Abstract
Objectives
To investigate the increase in the rates of OXA-48-like-producing isolates during 3 years of global surveillance.
Methods
Among 55?>162 Enterobacterales isolates, 354 carbapenem-resistant isolates carried genes encoding OXA-48-like enzymes. Isolates were susceptibility tested for ceftazidime/avibactam and comparators by broth microdilution methods. Analysis of β-lactam resistance mechanisms and MLST was performed in silico using WGS data.
Results
OXA-48-like-producing isolates increased from 0.5% (94/18 656) in 2016 to 0.9% (169/18?>808) in 2018. OXA-48 was the most common variant; isolates primarily were Klebsiella pneumoniae (318/354 isolates) from Europe and adjacent countries. MLST analysis revealed a diversity of STs, but K. pneumoniae belonging to ST395, ST23 and ST11 were observed most frequently. Thirty-nine isolates harboured MBLs and were resistant to most agents tested. The presence of blaCTX-M-15 (258 isolates), OmpK35 nonsense mutations (232) and OmpK36 alterations (316) was common among OXA-48 producers. Ceftazidime, cefepime and aztreonam susceptibility rates, when applying CLSI breakpoints, were 12%–15% lower for isolates carrying ESBLs alone and with either or both OmpK35 stop codons and OmpK36 alterations. Meropenem and, remarkably, meropenem/vaborbactam were affected by specific OmpK36 alterations when a deleterious mutation also was observed in OmpK35. These mechanisms caused a decrease of 12%–42% in the susceptibility rates for meropenem and meropenem/vaborbactam. Ceftazidime/avibactam susceptibility rates were >98.9%, regardless of the presence of additional β-lactam resistance mechanisms.
Conclusions
Guidelines for the treatment of infections caused by OXA-48-producing isolates are scarce and, as the dissemination of these isolates continues, studies are needed to help physicians understand treatment options for these infections.
Introduction
Carbapenem -resistant Enterobacterales (CRE) have been highlighted as an important threat to patients by the WHO, CDC and other institutions that monitor human health.1,2 Among CRE isolates, the isolates that produce carbapenemases are especially worrisome. Carbapenemase-producing Enterobacterales (CPE) often are MDR; therefore, limited treatment options are available to treat the serious infections they cause.3 Additionally, CPE isolates might belong to high-risk clones that promote their greater dissemination and have virulence factors that make them more fit to cause serious infections.4
In surveys that assess global prevalence of CPE isolates, OXA-48 variants have been recognized as the second or third most common CPE variant.5 The most prevalent OXA-48 variants are, in order, OXA-48, OXA-181, OXA-232, OXA-204, OXA-162 and OXA-244, but at least 13 other OXA-48 variants have been described.6 While isolates producing OXA-48 variants are endemic in certain areas, they also are being introduced in other regions where they disseminate and can be sporadically observed.
Overall, oxacillinases hydrolyse oxacillin more efficiently than benzylpenicillin and are resistant to inhibition by clavulanate, tazobactam and sulbactam.7 OXA-48 variants share these characteristics and have activity against amino-, carboxy, ureidopenicillins and narrow-spectrum cephalosporins.7 These enzymes have limited activity against cephamycins, monobactams and broad-spectrum cephalosporins, especially ceftazidime.6 Notably, OXA-48 variants have weak activity against the carbapenems, except for imipenem.7
Studies performed before the approval of new β-lactam/β-lactamase inhibitor combinations demonstrated that patients with infections caused by OXA-48-producing organisms experienced high mortality rates. A study published in 2013 by Navarro-San Francisco et al.8 documented a 30 day mortality rate of 50% in patients with bacteraemia caused by OXA-48-producing isolates. Recently, treatment options for infections caused by isolates producing OXA-48 variants have been evaluated9 and the IDSA has recommended ceftazidime/avibactam as the treatment option for infections caused by these isolates.10 Drug combinations that included a carbapenem have shown poor efficacy in the treatment of infections caused by OXA-48-like producers, despite the low carbapenem MIC values that some of the OXA-48-producing strains might display against these agents.1,9 However, combinations including amikacin or colistin have achieved treatment success in a few case reports. Ceftazidime/avibactam has been considered an important treatment option for CRE isolates due to its activity against isolates producing KPC and/or OXA-48.9
We evaluated the occurrence of Enterobacterales isolates carrying OXA-48 enzymes over 3 years of the SENTRY Antimicrobial Surveillance Programme and then analysed the presence of other β-lactam resistance mechanisms in these isolates. We also reported the activity of ceftazidime/avibactam, meropenem/vaborbactam and comparator agents against 354 isolates carrying OXA-48-like genes.
Materials and methods
A total of 53?>200 Enterobacterales isolates were submitted to the SENTRY Antimicrobial Surveillance Programme during the years 2016, 2017 and 2018. These isolates were collected in 149 hospitals located in 31 countries from Asia-Pacific, Europe, Latin America and North America that participated in the programme during all 3 years. Only bacterial isolates determined to be significant by local criteria as the reported probable cause of an infection were included in this investigation.
Species identification was confirmed when necessary by MALDI-TOF mass spectrometry using the Bruker Daltonics MALDI Biotyper (Billerica, MA, USA), following the manufacturer’s instructions.
Antimicrobial susceptibility was evaluated by reference broth microdilution methods according to CLSI procedures (document M07).11 CLSI and susceptibility interpretive criteria were used to determine susceptibility/resistance rates for ceftazidime/avibactam, meropenem/vaborbactam and comparator agents.12 Quality control (QC) testing was performed to ensure proper test conditions and procedures. QC strains included Escherichia coli ATCC 25922 and NCTC 13353, Klebsiella pneumoniae ATCC 700603 and ATCC BAA-1705, and Pseudomonas aeruginosa ATCC 27853.
Isolates displaying an MIC value ≥2 mg/L against imipenem and/or meropenem or to any two of the following agents—ceftazidime, ceftriaxone, cefepime or aztreonam—were all identified using MALDI-TOF and then submitted for WGS. Genomic DNA was extracted and prepared using Nextera XT™ library construction protocol and index kit (Illumina, San Diego, CA, USA), following the manufacturer’s instructions. The sequencing was performed on a MiSeq instrument (Illumina) with a target coverage of 30×. Reads were assembled using de novo assembler SPAdes 3.11.113 with K-values of 21, 33, 55, 77 and 99, and careful mode on to reduce the number of mismatches, producing contiguous sequences with the best N50 value. Resistance determinants from the NCBI Bacterial Antimicrobial Resistance Reference Gene Database were aligned to assembled sequences to identify β-lactamase genes: hits with greater than 94% identity and 40% minimum coverage length were selected for further analysis. OmpK35/OmpF and OmpK36/OmpC sequences were analysed and compared with reference sequences.
For each assembly, a multilocus ST was determined using definitions from PubMLST.14,15 Relevant K. pneumoniae STs and metadata were used as input to PHYLOViZ16,17 to generate a full minimum spanning tree (MST) representation of a globally optimized (go) implementation of the updated (e) BURST (Based Upon Related Sequence Types) (goeBURST) algorithm.18,19
To assess the presence and identity of blaOXA-48-containing plasmids, sequencing reads for each of the 354 blaOXA-48-like-carrying isolates were trimmed using Sickle version 1.331,20 and mapped to a pOXA-48 reference sequence (GenBank # JN626286) from K. pneumoniae strain Kp119784,21 using Bowtie2 version 2.3.5.2,22 A consensus sequence for each of the reference-guided assemblies was built using SAMtools/BCFtools/HTSLib version 1.1023,24 and BEDTools version 2.27.1.6,25 These consensus sequences were aligned26 back to the pOXA-48 reference and analysed for percentage identity and percentage coverage. The presence of pOXA-48 was confirmed for reference-guided assemblies that covered 100% of the reference with greater than 95% identity. For isolates where the presence of the pOXA-48 plasmid could not be confirmed, we selected the blaOXA-48-like-containing contiguous sequence from each de novo assembly and employed PlasmidFinder version 2.127,28 to identify additional incompatibility types .
Results
Epidemiology of Enterobacterales isolates carrying genes encoding OXA-48 variants
A total of 354 isolates carrying OXA-48-like-encoding genes were detected among Enterobacterales isolates with MIC values ≥2 mg/L for any of the following agents: imipenem, meropenem, ceftazidime, cefepime, ceftriaxone or aztreonam. Most of the isolates carrying genes encoding OXA-48 variants were K. pneumoniae (318/354; 89.8%), but another six species also harboured these genes. Among these other Enterobacterales species, 12 were E. coli, 9 were Enterobacter cloacae species complex, 6 were Klebsiella oxytoca, 5 were Serratia marcescens, 3 were Citrobacter freundii species complex and 1 was a Raoultella ornithinolytica.
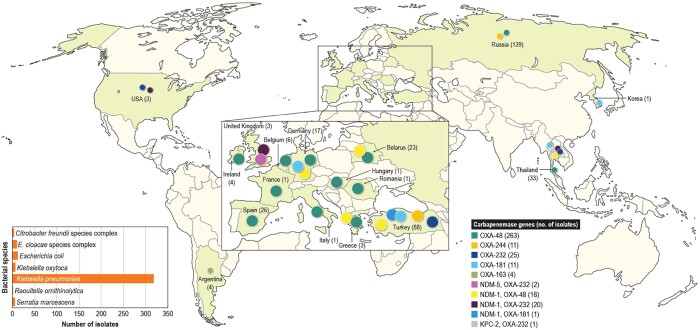
As observed by others, blaOXA-48 was the most common variant; it was detected in 279 isolates. Additionally, 48, 12, 11 and 4 isolates carried genes encoding OXA-232, OXA-181, OXA-244 and OXA-163, respectively. Forty isolates carried other carbapenemases alongside OXA-48-like-encoding genes (23 OXA-232, 16 OXA-48 and 1 OXA-181). Twenty of these 40 isolates carried genes encoding NDM-1 plus OXA-232, 16 had NDM-1 plus OXA-48, 2 isolates had NDM-5 plus OXA-232 and 1 each carried NDM-1 plus OXA-181 or KPC-2 plus OXA-232.
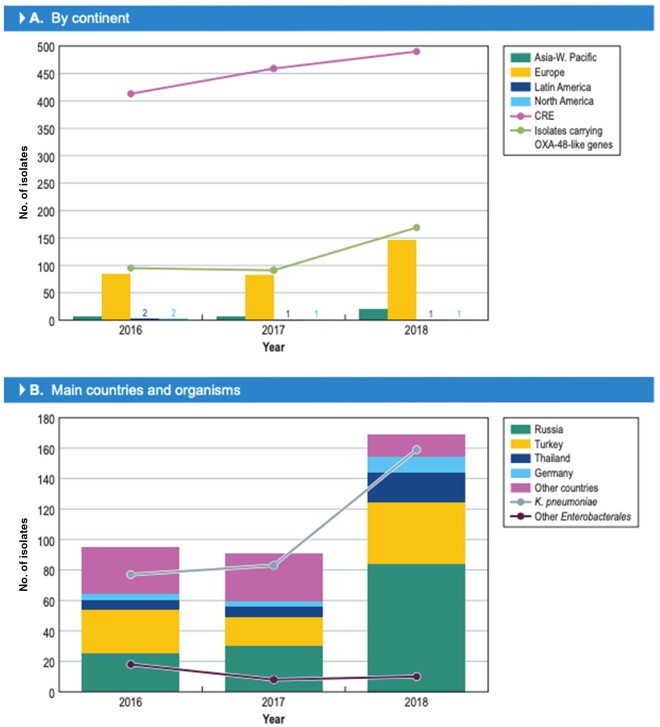
The number of isolates carrying OXA-48 variants significantly increased in 2018 (Figure 1a). In 2016 and 2017, 94 and 91 (185/36?>354 isolates) isolates harbouring these enzymes were detected, while 169 (out of 18 808) isolates were found in 2018 (P ≤ 0.0001; OR 0.565 [95% CI 0.4340, 0.7307]). Isolates carrying genes encoding OXA-48 variants were identified in 30 hospitals located in 17 countries. The countries with the greatest number of isolates producing OXA-48-like enzymes were Russia (139/670 isolates [denominator reflects the number of Enterobacterales isolates received during the study]), Turkey (88/1055 isolates), Thailand (33/320 isolates), Spain (26/1831 isolates), Belarus (23/294 isolates) and Germany (17/3110 isolates; Figure 2). The increase in the numbers of OXA-48-producing isolates was driven mainly by an increase in the numbers of K. pneumoniae isolates in Russia and Turkey (Figure 1b).
Figure 1 .
Increase in CRE and/or isolates carrying OXA-48-like genes in 2018 compared with 2016 and 2017.
Figure 2.
Distribution of OXA-48-like-encoding genes worldwide.
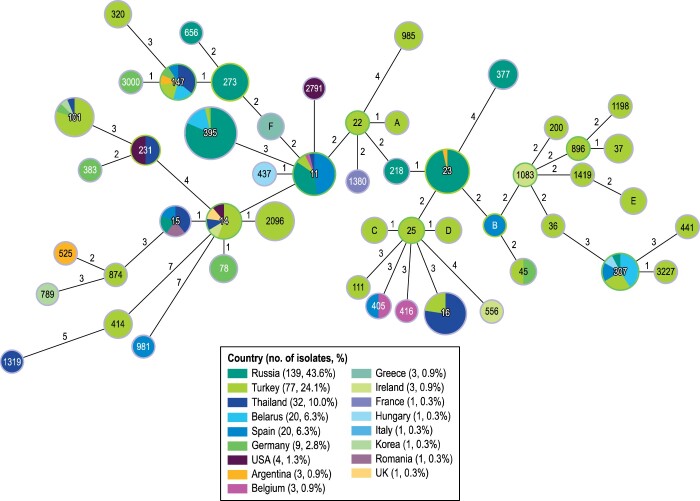
At least 59 MLST profiles were identified among the K. pneumoniae isolates carrying genes encoding OXA-48 variants (Figure 3). Despite the diversity of STs, 29.6% (94/318) of the isolates belonged to ST395 while 72.3% (230/318) of the isolates belonged to just 10 other STs. Of these, the most common STs were ST23 (31 isolates), ST11 (27 isolates) and ST16 (22 isolates). Most isolates belonging to ST395 were from Russia and Turkey (53 and 22 isolates, respectively), but these isolates also were observed in six other countries.
Figure 3.
MLST for K. pneumoniae isolates carrying OXA-48-like genes. The numbers in the circles represent the ST and the numbers in the lines represent the number of allele differences between groups of isolates. The lengths of the lines are not proportional to the number of differences. The colours indicate the origin of the isolates by country.
Among other Enterobacterales, six STs were noted among 12 E. coli isolates, five STs among 9 E. cloacae species complex and two STs among 3 C. freundii species complex isolates. Notably, 4 E. coli isolates belonged to ST410; these isolates were from Turkey and Thailand (3 and 1 isolates, respectively). Five K. oxytoca isolates belonging to the same ST were identified in a single hospital in Germany, but two other STs were observed in this bacterial species.
Over a third (126/354; 35.5%) of the isolates carried OXA-48 in the pOXA-48a IncL plasmid (GenBank #JN626286) associated with the global dissemination of these genes.6,7 A total of 52 (16.6%) isolates had the blaOXA-48-like genes embedded in the ColKP3 plasmid (#JN258). Despite many reports describing ColKP3 carrying blaOXA-232,23,29,30 we noted this plasmid also harboured blaOXA-48, blaOXA-181 and blaOXA-163 (20, 5 and 1 isolates, respectively). Lastly, six other isolates carried blaOXA-48-like genes embedded in three other plasmid structures: IncM2 (#AF55415; 3 isolates), IncC (#JN15784; 2 isolates) and IncM1 (#U27345; 1 isolate). We were unable to find plasmids associated with blaOXA-48-like among the remaining 168 isolates studied.
The presence of pOXA-48a and ColKP3 was not associated with a specific blaOXA-48 variant, ST, or country.
Other β-lactam resistance mechanisms
Beyond carbapenemases, OXA-48-producing isolates carried ESBLs, transferable cephalosporinases and other β-lactamase-encoding genes (Table 1). Among ESBLs, the gene encoding CTX-M-15 was observed among 258 isolates; of those 258 isolates, 78 carried this ESBL gene and blaOXA-48-like only. In all other instances, blaCTX-M-15 was accompanied by blaOXA-1 and/or blaTEM, blaSHV, blaCMY or blaDHA-1. Other CTX-M-encoding genes were noted among 19 isolates, including blaCTX-M-14, blaCTX-M-3, blaCTX-M-9, blaCTX-M-24 and blaCTX-M-55. Genes encoding SHV enzymes with ESBL spectrum were detected alone in six isolates and with blaCTX-M genes in another six isolates. Additionally, five isolates carried only transferable AmpC genes and one isolate harboured blaOXA-1 alone. A total of 54 isolates carried no other enzymes beyond OXA-48 and the intrinsic (chromosomal) cephalosporinase or ubiquitous (SHV-1-like in K. pneumoniae) enzymes for that bacterial species.
Table 1.
Additional β-lactam resistance mechanisms observed among 319 OXA-48-like-producing K. pneumoniae
| Organisms/OMP alterations | No. of isolates among all K. pneumoniae | No. of isolates with acquired broad-spectrum β-lactamases |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ESBLs |
MBLs | KPC-2 | transferable AmpC ± ESBL | only OXA-48-like | ||||||
| all ESBLs | CTX-M-14 | CTX-M-15 | CTX-M-15 + OXA-1 | other ESBLs | ||||||
| All K. pneumoniae | 319 | 241 | 9 | 57 | 156 | 19 | 36 | 1 | 4 | 37 |
| OMP alterations | ||||||||||
| OmpK35 stop codon | 212 | 152 | 6 | 44 | 92 | 10 | 25 | 1 | 2 | 32 |
| all OmpK36 alterations | 197 | 141 | 6 | 41 | 85 | 9 | 22 | 2 | 32 | |
| OmpK36 A183_T184insLSP | 67 | 50 | 3 | 46 | 1 | 4 | 2 | 11 | ||
| all OmpK36 G134_D135ins | 130 | 91 | 6 | 38 | 39 | 8 | 18 | 21 | ||
| OmpK36 G134_D135insD | 18 | 3 | 3 | 15 | ||||||
| OmpK36 G134_D135insDG | 20 | 15 | 5 | 1 | 8 | 1 | 2 | 3 | ||
| OmpK36 G134_D135insDG, A183_T184insLSP | 88 | 70 | 1 | 32 | 31 | 6 | 1 | 17 | ||
| OmpK36 G134_D135insDT, G182_A183insTS | 2 | 2 | 2 | |||||||
| OmpK36 stop codon | 2 | 1 | 1 | 1 | ||||||
| OmpK36 WT | 15 | 11 | 3 | 7 | 1 | 3 | 1 | |||
| OmpK35 WT | 107 | 89 | 3 | 13 | 64 | 9 | 11 | 2 | 5 | |
| all OmpK36 alterations | 76 | 64 | 1 | 10 | 50 | 3 | 8 | 2 | 2 | |
| OmpK36 A183_T184insLSP | 44 | 39 | 5 | 31 | 3 | 1 | 2 | 2 | ||
| all OmpK36 G134_D135ins | 32 | 25 | 1 | 5 | 19 | 7 | ||||
| OmpK36 G134_D135insD | 1 | 1 | 1 | |||||||
| OmpK36 G134_D135insDG | 23 | 16 | 16 | 7 | ||||||
| OmpK36 G134_D135insDG, A183_T184insLSP | 4 | 4 | 4 | |||||||
| OmpK36 stop codon | 4 | 4 | 1 | 3 | ||||||
| OmpK36 WT | 31 | 25 | 2 | 3 | 14 | 6 | 3 | 3 | ||
Analysis of outer membrane protein (OMP) sequences revealed that 232 isolates had a stop codon in OmpK35 or OmpF. Only 38 isolates had no insertions and deletions in the OmpK36/OmpC sequences.
Alterations in OMPs that have been described previously to be involved in β-lactam resistance were noted among 288 K. pneumoniae isolates. Among the alterations observed, the insertion of LSP in position 183 and the D/DG/DT insertion in position 134 (119 of the mature protein) were noted in 202 and 156 of the K. pneumoniae isolates, respectively. In 94 isolates, these alterations were observed together. Notably, 195 isolates had LSP or the D/DG/DT insertions in OmpK36 and a nonsense mutation in OmpK35.
Activity of ceftazidime/avibactam, meropenem/vaborbactam and other agents
The 39 OXA-producing isolates carrying MBLs (36 K. pneumoniae, 2 E. coli and 1 E. cloacae species complex) displayed remarkably low susceptibility rates for most agents tested (data not shown). Tigecycline was the only agent that displayed activity against all isolates (100.0% susceptibility). Amikacin and gentamicin inhibited 53.8% and 48.7% of these isolates, respectively, when CSLI breakpoints were applied. Intermediate colistin MIC values were observed among 61.5% of the isolates when recently revised CLSI breakpoints with no susceptible category were applied.
Among the remaining 315 isolates that did not carry an MBL, susceptibility rates were higher for most agents when compared with the isolates that co-produced MBLs (Table 2). Ceftazidime/avibactam and tigecycline were the most active agents against these isolates, inhibiting 99.0% and 95.6% of them when applying the CLSI and US FDA breakpoints, respectively. Amikacin and meropenem/vaborbactam inhibited 56.8% and 46.7% of these isolates, respectively, whereas 81.5% had an intermediate colistin MIC value.
Table 2.
Susceptibility profiles of 315 OXA-48-producing Enterobacterales without MBL genes
| Antimicrobial agent | MIC50 | MIC90 | Range | CLSIa |
EUCASTa |
||||
|---|---|---|---|---|---|---|---|---|---|
| %S | %I | %R | %S | %I | %R | ||||
| Ceftazidime/avibactam | 2 | 4 | 0.06 to >8 | 99.0 | 1.0 | 99.0 | 1.0 | ||
| Meropenem/vaborbactam | 16 | >16 | 0.06 to >16 | 46.7 | 3.2 | 50.2 | 49.8 | 50.2 | |
| Ceftazidime | >32 | >32 | 0.06 to >32 | 20.6 | 1.3 | 78.1 | 15.9 | 4.8 | 79.4 |
| Ceftriaxone | >8 | >8 | 0.25 to >8 | 7.6 | 5.7 | 86.7 | 7.6 | 5.7 | 86.7 |
| Cefepime | >16 | >16 | ≤0.12 to >16 | 15.2 | 6.0 | 78.7b | 10.8 | 8.6 | 80.6 |
| Aztreonam | >16 | >16 | ≤0.03 to >16 | 18.1 | 0.3 | 81.6 | 16.8 | 1.3 | 81.9 |
| Imipenem | 4 | >8 | ≤0.12 to >8 | 7.3 | 18.4 | 74.3 | 25.7 | 29.5 | 44.8 |
| Meropenem | >8 | >8 | 0.06 to >8 | 24.8 | 11.1 | 64.1 | 35.9 | 11.7 | 52.4 |
| Ampicillin/sulbactam | >32 | >32 | >32 to >32 | 0.0 | 0.0 | 100.0 | 0.0 | 100.0c | |
| Piperacillin/tazobactam | >128 | >128 | 2 to >128 | 0.6 | 0.3 | 99.0 | 0.6 | 0.0 | 99.4 |
| Ceftolozane/tazobactam | >8 | >8 | 0.25 to >8 | 10.2 | 9.8 | 80.0 | 10.2 | 89.8 | |
| Ciprofloxacin | >4 | >4 | ≤0.03 to >4 | 7.0 | 1.6 | 91.4 | 7.0 | 1.6 | 91.4 |
| Levofloxacin | >4 | >4 | ≤0.03 to >4 | 11.2 | 1.6 | 87.2 | 11.2 | 1.6 | 87.2 |
| Tetracycline | >16 | >16 | 1 to >16 | 21.7 | 10.5 | 67.8 | |||
| Minocycline | 8 | >32 | 0.5 to >32 | 46.7 | 23.2 | 30.2 | |||
| Doxycycline | >8 | >8 | 0.5 to >8 | 29.8 | 7.6 | 62.5 | |||
| Tigecycline | 1 | 2 | 0.12 to 8 | 95.6 | 3.8 | 0.6c | |||
| Amikacin | 8 | >32 | 0.5 to >32 | 56.8 | 1.9 | 41.3 | 51.1 | 48.9 | |
| Gentamicin | >8 | >8 | ≤0.12 to >8 | 35.9 | 1.0 | 63.2 | 35.9 | 64.1 | |
| Tobramycin | >8 | >8 | ≤0.12 to >8 | 17.1 | 9.5 | 73.3 | 14.9 | 85.1 | |
| Colistin | 0.25 | >8 | ≤0.06 to >8 | 81.5 | 18.5 | 81.5 | 18.5 | ||
| Trimethoprim/sulfamethoxazole | >4 | >4 | ≤0.5 to >4 | 21.0 | 79.0 | 21.0 | 6.7 | 72.3 | |
S, susceptible; I, intermediate; R, resistant.
Criteria as published by CLSI (2020)12 and EUCAST (2020) (https://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Breakpoint_tables/v_11.0_Breakpoint_Tables.pdf).
Intermediate is interpreted as susceptible-dose-dependent.
FDA breakpoints were published on 13 December 2017 (https://www.fda.gov/drugs/development-resources/antibacterial-susceptibility-test-interpretive-criteria).
The impact of OMP alterations and/or ESBLs on the susceptibility of β-lactam agents was evaluated for 282 OXA-48-like-carrying K. pneumoniae that did not harbour an MBL (Table 3). Among 12 isolates displaying OmpK35 nonsense mutations and intact OmpK36, the susceptibility of ceftazidime, cefepime and aztreonam was 13.1%–15.6% lower than for the 282 K. pneumoniae isolates. The susceptibility of these β-lactams was also reduced by 4.1%–6.6% in the 67 isolates that exhibited alterations in OmpK36 alone. Notably, both OMP alterations had a greater impact on the susceptibility of meropenem and meropenem/vaborbactam than seen in the other agents. These two agents had 10.8% and 12.9% lower susceptibility rates against the 175 isolates carrying a double OMP alteration compared with the 282 OXA-48-like-producing K. pneumoniae. Further analysis demonstrated that substitutions in position G134 (G119 of the mature protein; 110 isolates) caused a decrease in 19.0% and 41.7% of the susceptibility rates of meropenem and meropenem/vaborbactam, respectively, whereas alterations in position 183 (63 isolates) did not affect the susceptibility of any of the β-lactam agents analysed.
Table 3.
Susceptibility profiles of OXA-48-producing K. pneumoniae without MBL genes and carrying other resistance mechanisms against tested β-lactam agents
| Group of K. pneumoniae isolates (no. of isolates) | % Susceptibility applying CLSI breakpoints |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| ceftazidime/avibactam | meropenem/vaborbactam | imipenem | meropenem | ceftazidime | ceftriaxone | cefepime | aztreonam | piperacillin/tazobactam | |
| All K. pneumoniae (282) | 99.6 | 42.6 | 6.4 | 19.9 | 15.6 | 5.7 | 13.1 | 15.6 | 0.7 |
| OMPs | |||||||||
| OmpK35 stop codon (12) | 100.0 | 75.0 | 8.3 | 50.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| OmpK36 alterations (67) | 100.0 | 52.2 | 6.0 | 25.4 | 9.0 | 6.0 | 9.0 | 10.4 | 0.0 |
| OmpK35 stop codon/OmpK36 alteration (175) | 99.4 | 29.7 | 4.0 | 9.1 | 18.3 | 5.1 | 14.9 | 18.9 | 0.6 |
| OmpK35 stop codon/OmpK36 G134 alteration (110) | 99.1 | 0.9 | 0.9 | 0.9 | 18.2 | 5.5 | 13.6 | 18.2 | 0.9 |
| OmpK35 stop codon/OmpK36 A183 alteration (63) | 100.0 | 81.0 | 9.5 | 23.8 | 19.0 | 4.8 | 17.5 | 20.6 | 0.0 |
| OmpK35 WT/OmpK36 WT (28) | 100.0 | 85.7 | 21.4 | 60.7 | 21.4 | 10.7 | 17.9 | 14.3 | 3.6 |
| ESBLs/transferable AmpC | |||||||||
| ESBL/transferable AmpC (245) | 99.6 | 42.9 | 7.3 | 21.2 | 4.1 | 1.2 | 2.9 | 4.1 | 0.8 |
| CTX-M-15 plus OXA-1 (155) | 99.4 | 51.0 | 6.5 | 20.6 | 0.6 | 0.6 | 0.6 | 0.6 | 1.3 |
| CTX-M-15 alone (58) | 100.0 | 22.4 | 6.9 | 19.0 | 1.7 | 0.0 | 0.0 | 1.7 | 0.0 |
| other CTX-M enzymes (15) | 100.0 | 13.3 | 0.0 | 0.0 | 13.3 | 0.0 | 0.0 | 0.0 | 0.0 |
| other ESBLs/transferable AmpC (12) | 100.0 | 66.7 | 25.0 | 58.3 | 33.3 | 16.7 | 16.7 | 33.3 | 0.0 |
| absent of ESBL/transferable AmpC (37) | 100.0 | 40.5 | 0.0 | 10.8 | 91.9 | 35.1 | 81.1 | 91.9 | 0.0 |
| ESBL plus OmpK35 stop codon/OmpK36 G134 alteration (91) | 98.9 | 1.1 | 1.1 | 1.1 | 2.2 | 0.0 | 0.0 | 2.2 | 1.1 |
| Both resistance mechanisms | |||||||||
| ESBL plus OmpK35 stop codon (64) | 100.0 | 78.1 | 10.9 | 32.8 | 1.6 | 1.6 | 1.6 | 3.1 | 0.0 |
| ESBL plus OmpK36 G134 alteration (25) | 100.0 | 8.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| OmpK35 stop codon/OmpK36 G134 alteration without ESBL (21) | 100.0 | 0.0 | 0.0 | 0.0 | 85.7 | 28.6 | 71.4 | 85.7 | 0.0 |
| ESBL plus OmpK35 WT/OmpK36 WT (65) | 100.0 | 80.0 | 15.4 | 46.2 | 10.8 | 3.1 | 9.2 | 9.2 | 1.5 |
| OmpK35 stop codon without ESBL (11) | 100.0 | 90.9 | 100.0 | 18.2 | 90.9 | 100.0 | 0.0 | 0.0 | 0.0 |
| absent of ESBL plus OmpK35 WT/OmpK36 WT (5) | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 0.0 | 80.0 | 0.0 |
As expected, when an isolate produced an ESBL in addition to an OXA-48-like, it caused a decrease in the susceptibility rates of ceftazidime, cefepime and aztreonam. The efficacy of ceftazidime was affected by the presence of CTX-M-15 with or without OXA-1 (155 and 58 isolates, respectively; 14.9% decrease in susceptibility rates), whereas other ESBLs (CTX-Ms or SHVs; 15 isolates) caused a noticeable reduction in cefepime and aztreonam susceptibility. Susceptibility rates were 12.5%–13.1% lower for cefepime and 11.5%–15.6% lower for aztreonam when ESBL-producing isolates were compared with the overall K. pneumoniae collection.
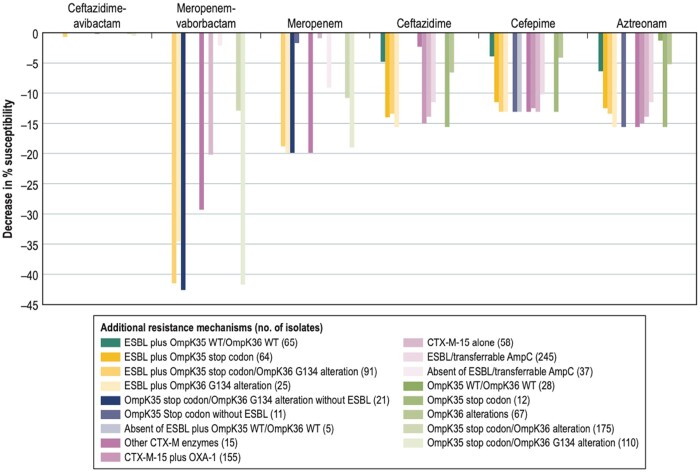
The presence of double alteration in the OMPs, regardless of the presence of an ESBL, decreased the susceptibility rates of meropenem and, more importantly, meropenem/vaborbactam, when compared with the overall isolates (Figure 4). Meropenem susceptibility rates were 18.8%–19.9% lower for isolates exhibiting mutations in OmpK36 or in both OMPs. The double OMP alterations were more impactful in the susceptibility rates for meropenem/vaborbactam, reducing the susceptibility against this combination by 41.5%–42.6% when these alterations were present. The presence of an ESBL and OmpK36 mutations reduced the susceptibility to the combination by 34.6%. The presence of an ESBL with changes in either OmpK35 (64 isolates), OmpK36 (25 isolates), or both OmpK35 and OmpK36 (91 isolates) reduced the susceptibility rates of ceftazidime, cefepime and aztreonam compared with the overall collection of K. pneumoniae isolates or the 5 isolates that only had an ESBL and no important OMP alteration. Notably, the susceptibility rates for ceftazidime/avibactam were unchanged, regardless of the presence of additional resistance mechanisms alone or in combination in these isolates (98.9%–100% susceptible).
Figure 4.
Decrease in susceptibility rates of β-lactam agents due to co-production of ESBLs and/or presence of outer membrane protein alterations compared with the overall K. pneumoniae isolates without MBLs (n = 282).
Ceftriaxone susceptibility rates were low overall, but reductions of 4.5%–5.7% in the susceptibility rates for this cephalosporin were noted in the presence of any ESBL or transferable AmpC, any CTX-M, with OmpK35 alterations alone, and in combinations of ESBL and one or both altered OMPs.
Discussion
In this study, we reported the occurrence of Enterobacterales isolates producing OXA-48-like enzymes collected during a 3 year period in hospitals worldwide. We documented a remarkable increase in OXA-48-like-producing isolates, mainly due to the increase of K. pneumoniae carrying these enzymes from Russia, Turkey and a few other countries in 2018.
Despite the limited hydrolytic spectrum of OXA-48 enzymes,6 isolates carrying these β-lactamases can be resistant to various β-lactams. Thus, we performed a detailed analysis of additional β-lactam resistance mechanisms among the OXA-48-like-carrying isolates to understand the role of these additional mechanisms in creating resistance to β-lactam agents. Our evaluation included looking for additional β-lactamase-encoding genes and alterations in OMPs. Genes encoding an MBL were detected among 39 isolates and, when compared with isolates that did not carry these genes, these isolates displayed lower susceptibility rates to most agents tested. The combination of OXA-48-like and MBL genes has been documented consistently across the literature.
Remarkable differences were observed when analysing the β-lactam susceptibility profile of the 282 OXA-48-like-carrying K. pneumoniae without MBLs. Isolates that harboured blaOXA-48-like but no other resistance mechanisms were resistant to carbapenems and ceftriaxone but exhibited high susceptibility rates to ceftazidime, cefepime and aztreonam. These isolates all were susceptible to ceftazidime/avibactam and most were susceptible to meropenem/vaborbactam. The activity of meropenem and meropenem/vaborbactam was much reduced (a decrease of >18% in susceptibility) in the presence of ESBLs with simultaneous alterations in OmpK35 and OmpK36.
Other in vitro studies have documented the activity of ceftazidime/avibactam against OXA-48-producing Enterobacterales isolates or the variable activity of meropenem/vaborbactam against these isolates.15,16,27,31–33 In this study, we evaluated a large collection of OXA-48-like-producing K. pneumoniae isolates harbouring additional resistance mechanisms in addition to the OXA-48-like encoding genes. The presence of β-lactamases and OMP alterations affected many of the β-lactams tested, but not ceftazidime/avibactam.
A recent study that exposed OXA-48-producing K. pneumoniae isolates in vitro to ceftazidime/avibactam demonstrated that mutations could occur that increase hydrolysis against ceftazidime or decrease the inhibitory potential of avibactam,18 similar to what has been demonstrated here regarding KPC enzymes.34 Thus, guidelines to prevent the emergence of these mutants, such as the use of combination therapy, need to be evaluated and implemented before isolates containing these alterations emerge in a clinical setting.
Studies documenting the clinical use of ceftazidime/avibactam against OXA-48 are still scarce due to the low prevalence of these organisms in many countries, the recent approval of this agent and the lack of the availability of this combination in countries where it has not been approved. Souza et al.35 evaluated 57 patients that received ceftazidime/avibactam for the treatment of infections caused by OXA-48-like-producing organisms. Despite the severity of the infections found in the patients included in the study, these infections were for indications that have not been approved, so several patients received this combination as monotherapy and as rescue therapy. Accordingly, the authors observed an all-cause mortality rate of 14% at 14 days and 22% at 30 days. De la Calle et al.36 also noted low 30 day and 90 day mortality rates when retrospectively analysing a smaller cohort of patients with infections caused by OXA-48-producing Enterobacterales treated with ceftazidime/avibactam.
The possible increase and dissemination of OXA-48-like-producing isolates has been highlighted in the past.7 These isolates can spread undetected due to the limited hydrolytic profile of OXA-48 enzymes that confer low resistance for many agents in isolates that do not co-produce other resistance mechanisms. We might be seeing the results of that silent spread, but the ongoing dissemination of these isolates in regions where these organisms are well established also have contributed to the number of OXA-48-like producing isolates we observed in 2018.
Lastly, clinical and in vitro data have provided evidence that ceftazidime/avibactam is a valuable therapeutic option to treat infections caused OXA-48-producing Enterobacterales without an MBL present. These organisms often have co-resistance mechanisms that did not affect the susceptibility of ceftazidime/avibactam.
Acknowledgements
We would like to thank the SENTRY Antimicrobial Surveillance Programme participants for their years of contributions and the staff at JMI Laboratories for performing the susceptibility testing, WGS and editorial support for this manuscript.
Funding
This study was performed by JMI Laboratories and supported by Pfizer .
Transparency declarations
M.C., T.B.D., T.D.C., H.S.S. and R.E.M. are employees of JMI Laboratories, which was a paid consultant to Pfizer in connection with the development of this manuscript.
JMI Laboratories contracted to perform services in 2019–20 for Achaogen, Inc., Albany College of Pharmacy and Health Sciences, Allecra Therapeutics, Allergan, AmpliPhi Biosciences Corp., Amicrobe Advanced Biomaterials, Amplyx, Antabio, American Proficiency Institute, Arietis Corp., Arixa Pharmaceuticals, Inc., Astellas Pharma Inc., Athelas, Basilea Pharmaceutica Ltd, Bayer AG, Becton, Dickinson and Company, bioMerieux SA, Boston Pharmaceuticals, Bugworks Research Inc., CEM-102 Pharmaceuticals, Cepheid, Cidara Therapeutics, Inc., CorMedix Inc., DePuy Synthes, Destiny Pharma, Discuva Ltd, Dr. Falk Pharma GmbH, Emery Pharma, Entasis Therapeutics, Eurofarma Laboratorios SA, US Food and Drug Administration, Fox Chase Chemical Diversity Center, Inc., Gateway Pharmaceutical LLC, GenePOC Inc., Geom Therapeutics, Inc., GlaxoSmithKline plc, Harvard University, Helperby, HiMedia Laboratories, F. Hoffmann-La Roche Ltd, ICON plc, Idorsia Pharmaceuticals Ltd, Iterum Therapeutics plc, Laboratory Specialists, Inc., Melinta Therapeutics, Inc., Merck & Co., Inc., Microchem Laboratory, Micromyx, MicuRx Pharmaceuticals, Inc., Mutabilis Co., Nabriva Therapeutics plc, NAEJA-RGM, Novartis AG, Oxoid Ltd, Paratek Pharmaceuticals, Inc., Pfizer, Inc., Polyphor Ltd, Pharmaceutical Product Development, LLC, Prokaryotics Inc., Qpex Biopharma, Inc., Roivant Sciences, Ltd, Safeguard Biosystems, Scynexis, Inc., SeLux Diagnostics, Inc., Shionogi and Co., Ltd, SinSa Labs, Spero Therapeutics, Summit Pharmaceuticals International Corp., Synlogic, T2 Biosystems, Inc., Taisho Pharmaceutical Co., Ltd, TenNor Therapeutics Ltd, Tetraphase Pharmaceuticals, Theravance Biopharma, University of Colorado, University of Southern California-San Diego, University of North Texas Health Science Center, VenatoRx Pharmaceuticals, Inc., Viosera Therapeutics, Vyome Therapeutics Inc., Wockhardt, Yukon Pharmaceuticals, Inc., Zai Lab and Zavante Therapeutics, Inc. There are no speakers’ bureaus or stock options to declare.
References
- 1. Doi Y. Treatment options for carbapenem-resistant Gram-negative bacterial infections. Clin Infect Dis 2019; 69: S565–S75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Livermore DM, Nicolau DP, Hopkins KL. et al. Carbapenem-resistant Enterobacterales, carbapenem resistant organisms, carbapenamase-producing Enterobacterales, and carbapenemase-producing organisms: terminology past its ‘sell-by-date’ in an era of new antibiotics and regional carbapenemase epidemiology. Clin Infect Dis 2020; 71: 1776–82. [DOI] [PubMed] [Google Scholar]
- 3. Tzouvelekis LS, Markogiannakis A, Piperaki E. et al. Treating infections caused by carbapenemase-producing Enterobacteriaceae. Clin Microbiol Infect 2014; 20: 862–72. [DOI] [PubMed] [Google Scholar]
- 4. Woodford N, Turton JF, Livermore DM.. Multiresistant Gram-negative bacteria: the role of high-risk clones in the dissemination of antibiotic resistance. FEMS Microbiol Rev 2011; 35: 736–55. [DOI] [PubMed] [Google Scholar]
- 5. Castanheira M, Doyle TB, Kantro V. et al. Meropenem-vaborbactam activity against carbapenem-resistant Enterobacterales isolates collected in U.S. hospitals during 2016 to 2018. Antimicrob Agents Chemother 2020; 64: e01951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pitout JDD, Peirano G, Kock MM. et al. The global ascendency of OXA-48-type carbapenemases. Clin Microbiol Rev 2019; 33: e00102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Poirel L, Potron A, Nordmann P.. OXA-48-like carbapenemases: the phantom menace. J Antimicrob Chemother 2012; 67: 1597–606. [DOI] [PubMed] [Google Scholar]
- 8. Navarro-San Francisco C, Mora-Rillo M, Romero-Gomez MP. et al. Bacteraemia due to OXA-48-carbapenemase-producing Enterobacteriaceae: a major clinical challenge. Clin Microbiol Infect 2013; 19: E72–E9. [DOI] [PubMed] [Google Scholar]
- 9. Stewart A, Harris P, Henderson A. et al. Treatment of infections by OXA-48-producing Enterobacteriaceae. Antimicrob Agents Chemother 2018; 62: e01195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Infectious Diseases Society of America guidance on the treatment of antimicrobial resistant Gram-negative infections. https://www.idsociety.org/practice-guideline/amr-guidance/.
- 11.CLSI. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically—Eleventh Edition: M07. 2018.
- 12.CLSI. Performance Standards for Antimcirobial Susceptibility Testing—Thirtieth Edition: M100. 2020.
- 13. Bankevich A, Nurk S, Antipov D. et al. SPAdes: a new genome assembly algorithm and its applications to single-cell sequencing. J Comput Biol 2012; 19: 455–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.GitHub. https://github.com/tseemann/mlst.
- 15. Both A, Buttner H, Huang J. et al. Emergence of ceftazidime/avibactam non-susceptibility in an MDR Klebsiella pneumoniae isolate. J Antimicrob Chemother 2017; 72: 2483–8. [DOI] [PubMed] [Google Scholar]
- 16. Pfaller MA, Huband MD, Mendes RE. et al. In vitro activity of meropenem-vaborbactam and characterisation of carbapenem resistance mechanisms among carbapenem-resistant Enterobacteriaceae from the 2015 meropenem/vaborbactam surveillance programme. Int J Antimicrob Agents 2018; 52: 144–50. [DOI] [PubMed] [Google Scholar]
- 17. Francisco AP, Vaz C, Monteiro PT. et al. PHYLOViZ: phylogenetic inference and data visualization for sequence based typing methods. BMC Bioinformatics 2012; 13: 87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Frohlich C, Sorum V, Thomassen AM. et al. OXA-48-mediated ceftazidime-avibactam resistance is associated with evolutionary trade-offs. mSphere 2019; 4: e00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Francisco AP, Bugalho M, Ramirez M. et al. Global optimal eBURST analysis of multilocus typing data using a graphic matroid approach. BMC Bioinformatics 2009; 10: 152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sickle: A sliding-window, adaptive, quality-based trimming tool for FastQ files (Version 1.33). https://github.com/najoshi/sickle.
- 21. Poirel L, Bonnin RA, Nordmann P.. Genetic features of the widespread plasmid coding for the carbapenemase OXA-48. Antimicrob Agents Chemother 2012; 56: 559–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Langmead B, Salzberg SL.. Fast gapped-read alignment with Bowtie 2. Nat Methods 2012; 9: 357–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lutgring JD, Zhu W, de Man TJB. et al. Phenotypic and genotypic characterization of Enterobacteriaceae producing oxacillinase-48-like carbapenemases, United States. Emerg Infect Dis 2018; 24: 700–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Li H, Handsaker B, Wysoker A. et al. The sequence alignment/map format and SAMtools. Bioinformatics 2009; 25: 2078–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Quinlan AR, Hall IM.. BEDTools: a flexible suite of utilities for comparing genomic features. Bioinformatics 2010; 26: 841–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Camacho C, Coulouris G, Avagyan V. et al. BLAST+: architecture and applications. BMC Bioinformatics 2009; 10: 421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Livermore DM, Meunier D, Hopkins KL. et al. Activity of ceftazidime/avibactam against problem Enterobacteriaceae and Pseudomonas aeruginosa in the UK, 2015-16. J Antimicrob Chemother 2018; 73: 648–57. [DOI] [PubMed] [Google Scholar]
- 28. Carattoli A, Zankari E, Garcia-Fernandez A. et al. In silico detection and typing of plasmids using PlasmidFinder and plasmid multilocus sequence typing. Antimicrob Agents Chemother 2014; 58: 3895–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Li X, Ma W, Qin Q. et al. Nosocomial spread of OXA-232-producing Klebsiella pneumoniae ST15 in a teaching hospital, Shanghai, China. BMC Microbiol 2019; 19: 235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Shu L, Dong N, Lu J. et al. Emergence of OXA-232 carbapenemase-producing Klebsiella pneumoniae that carries a pLVPK-like virulence plasmid among elderly patients in China. Antimicrob Agents Chemother 2019; 63: e02246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mavroidi A, Katsiari M, Likousi S. et al. Changing characteristics and in vitro susceptibility to ceftazidime/avibactam of bloodstream extensively drug-resistant Klebsiella pneumoniae from a Greek intensive care unit. Microb Drug Resist 2020; 26: 28–37. [DOI] [PubMed] [Google Scholar]
- 32. Kazmierczak KM, Bradford PA, Stone GG. et al. In vitro activity of ceftazidime-avibactam and aztreonam-avibactam against OXA-48-carrying Enterobacteriaceae isolated as part of the International Network for Optimal Resistance Monitoring (INFORM) Global Surveillance Program from 2012 to 2015. Antimicrob Agents Chemother 2018; 62: e00592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Castanheira M, Huband MD, Mendes RE. et al. Meropenem-vaborbactam tested against contemporary Gram-negative isolates collected worldwide during 2014, including carbapenem-resistant, KPC-producing, multidrug-resistant, and extensively drug-resistant Enterobacteriaceae. Antimicrob Agents Chemother 2017; 61: e00567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Shields RK, Chen L, Cheng S. et al. Emergence of ceftazidime-avibactam resistance due to plasmid-borne blaKPC-3 mutations during treatment of carbapenem-resistant Klebsiella pneumoniae infections. Antimicrob Agents Chemother 2017; 61: e02097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sousa A, Perez-Rodriguez MT, Soto A. et al. Effectiveness of ceftazidime/avibactam as salvage therapy for treatment of infections due to OXA-48 carbapenemase-producing Enterobacteriaceae. J Antimicrob Chemother 2018; 73: 3170–5. [DOI] [PubMed] [Google Scholar]
- 36. De la Calle C, Rodriguez O, Morata L. et al. Clinical characteristics and prognosis of infections caused by OXA-48 carbapenemase-producing Enterobacteriaceae in patients treated with ceftazidime-avibactam. Int J Antimicrob Agents 2019; 53: 520–4. [DOI] [PubMed] [Google Scholar]