the Netherlands); Health Research Fund (FIS) - Instituto de Salud Carlos III (ISCIII), Regional Governments of Andalucía, Asturias, Basque Country, Murcia and Navarra, and the Catalan Institute of Oncology—ICO (Spain); Swedish Cancer Society, Swedish Research Council and County Councils of Skåne and Västerbotten (Sweden); Cancer Research UK (14136 to EPIC-Norfolk; C8221/A29017 to EPIC-Oxford), Medical Research Council (1000143 to EPIC-Norfolk; MR/M012190/1 to EPIC-Oxford) (United Kingdom).
Notes
Role of the funders: The funders had no role in the design of the study; the collection, analysis, and interpretation of the data; the writing of the manuscript; or the decision to submit the manuscript for publication.
Disclosures: The authors have no conflicts of interest to disclose.
Author contributions: Conceptualization: SR, NMurphy, MJG; Lab analysis: PKR, AG, SR; Statistical analysis: NMori, DA; Supervision: NMurphy, MJG; Writing—original draft: NMori, SR, ND, SH, JH, AJC, NMurphy, MJG; Writing—review and editing: all authors.
Disclaimer: Where authors are identified as personnel of the International Agency for Research on Cancer/World Health Organization, the authors alone are responsible for the views expressed in this article and they do not necessarily represent the decisions, policy or views of the International Agency for Research on Cancer/World Health Organization.
Data Availability
For information on how to submit an application for gaining access to EPIC data and/or biospecimens, please follow the instructions at http://epic.iarc.fr/access/index.php.
Supplementary Material
References
- 1. Arnold M, Sierra MS, Laversanne M, Soerjomataram I, Jemal A, Bray F.. Global patterns and trends in colorectal cancer incidence and mortality. Gut. 2017;66(4):683–691. [DOI] [PubMed] [Google Scholar]
- 2. McMichael AJ, Potter JD.. Reproduction, endogenous and exogenous sex hormones, and colon cancer: a review and hypothesis. J Natl Cancer Inst. 1980;65(6):1201–1207. [PubMed] [Google Scholar]
- 3. Morch LS, Lidegaard O, Keiding N, Lokkegaard E, Kjaer SK.. The influence of hormone therapies on colon and rectal cancer. Eur J Epidemiol. 2016;31(5):481–489. [DOI] [PubMed] [Google Scholar]
- 4. Johnson JR, Lacey JV Jr, Lazovich D, et al. Menopausal hormone therapy and risk of colorectal cancer. Cancer Epidemiol Biomarkers Prev. 2009;18(1):196–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Simon MS, Chlebowski RT, Wactawski-Wende J, et al. Estrogen plus progestin and colorectal cancer incidence and mortality. J Clin Oncol. 2012;30(32):3983–3990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lin KJ, Cheung WY, Lai JY, Giovannucci EL.. The effect of estrogen vs. combined estrogen-progestogen therapy on the risk of colorectal cancer. Int J Cancer. 2012;130(2):419–430. [DOI] [PubMed] [Google Scholar]
- 7. Chlebowski RT, Wactawski-Wende J, Ritenbaugh C, et al. ; Women's Health Initiative Investigators. Estrogen plus progestin and colorectal cancer in postmenopausal women. N Engl J Med. 2004;350(10):991–1004. [DOI] [PubMed] [Google Scholar]
- 8. Lin JH, Zhang SM, Rexrode KM, et al. Association between sex hormones and colorectal cancer risk in men and women. Clin Gastroenterol Hepatol. 2013;11(4):419–424.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gunter MJ, Hoover DR, Yu H, et al. Insulin, insulin-like growth factor-I, endogenous estradiol, and risk of colorectal cancer in postmenopausal women. Cancer Res. 2008;68(1):329–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Clendenen TV, Koenig KL, Shore RE, Levitz M, Arslan AA, Zeleniuch-Jacquotte A.. Postmenopausal levels of endogenous sex hormones and risk of colorectal cancer. Cancer Epidemiol Biomarkers Prev. 2009;18(1):275–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Falk RT, Dallal CM, Lacey JV Jr, et al. ; B∼FIT Research Group. Estrogen metabolites are not associated with colorectal cancer risk in postmenopausal women. Cancer Epidemiol Biomarkers Prev. 2015;24(9):1419–1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Murphy N, Strickler HD, Stanczyk FZ, et al. A prospective evaluation of endogenous sex hormone levels and colorectal cancer risk in postmenopausal women. J Natl Cancer Inst. 2015;107(10):djv210. doi:10.1093/jnci/djv210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Simpson ER. Aromatization of androgens in women: current concepts and findings. Fertil Steril. 2002;77(suppl 4):S6–S10. [DOI] [PubMed] [Google Scholar]
- 14. Mori N, Sawada N, Iwasaki M, et al. Circulating sex hormone levels and colorectal cancer risk in Japanese postmenopausal women: the JPHC nested case-control study. Int J Cancer. 2019;145(5):1238–1244. [DOI] [PubMed] [Google Scholar]
- 15. Slimani N, Kaaks R, Ferrari P, et al. European Prospective Investigation into Cancer and Nutrition (EPIC) calibration study: rationale, design and population characteristics. Public Health Nutr. 2002;5(6B):1125–1145. [DOI] [PubMed] [Google Scholar]
- 16. Riboli E, Hunt KJ, Slimani N, et al. European Prospective Investigation into Cancer and Nutrition (EPIC): study populations and data collection. Public Health Nutr. 2002;5(6B):1113–1124. [DOI] [PubMed] [Google Scholar]
- 17. Riboli E, Kaaks R.. The EPIC Project: rationale and study design. European Prospective Investigation into Cancer and Nutrition. Int J Epidemiol. 1997;26(suppl 1):S6–S14. doi:10.1093/ije/26.suppl_1.s6. [DOI] [PubMed] [Google Scholar]
- 18. Hallmans G, Agren A, Johansson G, et al. Cardiovascular disease and diabetes in the Northern Sweden Health and Disease Study Cohort—evaluation of risk factors and their interactions. Scand J Public Health Suppl. 2003;61:18–24. [DOI] [PubMed] [Google Scholar]
- 19. Norberg M, Wall S, Boman K, Weinehall L.. The Västerbotten Intervention Programme: background, design and implications. Glob Health Action. 2010;3: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bergmann MM, Bussas U, Boeing H.. Follow-up procedures in EPIC-Germany—data quality aspects. European Prospective Investigation into Cancer and Nutrition. Ann Nutr Metab. 1999;43(4):225–234. [DOI] [PubMed] [Google Scholar]
- 21. Bergmann MM, Noethlings U, Eisinger B, et al. The importance of the common cancer registry for the identification of cancer cases in the EPIC Potsdam-study—results of the first record linkage [in German]. Gesundheitswesen. 2004;66(8-9):475–481. [DOI] [PubMed] [Google Scholar]
- 22.World Health Organization. International Classification of Diseases for Oncology. 3rd ed. Geneva, Switzerland: World Health Organization; 2000. [Google Scholar]
- 23. Gambineri A, Pelusi C.. Sex hormones, obesity and type 2 diabetes: is there a link? Endocr Connect. 2019;8(1):R1–R9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kim C, Halter JB.. Endogenous sex hormones, metabolic syndrome, and diabetes in men and women. Curr Cardiol Rep. 2014;16(4):467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Muka T, Nano J, Jaspers L, et al. Associations of steroid sex hormones and sex hormone-binding globulin with the risk of type 2 diabetes in women: a population-based cohort study and meta-analysis. Diabetes. 2017;66(3):577–586. [DOI] [PubMed] [Google Scholar]
- 26. Franke AA, Custer LJ, Morimoto Y, Nordt FJ, Maskarinec G.. Analysis of urinary estrogens, their oxidized metabolites, and other endogenous steroids by benchtop orbitrap LCMS versus traditional quadrupole GCMS. Anal Bioanal Chem. 2011;401(4):1319–1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Keski-Rahkonen P, Desai R, Jimenez M, Harwood DT, Handelsman DJ.. Measurement of estradiol in human serum by LC-MS/MS using a novel estrogen-specific derivatization reagent. Anal Chem. 2015;87(14):7180–7186. [DOI] [PubMed] [Google Scholar]
- 28. Södergård R, Bäckström T, Shanbhag V, Carstensen H.. Calculation of free and bound fractions of testosterone and estradiol-17 beta to human plasma proteins at body temperature. J Steroid Biochem. 1982;16(6):801–810. [DOI] [PubMed] [Google Scholar]
- 29. Vermeulen A, Verdonck L, Kaufman JM.. A critical evaluation of simple methods for the estimation of free testosterone in serum. J Clin Endocrinol Metab. 1999;84(10):3666–3672. [DOI] [PubMed] [Google Scholar]
- 30. Rinaldi S, Geay A, Déchaud H, et al. Validity of free testosterone and free estradiol determinations in serum samples from postmenopausal women by theoretical calculations. Cancer Epidemiol Biomarkers Prev. 2002;11(10 pt 1):1065–1071. [PubMed] [Google Scholar]
- 31. Benjamini Y, Hochberg Y.. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser B: Methodol. 1995;57(1):289–300. [Google Scholar]
- 32. Schafer JL. Analysis of Incomplete Multivariate Data. New York: Chapman & Hall; 1997. [Google Scholar]
- 33.Dempster AP, Laird NM, Rubin DB. Maximum likelihood from incomplete data via the EM algorithm. J R Stat Soc Ser B: Methodol. 1977;39(1):1–38.
- 34. Greenland S, Longnecker MP.. Methods for trend estimation from summarized dose-response data, with applications to meta-analysis. Am J Epidemiol. 1992;135(11):1301–1309. [DOI] [PubMed] [Google Scholar]
- 35. Hartman J, Edvardsson K, Lindberg K, et al. Tumor repressive functions of estrogen receptor beta in SW480 colon cancer cells. Cancer Res. 2009;69(15):6100–6106. [DOI] [PubMed] [Google Scholar]
- 36. Watts EL, Perez-Cornago A, Knuppel A, Tsilidis KK, Key TJ, Travis RC.. Prospective analyses of testosterone and sex hormone-binding globulin with the risk of 19 types of cancer in men and postmenopausal women in UK Biobank. Int J Cancer. 2021;149(3):573–584. doi:10.1002/ijc.33555] [33720423. [DOI] [PubMed] [Google Scholar]
- 37. Dimou N, Mori N, Harlid S, et al. Circulating levels of testosterone, sex hormone binding globulin and colorectal cancer risk: observational and Mendelian randomization analyses. Cancer Epidemiol Biomarkers Prev. 2021;30(7):1336–1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Rosner W. The functions of corticosteroid-binding globulin and sex hormone-binding globulin: recent advances. Endocr Rev. 1990;11(1):80–91. [DOI] [PubMed] [Google Scholar]
- 39. Simó R, Sáez-López C, Barbosa-Desongles A, Hernández C, Selva DM.. Novel insights in SHBG regulation and clinical implications. Trends Endocrinol Metab. 2015;26(7):376–383. [DOI] [PubMed] [Google Scholar]
- 40. Hankinson SE, Manson JE, Spiegelman D, Willett WC, Longcope C, Speizer FE.. Reproducibility of plasma hormone levels in postmenopausal women over a 2-3-year period. Cancer Epidemiol Biomarkers Prev. 1995;4(6):649–654. [8547832] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
For information on how to submit an application for gaining access to EPIC data and/or biospecimens, please follow the instructions at http://epic.iarc.fr/access/index.php.