Abstract
Background
Cannabis exposure during adolescence is associated with emotional and motivational alterations that may entail an enhanced risk of developing psychiatric disorders. In rodent models, exposure to cannabinoids during adolescence leads to increased self-administration of opiates and cocaine, however, the psychological and neural mechanisms and the sex-specificity of this phenomenon are largely unknown.
Methods
We exposed male and female adolescent rats to Δ9-tetrahydrocannabinol (THC) and studied at adulthood the effects of such treatment on psychological processes related to reward, such as Pavlovian conditioned approach, Pavlovian to instrumental transfer, habit formation and waiting impulsivity. In the light of these data and given the involvement of the nucleus accumbens in the processes examined, we performed an RNASeq transcriptomic study and assessed cocaine addiction-like behavior.
Results
THC exposure increased goal-tracking (in males and females) and enhanced Pavlovian to instrumental transfer (especially in males) but did not affect habit formation. THC-exposed rats exhibited subtle, state-dependent changes in premature responding in the 2-CSRTT task. RNASeq data showed gene expression alterations in a marked sex-specific manner. While no effects were found on the acquisition of cocaine self-administration or punished drug-seeking, rats exposed to THC self-administered more cocaine under a progressive ratio schedule (males), had a higher rebound upon returning to continuous access to the drug (females) and showed reduced drug-seeking after 30 days of withdrawal (females).
Conclusions
Adolescent THC affects specific aspects of reward- (and cocaine-) guided behavior and the function of a key brain region mediating these effects, in a remarkable sex-specific manner.
Keywords: Cannabis, cocaine, reward, RNAseq
Significance Statement.
Cannabis is one of the psychoactive drugs most widely used by adolescents. Although a great deal of research has been carried out regarding its long-term effects, it is unknown if a chronic cannabinoid exposure during adolescence could affect the psychological processes governing reward-guided actions and cocaine addiction, and what could be the underlying neurobiological mechanisms. Here, we found that THC-exposed male rats showed more potentiation of reward seeking by stimuli predictive of those rewards but were less attracted to them. THC also decreased the tolerance to delays, especially in females. All this was accompanied by sex-specific alterations in the activation or deactivation of different families of genes in the nucleus accumbens, a key region of the reward circuit. We also observed a potentiation but also reduction of specific aspects of cocaine addiction, so these results do not fully support the Gateway Hypothesis of drug use.
Introduction
Adolescence is a crucial period of development characterized by profound changes in psychological and neural processes (Spear, 2000; Paus et al., 2008; Blakemore, 2012). As a result, any insult such as stressful events or drug use during this period will have several consequences at the psychological and neurobiological levels. Cannabis is the drug of abuse—other than alcohol and tobacco—most widely consumed by adolescents (EMCDA, 2019), and the exposure to this drug during adolescence has profound consequences for the developing individual (Higuera-Matas et al., 2015; Rubino and Parolaro, 2016; Ferland and Hurd, 2020; Hurd, 2020; Stringfield and Torregrossa, 2021), which may be more severe than when exposure occurs exclusively during adulthood. A potential consequence of adolescent cannabinoid exposure (ACE) is an increase in the use and/or abuse liability of other drugs later in life (termed Gateway Hypothesis, which is under intense debate (Kandel et al., 1992, 2003; Fergusson et al., 2006; Tarter et al., 2006; Vanyukov et al., 2012; Kleinig, 2015; Mayet et al., 2016; Nkansah-Amankra and Minelli, 2016; Lynskey and Agrawal, 2018)). Previous experiments by our group and others have suggested that animals with ACE show increased morphine (Biscaia et al., 2008a), heroin (Ellgren et al., 2007; Tomasiewicz et al., 2012; Lecca et al., 2020), fentanyl (but not oxycodone) (Nguyen et al., 2020), and cocaine self-administration (SA) (Higuera-Matas et al., 2008; Friedman et al., 2019). However, others have reported a delayed cocaine SA acquisition (Kononoff et al., 2018) or no changes in heroin SA (Stopponi et al., 2014). In addition, these studies were typically performed on male rats, neglecting the sex-dependent effects that are common when both sexes are included (Biscaia et al., 2008b; Higuera-Matas et al., 2008). Thus, a more detailed examination of the psychological and neurobiological processes involved in the increased SA of cocaine and opiates after ACE is warranted.
Several reward-related processes may be responsible for the increased use of the drug or may facilitate the development of addictive behavioral patterns. These processes include Pavlovian to instrumental transfer (PIT; i.e., the ability of classically conditioned cues to affect instrumental responses) (Cartoni et al., 2016), Pavlovian conditioned approach (a measure of incentive salience) (Fitzpatrick and Morrow, 2016), habit formation (Belin et al., 2013; Everitt and Robbins, 2013), and impulsivity, a core endophenotype that predicts the development of cocaine addiction (Belin et al., 2008; Jupp and Dalley, 2014). Noteworthy, the nucleus accumbens (NAcc) participates in many of these processes, and prior studies of ACE have highlighted alterations in this structure (Higuera-Matas et al., 2015; Stringfield and Torregrossa, 2021). Recent evidence suggests that exposure to a cannabinoid agonist modifies the initial responses of this region to cocaine (Scherma et al., 2020). However, this study did not examine the potential sex-specific alterations induced by ACE in the transcriptomic landscape of the NAcc, and they used WIN 55,212-2 instead of the actual phytocannabinoid, Δ 9-tetrahydrocannabinol (THC). Therefore, to gain a deeper and broader understanding of the neurochemical alterations induced by adolescent THC in the NAcc and given the crucial role of sex differences in the effects of cannabinoids (Viveros et al., 2011), we performed an RNASeq study in the NAcc of adult male and female rats that had been exposed to THC as adolescents.
In addition to exploring the potential alterations in reward-related behaviors, impulsivity, and the potential accumbal alterations involved, we decided to explore in more depth several characteristics of cocaine SA that could indicate an altered tendency to develop cocaine addiction-like behavior in THC-exposed rats. Indeed, in spite of the initial findings previously mentioned regarding the increased SA of drugs in cannabinoid pre-exposed animals, these studies have not always examined the complex full array of behaviors that are indicative of addiction, especially compulsive seeking or taking (Deroche-Gamonet et al., 2004; Everitt et al., 2018), a cardinal feature of addiction-like behavior typically evaluated using punished CSA procedures (Deroche-Gamonet et al., 2004; Belin et al., 2008). Therefore, the last goal of the present work was to experimentally examine the different features of addiction-like behavior that may be potentiated by ACE.
Our results provide extensive evidence that exposure to THC during adolescence causes sex-dependent changes in reward processing, impulsivity, and specific features of addiction-like behavior, together with gene-expression alterations in the NAcc, providing additional experimental support to the data gathered in clinical and epidemiological studies.
METHODS
Animals and THC Treatment
Subjects were the offspring of Wistar albino rats (35 males and 35 females) from Charles River S.A. (Saint-Germain-sur-l’Arbresle, France) that were mated in our laboratory 2 weeks after their arrival. Different sets of animals, belonging to different litters, were randomly assigned to each experiment, thus minimizing litter effects. The final sample size for each experiment is indicated in the sections below.
Chronic Δ 9-tetrahydrocannabinol (THCPharm, Frankfurt, Germany) treatment took place every other day from postnatal day (PND) 28 to PND 44. THC (3 mg/kg; 1 mL/kg) or its vehicle (kolliphor:ethanol:saline; 1:1:18) were administered i.p. Animals were left undisturbed until PND 90.
All procedures involving laboratory rats were conducted in accordance with the European Union legislation on the protection of animals used for scientific purposes (2010/63/EU Directive) and approved by the Ethics Board of the National University of Distance Learning. Every attempt was made to minimize the pain and discomfort of the experimental animals. See supplementary Methods for more information.
Reward-Related Psychological Alterations Induced by Adolescent THC Exposure
Experiment 1. Pavlovian Conditioned Approach and Habit Formation.
Pavlovian Conditioned Approach
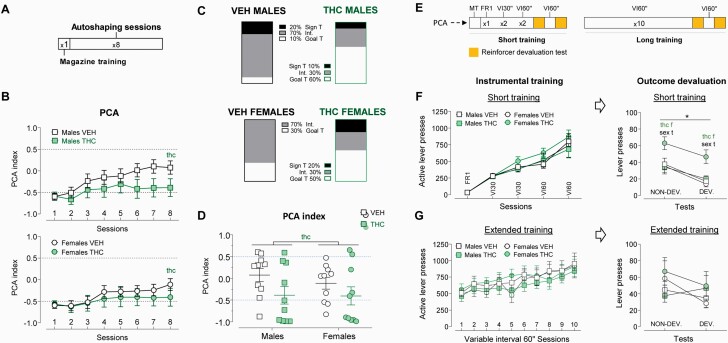
At approximately PND 90, the Pavlovian conditioned approach procedure began. The 8 daily training sessions consisted of 25 trials in which the feeder dispensed a pellet into the magazine under a variable interval 60-second schedule of reinforcement. A lever on one of the sides of the magazine (right or left, counterbalanced) was extended for 8 seconds before the pellet delivery and retracted right after it. The other lever was present during the whole session and served as a measure of general locomotor activity. None of the levers had programmed contingencies. Magazine-oriented behaviors are considered suggestive of goal-track while lever-oriented behaviors are associated with sign-track bias. During each session, an index ranging from 1 (absolute sign-tracking) to −1 (absolute goal-tracking) was calculated. See supplementary information for detailed methodological information. Sample sizes as follows: male VEH (n = 10), male THC (n = 10), female VEH (n = 10), and female THC (n = 10).
Habit Formation Studies
Ten days after the final Pavlovian conditioned approach session, animals began the habit training protocol. The rats performed a brief, in principle non–habit-forming, training, and an extended training scheme (supposed to induce habit-like responding). The brief training consisted of 5 consecutive daily sessions: 1 fixed-ratio 1 session, 2 variable-interval 30-second sessions, and 2 variable-interval 60-second sessions. After the training sessions, we subjected the animals to 2 counterbalanced, sensory-specific, satiety-based devaluation tests. For extended training, animals performed another 10 sessions (variable-interval 60 seconds) and then underwent the same counterbalanced devaluation tests as described before. See supplementary Methods for more information. Sample sizes as follows: male VEH (n = 10), male THC (n = 10), female VEH (n = 10), and female THC (n = 10).
Experiment 2. PIT and 2-Choice Serial Reaction Time Task
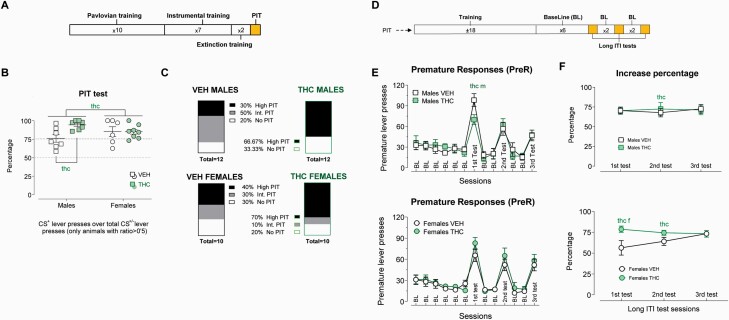
PIT
At approximately PND 90, animals were food restricted and their weight kept between 90% and 95% of the original in the free-feeding state. The PIT protocol consisted of 4 consecutive phases: (1) Pavlovian training, (2) instrumental training, (3) extinction, and (4) PIT test. The main PIT index considered was the percentage of active lever presses (ALP) during the conditioned stimulus (CS+) (%CS+ALPs). A %CS+ALPs between 50% and 75% was considered intermediate PIT and >75% was high PIT. See supplementary Methods for more information. Sample sizes as follows: male VEH (n = 12), male THC (n = 12), female VEH (n = 11), and female THC (n = 11).
Two-Choice Serial Reaction Time Task (2-CSRTT)
Ten days after the end of the PIT, animals were again food restricted, and the 2-CSRTT protocol began. The design of this task followed the protocol published in our previous report (Ucha et al., 2019). First, the rats went through 2 sessions of cue-lever training in which 1 of the cue lights over 1 of the levers (right or left) remained on, and lever presses on this lever were rewarded. 2-CSRTT training consisted of 12 phases with increasing demands (see supplementary Methods for more information). Animals progressively learn to wait a preset time (inter-trial interval) for the presentation of the cue over 1 of the levers before pressing that lever (correct lever press) and avoid responses in the other lever (incorrect lever press) or before cues were present (premature responses) to obtain a pellet. The omission of the response was also quantified. After reaching the desired performance level (phase 12 of training), 6 consecutive sessions with the same requirements were implemented to serve as a baseline. During the test session, the inter-trial interval duration was manipulated to last longer than usual (9 seconds instead of 5 seconds), provoking a relative enhancement of premature responses compared with baseline, which is the main impulsivity index considered. We performed 3 long inter-trial interval sessions with 2 phase-12 sessions between them. Sample sizes as follows: male VEH (n = 12), male THC (n = 12), female VEH (n = 12), and female THC (n = 12).
Transcriptomic Studies (Experiment 3)
RNA from the NAcc of P90 rats was extracted and sequenced in a 1 × 75 single-read sequencing run on a NextSeq500 sequencer. Sample sizes as follows: male VEH (n = 4), male THC (n = 4), female VEH (n = 4), and female THC (n = 4).
We used the Chipster analysis suite (Kallio et al., 2011) to perform data processing and analysis.
Differential gene expression analysis was performed using CUFFDIFF with replicates analysis to explore the differences in transcriptomic profiles between factor levels. Gene ontologies and pathways enrichment and overrepresentation were calculated with the online tools and databases of PANTHER Classification System (Mi et al., 2019) for every gene subset obtained in the differential analysis. See supplementary information for detailed methodological information.
Cocaine Addiction–Like Behavior (Experiment 4)
On PND 90, animals from a different batch underwent a single food-reinforced fixed ratio 1 instrumental training session limited to 10 reinforcers. After this, an i.v. polyvinylchloride tubing (0.064 mm i.d.) catheter was implanted into the right jugular vein.
The cocaine SA protocol was carried out in Coulbourn boxes. Cocaine (Alcaliber, Madrid, Spain) infusions (0.5 mg/kg in 100 µL of sterile saline solution) were administered by an electronic pump. The protocol consisted of 6 consecutive phases: (1) acquisition (12 daily 2-hour fixed ratio 1 sessions); (2) motivation for consumption (progressive ratio schedule (Sánchez-Cardoso et al., 2007); (3) rebound consumption: three 2-hour sessions under fixed ratio 1; (4) compulsive (punished) seeking: a single 1-hour session under a fixed ratio 3 schedule in which the animal randomly received an infusion or a 0.5-mA plant shock for 0.5 seconds; (5) extended access: 10 sessions of 6 hours each under fixed ratio 1; and (6) cue-induced reinstatement: 4 sessions of 1 hour each with response-contingent cues (same cues as those used during acquisition) but without drug delivery, occurring after 1, 30, 60, and 100 days of forced abstinence. See supplementary information for detailed methodological information. Initial sample sizes as follows: male VEH (n = 15), male THC (n = 18), female VEH (n = 15), and female THC (n = 15).
Statistical Analysis
In general, for the experiments involving repeated measures, we used a mixed ANOVA with 2 between-subject factors (sex and ACE) and 1 within-subject factor (session or test). For the indices without repeated measures, we used standard 2-way ANOVAs. Significant interactions were followed using simple effects analysis.
RESULTS
Reward-Related Psychological Alterations Induced by ACE
Pavlovian Conditioned Approach and Habit Formation
Pavlovian Conditioned Approach
The Pavlovian conditioned approach index changed across the sessions (F2.57, 107.61 = 5.827; P = .002; η p2 = 0.14) (Figure 1B), but no between-subject factor effects were detected. The analysis of the percentual distribution of the 3 different Pavlovian conditioned approach clusters for each group did not show significant differences (as assessed in a contingency table analysis) (Figure 1C). However, in the eighth session, THC-exposed rats (irrespective of the sex) were more biased to display goal-tracking behavior than their VEH-treated controls (F1,36 = 4.539; P = .04; η p2 = 0.11) (Figure 1D). See supplementary information for additional results.
Figure 1.
Pavlovian conditioned approach and habit formation experiments. (A) Timeline of experimental phases for the Pavlovian conditioned approach (PCA) experiment. (B) PCA index across the 8 auto-shaping sessions. Positive values indicate a bias to attribute incentive salience to outcome predictive signals, namely sign-tracking, while negative values indicate goal-tracking or the tendency to attribute salience to the goal (reward). Adolescent cannabinoid exposure (ACE) biased the index towards negative values, indicating increased goal-tracking. (C) Percentual distribution of the 3 different PCA clusters in each group. (D) PCA index obtained in the eighth autoshaping session. THC-exposed rats (irrespective of the sex) were more biased to display goal-tracking behavior than their vehicle (VEH)-treated-treated controls (F1,36 = 4.539; P = .04; η p2 = 0.11). Graphs represent mean ± SEM or individual values in the eighth session (D). Significant effects of ACE are represented by “THC.” (E) Timeline of experimental phases in the habit formation study. (F) Active lever presses during short training sessions and sensory-specific satiety outcome devaluation test. No effects of Sex or ACE were observed across training sessions. All the animals decreased their responses in the devalued condition (suggestive of goal-directed behavior and the absence of habit-like responding) (F1,36 = 30.976; P < .000; η p2 = 0.37). However, “THC”-exposed females showed a higher rate of lever pressing in both conditions compared with VEH-exposed females (F1,18 = 10.740; P = .004; η p2 = 0.37) and “THC”-exposed males (F1,18 = 9.526; P = .006; η p2 = 0.35). Significant effects of sex are represented by “sex,” ACE effects are represented by “thc,” and session effects by “*” A specific ACE effect in the females (after a significant sex × ACE interaction) is indicated by ”f” after the “THC” word. A specific sex effect in the “THC”-treated animals (after a significant sex × ACE interaction) is indicated by “t” after the “thc” word. (G) Extended training sessions and sensory-specific satiety outcome devaluation test. All groups progressively increased their responding across the training sessions (F4.48,147.72 = 21.575; P < .000; η p2 = 0.39). There were no session effects on lever pressing in the tests, indicating absence of devaluation and the development of a stimulus-response, habit-like behavior. There were no sex or adolescent treatment effects (F1,35 = 1.294; P = .263; η p2 = 0.03). Graphs represent mean ± SEM group values.
Habit Formation
No differences due to sex or ACE were detected during the short (non–habit-forming) training. All groups showed a reduction of ALPs in the devalued condition compared with the non-devalued condition during the test sessions (F1,36 = 30.98; P < .000; η p2 = 0.46), ruling out a potential acceleration of habit formation (Figure 1F). We found a sex × ACE interaction (F1,36 = 7.624; P = .009; η p2 = 0.18) due to a higher rate of lever presses in the THC-female group compared with all other groups. However, all groups had a similar slope; thus, the outcome devaluation test had an overall similar impact decreasing the instrumental when the reward was devalued (see Figure 1F). There were no differences during the extended (habit-forming) training. Subsequent testing showed no between-subject factor effects and no differences in lever presses between the test conditions (F1,35 = 1.294; P = .263; η p2 = 0.04), suggestive of habit-like responding in all rats irrespective of their THC history (see Figure 1G). Therefore, there is no evidence for acceleration or impairment of habit formation due to ACE.
PIT and Motor Impulsivity
PIT
A detailed exposition of training results is provided in the supplementary information. A majority of animals expressed PIT (percent ALPs during CS+ >50%) in all groups, but there were no clear significant differences in the phenotypic distribution of PIT expression profiles (see Figure 2C). However, during the PIT testing session, ACE was associated with higher percent ALPs during CS+ among subjects that actually expressed PIT (F1,25 = 4.685; P = .04; η p2 = 0.16) (see Figure 2B). We also observed a sex × ACE interaction that on further analysis showed that THC-exposed males expressed higher percent ALPs during CS+ compared with VEH males (F1,25 = 10.11; P = .004; η p2 = 0.29).
Figure 2.
Pavlovian to instrumental transfer (PIT) and 2-choice serial reaction time task (2-CSRTT). (A) Timeline of experimental phases in the PIT study. (B) % conditioned stimulus (CS+) Active lever presses during the PIT test. Higher percentages are obtained if instrumental responding is high during CS+ presentation and/or low in CS− (indicating increased PIT). Adolescent cannabinoid exposure (ACE) was associated with a higher PIT expression (F1,25 = 4.685; P = .04; η p2 = 0.16), particularly prevalent among THC-exposed males compared with VEH-treated males (F1,25 = 10.11; P = .004; η p2 = 0.29). The graph represents individual values (dots) and mean ± SEM (lines). Significant effects of the ACE factor are represented by “thc.” (C) Percentual distribution of the 3 different PIT clusters in each group. (D) Timeline of experimental phases in the 2-CSRTT. (E) Premature responses during baseline sessions and tests. There was a significant sessions × ACE interaction during baseline (F5,40 = 4.718; P = .002; η p2 = 0.37), especially notable in the females, which led us to compute the percentage of increment in responding against baseline performance. (F) Percentage of increment in premature responding against baseline. We found a significant effect of ACE in the females (F1,44 = 7.892; P = .007; η p2 = 0.15) in the first test session and a general ACE effect (regardless of the sex) in the second test session (F1,44 = 5.240; P = .027; η p2 = 0.11) whereby THC-exposed rats showed increased responding compared with baseline. Graphs represent mean ± SEM of the 4 groups. Significant effects of the ACE factor “thc.” A specific ACE effect in the males (after a significant sex × ACE interaction) is indicated by “m” after the “thc” word and, in the case of the females, by “f” after “thc”.
2-CSRTT
A detailed account of training results is provided in the supplementary information. Our initial analysis of the premature responses across the long inter-trial interval sessions revealed a sex × ACE interaction (F1.55,68.2 = 3.481; P = .048; η p2 = 0.07), and the individual analysis showed that in the first test session, THC males had fewer premature responses compared with VEH males (F1,44 = 5.740; P = .021; η p2 = 0.12) and VEH females also scored significantly under their male counterparts (F1,44 = 7.630; P = .008; η p2 = 0.15) (Figure 2E). During the second and third test sessions, there were no significant differences. However, on closer examination, we detected a significant sessions × ACE interaction during baseline (F5,40 = 4.718; P = .002; η p2 = 0.37) indicative of preexisting differences, so we decided to compute the percentage of increment in premature responses against the baseline for each subject. After correcting for these baseline differences, we found that there was a quasi-significant sex × ACE interaction (F1,44 = 4.034; P = .051; η p2 = 0.08) in the first test session that revealed a strongly significant effect of the ACE in the females (F1,44 = 7.892; P = .007; η p2 = 0.15) who showed a higher increase in premature responses compared with their performance during baseline. This effect was absent in the males. In the second test session, there was a significant effect of ACE (F1,44 = 5.240; P = .027; η p2 = 0.11) indicating a higher increase in premature responses compared with baseline due to ACE. These effects were no longer evident in the third test (see Figure 2F).
Transcriptome Profile in the Shell of the NAcc
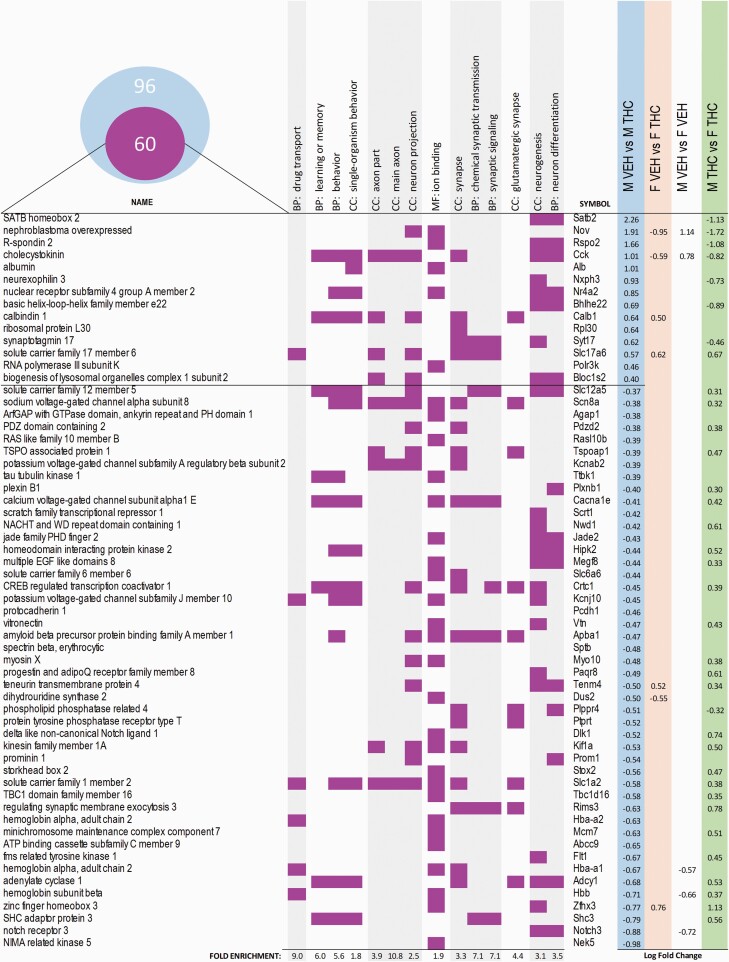
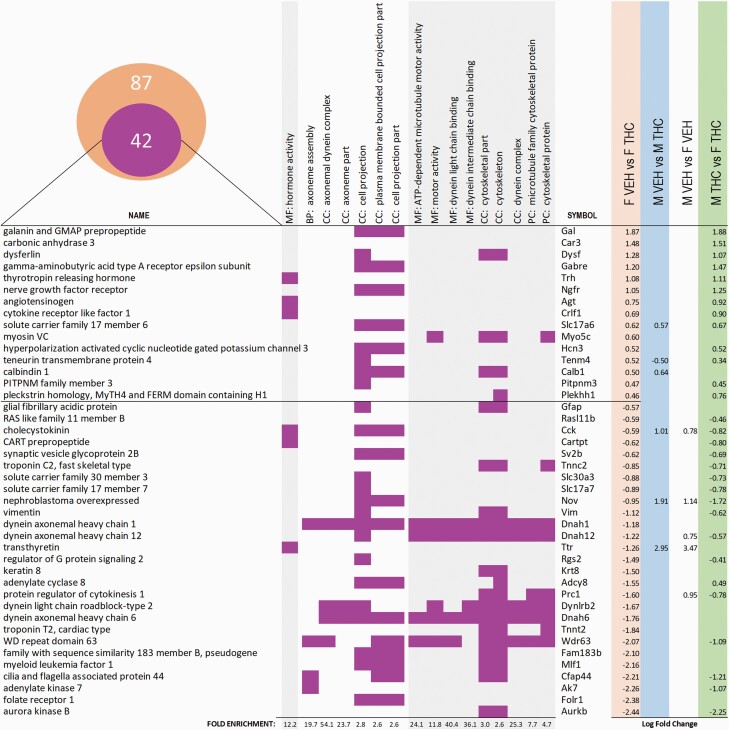
After the results obtained in all these reward-related processes, we decided to examine how ACE affected the transcriptome of the NAcc, a key region regulating reward-guided behavior and impulsivity. There were 95 differentially expressed genes (27 upregulated and 68 downregulated) in THC males compared with VEH males and 84 (30 upregulated and 54 downregulated) differentially expressed genes in the females’ comparison. Only 9 of these differentially expressed genes were present in both differential analyses (see Figures 3 and 4). In the males, the categories with higher fold enrichment included biological processes such as “drug transport,” “learning and memory,” and “chemical synaptic transmission” or were restricted to cell compartments such as the axon (see Figure 3). In the females, a completely different set of categories was affected. The ontologies with higher fold enrichment were related to “hormonal activity,” the organization of cellular projections (including the “axoneme”) and the “cytoskeleton” (see Figure 4).
Figure 3.
Main gene ontologies and differentially expressed genes DEG in the male comparison. DEGs obtained with Cuffdiff in the male-VEH vs male-THC comparison were submitted to PANTHER to perform the gene ontology (GO) analysis. In total, there were 96 DEGs in the male-VEH vs male-THC comparison. The Venn diagram represents the 60 DEGs (out of the total of 96) that compose the most representative GOs depicted in the graph. Each row represents a gene and its associated symbol, its presence in 1 of the GO terms (BP stands for biological process; CC for cellular component and MF for molecular function) highlighted with purple-colored squares, and the value of the corresponding log fold change (false discovery rate adjusted P < .05) in any of the Cuffdiff pairwise comparisons. Rows are arranged by the log fold change of the genes in the males comparison.
Figure 4.
Main GOs and DEGs in females. DEGs obtained with Cuffdiff in the female-VEH vs female-THC comparison were submitted to PANTHER to perform GO analysis. In total, there were 87 DE genes in the male-VEH vs male-THC comparison. The Venn diagram represents the 42 DEG (out of the total of 87) associated to the most representative GOs depicted in the graph. Each row represents a gene and their associated symbol, their presence in 1 of the GO terms (MF stands for molecular function, BP stands for biological process, CC for cellular component, and PC for protein class) highlighted with a purple-colored square, and the value of the log fold change if differentially expressed (false discovery rate-adjusted P < .05) in any of the Cuffdiff pairwise comparison. Rows are arranged by the log fold change of the genes in the female comparison.
Cocaine Addiction–Like Behavior
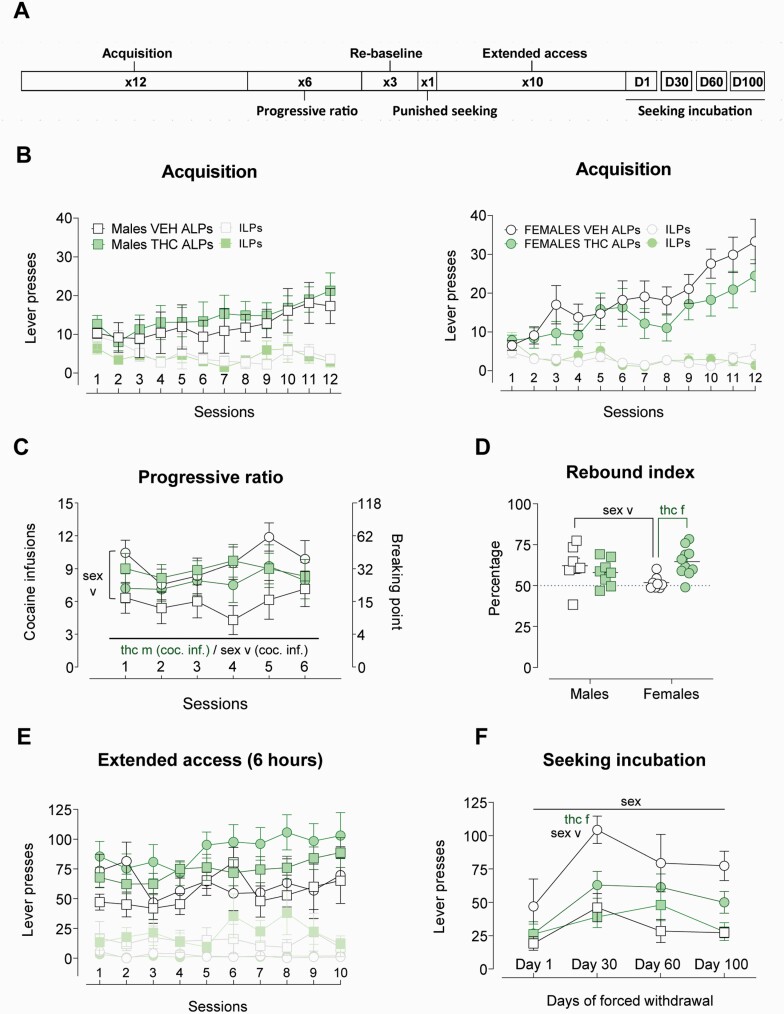
Following our behavioral and transcriptomic results, we proceeded to examine cocaine addiction–like behavior. All rats acquired cocaine SA in a similar way (Figure 5B). However, during progressive ratio sessions, a between-subjects analysis of infusions showed a sex × ACE interaction (F1,25 = 5.215; P = .031; η p2 = 0.173), revealing that THC-exposed males had a higher overall cocaine intake than VEH-treated males (F1,25 = 6.197; P = .032; η p2 = 0.382) and that VEH-treated females had a higher cocaine intake than VEH-exposed males (F1,25 = 7.717; P = .018; η p2 = 0.412) during these high-effort conditions (see Figure 5C). Interestingly, this sex difference was not observed among THC-treated rats. We then returned rats to continuous drug access (fixed ratio 1) for 3 days. When we compared the relative increase during these fixed ratio 1 sessions and the average of the last 3 acquisition sessions (also under fixed-ratio 1 schedule), we observe a significant sex × ACE interaction (F1,29 = 7.507; P = .010; η p2 = 0.21) that revealed that THC-exposed females had a higher rebound than VEH-exposed controls (F1,29 = 9.497; P = .004; η p2 = 0.25). We also observed that, among VEH-exposed rats, there was a significant effect of sex (with VEH-exposed males having higher rebound than VEH-exposed females [F1,29 = 5.165; P = .015; η p2 = 0.15]) (Figure 5D).
Figure 5.
Main indices in the cocaine addiction-like behavior study. (A) Timeline of the experimental phases. (B) Active (ALPs) and inactive lever presses (ILPs) across the twelve acquisition sessions. Data are plotted separated by sex for the sake of clarity. (C) Cocaine infusions and breaking points across the 6 progressive ratio sessions. VEH females consumed more than VEH males during the first session (F1,28 = 4.268; P = .048; η p2 = 0.13). Male rats exposed to THC during adolescence earned more cocaine infusions in average than their VEH-treated controls (F1,25 = 6.197; P = .032; η p2 = 0.382). (D) Rebound index: percentage of increase after returning to fixed ratio 1 conditions compared with the last 3 days of acquisition. Female rats exposed to THC had higher increase than their controls (F1,29 = 9.497; P = .004; η p2 = 0.25) and male rats exposed to VEH had higher increase than their female counterparts (F1,29 = 5.165; P = .015; η p2 = 0.15). (E) ALPs on FR1 and ILPs across the ten sessions of extended access. (F) Lever presses in the 4 extinction sessions as an index of seeking incubation during forced withdrawal. Females showed stronger seeking behavior (F1,21 = 11.607; P = .003; η p2 = 0.36). Graphs represent mean ± SEM and individual values in discrete session graph (D). Significant effects of the sex factor are represented by “sex,” ACE effects are denoted by “thc.” A specific ACE effect in the females is indicated by “f” after the “thc” word while “m” after “thc” indicates a significant effect of ACE among male rats.
During the punished seeking test, all rats reduced the number of infusions achieved compared with the last reacquisition session, but there were no effects due to sex or ACE (see supplementary information for additional measures and graphs). After this single session, we allowed the rats to self-administer cocaine for 6 h/d under a fixed-ratio 1 schedule of reinforcement for 10 days. All groups similarly escalated their intake (F1,20 = 4.349; P = .05; η p2 = 0.179) (see Figure S5D). We did not observe a significant effect of ACE on total cocaine intake across sessions across all 10 extended-access sessions (see Figure 5E).
We then withdrew the rats from cocaine and analyzed their (non-reinforced) seeking responses after 1, 30, 60, and 100 days of forced withdrawal. There was a progression of seeking responses increasing from withdrawal day 1 and peaking around withdrawal day 30—reproducing the incubation of seeking phenomenon—statistically evidenced by the significant effect of session (F2.05,43.05 = 6.618; P = .003; η p2 = 0.24). Noteworthy, females showed a more robust seeking behavior (significant effect of sex) (F1,21 = 11.607; P = .003; η p2 = 0.36) (see Figure 5F). We did not obtain a significant sessions × sex × ACE interaction, but the ad hoc analysis of the withdrawal day-30 session showed a sex × ACE interaction (F1,22 = 4.847; P = .038; η p2 = 0.18) with significant simple effects suggesting a significantly lower seeking behavior of THC-exposed females compared with VEH females (F1,22 = 11.924; P = .002; η p2 = 0.35) and also a significantly higher seeking VEH females compared with VEH males (F1,22 = 17.751; P < .000; η p2 = 0.45).
Discussion
We have provided evidence for a causal relationship between the exposure to THC, the main psychoactive component of cannabis, during adolescence and alterations at adulthood in a set of psychological mechanisms related to reward processing, impulsivity, and some features of cocaine addiction–like behavior.
Reward-Related Psychological Alterations Induced by Adolescent THC Exposure
The increased goal-tracking bias found in rats with ACE is consistent with a prior report showing that adolescent exposure to the CB1/CB2 receptor agonist WIN 55,512-2 altered the normal proportion of sign-tracking/goal-tracking in rats, creating an intermediate phenotype in cannabinoid-exposed animals that was not evident in vehicle-treated rats (biased towards sign-tracking in this study) (Schoch et al., 2017). Our data expand these findings and suggest that adolescent exposure to THC (rather than WIN) affects Pavlovian conditioned approach in both sexes and not only in males. To potentiate goal tracking, cannabinoids might be interfering with the dopamine signal (see below), since sign-tracking behavior, indicative of incentive salience, seems to be more dopamine dependent (Flagel et al., 2011; Saunders and Robinson, 2012).
We also examined habit formation tendency by using a sensory-specific satiation paradigm (Hogarth et al., 2013). We found no differences in the tendency to form habit-like responses in our adult rats with ACE. This is interesting in the general context of the involvement of the endocannabinoid systems in habit formation (Hilário et al., 2007; Nazzaro et al., 2012; Gremel et al., 2016) and the effects of THC in adults animals, which has been shown to accelerate habit formation (Nazzaro et al., 2012). However, when the treatment occurred during adolescence, we observed no such behavioral effects. This is in accordance with the differential effects that cannabinoids exert in the adolescent brain compared with the adult brain.
PIT, also known as Pavlovian motivation, was potentiated by ACE, especially in males. To our knowledge, there are no previous studies specifically ascertaining the effects of cannabinoids on PIT. Regarding substance use disorder liability, Takahashi et al. (2019) reported that the strength of PIT correlates with increased cocaine SA behavior. We did not see differences in cocaine SA acquisition due to THC or detect a subgroup of THC animals with an enhanced cocaine SA acquisition. However, in our experiments, different sets of animals underwent PIT, Pavlovian conditioned approach, and cocaine SA, so future experiments should be performed to directly check this correlation in the same subjects. Given that dopamine transmission in the NAcc is crucial for the PIT phenomenon, with a particular role of D1 receptors (Lex and Hauber, 2008), the specific enhancement of PIT in the THC-exposed males observed here may be related to the hyperdopaminergic state induced by ACE in specific circuits (De Felice and Laviolette, 2021), especially the increment in D1 receptors in the NAcc shell in males but not females after ACE observed by us in a previous report (Higuera-Matas et al., 2010).
Lastly, we have analyzed motor impulsivity using the 2-CSRTT. Adult animals with ACE have shown an increased preference for large, risky rewards (compared with small, certain ones) (Jacobs-Brichford et al., 2019) and a preference bias for small, immediate reinforcers (compared with large, delayed ones) (Johnson et al., 2019). We now expand these findings with our results in males and females and this form of impulsivity, suggesting that ACE not only affects the form of impulsivity capture by delay discounting tasks but also the kind of waiting impulsivity present in the 2-CSRTT, with a stronger effect in the females. This effect is consistent with the involvement of the endocannabinoid system in this variety of impulsivity (Pattij et al., 2007). However, given that this effect was transient, we suggest that ACE would be rather influencing state-like impulsivity and not a stable impulsiveness trait.
Transcriptome Profile in the Shell of the NAcc
Our RNA-seq study provides, for the first time, an exploration of the sex-dependent differential effects of ACE on the striatal transcriptome. We will focus our discussion on reward processes, response to drugs, and substance use disorders, which is the aim of the present work. However, given the importance of some of the transcriptional signatures obtained for schizophrenia, an important comorbid condition of substance used disorders (Khokhar et al., 2018), we also provide some discussion of the relevance of our findings to this disorder in the supplementary Discussion.
In male rats, the upregulated gene with the highest fold change was Satb2 (SATB homeobox 2), involved in transcription regulation and chromatin remodeling. CB1 receptors are coupled to the regulation of the Ctip2–Satb2 transcriptional regulatory code (Diaz-Alonso et al., 2012), and in so doing, they guide corticospinal motor neuron differentiation. The alteration of the Satb2 gene in the NAcc of our animals could also have developmental consequences in the morphology or function of accumbal neurons as suggested by the ontologies affected by THC treatment. Moreover, Satb2 in the paraventricular thalamus is also sensitive to cocaine-rewarding actions (Salti et al., 2018), so it could be speculated that this upregulated gene in accumbal cells may affect the rewarding actions of cocaine under specific circumstances (such as progressive ratio schedules; see below). Another gene with potential implications for our behavioral results was Notch3 (notch receptor 3), which was downregulated. This gene belongs to the notch signaling pathway that is also involved in brain development (Androutsellis-Theotokis et al., 2006). Interestingly, Notch3 is downregulated in striatal territories in spontaneously hypertensive rats treated with methylphenidate during adolescence (and that further self-administered methylphenidate as adults) (dela Peña et al., 2014), suggesting that this gene is responsive to several pharmacological challenges during adolescence (not just cannabinoids), with dopamine acting as a potential common link (Gottlieb, 2001; Bossong et al., 2009; Wahlstrom et al., 2010), and also that its downregulation may predispose to psychostimulant consumption.
In addition, we found transcriptional and translational alterations in adult animals exposed to adolescent cannabinoids that may influence, in a sex-dependent manner, elements of the dopaminergic signaling pathway and shape drug-related behaviors. In this regard, the transcriptional factor Zinc Finger Homeobox 3, Zfhx3 (downregulated in THC males, upregulated in THC females and associated with neurogenesis and ion-binding Gene Ontologies extracted from the list of differentially expressed genes in the males), is distinctively present in a subtype of D2-expressing neurons of the adult midbrain (Poulin et al., 2014). Thus, this difference may suggest a potential modulation of this specific subtype of D2-expressing neurons in the NAcc shell of rats exposed to THC. Additionally, THC males showed an upregulation of the nuclear receptor gene Nr4a2 involved in behavior and neuron differentiation gene ontologies within the male differentially expressed genes. Nr4a2 can be modulated by neuronal firing and dopamine signaling; moreover, the loss of D2 signaling also contributes to Nr4a2 upregulation (Tseng et al., 2000).
ACE also altered several elements belonging to glutamate, GABA signaling, and other ion channels relevant for the expression of motivated behaviors and drug use. Among the glutamatergic alterations, we find it relevant to highlight the upregulation in both males and females of the solute carrier Slc17a6, which encodes the vesicular glutamate transporter 2(VGlu2) protein involved in glutamate uptake into synaptic vesicles at presynaptic nerve terminals. Noteworthy, dopamine neuronal subtypes express this protein, and the presence of VGlu2 seems to be required for psychostimulant-induced behavioral activation (Birgner et al., 2010).
In addition, there are several relevant changes in the male NAcc shell ion channel expression profile. In this regard, THC produced a protracted downregulation in the male NAcc shell of the solute carrier Slc1a2, a glial transporter that clears glutamate from the synaptic cleft. The expression of this gene is altered by many drugs of abuse (cocaine, amphetamines, nicotine, opioids, ethanol, and cannabinoids), and it has received attention as a potential target for pharmacological interventions in substance use disorders (Roberts-Wolfe and Kalivas, 2015); the voltage-gated potassium channel subunit beta-2 Kcnab2, which is similarly depleted after chronic morphine exposure (Mazei-Robison et al., 2011) and has been involved in motivated behaviors (O’Donovan et al., 2019); the ATP-sensitive inward rectifier potassium channel 10 Kcnj10, also involved in substance use disorders and ethanol preference (Zou et al., 2009); the sodium channel protein type 8 subunit alpha, Scn8a, which plays an important role in regulating excitability in the brain; and the potassium-chloride transporter member 5, Slc12a5, associated with the formation and maturation of glutamatergic and GABAergic synaptic connections (Medina et al., 2014).
In the females, ACE was associated with upregulation of the GABA A Receptor Epsilon Subunit, Gabre. In the context of substance use disorders, rats with a genetic predisposition to alcohol consumption showed a Gabre upregulation (Spence et al., 2018). The hormone activity gene ontology was enriched in the female subset of DEGs, and, noteworthy, the neuropeptides included in this subset may be determining the dopaminergic activity in the NAcc shell of THC-treated females. Thyrotropin-releasing hormone Trh, upregulated by THC in females, participates in energy metabolism and affects different hormonal functions but also enhances dopamine release in the NAcc (Puga et al., 2016). We also detected an upregulation of Agt, which encodes angiotensinogen, the precursor protein of angiotensin I, which is further converted to the peptide angiotensin II. Reductions of angiotensin II, and consequent less activation of the angiotensin II type 1 and type 2 receptors are associated with lower levels of dopamine in the ventral tegmental area and linked to lower alcohol consumption (Maul et al., 2005). Similarly, the cocaine- and amphetamine-regulated transcript seems to exert a neuromodulatory role in the NAcc attenuating dopamine release (Rakovska et al., 2017), and cocaine- and amphetamine-regulated transcript injections into NAcc inhibit the behavioral effects of cocaine (Yu et al., 2017).
We have also detected an upregulation of the Gal gene, encoding the neuropeptide galanin. Noteworthy, galanin has been involved in pathological food consumption and addiction (Gosnell et al., 1986a, 1986b; Sandi et al., 1988). In this regard, an overabundance of galanin has been shown to decrease the sensitivity to amphetamine-induced behavioral effects (Clarke et al., 1988). It is also important to mention that agonists of the galanin receptor can reduce reinstatement of cocaine-seeking (Ogbonmwan et al., 2015) and cocaine-conditioned place preference (Narasimhaiah et al., 2009), which is important considering the sex-specific effects in cocaine SA after ACE (Higuera-Matas et al., 2008). Finally, THC produced a sex-dependent change in the expression of the Cck gene (upregulated in males and downregulated in females treated with THC), which encodes the peptide hormone cholecystokinin. Cholecystokinin (Cck) signaling pathways have been related to food intake, but also with reward and anxiety and even panic (Bradwejn and Vasar, 1995; Rotzinger and Vaccarino, 2003), and have also been studied in the context of drug-related behaviors (Lu et al., 2001, 2002; Wunderlich et al., 2004). Moreover, cocaine behavioral sensitization is accompanied by increasing levels of Cck in the NAcc shell (Beinfeld et al., 2002).
Cocaine Addiction–Like Behavior
In accordance with a previous study (Kononoff et al., 2018), we found no differences in the acquisition of cocaine SA between THC- or vehicle-exposed rats. We have used an intermediate dose of cocaine (0.5 mg/kg), which may explain the divergence between our results and those of Friedman and colleagues (Friedman et al., 2019), who found potentiated cocaine SA with lower doses (0.1 mg/kg) but not with a higher dose (0.32 mg/kg).
ACE was associated with higher intake during progressive ratio sessions in males but not females and effect that may rely on the previously mentioned increase of D1 receptors in the NAcc shell after ACE specifically in the males (Bari and Pierce, 2005; Higuera-Matas et al., 2010). Previous findings showed by Friedman and Kononoff showed unaltered motivation for consumption, although the lower doses (0.1 and 0.32 mg/kg) employed by Friedman et al. (Friedman et al., 2019) and the different timing of the cannabinoid treatment or the cannabinoid agent (WIN 55,512-2) in the study of Kononoff et al. (Kononoff et al., 2018) are 2 probable sources of this divergence. After returning to continuous access to the drug, females showed an increased rebound in consumption compared with the last cocaine SA sessions. This enhanced vulnerability may be relevant for situations of difficult drug access followed by resumption of availability (such as the transition from lock-down in the COVID-19 pandemic to a normal situation).
All the rats diminished their cocaine intake in a similar proportion during compulsive drug taking, ruling out potential changes in compulsivity, although this feature may require further investigation using repeated testing sessions to reveal the effect.
As far as we know, we are the first to explore cocaine-seeking incubation after ACE and to include females in the study. Our data suggesting higher incubation in the females are consistent with previous research showing more robust incubation in females and a higher tendency to reinstate seeking by conditioned cues and drug priming (Lynch and Carroll, 1999; Kerstetter et al., 2008; Nicolas et al., 2019). Previous ACE studies with cocaine showed that adult mice with an adolescent exposition to WIN55,212-2 were less susceptible to the anxiogenic effects of cocaine abstinence (Aguilar et al., 2017), suggesting a potential mechanism for our effects that needs to be further explored, especially concerning its potential sex-specific nature.
CAVEATS AND CONCLUDING REMARKS
An important caveat to consider in this work is the fact that we have opted for a passive i.p. administration route. We chose this route of administration to ensure a homogenous and comparable exposure with the THC across subjects, something that may have been difficult to achieve using i.v. SA procedures or operant vapor SA protocols; however, this is a limitation that should be kept in mind when considering the general translatability of our results
In this work, we have shown that exposure to THC during adolescence profoundly affects the transcriptomic programs of the NAcc and, concomitantly, modulates the influence of rewards and reward-related cues on behavior with a subtle and transient impact on a specific form impulsiveness. Exposure to the main active psychoactive component of cannabis during adolescence also affects certain aspects of addiction-like behavior differentially in males and females and may protect females from the incubation of seeking. These results should be taken into consideration for the ongoing debate about the validity of the Gateway Hypothesis, for tailoring sex-specific treatment approaches for cocaine use disorder depending on previous cannabis consumption during adolescence and, in general, for the evaluation of the long-term consequences of cannabis use by adolescents, an especially vulnerable population.
Supplementary Material
Acknowledgments
We thank Alberto Marcos, Rosa Ferrado Luis Carrillo, Luis Troca, and Gonzalo Moreno for their excellent technical assistance. This manuscript was prepared during the COVID-19 pandemic and is dedicated to those who helped our society. We honor them all.
This work was supported by the Spanish Ministry of Health, Social Services and Equality (Network of Addictive Disorders – Project no: RTA-RD16/0017/0022 of the Institute of Health Carlos III to E.A. and Plan Nacional Sobre Drogas, Project no: 2016I073 to E.A. and 2017I042 to A.H.-M.); Ministry of Science (PID2019-104523RB-I00 to A.-H.M. and PID2019-111594RB-100 to E.A.); UNED (Plan for the Promotion of Research to E.A. and A.H.-M.); the European Union (Project no: JUST/2017/AG-DRUG-806996-JUSTSO) and the BBVA Foundation (2017 Leonardo Grant for Researchers and Cultural Creators to A.H.-M.). These agencies funded the study but had no further role in the study design; the collection, analysis, and interpretation of data; the writing of the report; or the decision to submit the paper for publication. J.O. received funding from Instituto de Salud Carlos III and is now an alumnus of the graduate program of the International Graduate School of UNED. D.-R.M. received a predoctoral fellowship granted by UNED and M.U. received a predoctoral fellowship awarded by the Ministry of Science and Innovation (BES-2011-043814).
Interest Statement
The authors have no conflict of interest that may affect the results or conclusions reported in this work.
References
- Aguilar MA, Ledesma JC, Rodríguez-Arias M, Penalva C, Manzanedo C, Miñarro J, Arenas MC (2017) Adolescent exposure to the synthetic cannabinoid WIN 55212-2 modifies cocaine withdrawal symptoms in adult mice. Int J Mol Sci 18:1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Androutsellis-Theotokis A, Leker RR, Soldner F, Hoeppner DJ, Ravin R, Poser SW, Rueger MA, Bae SK, Kittappa R, McKay RD (2006) Notch signaling regulates stem cell numbers in vitro and in vivo. Nature 442:823–826. [DOI] [PubMed] [Google Scholar]
- Bari AA, Pierce RC (2005) D1-like and D2 dopamine receptor antagonists administered into the shell subregion of the rat nucleus accumbens decrease cocaine, but not food, reinforcement. Neuroscience 135:959–968. [DOI] [PubMed] [Google Scholar]
- Beinfeld MC, Connolly KJ, Pierce RC (2002) Cocaine treatment increases extracellular cholecystokinin (CCK) in the nucleus accumbens shell of awake, freely moving rats, an effect that is enhanced in rats that are behaviorally sensitized to cocaine. J Neurochem 81:1021–1027. [DOI] [PubMed] [Google Scholar]
- Belin D, Mar AC, Dalley JW, Robbins TW, Everitt BJ (2008) High impulsivity predicts the switch to compulsive cocaine-taking. Science 320:1352–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belin D, Belin-Rauscent A, Murray JE, Everitt BJ (2013) Addiction: failure of control over maladaptive incentive habits. Curr Opin Neurobiol 23:564–572. [DOI] [PubMed] [Google Scholar]
- Birgner C, Nordenankar K, Lundblad M, Mendez JA, Smith C, le Grevès M, Galter D, Olson L, Fredriksson A, Trudeau LE, Kullander K, Wallén-Mackenzie A (2010) VGLUT2 in dopamine neurons is required for psychostimulant-induced behavioral activation. Proc Natl Acad Sci U S A 107:389–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biscaia M, Fernández B, Higuera-Matas A, Miguéns M, Viveros MP, García-Lecumberri C, Ambrosio E (2008a) Sex-dependent effects of periadolescent exposure to the cannabinoid agonist CP-55,940 on morphine self-administration behaviour and the endogenous opioid system. Neuropharmacology 54:863–873. [DOI] [PubMed] [Google Scholar]
- Biscaia M, Fernández B, Higuera-Matas A, Miguéns M, Viveros MP, García-Lecumberri C, Ambrosio E (2008b) Sex-dependent effects of periadolescent exposure to the cannabinoid agonist CP-55,940 on morphine self-administration behaviour and the endogenous opioid system. Neuropharmacology 54:863–873. [DOI] [PubMed] [Google Scholar]
- Blakemore SJ (2012) Imaging brain development: the adolescent brain. Neuroimage 61:397–406. [DOI] [PubMed] [Google Scholar]
- Bossong MG, van Berckel BN, Boellaard R, Zuurman L, Schuit RC, Windhorst AD, van Gerven JM, Ramsey NF, Lammertsma AA, Kahn RS (2009) Delta 9-tetrahydrocannabinol induces dopamine release in the human striatum. Neuropsychopharmacology 34:759–766. [DOI] [PubMed] [Google Scholar]
- Bradwejn J, Vasar E (1995) Cholecystokinin and anxiety: from neuron to behavior. Berlin, Heidelberg, Germany: Springer Berlin Heidelberg. [Google Scholar]
- Cartoni E, Balleine B, Baldassarre G (2016) Appetitive Pavlovian-instrumental transfer: a review. Neurosci Biobehav Rev 71:829–848. [DOI] [PubMed] [Google Scholar]
- Clarke PB, Jakubovic A, Fibiger HC (1988) Anatomical analysis of the involvement of mesolimbocortical dopamine in the locomotor stimulant actions of d-amphetamine and apomorphine. Psychopharmacology 96:511–520. [DOI] [PubMed] [Google Scholar]
- De Felice M, Laviolette SR (2021) Reversing the psychiatric effects of neurodevelopmental cannabinoid exposure: exploring pharmacotherapeutic interventions for symptom improvement. Int J Mol Sci 22:7861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- dela Peña I, Kim HJ, Sohn A, Kim BN, Han DH, Ryu JH, Shin CY, Noh M, Cheong JH (2014) Prefrontal cortical and striatal transcriptional responses to the reinforcing effect of repeated methylphenidate treatment in the spontaneously hypertensive rat, animal model of attention-deficit/hyperactivity disorder (ADHD). Behav Brain Funct 10:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deroche-Gamonet V, Belin D, Piazza PV (2004) Evidence for addiction-like behavior in the rat. Science 305:1014–1017. [DOI] [PubMed] [Google Scholar]
- Diaz-Alonso J, Aguado T, Wu CS, Palazuelos J, Hofmann C, Garcez P, Guillemot F, Lu HC, Lutz B, Guzmán M, Galve-Roperh I (2012) The CB(1) cannabinoid receptor drives corticospinal motor neuron differentiation through the Ctip2/Satb2 transcriptional regulation axis. J Neurosci 32:16651–16665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellgren M, Spano SM, Hurd YL (2007) Adolescent cannabis exposure alters opiate intake and opioid limbic neuronal populations in adult rats. Neuropsychopharmacology 32:607–615. [DOI] [PubMed] [Google Scholar]
- EMCDA (2019) European Drug Report. Available at http://www.emcdda.europa.eu/edr2019_en. Accessed 2 April 2021.
- Everitt BJ, Robbins TW (2013) From the ventral to the dorsal striatum: devolving views of their roles in drug addiction. Neurosci Biobehav Rev 37:1946–1954. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Giuliano C, Belin D (2018) Addictive behaviour in experimental animals: prospects for translation. Philos Trans R Soc B Biol Sci. 373:20170027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fergusson DM, Boden JM, Horwood LJ (2006) Cannabis use and other illicit drug use: testing the cannabis gateway hypothesis. Addiction 101:556–569. [DOI] [PubMed] [Google Scholar]
- Ferland JN, Hurd YL (2020) Deconstructing the neurobiology of cannabis use disorder. Nat Neurosci 23:600–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzpatrick CJ, Morrow JD (2016) Pavlovian conditioned approach training in rats. J Vis Exp 2016:e53580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flagel SB, Clark JJ, Robinson TE, Mayo L, Czuj A, Willuhn I, Akers CA, Clinton SM, Phillips PE, Akil H (2011) A selective role for dopamine in stimulus-reward learning. Nature 469:53–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman AL, Meurice C, Jutkiewicz EM (2019) Effects of adolescent Δ9-tetrahydrocannabinol exposure on the behavioral effects of cocaine in adult Sprague-Dawley rats. Exp Clin Psychopharmacol 27:326–337. [DOI] [PubMed] [Google Scholar]
- Gosnell BA, Levine AS, Morley JE (1986a) The stimulation of food intake by selective agonists of mu, kappa and delta opioid receptors. Life Sci 38:1081–1088. [DOI] [PubMed] [Google Scholar]
- Gosnell BA, Morley JE, Levine AS (1986b) Opioid-induced feeding: localization of sensitive brain sites. Brain Res 369:177–184. [DOI] [PubMed] [Google Scholar]
- Gottlieb S (2001) Methylphenidate works by increasing dopamine levels. BMJ 322:259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gremel CM, Chancey JH, Atwood BK, Luo G, Neve R, Ramakrishnan C, Deisseroth K, Lovinger DM, Costa RM (2016) Endocannabinoid modulation of orbitostriatal circuits gates habit formation. Neuron 90:1312–1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higuera-Matas A, Soto-Montenegro ML, del Olmo N, Miguéns M, Torres I, Vaquero JJ, Sánchez J, García-Lecumberri C, Desco M, Ambrosio E (2008) Augmented acquisition of cocaine self-administration and altered brain glucose metabolism in adult female but not male rats exposed to a cannabinoid agonist during adolescence. Neuropsychopharmacology 33:806–813. [DOI] [PubMed] [Google Scholar]
- Higuera-Matas A, Botreau F, Del Olmo N, Miguéns M, Olías O, Montoya GL, García-Lecumberri C, Ambrosio E (2010) Periadolescent exposure to cannabinoids alters the striatal and hippocampal dopaminergic system in the adult rat brain. Eur Neuropsychopharmacol 20:895–906. [DOI] [PubMed] [Google Scholar]
- Higuera-Matas A, Ucha M, Ambrosio E (2015) Long-term consequences of perinatal and adolescent cannabinoid exposure on neural and psychological processes. Neurosci Biobehav Rev 55:119–146. [DOI] [PubMed] [Google Scholar]
- Hilário MR, Clouse E, Yin HH, Costa RM (2007) Endocannabinoid signaling is critical for habit formation. Front Integr Neurosci 1:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogarth L, Balleine BW, Corbit LH, Killcross S (2013) Associative learning mechanisms underpinning the transition from recreational drug use to addiction. Ann N Y Acad Sci 1282:12–24. [DOI] [PubMed] [Google Scholar]
- Hurd YL (2020) Cannabis and the developing brain challenge risk perception. J Clin Invest 130:3947–3949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs-Brichford E, Manson KF, Roitman JD (2019) Effects of chronic cannabinoid exposure during adolescence on reward preference and mPFC activation in adulthood. Physiol Behav 199:395–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson KR, Boomhower SR, Newland MC (2019) Behavioral effects of chronic WIN 55,212-2 administration during adolescence and adulthood in mice. Exp Clin Psychopharmacol 27:348–358. [DOI] [PubMed] [Google Scholar]
- Jupp B, Dalley JW (2014) Behavioral endophenotypes of drug addiction: etiological insights from neuroimaging studies. Neuropharmacology 76 Pt B:487–497. [DOI] [PubMed] [Google Scholar]
- Kallio MA, Tuimala JT, Hupponen T, Klemelä P, Gentile M, Scheinin I, Koski M, Käki J, Korpelainen EI (2011) Chipster: user-friendly analysis software for microarray and other high-throughput data. BMC Genomics 12:507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandel DB (2003) Does marijuana use cause the use of other drugs? JAMA 289:482–483. [DOI] [PubMed] [Google Scholar]
- Kandel DB, Yamaguchi K, Chen K (1992) Stages of progression in drug involvement from adolescence to adulthood: further evidence for the gateway theory. J Stud Alcohol 53:447–457. [DOI] [PubMed] [Google Scholar]
- Kerstetter KA, Aguilar VR, Parrish AB, Kippin TE (2008) Protracted time-dependent increases in cocaine-seeking behavior during cocaine withdrawal in female relative to male rats. Psychopharmacology 198:63–75. [DOI] [PubMed] [Google Scholar]
- Khokhar JY, Dwiel LL, Henricks AM, Doucette WT, Green AI (2018) The link between schizophrenia and substance use disorder: a unifying hypothesis. Schizophr Res 194:78–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinig J (2015) Ready for retirement: the gateway drug hypothesis. Subst Use Misuse 50:971–975. [DOI] [PubMed] [Google Scholar]
- Kononoff J, Melas PA, Kallupi M, de Guglielmo G, Kimbrough A, Scherma M, Fadda P, Kandel DB, Kandel ER, George O (2018) Adolescent cannabinoid exposure induces irritability-like behavior and cocaine cross-sensitization without affecting the escalation of cocaine self-administration in adulthood. Sci Rep 8:13893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecca D, Scifo A, Pisanu A, Valentini V, Piras G, Sil A, Cadoni C, Di Chiara G (2020) Adolescent cannabis exposure increases heroin reinforcement in rats genetically vulnerable to addiction. Neuropharmacology 166:107974. [DOI] [PubMed] [Google Scholar]
- Lex A, Hauber W (2008) Dopamine D1 and D2 receptors in the nucleus accumbens core and shell mediate Pavlovian-instrumental transfer. Learn Mem 15:483–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L, Huang M, Ma L, Li J (2001) Different role of cholecystokinin (CCK)-A and CCK-B receptors in relapse to morphine dependence in rats. Behav Brain Res 120:105–110. [DOI] [PubMed] [Google Scholar]
- Lu L, Zhang B, Liu Z, Zhang Z (2002) Reactivation of cocaine conditioned place preference induced by stress is reversed by cholecystokinin-B receptors antagonist in rats. Brain Res 954:132–140. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Carroll ME (1999) Sex differences in the acquisition of intravenously self-administered cocaine and heroin in rats. Psychopharmacology 144:77–82. [DOI] [PubMed] [Google Scholar]
- Lynskey MT, Agrawal A (2018) Denise Kandel’s classic work on the gateway sequence of drug acquisition. Addiction 113:1927–1932. [DOI] [PubMed] [Google Scholar]
- Maul B, Krause W, Pankow K, Becker M, Gembardt F, Alenina N, Walther T, Bader M, Siems WE (2005) Central angiotensin II controls alcohol consumption via its AT1 receptor. Faseb J 19:1474–1481. [DOI] [PubMed] [Google Scholar]
- Mayet A, Legleye S, Beck F, Falissard B, Chau N (2016) The gateway hypothesis, common liability to addictions or the route of administration model a modelling process linking the three theories. Eur Addict Res 22:107–117. [DOI] [PubMed] [Google Scholar]
- Mazei-Robison MS, et al. (2011) Role for mTOR signaling and neuronal activity in morphine-induced adaptations in ventral tegmental area dopamine neurons. Neuron 72:977–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina I, Friedel P, Rivera C, Kahle KT, Kourdougli N, Uvarov P, Pellegrino C (2014) Current view on the functional regulation of the neuronal K(+)-Cl(-) cotransporter KCC2. Front Cell Neurosci 8:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mi H, Muruganujan A, Ebert D, Huang X, Thomas PD (2019) PANTHER version 14: more genomes, a new PANTHER GO-slim and improvements in enrichment analysis tools. Nucleic Acids Res 47:D419–D426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narasimhaiah R, Kamens HM, Picciotto MR (2009) Effects of galanin on cocaine-mediated conditioned place preference and ERK signaling in mice. Psychopharmacology 204:95–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nazzaro C, Greco B, Cerovic M, Baxter P, Rubino T, Trusel M, Parolaro D, Tkatch T, Benfenati F, Pedarzani P, Tonini R (2012) SK channel modulation rescues striatal plasticity and control over habit in cannabinoid tolerance. Nat Neurosci 15:284–293. [DOI] [PubMed] [Google Scholar]
- Nguyen JD, Creehan KM, Kerr TM, Taffe MA (2020) Lasting effects of repeated ∆9 -tetrahydrocannabinol vapour inhalation during adolescence in male and female rats. Br J Pharmacol 177:188–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolas C, Russell TI, Pierce AF, Maldera S, Holley A, You ZB, McCarthy MM, Shaham Y, Ikemoto S (2019) Incubation of cocaine craving after intermittent-access self-administration: sex differences and estrous cycle. Biol Psychiatry 85:915–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nkansah-Amankra S, Minelli M (2016) “Gateway hypothesis” and early drug use: additional findings from tracking a population-based sample of adolescents to adulthood. Prev Med Rep 4:134–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donovan B, Adeluyi A, Anderson EL, Cole RD, Turner JR, Ortinski PI (2019) Altered gating of Kv1.4 in the nucleus accumbens suppresses motivation for reward. Elife 8:e4787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogbonmwan YE, Sciolino NR, Groves-Chapman JL, Freeman KG, Schroeder JP, Edwards GL, Holmes PV, Weinshenker D (2015) The galanin receptor agonist, galnon, attenuates cocaine-induced reinstatement and dopamine overflow in the frontal cortex. Addict Biol 20:701–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattij T, Janssen MC, Schepers I, González-Cuevas G, de Vries TJ, Schoffelmeer AN (2007) Effects of the cannabinoid CB1 receptor antagonist rimonabant on distinct measures of impulsive behavior in rats. Psychopharmacology 193:85–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paus T, Keshavan M, Giedd JN (2008) Why do many psychiatric disorders emerge during adolescence? Nat Rev Neurosci 9:947–957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulin JF, Zou J, Cicchetti F, Awatramani RB (2014) Defining midbrain dopaminergic neuron diversity by single-cell gene expression profiling correspondence. Cell Rep 9:930–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puga L, Alcántara-Alonso V, Coffeen U, Jaimes O, de Gortari P (2016) TRH injected into the nucleus accumbens shell releases dopamine and reduces feeding motivation in rats. Behav Brain Res 306:128–136. [DOI] [PubMed] [Google Scholar]
- Rakovska A, Baranyi M, Windisch K, Petkova-Kirova P, Gagov H, Kalfin R (2017) Neurochemical evidence that cocaine- and amphetamine-regulated transcript (CART) 55-102 peptide modulates the dopaminergic reward system by decreasing the dopamine release in the mouse nucleus accumbens. Brain Res Bull 134:246–252. [DOI] [PubMed] [Google Scholar]
- Roberts-Wolfe DJ, Kalivas PW (2015) Glutamate transporter GLT-1 as a therapeutic target for substance use disorders. CNS Neurol Disord Drug Targets 14:745–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotzinger S, Vaccarino FJ (2003) Cholecystokinin receptor subtypes: role in the modulation of anxiety-related and reward-related behaviours in animal models. J Psychiatry Neurosci 28:171–181. [PMC free article] [PubMed] [Google Scholar]
- Rubino T, Parolaro D (2016) The impact of exposure to cannabinoids in adolescence: insights from animal models. Biol Psychiatry 79:578–585. [DOI] [PubMed] [Google Scholar]
- Salti A, Apostolova G, Kummer KK, Lemos C, Dechant G, El Rawas R (2018) Cocaine paired environment increases SATB2 levels in the rat paraventricular thalamus. Front Behav Neurosci 12:224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez-Cardoso P, Higuera-Matas A, Martín S, del Olmo N, Miguéns M, García-Lecumberri C, Ambrosio E (2007) Modulation of the endogenous opioid system after morphine self-administration and during its extinction: a study in Lewis and Fischer 344 rats. Neuropharmacology 52:931–948. [DOI] [PubMed] [Google Scholar]
- Sandi C, Borrell J, Guaza C (1988) Involvement of kappa type opioids on ethanol drinking. Life Sci 42:1067–1075. [DOI] [PubMed] [Google Scholar]
- Saunders BT, Robinson TE (2012) The role of dopamine in the accumbens core in the expression of Pavlovian-conditioned responses. Eur J Neurosci 36:2521–2532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherma M, Qvist JS, Asok A, Huang SC, Masia P, Deidda M, Wei YB, Soni RK, Fratta W, Fadda P, Kandel ER, Kandel DB, Melas PA (2020) Cannabinoid exposure in rat adolescence reprograms the initial behavioral, molecular, and epigenetic response to cocaine. Proc Natl Acad Sci U S A 117:9991–10002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoch H, Huerta MY, Ruiz CM, Farrell MR, Jung KM, Huang JJ, Campbell RR, Piomelli D, Mahler SV (2017) Adolescent cannabinoid exposure effects on natural reward seeking and learning in rats. Psychopharmacology 235:121–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear LP (2000) The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev 24:417–463. [DOI] [PubMed] [Google Scholar]
- Spence JP, Reiter JL, Qiu B, Gu H, Garcia DK, Zhang L, Graves T, Williams KE, Bice PJ, Zou Y, Lai Z, Yong W, Liang T (2018) Estrogen-dependent upregulation of Adcyap1r1 expression in nucleus accumbens is associated with genetic predisposition of sex-specific QTL for alcohol consumption on rat chromosome 4. Front Genet 9:513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stopponi S, Soverchia L, Ubaldi M, Cippitelli A, Serpelloni G, Ciccocioppo R (2014) Chronic THC during adolescence increases the vulnerability to stress-induced relapse to heroin seeking in adult rats. Eur Neuropsychopharmacol 24:1037–1045. [DOI] [PubMed] [Google Scholar]
- Stringfield SJ, Torregrossa MM (2021) Disentangling the lasting effects of adolescent cannabinoid exposure. Prog Neuropsychopharmacol Biol Psychiatry 104:110067. [DOI] [PubMed] [Google Scholar]
- Takahashi TT, Vengeliene V, Enkel T, Reithofer S, Spanagel R (2019) Pavlovian to instrumental transfer responses do not correlate with addiction-like behavior in rats. Front Behav Neurosci 13:129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarter RE, Vanyukov M, Kirisci L, Reynolds M, Clark DB (2006) Predictors of marijuana use in adolescents before and after licit drug use: examination of the gateway hypothesis. Am J Psychiatry 163:2134–2140. [DOI] [PubMed] [Google Scholar]
- Tomasiewicz HC, Jacobs MM, Wilkinson MB, Wilson SP, Nestler EJ, Hurd YL (2012) Proenkephalin mediates the enduring effects of adolescent cannabis exposure associated with adult opiate vulnerability. Biol Psychiatry 72:803–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng KY, Roubert C, Do L, Rubinstein M, Kelly MA, Grandy DK, Low MJ, Gershanik OS, Murer MG, Giros B, Raisman-Vozari R (2000) Selective increase of Nurr1 mRNA expression in mesencephalic dopaminergic neurons of D2 dopamine receptor-deficient mice. Brain Res Mol Brain Res 80:1–6. [DOI] [PubMed] [Google Scholar]
- Ucha M, Roura-Martínez D, Contreras A, Pinto-Rivero S, Orihuel J, Ambrosio E, Higuera-Matas A (2019) Impulsive action and impulsive choice are differentially associated with gene expression variations of the GABAA receptor Alfa 1 subunit and the CB1 receptor in the lateral and medial orbitofrontal cortices. Front Behav Neurosci 13:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanyukov MM, Tarter RE, Kirillova GP, Kirisci L, Reynolds MD, Kreek MJ, Conway KP, Maher BS, Iacono WG, Bierut L, Neale MC, Clark DB, Ridenour TA (2012) Common liability to addiction and “gateway hypothesis”: theoretical, empirical and evolutionary perspective. Drug Alcohol Depend 123 (Suppl 1):S3–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viveros MP, Marco EM, López-Gallardo M, Garcia-Segura LM, Wagner EJ (2011) Framework for sex differences in adolescent neurobiology: a focus on cannabinoids. Neurosci Biobehav Rev 35:1740–1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahlstrom D, Collins P, White T, Luciana M (2010) Developmental changes in dopamine neurotransmission in adolescence: behavioral implications and issues in assessment. Brain Cogn 72:146–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wunderlich GR, Rotzinger S, Bush DE, DeSousa NJ, Vaccarino FJ (2004) Cholecystokinin modulation of locomotor behavior in rats is sensitized by chronic amphetamine and chronic restraint stress exposure. Brain Res 1001:95–107. [DOI] [PubMed] [Google Scholar]
- Yu CP, Zhou XY, Fu Q, Peng QH, Oh KW, Hu ZZ (2017) A new insight into the role of CART in cocaine reward: involvement of CaMKII and inhibitory G-protein coupled receptor signaling. Front Cell Neurosci 11:244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou SB, Weng J, Symons MN, Singh SM (2009) Role of potassium channel gene Kcnj10 in ethanol preference in C57bl/6J and DBA/2J mice. Alcohol Clin Exp Res 33:394–399. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.