Abstract
Roots are central to the function of natural and agricultural ecosystems by driving plant acquisition of soil resources and influencing the carbon cycle. Root characteristics like length, diameter and volume are critical to measure to understand plant and soil functions. RhizoVision Explorer is an open-source software designed to enable researchers interested in roots by providing an easy-to-use interface, fast image processing and reliable measurements. The default broken roots mode is intended for roots sampled from pots and soil cores, washed and typically scanned on a flatbed scanner, and provides measurements like length, diameter and volume. The optional whole root mode for complete root systems or root crowns provides additional measurements such as angles, root depth and convex hull. Both modes support providing measurements grouped by defined diameter ranges, the inclusion of multiple regions of interest and batch analysis. RhizoVision Explorer was successfully validated against ground truth data using a new copper wire image set. In comparison, the current reference software, the commercial WinRhizo™, drastically underestimated volume when wires of different diameters were in the same image. Additionally, measurements were compared with WinRhizo™ and IJ_Rhizo using a simulated root image set, showing general agreement in software measurements, except for root volume. Finally, scanned root image sets acquired in different labs for the crop, herbaceous and tree species were used to compare results from RhizoVision Explorer with WinRhizo™. The two software showed general agreement, except that WinRhizo™ substantially underestimated root volume relative to RhizoVision Explorer. In the current context of rapidly growing interest in root science, RhizoVision Explorer intends to become a reference software, improve the overall accuracy and replicability of root trait measurements and provide a foundation for collaborative improvement and reliable access to all.
Keywords: Ground truth, phenomics, phenotyping, rhizosphere, root system architecture, traits
RhizoVision Explorer is a new, easy-to-use software that measures root traits like length, diameter, area and volume from images. The software is free and open-source so that future improvements are possible. RhizoVision Explorer was validated using a new and freely available image set containing different sizes of measured copper wires. The software is intended to democratize access to root biology and to be the standard for validated measurements.
Introduction
Roots are central to understanding and providing answers to pressing global challenges like climate change’s impact on plant communities, ecosystem services and food security. From an ecological perspective, roots are an integral component of global biogeochemical processes, accounting for 46 % of the net primary productivity of the terrestrial biosphere globally, ranging from 32 % in croplands, 41–58 % in various forest types and 64 % in grasslands (Gherardi and Sala 2020). They have demonstrated influence on countless numbers of plant and ecosystem functions (Bardgett et al. 2014; Freschet et al. 2020). As such, on an ecosystem level, conceptual understanding, parameterization and spatial scaling of various root processes are critical for quantifying carbon, water and nutrient fluxes (Warren et al. 2015). From an agricultural perspective, rising demand for food, increased cost of production, agricultural intensification and depletion of finite resources in the face of changing climates have adverse effects on food security prospects (Godfray et al. 2010). Crops with optimized root traits are considered an important determinant of future food security, and the key to the second green revolution that can improve farm productivity and sustainability (Lynch 2007). Additionally, the root–soil rhizosphere is regarded as the keyspace for the future discovery of novel root traits relevant to increasing crop and farm productivity (Tracy et al. 2020). Hence, a better understanding and characterization of below-ground plant components will enable a greater knowledge of the underlying ecosystem processes in both natural and agricultural systems.
Due to their vital roles in anchorage, acquisition of water and nutrients and driving soil biology, plant root systems have been studied by the scientific community for over 100 years (Lux and Rost 2012). Roots still remain the least understood plant organ, partly because their underground growth is obscured by the opaque soil matrix (Eshel and Beeckman 2013; Hochholdinger 2016). This below-ground life leads to the need to sample roots by excavation or soil coring, followed by washing, which requires substantial effort. However, the rewards for measuring roots are great, with substantial evidence presented for how numerous root traits directly influence processes like nitrogen uptake and soil reinforcement (Freschet et al. 2020). The term ‘phenotyping’ has become commonly used to mean measuring traits in the context of screening crops for genetic mapping. Typically, these applications require measuring hundreds or thousands of samples, and so high-throughput phenotyping is the goal (Fiorani and Schurr 2013).
From a historical perspective, some of the primary root measurements used have included root dry weight, number, volume, surface area and length. As explained by Böhm (1979), the dry weight is commonly used due to its relative ease and, when measured on distinct organs of a plant, is a good indicator of carbon allocation in the plant. Volume is known to correlate with root dry weight in many circumstances and is relatively easy to measure by water displacement methods (Harrington et al. 1994; Pang et al. 2011). Although root mass or volume measurements are relatively easy to measure, they cannot adequately explain many root functions in the plant–soil continuum (Costa et al. 2000). Böhm (1979) explained that volume did not become widely used because these parameters cannot distinguish samples with few thick roots from those with many fine roots, nor can dry weight. By 1970, Gardner (1964) and others had shown that root length was the best predictor of water uptake, and so was highly desirable to measure. Throughout the 20th century, root length was typically measured by placing roots on graph paper, using tacks or gum to hold the ends in place to stretch out, and lateral roots had to be cut away to measure separately (Böhm 1979). Therefore, any method for measuring the length and other parameters with higher throughput and precision was highly sought.
Methods designed to overcome the barrier in measuring root length using a ruler or graph paper date back at least to Newman’s (1966) ‘line-intersection’ method, assuming random placement of lines and its variant by Tennant using a grid of lines (1975). In these methods, total root length is estimated by manual counting of roots that intersect a line marker and a formula to convert to root length. Subsequently, the line-intersection method was digitized using a photo-electric sensor that travelled over the top of a backlit root sample to count transitions from white to black using a scaler or electronic counter (Rowse and Phillips 1974). Image-based methods were employed to capture minirhizotron images starting in the 1970s, and these images were processed with the line-intersection method, either manually or with the digitized version (Upchurch and Ritchie 1983). Smucker et al. (1987) described a complete algorithm for thresholding a root image, thinning to produce a skeleton, using successive boundary erosion to compute a distance map, and calculating length, surface area and volume from images acquired using a videotape camera. In parallel, more advanced digitized methods for the line-intersection method were still in development in 1989 using specialized counter devices as described above (Harris and Campbell 1989). This same type of counting algorithm was also applied through image analysis to count from images acquired by a desktop document scanner (Krstansky and Henderson 1989). The business applications of desktop scanners led to their wider availability and lower costs, while the optical arrangement made the method superior relative to using cameras; leading to higher resolution images with less distortion. The first reported use of a desktop scanner for acquiring images of spread roots followed by image analysis of the entire root objects (rather than line-intersection) was described by Pan and Bolton (1991), with the algorithm determining the perimeter of root objects and then deriving root length, area and average diameter from that single measurement. WinRhizo™ is a commercial and closed-source software released in 1993 (Arsenault et al. 1995) based on the principle of standardizing the use of desktop scanners and image analysis using similar algorithms as described by Smucker et al. (1987) to measure root length, diameters, areas and volumes. ROOTEDGE is free and open-source software developed to measure root length from scanned images using the edge-chord algorithm (Kaspar and Ewing 1997), for which a legacy DOS program was still available for download as of the writing of this article (https://www.ars.usda.gov/midwest-area/ames/nlae/docs/software-available-from-nlae/). The edge-chord algorithm efficiently measured the perimeter by summing short chord segments, and then used the total root pixel count for area to derive length. However, skeletonization routines as proposed by Smucker et al. (1987) became the preferred method for measuring length because of their simplicity and fewer assumptions. Kimura and Yamasaki (2003) provided a useful refinement of the algorithms to measure length and diameters more accurately from skeletons, using the NIH Image software (now ImageJ).
Since then, advances in image capture and image processing algorithms have continued and been widely utilized to measure various root metrics. Roots are imaged across a wide degree of modalities, such as in situ with minirhizotrons or soil pits, in rhizoboxes, on coloured backgrounds, or with flatbed scanners (Topp et al. 2016). Therefore, image analysis approaches are often specific to particular types of collected images. At the time of writing, there are 17 root image analysis tools for single root images and 42 tools for root system images listed on Quantitiative-Plant.org, each with varying features and assumptions for input image type (Lobet et al. 2013). At present, 2D root imaging and image analysis is the most popular root phenotyping approach, after root dry weight determination, as it is the most accessible and simplest image data to acquire and analyse. Software that work with 2D branched and connected root system images such as root crowns or seedling root systems grown on blue paper included EZ-Rhizo (Armengaud et al. 2009), SmartRoot (Lobet et al. 2011), RootNav (Pound et al. 2013), ARIA (Pace et al. 2014), DIRT (Das et al. 2015) and RhizoVision Analyser (Seethepalli et al. 2020). In many cases, roots are not connected as they have been excavated from the field or pots, and these roots are typically imaged on a flatbed scanner then analysed using the commercial WinRhizo™ software (Regent Instrument Inc., Quebec, Canada) or free software including IJ_Rhizo (Pierret et al. 2013; requiring ImageJ), GiA-Roots (Galkovskyi et al. 2012; no longer available for download) and saRIA (Narisetti et al. 2019; requiring Matlab runtime). While the growing numbers of image analysis tools for root phenotyping have been really useful to the root science community, these have also introduced a range of biases and inconsistencies among studies such as the use of pixel counts for length that do not account for diagonal lengths and problems with the calculation of volume (e.g. Rose and Lobet 2019). The different software tools and measured properties likely reinforced the conceptual barriers between root researcher communities (see Freschet et al. 2020). Currently, there is a need to bring these tools together into a user-friendly, generalist, all-inclusive package that has wide utility across plant science, provides standardized results and is open-source.
As presented in detail below, RhizoVision Explorer fills the gap for generalized root image analysis, with modes to extract several root traits from both connected and unconnected plant root systems. Here we describe aspects of the user-friendly, lightweight and open-source graphical user interface (GUI) that facilitate high-throughput root phenotyping, such as its ease to install and use, support of multiple image formats, the inclusion of image preprocessing and batch analysis mode. Additionally, we present the methods and outcomes of the software validation using a novel copper wire image set and simulated roots with ground truth measurements. For scanned roots, the software was further compared with WinRhizo™ using images from several plant species and functional categories.
Materials and Methods
RhizoVision Explorer (Fig. 1) builds upon the functionality and codebase of RhizoVision Analyser (Seethepalli and York 2019) to analyse not only root crown images but also broken roots that are imaged on a flatbed scanner. The term ‘Explorer’ indicates the user has more freedom to understand the analysis process and work interactively with the GUI to optimize the desired outcome. The program adds functionality such as greater interaction with the raw, segmented or analysed image, performing root pruning, and support for the region of interest (ROI). The minimum-tested system requirements for the RhizoVision Explorer are an Intel or AMD x86 64-bit processor and a minimum of 4 GB of RAM. If the processor supports Intel AVX 2.0, the program is accelerated using AVX 2 vector processing instructions. The program does not require any installation or external dependencies and runs locally on Windows 8.1 or higher, but could be compiled to run on other operating systems in the future. The program is open-source, and the zipped binary files for simple installation are available for download at https://doi.org/10.5281/zenodo.3747697 (Seethepalli and York 2020).
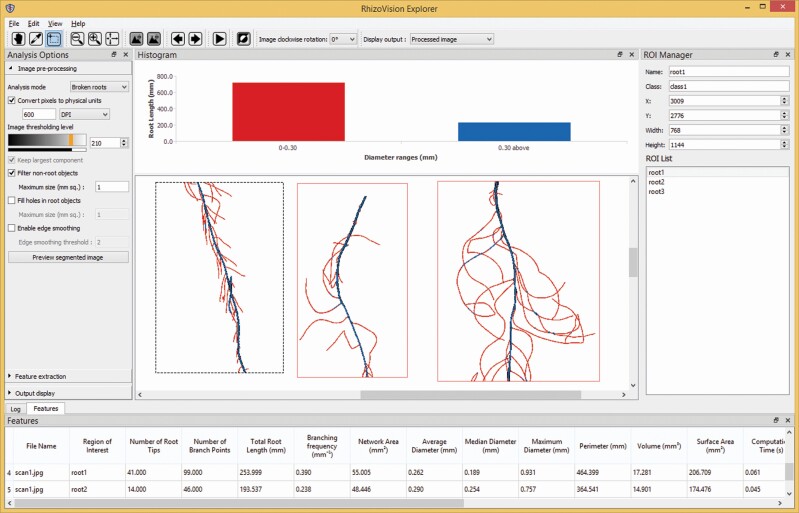
Figure 1.
The RhizoVision Explorer application window consists of menus (top), toolbar under menus, Analysis Options pane (left), image window (centre), Histogram pane (over image window), ROI Manager (right) and the Features or Log pane (bottom).
Software architecture
RhizoVision Explorer is written in C++ (https://github.com/noble-research-institute/RhizoVisionExplorer) and uses cvutil (https://github.com/noble-research-institute/cvutil), a C++ library that includes several helper functions that extend the OpenCV library (www.opencv.org) and was developed by the same team in parallel. These functions include determining the distance map, creating the skeletal structure and smoothing the contours of a segmented image. Additionally, the cvutil library supports adding a user interface to an application with the main window, extending the user interface with the plug-in system and optionally enabling the ROI system that can be utilized by the plug-ins loaded by the application. Finally, the library includes several plotting functions for data visualization. The cvutil library utilizes the platform-independent Qt C++ library (www.qt.io) to generate the required GUIs. Using OpenCV and Qt enables extending the software and required libraries to be deployed on operating systems other than Windows in the future.
The primary algorithms for extracting features, or measurements, from root images, were implemented within a plug-in to the image processing window. The plug-in also includes the logic for identifying the root topology and generating the feature image with visual elements such as the skeleton or convex hull overlaid on the segmented image. When the user starts analysis from RhizoVision Explorer, the main window checks if the image has any ROIs drawn by the user. If any ROIs are found, then the main window invokes the plug-in for extracting features for every sub-image given by the ROI set. Otherwise, the whole image is passed to the plug-in for analysis. The plug-in then analyses the image and returns the extracted features and the segmented and processed images to the main window. The main window then updates the features table with the extracted features, displays the processed image in the image pane and updates the bar plot root length histogram that groups length measurements based on the diameter ranges given by the user before analysis.
The main window allows the user to change the analysis options interactively and run the analysis, thereby allowing the user to find the optimal analysis options for an image. These analysis options can be saved in a CSV file and loaded later. Similarly, the main window also supports loading and saving the ROIs as annotation text files, so that these annotations can be reused later.
Description of analysis tools in RhizoVision Explorer
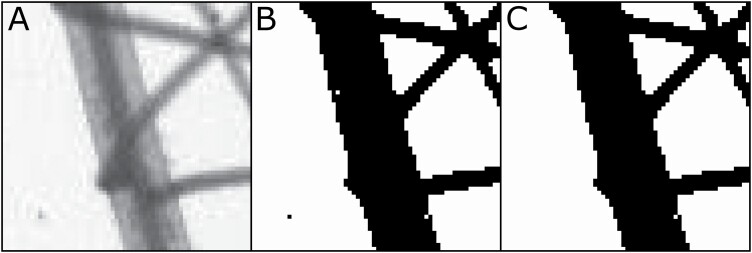
A typical workflow for analysing a plant root image is to load the image in RhizoVision Explorer by drag-and-drop or through the file menu. Most common image formats are supported, including PNG, JPEG, BMP and TIFF, in both greyscale and colour, but note only the red channel is used for colour images. The Analysis Options pane on the left side of the program lists the options for image preprocessing, feature extraction and how the output of the analysed image is displayed to the user. The user needs to set the options to be appropriate for any given experiment, preview segmentation and then run the analysis. Upon pressing the ‘run analysis’ arrow button, the program extracts relevant features from the plant root image, displaying the processed image at the centre of the window. The default analysis mode is for broken roots, but the software also has a whole root mode that will perform an analysis similar to RhizoVision Analyser including extra features such as the convex hull, root system width and depth, and angle measurements. The user may convert pixels to physical dimensions in units of either pixels per mm or DPI (dots per inch) as typically used when scanning. Apart from the thresholding and colour inversion options, which are present in the RhizoVision Analyser, the program also supports filtering non-root objects from the background and filling holes in root objects. Non-root object filtering enables soil particles or other debris, which may be present after root washing, to be filtered from the image. Hole filling is useful in cases where the root region in the image may contain bright spots that may be identified as background after thresholding, and allows the small areas to be filled in that are surrounded by root pixels. The filtering is performed based on the user-provided maximum size of a filtered component for the background and the root portions of the image. The maximum size is provided in sq. mm if pixel to millimetre conversion is specified in the program. The program then filters the connected components based on the size of each component. Figure 2 shows the thresholded images of a root image patch before and after filtering noisy components. Once the user is satisfied with the various options, they may also use the batch analysis mode which will apply the current settings to all images in a selected folder and output a metadata settings file and the tabular data file.
Figure 2.
(A) Selected region of a plant root image taken from a flatbed scanner with a transparency unit. (B) Segmented image without noise filtering. We observe that the dark component on the white background is caused due to soil particles and a white component in the root portion is caused due to brighter root pixel. (C) Segmented image with noise filtering. The white pixel at the bottom is not considered as a hole because the pixel is only surrounded by seven root pixels, rather than eight.
The contours, or root surfaces, of the thresholded and filtered image, can be optionally simplified using the Ramer–Douglas–Peucker line simplifier (Ramer 1972; Douglas and Peucker 1973). The procedure simplifies the contours of the thresholded image within a user-provided pixel distance threshold. Small contours of the segmented root can lead to the formation of short lateral roots during skeletonization that are not valid. Therefore, smoothing the surface using line simplification can reduce the number of these unwanted invalid lateral roots. It is recommended not to set values higher than 2.0 for pixel distance threshold for line simplification, because it may alter the root topology, leading to an angular appearance.
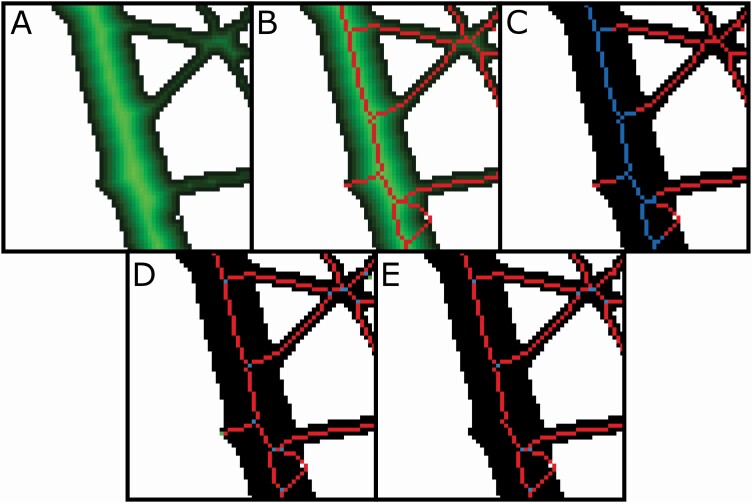
Using the segmented image generated using the methods described above, a precise Euclidean distance transform (Felzenszwalb and Huttenlocher 2012) is computed for the image (Fig. 3A). The distance transform at any pixel is the shortest distance from the pixel to a background pixel. The skeletonization of the segmented image is performed by the identification of ridges of the distance transform (Fig. 3B). This is done by checking each distance transform pixel if it is greater than two neighbouring pixels of opposite directions. The ridges so identified are not always connected throughout the root system. This is because the distance transforms pixels at the junction of the main root and its lateral root increase towards the ridge of the main root. Hence, no ridge pixels can be found that can connect the lateral root to the main root. These ridges are connected in the steepest ascent algorithm to form a connected skeletal structure. The ridge may be two pixels wide in some places which may be formed in the ridge detection procedure. In such a case, the ridge is thinned using the Guo–Hall thinning algorithm (Lam et al. 1992) to one-pixel width while preserving the connectivity of the skeletal structure. The skeletal structure is internally stored as an image where pixel values take on the value computed from the distance map for that pixel and use it for computing the average diameter of all roots and to classify root segments into diameter bins as described below.
Figure 3.
(A) Distance transform of the segmented image of the selected region shown in Fig. 2A. (B) The skeletonization procedure finds the ridges and connects them using the steepest ascent algorithm. (C) Skeletal pixels coloured based on the diameter ranges given in the feature extraction options. (D) Root topology without root pruning. The blue pixels are the branch points, and the green pixels are root tips. (E) Root topology with root pruning. Note two lateral roots in the bottom right of the image are not pruned because they form a single segment connecting two branch points.
After skeletonizing the segmented image, the topology of the root is identified, which involves finding the topological branch points, end points and the root segments between any two branch points or a branch point and an end point (Fig. 3D). The end points, here on called root tips, are identified as any skeletal pixel having only a single neighbouring pixel and branch points have three or four neighbouring pixels. This topology is stored as a copy of the skeletal image where pixels can take discrete values that encode the type, tip, branch point or segment. Using this topological image and the skeletal image, another method for decreasing the number of short, invalid lateral roots was devised as an analysis option called ‘root pruning’ as an alternative to using line simplification of the segmented image. The basic premise of root pruning is that the length of invalid lateral roots will be no longer than the radius of the parent root from which they branch. When the root pruning option is selected, the software evaluates the topological image to analyse all root segments starting from a branch point and ending in a tip. The software determines whether the length from the branch to the tip is longer than the radius of the branch point plus an additional number of pixels supplied by the user (Root pruning threshold). If a given lateral root is too short, then its tip and segment are deleted from the topological image and the skeletal image. If the resulting parent branch point has one neighbour, it becomes a tip; if two neighbours, it becomes merged with the segment to which it belongs; and if it still has three or more, it remains a branch point. Since new tips can emerge during this process, root pruning is iterative until no invalid lateral roots remain. Unlike line smoothing, root pruning does not alter the shape of the segmented root image. Root pruning removes the invalid lateral roots that can inaccurately affect the extracted features, such as the total root length (Fig. 3E). The resulting pruned topological image is used for extracting features requiring lengths and diameters. Features extracted in RhizoVision Explorer that rely on the segmented image, the skeletal image and the topological image are defined in Table 1. Surface area and volume are calculated assuming that every pixel in the skeleton is a cylinder, calculating based on its height and radius, and summing those for the entire image or diameter range.
Table 1.
List of features extracted from a plant root image. Features extracted from broken roots only are marked with (*), and from whole root images only are marked with (**).
| Feature extracted | Description |
|---|---|
| Number of Root Tips | The number of root tips is pixels in identified root topology that have only one neighbouring skeletal pixel. |
| Total Root Length (px, mm) | The sum of the Euclidean distances between the connected skeletal pixels in the entire root topology of the plant root image. |
| Network Area (px2, mm2) | The total number of pixels in the segmented image. |
| Average, Median and Maximum Diameter (px, mm) | The distance transform value at each skeletal pixel is the radius at that pixel and is doubled to give the diameter. The average, median and maximum diameters are computed across all these skeleton pixels. |
| Perimeter (px, mm) | The sum of the Euclidean distances between the connected contour pixels in the entire segmented image of the plant root. |
| Volume (px3, mm3) and Surface Area (px2, mm2) | Using the radii for each skeletal pixel determined earlier, the volume at the skeletal pixel is calculated as the length of the pixel multiplied by the cross-sectional area of the root at that pixel. Similarly, the surface area is calculated as the length of the pixel multiplied by the circumference of the cross-section of the root at that pixel. Volume and surface area are calculated as the sum of values from all skeletal pixels. |
| Computation Time (s) | The time taken to analyse a plant root image is noted as computation time in seconds. |
| Root Length, Projected Area, Surface Area and Volume histograms | For each skeletal pixel, the root length, projected area on the surface of the image plane, surface area and volume of the root at that pixel are computed. These values are binned according to the diameter ranges specified by the user. |
| Number of Branch Points* | The number of branch points is pixels in the identified root topology that have at least three neighbouring skeletal root segments. |
| Branching Frequency (px−1, mm−1)* | The number of branch points per unit root length. |
| Median and Maximum number of roots** | For each row in the segmented image, a horizontal line scanning is performed that records the number of pixel transitions from a background to a root pixel. This list of pixel transitions is sorted and the median and the maximum number of roots are noted. |
| Depth, Maximum Width (px, mm)** | The maximum vertical and horizontal distance the root crown grew at the time of imaging are noted as Depth and Maximum Width, respectively. |
| Width-to-Depth Ratio** | The ratio of Maximum Width to Depth is noted as the Width-to-Depth ratio. |
| Convex Area (px2, mm2) and Solidity** | The area of the convex hull that is fit to include the entire root crown system is noted as Convex Area. Solidity is the ratio of the Network Area to Convex Area. |
| Lower Root Area (px2, mm2)** | For a root crown image, the skeletal pixel that has the maximum radius is located. The network area of the root system that is below the skeletal pixel located above is noted as Lower Root Area. |
| Holes and Average Hole Size (px2, mm2)** | Holes are background components between roots in a segmented image. These holes are counted, and their average size is determined. |
| Steep Angle Frequency, Medium Angle Frequency, Shallow Angle Frequency and Average Root Orientation** | Within 40 × 40 pixel locality for every skeletal pixel in the centre, we get the coordinates of all the skeletal pixels in that locality and compute the angular orientation for the locality. We group these orientations in bins of 0–30°, 30–60° and 60–90° and note the frequencies as Steep, Medium and Shallow Angle Frequencies. The average of these orientations is noted as Average Root Orientation. |
Apart from extracting the root features, the program can also be used to visualize the analysed root interactively. Further, after running the analysis, a histogram appears of root length grouped by different root diameter ranges provided by the user (Fig. 1). Figure 3 shows different ways a processed root can be visualized. The visualization of a processed image contains options for displaying the distance transform map of the segmented image and generated skeletal structure, or medial axis. Additionally, for root crown images, the processed image can be optionally displayed with a convex hull that fits the whole root system, the holes or the background components separated by the root system and contour of the segmented image. The skeletal structure can be displayed based on the user-specified diameter ranges (Fig. 3C) or based on the topology of the skeletal structure (Fig. 3D and E). When a root skeletal structure is visualized based on diameter ranges, the ranges can be selected by the user to distinguish the first- and second-order lateral roots (Fig. 3C). This also changes the histogram features that are extracted, to get useful information such as the root length of the main roots and the lateral roots separately. When generating the skeletal structure based on root topology, the branch points and root tips are highlighted.
RhizoVision Explorer also supports several tools that dramatically improve the usability of the software. One such tool is the ROI system, termed the ROI Manager. The ROI tool on the toolbar allows drawing multiple ROIs, and the ROI Manager allows custom naming of a selected ROI; manually changing XY coordinate position, width and height; and maintains a list of ROIs in the image. For each ROI in an image, the roots within that ROI are analysed separately and reported as a unique row of data, including a column for the name of the ROI. Region of interests can be saved and loaded as an annotation file and are honoured for batch processing.
Validation data sets and procedures
For validation of data extracted from RhizoVision Explorer, known gauges of copper wire (Fig. 4B) were used. Copper wire samples with average lengths of 30.48 cm and various diameter wire gauges (American Wire Gauge 40, 32, 28, 22, 16 and 10; with the diameter ranging from 0.07 to 2.58 mm) were used for the validation. For each gauge, two wires were used for a total of 12 wires. The diameter of each wire was measured with a digital micrometer and the length was measured by fitting string to the length of the wire, then measured with a ruler. The surface area and volume for each wire were calculated assuming the wires were cylinders. The average diameter for scans containing multiple wires was calculated as the weighted diameter based on the length of each wire in the scan. The wires were then scanned on an Epson Expression 12000XL flatbed scanner with a transparency unit (overhead light), as common practice for root imaging. The images were digitized at 600 DPI as 16-bit greyscale images and then saved as TIFF (tagged image file format). Scans were taken in 28 combinations from individual wires to all as shown in Supporting Information—Table S1 in order to achieve variation in total length, average diameter, total surface area and total volume. The copper wire image set used here is available in a public repository and can be downloaded at http://doi.org/10.5281/zenodo.4677546 (Dhakal et al. 2021a).
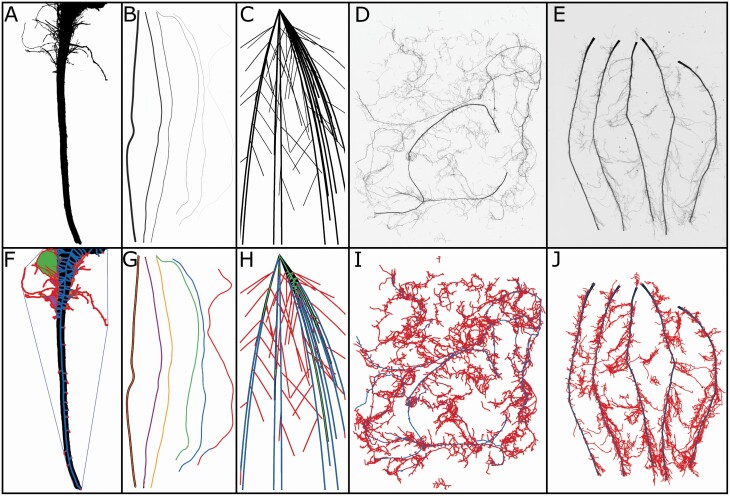
Figure 4.
RhizoVision Explorer works with images that have a high contrast of roots with the background. (A) Whole root mode is intended to be used with connected root systems such as this soybean root crown image taken using the RhizoVision Crown platform. (B) A new image set containing copper wires of various diameters and combinations was created for validation. (C) Simulated root systems were analysed from Rose and Lobet (2018). (D) Complex root samples in broken root mode such as from the herbaceous species data set can be accommodated. (E) Maize nodal roots exhibit a substantial difference in diameter between nodal roots and their lateral roots. F, G, H, I and J depict corresponding feature images highlighting the additional measures in whole root mode in F and the ability to use diameter bins to accurately classify wires and roots (G, H, I and J).
The wire images were processed in RhizoVision Explorer v2.0.2 (Fig. 4G) using the following analysis options: Root type was chosen as broken roots, image thresholding level was set at 191, filter noisy components on the background was set as true, with the maximum noisy component size of 8 mm2. The threshold value was adjusted until the copper wires were fully visual, without introducing any deviation in the shape of the wires. The Root pruning threshold was set to five pixels. Images were then processed in batch mode. The same images were processed in WinRhizo™ v2019a using the following analysis options: root detection was based on grey level and thresholding was set manually at 191 to match the options chosen for RhizoVision Explorer. Roots were chosen as darker than the background. Debris and rough edges removal were selected as low.
For comparison of performance metrics of RhizoVision Explorer, we chose two popular software for root phenotyping, WinRhizo™, and IJ_Rhizo—the former being a commercial software and the latter being an open-source, freeware. We used simulated root images of known measurements (http://doi.org/10.5281/zenodo.1159845; Rose and Lobet 2018) which were previously used in Rose and Lobet (2019). A Root System Markup Language file had been previously generated using the root model ArchiSimple (Pagès et al. 2014) for 50 taproot and 50 fibrous root systems which were then stored as black and white JPEG image files (resolution of 1200 DPI) (Rose and Lobet 2019). Images were analysed with a thresholding value set to 144 for the three software. Values for length, average diameter, surface area and volume for the root images for IJ_Rhizo and WinRhizo™ were used from Rose and Lobet (2018) and batch analysed to get the corresponding measurements for RhizoVision Explorer.
To compare the efficacy of RhizoVision Explorer, scanned greyscale root images of maize (Zea mays), wheat (Triticum aestivum) and various herbaceous and tree species were analysed and compared with the image analysis results from WinRhizo™. The herbaceous species (Fig. 4D) include nine species sampled at ca. 115 days old: (Bromus erectus, Dactylis glomerata, Holcus lanatus, Plantago lanceolata, Sanguisorba minor, Taraxacum officinale, Lotus corniculatus, Medicago sativa, Trifolium repens) (Freschet et al. 2018). The maize (Z. mays) roots included scans (Fig. 4E) from 40-day-old plants across 30 cm depths for several nodes of roots (Guo and York 2019). The wheat (T. aestivum) roots are entire root systems from 10-day-old wheat seedlings grown in hydroponics (Guo et al. 2020). The tree roots are from the unpublished work of M. Luke McCormack and include root branches from 14 gymnosperm species sampled from the field (Cephalotaxus harringtonii, Ephedra distachya, Chamaecyparis pisifera, Ginkgo biloba, Juniperus chinensis, Larix decidua, Metasequoia glyptostroboides, Picea abies, Pinus resinosa, Pinus strobus, Sciadopitys verticillata, Taxus cuspidata, Taxodium distichum, Tsuga canadensis). All these image sets were scanned separately in different labs using Epson scanners with transparency units at resolutions of 600 DPI for maize, wheat and herbaceous species, and 800 DPI for the tree species. Measurements were extracted for length, average diameter, surface area and volume. In RhizoVision Explorer, the images were analysed with the following analysis options: (i) Analysis mode as ‘Broken roots’, (ii) Image thresholding level of 205, (iii) Filter non-root objects as 8 mm2, (iv) Filter holes in root objects set to false, (v) Enable edge smoothing set to false and (vi) Root pruning threshold set to 5. In the case of WinRhizo™, parameters were kept as the original used by the respective image providers, thresholding values were set as 205 in the manual mode for maize and wheat roots, automatic for herbaceous and global Lagarde with a 64-pixel region size for the tree roots. In both software, the respective resolution was used. Figure 4 shows the input images including a root crown image as well as the corresponding processed images highlighting the skeletal structure of the roots that are coloured based on various diameter ranges. These four image sets of roots from several plant species are available in a public repository and can be downloaded at http://doi.org/10.5281/zenodo.4677751 (Dhakal et al. 2021b).
Statistical analysis
Statistical analyses were performed using R (R Core Team 2020) through RStudio (RStudio Team 2020). The R package ‘ggplot2’ (Wickham 2016) was used for data visualization. Other packages used in the analysis were ‘ggpmisc’ (Pedro 2020), ‘tidyverse’ (Wickham et al. 2019), ‘ggpubr’, ‘magrittr’, ‘readxl’ and ‘Metrics’ (Hamner and Frasco 2018). The R code and tabular data used in this study are available in a public repository and can be downloaded at http://doi.org/10.5281/zenodo.4677553 (Dhakal et al. 2021c).
Linear regression was used to compare paired measurements for ground truth data and estimated measurements. Slope (α), intercept (β) and coefficient of determination (R2) of the linear regression between the ground truth and the estimated values were calculated. In order to quantify the prediction error for the software, various performance metrics such as mean bias error (MBE), root mean square error (RMSE) and determination coefficient (R2) were used. The R package ‘Metrics’ was used for calculating the prediction errors. Root mean square error is the measure of the standard deviation of the residuals. Mean bias error is an index to quantify whether the estimates are under- or overestimated. A good prediction results in the narrow spread of the residuals. Likewise, MBE also informs the direction and magnitude of the bias.
| (1) |
| (2) |
| (3) |
where Pi is the estimated value, Oi is the measured value and N is the sample size. Lower values for both MBE and RMSE indicate a more accurate prediction.
Results
Validation with copper wires
Length.
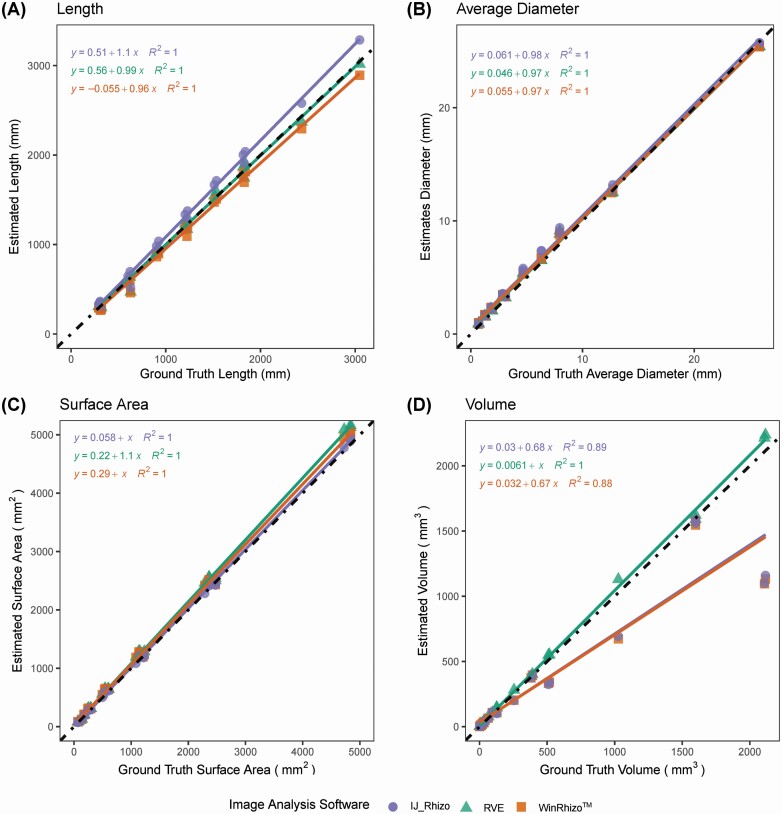
All IJ_Rhizo, RhizoVision Explorer and WinRhizo™ generated accurate length data for the copper wires. Determination coefficients were 1 for all software (P < 0.001), indicating a strong correlation between the ground truth measurements and the estimated length (Fig. 5A). Root mean square error for estimated length for scanned copper wire images for IJ_Rhizo, RhizoVision Explorer and WinRhizo™ was 10.74, 4.56 and 6.67 mm, respectively. Results show that IJ_Rhizo overestimated length (MBE = 7.84 mm), RhizoVision Explorer slightly overestimated length (MBE = 0.02 mm) and WinRhizo™ slightly underestimated the length (−4.2 mm) (Table 2).
Figure 5.
Comparison of physical measurements (length, average diameter, surface area and volume) of various gauges of copper wires to the trait estimates generated from scanned images acquired at 600 DPI and analysed using IJ_Rhizo, RhizoVision Explorer (RVE) and WinRhizo™, at a threshold value of 191. The fitted regression line for RVE and WinRhizo™ are shown in green and orange, respectively. The dotted black line represents a 1:1 relationship. A, B, C and D show length, average diameter, surface area and volume, respectively.
Table 2.
Comparison of various traits measured by IJ_Rhizo, RhizoVision Explorer (RVE) and WinRhizo™ using scanned copper wires images (n = 54) of known measurements. Length and average diameter are given in millimetres, and surface area and volume are given in squared millimetres and cubic millimetres, respectively.
| Traits | RMSE | MBE | Coefficient of determination | P-value | Software |
|---|---|---|---|---|---|
| Length (mm) | 10.74 | 7.84 | 1 | <0.001 | IJ_Rhizo |
| 4.56 | 0.02 | 1 | <0.001 | RVE | |
| 6.67 | −4.2 | 1 | <0.001 | WinRhizo™ | |
| Avg. diameter (mm) | 0.06 | 0.05 | 1 | <0.001 | IJ_Rhizo |
| 0.05 | 0.03 | 1 | <0.001 | RVE | |
| 0.05 | 0.04 | 1 | <0.001 | WinRhizo™ | |
| Surface area (mm2) | 0.4 | 0.2 | 1 | <0.001 | IJ_Rhizo |
| 1.42 | 1.03 | 1 | <0.001 | RVE | |
| 1 | 0.72 | 1 | <0.001 | WinRhizo™ | |
| Volume (mm3) | 0.28 | −0.1 | 0.89 | <0.001 | IJ_Rhizo |
| 0.04 | 0.02 | 1 | <0.001 | RVE | |
| 0.28 | −0.1 | 0.88 | <0.001 | WinRhizo™ |
Average diameter.
For average diameter, all three root image analysis software (IJ_Rhizo, RhizoVision Explorer and WinRhizo™) returned nearly perfect fits (R2 = 1; P < 0.001) with similar RMSE values (Fig. 5B). IJ_Rhizo, RhizoVision Explorer and WinRhizo™, all slightly overestimated average diameter values (MBE 0.03–0.05 mm, respectively). RhizoVision Explorer had the lowest MBE (0.03 mm) among the three software (Table 2).
Surface area.
The surface area for the scanned copper wire images was accurately estimated with all three software IJ_Rhizo, RhizoVision Explorer and WinRhizo™ (R2 = 1) and RMSE values of 0.40, 1.42 and 1 mm2, respectively (Fig. 5C). All of these software overestimated surface area (MBE 0.2, 1.03 and 0.72 mm2, respectively) (Table 2).
Volume.
RhizoVision Explorer had a strong agreement between the ground truth measurements and estimated volume from the scanned copper wire images (R2 = 1, P < 0.001). Root mean square error for RhizoVision Explorer volume estimates was 0.04 mm3 with slight volume overestimation (MBE = 0.02 mm3). Both IJ_Rhizo and WinRhizo™ had a substantially lower agreement between the measured and estimated volume (R2 of 0.88 and 0.89, respectively; P < 0.001) with an RMSE value of 0.28 mm3. The MBE value for both WinRhizo™ and IJ_Rhizo is −0.1 mm3, a slight underestimation (MBE = −0.1 mm3), as supported by the slope of the regression line 0.67 (Fig. 5D; Table 2).
Performance of IJ_Rhizo, RhizoVision Explorer and WinRhizo™ using simulated root systems
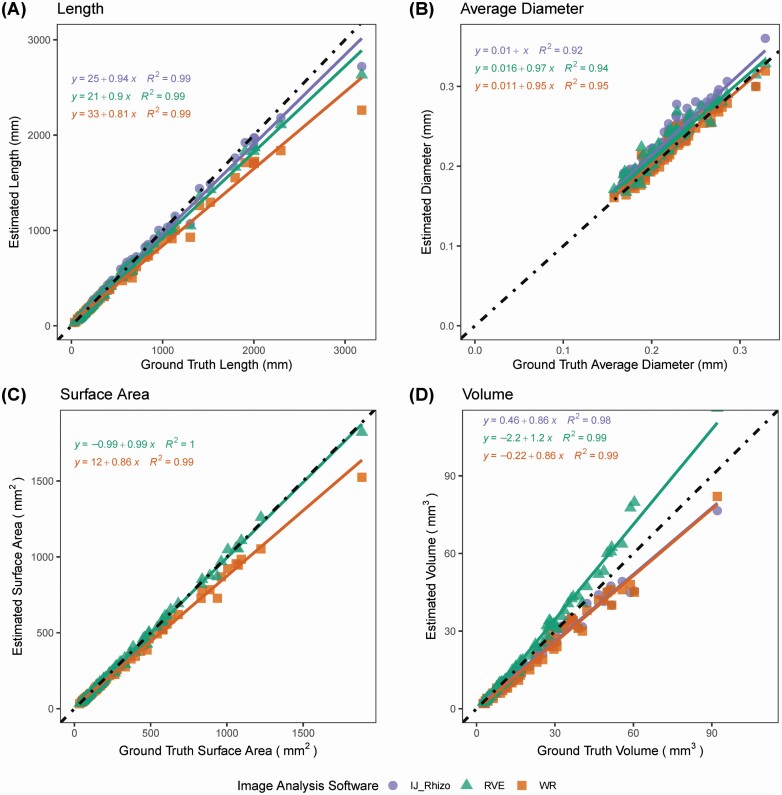
The determination coefficients obtained for the regression between estimated length and ground truth values for IJ_Rhizo, RhizoVision Explorer and WinRhizo™ indicated a strong agreement between the estimated length and ground truth measurements (R2 = 0.99, P < 0.001) (Fig. 6A).
Figure 6.
Comparison among IJ_Rhizo, RhizoVision Explorer (RVE) and WinRhizo™ for various trait estimates (n = 294) against ground truth measurements obtained from simulated root images. For each trait, a linear regression model is fitted for IJ_Rhizo, RVE and WinRhizo™ as shown in purple, orange and green, respectively. The dotted black line represents a 1:1 relationship. A, B, C and D show length, average diameter, surface area and volume, respectively.
IJ_Rhizo had the lowest RMSE (58.18 mm) and MBE (−5.46) values, followed by RhizoVision Explorer (RMSE = 74.86 mm, MBE = −29.14 mm) and WinRhizo™ (RMSE = 137.72 mm, MBE = −64.50 mm), respectively (Table 3). The MBEs are positive indicating a tendency to underestimate length for the three software.
Table 3.
Comparison of various traits measured by IJ_Rhizo, RhizoVision Explorer (RVE) and WinRhizo™ using simulated root images of dicot and monocot root systems (n = 294) of known measurements. Length and average diameter are given in millimetres, and surface area and volume are given in squared millimetres and cubic millimetres, respectively.
| Traits | RMSE | MBE | Coefficient of determination | P-value | Software |
|---|---|---|---|---|---|
| Length (mm) | 58.18 | −5.46 | 0.99 | <0.001 | IJ_Rhizo |
| 74.86 | −29.14 | 0.99 | <0.001 | RVE | |
| 137.72 | −64.50 | 0.99 | <0.001 | WinRhizo™ | |
| Avg. diameter (mm) | 0.02 | 0.01 | 0.92 | <0.001 | IJ_Rhizo |
| 0.01 | 0.01 | 0.94 | <0.001 | RVE | |
| 0.01 | 0.01 | 0.95 | <0.001 | WinRhizo™ | |
| Surface area (mm2) | NA | NA | NA | NA | IJ_Rhizo |
| 15.19 | −2.59 | 1 | <0.001 | RVE | |
| 58.86 | −31.91 | 0.99 | <0.001 | WinRhizo™ | |
| Volume (mm3) | 3.68 | −5.46 | 0.98 | <0.001 | IJ_Rhizo |
| 4.58 | 1.79 | 0.99 | <0.001 | RVE | |
| 3.93 | −2.71 | 0.99 | <0.001 | WinRhizo™ |
Likewise, the determination coefficients obtained for the regression between estimated average diameter and ground truth average diameter values for IJ_Rhizo, RhizoVision Explorer and WinRhizo™ indicated a strong agreement between the estimated length and ground truth measurements (R2 > 0.9, P < 0.001) (Fig. 6B). RhizoVision Explorer and WinRhizo™ had lower RMSE values (0.02 mm) compared to IJ_Rhizo. A negative MBE (0.01) indicated a tendency to overestimate diameter for the three software slightly (Table 3).
For surface area, because Rose and Lobet (2019) did not include the analysis, the data were not presented for IJ_Rhizo. Determination coefficients were high for both RhizoVision Explorer and WinRhizo™ (R2 > 0.99, P < 0.001) (Fig. 6C). WinRhizo™, however, returned the highest RMSE (58.86 mm2) and MBE values (−31.91 mm) compared to RhizoVision Explorer (RMSE = 15.19 mm and MBE = −2.59 mm) (Table 3).
Linear regression analysis for volume confirms a strong correlation between the ground truth measurement and the estimated volume for the three software (R2 > 0.98, P < 0.001) (Fig. 6D). Although, the RMSE values were highest for RhizoVision Explorer (4.58 mm3), it had the lowest MBE (1.79 mm3) compared to IJ_Rhizo (RMSE = 3.68 mm3, MBE = −5.46 mm3) and WinRhizo™ (RMSE = 3.93 mm3, MBE = −2.71 mm3) (Table 3).
Comparisons across roots of various plant species
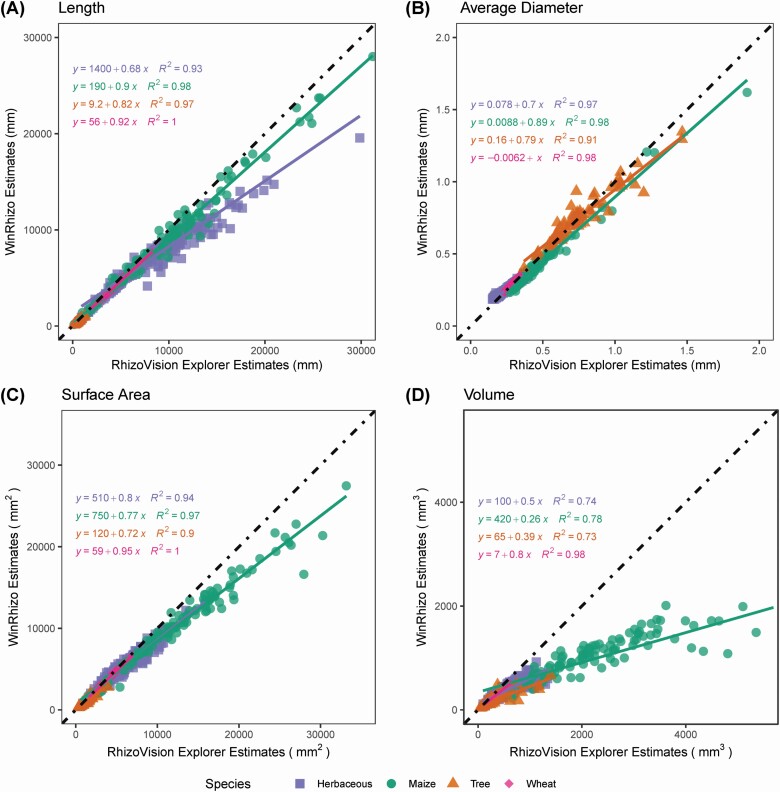
For length, average diameter, surface area and volume, WinRhizo™ trait values were typically underestimated relative to RhizoVision Explorer across all the species based on intercept and slope estimates from linear regression (Fig. 7). However, typical R2 values greater than 0.9 indicate that the relative ranking of individual data points was relatively consistent.
Figure 7.
Comparison between RhizoVision Explorer and WinRhizo™ for various trait estimates across four image sets representing diverse plant species. For each trait, a linear regression model is fit for herbaceous species, maize, tree species and wheat as shown in purple, green, orange and pink, respectively. The dotted black line represents a 1:1 relationship. A, B, C and D show length, average diameter, surface area and volume, respectively.
Discussion
In this paper we have introduced RhizoVision Explorer, a freely available, easy-to-use, GUI-based, precompiled executable software for personal computer (PC) users. To facilitate adoption, the open-source software can be executed easily on most Windows PCs (Windows 8.1 or 10) without the need for dedicated GPUs or cloud-hosted services. The program is developed for a modular approach with the implementation of open-source libraries such as Qt, OpenCV and a plug-in system. Developers can easily build upon the existing open-source software codebase to fit their root research pipeline and share the software with the root phenotyping community. The software provides interactive and batch processing modes, ROI functionality and numerous options for segmentation, filtering, feature extraction and output display.
To validate the physical measures provided by the software, a novel image set was created using six diameters of copper wires with known lengths imaged in 28 combinations and scanned with a similar protocol as for plant roots. RhizoVision Explorer showed excellent agreement with the ground truth values for total length, average diameter, surface area and volume. In some cases, reflections or glare from the smooth copper wire produced non-ideal images with minor imperfections. A published image and data set were used to compare RhizoVision Explorer with previous results obtained for IJ_Rhizo and WinRhizo™. In general, the three software performed similarly and their inaccuracies can largely be attributed to the ground truth data was based on simulated 3D root systems, but that image analysis results are based on single perspective 2D projections that increase root occlusion and overlap. When the original 3D images are flattened, many of the fine roots are effectively combined into larger diameter structures while simultaneously decreasing root length, as seen in Fig. 6A showing underestimation of length by all software. Larger diameter structures would tend to inflate volume estimates, derived from the squared radius in a cylinder formula, while decreased length would tend to decrease the volume. Therefore, RhizoVision Explorer may overestimate the volume (Fig. 6D) due to the increased apparent diameters observable in the crown of the simulated root images (see green medial axis visible in the upper right of Fig. 4H), while WinRhizo and IJ_Rhizo underestimate volume because of their bias in using the average diameter and length as discussed below. In practice, this type of root occlusion may be minimized at the imaging stage by ensuring roots are spread with minimal overlap. The root image analysis community would benefit from further ground truth data sets that correct some of these issues, such as 2D simulated roots of both connected and unconnected types, with various overlap, root hair presence, distortion and diameter heterogeneity.
For performance evaluation of RhizoVision Explorer with typical scanned root images, and to aid future root algorithm development, another image set containing wheat, maize, wild herbaceous species and tree species was created. In this case, only a comparison with the commonly used WinRhizo™ was conducted. Overall, the results show good agreement between the two software, except WinRhizo™ substantially underestimated root volume relative to RhizoVision Explorer. Previous work had indicated that WinRhizo™ and IJ_Rhizo inaccurately calculate volume based on the average diameter of all roots in an image and the total length of all roots in the image (Delory et al. 2017; Rose and Lobet 2019). This method assumes all roots are of the same (average) diameter, but diameters of roots within a sample can vary 10-fold. In such samples with a great variation of diameter, the average diameter will be biased by the smaller diameter roots and the volume will be underestimated. RhizoVision Explorer does not suffer from this inaccurate method, because surface area and volume are calculated by assuming that every pixel in the skeleton is a cylinder and summing across the image or diameter range. The severe volume underestimation possible when using WinRhizo™ or IJ_Rhizo is readily observable in the copper wire image set (Fig. 4B and G), where RhizoVision Explorer gave the correct result, but WinRhizo™ or IJ_Rhizo only reported half of the volume in the most extreme cases (Fig. 5D). When comparing results between WinRhizo™ and RhizoVision Explorer across the image sets of various crop and wild species, reasonable agreement is seen for length, average diameter and surface area, but in some maize root images with significant diameter heterogeneity between nodal and lateral root diameters (see Fig. 4E and J), the WinRhizo™ volume estimate was 5-fold less (Fig. 7D). Volume is an important measurement, typically used as a proxy for root mass and also for the calculation of root tissue density. These measures relying on volume that exist in the literature and in public root databases like FRED (Iversen et al. 2017) or GRoot (Guerrero-Ramírez et al. 2021) should be carefully evaluated before use.
A subset of 20 images from the herbaceous species image set were analysed in batch mode in both RhizoVision Explorer and WinRhizo™ on a Windows computer with an Intel 1.90 GHz i7 processor and 16 GB RAM. Thresholding values of 220, no filtering and no diameter bins were used in both software. RhizoVision Explorer completed the task in 55 s, and WinRhizo™ in 185 s. These results would depend on computer types and images used but are consistent with general experience. RhizoVision Explorer can be improved for speed and memory usage by reducing the number of internal images stored during analysis and optimizing code.
RhizoVision Explorer uses global greyscale thresholding to encourage imaging platforms that maximize the contrast of roots with the background, such as by using the RhizoVision Crown platform for root crown imaging using a backlight (Seethepalli et al. 2020), or use of a flatbed scanner with a transparency unit (or top light). Therefore, many types of complex images produced with blue paper screens, rhizoboxes or minirhizotrons may not work directly, which presents a limitation of the software. Image analysis tools have built-in assumptions of the input image on which the analysis operations are based, such as high contrast between the roots and the background. Users of these tools should carefully consider each tool before collecting or processing image data to ensure accurate and representative results. Image preprocessing steps such as cropping, filtering or thresholding are often required separately before analysis in software such as ImageJ. However, several software tools have become available over the past few years for machine learning approaches for root segmentation from complex backgrounds, with RootPainter being a GUI-based example (Smith et al. 2020). In addition, ImageJ has many thresholding options, including colour, which could be applied in batch processing. Segmented images can be produced in such a companion software, and then processed in RhizoVision Explorer for feature extraction.
Future development of RhizoVision Explorer will focus on correcting any bugs identified, improving the user interface for convenience and expanding features measured, especially for differentiating root classes such as laterals and axial roots or branch orders. In addition, the presence of root hairs in images can drastically increase all measures but this issue has rarely been discussed in the literature. Methods to address problems from root hairs may include blurring routines before image segmentation, or additional methods to prune the skeleton. In order to generate a skeleton that encodes a more accurate topology, in the future further refinements to define branch points and delete invalid root tips will need to accommodate the false loops as seen in Fig. 3. Most topological analysis is based on simple considerations of the skeleton branching; however, other considerations such as child and parent root diameters may help to refine the correct topology. As improvements are made in identifying root type or order, more measurements at the level of individual roots may be beneficial such as lateral root insertion angles or diameters. The software is open-source and community development is highly encouraged. RhizoVision Explorer is intended for roots; however, it is suitable for measuring leaf area from scanned images, and the plug-in-based software architecture could be extended for other phenotyping needs.
Conclusion
RhizoVision Explorer is an image analysis tool that is intended to enable many more researchers to be able to routinely measure plant roots to answer a variety of biological questions. The GUI and ready-to-run download for Windows, as seen in other popular image analysis software (Pound et al. 2013), lowers barriers and increases accessibility to image analysis. The underlying software architecture is modular and with plug-in support that is suitable for modifying for other phenotyping, or other image analysis needs. Future improvements include greater topology analysis with the ability to predict root order, to provide more features at the diameter bin or order level such as average diameter and angle. In summary, this open-source software builds on a range of previous software to propose a user-friendly, fast, generalist, collectively improvable and all-inclusive tool that will facilitate the standardization of root architectural and morphological measures.
Supporting Information
The following additional information is available in the online version of this article—
Table S1. Combinations of 12 wires used for the copper wire image data set.
Acknowledgements
The authors acknowledge nearly 30 beta testers not listed here that provided helpful feedback to improve the software before the public release, including in a survey answered by 20 testers. Especially, the authors thank Arthur Villordon both for his suggestions and his promotion of the software in the root crop community. The authors thank M. Luke McCormack for providing the tree root images. Meredith Hanlon provided the speed test results comparing RhizoVision Explorer to WinRhizo™. This manuscript has been authored by UT-Battelle, LLC, under contract DE-AC05-00OR22725 with the US Department of Energy (DOE). The US government retains and the publisher, by accepting the article for publication, acknowledges that the US government retains a non-exclusive, paid-up, irrevocable, worldwide license to publish or reproduce the published form of this manuscript, or allow others to do so, for US government purposes. DOE will provide public access to these results of federally sponsored research in accordance with the DOE Public Access Plan (http://energy.gov/downloads/doe-public-access-plan).
Form & Function. Chief Editor: Kate McCulloh
Sources of Funding
The research and software development were funded by Noble Research Institute, LLC and the Center for Bioenergy Innovation, a U.S. Department of Energy Bioenergy Research Center supported by the Office of Biological and Environmental Research in the DOE Office of Science. Oak Ridge National Laboratory is managed by UT-Battelle, LLC under contract DE-AC05-00OR22725 with the U.S. Department of Energy.
Contributions by the Authors
A.S. and L.M.Y co-developed RhizoVision Explorer and planned the manuscript. K.D. created the wire image set and conducted statistical analysis with input from all authors. All authors contributed to designing appropriate validation, writing the manuscript, and approved the final version.
Conflict of Interest
None declared.
Data Availability
RhizoVision Explorer is available as a ready-to-run executable for Windows at https://doi.org/10.5281/zenodo.3747697 (Seethepalli and York 2020). The open-source code for RhizoVision Explorer written in C++ is available at https://github.com/noble-research-institute/RhizoVisionExplorer on GitHub. The copper wire image set is available in a public repository and can be downloaded at http://doi.org/10.5281/zenodo.4677546 (Dhakal et al. 2021a). These four image sets of roots from several plant species are available in a public repository and can be downloaded at http://doi.org/10.5281/zenodo.4677751 (Dhakal et al. 2021b). The statistical R code and tabular data used in this study are available in a public repository and can be downloaded at http://doi.org/10.5281/zenodo.4677553 (Dhakal et al. 2021c).
Literature Cited
- Armengaud P, Zambaux K, Hills A, Sulpice R, Pattison RJ, Blatt MR, Amtmann A. 2009. EZ-Rhizo: integrated software for the fast and accurate measurement of root system architecture. The Plant Journal 57:945–956. [DOI] [PubMed] [Google Scholar]
- Arsenault JL, Poulcur S, Messier C, Guay R. 1995. WinRhizo™, a root-measuring system with a unique overlap correction method. HortScience 30:906D–906. [Google Scholar]
- Bardgett RD, Mommer L, De Vries FT. 2014. Going underground: root traits as drivers of ecosystem processes. Trends in Ecology & Evolution 29:692–699. [DOI] [PubMed] [Google Scholar]
- Böhm W. 1979. Root parameters and their measurement. In: Billings WD, Golley F, Lange OL, Olson JS, eds. Methods of studying root systems. New York: Springer, 125–138. [Google Scholar]
- Costa C, Dwyer LM, Hamilton RI, Hamel C, Nantais L, Smith DL. 2000. A sampling method for measurement of large root systems with scanner-based image analysis. Agronomy Journal 92:621–627. [Google Scholar]
- Das A, Schneider H, Burridge J, Ascanio AK, Wojciechowski T, Topp CN, Lynch JP, Weitz JS, Bucksch A. 2015. Digital imaging of root traits (DIRT): a high-throughput computing and collaboration platform for field-based root phenomics. Plant Methods 11:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delory BM, Weidlich EWA, Meder L, Lütje A, van Duijnen R, Weidlich R, Temperton VM. 2017. Accuracy and bias of methods used for root length measurements in functional root research. Methods in Ecology and Evolution 8:1594–1606. [Google Scholar]
- Dhakal K, Seethepalli A, Griffiths M, Guo H, Freschet GT, York LM. 2021a. Images of copper wires with various diameters for validating root image analysis. Zenodo. doi: 10.5281/zenodo.4677546. [DOI] [Google Scholar]
- Dhakal K, Seethepalli A, Griffiths M, Guo H, Freschet GT, York LM. 2021b. Scanned images of roots from wild herbaceous species, trees, maize, and wheat. Zenodo. doi: 10.5281/zenodo.4677751. [DOI] [Google Scholar]
- Dhakal K, Seethepalli A, Griffiths M, Guo H, Freschet GT, York LM. 2021c. Data and statistical code for the manuscript on RhizoVision Explorer for root image analysis. Zenodo. doi: 10.5281/zenodo.4677553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas DH, Peucker TK. 1973. Algorithms for the reduction of the number of points required to represent a digitized line or its caricature. Cartographica 10:112–122. [Google Scholar]
- Eshel A, Beeckman T. 2013. Plant roots: the hidden half, 4th edn. Boca Raton, FL: CRC Press. [Google Scholar]
- Felzenszwalb PF, Huttenlocher DP. 2012. Distance transforms of sampled functions. Theory of Computing 8:415–428. [Google Scholar]
- Fiorani F, Schurr U. 2013. Future scenarios for plant phenotyping. Annual Review of Plant Biology 64:267–291. [DOI] [PubMed] [Google Scholar]
- Freschet GT, Roumet C, Comas LH, Weemstra M, Bengough AG, Rewald B, Bardgett RD, De Deyn GB, Johnson D, Klimešová J, Lukac M. 2020. Root traits as drivers of plant and ecosystem functioning: current understanding, pitfalls and future research needs. New Phytologist. doi: 10.1111/nph.17072. [DOI] [PubMed] [Google Scholar]
- Freschet GT, Violle C, Bourget MY, Scherer-Lorenzen M, Fort F. 2018. Allocation, morphology, physiology, architecture: the multiple facets of plant above- and below-ground responses to resource stress. New Phytologist 219:1338–1352. [DOI] [PubMed] [Google Scholar]
- Galkovskyi T, Mileyko Y, Bucksch A, Moore B, Symonova O, Price CA, Topp CN, Iyer-Pascuzzi AS, Zurek PR, Fang S, Harer J, Benfey PN, Weitz JS. 2012. GiA Roots: software for the high throughput analysis of plant root system architecture. BMC Plant Biology 12:116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner WR. 1964. Relation of root distribution to water uptake and availability. Agronomy Journal 56:41–45. [Google Scholar]
- Gherardi LA, Sala OE. 2020. Global patterns and climatic controls of belowground net carbon fixation. Proceedings of the National Academy of Sciences of the United States of America 117:20038–20043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godfray HC, Beddington JR, Crute IR, Haddad L, Lawrence D, Muir JF, Pretty J, Robinson S, Thomas SM, Toulmin C. 2010. Food security: the challenge of feeding 9 billion people. Science 327:812–818. [DOI] [PubMed] [Google Scholar]
- Guerrero-Ramírez NR, Mommer L, Freschet GT, Iversen CM, McCormack ML, Kattge J, Poorter H, van Der Plas F, Bergmann J, Kuyper TW, York LM. 2021. Global root traits (GRooT) database. Global Ecology and Biogeography 30:25–37. [Google Scholar]
- Guo H, Habtamu A, Seethepalli A, Dhakal K, Griffiths M, Ma XF, York LM. 2020. Functional phenomics and genetics of the root economics space in winter wheat using high-throughput phenotyping of respiration and architecture. New Phytologist. doi: 10.1101/2020.11.12.380238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo H, York LM. 2019. Maize with fewer nodal roots allocates mass to more lateral and deep roots that improve nitrogen uptake and shoot growth. Journal of Experimental Botany 70:5299–5309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamner B, Frasco M. 2018. Metrics: evaluation metrics for machine learning. R package version 0.1.4. [Google Scholar]
- Harrington JT, Mexal JG, Fisher JT. 1994. Volume displacement provides a quick and accurate way to quantify new root production. Tree Planter’s Notes 45:121–124. [Google Scholar]
- Harris GA, Campbell GS. 1989. Automated quantification of roots using a simple image analyzer. Agronomy Journal 81:935–938. [Google Scholar]
- Hochholdinger F. 2016. Untapping root system architecture for crop improvement. Journal of Experimental Botany 67:4431–4433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iversen CM, McCormack ML, Powell AS, Blackwood CB, Freschet GT, Kattge J, Roumet C, Stover DB, Soudzilovskaia NA, Valverde-Barrantes OJ, van Bodegom PM, Violle C. 2017. A global Fine-Root Ecology Database to address below-ground challenges in plant ecology. New Phytologist 215:15–26. [DOI] [PubMed] [Google Scholar]
- Kaspar TC, Ewing RP. 1997. ROOTEDGE: software for measuring root length from desktop scanner images. Agronomy Journal 89:932–940. [Google Scholar]
- Kimura K, Yamasaki S. 2003. Accurate root length and diameter measurement using NIH Image: use of Pythagorean distance for diameter estimation. Plant and Soil 254:305–315. [Google Scholar]
- Krstansky JJ, Henderson GS. 1989. Computerized measurement of fine root length using a desktop image scanner. In Agronomy abstracts: 307. Madison, WI: ASA. [Google Scholar]
- Lam L, Lee S, Suen CY. 1992. Thinning methodologies-a comprehensive survey. IEEE Transactions on Pattern Analysis and Machine Intelligence 14:869–885. [Google Scholar]
- Lobet G, Draye X, Périlleux C. 2013. An online database for plant image analysis software tools. Plant Methods 9:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobet G, Pagès L, Draye X. 2011. A novel image-analysis toolbox enabling quantitative analysis of root system architecture. Plant Physiology 157:29–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lux A, Rost TL. 2012. Plant root research: the past, the present and the future. Annals of Botany 110:201–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch JP. 2007. Roots of the second green revolution. Australian Journal of Botany 55:493. [Google Scholar]
- Narisetti N, Henke M, Seiler C, Shi R, Junker A, Altmann T, Gladilin E. 2019. Semi-automated Root Image Analysis (saRIA). Scientific Reports 9:19674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman EI. 1966. A method of estimating the total length of root in a sample. Journal of Applied Ecology 3:139–145. [Google Scholar]
- Pace J, , Lee N,, Naik HS,, Ganapathysubramanian B,, Lubberstedt T. 2014. Analysis of maize (Zea mays L.) seedling roots with the high-throughput image analysis tool ARIA (Automatic Root Image Analysis). PLoS One 9:e108255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagès L, Bécel C, Boukcim H, Moreau D, Nguyen C, Voisin A. 2014. Calibration and evaluation of ArchiSimple, a simple model of root system architecture. Ecological Modelling 290:76–84. [Google Scholar]
- Pan WL, Bolton RP. 1991. Root quantification by edge discrimination using a desktop scanner. Agronomy Journal 83:1047–1052. [Google Scholar]
- Pang W, Crow WT, Luc JE, McSorley R, Giblin-Davis RM, Kenworthy KE, Kruse JK. 2011. Comparison of water displacement and WinRHIZO software for plant root parameter assessment. Plant Disease 95:1308–1310. [DOI] [PubMed] [Google Scholar]
- Pedro JA. 2020. ggpmisc: miscellaneous extensions to ‘ggplot2’. R package version 0.3.7. [Google Scholar]
- Pierret A, Gonkhamdee S, Jourdan C, Maeght J. 2013. IJ_Rhizo: an open-source software to measure scanned images of root samples. Plant and Soil 373:531–539. [Google Scholar]
- Pound MP, French AP, Atkinson JA, Wells DM, Bennett MJ, Pridmore T. 2013. RootNav: navigating images of complex root architectures. Plant Physiology 162:1802–1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team . 2020. R: a language and environment for statistical computing. 4.0.1. [Google Scholar]
- Ramer U. 1972. An iterative procedure for the polygonal approximation of plane curves. Computer Graphics and Image Processing 1:244–256. [Google Scholar]
- Rose L, Lobet G. 2018. Dataset of 100 simulated root images, with different resolutions. Zenodo. doi: 10.5281/zenodo.1159846. [DOI] [Google Scholar]
- Rose L, Lobet G. 2019. Accuracy of image analysis tools for functional root traits: a comment on Delory et al. (2017). Methods in Ecology and Evolution 10:702–711. [Google Scholar]
- Rowse HR, Phillips DA. 1974. An instrument for estimating the total length of root in a sample. Journal of Applied Ecology 11:309–314. [Google Scholar]
- RStudio Team . 2020. RStudio: integrated development for R. 1.3.959. [Google Scholar]
- Seethepalli A, Guo H, Liu X, Griffiths M, Almtarfi H, Li Z, Liu S, Zare A, Fritschi FB, Blancaflor EB, Ma XF, York LM. 2020. RhizoVision Crown: an integrated hardware and software platform for root crown phenotyping. Plant Phenomics 2020:3074916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seethepalli A, York LM. 2019. RhizoVision Analyzer: software for high-throughput measurements from images of crop root crowns. 1.0. Zenodo. doi: 10.5281/zenodo.2585892. [DOI] [Google Scholar]
- Seethepalli A, York LM. 2020. RhizoVision Explorer - interactive software for generalized root image analysis designed for everyone. 2.0.2. Zenodo. doi: 10.5281/zenodo.4095629. [DOI] [Google Scholar]
- Smith AG, Han E, Petersen J Olsen NAF Giese C Athmann M Dresbøll DB Thorup-Kristensen K. 2020. RootPainter: deep learning segmentation of biological images with corrective annotation. bioRxiv, doi: 10.1101/2020.04.16.044461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smucker AJM, Ferguson JC, DeBruyn WP, Belford RK, Ritchie JT. 1987. Image analysis of video-recorded plant root systems. In: Minirhizotron observation tubes: methods and applications for measuring rhizosphere dynamics. 67–80. doi: 10.2134/asaspecpub50.c6. [DOI] [Google Scholar]
- Tennant D. 1975. A test of a modified line intersect method of estimating root length. Journal of Ecology 63:995–1001. [Google Scholar]
- Topp CN, Bray AL, Ellis NA, Liu Z. 2016. How can we harness quantitative genetic variation in crop root systems for agricultural improvement? Journal of Integrative Plant Biology 58:213–225. [DOI] [PubMed] [Google Scholar]
- Tracy SR, Nagel KA, Postma JA, Fassbender H, Wasson A, Watt M. 2020. Crop improvement from phenotyping roots: highlights reveal expanding opportunities. Trends in Plant Science 25:105–118. [DOI] [PubMed] [Google Scholar]
- Upchurch DR, Ritchie JT. 1983. Root observations using a video recording system in mini-rhizotrons. Agronomy Journal 75:1009–1015. [Google Scholar]
- Warren JM, Hanson PJ, Iversen CM, Kumar J, Walker AP, Wullschleger SD. 2015. Root structural and functional dynamics in terrestrial biosphere models - evaluation and recommendations. New Phytologist 205:59–78. [DOI] [PubMed] [Google Scholar]
- Wickham H. 2016. ggplot2: elegant graphics for data analysis, 2nd edn. New York: Springer-Verlag, Springer International Publishing. [Google Scholar]
- Wickham H, Averick M, Bryan J, Chang W, McGowan LD, François R, Grolemund G, Hayes A, Henry L, Hester J, Kuhn M. 2019. Welcome to the tidyverse. Journal of Open Source Software 4:1686. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
RhizoVision Explorer is available as a ready-to-run executable for Windows at https://doi.org/10.5281/zenodo.3747697 (Seethepalli and York 2020). The open-source code for RhizoVision Explorer written in C++ is available at https://github.com/noble-research-institute/RhizoVisionExplorer on GitHub. The copper wire image set is available in a public repository and can be downloaded at http://doi.org/10.5281/zenodo.4677546 (Dhakal et al. 2021a). These four image sets of roots from several plant species are available in a public repository and can be downloaded at http://doi.org/10.5281/zenodo.4677751 (Dhakal et al. 2021b). The statistical R code and tabular data used in this study are available in a public repository and can be downloaded at http://doi.org/10.5281/zenodo.4677553 (Dhakal et al. 2021c).