Abstract
Background
Rifampicin doses of 40 mg/kg in adults are safe and well tolerated, may shorten anti-TB treatment and improve outcomes, but have not been evaluated in children.
Objectives
To characterize the pharmacokinetics and safety of high rifampicin doses in children with drug-susceptible TB.
Patients and methods
The Opti-Rif trial enrolled dosing cohorts of 20 children aged 0–12 years, with incremental dose escalation with each subsequent cohort, until achievement of target exposures or safety concerns. Cohort 1 opened with a rifampicin dose of 15 mg/kg for 14 days, with a single higher dose (35 mg/kg) on day 15. Pharmacokinetic data from days 14 and 15 were analysed using population modelling and safety data reviewed. Incrementally increased rifampicin doses for the next cohort (days 1–14 and day 15) were simulated from the updated model, up to the dose expected to achieve the target exposure [235 mg/L·h, the geometric mean area under the concentration–time curve from 0 to 24 h (AUC0–24) among adults receiving a 35 mg/kg dose].
Results
Sixty-two children were enrolled in three cohorts. The median age overall was 2.1 years (range = 0.4–11.7). Evaluated doses were ∼35 mg/kg (days 1–14) and ∼50 mg/kg (day 15) for cohort 2 and ∼60 mg/kg (days 1–14) and ∼75 mg/kg (day 15) for cohort 3. Approximately half of participants had an adverse event related to study rifampicin; none was grade 3 or higher. A 65–70 mg/kg rifampicin dose was needed in children to reach the target exposure.
Conclusions
High rifampicin doses in children achieved target exposures and the doses evaluated were safe over 2 weeks.
Introduction
TB remains an important cause of childhood morbidity and mortality globally, with an estimated 1.19 million incident cases in children <15 years of age in 2019, resulting in an estimated 230 000 deaths.1 Treatment of drug-susceptible pulmonary TB (PTB) in adults and children continues to mainly rely on a 2 month intensive phase of isoniazid, rifampicin and pyrazinamide, with or without ethambutol, and a 4 month continuation phase of isoniazid and rifampicin. Although outcomes among adults and children treated with this regimen are good in routine care, shorter effective regimens are urgently needed.
Rifampicin, with its distinctive ability to sterilize lesions and prevent relapse, has been a critical component of short-course TB regimens since its introduction in the 1970s. The optimal rifampicin dose was never rigorously established and the currently recommended adult dose of 600 mg (10 mg/kg) once daily results in exposures at the low end of the exposure–response curve.2 In mice, higher doses of rifampicin result in more rapid sterilization and potentially shorter treatment.3–5
Higher rifampicin doses have been explored in multiple adult trials. The HIGHRIF1 study was a dose-ranging trial in adults with drug-susceptible TB that evaluated the pharmacokinetics, safety and extended early bactericidal activity (EBA) of rifampicin doses of 20, 25, 30 and 35 mg/kg over 14 days compared with the standard 10 mg/kg dose.6 An extension trial subsequently evaluated doses of 40 and 50 mg/kg.7 Higher rifampicin doses resulted in more than proportional increases in plasma exposures and were safe and well tolerated through 40 mg/kg.6 A more rapid fall in bacterial load with higher doses was observed and secondary analyses confirmed a statistically significant linear exposure–response relationship between rifampicin exposure and greater EBA over the doses studied.8 Another trial showed that a 35 mg/kg daily dose of rifampicin was safe and tolerable over 3 months in adults9 and an association was demonstrated between higher rifampicin exposures and shortened time to sputum culture conversion, suggesting the potential for treatment shortening.10
Rifampicin is readily absorbed after oral administration. Co-administration with food delays absorption by 1–2 h and reduces bioavailability by 6%–26%.11,12 Rifampicin is eliminated primarily in the bile from where it undergoes progressive enterohepatic recirculation and deacetylation in the liver to its primary metabolite desacetyl-rifampicin.11 It induces its own clearance, with clearance approximately doubling due to this autoinduction; 90% of the induction occurs by 2 weeks.13,14 As the rifampicin dose increases there is a more than proportional increase in exposure, likely due to saturation of rifampicin transport into the bile affecting both hepatic clearance and first-pass extraction.13–15 In children, both body size and age impact drug exposures.16 Although rifampicin has been studied extensively in children, to the best of our knowledge, doses as high as those studied in HIGHRIF1 have not yet been evaluated in children.
The objectives of this study were to establish the rifampicin doses in children required to match the rifampicin exposure in adults receiving a 35 mg/kg daily dose and to evaluate the safety and tolerability of these doses over 15 days.
Patients and methods
Trial design
The Opti-Rif trial was an open-label multiple dose-escalation study (South African National Clinical Trials Registry, number 27–0117-5411).
Study setting and participants
Eligible children at two South African sites (Cape Town and Johannesburg) were identified and screened. Children were eligible if they were 0 to <12 years of age, HIV-negative and routinely treated for pulmonary and/or extrapulmonary drug-susceptible TB. Children 12–17 years of age have pharmacokinetics very similar to adults, so were not included in this study, in order to focus limited resources on children with the most expected differences in rifampicin pharmacokinetics. Initially, participants were required to be on treatment for <28 days at enrolment, which was amended to 42 days for cohorts 2 and 3 to improve study feasibility. Exclusion criteria included rifampicin-resistant TB exposure or disease, weight <2.5 or >40 kg, severe comorbid illness, history or family history of rifampicin hypersensitivity, grade 2 or higher ALT, AST or bilirubin, or evidence of acute viral hepatitis.
Sample size
Three to five dosing cohorts of 20 children each (total n = 100) were allowed to be enrolled sequentially, until the rifampicin target exposure was achieved, as long as rifampicin doses were safe in each dosing cohort. This sample size was based on prior knowledge of rifampicin pharmacokinetics in children.17 Twenty children were assumed to be sufficient to achieve the 95% CIs of the typical clearance value within 60% and 140% of the geometric mean estimates of clearance in children with at least 80% power.
Interventions
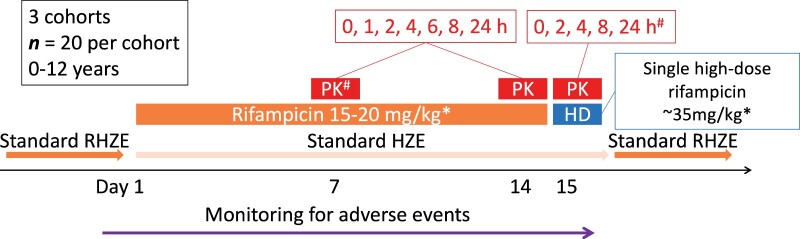
In South Africa, children with drug-susceptible TB are currently routinely treated according to WHO guidelines for 6 months with a rifampicin dose of 10–20 mg/kg/day. After enrolment, participants received study-specified rifampicin doses as a single-drug formulation for 14 days and the other TB medications at recommended routine doses using routinely available single-drug formulations. On day 15, a single higher rifampicin dose was administered (Figure 1). The rifampicin study formulation used was a 150 mg rifampicin powder in capsule form [Rimactane, Sandoz SA (Pty) Limited, Johannesburg, South Africa]. After day 15, each participant restarted standard recommended doses of all TB drugs with routine formulations to complete their treatment. Rifampicin dosing and administration are described in the Supplementary Methods (available as Supplementary data at JAC Online). There was no randomization or blinding. On non-pharmacokinetic sampling days, caregivers prepared and administered the rifampicin dose at home. To support adherence, caregivers were trained on preparing the dose and the importance of adherence and a treatment diary was provided in which they documented that the dose was administered, the dose time and any vomiting.
Figure 1.
Opti-Rif study schematic. *The doses for days 1–14 and day 15 are the cohort 1 starting doses. The doses for subsequent cohorts were higher and were selected as described in the Methods section, based on the previous cohort’s modelled data and simulated doses. #Following a protocol amendment during cohort 2, the day 7 pharmacokinetic (PK) sampling was dropped and additional 1 and 6 h timepoints were added to the day 15 sampling, so that most participants in cohort 2 and all participants in cohort 3 had PK sampling on days 14 and 15 at 0, 1, 2, 4, 6, 8 and 24 h post-dose. HD, high-dose; RHZE, rifampicin/isoniazid/pyrazinamide/ethambutol; HZE, isoniazid/pyrazinamide/ethambutol. This figure appears in colour in the online version of JAC and in black and white in the print version of JAC.
Cohort management and dose selection
Each dosing cohort was stratified by age targeting approximately the following: ages 0 to <2 years (n = 10), 2 to <6 years (n = 5) and 6 to <12 years (n = 5). Age groups were enrolled in parallel within each dosing cohort without age de-escalation. Participants withdrawing prior to pharmacokinetic sampling on day 14 were replaced and their data included in analyses. There was incremental dose escalation from each cohort to the next and single-dose within-cohort dose escalation (day 15). Dosing cohort 1 opened using the standard WHO-recommended paediatric rifampicin dose (15 mg/kg; maximum 20 mg/kg) for 14 days, with a single higher dose (35 mg/kg) on day 15. Following completion of cohort 1, pharmacokinetic data were modelled and safety data reviewed. Rifampicin doses for cohort 2 (days 1–14 and day 15) were based on simulations from the updated model. For cohort 3, rifampicin doses were based on simulations from the model additionally updated with cohort 2 data. Doses were incrementally increased up to the dose expected to achieve the target exposure, but were limited by a maximally allowed increase (100%) from the dose given on day 14, from one cohort to the next. A Trial Steering Committee (TSC) oversaw the trial (see the Supplementary Methods).
Pharmacokinetic sampling and assays
Pharmacokinetic sampling was conducted just before an observed dose and 1, 2, 4, 6, 8 and 24 h after dosing on days 7 and 14, and just before dosing and 2, 4, 8 and 24 h after dose on day 15. After completion of data analysis for cohort 1, the day 7 sampling was omitted for cohort 2 and cohort 3 as it did not contribute meaningfully to the model and samples at 1 and 6 h were added to day 15 to mirror the day 14 sampling schedule.
Plasma rifampicin concentrations were determined with a validated LC-tandem MS assay developed at the University of Cape Town according to international guidelines (see the Supplementary Methods).18,19
Safety evaluation
Participants had regular clinical and laboratory safety assessments, including chemistries, liver enzymes and full blood picture. All events occurring after the first study rifampicin dose were considered adverse events (AEs). AEs were graded according to standard DAIDS grading20 and attribution to study rifampicin was assessed by a study investigator.
Outcomes
The primary outcome was the weight-banded rifampicin dose across the weight spectrum that achieved target rifampicin exposures [the day 14 area under the concentration–time curve from 0 to 24 h (AUC0–24) target was 235 mg/L·h].6 The co-primary safety outcome was the frequency and proportion of participants discontinuing study drug due to an AE at least possibly related to study rifampicin dosing. The secondary pharmacokinetic outcomes were the primary model parameters describing the population pharmacokinetics of high-dose rifampicin and the contribution of key covariates. Secondary safety outcomes included the frequency and proportion of participants within the 15 day study dosing period by dosing cohort with any grade 3 or higher AEs, study drug-related grade 3 or higher AEs, all AEs of any grade and study drug-related AEs of any grade.
Statistical methods
Population pharmacokinetic methods
An existing paediatric population pharmacokinetic model with saturable hepatic extraction was updated with the observed data in interim analyses between each cohort.21 The model was used to derive AUC0–24 and predict doses for the subsequent cohort. It was further refined in the final analysis including all data collected and evaluated using the objective function value, standard goodness-of-fit plots and visual predictive checks. Samples with non-detectable concentrations were excluded, since these almost exclusively occurred at timepoints where very low drug levels were expected and hence would not contribute pharmacokinetic information. Parameter precision was determined using the sampling importance re-sampling (‘SIR’) methodology.22 The dosing simulations approach is described in the Supplementary Methods.
Data management, plotting and post-processing of results were performed in R (R Foundation for Statistical Computing, Vienna, Austria). The modelling and simulations were performed in NONMEM 7.4 (Icon Development Solutions, Ellicott City, MD, USA), aided by PsN (Department of Pharmaceutical Biosciences, Uppsala University, Uppsala, Sweden) and Pirana (Certara, Princeton, NJ, USA).23
Other statistical methods
Weight-for-age and height-for-age z-scores were calculated using British reference values, as WHO references only include children <10 years of age.24 Duration on rifampicin before study enrolment was compared by study cohort using the Kruskal–Wallis test. An exploratory analysis was done to determine if a trend was present in AEs across dosing cohorts using the Cochran–Armitage trend test. These analyses were completed using Stata 16.0 special edition software (StataCorp. Stata Statistical Software: Release 16. 2019).
Ethics
Approval for the study was provided by the Stellenbosch University Health Research Ethics Committee (M16/05/019) and the University of the Witwatersrand Human Research Ethics Committee (160804). Written informed consent was provided by the parent or legal guardian for all participants and informed assent provided by participants ≥7 years of age.
Results
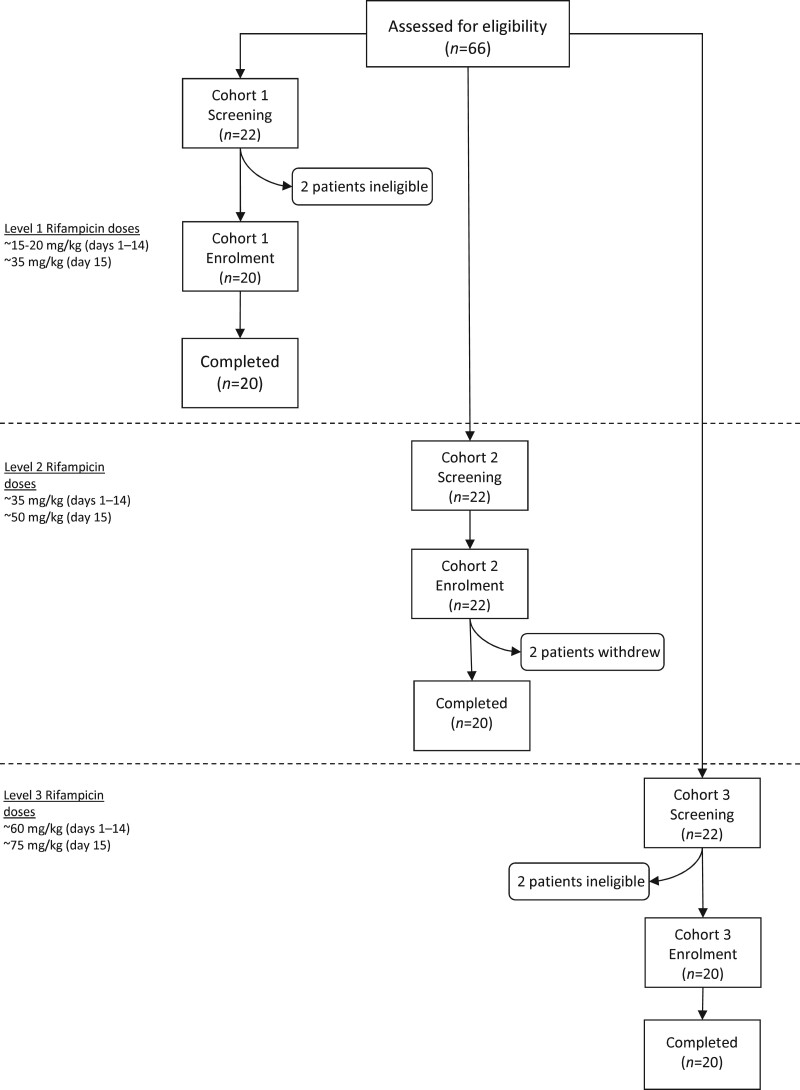
Sixty-two children were enrolled in three cohorts between March 2017 and September 2019 (Figure 2). All enrolled children completed the 15 day dosing period, with the exception of two children in cohort 2 who withdrew prior to day 15 and were replaced. Table 1 shows baseline characteristics by cohort. The median age overall was 2.1 years (range = 0.4–11.7), similar across cohorts. Median (IQR) time on rifampicin prior to enrolment (Table 1) differed significantly (P = 0.0068), due to the change in eligibility criteria. Only two children required nasogastric tube dosing of rifampicin on pharmacokinetic sampling days and 10 children were able to swallow whole capsules.
Figure 2.
Flow diagram demonstrating enrolment of children with drug-susceptible TB into sequential dosing cohorts in the Opti-Rif trial. The ∼ symbol indicates ‘approximately’.
Table 1.
Baseline demographic and clinical characteristics of children with drug-susceptible TB in the Opti-Rif trial
| Cohort 1, N = 20 | Cohort 2, N = 22 | Cohort 3, N = 20 | Total, N = 62 | |
|---|---|---|---|---|
| Male, n (%) | 10 (50) | 10 (45.5) | 12 (60) | 32 (51.6) |
| Age (years), median (IQR; range) | 2.0 (IQR = 1.2–3.4; range = 0.4–11.4) | 2.0 (IQR = 1.1–3.9; range = 0.5–9.4) | 2.8 (IQR = 1.0–5.5; range = 0.4–11.7) | 2.1 (IQR = 1.1–4.4; range = 0.4–11.7) |
| Age group categories, n (%) | ||||
| 0 to <2 years | 10 (50) | 11 (50) | 8 (40) | 29 (46.8) |
| 2 to <6 years | 8 (40) | 8 (36.4) | 7 (35) | 23 (37.1) |
| 6 to <12 years | 2 (10) | 3 (13.6) | 5 (25) | 10 (16.1) |
| Weight (kg), median (IQR) | 10.6 (8.7–14.2) | 10.9 (9.3–14.1) | 12.5 (8.0–17.4) | 10.6 (8.6–15.9) |
| Weight-for-age z-score, median (IQR) | −1.42 (−2.13 to −0.73) | −0.80 (−1.96 to −0.26) | −1.60 (−2.40 to −0.83) | −1.37 (−2.10 to −0.55) |
| Height/length (cm), median (IQR) | 80.5 (70.5–95.0) | 80.9 (72.0–98.5) | 90.0 (71.8–108.1) | 83.4 (71.0–105.1) |
| Height/length-for-age z-score, median (IQR) | −1.30 (−2.34 to −0.17) | −0.93 (−2.08 to −0.39) | −1.13 (−2.19 to −0.26) | −1.18 (−2.23 to −0.34) |
| Type of TB disease, n (%) | ||||
| PTB only | 16 (80) | 21 (95.5) | 8 (40) | 45 (72.6) |
| EPTB only | 0 (0) | 1 (4.6) | 1 (5) | 2 (3.2) |
| PTB and EPTB | 4 (20) | 0 (0) | 11 (55) | 15 (24.2) |
| EPTB typea, n (% of those with EPTB) | ||||
| abdominal TB | 2 (50) | 1 (100) | 2 (17) | 5 (29) |
| peripheral lymph node TB | 1 (25) | 0 (0) | 1 (8) | 2 (12) |
| pleural effusion | 2 (50) | 0 (0) | 2 (17) | 4 (24) |
| skin | 0 (0) | 0 (0) | 1 (8) | 1 (6) |
| TB meningitis | 1 (25) | 0 (0) | 6 (50) | 7 (41) |
| TB disease classification, n (%) | ||||
| confirmed TB | 5 (25) | 3 (13.6) | 5 (25) | 9 (14.5) |
| probable TB | 15 (75) | 18 (81.8) | 13 (65) | 50 (80.6) |
| possible TB | 0 (0) | 1 (4.5) | 2 (10) | 3 (4.8) |
| Children <5 years of age, n (%) | 16 (80) | 19 (86.4) | 15 (75) | 50 (80.7) |
| Gestational status for children <5 years of age at enrolment | ||||
| number of pre-term births (%) | 4 (25) | 4 (21.1) | 3 (20) | 11 (22) |
| number of term births (%) | 7 (43.8) | 13 (68.4) | 10 (66.7) | 30 (60) |
| number unknown (%) | 5 (31.3) | 2 (10.5) | 2 (13.3) | 9 (18) |
| Duration on rifampicin (days), median (IQR)b | 17.0 (12.5–26.5) | 26.0 (18.0–27.0) | 32.5 (22.0–38.0) | 25.5 (16.0–30.0) |
EPTB, extrapulmonary TB.
One child in cohort 1 with EPTB had TB meningitis, abdominal TB and a pleural effusion, all others had only one form of EPTB.
Median duration on rifampicin calculated from the date of starting TB treatment until the date of starting study doses of rifampicin.
Pharmacokinetics
For cohort 2, doses were approximately 35 mg/kg (days 1–14) and 50 mg/kg (day 15). For cohort 3, doses were approximately 60 mg/kg (days 1–14) and 75 mg/kg (day 15). Exact doses are shown in Table S1 (available as Supplementary data at JAC Online).
A total of 141 samples had non-detectable concentrations, of which all but one were pre-dose or 24 h samples. The analysis dataset included 693 concentration measurements. The structure and covariate relations in the existing model fitted the observed data well. The covariate relations included were: allometric scaling with fat-free mass25 and the fixed coefficient 0.75 for clearances and total body weight and the fixed coefficient 1 for volumes, maturation of intrinsic clearance with age according to a Hill equation26 and age decreasing bioavailability linearly under 3 years. Administration method (capsules swallowed whole versus opened) was highly correlated with age and its inclusion did not improve model fit. A visual predictive check of the final model together with plots of predictions versus observations showed adequate fit to the data (Figure S1 and Figure S2) and final parameter estimates with their uncertainty are presented in Table 2. The derived exposure parameters AUC0–24 and maximum concentration (Cmax) per cohort and sampling day are presented in Table 3. The median rifampicin AUC0–24 associated with doses of 15, 35 and 60 mg/kg were 37.5, 70.3 and 183 mg/L·h, respectively, and the day 15 dose of 75 mg/kg resulted in a median AUC0–24 of 260 mg/L·h, consistent with the pre-specified target.
Table 2.
Final model parameter estimates for high-dose rifampicin pharmacokinetics in children <12 years of age with TB in the Opti-Rif trial
| Parameter | Value | 95% CI |
|---|---|---|
| Intrinsic clearancea (L/h) | 54.4 | 44.0–68.4 |
| Volume of distributionb (L) | 15.0 | 13.5–16.5 |
| Rate of absorption (1/h) | 2.06 | 1.55–2.96 |
| Mean absorption transit time (h) | 0.368 | 0.252–0.478 |
| Number of absorption transit compartments | 13.0 | 10.5–16.7 |
| Liver blood flowc (L/h) | 90 FIXED | |
| Liver volumec (L) | 1 FIXED | |
| Rifampicin fraction unbound in plasma | 0.2 FIXED | |
| K m – Michaelis constant for saturation of clearance (mg/L) | 9.48 | 6.69–14.3 |
| Post-menstrual age with 50% clearance maturation (years) | 1.24 | 1.15–1.31 |
| Shape factor for clearance maturation function | 10.2 | 5.27–16.7 |
| Bioavailability at birth relative to 1 | 0.689 | 0.561–0.831 |
| Age when bioavailability reaches maximum (years) | 3 FIXED | |
| Inter-individual variability intrinsic clearance (CV%) | 25.3% | 19.5%–31.9% |
| Inter-occasional variability bioavailability (CV%) | 31.2% | 25.9%–37.6% |
| Inter-occasional variability rate of absorption (CV%) | 152% | 112%–224% |
| Inter-occasional variability mean transit time (CV%) | 132% | 94.3%–214% |
| Proportional error (%) | 21.1 | 19.9–24.4 |
| Additive error (mg/L) | 0.0766 | 0.0618–0.0901 |
CV%, coefficient of variation.
For a child with a fat-free mass of 9 kg.
For a child with total body weight of 12 kg.
Values for an adult with a fat-free mass of 56 kg, scaled allometrically with fat-free mass.
Table 3.
Model-derived rifampicin exposure individual parameters in children with drug-susceptible TB in the Opti-Rif trial
| Cohort | Day | Approximate dose (once daily) | n | AUC0–24 (mg/L·h), median (range)a | C max (mg/L), median (range) |
|---|---|---|---|---|---|
| 1 | 7 | 15 mg/kg | 20 | 37.4 (14.5–72.3) | 9.5 (4.2–16) |
| 14 | 15 mg/kg | 20 | 37.2 (11.1–75.1) | 8.4 (3.3–15) | |
| 15 | 35 mg/kg | 19 | 80.7 (30.0–151) | 17 (5.1–27) | |
| 2 | 7 | 35 mg/kg | 7b | 60.4 (17.5–157) | 15 (5.0–25) |
| 14 | 35 mg/kg | 20 | 70.3 (19.1–161) | 14 (5.3–31) | |
| 15 | 50 mg/kg | 17 | 120 (40.3–239) | 29 (8.4–41) | |
| 3 | 7 | NA | 0b | NA | NA |
| 14 | 60 mg/kg | 19c | 187 (28.1–430) | 28 (7.5–52) | |
| 15 | 75 mg/kg | 19c | 236 (46.6–375) | 34 (9.4–57) |
NA, not applicable.
Target adult AUC0–24 = 235 mg/L·h.
Protocol amendment during enrolment of cohort 2 removed sampling on day 7 based on results from cohort 1.
One participant vomited during days 14 and 15 and did not contribute a full pharmacokinetic profile.
Safety
Table 4 shows key trial safety endpoints. Two participants in dosing cohort 2, a 1.2-year-old and a 9.4-year-old, discontinued study rifampicin because of AEs. Both participants’ caregivers voluntarily withdrew during the 14 day dosing period because of grade 1 vomiting assessed as possibly related to study rifampicin. There were three grade 3 AEs and one serious AE, none attributed to study rifampicin, and no grade 4 AEs or deaths.
Table 4.
Summary of AEs in children with drug-susceptible TB treated with high doses of rifampicin
| Cohort 1, n (%) | Cohort 2, n (%) | Cohort 3, n (%) | All patients, n (%) | |
|---|---|---|---|---|
| Participants stopping study rifampicin due to any drug-related AE | 0 | 2 (9.1) | 0 | 2 (3.2) |
| Participants with any AE | 13 (65) | 20 (90.9) | 17 (85) | 50 (80.7) |
| participants with any grade 1 or 2 AE | 12 (60) | 20 (90.9) | 17 (85) | 49 (79) |
| participants with grade 1 or 2 AE at least possibly related to study rifampicin | 7 (35) | 9 (40.9) | 12 (60) | 28 (45.2) |
| participants with any grade 3 or 4 AEa | 1 (5) | 2 (9.1) | 0 | 3 (4.8) |
| participants with any grade 3 or 4 AE at least possibly related to study rifampicin | 0 | 0 | 0 | 0 |
| Participants with any serious AEb | 1 (5) | 0 | 0 | 1 (1.6) |
| participants with serious AE at least possibly related to study rifampicin | 0 | 0 | 0 | 0 |
| Deaths | 0 | 0 | 0 | 0 |
All three grade 3 or 4 AEs were worsening anaemia that were not attributed to study rifampicin.
The single serious AE was due to a hospitalization after an injury sustained from an auto-pedestrian accident unrelated to study rifampicin.
Table S2 and Table S3 show a more detailed breakdown of grade 1 and grade 2 AEs. The most frequent were mild gastrointestinal disorders. The most common AE related to study rifampicin was vomiting, with 13 participants having a grade 1 event [n = 2 (10%), n = 4 (19%) and n = 7 (35%) in cohort 1, cohort 2 and cohort 3, respectively] and 1 in cohort 3 with grade 2 vomiting. There was a significant trend in the proportion of participants with vomiting of any grade associated with study drug and cohort, which increased from 10.0% to 40.0% across dosing cohorts (χ2= 5.148, P = 0.0233). Only four participants had elevated ALT or AST, all grade 1
Dosing simulations
The model predicted a significant impact of age on apparent clearance and thereby on expected exposures for children <6 months of age (see Figure S3). However, only two children <6 months were included, the youngest almost 5 months old; hence the model prediction in this age range was highly uncertain. We therefore only simulated doses for children ≥6 months, which also allowed for dosing only according to body size.
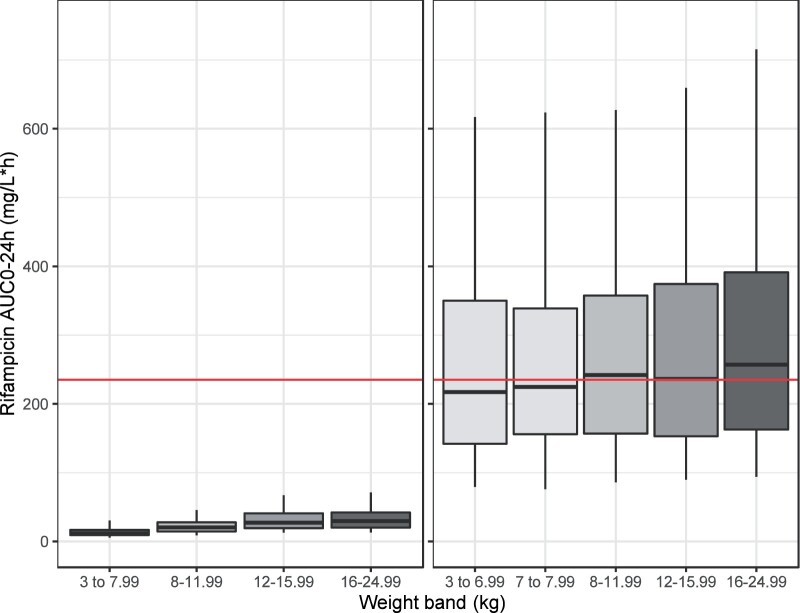
A daily dose of 65–70 mg/kg rifampicin was needed in children to reach the target exposure. The suggested rifampicin dosing per weight band in children is presented in Table 5, with expected exposures compared with current standard dosing shown in Figure 3.
Table 5.
Rifampicin doses evaluated with simulations using the final model and virtual paediatric population (n = 5000; >6 months and <25 kg)
| Weight band | Weight range (kg) | Current paediatric dose recommendation (mg) | Dose for target exposure of 235 mg/L·h (mg) |
|---|---|---|---|
| 1 | 4–7.99 | 75 | <7 kg: 450; 7–7.99 kg: 600 |
| 2 | 8–11.99 | 150 | 750 |
| 3 | 12–15.99 | 225 | 900 |
| 4 | 16–24.99 | 300 | 1200 |
Figure 3.
Expected exposures (AUC0–24) with the currently approved rifampicin dosing and the suggested dosing to match a target from adults (235 mg/L·h, corresponding to 35 mg/kg, red line) in a virtual paediatric population (n = 5000; >6 months and <25 kg). The box represents the median and upper and lower quartiles and the whiskers correspond to the 5th and 95th percentiles. This figure appears in colour in the online version of JAC and in black and white in the print version of JAC.
Discussion
We present data in children demonstrating that rifampicin daily doses of approximately 65–70 mg/kg are required to approximate target exposures in adults receiving 35 mg/kg and that the doses evaluated were safe over 14 days.
Much higher mg/kg doses of rifampicin are needed in children compared with adults to achieve similar exposures. This is not unexpected and is a known consequence of allometric scaling, which predicts that clearance relative to body size is more rapid, especially in smaller children.27–29 However, higher doses than we had originally anticipated were required in children. This may be due to reduced rifampicin bioavailability in children <3 years of age. While this could be a function of the formulation used in the study, which required manipulation of the capsules to administer the doses, it may also be due to reduced rifampicin absorption in young children. Rifampicin absorption is reduced at lower pH and children have a lower gastric pH than adults.30 As expected, we observed a more than proportional increase in rifampicin exposure with dose, especially beyond 35 mg/kg, without a ceiling effect. There was large inter-individual variability in rifampicin pharmacokinetics, which has been observed previously in both children and adults.6,7,28 A small part of the variability can be explained by characteristics, such as body size and composition, age, genetic factors, malnutrition or comorbidities,12 but the majority of the inter-individual variability appears random.
Overall, the ∼35 and ∼60 mg/kg daily rifampicin doses evaluated in this study were safe over 14 days. The final simulated doses achieving target exposures (65–70 mg/kg) were not evaluated over 14 days, although cohort 3 received similar mg/kg doses. High-dose rifampicin was also well tolerated, with only two participants (3.3%) interrupting treatment due to vomiting. We observed more gastrointestinal AEs, including vomiting, nausea and abdominal pain, with higher doses of rifampicin. Other possible contributors to gastrointestinal effects include the large pill burden at higher doses and the formulation, as the suspension administered to children unable to swallow tablets was gritty and likely not very palatable. It is difficult to separate out the effects of rifampicin itself from the effects of the formulation and administration method; however, gastrointestinal AEs may be an impediment to high rifampicin doses in children that should be carefully evaluated in future studies. The lack of elevated transaminases is reassuring. Hyperbilirubinaemia without transaminitis, occurring in the first few days after initiating rifampicin, was observed in adults receiving a 50 mg/kg dose.7 We did not observe jaundice or elevated bilirubin in our study, possibly due to no scheduled evaluations in the days immediately after starting high-dose rifampicin. The clinical relevance of such potential asymptomatic bilirubin abnormalities is questionable, but should be evaluated in future studies.
Rifampicin bioavailability varies substantially by formulation.31,32 This has important implications for extrapolation of this trial’s findings to different formulations, especially given the opening and splitting of capsules required for dosing in our study. As rifampicin is quite hydrophobic, sprinkling the rifampicin powder on a small amount of pureed food for oral administration would be a consideration if a capsule formulation is evaluated in future studies. The formulation may also have contributed to the observed gastrointestinal AEs. A more acceptable formulation, such as a scored dispersible tablet with a more appropriate strength to reduce the pill burden, would ideally be needed for future trials evaluating high rifampicin doses and for any eventual routine use. Although there were not substantial issues with adherence to the study rifampicin, there may have been some unidentified missed doses, as observed dosing was not done other than on pharmacokinetic sampling days. However, as rifampicin accumulates very little, the risk that this increased the variability of rifampicin exposure is limited, unless there were many missed doses that may have impacted on induction.
An additional limitation is that, at the time of study enrolment, participants had already been on routine rifampicin doses for weeks. Substantial rifampicin induction would have been present already, reducing exposures at the studied doses compared with exposures in rifampicin-naive patients. This would need to be modelled and carefully evaluated in future studies.
The results of this study have important implications for improving TB treatment strategies for children. High rifampicin doses have the potential to shorten treatment across the spectrum of disease and bacillary burden in children and this is one approach being evaluated in trials in adults concerning TB treatment shortening. Our data will enable evaluation of similar strategies in children and facilitate extrapolation of adult findings to paediatric populations. Recent studies in adults have identified a 40 mg/kg rifampicin dose as the maximal tolerated dose.7 Rifampicin doses in children slightly higher than those studied here would be necessary to achieve those higher target exposures. Studies of such doses over a longer duration, such as the 8 week intensive phase, should be considered in children with both minimal and more extensive TB for the purpose of treatment shortening. Such studies would require child-friendly high-quality formulations with close adherence monitoring. The HighRIF-C trial (NCT04437836), now enrolling in Tanzania, is examining rifampicin doses of 30 and 40 mg/kg in addition to standard doses among children 1–14 years of age and will contribute important additional data on optimal paediatric rifampicin dosing.
TB meningitis, which causes a large proportion of the paediatric TB-related morbidity and mortality, may benefit from high rifampicin doses. A pooled analysis from three trials of different rifampicin-containing treatment regimens for adults with TB meningitis found that an oral rifampicin dose of 30 mg/kg compared with the standard 10 mg/kg would be expected to increase 6 month survival from 50% to 70%.33 HIV-positive children with TB are another important group that may benefit from higher rifampicin doses.
Optimizing rifampicin doses may be a promising strategy for shortening TB treatment and improving outcomes in children across the spectrum of TB disease, although careful monitoring of gastrointestinal AEs and tolerability would be needed. This study substantially advances our understanding of higher-dose rifampicin in children, who urgently need to benefit from improved TB treatment strategies.
Data sharing
Data underlying these findings may be requested from the trial Principal Investigator (A.C.H.).
Supplementary Material
Acknowledgements
We thank the patients and caregivers who participated in the study and the study teams at the Desmond Tutu TB Centre and Shandukani who supported the study. We thank the Trial Steering Committee members: Peter Donald, James Seddon, Kelly Dooley, Carole Mitnick, Grace Montepiedra and Susan Abdel-Rahman.
Funding
Funding for this study was provided by the TB Alliance through the Unitaid-funded STEP-TB project. A.C.H. was supported through a South African National Research Foundation SARChi Chair in Paediatric Tuberculosis. The University of Cape Town Clinical PK Laboratory is supported in part by the Adult Clinical Trial Group (ACTG) via the National Institute of Allergy and Infectious Diseases (NIAID) of the National Institutes of Health under award numbers UM1 AI068634, UM1 AI068636 and UM1 AI106701, as well as the Infant Maternal Pediatric Adolescent AIDS Clinical Trials Group (IMPAACT), with funding provided by the National Institute of Allergy and Infectious Diseases (U01 AI068632), the Eunice Kennedy Shriver National Institute of Child Health and Human Development and the National Institute of Mental Health grant AI068632.
Transparency declarations
None to declare.
Supplementary data
Supplementary Methods, Tables S1 to S3 and Figures S1 to S3 are available as Supplementary data at JAC Online.
References
- 1.WHO. Global Tuberculosis Report 2020. https://apps.who.int/iris/bitstream/handle/10665/336069/9789240013131-eng.pdf (26 January 2021).
- 2. van Ingen J, Aarnoutse RE, Donald PR. et al. Why do we use 600 mg of rifampicin in tuberculosis treatment? Clin Infect Dis 2011; 52: e194–9. [DOI] [PubMed] [Google Scholar]
- 3. Jayaram R, Gaonkar S, Kaur P. et al. Pharmacokinetics-pharmacodynamics of rifampin in an aerosol infection model of tuberculosis. Antimicrob Agents Chemother 2003; 47: 2118–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. de Steenwinkel JE, Aarnoutse RE, de Knegt GJ. et al. Optimization of the rifampin dosage to improve the therapeutic efficacy in tuberculosis treatment using a murine model. Am J Respir Crit Care Med 2013; 187: 1127–34. [DOI] [PubMed] [Google Scholar]
- 5. Hu Y, Liu A, Ortega-Muro F. et al. High-dose rifampicin kills persisters, shortens treatment duration, and reduces relapse rate in vitro and in vivo. Front Microbiol 2015; 6: 641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Boeree MJ, Diacon AH, Dawson R. et al. A dose-ranging trial to optimize the dose of rifampin in the treatment of tuberculosis. Am J Respir Crit Care Med 2015; 191: 1058–65. [DOI] [PubMed] [Google Scholar]
- 7. Te Brake LHM, de Jager V, Narunsky K. et al. Increased bactericidal activity but dose-limiting intolerability at 50 mg·kg−1 rifampicin. Eur Respir J 2021; 58: 2000955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Svensson RJ, Svensson EM, Aarnoutse RE. et al. Greater early bactericidal activity at higher rifampicin doses revealed by modeling and clinical trial simulations. J Infect Dis 2018; 218: 991–9. [DOI] [PubMed] [Google Scholar]
- 9. Boeree MJ, Heinrich N, Aarnoutse R. et al. High-dose rifampicin, moxifloxacin, and SQ109 for treating tuberculosis: a multi-arm, multi-stage randomised controlled trial. Lancet Infect Dis 2017; 17: 39–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Svensson EM, Svensson RJ, Te Brake LHM. et al. The potential for treatment shortening with higher rifampicin doses: relating drug exposure to treatment response in patients with pulmonary tuberculosis. Clin Infect Dis 2018; 67: 34–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rifampin U.S. Food and Drug Administration (FDA) Label. https://www.accessdata.fda.gov/drugsatfda_docs/label/2010/050420s073,050627s012lbl.pdf (3 August 2020).
- 12. Abulfathi AA, Decloedt EH, Svensson EM. et al. Clinical pharmacokinetics and pharmacodynamics of rifampicin in human tuberculosis. Clin Pharmacokinet 2019; 58: 1103–29. [DOI] [PubMed] [Google Scholar]
- 13. Chirehwa MT, Rustomjee R, Mthiyane T. et al. Model-based evaluation of higher doses of rifampin using a semimechanistic model incorporating autoinduction and saturation of hepatic extraction. Antimicrob Agents Chemother 2016; 60: 487–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Svensson RJ, Aarnoutse RE, Diacon AH. et al. A population pharmacokinetic model incorporating saturable pharmacokinetics and utoinduction for high rifampicin doses. Clin Pharmacol Ther 2018; 103: 674–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Acocella G. Pharmacokinetics and metabolism of rifampin in humans. Rev Infect Dis 1983; 5 Suppl 3: S428–32. [DOI] [PubMed] [Google Scholar]
- 16. Anderson BJ, Holford NH.. Understanding dosing: children are small adults, neonates are immature children. Arch Dis Child 2013; 98: 737–44. [DOI] [PubMed] [Google Scholar]
- 17. Zvada SP, Denti P, Donald PR. et al. Population pharmacokinetics of rifampicin, pyrazinamide and isoniazid in children with tuberculosis: in silico evaluation of currently recommended doses. J Antimicrob Chemother 2014; 69: 1339–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Food and Drug Administration, Center for Drug Evaluation and Research. Guidance for Industry: Bioanalytical Method Validation. https://www.fda.gov/files/drugs/published/Bioanalytical-Method-Validation-Guidance-for-Industry.pdf.
- 19.EMA. Guideline on Bioanalytical Method Validation. http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2011/08/WC500109686.pdf.
- 20.U.S. Department of Health and Human Services, National Institutes of Health, National Institute of Allergy and Infectious Diseases, Division of AIDS. Division of AIDS (DAIDS) Table for Grading the Severity of Adult and Pediatric Adverse Events, Corrected Version 2.0. November 2014. https://rsc.niaid.nih.gov/sites/default/files/daids-ae-grading-table-v2-nov2014.pdf (3 August 2020).
- 21. Denti P, Gonzalez-Martinez C, Winckler J. et al. Pharmacokinetics of rifampicin in African children: evaluation of the new WHO dosing guidelines. Forty-Eighth Union World Conference on Lung Health, Guadalajara, Mexico, 2017. Abstract A0156-13.
- 22. Dosne AG, Bergstrand M, Harling K. et al. Improving the estimation of parameter uncertainty distributions in nonlinear mixed effects models using sampling importance resampling. J Pharmacokinet Pharmacodyn 2016; 43: 583–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Keizer RJ, Karlsson MO, Hooker A.. Modeling and simulation workbench for NONMEM: tutorial on Pirana, PsN, and Xpose. CPT Pharmacometrics Syst Pharmacol 2013; 2: e50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cole TJ, Freeman JV, Preece MA.. British 1990 growth reference centiles for weight, height, body mass index and head circumference fitted by maximum penalized likelihood. Stat Med 1998; 17: 407–29. [PubMed] [Google Scholar]
- 25. Al-Sallami HS, Goulding A, Grant A. et al. Prediction of fat-free mass in children. Clin Pharmacokinet 2015; 54: 1169–78. [DOI] [PubMed] [Google Scholar]
- 26. Anderson BJ, Holford NH.. Mechanism-based concepts of size and maturity in pharmacokinetics. Annu Rev Pharmacol Toxicol 2008; 48: 303–32. [DOI] [PubMed] [Google Scholar]
- 27. Bekker A, Schaaf HS, Draper HR. et al. Pharmacokinetics of rifampin, isoniazid, pyrazinamide, and ethambutol in infants dosed according to revised WHO-recommended treatment guidelines. Antimicrob Agents Chemother 2016; 60: 2171–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Schipani A, Pertinez H, Mlota R. et al. A simultaneous population pharmacokinetic analysis of rifampicin in Malawian adults and children. Br J Clin Pharmacol 2016; 81: 679–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hiruy H, Rogers Z, Mbowane C. et al. Subtherapeutic concentrations of first-line anti-TB drugs in South African children treated according to current guidelines: the PHATISA study. J Antimicrob Chemother 2015; 70: 1115–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wilkins JJ, Savic RM, Karlsson MO. et al. Population pharmacokinetics of rifampin in pulmonary tuberculosis patients, including a semimechanistic model to describe variable absorption. Antimicrob Agents Chemother 2008; 52: 2138–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. McIlleron H, Hundt H, Smythe W. et al. Bioavailability of two licensed paediatric rifampicin suspensions: implications for quality control programmes. Int J Tuberc Lung Dis 2016; 20: 915–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Court R, Chirehwa MT, Wiesner L. et al. Quality assurance of rifampicin-containing fixed-drug combinations in South Africa: dosing implications. Int J Tuberc Lung Dis 2018; 22: 537–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Svensson EM, Dian S, Te Brake L. et al. Model-based meta-analysis of rifampicin exposure and mortality in Indonesian tuberculosis meningitis trials. Clin Infect Dis 2020; 71: 1817–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.