Abstract
Background
Therapeutic failure is a frequent issue in the management of post-operative peritonitis.
Objectives
A post hoc analysis of the prospective, multicentre DURAPOP trial analysed the risk factors for failures in post-operative peritonitis following adequate source control and empirical antibiotic therapy in critically ill patients.
Patients and methods
Overall failures assessed post-operatively between Day 8 and Day 45 were defined as a composite of death and/or surgical and/or microbiological failures. Risk factors for failures were assessed using logistic regression analyses.
Results
Among the 236 analysed patients, overall failures were reported in 141 (59.7%) patients, including 30 (12.7%) deaths, 81 (34.3%) surgical and 95 (40.2%) microbiological failures. In the multivariate analysis, the risk factors associated with overall failures were documented piperacillin/tazobactam therapy [adjusted OR (aOR) 2.10; 95% CI 1.17–3.75] and renal replacement therapy on the day of reoperation (aOR 2.96; 95% CI 1.05–8.34). The risk factors for death were age (aOR 1.08 per year; 95% CI 1.03–1.12), renal replacement therapy on reoperation (aOR 3.95; 95% CI 1.36–11.49) and diabetes (OR 6.95; 95% CI 1.34–36.03). The risk factors associated with surgical failure were documented piperacillin/tazobactam therapy (aOR 1.99; 95% CI 1.13–3.51), peritoneal cultures containing Klebsiella spp. (aOR 2.45; 95% CI 1.02–5.88) and pancreatic source of infection (aOR 2.91; 95% CI 1.21–7.01). No specific risk factors were identified for microbiological failure.
Conclusions
Our data suggest a predominant role of comorbidities, the severity of post-operative peritonitis and possibly of documented piperacillin/tazobactam treatment on the occurrence of therapeutic failures, regardless of their type.
Introduction
Therapeutic failure is a frequent issue in the management of intra-abdominal infection. In post-operative peritonitis, this complication can lead to life-threatening issues marked by organ dysfunction and complex source control.1 In recent studies, up to half of intra-abdominal infection cases experienced therapeutic failure2,3 associated with increased morbidity or fatality rates.3–6
Risk factors have been largely described for these complications, most of which are due to an underlying disease, severity of infection at the time of diagnosis, inadequate source control and/or inadequate antibiotic therapy.2 These last two conditions are the only ones with which clinicians can interact to limit the risk of therapeutic failure.7–11 Most published data come from retrospective single-centre studies and focus on microbiological and anti-infective issues while data analysing source control problems are scarce.2,3,6,12 Interestingly, the respective weight of antibiotic therapy and source control management at the onset of therapeutic failure is difficult to assess, as these two conditions are closely associated.
The DURAPOP trial, which aimed to assess short-course antibiotic therapy for critically ill patients treated for post-operative peritonitis, did not demonstrate any influence of the duration of antibiotic therapy on the outcome or mortality rate.13 In that trial, adequate source control and antibiotic therapy were mandatory inclusion criteria, which allowed us to perform post hoc analyses of the risk factors for therapeutic failure in the management of ICU patients treated for post-operative peritonitis.13 Risk factors associated with death and/or surgical failure and/or microbiological failure were assessed within the first 45 post-operative days following post-operative peritonitis.
Patients and methods
Study design
The randomized, open-label, multicentre, prospective DURAPOP trial (Clinicaltrials.gov NCT01311765) conducted in 21 French ICUs between May 2011 and February 2015 compared the efficacy and safety of 8 day versus 15 day antibiotic therapy in 236 critically ill patients with post-operative peritonitis.13 All patients required surgical intervention to halt the infectious process and to control the foci of infection.1 The eligible patients had operative samples with positive microbiological cultures, adequate source control as assessed by the surgeon, and adequate empirical antibiotic therapy initiated within 24 h after surgery. These patients were randomly assigned on Day 8 to either stop or to continue antibiotic therapy until Day 15. Inadequate empirical antibiotic therapy not targeting all organisms cultured from blood and surgical samples within the first 24 h after surgery, early death (before Day 8), inadequate source control and/or any reoperation before Day 8 of antibiotic therapy were exclusion criteria (Supplementary methods, available as Supplementary data at JAC Online). The primary endpoint was the number of antibiotic-free days between Day 8 (randomization) and Day 28. Secondary outcomes were death, ICU and hospital length of stay, emergence of MDR bacteria and reoperation rate, with follow-up at Day 45. Patients treated for 8 days had a higher median number of antibiotic-free days than those treated for 15 days (P < 0.0001) while the mortality rate at Day 45 was similar in both groups. Treatments did not differ in terms of ICU and hospital length of stay, emergence of MDR bacteria or reoperation rate, while increased rates of subsequent drainages and positive blood cultures between Day 8 and Day 45 were observed following short-course antibiotic therapy.
The study received approval from the CPP Ile de France I Ethics Committee (reference 2010-08-12392, EudraCT No.: 2010-022059-47). Written informed consent was obtained from the patients or their legal representative before study inclusion. In the case of impaired decision-making capacity without a legal representative available, the patient’s informed consent was obtained after enrolment (emergency inclusion).
Surgical observations (source, cause and extent of peritoneal contamination) and procedures performed during the index surgery were prospectively collected (additional details on the definitions used for surgical procedures are given in the Supplementary methods). Antibiotic selection according to French guidelines was left to the discretion of the attending physician, including therapeutic drug monitoring and any adaptation of the documented therapy considered necessary as a function of the definitive microbiological results identifying the pathogens and their susceptibility patterns.9 Based on culture results obtained within 48–72 h after surgery, the investigators were encouraged to de-escalate the empirical regimen to a narrow-spectrum documented antibiotic therapy.
The following variables were recorded from the day of randomization until Day 45: death, time to discharge from the ICU and hospital, need for reoperation or percutaneous abdominal drainage for any reason, need for another course of antibiotic therapy for any reason (including extra-abdominal causes), bacteraemia and microbiological recurrence in peritoneal cultures obtained from surgical or percutaneous samples. The latter was defined as at least one of the initial causative microorganisms (the same genus, species and serotype, when available) that were cultured from an abdominal sample obtained from reoperation or percutaneous drainage; otherwise, it was considered a superinfection.
MDR microorganisms were defined as one of the following organisms according to expert recommendations:14Pseudomonas aeruginosa, Acinetobacter baumannii, ESBL-producing Enterobacterales, AmpC-hyperproducing Enterobacterales and MRSA. The emergence of MDR bacteria in surveillance samples was assessed on a weekly basis from ICU admission to discharge (Supplementary methods).
The daily dose and duration of the three most frequently administered documented antibiotic therapies (piperacillin/tazobactam, vancomycin and carbapenems) were recorded up to Day 15.
Definitions
The following therapeutic failures at Day 45 were analysed in the present work: death (from any cause), surgical failure (need for reoperation and/or additional abdominal drainage for any reason), microbiological failure (another course of antibiotic therapy at least 48 h after stopping antibiotics in order to treat infection recurrence or superinfection between Day 8 and Day 45) and overall failure (a composite of death and/or surgical failure and/or microbiological failure).13
Statistical analysis
The dataset of the DURAPOP trial, composed of 236 patients, was used for all analyses. Categorical variables are reported as the frequency and percentage, n (%); continuous variables are expressed as the median and IQR.
Comparisons between groups were performed using the Wilcoxon or chi-squared test or Fisher’s exact test, as appropriate. After a clinical evaluation of variables to introduce into the models, potential risk factors were identified using univariate logistic regressions, and those identified at the 0.05 significance level were selected.
Collinearity and strong associations were evaluated for all variables. Stepwise methods based on the Akaike information criterion were used to select the best fit.15 The two-by-two interactions were tested for all variables selected for the final models to exclude the possibility that the effect of one variable did not depend on the value of other selected variables.
Following the TRIPOD guidelines, we assessed goodness of fit of the models.16 Discrimination was assessed with the C-statistic (identical to the AUC in the context of logistic regressions) and calibration was assessed with the Hosmer–Lemeshow test. The results are presented as OR for univariate analyses and adjusted OR (aOR) for multivariate analyses and are presented together with their 95% CIs. All analyses were performed with R software (version 4.0.5). The two-sided alpha level was set at a significance of 0.05.
Results
Overall failure (a composite of death and/or surgical failure and/or microbiological failure)
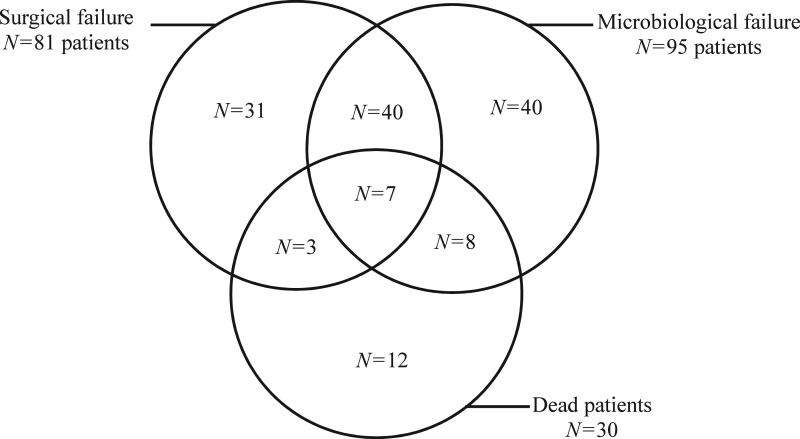
Among the 236 analysed patients, overall failures were reported in 141 (59.7%) patients, including 30 (12.7%) deaths, 81 (34.3%) surgical failures and 95 (40.2%) microbiological failures (Figure 1). The clinical characteristics of the population are presented in Table S1. No differences were observed between the 141 patients with overall failures and the 95 others in terms of underlying disease, type of initial surgery, clinical characteristics leading to reoperation, surgical observations during reoperation or surgical management.
Figure 1.
Subpopulations of the 141 patients in the DURAPOP trial classified as having overall failure, analysed by failure cause.
The risk factors for overall failures identified in the univariate analysis are presented in Table 1. In the multivariate analysis, risk factors associated with failure were documented piperacillin/tazobactam therapy (aOR 2.10; 95% CI 1.17–3.75; P = 0.012) and renal replacement therapy on the day of reoperation (aOR 2.96; 95% CI 1.05–8.34; P = 0.040) (C-statistic 0.70; 95% CI 0.62–0.82; Hosmer–Lemeshow test, P > 0.99). The somewhat large 95% CI for renal replacement therapy was due to a lower number of renal replacements in the success group than in the overall failure group: 5 (5.5%) versus 19 (14.4%). Additional criteria overlapping with the selected variables were not used in the multivariate analysis (Table S1).
Table 1.
Determinants of overall failure at discharge or at Day 45 from the univariate and multivariate analyses (variables with a P value of <0.05 in the univariate analysis)
| Significant risk factors in univariate analyses | Success | Overall failure | Univariate |
Multivariate |
||
|---|---|---|---|---|---|---|
| OR (95% CI) | P value | aOR (95% CI) | P value | |||
| Documented piperacillin/tazobactam therapy, n/N (%) | 26/95 (27.4) | 58/141 (41.1) | 1.85 (1.06–3.25) | 0.031 | 2.10 (1.17–3.75) | 0.012 |
| Renal replacement therapy on the day of reoperation, n/N (%) | 5/91 (5.5) | 19/132 (14.4) | 2.89 (1.04–8.05) | 0.042 | 2.96 (1.05–8.34) | 0.040 |
| Bacteraemia between Day 8 and Day 45, n/N (%) | 3/95 (3.2) | 15/141 (10.6) | 3.65(1.03–12.98) | 0.045 | ||
Risk factors for death (from any cause)
Overall, 30/256 (12.7%) patients died, with a median delay of 19.5 (IQR 15.8–31) days after reoperation for post-operative peritonitis. Seven (23.3%) patients had both surgical and microbiological failure. The risk factors for death at Day 45 identified in the univariate analyses are presented in Table 2. The type of initial surgery, clinical characteristics leading to reoperation, surgical observations during reoperation, surgical management, evaluation of source control at the end of surgery, microbiological characteristics, empirical and documented antibiotic therapy, subsequent reoperation and/or drainages, and need for new antibiotic therapy did not differ between groups (Table S2). In the multivariate analysis, the risk factors for death were age (aOR 1.08 per year; 95% CI 1.03–1.12; P = 0.0005), renal replacement therapy on the day of reoperation (aOR 3.95; 95% CI 1.36–11.49; P = 0.012) and complicated diabetes mellitus (aOR 6.95; 95% CI 1.34–36.03; P = 0.021). The C-statistic was 0.78 (95% CI 0.70–0.88) and the result of the Hosmer–Lemeshow test was not significant (P = 0.44).
Table 2.
Determinants of death at Day 45 from univariate and multivariate analyses (variables with a P value of <0.05 in the univariate analysis)
| Significant risk factors in univariate analyses | Survivors | Deceased | Univariate |
Multivariate |
||
|---|---|---|---|---|---|---|
| OR (95% CI) | P value | aOR (95% CI) | P value | |||
| Rapidly fatal underlying disease (survival <1 year), n/N (%) | 27/205 (13.2) | 9/30 (30) | 2.83 (1.17–6.81) | 0.021 | ||
| Heart disease, n/N (%) | 12/206 (5.8) | 5/30 (16.7) | 3.23 (1.05–9.94) | 0.041 | ||
| Complicated diabetes mellitus, n/N (%) | 5/206 (2.4) | 3/30 (10) | 4.47 (1.01–19.75) | 0.048 | 6.95 (1.34–36.03) | 0.021 |
| Pulmonary disease, n/N (%) | 16/206 (7.8) | 6/30 (20) | 2.97 (1.06–8.31) | 0.038 | ||
| CVA or hemiplegia, n/N (%) | 11/206 (5.3) | 5/30 (16.7) | 3.55 (1.14–11.04) | 0.029 | ||
| Renal replacement therapy on the day of reoperation, n/N (%) | 17/206(8.3) | 7/30 (23.3) | 3.49 (1.30–9.39) | 0.013 | 3.95 (1.36–11.49) | 0.012 |
| Emergence of MDR organisms in clinical or surveillance samples, n/N (%) | 80/185 (43.2) | 18/27 (66.6) | 2.62 (1.12–6.15) | 0.026 | ||
| Age, years, median (IQR) | 65 (57–74) | 74 (64.5–81) | 1.06 (1.02–1.09) | 0.002 | 1.08 (1.03–1.12) | 0.0005 |
| BMI, kg/m2, median (IQR) | 27.8 (24.0–32.8) | 25.4 (21.4–30.0) | 0.94 (0.88–1) | 0.046 | ||
| Charlson score, median (IQR) | 4 (2–7) | 6 (3.3–8.8) | 1.2 (1.06–1.36) | 0.004 | ||
| SAPS II score on the day of reoperation, median (IQR) | 43 (33–52) | 50 (43.3–59.3) | 1.03 (1–1.06) | 0.024 | ||
CVA, cerebrovascular accident.
Risk factors for surgical failure (need for reoperation and/or additional abdominal drainage for any reason)
Surgical failures were observed in 81/236 (34.3%) patients. Reoperations were performed in 58/236 (24.6%) patients, with a median delay of 14 (IQR 10–14) days after surgery for post-operative peritonitis, including 16 patients who required several procedures [median 2 (IQR 2–3)] (additional details on the procedures are given in Figures S1 and S2). Additional percutaneous drainages were needed in 44 patients, with a median delay of 13 (IQR 10–21) days after initial source control, including multiple drainages [median 2 (IQR 2–2)] in 16 patients. Combined additional percutaneous drainages and surgical reoperations were performed in 20/58 (35%) reoperated patients.
The risk factors for surgical failure identified in the univariate analyses are presented in Table 3. Demographic characteristics and underlying disease, clinical characteristics leading to reoperation, surgical observations and surgical management during reoperation, evaluation of source control at the end of surgery and clinical severity on the day of reoperation did not differ between the patients who had an uneventful post-operative course and those who required additional source control (Table S3). In the multivariate analysis, the risk factors associated with surgical failure were pancreatic source of contamination (aOR 2.91; 95% CI 1.21–7.01; P = 0.017), documented piperacillin/tazobactam therapy (aOR 1.99; 95% CI 1.13–3.51; P = 0.018) and the presence of Klebsiella spp. in peritoneal samples (aOR 2.45; 95% CI 1.02–5.88; P = 0.044). The C-statistic was 0.70 (95% CI 0. 61–0.79) and the result of the Hosmer–Lemeshow test was not significant (P = 0.98).
Table 3.
Determinants of surgical failure at Day 45 from the univariate and multivariate analyses (variables with a P value of <0.05 in the univariate analysis)
| Significant risk factors in univariate analyses | No surgical failure | Surgical failure | Univariate |
Multivariate |
||
|---|---|---|---|---|---|---|
| OR (95% CI) | P value | aOR (95% CI) | P value | |||
| Small bowel source of contamination, n/N (%) | 57/155 (36.8) | 19/81 (23.5) | 0.53 (0.29–0.97) | 0.039 | ||
| Pancreas source of contamination, n/N (%) | 10/155 (6.5) | 14/81 (17.3) | 3.03 (1.28–7.17) | 0.012 | 2.91 (1.21–7.01) | 0.017 |
| Presence of Klebsiella spp. in peritoneal samples, n/N (%) | 11/155 (7.1) | 13/81 (16.1) | 2.50 (1.07–5.87) | 0.035 | 2.45 (1.02–5.88) | 0.044 |
| Documented piperacillin/tazobactam therapy, n/N (%) | 47/155 (30.3) | 37/81 (45.7) | 1.93 (1.11–3.37) | 0.020 | 1.99 (1.13–3.51) | 0.018 |
In the subgroup of patients with documented piperacillin/tazobactam therapy, neither the daily dose of piperacillin/tazobactam nor its duration differed between patients with or without surgical failure (Table 4). When comparing patients with surgical failure with the others, the delay to ICU discharge of survivors was significantly increased from 10 (IQR 7–15) to 13 (IQR 7–25) days (P < 0.018).
Table 4.
Daily dose and duration of therapy of piperacillin/tazobactam, vancomycin and carbapenems in the study population analysed in terms of overall failure or successful outcome
| Antibiotic regimen | Missing data | Successful outcome (n = 95) | Overall failure (n = 141) | P value |
|---|---|---|---|---|
| Piperacillin/tazobactam | n = 25 | n = 58 | ||
| Daily dose (g), median (IQR) | 1/0 | 11.5 (10.6–14.8) | 12 (10.7–13.5) | 0.81 |
| Duration of therapy (days), median (IQR) | 1/0 | 13 (8–15) | 11 (8–14) | 0.34 |
| Vancomycin | n = 11 | n = 32 | ||
| Daily dose (mg), median (IQR) | 1/2 | 2575 (1725–2980) | 2020 (1430–3060) | 0.33 |
| Duration of therapy (days), median (IQR) | 1/2 | 8 (5–12) | 9 (7–11.8) | 0.53 |
| Carbapenems | n = 13 | n = 31 | ||
| Daily dose (mg), median (IQR) | 0/0 | 2430 (1790–2730) | 2200 (1830–2750) | 0.64 |
| Duration of therapy (days), median (IQR) | 0/0 | 9 (7–11) | 12 (8–15) | 0.039 |
Risk factors for microbiological failure (another course of antibiotic therapy at least 48 h after stopping antibiotics in order to treat infection recurrence or superinfection between Day 8 and Day 45)
Demographic characteristics, underlying diseases, clinical characteristics leading to reoperation, surgical observations and surgical management, evaluation of source control at the end of surgery, clinical severity on the day of reoperation and microbiological results (initial blood cultures and surgical samples) did not differ between the 95 patients who had microbiological failure and the other patients (Table S4).
When comparing patients with microbiological failure with those who did not have microbiological failure, the median time to ICU discharge of survivors was increased from 9 (IQR 6–15) to 14 (IQR 7–22) days (P = 0.017).
Additional antibiotic therapies were initiated with a median delay of 18 (IQR 14–25) days for a median duration of 10 (IQR 5–14) days in 34 patients who needed reoperation, 26 patients with additional drainage (including 13 patients requiring both surgery and drainage) and 48 patients who did not require any additional source control. A subgroup analysis among these patients with microbiological failure comparing those with and without additional source control did not show any significant difference (Table S5).
Documented piperacillin/tazobactam therapy
Comparisons between the 84 patients receiving documented piperacillin/tazobactam therapy versus the 152 patients receiving other regimens demonstrated that the former were more frequently treated for a monomicrobial peritoneal infection [14/84 (16.6%) patients versus 52/152 (34.2%) patients; P = 0.004], more often had involvement of streptococci [20/84 (23.8%) versus 17/152 (11.2%); P = 0.0106] and more often had P. aeruginosa involvement [20/84 (23.8%) versus 14/152 (9.2%); P = 0.0022]. On Day 8 post-reoperation, a renal SOFA score ≥3 was observed in 11 patients treated with piperacillin/tazobactam [median (IQR) daily dose of 10.8 (9.6–12.8) g], including 10 patients who experienced therapeutic failure (P = 0.09). The daily dose and duration of piperacillin/tazobactam therapy were similar in patients with successful outcomes and those with overall failures (Table 4).
Among the 18 patients who had bacteraemia between Day 8 and Day 45, 11 (61.1%) received documented piperacillin/tazobactam treatment, while this drug was given to only 73/218 (33.4%) non-bacteraemic patients (OR 3.12; 95% CI 1.16–8.38; P = 0.018). Among the 95 patients who had microbiological failure, 40 (42.1%) patients received documented piperacillin/tazobactam treatment versus 55/95 (57.9%) who were treated with other agents (OR 1.60; 95% CI 0.93–2.75; P = 0.08).
Finally, the comparison between the 84 patients who received documented piperacillin/tazobactam therapy and the 152 patients who received another therapy demonstrated a decreased proportion of clinical success among those treated with piperacillin/tazobactam [26/84 (30.9%) patients who received documented piperacillin/tazobactam therapy versus 69/152 (45.4%) patients who received another regimen (OR 0.53; 95% CI 0.31–0.95; P = 0.031)].
Discussion
In this post hoc analysis of a multicentre prospective trial in ICU patients with post-operative peritonitis adequately treated for source control and antibiotic therapy, we did not observe any relationship between the characteristics of infection, the methods applied for source control and the diagnosis of therapeutic failure. In the study population (n = 236), the documented use of piperacillin/tazobactam and renal replacement therapy on the day of reoperation were identified as risk factors for failure. In patients with surgical failure, documented piperacillin/tazobactam treatment was also a risk factor, similar to pancreatic sources of infection and the presence of Klebsiella spp. in peritoneal cultures. Neither surgical failure nor microbiological failure were identified as risk factors for death. The risk factors associated with fatality were age, underlying disease, and severity of the disease on the day of reoperation. Finally, in the multivariate analysis, we did not identify any specific risk factors for microbiological failure.
The high rate of overall failures, representing 60% in our population, is largely influenced by the patient’s profile and the definitions used. Recent publications have reported similar proportions, ranging between 50% and 68% of cases.2,3 Various definitions have been used to describe therapeutic failure, such as death, additional procedures for source control, and the need for additional antibiotic therapy; however, these last two conditions are frequently combined. Based on this complex picture, we decided first to perform a global analysis of the population in which the procedures failed and then detailed the various causes of therapeutic failure.
The analysis of the risk factors for death could be considered biased because only late fatalities were recorded in our study. According to our observations, when the key drivers of success were achieved, such as adequate source control and antibiotic therapy, a lower fatality rate was observed in critically ill patients. Not surprisingly, comorbidities and the severity of infection were identified as important risk factors for death, as has been reported in other studies.17–19
The inadequacy of source control is frequently reported in the literature, with proportions ranging from 38% to 68% of cases.2,4,6,19 Because inadequate source control and reoperation before Day 8 of anti-infective therapy were exclusion criteria, we assume that our rates of surgical failure could be considered the lowest expected proportions of reoperations for surgical complications. In view of our results, the surgeon’s clinical judgement at the end of the procedure seems to be moderately relevant for predicting failure. Interestingly, we only identified risk factors related to anatomical and microbiological data and anti-infective characteristics, suggesting intimate relationships between microbiological data and surgical management. Various procedures aimed at controlling the infectious process are available, depending on the anatomical location and the diagnostic findings.3 As observed in our ICU population, additional percutaneous drainages have grown in popularity over the last decade, especially for intra-abdominal abscesses.3,6 Inadequate source control has been repeatedly shown to be a key determinant of death and increased morbidity, a point also confirmed in our cohort.2,4–6,12,19
Inadequate empirical antibiotic therapy is the main driver of failures reported in 32% to 44% of ICU cases.2,6,17 Concurrent infections (including pneumonia and urinary tract infection, among others) have also been reported in 15% to 20% of cases to explain the use of additional antibiotic therapies.3 However, the consequences of documented antibiotic therapy on the outcomes of post-operative peritonitis have not been reported, assuming that identification and susceptibility testing of surgical samples allow clinicians to correct for inadequate initial antibiotic therapy. Interestingly, the use of piperacillin was associated with therapeutic failure in a retrospective cohort of post-operative peritonitis.20 The authors reported statistical links between mortality and resistant microorganisms involving not only piperacillin/sulbactam but also meropenem, tigecycline, cefotaxime/metronidazole and meropenem/vancomycin. However, several methodological issues limit the value of these observations, and it is difficult to extrapolate these conclusions to our current observations.
The pharmacokinetic features of antibiotic therapy and MICs of microorganisms are major determinants of success. The daily dose, total dose and duration of antibiotic treatments were similar in patients with and without failures, which limits the possibility of observing pharmacokinetic issues. In addition, the type of pathogens in patients who received documented piperacillin/tazobactam was similar to those who did not receive this treatment, and the selection of this drug was based on susceptibility testing, suggesting that the prescribers were confident in their choice. However, our observations deserve additional investigation in light of the detrimental effects of definitive therapy with piperacillin/tazobactam compared with meropenem, as reported in the Merino trial.21
Our investigation has some limitations linked to the design of the study. First, it is impossible to draw conclusions for the most severe cases who died in the first days after surgery, nor can we do this for immunocompromised patients, who were excluded from the study population. Second, delays in reoperation and the onset of empirical antibiotic therapy may have played a role in post-operative complications. However, in these well-matched populations comprising the control and experimental groups, the physicians followed the current recommendations for the early and timely management of septic patients with post-operative peritonitis.8–11 Third, the adequacy of source control and antibiotic therapy was assessed by the surgeon and the intensivist in charge. Nevertheless, we consider our study population to be homogeneous in terms of adequacy of source control and documented antibiotic therapy. In addition, MICs for the microorganisms targeted by the documented antibiotic therapy were not recorded. This issue is of peculiar relevance for patients receiving piperacillin/tazobactam in whom it cannot be excluded that microbiological failure might be associated with difficult-to-treat microorganisms with high MICs. However, we did not observe any significant difference in the proportions of MDR bacteria, either at the time of admission or during the ICU stay, between those having an uneventful course and those with failure. Finally, therapeutic drug monitoring was not recorded, even if it was performed on a routine basis. We cannot exclude that underdosage could have occurred in patients receiving renal replacement therapy. Similar observations have been recently made with new antibiotic agents with an increased risk of therapeutic failure in patients undergoing renal replacement therapy.22,23 However, we did not observe any significant differences in the daily dose or duration of antibiotic therapy in either group.
In summary, convergent observations in our population in the various types of failure suggest a predominant role of comorbidities, severity of post-operative peritonitis and some specific antibiotic regimens on the emergence of therapeutic failure. Because of our highly selected population, a cautious interpretation of these results is required, especially for piperacillin/tazobactam, whose role must be clarified by further investigations.
Supplementary Material
Acknowledgements
Clinical investigators of the DURAPOP trial group (named with permission)
CHU Bichat-Claude Bernard, Paris: Philippe Montravers, Regis Bronchard and Mathieu Desmard; CHU Amiens, Amiens: Herve Dupont, Melanie Levrard and Yazine Mahjoub; CHU Angers, Angers: Sigismond Lasocki, Soizic Gergaud and Thomas Gaillard; CH Victor Dupouy, Argenteuil: Gaetan Plantefeve and Olivier Pajot; CHU Besançon, Besançon: Gilles Blasco; Emmanuel Samain; Guillaume Besch and Sebastien Pily-Floury; CHU Beaujon, Clichy: Catherine Paugam, Sebastien Pease and Paer Abback; CHU Dijon, Dijon: Claude Girard; CHU Grenoble, Grenoble: Jean-Francois Payen and Marie-Christine Herault; CHU Montpellier, Montpellier: Sami Jaber, Boris Jung and Jean-Marc Delay; CH Mulhouse, Mulhouse: Josette Gally; CHU Brabois, Nancy: Claude Meistelman and Jean-François Perrier; CHU Nantes, Nantes: Karim Asehnoune and Raphael Cinotti; CHU Cochin, Paris: Antoine Tesniere and Alexandre Mignon; CHU St. Antoine, Paris: Thomas Lescot, Nouria Belhadj-Tahar and Marc Beaussier; CHU Lyon Sud, Pierre Bénite: Alain Lepape, Vincent Piriou, Florent Wallet and Candice Tassin; CHU Reims, Reims: Joel Cousson, Pascal Raclot and Thierry Floch; CHU Rennes, Rennes: Philippe Seguin and Yoann Launey; CHU Rouen, Rouen: Benoit Veber, Philippe Gouin and Thomas Clavier; CHU St-Etienne, St-Etienne: Christian Auboyer; CHRU Strasbourg, Strasbourg: Olivier Collanges; and CH Intercommunal Villeneuve-St-George, Villeneuve-St-George: Jean-François Georger.
Contributor Information
the DURAPOP trial group:
Philippe Montravers, Regis Bronchard, Mathieu Desmard, Herve Dupont, Melanie Levrard, Yazine Mahjoub, Sigismond Lasocki, Soizic Gergaud, Thomas Gaillard, Gaetan Plantefeve, Olivier Pajot, Gilles Blasco, Emmanuel Samain, Guillaume Besch, Sebastien Pily-Floury, Catherine Paugam, Sebastien Pease, Paer Abback, Claude Girard, Jean-Francois Payen, Marie-Christine Herault, Sami Jaber, Boris Jung, Jean-Marc Delay, Josette Gally, Claude Meistelman, Jean-François Perrier, Karim Asehnoune, Raphael Cinotti, Antoine Tesniere, Alexandre Mignon, Thomas Lescot, Nouria Belhadj-Tahar, Marc Beaussier, Alain Lepape, Vincent Piriou, Florent Wallet, Candice Tassin, Joel Cousson, Pascal Raclot, Thierry Floch, Philippe Seguin, Yoann Launey, Benoit Veber, Philippe Gouin, Thomas Clavier, Christian Auboyer, Olivier Collanges, and Jean-François Georger
Funding
This work was supported by the Programme Hospitalier de Recherche Clinique (PHRC) National, 2009 (grant number AOM 09024), funded by the French Ministry of Health. The sponsor was the Direction de la Recherche Clinique de l’Assistance Publique des Hôpitaux de Paris (France).
Transparency declarations
P.M. reports personal fees and non-financial support from Astellas, AstraZeneca, Basilea, Bayer, Cubist, Menarini, MSD, Parexel, Pfizer, Tetraphase and The Medicines Company unrelated to the submitted work. S.L. reports research grants from Vifor Pharma and grants from LFB and Astellas unrelated to the submitted work. H.D. reports personal fees from Astellas, Pfizer, Gilead, AstraZeneca, Novartis, Merck, Cubist and Paratek unrelated to the submitted work. No other disclosures were reported.
Author contributions
Concept and design: P.M. and H.D. Acquisition, analysis or interpretation of data: all authors.
Drafting of the manuscript: P.M., M.E.F., N.G., P.E. and H.D. Critical revision of the manuscript for important intellectual content: all authors. Statistical analysis: P.M., M.E.F., P.E. and H.D. Study supervision: P.M., B.V., E.W., P.S., S.L. and H.D.
Supplementary data
Tables S1 to S5, Figures S1 and S2 and Supplementary methods, result and references are available as Supplementary data at JAC Online.
References
- 1. Montravers P, Blot S, Dimopoulos G. et al. Therapeutic management of peritonitis: a comprehensive guide for intensivists. Intensive Care Med 2016; 42: 1234–47. [DOI] [PubMed] [Google Scholar]
- 2. De Pascale G, Carelli S, Vallecoccia MS. et al. Risk factors for mortality and cost implications of complicated intra-abdominal infections in critically ill patients. J Crit Care 2019; 50: 169–76. [DOI] [PubMed] [Google Scholar]
- 3. van de Groep K, Verhoeff TL, Verboom DM. et al. Epidemiology and outcomes of source control procedures in critically ill patients with intra-abdominal infection. J Crit Care 2019; 52: 258–64. [DOI] [PubMed] [Google Scholar]
- 4. Bassetti M, Righi E, Ansaldi F. et al. A multicenter multinational study of abdominal candidiasis: epidemiology, outcomes and predictors of mortality. Intensive Care Med 2015; 41: 1601–10. [DOI] [PubMed] [Google Scholar]
- 5. Bloos F, Thomas-Ruddel D, Ruddel H. et al. Impact of compliance with infection management guidelines on outcome in patients with severe sepsis: a prospective observational multi-center study. Crit Care 2014; 18: R42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tellor B, Skrupky LP, Symons W. et al. Inadequate source control and inappropriate antibiotics are key determinants of mortality in patients with intra-abdominal sepsis and associated bacteremia. Surg Infect 2015; 16: 785–93. [DOI] [PubMed] [Google Scholar]
- 7. Marshall JC, Maier RV, Jimenez M. et al. Source control in the management of severe sepsis and septic shock: an evidence-based review. Crit Care Med 2004; 32: S513–26. [DOI] [PubMed] [Google Scholar]
- 8. Mazuski JE, Tessier JM, May AK. et al. The Surgical Infection Society revised guidelines on the management of intra-abdominal infection. Surg Infect (Larchmt) 2017; 18: 1–76. [DOI] [PubMed] [Google Scholar]
- 9. Montravers P, Dupont H, Leone M. et al. Guidelines for management of intra-abdominal infections. Anaesth Crit Care Pain Med 2015; 34: 117–30. [DOI] [PubMed] [Google Scholar]
- 10. Sartelli M, Catena F, Abu-Zidan FM. et al. Management of intra-abdominal infections: recommendations by the WSES 2016 consensus conference. World J Emerg Surg 2017; 12: 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Solomkin JS, Mazuski JE, Bradley JS. et al. Diagnosis and management of complicated intra-abdominal infection in adults and children: guidelines by the Surgical Infection Society and the Infectious Diseases Society of America. Clin Infect Dis 2010; 50: 133–64. [DOI] [PubMed] [Google Scholar]
- 12. Martínez ML, Ferrer R, Torrents E. et al. Impact of source control in patients with severe sepsis and septic shock. Crit Care Med 2017; 45: 11–9. [DOI] [PubMed] [Google Scholar]
- 13. Montravers P, Tubach F, Lescot T. et al. Short-course antibiotic therapy for critically ill patients treated for postoperative intra-abdominal infection: the DURAPOP randomised clinical trial. Intensive Care Med 2018; 44: 300–10. [DOI] [PubMed] [Google Scholar]
- 14. Magiorakos AP, Srinivasan A, Carey RB. et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect 2012; 18: 268–81. [DOI] [PubMed] [Google Scholar]
- 15. Akaike H. A new look at the statistical model identification. IEEE Trans Automat Contr 1974; 19: 716–23. [Google Scholar]
- 16. Moons KG, Altman DG, Reitsma JB. et al. Transparent Reporting of a multivariable prediction model for Individual Prognosis Or Diagnosis (TRIPOD): explanation and elaboration. Ann Intern Med 2015; 162: W1–73. [DOI] [PubMed] [Google Scholar]
- 17. Alqarni A, Kantor E, Grall N. et al. Clinical characteristics and prognosis of bacteraemia during postoperative intra-abdominal infections. Crit Care 2018; 22: 175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Blot S, Antonelli M, Arvaniti K. et al. Epidemiology of intra-abdominal infection and sepsis in critically ill patients: “AbSeS”, a multinational observational cohort study and ESICM Trials Group Project. Intensive Care Med 2019; 45: 1703–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lagunes L, Rey-Pérez A, Martín-Gómez MT. et al. Association between source control and mortality in 258 patients with intra-abdominal candidiasis: a retrospective multi-centric analysis comparing intensive care versus surgical wards in Spain. Eur J Clin Microbiol Infect Dis 2017; 36: 95–104. [DOI] [PubMed] [Google Scholar]
- 20. Grotelueschen R, Luetgehetmann M, Erbes J. et al. Microbial findings, sensitivity and outcome in patients with postoperative peritonitis a retrospective cohort study. Int J Surg 2019; 70: 63–9. [DOI] [PubMed] [Google Scholar]
- 21. Harris PNA, Tambyah PA, Lye DC. et al. Effect of piperacillin-tazobactam vs meropenem on 30-day mortality for patients with E coli or Klebsiella pneumoniae bloodstream infection and ceftriaxone resistance: a randomized clinical trial. JAMA 2018; 320: 984–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Haidar G, Philips NJ, Shields RK. et al. Ceftolozane-tazobactam for the treatment of multidrug-resistant Pseudomonas aeruginosa infections: clinical effectiveness and evolution of resistance. Clin Infect Dis 2017; 65: 110–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Shields RK, Nguyen MH, Chen L. et al. Pneumonia and renal replacement therapy are risk factors for ceftazidime-avibactam treatment failures and resistance among patients with carbapenem-resistant Enterobacteriaceae infections. Antimicrob Agents Chemother 2018; 62: e02497-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.