Graphical abstract
Keywords: SARS-CoV-2 infection, COVID-19, Gastrointestinal tract, Sialic acid
Abstract
Background
It is known that SARS-CoV-2 mostly infects the respiratory system causing pneumonia; although it can also affect the gastrointestinal tract (GIT), which covered with a bi-layer of mucus rich in glycosylated proteins that terminated by sialic acid. Therefore; this study aimed to evaluate serum total sialic acid (TSA) in moderate COVID-19 patients with and without GIT manifestations.
Methods
A total of 161 moderate COVID-19 patients without and with GIT manifestations and 50 controls were enrolled into our study. Serum electrolytes levels were measured by using colorimetric or turbidmetric commercial assay kits, while the level of serum TSA was measured by using a commercial ELISA kit.
Results
Our results showed that serum TSA level was highly significantly increased in moderate COVID-19 patients with GIT manifestations (81.43 ± 8.91) when compared with controls (61.24 ± 6.41) or even moderate COVID-19 patients without GIT manifestations (69.46 ± 7.03). ROC curve analysis showed that AUC for TSA is 0.84 with 76.2 % sensitivity and 73.7 % specificity in discrimination between moderate COVID-19 patients with and without GIT manifestations. Serum potassium and sodium levels were highly significantly decreased in moderate COVID-19 patients with GIT manifestations when compared with controls or even moderate COVID-19 patients without GIT manifestations; while serum calcium level was found to be significantly decreased in moderate COVID-19 patients with GIT manifestations when compared with controls.
Conclusion
Finally, we can conclude that SA plays a crucial role in the pathogenesis of GIT complications associated with COVID-19 and could be a potential biomarker for the COVID-19 gastrointestinal complications.
1. Introduction
Coronavirus disease (COVID-19), is a rapidly expanding global pandemic caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and first emerged in Wuhan-China in December 2019, was declared as a global pandemic by WHO on 11 March 2020 (WHO, 2020; Huang et al., 2020). It has had devastating effects on economic growth, social structures, and populations, becoming a catastrophic public health crisis affecting many people; as till 12 July 2021, there have been 187,916,376 confirmed cases and about 4,053,093 deaths were reported in about 221 countries (COVID-19 coronavirus pandemic: Worldometer, 2021). It is known that SARS-CoV-2 mostly infect the respiratory system causing pneumonia; although, it can also affect the gastrointestinal tract (GIT) (Chen et al., 2020a,b). Diarrhea, anorexia, vomiting, nausea, and abdominal pain are the most reported GIT manifestations in COVID-19 patients (Perisetti et al., 2020). Also, acute pancreatitis, GIT bleeding and colitis have been observed (Lin et al., 2020). It was found that nearly one-fifth of COVID-19 patients recorded to have GIT manifestations (Henry et al., 2020).
Sialic acid (SA), also known as N-acetyl neuraminic acid (NANA), is a generic term for a family of monosaccharides with nine-carbon backbone and high structural diversity (Zhang et al., 2019). It located as the terminal of side chains of glycoproteins and glycolipids, which are the main components of cell membranes (Chittemsetti et al., 2019). SA serves an important role in cell aggregation, cell signaling, immune response, tissue regeneration, and also in human diseases, including in the infection process, as there is a complex array of glycans at the surface of animal cells and microorganisms mediating specific roles in health and disease state (Olaru et al., 2020). Serum proteins are mostly glycoproteins, in which glycans are terminated with sialic acid residues (Gruszewska et al., 2014). Several studies reported changes in the glycoproteins concentrations in the blood among COVID-19 patients (d’Alessandro et al., 2020; Sun et al., 2020a,b). These changes should affect the concentration of total sialic acid. Also, the binding capacity to sialic acid is one of many determinants of biological diversity in coronaviruses family (Matrosovich et al., 2015), as it was reported that SARS-CoV-2 could attach to sialic acid, while SARS-CoV couldn’t, and the sialic acid may be a key player of cytokine storm associated with SARS-CoV-2 infection (Wielgat et al., 2020).
Therefore; this study aimed to evaluate the level of serum total sialic acid (TSA) among COVID-19 patients with and without GIT manifestations to investigate its roles in the pathogenesis of COVID-19 disease and its GIT manifestations.
2. Materials and methods
2.1. Human subjects
The present study was conducted on 50 healthy subjects and 161 moderate COVID-19 patients without (98) and with (63) GIT manifestations recruited from the isolation hospitals in Port-Said; Egypt. The present study was approved by the ethics committees of Port-Said Hospital, Port-Said University; Egypt (ERN MED (23/04/2020)S.no(5)MED). Informed consents were obtained from all patients.
Sputum and throat swab specimens (for qPCR for SARS-Cov-2 RNA test) and blood samples were collected from all patients. Laboratory tests were conducted at admission, including a complete blood count, liver function tests (ALT & AST), kidney function tests (urea & creatinine), CRP, and ferritin. Our moderate COVID-19 patients were defined as patients with fever, respiratory manifestations, and radiological findings indicative of pneumonia. The levels of serum total sialic acid, electrolytes and other parameters were done at admission and before any treatment, and then all patients received antiviral medication, liquid supplements and other supportive treatments according to Suspected COVID- 19 Cases Management in Triage Hospitals by Ministry of Health and Population of Egypt.
2.2. Level of total sialic acid in serum
Level of serum total sialic acid was measured by using EnzyChrom™ Sialic Acid Assay Kit (cat# ESLA-100) (Bioassay system, Inc., USA) according to the protocol of manufacturer.
2.3. Levels of serum electrolytes
Levels of serum sodium, total calcium, magnesium and chloride were measured by using a colorimetric commercial assay kits (bio-diagnostic, Inc., Egypt), while serum potassium level was measured by using a turbidmetric commercial assay kits (bio-diagnostic, Inc., Egypt) according to the protocol of manufacturer.
2.4. Statistical analysis
Statistical analysis was performed using IBM SPSS software (version 23.0; IBM Corp., Armonk, NY, USA), and data were presented as mean ± S.D. One-way ANOVA was used to determine statistically significant difference between group's means. The Receiver Operating Characteristic curve (ROC curve) was used to calculate the area under the curve (AUC), sensitivity and specificity of serum TSA in differentiation between moderate COVID-19 patients with and without GIT manifestations. The criterion for significance was p < 0.05.
3. Results
3.1. Demographic and biochemical data of moderate COVID-19 patients with and without GIT manifestations
The present study included 50 healthy subjects and 161 moderate COVID-19 patients without (98) and with (63) GIT manifestations; the clinicopathological data of healthy subjects and moderate COVID-19 patients with and without GIT manifestations are summarized in Table 1 ).
Table 1.
Clinicopathological characteristics moderate COVID-19 patients and controls.
| Group | Variable |
||
|---|---|---|---|
| Control (n = 50) (Mean ± SD) | Moderate COVID-19 without GIT (n = 98) (Mean ± SD) | Moderate COVID-19 with GIT (n = 63) (Mean ± SD) | |
| Age (yrs) | 45.80 ± 8.82 | 48.36 ± 9.14 | 51.54 ± 9.15*, a |
| Gender (n (%)) | |||
| Male | 32(64 %) | 56(57.2 %) | 42(66.7 %) |
| Female | 18(36 %) | 42(42.8 %) | 21(33.3 %) |
| AST (U/L) | 22.05 ± 9.37*, b,c | 33.15 ± 21.97*, a | 36.60 ± 20.07*, a |
| ALT (U/L) | 19.35 ± 7.71*, b,c | 34.44 ± 23.56*, a | 34.45 ± 19.26*, a |
| Urea (mg/dl) | 32.05 ± 6.57*, b,c | 40.71 ± 14.48*, a | 42.88 ± 5.02*, a |
| Creatinine (mg/dl) | 0.93 ± 0.22*, b | 1.22 ± 0.35*, a | 1.0 ± 0.16 |
| CRP (mg/dl) | 4.99 ± 1.57**, b,c | 75.04 ± 39.50**, a | 76.35 ± 12.9**, a |
| WBCs (10^3/μl) | 7.47 ± 2.05*, b,c | 10.03 ± 4.17*, a | 10.13 ± 4.62*, a |
| Neutrophils (%) | 65.00 ± 5.06**, b,*, c | 73.76 ± 7.19**, a | 76.95 ± 5.57**, a |
| Lymphocytes (%) | 29.60 ± 5.30**, b,c | 19.89 ± 6.94**, a | 20.41 ± 6.13**, a |
| Ferritin (ng/mL) | 104.35 ± 50.85*, b,c | 213.86 ± 135.24*, a | 221.85 ± 186.49*, a |
| Serum electrolytes | |||
|
8.06 ± 0.68**, b,c | 3.81 ± 0.38**, a,c | 2.68 ± 0.89**, a,b |
|
143.7 ± 7.50*, b,**, c | 138.76 ± 4.73*, a, **, c | 127.95 ± 11.27**, a,b |
|
9.06 ± 0.69*, b,c | 8.039 ± 0.80*, a | 8.095 ± 1.029*, a |
|
0.86 ± 0.08 | 0.86 ± 0.12 | 0.87 ± 0.11 |
|
99.42 ± 3.45 | 98.82 ± 3.35 | 99.23 ± 4.48 |
| GIT manifestations | |||
|
----------- | ----------- | 38.1 % (24/63) |
|
31.7 % (20/63) | ||
|
20.6 % (13/63) | ||
|
9.5 % (6/63) | ||
Significant at p-value < 0.05.
Highly significant at p-value < 0.001.
Significant difference versus Control group.
Significant difference versus Without GIT group.
Significant difference versus With GIT group.
Our results revealed that there were highly significant (p <0.001) increase in serum CRP and whole blood neutrophils percentage levels, significant (p <005) increase in serum AST, ALT, urea, ferritin and whole blood WBCs levels and highly significant (p <0.001) decrease in whole blood lymphocytes percentage in both moderate COVID-19 patients with and without GIT manifestations as compared with controls; while there is no significant (p >005) change in these parameters when compared with each other. Serum creatinine levels were found to significantly (p <0.001) increased only in moderate COVID-19 patients without GIT manifestations as compared to controls; as shown in Table 1.
Data obtained in this study showed that 38.1 % (24/63) of moderate COVID-19 patients with GIT manifestations had anorexia, 31.7 % (20/63) had diarrhea, and 20.6 % (13/63) had vomiting and nausea; while only 9.5 % (6/63) had epigastric or abdominal pain; as shown in Table 1.
3.2. Levels of serum TSA in moderate COVID-19 patients with and without GIT manifestations
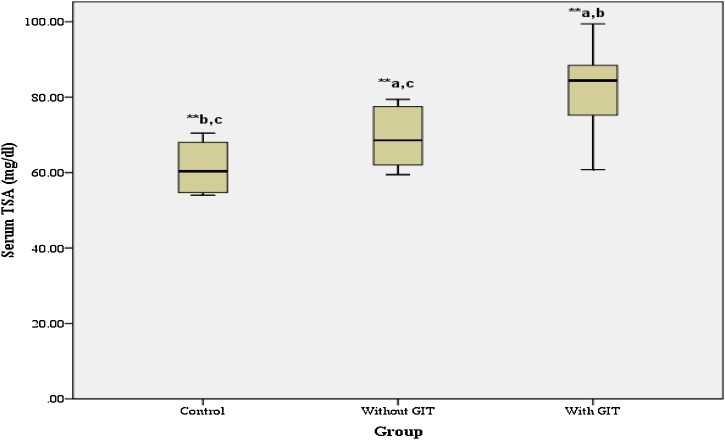
As depicted from Fig. 1 , the levels of serum TSA were highly significantly (p < 0.001) increased in moderate COVID-19 patients with GIT manifestations (81.43 ± 8.91) when compared with control group (61.24±6.41) or even moderate COVID-19 patients without GIT manifestations (69.46±7.03).
Fig. 1.
Mean level of serum TSA in controls and moderate COVID-19 patients with and without GIT manifestations. Results are given as mean ± SD. * Significant at p-value < 0.05, ** Highly significant at p-value < 0.001, a Significant difference versus Control group, b Significant difference versus Without GIT group, c Significant difference versus With GIT group.
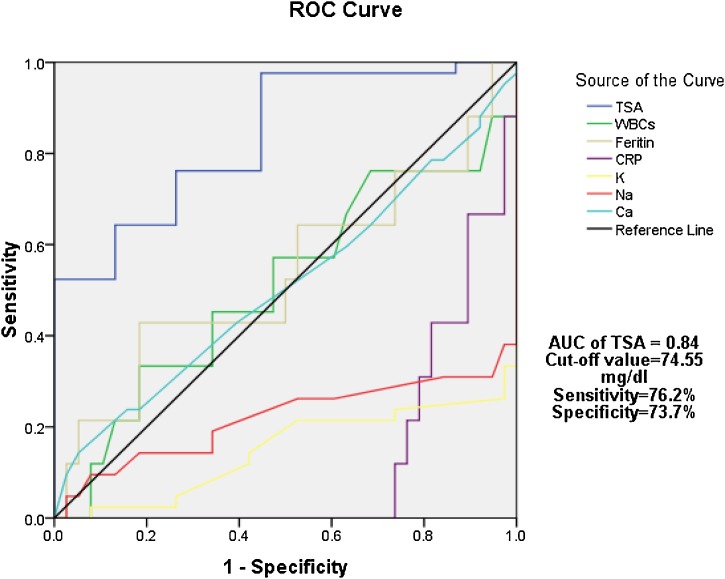
Authors used ROC curve analysis to calculate the sensitivity and specificity of TSA to differentiate between moderate COVID-19 patients with and without GIT manifestations. Our results showed that AUC for TSA is 0.84 with 76.2 % sensitivity and 73.7 % specificity at cut-off value 74.55 mg/dl; as shown in Fig. 2 .
Fig. 2.
ROC curve of serum TSA and other parameters in discrimination between moderate COVID-19 patients with and without GIT manifestations.
3.3. Levels of serum electrolytes in moderate COVID-19 patients with and without GIT manifestations
Our results showed that the levels of serum potassium (2.68 ± 0.89) and sodium (127.95 ± 11.27) were found to be highly significantly (p <0.001) decreased in moderate COVID-19 patients with GIT manifestations when compared with healthy controls (8.06 ± 0.68 & 143.7 ± 7.50; respectively) or even moderate COVID-19 patients without GIT manifestations (3.81 ± 0.38 & 138.76 ± 4.73; respectively); while the level of serum calcium (8.095 ± 1.029) was found to be significantly (p <0.05) decreased in moderate COVID-19 patients with GIT manifestations when compared with healthy controls only (9.06 ± 0.69). The levels of serum magnesium and chloride were found to be nonsignificantly changed in moderate COVID-19 patients with GIT manifestations when compared with healthy controls or moderate COVID-19 patients without GIT manifestations; as shown in Table 1.
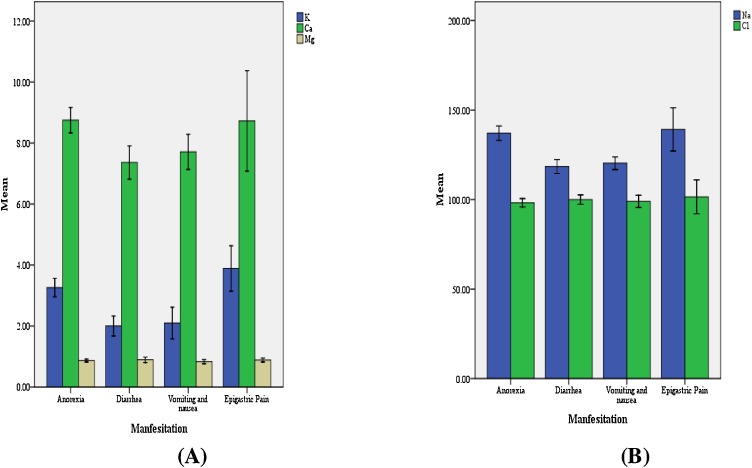
By investigating the levels of serum electrolytes among moderate COVID-19 patients with GIT manifestations only, our results showed that patients with diarrhea or vomiting and nausea had significant decrease of serum potassium (2.003 ± 0.54 & 2.10 ± 0.68; respectively), sodium (118.46 ± 6.41& 120.33 ± 4.58; respectively), and calcium (7.36 ± 0.90 & 7.71 ± 0.75; respectively) when compared with patients with anorexia (3.26 ± 0.57; 137.12 ± 7.45 & 8.75 ± 0.78; respectively) or patients with abdominal pain (3.89 ± 0.47; 139.25 ± 7.59 & 8.72 ± 1.03; respectively), while serum magnesium and chloride levels were nonsignificantly changed; as shown in Fig. 3 A & B.
Fig. 3.
Mean level of serum electrolytes in moderate COVID-19 patients with GIT manifestations: (A) Mean levels of serum of K (mmol/L), Ca (mg/dl) and Mg (mmol/L); (B) Mean levels of serum of Na (mmol/L) and Cl (mmol/L).
4. Discussion
Authors concerning mainly with the pathogenesis of COVID-19, as our previous work (Haroun et al., 2021a,b) showed that the increased levels of interferon-γ-induced protein-10 (IP-10), serum amyloid A (SAA), and miRNA-155 among COVID-19 patients. Here, we intended to measure serum TSA in moderate COVID-19 patients with and without GIT manifestations to investigate its roles in the pathogenesis of COVID-19 disease and its GIT complications. Our results revealed that the mean levels of TSA were significantly increased among moderate COVID-19 patients with GIT manifestations as compared with controls or even with mild COVID-19 patients without GIT manifestations.
The epithelium of GIT, especially colon, is covered with a bi-layer of mucus which rich in highly glycosylated proteins that usually terminated by fucose, sialic acid residues and sulfate (Bell et al., 2019). It known that sialic acids are often used as receptors by a wide range of viruses (Li, 2016; Wasik et al., 2016) including SARS-CoV-2 (Robson, 2020). Engin et al. (2020) demonstrated that by interacting with sialo-glycoconjugates, the SARS-CoV-2 can move along the intestinal lumen and migrate through the SA-rich mucus layer that coats and protects the enterocytes from viral attack, therefore SA moieties on glycoproteins are crucial, and essential receptor determinants for SARS-CoV-2 infection. From these facts, along with our results, authors believed that SA plays a pivotal role in the pathogenesis of COVID-19 disease specially its GIT manifestations. Also, by using ROC curve TSA level could differentiate between moderate COVID-19 patients with and without GIT with 76.2 % sensitivity and 73.7 % specificity at cut-off value 74.55 mg/dl, therefore TSA could be a potential biomarker for the COVID-19 gastrointestinal complications.
Anorexia and diarrhea are considered as the most frequent GIT manifestations in COVID-19 patients, and its prevalence are 12.2–50.2 % (Chen et al., 2020a,b; Wang et al., 2020) and 2–50 % (Cheung et al., 2020; Hajifathalian et al., 2020); respectively. According to our results the prevalence of anorexia is 38.1 % (24/63); while for diarrhea, the prevalence is 31.7 % (20/63). In addition, vomiting and nausea have been also observed among patients with COVID-19, either as their only manifestations or in combined with other GIT manifestations, with prevalence of 5.2–28 % (Cheung et al., 2020; Hajifathalian et al., 2020; Nobel et al., 2020). Also, patients with COVID-19 may have abdominal pain at presentation, however it is less common when compared to diarrhea, anorexia, nausea or vomiting, and its prevalence is 3.9–6.8% (Hajifathalian et al., 2020; Xing et al., 2020). Results of the current study revealed that the prevalence of vomiting and nausea is 20.6 % (13/63); while for the abdominal pain, the prevalence is 9.5 % (6/63).
Electrolyte imbalances, including decreased serum levels of potassium, sodium and calcium, have been reported in severe COVID-19 patients (Lippi et al., 2020). Results of the current study reveal that the level of serum potassium is highly significantly decreased either in moderate COVID-19 patients with and without GIT manifestations as compared to controls, and in moderate COVID-19 patients with GIT manifestations as compared with moderate COVID-19 patients without GIT manifestations.
It is known that hypokalemia is a frequent disorder in patients with COVID-19 (Alfano et al., 2021). Early studies suggest that hypokalaemia associated with SARS-CoV-2 infection may be due to gastrointestinal potassium loss, diuretic-induced potassium wasting, renin-angiotensin-aldosterone system (RAS) activation, or renal tubulopathy (Mabillard et al., 2020). As SARS-CoV-2 enters to host cells mainly by binding to angiotensin-converting enzyme 2 (ACE2) resulting in the reduction of angiotensin II decay, and therefore increase of aldosterone secretion, which in turn causes loss of potassium in the urine (Pal and Bhansali, 2020). Our results also indicate that the levels of serum sodium and calcium were highly significantly decreased in moderate COVID-19 patients with GIT manifestations and significantly decreased in moderate COVID-19 patients without GIT manifestations as compared with controls. Hyponatremia is from the most common electrolyte disorders reported in COVID-19 patients (Zhang et al., 2020). Berni et al. (2020) suggested that the cause behind the hyponatremia is that SARS-CoV-2 infection is associated with the increase of IL-6, which causes electrolyte disorder by inducing the vasopressin non-osmotic release. As consistent with our results, hypocalcemia was reported in COVID-19 patients (Di Filippo et al., 2020; Liu et al., 2020; Sun et al., 2020a,b). Pal et al. (2021) concluded that mild or moderate COVID-19 patients tend to have low serum total calcium levels at initial presentation, indicating that hypocalcemia may be intrinsic to the disease per se. Also, our results revealed that there was no significant change in magnesium and chloride.
In conclusion our results showed that the levels of serum TSA were highly significantly increased in moderate COVID-19 patients with and without GIT manifestations when compared with control group, and also in moderate COVID-19 patients with GIT manifestations when compared with moderate COVID-19 patients without GIT. Moreover, by using ROC curve TSA level could discriminate between moderate COVID-19 patients with and without GIT. Therefore we can conclude that SA plays a crucial role in the pathogenesis of GIT complications associated with COVID-19 and could be a potential biomarker for the COVID-19 gastrointestinal complications.
Author statement
All authors would like to acknowledge the patients for participation in this work. Also, we want to thank the great reviewers for revision of our manuscript; thank you so much for your valuable reviewing of our manuscript to be better.
Ethics statement
The present study was approved by the ethics committees of Port-Said Hospital, Port-Said University; Egypt (ERN MED (23/04/2020)S.no(5)MED). Informed consents were obtained from all patients. All procedures performed in this study were in accordance with the ethical standards of the Helsinki Declaration.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for- profit sectors.
Availability of data of material
All data generated or analyzed during this study are included in this published article.
Declaration of Competing Interest
The authors report no declarations of interest.
References
- Alfano G., Ferrari A., Fontana F., Perrone R., Mori G., Ascione E., Magistroni R., Venturi G., Pederzoli S., Margiotta G., Romeo M., Piccinini F., Franceschi G., Volpi S., Faltoni M., Ciusa G., Bacca E., Tutone M., Raimondi A., Menozzi M., Franceschini E., Cuomo G., Orlando G., Santoro A., Di Gaetano M., Puzzolante C., Carli F., Bedini A., Milic J., Meschiari M., Mussini C., Cappelli G., Guaraldi G. Modena covid-19 working group (MoCo19). Hypokalemia in patients with COVID-19. Clin. Exp. Nephrol. 2021;25(April (4)):401–409. doi: 10.1007/s10157-020-01996-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell A., Brunt J., Crost E., Vaux L., Nepravishta R., Owen C.D., Latousakis D., Xiao A., Li W., Chen X., Walsh M.A., Claesen J., Angulo J., Thomas G.H., Juge N. Elucidation of a sialic acid metabolism pathway in mucus-foraging Ruminococcus gnavus unravels mechanisms of bacterial adaptation to the gut. Nat. Microbiol. 2019;4(December (12)):2393–2404. doi: 10.1038/s41564-019-0590-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berni A., Malandrino D., Parenti G., Maggi M., Poggesi L., Hyponatremia Peri A. IL-6, and SARS-CoV-2 (COVID-19) infection: may all fit together? J. Endocrinol. Invest. 2020;43(August (8)):1137–1139. doi: 10.1007/s40618-020-01301-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen T., Wu D., Chen H., Yan W., Yang D., Chen G., Ma K., Xu D., Yu H., Wang H., Wang T., Guo W., Chen J., Ding C., Zhang X., Huang J., Han M., Li S., Luo X., Zhao J., Ning Q. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. 2020;368(March (26)):m1091. doi: 10.1136/bmj.m1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Chen L., Deng Q., Zhang G., Wu K., Ni L., Yang Y., Liu B., Wang W., Wei C., Yang J., Ye G., Cheng Z. The presence of SARS-CoV-2 RNA in the feces of COVID-19 patients. J. Med. Virol. 2020;92(July(7)):833–840. doi: 10.1002/jmv.25825. [DOI] [PubMed] [Google Scholar]
- Cheung K.S., Hung I.F.N., Chan P.P.Y., Lung K.C., Tso E., Liu R., Ng Y.Y., Chu M.Y., Chung T.W.H., Tam A.R., Yip C.C.Y., Leung K.H., Fung A.Y., Zhang R.R., Lin Y., Cheng H.M., Zhang A.J.X., To K.K.W., Chan K.H., Yuen K.Y., Leung W.K. Gastrointestinal manifestations of SARS-CoV-2 infection and virus load in fecal samples from a Hong Kong cohort: systematic review and meta-analysis. Gastroenterology. 2020;159(July (1)):81–95. doi: 10.1053/j.gastro.2020.03.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chittemsetti S., Manchikatla P.K., Guttikonda V. Estimation of serum sialic acid in oral submucous fibrosis and oral squamous cell carcinoma. J. Oral Maxillofac. Pathol. 2019;23(January-April (1)):156. doi: 10.4103/jomfp.JOMFP_239_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COVID-19 coronavirus pandemic: Worldometer, 2020. Available: https://www.worldometers.info/coronavirus/. (Accessed 12 July 2021).
- d’Alessandro M., Cameli P., Refini R.M., Bergantini L., Alonzi V., Lanzarone N., Bennett D., Rana G.D., Montagnani F., Scolletta S., Franchi F., Frediani B., Valente S., Mazzei M.A., Bonella F., Bargagli E. Serum KL-6 concentrations as a novel biomarker of severe COVID-19. J. Med. Virol. 2020;92(October (10)):2216–2220. doi: 10.1002/jmv.26087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Filippo L., Formenti A.M., Rovere-Querini P., Carlucci M., Conte C., Ciceri F., Zangrillo A., Giustina A. Hypocalcemia is highly prevalent and predicts hospitalization in patients with COVID-19. Endocrine. 2020;68(June (3)):475–478. doi: 10.1007/s12020-020-02383-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engin A.B., Engin E.D., Engin A. Dual function of sialic acid in gastrointestinal SARS-CoV-2 infection. Environ. Toxicol. Pharmacol. 2020;79(October) doi: 10.1016/j.etap.2020.103436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruszewska E., Cylwik B., Panasiuk A., Szmitkowski M., Flisiak R., Chrostek L. Total and free serum sialic acid concentration in liver diseases. Biomed Res. Int. 2014;2014:876096. doi: 10.1155/2014/876096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajifathalian K., Krisko T., Mehta A., Kumar S., Schwartz R., Fortune B., Sharaiha R.Z., WCM-GI research group∗ Gastrointestinal and hepatic manifestations of 2019 novel coronavirus disease in a large cohort of infected patients from New York: clinical implications. Gastroenterology. 2020;159(September (3)):1137–1140. doi: 10.1053/j.gastro.2020.05.010. e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haroun R.A., Osman W.H., Abo-Shanab W.S., Eessa A.M. Circulating plasma miR-155 is a potential biomarker for the detection of SARS-CoV-2 infection. Pathology. 2021:0031–3025. doi: 10.1016/j.pathol.2021.09.006. ISSN. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haroun R.A., Osman W.H., Eessa A.M. Interferon-γ-induced protein 10 (IP-10) and serum amyloid A (SAA) are excellent biomarkers for the prediction of COVID-19 progression and severity. Life Sci. 2021;269(March (15)):119019. doi: 10.1016/j.lfs.2021.119019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry B.M., de Oliveira M.H.S., Benoit J., Lippi G. Gastrointestinal symptoms associated with severity of coronavirus disease 2019 (COVID-19): a pooled analysis. Intern. Emerg. Med. 2020;15(August (5)):857–859. doi: 10.1007/s11739-020-02329-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X., Cheng Z., Yu T., Xia J., Wei Y., Wu W., Xie X., Yin W., Li H., Liu M., Xiao Y., Gao H., Guo L., Xie J., Wang G., Jiang R., Gao Z., Jin Q., Wang J., Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(February (10223)):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F. Structure, Function, and Evolution of Coronavirus Spike Proteins. Annu. Rev. Virol. 2016;3(September (1)):237–261. doi: 10.1146/annurev-virology-110615-042301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin L., Jiang X., Zhang Z., Huang S., Zhang Z., Fang Z., Gu Z., Gao L., Shi H., Mai L., Liu Y., Lin X., Lai R., Yan Z., Li X., Shan H. Gastrointestinal symptoms of 95 cases with SARS-CoV-2 infection. Gut. 2020;69(June (6)):997–1001. doi: 10.1136/gutjnl-2020-321013. [DOI] [PubMed] [Google Scholar]
- Lippi G., South A.M., Henry B.M. Electrolyte imbalances in patients with severe coronavirus disease 2019 (COVID-19) Ann. Clin. Biochem. 2020;57(May (3)):262–265. doi: 10.1177/0004563220922255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Han P., Wu J., Gong J., Tian D. Prevalence and predictive value of hypocalcemia in severe COVID-19 patients. J. Infect. Public Health. 2020;13(September (9)):1224–1228. doi: 10.1016/j.jiph.2020.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mabillard H., Tedd H., Speight A., Duncan C., Price D.A., Sayer J.A. Case report: renal potassium wasting in SARS-CoV-2 infection. F1000Res. 2020;9(June (30)):659. doi: 10.12688/f1000research.24621.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matrosovich M., Herrler G., Klenk H.D. Sialic acid receptors of viruses. Top. Curr. Chem. 2015;367:1–28. doi: 10.1007/128_2013_466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobel Y.R., Phipps M., Zucker J., Lebwohl B., Wang T.C., Sobieszczyk M.E., Freedberg D.E. Gastrointestinal symptoms and coronavirus disease 2019: a case-control study from the United States. Gastroenterology. 2020;159(July (1)):373–375. doi: 10.1053/j.gastro.2020.04.017. e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olaru O.G., Constantin G.I., Pena C.M. Variation of total serum sialic acid concentration in postmenopausal women. Exp. Ther. Med. 2020;20(September (3)):2455–2459. doi: 10.3892/etm.2020.8873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal R., Bhansali A. COVID-19, diabetes mellitus and ACE2: the conundrum. Diabetes Res. Clin. Pract. 2020;162(April) doi: 10.1016/j.diabres.2020.108132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal R., Ram S., Zohmangaihi D., Biswas I., Suri V., Yaddanapudi L.N., Malhotra P., Soni S.L., Puri G.D., Bhalla A., Bhadada S.K. High prevalence of Hypocalcemia in non-severe COVID-19 patients: a retrospective case-control study. Front. Med. (Lausanne) 2021;7(January (7)):590805. doi: 10.3389/fmed.2020.590805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perisetti A., Goyal H., Gajendran M., Boregowda U., Mann R., Prevalence Sharma N. Mechanisms, and implications of gastrointestinal symptoms in COVID-19. Front. Med. (Lausanne) 2020;7(October (30)):588711. doi: 10.3389/fmed.2020.588711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robson B. Bioinformatics studies on a function of the SARS-CoV-2 spike glycoprotein as the binding of host sialic acid glycans. Comput. Biol. Med. 2020;122(July):103849. doi: 10.1016/j.compbiomed.2020.103849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun B., Feng Y., Mo X., Zheng P., Wang Q., Li P., Peng P., Liu X., Chen Z., Huang H., Zhang F., Luo W., Niu X., Hu P., Wang L., Peng H., Huang Z., Feng L., Li F., Zhang F., Li F., Zhong N., Chen L. Kinetics of SARS-CoV-2 specific IgM and IgG responses in COVID-19 patients. Emerg. Microbes Infect. 2020;9(December (1)):940–948. doi: 10.1080/22221751.2020.1762515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J.K., Zhang W.H., Zou L., Liu Y., Li J.J., Kan X.H., Dai L., Shi Q.K., Yuan S.T., Yu WK Xu H.Y., Gu W., Qi J.W. Serum calcium as a biomarker of clinical severity and prognosis in patients with coronavirus disease 2019. Aging (Albany NY) 2020;12(June (12)):11287–11295. doi: 10.18632/aging.103526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J., Wang B., Xiang H., Cheng Z., Xiong Y., Zhao Y., Li Y., Wang X., Peng Z. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323(March (11)):1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasik B.R., Barnard K.N., Parrish C.R. Effects of sialic acid modifications on virus binding and infection. Trends Microbiol. 2016;24(December (12)):991–1001. doi: 10.1016/j.tim.2016.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO . World Health Organization; 2020. Director-General’s Opening Remarks at the Media Briefing on COVID-19 -. 11 March 2020. Available: https://www.who.int/director-general/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19-.--11-march-2020. (Accessed 26 Jun 2021) [Google Scholar]
- Wielgat P., Rogowski K., Godlewska K., Coronaviruses Car H. Is sialic acid a gate to the eye of cytokine storm? From the entry to the effects. Cells. 2020;9(August (9)):1963. doi: 10.3390/cells9091963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing Y.H., Ni W., Wu Q., Li W.J., Li G.J., Wang W.D., Tong J.N., Song X.F., Wing-Kin Wong G., Xing Q.S. Prolonged viral shedding in feces of pediatric patients with coronavirus disease 2019. J. Microbiol. Immunol. Infect. 2020;53(June (3)):473–480. doi: 10.1016/j.jmii.2020.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C., Chen J., Liu Y., Xu D. Sialic acid metabolism as a potential therapeutic target of atherosclerosis. Lipids Health Dis. 2019;18(September (1)):173. doi: 10.1186/s12944-019-1113-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Cai H., Hu J., Lian J., Gu J., Zhang S., Ye C., Lu Y., Jin C., Yu G., Jia H., Zhang Y., Sheng J., Li L., Yang Y. Epidemiological, clinical characteristics of cases of SARS-CoV-2 infection with abnormal imaging findings. Int. J. Infect. Dis. 2020;94(May):81–87. doi: 10.1016/j.ijid.2020.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]