Abstract
Some chemicals commonly used in personal care products, household items, food vessels, cosmetics, and other consumer products are potentially harmful, and several reviews of epidemiological studies have suggested the associations between the chemical exposure from consumer products, and respiratory diseases, skin sensitization, and reproductive problems. Therefore, risk assessment is essential for management of consumer products safety. Necessarily, the estimation of human exposure is an essential step in risk assessment, and the absorption rate of those chemicals via the gastrointestinal tract, respiratory tract, and skin are very critical in determining the internal dose of the exposed chemicals. In this study, parallel artificial membrane permeability assays (PAMPA) for the gastrointestinal tract and skin were performed to evaluate the permeability of parabens (4-hydroxybenzoic acid, methyl-, propyl-, and butyl paraben), bisphenols (bisphenol A, bisphenol F, and bisphenol S), isothiazolinones (methyl-, chloromethyl-, benz-, octyl-, and dichlorooctyl isothiazolinone), and phthalates [diethyl-, dibutyl-, Di-isononyl-, and bis(2-ethylhexyl) phthalate]. Lipid solubility of test chemicals indicated by log P values was shown as the most critical factor and showed a positive association with the permeability of parabens, bisphenols, and isothiazolinones in PAMPA assay. However, phthalate showed a reverse-association between lipophilicity and permeability. The permeability of all the tested chemicals was higher in the gastrointestinal tract membrane than in the skin membrane. The pH in donor solution did not show significant effects on the permeability in all the chemicals, except the chemicals with a free hydrophilic moiety in their chemical structures.
Keywords: Permeability, PAMPA, Parabens, Bisphenols, Isothiazolinones, Phthalates
Introduction
Some chemicals commonly found in personal care products, household items, food vessels, cosmetics, and other consumer products are potentially harmful either through direct exposure during use or indirectly via subsequent environmental emissions. Several reviews of epidemiological studies have suggested an association between the exposure to some consumer products and respiratory diseases, skin sensitization, and reproductive system problems [1,2]. For example, isothiazolinones which are used as biocides may cause skin irritation and/or eye irritation. Parabens used as preservatives in cosmetics are absorbed into the skin causing an estrogenic effect. Phthalates used in plastics to give flexibility and transparency are capable of endocrine disruption leading to disorders such as neurobehavioral disorder [3,4]. However, the exact category of harmful chemicals in consumer products and the routes of exposure remain unclear. Therefore, a risk assessment is essential for management of consumer products safety. Necessarily, estimation of human exposure is a key factor in the process of risk assessment, and the absorption rates are very critical in determining the internal dose of the exposed chemicals. A study on the chemical exposure from consumer products demonstrated that use-stage exposure may exceed environmentally mediated exposures. This highlights the importance of the usage patterns, exposure route, and absorption rate in the risk assessment of chemicals in consumer products [5,6].
Internal dose is the amount of a chemical that is absorbed by the body by penetrating an epithelial barrier such as the gastrointestinal tract, respiratory tract, and skin. The body burden of chemicals in risk assessment is a determinant of the toxicological threshold and it mainly depends on the absorption rate. However, there is a scarcity of information on chemical permeability because in vivo studies for pharmacokinetic parameters are done using physiologically based pharmacokinetic models which are costly and time-consuming [7]. As an easier alternative, in vitro permeability assays are used to predict chemical permeability. Among them, the parallel artificial membrane permeability assay (PAMPA) is prominent owing to its high-throughput screening technique developed to predict passive permeability through a wide range of biological membranes. It can be used to predict passive absorption of a diverse range of chemicals through the gastrointestinal tract, blood-brain barrier, and skin layer [8–10]. PAMPA was introduced as a tool for rapid determination of passive membrane permeability of chemicals. Expectedly, due to its low cost and high throughput performance, it is widely used in the pharmaceutical and cosmetic industries to determine the gastrointestinal tract and skin absorption rate in the discovery stage of development. In this study, several chemicals used in consumer products were applied to PAMPA to screen the absorption rate in gastrointestinal tract and skin.
Materials and Methods
PAMPA kit and reference chemicals
The assay kits were purchased from Pion Inc. (Billerica, MA, USA). Reference chemicals for gastrointestinal tract (GIT), ketoprofen, verapamil, carbamazepine, propranolol, metoprolol, antipyrine, and ranitidine, were supplied with the assay kit; their physico-chemical information is provided in (Table 1). Reference chemicals for skin, chlorpromazine, verapamil, warfarin, piroxicam, progesterone, niflumic acid, and atenolol, were also supplied with the assay kit; listed in (Table 2).
Table 1.
The permeability means values of 7 reference chemicals in GIT PAMPA.
| Compound | CAS No. | Test conc. | BCS class1 | pKa | Uncharged pH range | pH | Permeability (10−6 cm/s) (Mean±S.D.) | |
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Expected from supplier2 | Measured in this lab3 | |||||||
| Ketoprofen | 22071-15-4 | 50 μM | 2 | 4.1 | <4.1 | 5.0 | 41.2±5.0 | 61.5±15.9 |
| 6.2 | 15.8±2.0 | 15.4±1.9 | ||||||
| 7.4 | 1.7±0.5 | 1.3±0.1 | ||||||
|
| ||||||||
| Verapamil HCl | 152-11-4 | 50 μM | 1 | 9.1 | >9.1 | 5.0 | 47.5±5.0 | 60.4±15.8 |
| 6.2 | 60.5±10.0 | 66.5±21.8 | ||||||
| 7.4 | 61.3±10.0 | 67.0±28.7 | ||||||
|
| ||||||||
| Carbamazepine | 298-46-4 | 50 μM | 2 | 1.4 | >1.4 | 5.0 | 41.8±5.0 | 44.1±7.0 |
| 6.2 | 36.6±5.0 | 42.9±6.3 | ||||||
| 7.4 | 42.9±5.0 | 40.9±7.7 | ||||||
|
| ||||||||
| Propranolol | 318-98-9 | 50 μM | 1 | 9.5 | >9.5 | 5.0 | 44.2±6.5 | 49.9±11.0 |
| 6.2 | 66.7±10.3 | 80.8±25.1 | ||||||
| 7.4 | 63.9±10.1 | 83.1±30.1 | ||||||
|
| ||||||||
| Metoprolol Tartrate | 56392-17-7 | 50 μM | 1 | 9.6 | >9.6 | 5.0 | 4.9±0.5 | 4.1±0.2 |
| 6.2 | 19.1±3.0 | 29.1±4.0 | ||||||
| 7.4 | 45.7±5.0 | 59.0±21.6 | ||||||
|
| ||||||||
| Antipyrine | 60-80-0 | 250 μM | 1 | 1.4 | >1.4 | 5.0 | 2.9±0.3 | 2.0±0.2 |
| 6.2 | 2.8±0.3 | 2.0±0.2 | ||||||
| 7.4 | 2.4±0.2 | 1.9±0.1 | ||||||
|
| ||||||||
| Ranitidine | 66357-59-3 | 50 μM | 3 | 2.0, 8.3 | >8.3 | 5.0 | <0.1 | 0.1±0.1 |
| 6.2 | <0.1 | 0.0±0.0 | ||||||
| 7.4 | 0.1±0.5 | 0.3±0.1 | ||||||
BCS represents biopharmaceutics classification system that classifies the drugs/chemicals on the basis of their solubility and permeability.
The expected permeability values of reference chemicals were provided by the supplier of PAMPA.
The measured permeability values in this study were generated from three different experiments.
Table 2.
The permeability means values of 7 reference chemicals in Skin PAMPA.
| Compound | CAS No. | Test conc. | pH | Permeability (10−6 cm/s) (Mean±S.D.) | |
|---|---|---|---|---|---|
|
| |||||
| Expected1 | Measured2 | ||||
| Chlorpromazine | 69-09-0 | 50 μM | 6.5 | - | 2.5±0.4 |
| 7.4 | 10.7 | 9.5±0.2 | |||
|
| |||||
| Verapamil HCl | 152-11-4 | 50 μM | 6.5 | - | 0.2±0.2 |
| 7.4 | 3.0 | 2.6±1.2 | |||
|
| |||||
| Warfarin | 81-81-2 | 50 μM | 6.5 | 1.0 | 1.2±0.1 |
| 7.4 | - | 0.6±0.2 | |||
|
| |||||
| Piroxicam | 36322-90-4 | 50 μM | 6.5 | 2.1 | 3.1±0.5 |
| 7.4 | - | 1.3±0.3 | |||
|
| |||||
| Progesterone | 57-83-0 | 50 μM | 6.5 | 12.0 (pH 5.0, 9.0) | 9.1±2.0 |
| 7.4 | - | 10.7±3.3 | |||
|
| |||||
| Niflumic acid | 4394-00-7 | 50 μM | 6.5 | 2.2 | 2.6±0.6 |
| 7.4 | - | 1.1±0.3 | |||
|
| |||||
| Atenolol | 29122-68-7 | 50 μM | 6.5 | <1 | 0.1±0.2 |
| 7.4 | 0.3±0.4 | ||||
The expected permeability values of reference chemicals were provided by the supplier of PAMPA.
The measured permeability values in this study were generated from three different experiments.
Test chemicals
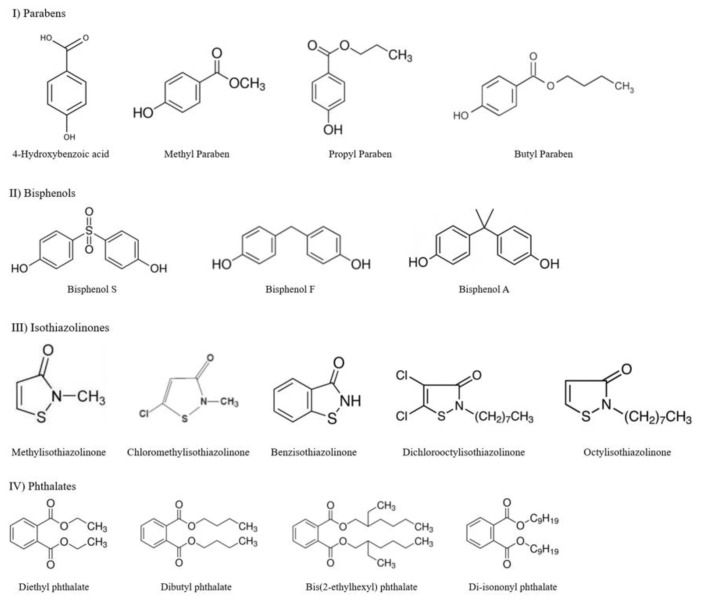
The GIT and skin permeation of parabens (4-hydroxybenzoic acid, methyl paraben, propyl paraben, and butyl paraben), bisphenols (bisphenol A, bisphenol S, and bisphenol F), isothiazolinones (methylisothiazolinone, chloromethylisothiazolinone, benzisothiazolinone, octylisothiazolinone, and dichlorooctylisothiazolinone), and Phthalates [diethyl phathalate, dibutyl phthalate, Di-isononyl phthalate, and bis (2-ethylhexyl) phthalate] were tested in this study. They are all used as preservatives, food contact applications, plastic raw materials, and biocides in consumer products, and are potentially absorbed through the oral route or skin. The information on the test chemicals including suppliers and physico-chemical properties is listed in (Table 3), and the chemical structures are shown in (Figure 1).
Table 3.
The information on the physico-chemical properties and test concentrations of 16 test chemicals.
| Compound | CAS No. | Molecular weight (g/mol) | pKa | Log P | Test conc. | Supplier |
|---|---|---|---|---|---|---|
| 4-Hydroxybenzoic acid (4-HBA) | 99-96-7 | 138.1 | 4.54 | 1.58 | 250 μM | SIGMA-ALDRICH |
|
|
||||||
| Methyl paraben (MeP) | 99-76-3 | 152.2 | 8.4, 8.5 | 1.96 | 75 μM | |
|
|
||||||
| Propyl paraben (PrP) | 94-13-3 | 180.2 | 8.5 (phenol) | 3.04 | 75 μM | |
|
|
||||||
| Butyl paraben (BuP) | 94-26-8 | 194.23 | 8.47 | 3.57 | 75 μM | |
|
| ||||||
| Bisphenol S (BPS) | 80-09-1 | 250.3 | 8.2 | 1.65 | 125 μM | SIGMA-ALDRICH |
|
|
||||||
| Bisphenol F (BPF) | 620-92-8 | 200.2 | 7.55, 10.80 | 2.91 | 250 μM | |
|
|
||||||
| Bisphenol A (BPA) | 80-05-7 | 228.3 | 9.6 | 3.40 | 75 μM | |
|
| ||||||
| Methylisothiazolinone (MIT) | 2682-20-4 | 115.2 | - | −0.83 | 350 μM | AK scientific |
|
|
||||||
| Chloromethylisothiazolinone (CMIT) | 26172-55-4 | 149.6 | - | 0.40 | 250 μM | LGC |
|
|
||||||
| Benzisothiazolinone (BIT) | 2634-33-5 | 151.2 | - | 0.76 | 100 μM | SIGMA-ALDRICH |
|
|
||||||
| Dichlorooctylisothiazolinone (DCOIT) | 64359-81-5 | 282.2 | - | 2.85 | 125 μM | Tokyo Chemical Industry |
|
|
||||||
| Octylisothiazolinone (OIT) | 26530-20-1 | 213.3 | - | 5.00 | 125 μM | |
|
| ||||||
| Diethyl phthalate (DEP) | 84-66-2 | 222.2 | - | 2.20 | 250 μM | SIGMA-ALDRICH |
|
|
||||||
| Dibutyl phthalate (DBP) | 84-74-2 | 278.3 | - | 4.40 | 250 μM | |
|
|
||||||
| Bis(2-ethylhexyl) phthalate (DEHP) | 117-81-7 | 390.6 | - | 7.60 | 250 μM | |
|
|
||||||
| Di-isononyl phthalate (DINP) | 28553-12-0 | 418.6 | - | 9.37 | 250 μM | |
The sources of physico-chemical properties of test chemicals were materials safety data sheet (MSDS) and PubChem of National Library of Medicine.
Figure 1.
Chemical structures of 16 test chemicals.
Validation of PAMPA
The permeability of seven reference chemicals listed in (Table 1) was tested to evaluate the integrity of GIT PAMPA, and the seven reference chemicals listed in (Table 2) were tested to evaluate the integrity of skin PAMPA. The absorption coefficient Pe values obtained from the three separate experiments in this study were compared to the expected values provided by the PAMPA kit supplier.
PAMPA experiment
The PAMPA kit was purchased from Pion Inc. The stock solutions of the test chemicals and reference chemicals were prepared at respective concentrations in dimethyl sulfoxide (DMSO) and stored at room temperature before use. On the day of the test, the stock solution was diluted first with the buffer to achieve a final sample concentration described in (Table 1–3). The final concentration of DMSO in the test preparations was 0.5%. For the GIT permeation test, the donors of a 96-well plate were filled with a 200 μL diluted test solution at three different pH conditions, pH5.0, pH6.2, and pH7.4. The artificial membrane on the acceptor plate was wetted with 5μL 20% w/v phospholipid compound solution in n-dodecane and the acceptor wells were filled with 200 μL buffer solution. Then, the acceptor plate was assembled with the donor plate to make a sandwich. The assembled sandwich plate was incubated at 25 °C for 4 h. After reaching the permeation time, the PAMPA sandwich plate was disassembled and the volume of test chemicals in both donor wells and acceptor wells were measured by UV spectrum at 250 nm ~ 500 nm using SPARK (Tecan, Zurich, Switzerland). Experiments were performed using an aqueous buffer solution. The process of the skin permeation test was similar to that of the GIT test but without the pH of buffer condition (pH 6.5 and pH7.4.) and incubation time (5 h). The artificial membranes that were used for the skin test were hydrated overnight before the permeation test [11,12].
Software and data analysis
The absorption coefficient Pe data for the test chemicals and reference chemicals were calculated using PAMPA Explorer Command Software (Ver 3.8) provided by the supplier. Three separate experiments were performed, and the average and standard deviation of Pe were represented.
Results
Validation of PAMPA
GIT PAMPA was validated by comparing the expected values suggested by the PAMPA kit supplier and the measured values obtained in the author’s laboratory for the 7 reference chemicals. They were compared at three different pH conditions, pH5.0, pH6.2, and pH7.4. Biopharmaceutics classification system (BCS) is a scientific framework for classifying drug substances according to their aqueous solubility and their intestinal permeability. BCS class 1 means high permeability and high solubility, and BCS class 2 means high permeability and low solubility, and BCS class 3 means low permeability and high solubility. As shown in (Table 1), the values obtained in this experiment were in the range of the expected values. The permeability patterns (increase/decrease) according to the pHs of donor solution were similar to those of the supplier’s expected values. The BCS 1 chemicals of verapamil, propranolol, and metoprolol showed high permeability values in a pH dependent manner both in expected values and in measured values. The BCS 2 chemicals of ketoprofen and carbamazepine also showed comparatively high permeability. The permeability value of ranitidine, chemicals of BCS 3 showed very low permeability in all the test pH ranges. Antipyrine, although it was categorized as BCS class 1 chemical, showed low permeability in both the expected value and measured value. The reference chemicals selected for the skin permeability test were chlorpromazine, verapamil, warfarin, piroxicam, progesterone, niflumic acid, and atenolol. Although the expected values for skin permeability of the seven reference chemicals suggested by the supplier were not provided in detail compared to those of GIT PAMPA, the measured values obtained in this study were very similar to those of expected values as shown in (Table 2). It means that the integrity of the GIT and skin PAMPA kits used in these tests was intact and the conditions of PAMPA experiments in the author’s laboratory were as required.
GIT permeation
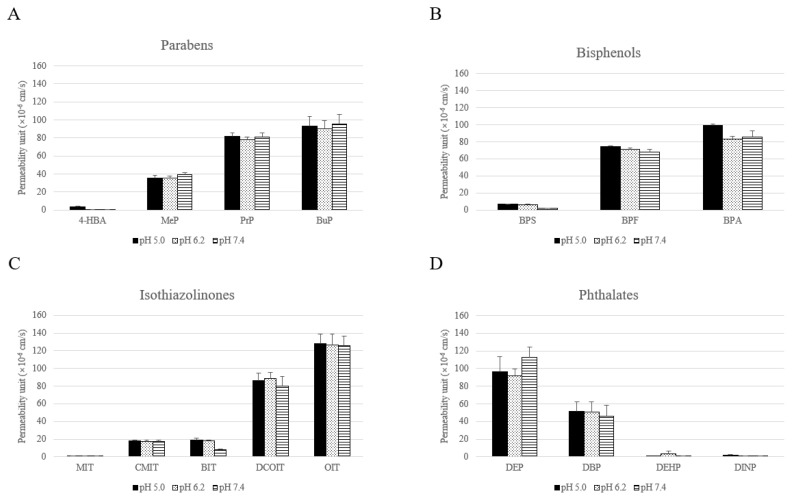
The general information of the test chemicals and structures is shown in (Table 3) and (Figure 1). 4-Hydroxybenzoic acid, methyl-, propyl-, and butyl paraben are used as preservatives in food, cosmetics, and other consumer products. The permeability (×10−6 cm/s) of 4-hydroxybenzoic acid (4-HBA) was lowest among the parabens, 0.15±0.11 and those of methyl paraben (MeP), propyl paraben (PrP), and butyl paraben (BuP) were increased to 39.12±2.58, 81.32±4.36, and 95.14±11.31, respectively at pH 7.4. Based on the present study findings, 4-HBA was slightly permeable in pH 5.0 (3.56±0.71) and almost non-permeable in pH 7.4. This can be attributed to the free carboxylic acid moiety in the 4-HBA chemical structure that is easily ionized in higher pH consequently decreasing its permeability, while the non-ionized form of 4-HBA passed the lipid barrier easily in low pH. The permeability of alkyl ester parabens showed dependency on the log P values, which indicates the lipid solubility of the chemicals. However, the permeability of alkyl ester parabens (MeP, PrP, and BuP) showed no dependency on pH.
The permeability of bisphenols also seemed to be depended on the log P values. The permeability (×10−6 cm/s) of BPA, BPF, and BPS was 85.28±7.45, 68.22±2.63, and 2.26±0.08 at pH 7.4, respectively. The log P value of BPS permeability (1.65) was significantly lower than that of BPA (3.40). The permeability difference in different pH conditions was not evident, although a slight decrease was observed with the increase in pH. This pattern was especially observed in BPS.
For isothiazolinones, the permeability of octylisothiazolinone (OIT) and dichlorooctylisothiazolinone (DCOIT) was higher than that of benzisothiazolinone (BIT), chloromethylisothiazolinone (CMIT), and methylisothiazolione (MIT). Based on our findings the permeability of isothiazolinones was also dependent on the lop P values. The permeability (×10−6 cm/s) of OIT (log P; 5.00) was 125.76±10.99 while that of MIT (log P; −0.83) was 0.85±0.11 at pH7.4. The permeability of phthalates did not show association with log P values, but reversed dependency was observed. The log P value of diisononyl phthalate (DINP) was the highest (log P; 9.37) among the tested phthalates but its permeability (×10−6 cm/s) was the lowest; almost non-permeable, 0.37 ±0.64 at pH7.4. Diethylphthalae (DEP) which has the shortest alkyl chain and lowest log P values (2.20) among the tested phthalates, showed the highest permeability, 112.78±11.39 at pH7.4. The test chemicals permeability results are shown in (Figure 2).
Figure 2.
The permeability means values of 16 test chemicals in GIT PAMPA. GIT PAMPA was performed under three different pH conditions and mean ±S.D was obtained from three different experiments. The y-axis represents the permeability unit (x 10−6 cm/s).
Skin permeation
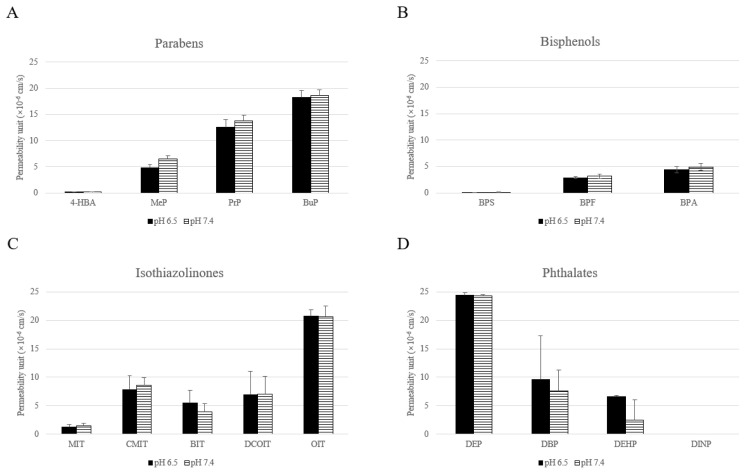
In this study, the chemicals used in GIT PAMPA were the same as those used in skin PAMPA. The relationship between the parabens permeability and log P values was similar to that of GIT PAMPA, while the absolute permeability rate was significantly lower than in the GIT test (Figure 3). The permeability increased as the carbon number of parabens alkyl ester increased, similar to the GIT test. The permeability (×10−6 cm/s) of 4-HBA was 0.18±0.04, and the permeability of MeP, PrP, and BuP were 6.55±0.56, 13.83±1.01 and 18.64±1.13, respectively, at pH 7.4. The permeability of BuP was approximately 1,000 fold higher than that of 4-HBA. Based on our findings, the permeability of parabens was log P values dependent. For skin PAMPA, the tests were done under two pH conditions (pH 6.5 and pH 7.4), and all the tested chemicals showed no permeability difference between the two pH conditions (Figure 3). The permeability of bisphenols was also the log P value dependent. The permeability (×10−6 cm/s) of BPA was 4.91±0.62, while that of BPS was 0.13±0.04. The permeability (×10−6 cm/s) of BPA in GIT was 85.28 ±7.45 at pH 7.4, but in the skin, it was only approximately 5.8% that of GIT, 4.91±0.62. For isothiazolinones, the permeability rates of OIT and DCOIT were higher than that of BIT, CMIT, and MIT as shown in the GIT. The isothiazolinones skin and GIT permeability rates were log P value dependent, however, the magnitude of skin permeability was comparatively very low. OIT permeability was 20.59±1.99 while that of MIT was 1.41±0.48 at pH7.4. The skin permeability of phthalates did not follow the positive but the reverse relationship between log P and membrane permeability.
Figure 3.
The permeability means values of 16 test chemicals in Skin PAMPA. Skin PAMPA was performed in two different pH conditions and mean ±S.D was obtained from three different experiments. The y-axis represents the permeability unit (x 10−6 cm/s).
Discussion
The alkyl esters of p-hydroxybenzoic acid are extensively used as preservatives in consumer products. According to the surveys of consumer products, methyl parabens were most widely found in the major cosmetics for body care, and the most common chemical detected in human samples of breast tissue, urine, and serum [13]. In this study, parabens showed a positive relationship between log P values and apparent permeability in GIT and skin. In vitro tests using artificial membranes, pig ear, and human skin, showed that the permeation of parabens increased as the length of the ester carbon chain increased [14,15]. Whitworth and Jun investigated the absorption of parabens in frogs and showed that the greater the lipid solubility of the parabens, the higher the rate of absorption [16]. However, inconsistent results were also published by other studies; Paraben permeability test performed using biomimetic artificial membrane showed that the low log P compound (4-HBA) and high log P compounds (heptyl- and octyl parabens) had a low permeability, while intermediate log P compound (ethyl paraben) showed a maximal permeability. The results showed a parabolic curve. The artificial membranes used in the test were constructed by impregnating a lipid solution on a hydrophobic filter [17]. In another study of skin PAMPA which used artificial membrane constructed using free fatty acid, cholesterol, and synthetic ceramide analogs to mimic the stratum cornea, permeation order of the parabens was: methyl paraben > ethyl paraben > propyl parabens, and the permeation was reversely related with the log P values [18]. Evaluation of the transdermal permeation of different parabens through a pig ear skin model also showed a reverse relationship [19].
Bisphenol A (BPA) is the most widely used color developer in thermal paper for cashiers receipts, labels, and tickets. BPA on the paper can be absorbed through the skin during contact. BPA is known to have endocrine disrupting effects and it is now being replaced by alternatives such as bisphenol S (BPS). As shown in (Table 3), BPS has a lower log P value than BPA and bisphenol F (BPF) due to its hydrophilic moiety. A study using reconstructed human epidermis to compare the percutaneous absorption of BPA and BPS revealed that the permeability coefficient of BPS was significantly lower than that of BPA [20–22]. These findings were similar to the findings of the present study, the log P values of BPA, BPF, and BPS were 3.40, 2.91, and 1.65, respectively. The permeation order was BPA > BPF > BPS, which showed a close relationship between the permeability and log P values. Although the skin permeability was lower than GIT, the relationship was similar in both GIT and skin (Figure 2,3). The information on the relative order of permeability would be useful for the development of alternative bisphenols with low risk to human health.
Isothiazolinones are the most commonly used biocides in consumer products; CMIT and MIT are used as a mixture in the ratio of 3:1, and in some cases, MIT are used as a single substance. Other isothiazolinones including BIT, OIT, and DCOIT are also widely used in consumer products. All the isothiazolinones are classified as skin sensitizers in the harmonized classification of the Regulation European Commission, and they are known to cause allergic contact dermatitis. The information on their pharmacokinetics were not inadequate and no data on in vitro permeation tests were found. A study performed by Garcia-Hidalgo et al estimated individual-based aggregate dermal exposure to four isothiazolinones by modeling, and the exposure order was suggested as BIT > OIT > MIT > CMIT. Furthermore, their skin permeability coefficient was similar to that of four isothiazolinones estimated by previous reports. The permeability coefficient through stratum corneum (cm/h) of OIT was the highest followed by BIT, CMIT, and MIT [23]. The estimated coefficient which was dependent on log P values supported our results, shown in (Figure 2,3).
Phthalates are used in plastic products, cosmetics, and personal care products. According to a study on phthalates human exposure, DEP was detected in almost all types of surveyed products with the highest levels of daily exposure of 78 μg/kg bw/d. The human exposure level of the other phthalates including DEHP, DBP, and DMP was much lower compared to DEP [24]. In vitro absorption of some o-phthalate diesters was investigated by Scott et al [25]. In the study, they used human abdominal skin obtained from cadavers and rat dorsal skin. The absorption rate was found to be higher in rat skin than in human skin. The permeability constant (×10−5 cm/hr) of phthalates were as follows; DMP: 3.32±0.54, DEP 1.14±0.10, DBP: 0.23±0.06, and DEHP 0.57±0.12 in human skin. The permeability constant order in rat skin was similar to human skin, although the permeability was more than 10-fold higher in rat skin compared to human skin, and reversely dependent on the log P values. In the author’s results as shown in (Figure 2), DEP had the highest permeability constant compared to DBP, DINP, and DEHP. The log P value of DEP was the smallest compared to the other phthalates, but its permeability was highest among the phthalates tested in this experiment. Although MEP was not tested in the present study, the results of the relative permeability of the tested phthalates were similar to the results of Scott’s group [25]. Based on the phthalates results, it seems that log P is not the only factor that determines the permeability of the artificial membrane. Molecular weight, pKa, structural characteristics are also key factors in the permeability of artificial membranes. The interaction of chemicals with lipid structure in artificial membrane also plays a key role in chemicals permeability. For example, the low permeability of DEHP was due to its interaction with the phosphatidyl choline of the membrane. The interaction of chemicals with a lipid component in the membrane may deter the transport of the chemicals and increase the retention on the membrane [26,27].
Conclusions
In conclusion, the permeability rates of parabens, bisphenols, and isothiazolinones were log P value dependent in both the gastrointestinal tract and skin while a reversely related pattern was observed in phthalates. Among the key factors determining the permeability through the artificial lipid membrane, log P values was demonstrated as the most important. However, molecular weight, pKa, structural characteristics, and the interaction of chemicals with lipid molecules also determine the permeability rate of chemicals. The data in this experiment showed that permeability through the gastrointestinal tract may be higher than the skin.
Acknowledgement
This work was supported by Korea Environment Industry & Technology Institute (KEITI) through Technology Development Project for Safety Management of Household Chemical Products, funded by the Korea Ministry of Environment (MOE) (2020002970001)
Footnotes
The authors have no conflicts of interest associated with this study.
CRediT author statement
JP: Methodology, Resources, Validation, Formal analysis; Writing-Original draft preparation; HL: Methodology, Writing-Review & Editing, Resource, Investigation, KP: Supervision, Writing-Review & Editing, Project administration
References
- 1.Li D, Suh S. Health risks of chemicals in consumer products: A review. Environ Int. 2019;123:580–587. doi: 10.1016/j.envint.2018.12.033. [DOI] [PubMed] [Google Scholar]
- 2.Trantallidi M, Dimitroulopoulou C, Wolkoff P, Kephalopoulos S, Carrer EP. EPHECT III: Health risk assessment of exposure to household consumer products. Sci Total Environ. 2015;536:903–913. doi: 10.1016/j.scitotenv.2015.05.123. [DOI] [PubMed] [Google Scholar]
- 3.Silva V, Silva C, Soares P, Garrido EM, Borges F, Garrido J. Isothiazolinone biocides: chemistry, biological, and toxicity profiles. Molecules. 2020;25(4):991. doi: 10.3390/molecules25040991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Harley KG, Berger KP, Kogut K, Parra K, Lustig RH, Greenspan LC, et al. Association of phthalates, parabens and phenols found in personal care products with pubertal timing in girls and boys. Hum Reprod. 2019;34(1):109–117. doi: 10.1093/humrep/dey337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jolliet O, Ernstoff AS, Csiszar SA, Fantke P. Defining product intake fraction to quantify and compare exposure to consumer products. Environ Sci Technol. 2015;49(15):8924–8931. doi: 10.1021/acs.est.5b01083. [DOI] [PubMed] [Google Scholar]
- 6.Shaaban H, Alhajri W. Usage patterns of cosmetic and personal care products among female population in saudi arabia: Important factors for exposure and risk assessment. J Environ Public Health. 2020 doi: 10.1155/2020/8434508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Katsikantami I, Colosio C, Alegakis A, Tzatzarakis MN, Vakonaki E, Rizos AK, et al. Estimation of daily intake and risk assessment of organophosphorus pesticides based on biomonitoring data – the internal exposure approach. Food chem toxicol. 2019;123:57–71. doi: 10.1016/j.fct.2018.10.047. [DOI] [PubMed] [Google Scholar]
- 8.Sinkó B, Vizserálek G, Takács-Novák K. Skin PAMPA: Application in practice. ADMET&DMPK. 2014;2(4):191–198. doi: 10.5599/admet.2.4.150. [DOI] [Google Scholar]
- 9.Oh MH, Lee HJ, Jo SH, Park BB, Park SB, Kim EY, et al. Development of cassette PAMPA for permeability screening. Biol Pharm Bull. 2017;40(4):419–424. doi: 10.1248/bpb.b16-00755. [DOI] [PubMed] [Google Scholar]
- 10.Bujard A, Petit C, Carrupt PA, Rudaz S, Schappler J. HDM-PAMPA to predict gastrointestinal absorption, binding percentage, equilibrium and kinetics constants with human serum albumin and using 2 end-point measurements. Eur J Pharm Sci. 2017;97:143–150. doi: 10.1016/j.ejps.2016.11.001. [DOI] [PubMed] [Google Scholar]
- 11.Li H, Zeng H, He D, Wang M, Liu L, Liang W, et al. A new approach to examining the extraction process of Zhishi and Zhiqiao considering the synergistic effect of complex mixtures by PAMPA. J Chromatogr B. 2018;1099:10–17. doi: 10.1016/j.jchromb.2018.09.017. [DOI] [PubMed] [Google Scholar]
- 12.Yu H, Wang Q, Sun Y, Shen M, Li H, Duan Y. A New PAMPA model proposed on the basis of a synthetic phospholipid membrane. PLoS One. 2015;10(2):e0116502. doi: 10.1371/journal.pone.0116502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Darbre PD, Harvey PW. Paraben esters: review of recent studies of endocrine toxicity, absorption, esterase and human exposure, and discussion of potential human health risks. J appl toxicol. 2008;28(5):561–578. doi: 10.1002/jat.1358. [DOI] [PubMed] [Google Scholar]
- 14.Martins I, Lambert M, Pereira AFS, Faria HDD, Lima ECD, Pereira GR. Serum analysis in women and in vitro skin assay for the assessment of exposure to parabens in antiperspirants. Environ Sci Pollut Res Int. 2020;27(4):4219–4226. doi: 10.1007/s11356-019-07038-1. [DOI] [PubMed] [Google Scholar]
- 15.Akomeah F, Nazir T, Martin GP, Brown MB. Effect of heat on the percutaneous absorption and skin retention of three model penetrants. Eur J Pharm Sci. 2004;21(2–3):337–345. doi: 10.1016/j.ejps.2003.10.025. [DOI] [PubMed] [Google Scholar]
- 16.Whitworth CW, Jun HW. Influence of polysorbate 20 and sodium cholate on uptake of p-hydroxybenzoates by the frog, Rana pipiens. Journal Pharmaceutical Sciences. 1973;62(11):1890–1891. doi: 10.1002/jps.2600621139. [DOI] [PubMed] [Google Scholar]
- 17.Lakeram M, Lockley DJ, Sanders DJ, Pendlington R, Forbes B. Paraben transport and metabolism in the biomimetic artificial membrane permeability assay (BAMPA) and 3-Day and 21-Day Caco-2 cell systems. J biomol screen. 2007;12(1):84–91. doi: 10.1177/1087057106295383. [DOI] [PubMed] [Google Scholar]
- 18.Köllmer M, Mossahebi P, Sacharow E, Gorissen S, Gräfe N, Evers DH, et al. Investigation of the compatibility of the skin PAMPA model with topical formulation and acceptor media additives using different assay setups. AAPS PharmSciTech. 2019;20(2):1–10. doi: 10.1208/s12249-019-1305-3. [DOI] [PubMed] [Google Scholar]
- 19.Caon T, Costa ACO, Oliveira MALD, Micke GA, Simões CMO. Evaluation of the transdermal permeation of different paraben combinations through a pig ear skin model. Int J Pharm. 2010;391(1–2):1–6. doi: 10.1016/j.ijpharm.2010.02.006. [DOI] [PubMed] [Google Scholar]
- 20.Reale E, Vernez D, Hopf NB. Skin absorption of Bisphenol A and its alternatives in thermal paper. Ann Work Expo Health. 2021;65(2):206–218. doi: 10.1093/annweh/wxaa095. [DOI] [PubMed] [Google Scholar]
- 21.Liu J, Martin JW. Comparison of bisphenol A and bisphenol S percutaneous absorption and biotransformation. Environ Health Perspect. 2019;127(6):67008. doi: 10.1289/EHP5044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Champmartin C, Marquet F, Chedik L, Décret MJ, Aubertin M, Ferrari E, et al. Human in vitro percutaneous absorption of bisphenol S and bisphenol A: A comparative study. Chemosphere. 2020;252:126525. doi: 10.1016/j.chemosphere.2020.126525. [DOI] [PubMed] [Google Scholar]
- 23.Garcia-Hidalgo E, Schneider D, Goetz NV, Delmaar C, Siegrist M, Hungerbühler K. Aggregate consumer exposure to isothiazolinones via household care and personal care products: Probabilistic modelling and benzisothiazolinone risk assessment. Environ Int. 2018;118:245–256. doi: 10.1016/j.envint.2018.05.047. [DOI] [PubMed] [Google Scholar]
- 24.Koniecki D, Wang R, Moody RP, Zhu J. Phthalates in cosmetic and personal care products: concentrations and possible dermal exposure. Environ Res. 2011;111(3):329–336. doi: 10.1016/j.envres.2011.01.013. [DOI] [PubMed] [Google Scholar]
- 25.Scott RC, Dugard PH, Ramsey JD, Rhodes C. In vitro absorption of some o-phthalate diesters through human and rat skin. Environ Health Perspect. 1987;74:223–227. doi: 10.1289/ehp.8774223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bailey-Hytholt CM, Puranik T, Tripathi A, Shukla A. Investigating interactions of phthalate environmental toxicants with lipid structures. Colloids Surf B Biointerfaces. 2020;190:110923. doi: 10.1016/j.colsurfb.2020.110923. [DOI] [PubMed] [Google Scholar]
- 27.Kwon JH, Katz LE, Liljestrand HM. Use of a parallel artificial membrane system to evaluate passive absorption and elimination in small fish. Environ Toxicol Chem. 2006;25(12):3083–3092. doi: 10.1897/06-013R.1. [DOI] [PubMed] [Google Scholar]