ABSTRACT
Background
There are limited data from randomized control trials to support or refute the contention that whole-grains can enhance protein metabolism in humans.
Objectives
To examine: 1) the clinical effects of a whole-grain diet on whole-body protein turnover; 2) the cellular effects of whole-grains on protein synthesis in skeletal muscle cells; and 3) the population effects of whole-grain intake on age-related muscle loss.
Methods
Adults with overweight/obesity (n = 14; age = 40 ± 7 y; BMI = 33 ± 5 kg/m2) were recruited into a crossover, randomized controlled trial (NCT01411540) in which isocaloric, macronutrient-matched whole-grain and refined-grain diets were fully provisioned for two 8-wk periods. Diets differed only in the presence of whole-grains (50 g/1000 kcal). Whole-body protein kinetics were assessed at baseline and after each diet in the fasted-state (13C-leucine) and integrated over 24 h (15N-glycine). In vitro studies using C2C12 cells assessed global protein synthesis by surface sensing of translation and anabolic signaling by Western blot. Complementary epidemiological assessments using the NHANES database assessed the effect of whole-grain intake on muscle function assessed by gait speed in older adults (n = 2783).
Results
Integrated 24-h net protein balance was 3-fold higher on a whole-grain diet compared with a refined-grain diet (P = 0.04). A whole-grain wheat extract increased submaximal rates of global protein synthesis (27%, P < 0.05) in vitro. In a large sample of older adults, whole-grain intake was associated with greater muscle function (OR = 0.92; 95% CI: 0.86, 0.98).
Conclusions
Consuming 50 g/1000 kcal whole-grains per day promotes greater protein turnover and enhances net protein balance in adults. Whole-grains impact skeletal muscle at the cellular level, and are associated with greater muscle function in older adults. Collectively, these data point to a new mechanism whereby whole-grain consumption favorably enhances protein turnover and improves health outcomes.
This clinical trial is registered on clinicaltrials.gov (identifier: NCT01411540).
Keywords: grain, polyphenol, phytonutrient, phytochemical, botanical, sarcopenia, muscle function, processed food, nitrogen, walking speed
Whole-body protein balance improves when consuming a whole-grain diet compared with a macronutrient-matched refined-grain diet. Further, whole-grains increase protein synthesis in muscle cells and are related to greater muscle function in older adults.
Introduction
The goal of replacing refined-grains with whole-grains is a pillar of many national dietary guidelines (1). Strong epidemiological evidence across diverse populations and cultures suggests whole-grains improve body composition (2–4). Body composition, as determined by the proportion of lean body mass, is regulated by whole-body protein turnover (WBPT) (5, 6). A small intervention study intriguingly found that a whole-grain–rich diet had a favorable effect on protein turnover compared with a refined-grain diet (7). Whole-grains have also been found to impact blood glucose regulation and insulin secretion (8, 9), with insulin being a key regulator of protein turnover (10). Intervention trial results on body composition (11, 12), blood glucose and insulin (9, 13), and metabolomic markers of protein metabolism all point to a potential acute benefit of replacing refined-grains with whole-grains on protein turnover and lean mass; however, this remains to be empirically confirmed. Indeed, well-controlled clinical trials investigating the effect of whole-grains on muscle mass or protein turnover are sparse and inconclusive (7, 11, 12, 14).
Despite the prevailing epidemiological data that whole-grains improve body composition, it is less clear whether whole-grain intake per sehas a physiologically meaningful impact on muscle function. The age-related loss of muscle function is an increasing clinical concern and negatively impacts mobility (15), quality of life (16), and mortality (17). This age-related loss of muscle function results from chronic, imbalanced protein turnover, where rates of protein breakdown chronically surpass rates of protein synthesis, culminating in a negative net protein balance and loss of body protein (18). Novel approaches to address the age-related loss of muscle function are a clinical and research need, because classical nutritional interventions that positively impact protein turnover (e.g., increasing protein and energy intake) have shown little effect on muscle function (19–21).
Given the absence of level 1 evidence that whole-grains can positively impact protein turnover, we implemented a multilevel approach, which used a randomized controlled feeding trial, mechanistic cell culture studies, and epidemiology to understand the effect of whole-grains on protein turnover. We hypothesized a priori that when whole-grain intake meets the USDA dietary recommendations (22), protein turnover would be increased along with net protein balance when compared with a macronutrient-matched refined-grain diet. Following with anin vitro approach, we hypothesized that a whole-grain wheat extract would increase skeletal muscle protein synthesis at the cellular level by activating anabolic signaling pathways [e.g., protein kinase B (Akt)/mammalian target of rapamycin (mTOR)]. We then tested an exploratory hypothesis that whole-grains would be associated with greater muscle function in older adults.
Methods
Study design
This human clinical trial involved a double-blind, crossover, randomized controlled trial, to determine the effect of a whole-grain or refined-grain diet on total protein turnover in adults with overweight/obesity using 2 different stable isotope tracers. The study diets were fully provided and consumed in free-living conditions. This study was approved by the Institutional Review Board at the Cleveland Clinic and registered at clinicaltrials.gov (NCT01411540). The trial included a multilevel evaluation of cardiovascular (23), glucose metabolism (9, 13), and protein metabolism outcomes. Cell culture studies were performed in muscle cell lines (C2C12 myotubes) using the surface sensing of translation (SUnSET) method (24) in combination with in-cell Western and dual-channel near-infrared imaging to investigate global protein synthesis along with standard Western blotting to investigate protein synthesis signaling pathways. We used the publicly available NHANES database to examine whole-grain intake in a clinically relevant population at risk of developing impaired muscle function, a symptom of chronic, imbalanced protein turnover.
Human clinical trial
Recruitment and group designations
Participants (n = 14) were recruited from the Greater Cleveland area (Cleveland, OH). Inclusion criteria were: age 20–50 y, BMI 25–38 kg/m2, low normal whole-grain intake [<16 g whole-grain ingredients per day, consistent with the average whole-grain intake of the US population (25)], weight stable within 2 kg over the previous 6 mo, and a sedentary lifestyle (<1 h physical activity per week). Additionally, women were premenopausal and measures were obtained during their midfollicular phase (26). Exclusion criteria included smoking, fasting blood glucose >126 mg/dL, prior diagnosis of chronic illness (e.g., kidney, liver, pulmonary diseases), alcohol intake >7 drinks/wk, and food allergies not compatible with the study diets (e.g., peanut allergy). Participants were randomly assigned a priori to protein turnover assessments as described below, and were also part of the cardiovascular outcomes assessment as previously described (23). All participants signed informed consent prior to the initiation of the study procedures.
Study diets
Participants were randomly allocated to either a whole-grain–enriched or refined-grain diet for 8 wk, followed by a washout period of ≥10 wk prior to receiving the alternate diet. Diets were matched for macronutrient composition and were isocaloric for each individual participant. Caloric needs were calculated from resting energy requirements measured by indirect calorimetry (Vmax Encore; Viasys SensorMedics) multiplied by a sedentary physical activity factor of 1.3. The provisional diets consisted of nonwater beverages, fresh produce, prepackaged items, and frozen meals. Diet compliance was monitored by food weigh-backs.
The study diets were designed to meet the dietary guidelines for macronutrient, vitamin, and mineral intake for adults. Diets were closely matched for macronutrient intake, differing primarily in the presence of whole-grains at 50 g/1000 kcal in the whole-grain diet and 0 g/1000 kcal in the refined-grain diet. This level of whole-grain intake represents the highest level of intake recommended by the USDA (22) (100 g or 6 servings on a 2000 kcal/d diet). Whole-grain intake was modified through entrées, breakfast cereals, and cereal bars that differed in whole-grain or refined-grain content between each diet. Entrées were sourced from Nestlé Product Technology Center. Macronutrient and energy contents of the diets have been previously published for other study outcomes (13, 23). The main grains included wheat (57%), rice (21%), and oats (16%). Diet analysis was performed using the Food Processor Nutritional Analysis Pro version 10.80 (ESHA Research).
WBPT
WBPT was determined in both the fasted state and integrated over 24 h. Prior to WBPT assessment, subjects arrived at the Clinical Research Unit for a 3-d inpatient stay. Fasted-state WBPT was determined from leucine kinetics using a primed, constant infusion of l-[1-13C]-leucine (0.32 mg/kg/h). Leucine flux was determined from the plasma enrichment of labeled α-ketoisocaproic acid measured by GC-MS. Oxidation of leucine was measured in breath samples using isotope ratio MS following an NaH13CO3 bolus to prime body carbon dioxide pools. The rate of leucine incorporation into protein was calculated from total leucine flux minus leucine oxidation (27). Leucine kinetics were measured from samples obtained during the steady state of the infusion protocol (5 plasma samples, 3.0 h, 3.25 h, 3.5 h, 3.75 h, 4.0 h) and calculated as described by Matthews et al. (27) by determining leucine turnover, leucine incorporated into protein (as a measure of whole-body protein synthesis), and leucine release from protein (as a measure of whole-body protein breakdown). These outputs were reported as micromoles leucine per kilogram fat-free mass (FFM) per hour. Integrated WBPT was determined from glycine kinetics using a 15N-glycine–enriched glucose solution (80 mg labeled glycine). Following an overnight fast, at 06:30, subjects were weighed and provided baseline urine samples. At 07:00 subjects consumed the 15N-glycine–enriched glucose solution and were subsequently provided an isocaloric breakfast, lunch, and dinner. Urine for integrated WBPT was collected and pooled. Glycine kinetics were determined by enrichment of urea and ammonia and calculated using the end-product method (28). Rates of protein turnover, synthesis, and breakdown are reported as milligrams protein per kilogram FFM per hour, using the conversion factor of 1 g nitrogen per 6.25 g protein. Note: due to difficulties in sample collection and analysis, glycine flux data were not available for 3 of the participants.
In vitro experiments
SUnSET
Because wheat was the main grain consumed in the clinical trial, we investigated the effect of a whole-grain wheat extract on skeletal muscle protein synthesis in C2C12 skeletal muscle cells using the SUnSET method in combination with the in-cell Western technique (24). C2C12 cells were placed in growth medium (DMEM, 10% FBS, 1% antibiotic) on a 96-well plate (Nunc MicroWell 96-Well, Poly-D Lysine-Treated, Flat-Bottom, Optical Polymer Base Microplate; Thermo Scientific) (29). When cells reached ∼80% confluence, growth medium was replaced with differentiation medium (DMEM supplemented with 2% horse serum and 1% antibiotic, 0.1% BSA) for 48 h, which is sufficient to produce myotubes throughout the culture (30, 31). After 48 h differentiation, myotubes were serum-starved in DMEM without amino acids for 1 h followed by an additional 2-h treatment under the following conditions: Control (HBSS), Low (DMEM without amino acids), Medium (DMEM with amino acids), or Max (DMEM with amino acids, supplemented with 4 mM leucine, 2 mM isoleucine, 2 mM valine, and 1.5 nM insulin). Conditions for stimulating protein synthesis in vitro were selected based on previously published reports (32–35). All conditions were repeated with the addition of a whole-grain botanical wheat bran extract for the treatment duration (0.25% v/v, supercritical carbon dioxide extract of wheat-bran, 180.003; FLAVEX). Experiments were conducted on 3 separate occasions with 4 technical replicates in each batch. For the final 30 min of treatment, puromycin was added to a final concentration of 1 µM. Cells were washed in PBS and fixed in 150 µL formalin (4% formaldehyde) for 20 min at room temperature. Fixed cells were washed in PBS and incubated in 200 µL of a permeabilization and blocking solution [Odyssey Blocking Buffer (OBB) 927-50000; LI-COR Biosciences) with 0.3% Triton-X for 30 min. Cells were incubated overnight at 4°C on a plate shaker (low setting) in the primary antibody solution (OBB, 1:5000 antipuromycin, 1:2500 anti-GAPDH). After overnight incubation, cells were washed 3 × 5 min in Tris-buffered saline with 0.1% Tween 20 (TBST) and placed in secondary antibody solution (OBB, 1:10,000 anti-rabbit, 1:20,000 anti-mouse) for 60 min. Cells were washed 3 × 5 min in TBST followed by 15 min in TBS to remove residual Tween. Puromycin protein labeling was quantified with near-infrared fluorescence imaging (Odyssey Clx; LI-COR Biosciences) using Image Studio software (v5.2, LI-COR Biosciences).
Intracellular signaling
Protein synthesis is regulated at the cellular level through Akt/mTOR signaling. Akt/mTOR signaling in skeletal muscle was investigated using standard Western blotting procedures. C2C12 cells were grown, differentiated, and treated in the same conditions described above for the SUnSET assay with the following modifications: cells were cultured in 12-well plates, and experiments were repeated on 4 separate occasions with 3 technical replicates in each batch. After treatment, cells were harvested at 30 and 120 min in ice-cold lysis buffer (C3228; Sigma-Aldrich) with 1:300 protease and phosphatase inhibitor (Halt Protease and Phosphatase Inhibitor Cocktail; Thermo Scientific) using the cell scraping method. Total protein concentration was determined by BCA Protein Assay (Pierce Biotechnology). For each condition, 20 µg protein was separated via SDS-PAGE, transferred to nitrocellulose membranes, and blocked in OBB prior to incubating in primary and secondary antibodies. Gel electrophoresis, transfer, and blotting conditions were optimized individually for each protein of interest and loading controls; a list of antibodies is provided in Supplemental Table 1. Protein visualization and quantification were performed on the Odyssey CLx System (LI-COR Biosciences).
Ultra performance liquid chromatography/MS analysis of the whole-grain wheat extract
Ultra performance liquid chromatography (UPLC)/MS analysis was conducted as previously reported (36). Compounds in samples were separated and analyzed by a UPLC/MS system including the Dionex UltiMate 3000 RSLC ultra-high-pressure LC system, consisting of a workstation with Xcalibur v. 4.0 software package (ThermoFisher Scientific) combined with SII LC control software, solvent rack/degasser SRD-3400, pulseless chromatography pump HPG-3400RS, autosampler WPS-3000RS, column compartment TCC-3000RS, and photodiode array detector DAD-3000RS (all by Dionex). After the photodiode array detector, the eluent flow was guided to a Q Exactive Plus Orbitrap high-resolution high-mass-accuracy mass spectrometer. Mass detection was based on a full MS scan with low-energy collision-induced dissociation from 100 to 1000 m/z in negative ionization mode with electrospray interface. Sheath gas flow rate was 30 arbitrary units, auxiliary gas flow rate was 7, and sweep gas flow rate was 1. The spray voltage was −3500 V with a capillary temperature of 275°C. The mass resolution was ≥70,000. Substances were separated on a Kinetex C8 reverse-phase column (Phenomenex), size 100 × 2 mm, particle size 2.6 mm, pore size 100 Å. The mobile phase consisted of 2 components: solvent A [0.5% American Chemical Society (ACS) grade acetic acid in LC-MS grade water, pH 3–3.5), and solvent B (100% acetonitrile, LC-MS grade]. The mobile phase flow was 0.20 mL/min, and a gradient mode was used for all analyses. The initial conditions of the gradient were 95% A and 5% B; for 30 min the proportion reached 5% A and 95% B, which was kept for the next 8 min, and during the subsequent 4 min the ratio was brought to initial conditions. An 8-min equilibration interval was included between injections. The average pump pressure using these parameters was typically ∼3900 psi for the initial conditions. Samples were prepared by dissolving 10 mg wheat oil in 1 mL 90% ethanol. 1 µL of the sample was injected for the UPLC-MS analysis. Putative formulae of wheat oil components were determined by performing isotope abundance analysis on the high-resolution mass spectral data with Xcalibur v. 4.0 software and reporting the best fitting empirical formula. Database searches were performed using www.reaxys.com (Elsevier RELX Intellectual Properties SA) and SciFinder (ACS).
Epidemiological investigation
The NHANES database was interrogated for the effect of whole-grain intake on muscle function. NHANES is a nationally representative survey of the US population conducted as approved by the National Center for Health Statistics Research Ethics Review Board; subjects provided informed consent to participate and the data are publicly available (37). We assessed deidentified data from 2783 older adults (age >65 y) participating in NHANES that provided both 24-h dietary recalls and gait speed assessments along with demographic and anthropometric information, including age, weight, sex, and race/ethnicity according to NHANES protocols. Based on these criteria, the NHANES cohorts were limited to the years 1999–2000 and 2001–2002. Participants were categorized into 2 groups according to gait speed: Impaired Muscle Function, gait speed <1 m/s; and Healthy, gait speed >1 m/s (38, 39). Gait speed output was converted to meters per second from NHANES data (time to complete a 20 ft walk (s): MSXWTIME for 1999–2000 and MSXW20TM for 2001–2002). Due to the well-established effect of amino acid availability on protein synthesis (40), emerging evidence that high-protein meal intake impacts skeletal muscle protein turnover (41), and our in vitro results suggesting whole-grains potentiate responses to protein (amino acid) stimuli, we selected to control for protein intake by quantifying habitual high-protein meal intake (>10 g protein per meal; 3 high-protein meals per day used as the reference). Multiple logistic regression modeling was used to evaluate muscle function, while accounting for age, sex, high-protein meal intake, and total energy intake. Data are reported as ORs with 95% CIs.
Statistical analysis
Statistical analyses were performed using PRISM8 (GraphPad) and SAS version 9.4 (SAS Institute). Linear mixed model regression was used to detect the effect of a whole-grain compared with a refined-grain diet on the outcomes of interest, while adjusting for period, age, sex, race, and body weight change. A carryover effect was not found to be significant for any of the outcome variables evaluated. Within-diet effects were assessed using paired Student's t-test. The effect of a whole-grain extract in vitro was assessed by 1-factor ANOVA with Fisher's least significant difference post hoc test. The effect of whole-grain intake on muscle function was determined using the NHANES database and multiple logistic regression modeling. Logistic regression model assumptions were not externally validated. Data are expressed as mean ± SD. Significance was accepted at P < 0.05.
Results
Subject characteristics, diet, and compliance
Subject characteristics and baseline measures are presented in Table 1. Study diets were well matched for total energy, macronutrients, and sugar content, whereas fiber intake was higher when consuming the whole-grain diet (Table 2). Compliance was high and equivalent between both diets. Participants in the 2 diet groups exhibited similar weight and body composition changes through each diet arm (Table 3). Both diets resulted in a mild weight loss over 8 wk (whole-grain: −1.8 ± 2.4 kg; refined-grain: −3.1 ± 3.8 kg; both P = 0.01 compared with week 0). The magnitude of weight change was not different between the 2 diets (P = 0.36).
TABLE 1.
Baseline subject characteristics1
| Characteristic | Value |
|---|---|
| n (sex) | 14 (3M, 11F) |
| Race H/AA/W | 1/9/4 |
| Age, y | 40 ± 7 |
| BMI, kg/m2 | 33.2 ± 4.7 |
| Glucose, mg/dL | 95 ± 20 |
| HbA1c, % | 5.9 ± 0.7 |
| Total cholesterol, mg/dL | 166 ± 20 |
| Triglycerides, mg/dL | 98 ± 45 |
| Total protein, g/dL | 6.9 ± 0.3 |
| Albumin, g/dL | 4.0 ± 0.2 |
| Blood urea nitrogen, mg/dL | 13 ± 2 |
| Creatinine, mg/dL | 0.7 ± 0.1 |
| Whole-body protein turnover, fasted state, µmol Leu/kgFFM/h | |
| Synthesis | 190 ± 39 |
| Breakdown | 217 ± 44 |
| Turnover | 222 ± 44 |
| Net balance | −28 ± 6 |
| Integrated,2 mg protein/kgFFM/h | |
| Synthesis | 289 ± 105 |
| Breakdown | 266 ± 97 |
| Turnover | 370 ± 106 |
| Net balance3 | 23 ± 21 |
Data are presented as mean ± SD. Baseline data represent the mean values of measures collected prior to the initial randomization diet and after the washout period prior to the crossover diet. AA, African American; H, Hispanic; HbA1c, glycated hemoglobin; kgFFM, kilograms fat-free mass; W, white.
Data from 3 participants were not available for integrated protein synthesis.
Net balance is net protein balance determined by subtracting protein breakdown from protein synthesis rates.
TABLE 2.
Study diets and compliance1
| Whole-grain | Refined-grain | P value | |
|---|---|---|---|
| Food energy, kcal | 2029 ± 306 | 1962 ± 266 | 0.54 |
| Protein, g | 90 ± 12 | 88 ± 11 | 0.56 |
| Fat, g | 65 ± 11 | 64 ± 10 | 0.79 |
| Saturated fat, g | 21 ± 4 | 22 ± 4 | 0.68 |
| Carbohydrates, g | 284 ± 44 | 270 ± 39 | 0.35 |
| Sugar, g | 130 ± 23 | 123 ± 25 | 0.45 |
| Fiber, g | 28 ± 4 | 20 ± 3 | <0.01 |
| Whole-grains, g | 89 ± 15 | — | — |
| Compliance, % | 97 ± 4 | 95 ± 3 | 0.09 |
Data are presented as mean ± SD. Paired t tests were used to assess differences between diets.
TABLE 3.
Body weight and composition1
| Whole-grain | Refined-grain | |||
|---|---|---|---|---|
| Week 0 | Week 8 | Week 0 | Week 8 | |
| Weight, kg | 91.1 ± 17.6 | 89.3 ± 17* | 93.2 ± 19 | 90.1 ± 17.4* |
| Fat mass, kg | 37.1 ± 8.9 | 35.8 ± 7.9t | 38.5 ± 10.3 | 36.3 ± 8.8* |
| Fat-free mass, kg | 53.6 ± 10.4 | 53.3 ± 11.4 | 54.3 ± 11.1 | 53.6 ± 10.3t |
Data are presented as mean ± SD. *,t, Different within-diet from week 0: *P < 0.05; t, P < 0.10.
Clinical trial: effect of whole-grains on WBPT
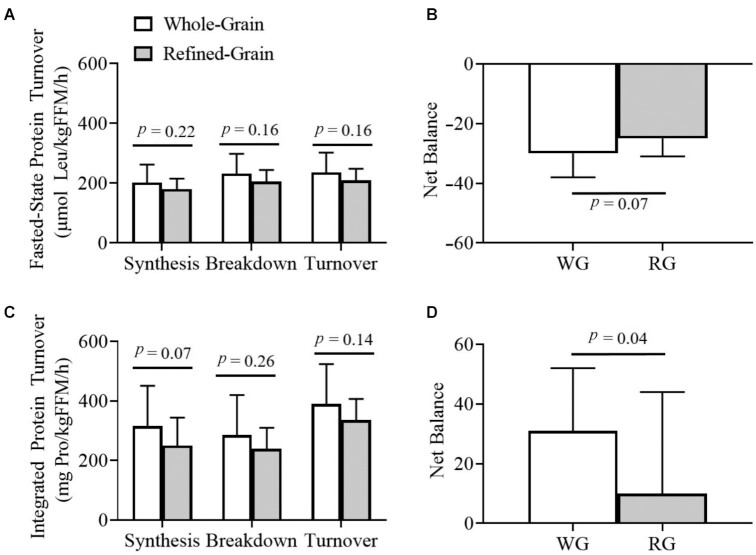
In the fasted state, WBPT, whole-body protein synthesis, and whole-body protein breakdown were 12.3–13.0% higher when consuming a whole-grain diet, but did not reach statistical significance (Figure 1A). The resultant fasted-state net balance showed a trend toward being more negative when consuming the whole-grain diet (Figure 1B). Similarly, 24-h integrated protein turnover, incorporating both fasted and fed states, was 16.1–26.5% higher when consuming the whole-grain diet, but again did not reach statistical significance (Figure 1C). Here importantly, the resultant 24-h integrated net balance was significantly greater for the whole-grain diet (Figure 1D). These data suggest that whole-grains impact WBPT primarily in response to the fed state (an anabolic stimulus).
FIGURE 1.
Fasted-state and integrated whole-body protein turnover. (A) Fasted-state leucine kinetics revealed greater turnover (WG: 235.1 ± 66.2 µM Leu/kgFFM/h; RG: 209.2 ± 39.4 µM Leu/kgFFM/h, P = 0.16, n = 14), synthesis (WG: 202.1 ± 59.8 µM Leu/kgFFM/h; RG: 179.9 ± 35.2 µM Leu/kgFFM/h, P = 0.22, n = 14), and breakdown (WG: 231.8 ± 66.2 µM Leu/kgFFM/h; RG: 205.1 ± 39.4 µM Leu/kgFFM/h, P = 0.16, n = 14) on a whole-grain diet, although these did not reach statistical significance. (B) This resulted in a trend for a more negative net protein balance on the WG diet (WG: −29.7 ± 7.8 µM Leu/kgFFM/h; RG: −25.2 ± 5.7 µM Leu/kgFFM/h, P = 0.07, n = 14). (C) Integrated glycine kinetics revealed a similar pattern for turnover (WG: 390.4 ± 134.0 mg Pro/kgFFM/h; RG: 336.4 ± 70.1 mg Pro/kgFFM/h, P = 0.14), synthesis (WG: 315.6 ± 134.7 mg Pro/kgFFM/h; RG: 249.5 ± 93.8, P = 0.07, n = 11), and breakdown (WG: 284.5 ± 135.5 mg Pro/kgFFM/h; RG: 239.3 ± 71.5 mg Pro/kgFFM/h, P = 0.26, n = 11), again not reaching statistical significance. (D) This resulted in a substantially more positive net protein balance on the WG diet (WG: 31.1 ± 21.5 mg Pro/kgFFM/h; RG: 10.3 ± 34.3 mg Pro/kgFFM/h, P = 0.04, n = 11). Data from 3 participants were not available for integrated protein synthesis. Net Balance denotes net protein balance determined by subtracting protein breakdown from protein synthesis rates. This figure represents data obtained after consuming each diet for 8 weeks. Data are presented as mean ± SD. P values were obtained from ANOVA with linear mixed model effects and were adjusted for period, age, sex, race, and body weight change. kgFFM, kilograms fat-free mass; Leu, leucine; Pro, protein; RG, refined-grain; WG, whole-grain.
In vitro experiments: effect of whole-grains on skeletal muscle protein synthesis
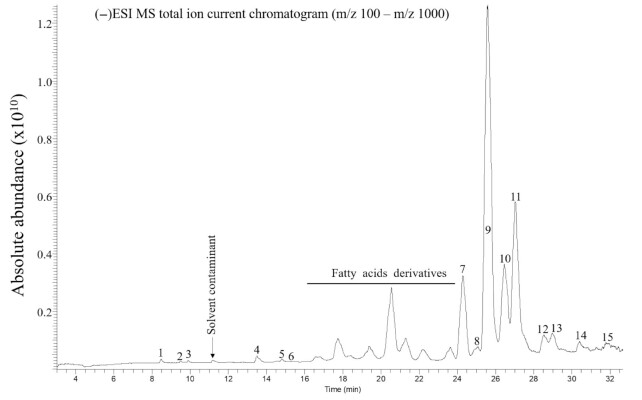
Global protein synthesis expectedly increased in response to anabolic stimuli (Low—DMEM without amino acids; Medium—DMEM with amino acids; and Max—DMEM with amino acids, plus additional branched-chain amino acids and insulin; Figure 2). With the addition of the whole-grain wheat extract, global protein synthesis was further elevated in the Low condition and showed a trend to increase in the Medium condition, but no additional increase was observed in the Max condition. MS analysis allowed us to confirm that the most abundant compounds present in the whole-grain wheat extract were linoleic, linolenic, and oleic acids along with fatty acid derivatives (Figure 3). We note that a full characterization of the extract was not carried out and therefore we cannot exclude that other compounds in the extract might also impact protein synthesis.
FIGURE 2.
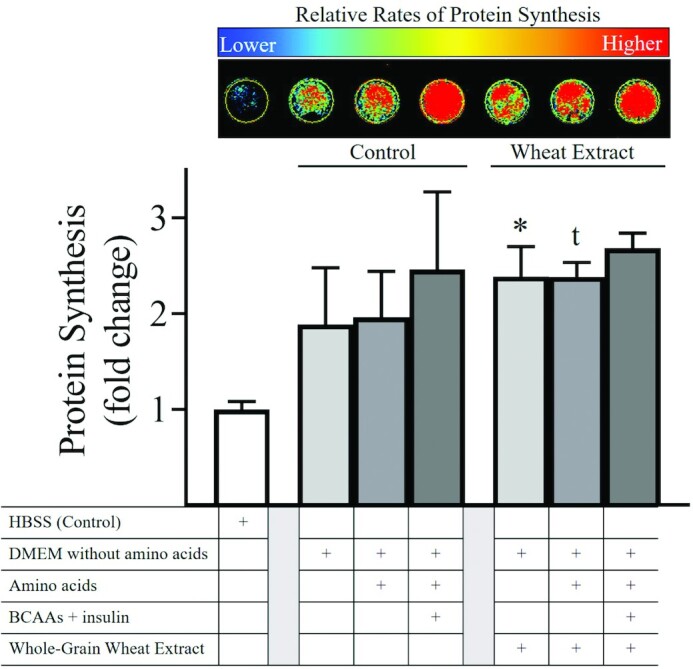
Global protein synthesisin vitro. Global protein synthesis quantified by the surface sensing of translation (SUnSET) method increased in stepwise fashion with Low, Medium, and Max conditions in relation to the HBSS in C2C12 skeletal muscle cells. The addition of a whole-grain wheat extract at 0.25% v/v further increased global protein synthesis in the Low condition, with a trend for an increase in the Medium condition, but no additional increase was observed in the Max condition. HBSS, Hanks' Balanced Salt Solution; DMEM, Dulbecco's Modified Eagle Medium; BCAA, branched-chain amino acid; Low, amino acid–free DMEM; Medium, DMEM; Max, DMEM supplemented with 4 mM leucine, 2 mM isoleucine, 2 mM valine, and 1.5 nM insulin. The whole-grain wheat extract and amino acid doses were selected based upon results from dose-response curves that showed the greatest signal for global protein synthesis quantified by the SUnSET method (data not presented). Data represent mean ± SD of the fold-change compared with the HBSS condition. *,t Different compared with matched Control condition using 1-factor ANOVA with Fisher's least significant difference post hoc test: *P < 0.05; t, P < 0.10.
FIGURE 3.
Chromatogram of whole-grain wheat extract. A sample of the whole-grain wheat extract was run in negative ionization mode [(−)ESI]. Molecules were identified putatively: 1, C26H44O6, or C27H48O5, glycosylated sesquiterpene, and/or polyhydroxylated steroid; 2, C8H12O5, shikimic acid, or quinic acid derivative; 3, C39H66O9, glycosylated sesquiterpene, or a steroid; 4–6, C18H34O5, polyhydroxylated fatty acids; 7, C18H30O2, linolenic acid; 8, C16H30O2, hexadecenoic acid/palmitoleic acid; 9, C18H32O2, linoleic acid; 10, C16H32O2, palmitic acid; 11, C18H34O2, octadecenoic acid/oleic acid; 12, C18H36O2, stearic acid; 13, C20H38O2, eicosenoic acid; 14, C25H44O2, alkylresorcinol; 15, C27H48O2, alkylresorcinol; ESI, electrospray ionization.
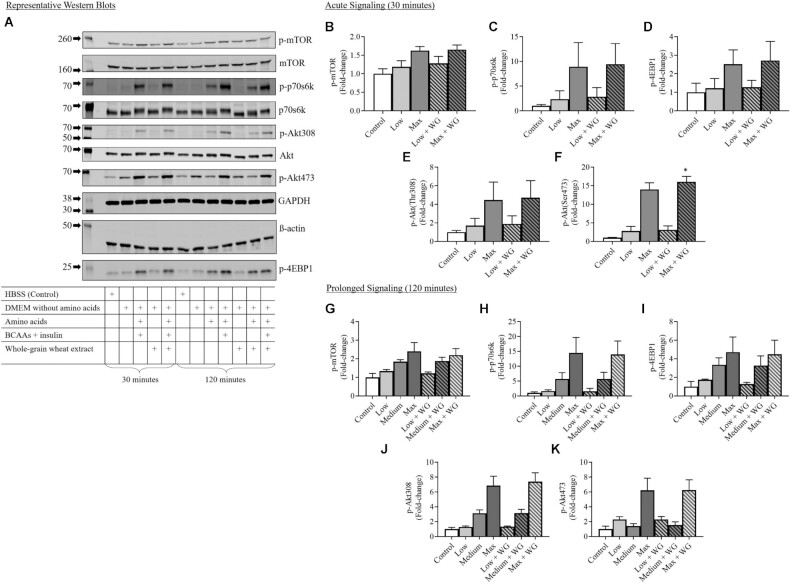
Representative western blots showing the effect of the extract on the skeletal muscle Akt/mTOR signaling pathway are presented in Figure 4A. Phosphorylated proteins are presented as the ratio of phosphorylated to total signal for each target, with the exception of phosphorylated eukaryotic translation initiation factor 4E-binding protein 1 (p-4EBP1), which was normalized to GAPDH. Acute signaling events (30-min treatment) increased in stepwise fashion with Low and Max protein synthesis stimuli and were not further elevated by the addition of the whole-grain extract (Figure 4B–E) The exception was Akt(Ser473), which was further elevated in the Max condition with the addition of a whole-grain extract (Figure 4F). Prolonged signaling events (120-min treatment) increased in a similar stepwise fashion with Low, Medium, and Max protein synthesis stimuli and were not further elevated by the addition of the whole-grain extract (Figure 4G–K).
FIGURE 4.
Intracellular Akt/mTOR signaling pathway. (A) Representative western blot images for proteins of interest. (B–F) Acute (30-min) Akt/mTOR signaling. (G–K) Prolonged (120-min) Akt/mTOR signaling. The effect of a whole-grain extract in vitro was assessed by 1-factor ANOVA with Fisher’s LSD post hoc test. *, P < 0.05 compared with the same condition without whole-grain wheat extract. WG, whole-grain wheat extract supplemented at 0.25% v/v; HBSS, Hanks' Balanced Salt Solution; DMEM, Dulbecco's Modified Eagle Medium; BCAA, branched-chain amino acids supplemented at 4 mM leucine, 2 mM isoleucine, 2 mM valine; Low, DMEM without amino acids; Medium, DMEM; Max, DMEM supplemented with BCAA and 1.5 nM insulin; Akt, protein kinase B; mTOR, mammalian target of rapamycin; p70s6k, ribosomal protein S6 kinase beta-1; 4EBP1, eukaryotic translation initiation factor 4E-binding protein 1; p-, phosphorylated.
Whole-grain intake and muscle function in older adults
Physical characteristics and nutrition information for the participants are presented in Table 4. Records of 2783 individuals were identified for adults aged >65 y in the NHANES database where diet records and gait speed data were available for each participant. Average whole-grain intake in all groups was less than what is recommended in the USDA Dietary Guidelines for Americans (Male, Healthy: 0.97 ± 1.43 servings/d; Male, Impaired Muscle Function: 0.79 ± 1.31 servings/d; Female, Healthy: 0.77 ± 1.11 servings/d; Female, Impaired Muscle Function: 0.68 ± 1.05 servings/d; conversion factor, 1 serving = 16 g whole-grains) (22). The logistic regression model to assess the effect of whole-grain intake on muscle function is presented in Table 5. Expectedly, age (OR = 1.09; 95% CI: 1.08, 1.11), male sex (OR = 1.20; 95% CI: 1.01, 1.43), and consuming <3 high-protein meals per day [2: OR = 1.64 (95% CI: 1.26, 2.14); 1: OR = 2.17 (95% CI: 1.61, 2.92); 0: OR = 3.83 (95% CI: 2.09, 7.03)] were all associated with slower gait speed, whereas higher daily energy intake (OR = 0.87; 95% CI: 0.76, 0.99) and whole-grain intake (OR = 0.92; 95% CI: 0.86, 0.98) were associated with faster gait speed.
TABLE 4.
NHANES subject characteristics1
| Male | Female | |||
|---|---|---|---|---|
| Healthy | Impaired muscle function | Healthy | Impaired muscle function | |
| n | 558 | 829 | 461 | 935 |
| Age, y | 68.5 ± 6.6 | 72.9 ± 7.8 | 68.1 ± 6.5 | 72.7 ± 8.1 |
| BMI, kg/m2 | 27.9 ± 4.4 | 27.8 ± 5 | 27.5 ± 5.1 | 29.2 ± 6.5 |
| Gait speed, m/s | 1.2 ± 0.1 | 0.8 ± 0.2 | 1.2 ± 0.2 | 0.7 ± 0.2 |
| Protein intake, g/d | 82.8 ± 36.6 | 74.0 ± 36.0 | 61.5 ± 26.8 | 56.3 ± 24.5 |
| Energy, kcal/d | 2116.7 ± 800.5 | 1859.1 ± 882.1 | 1583.1 ± 586.5 | 1445.9 ± 566.6 |
| Whole-grain intake, servings/d | 0.97 ± 1.43 | 0.79 ± 1.31 | 0.77 ± 1.11 | 0.68 ± 1.05 |
Data are presented as mean ± SD.
TABLE 5.
Effect of whole-grain intake on impaired muscle function1
| Factor | OR (95% CI) |
|---|---|
| Age | 1.09 (1.08, 1.11) |
| Sex | 1.20 (1.01, 1.43) |
| BMI | 1.06 (1.04, 1.08) |
| Daily meal(s) with high protein intake (>10 g) | |
| Three meals | Reference |
| None | 3.83 (2.09, 7.03) |
| One meal | 2.17 (1.61, 2.92) |
| Two meals | 1.64 (1.26, 2.14) |
| Energy | 0.87 (0.76, 0.99) |
| Whole-grains | 0.92 (0.86, 0.98) |
Male sex and 3 daily meals with high protein intake (>10 g) were used as reference values for categorical variables.
Discussion
Our randomized controlled trial revealed that when adults with overweight/obesity consume a whole-grain diet, WBPT and 24-h integrated net protein balance are increased compared with a carefully matched refined-grain diet. To support these findings, we obtained in vitro evidence that a whole-grain wheat extract increases skeletal muscle global protein synthesis rates in response to anabolic stimuli. To complement these 2 sets of data we found that whole-grain intake is associated with greater muscle function in older adults. Together, these data corroborate the view that whole-grain intake positively impacts short-term WBPT with a potential to impart long-term physiological benefits.
The comparison of WBPT between whole-grain and refined-grain diets is novel and important. To the best of our knowledge, this report is the first to characterize fasted- and fed-state WBPT using 2 stable isotope tracers in combination with a highly controlled, fully provisioned crossover feeding trial design. When consuming the whole-grain diet, WBPT, whole-body protein synthesis, and whole-body protein breakdown rates were all consistently elevated by ∼10–20% in both the fasted-state and integrated over fed-fasted cycles compared with the refined-grain diet, although none of these differences reached statistical significance. The resultant net protein balance showed a trend to be more negative in the fasted-state, but was significantly more positive when integrated over fed-fasted cycles when consuming the whole-grain diet. Although this phenomenon might appear counterintuitive, it is observed in protein turnover studies where exercise was used to stimulate protein synthesis (42–44), and suggests a physiological response, whereby a protein synthesis stimulus increases net protein balance only when sufficient dietary protein is also available, as is the case in the fed, but not fasted, states (45, 46). This concept has precedent in the literature, because diets that result in a more positive integrated net balance also display greater fasted-state losses and accentuated fed-state gains (47). Analyzing our results in this context suggests that whole-grain intake increases WBPT, manifesting in an augmented positive net protein balance only when the response to meals (fed state) is considered. It is therefore intriguing to postulate that whole-grain intake can increase whole-body protein balance by accentuating the anabolic response to a meal; however, this remains to be empirically tested.
The central finding of the clinical trial was the elevation of net protein balance when consuming a whole-grain diet, in agreement with our hypothesis. However, these results should be interpreted in the context of several limitations. Mild weight loss (∼0.3 kg/wk) was observed on both whole-grain and refined-grain diets, indicating that the participants were in a slight energy deficit throughout the study. Body composition was similar between diets in this trial, and although other clinical trials support improved body composition when consuming a whole-grain diet (11, 14), a meta-analysis of relevant clinical trials was inconclusive (48). Our study population had more females, but based on available literature (49, 50), we do not anticipate any sex-related differences on protein turnover. However, due to our small sample size and the predominance of female participants, we are unable to assess this directly. We also used an end point analysis for protein turnover because protein intake of the participants’ habitual diets was not quantified prior to study enrollment or during the washout period; habitual diet protein intake might independently impact WBPT and urea space (47, 51, 52) (which we assumed to be constant). Despite these limitations, this clinical feeding trial provides important advances regarding a novel role of whole-grains on whole-body protein metabolism.
We also hypothesized that the underlying biological mechanism by which whole-grains impact protein turnover should involve skeletal muscle protein synthetic responses to anabolic stimuli. Using a representative whole-grain wheat extract we found that global protein synthesis was increased when a nutritional stimulus of protein synthesis was applied in combination with the extract. Notably, the extract did not augment skeletal muscle protein synthesis when both amino acids and insulin were used as stimuli. This suggests maximal protein synthesis rates were not further increased by whole-grains and that the effect of whole-grains on protein synthesis could have an upper limit. We conducted these experiments using an immortalized C2C12 skeletal muscle cell line, which might limit the translation to human skeletal muscle cells due to phenotypic and metabolic differences between C2C12 and primary human skeletal muscle cells (53). Still, these findings are important, because the classical view of the nutritional regulators of skeletal muscle growth and protein synthesis are macronutrient-focused (total energy and protein intake) (54–56), whereas our data suggest that nonmacronutrient factors present in whole-grains could also play a role.
Whole-grains are composed of 3 primary components: the bran, germ, and the starchy endosperm (57). The bran and germ are rich sources of proteins, lipids, fiber, and a wide range of phytochemicals. The bran and germ are removed from whole-grains during conventional milling practices in the processing of refined-grain products, leaving only the starchy endosperm. Phytochemicals present in the whole-grain's bran and germ are also removed in this milling process. However, because fiber is the most abundant nutrient lost during the processing of whole-grain to refined-grain flour, research attention on whole-grains has focused on health effects associated with dietary fiber (23, 58–60). A provocative implication is that our results support the view that phytochemicals present in whole-grains impact protein turnover. Our rationale is built on evidence from the clinical trial, which matched diets for protein and energy content, and thein vitro experiments, which used phytochemicals from a whole-grain wheat extract. There are hundreds of phytochemical components in whole-grains, and these can be broadly categorized into: alkylresorcinols, benzoxazinoids, phytosterols, sphingolipids, lignans, flavonoids, phenolic acids, tocols, carotenoids, fatty acids, betaines, and glycolipids (61). Some of these phytochemicals have shown a positive impact on lean body mass and skeletal muscle protein turnover (62, 63). In parallel, a growing body of literature evidences that many previously unexplored nutritional factors impact health and disease (64–68). However, due to a paucity of well-controlled research in this area, it is currently unclear which specific whole-grain components might contain the biological activity necessary to impact protein turnover. For this reason, additional research is warranted to identify the specific whole-grain phytochemicals that might impact protein turnover and the cellular mechanisms that govern this effect.
Because our human and in vitro data suggest that whole-grains positively impact protein turnover, we turned to an epidemiological approach to assess the clinical relevance of these results in the context of a contemporary issue in older adults—the age-related loss of muscle function consistent with sarcopenia (69). Loss of muscle function is well characterized in aging (70), and results primarily from imbalanced protein turnover (71) and reduced protein synthesis signaling (72, 73). Here, we show that whole-grain intake is independently associated with a faster gait speed, suggesting better muscle function. This is consistent with the literature, because dietary patterns high in whole-grains (e.g., Mediterranean diet) have been associated with greater muscle function across aging populations throughout the developed world (74–76). Taking these epidemiological findings together with our clinical trial and in vitro data, it is reasonable to speculate that the habitual consumption of whole-grains contributes to preservation of muscle function by favorably impacting WBPT by augmenting the protein synthetic response to dietary protein. Current nutrition approaches to counter the age-related loss of muscle function are focused on protein and energy intake. These recommendations are effective and consistent with our classical understanding of the nutritional regulators of muscle mass and function (77). Unfortunately, habitual protein and energy intake in older adults remains low (78) and it has been speculated that the latter likely contributes to impaired muscle function in aging (18). Meanwhile, whole-grain intake in older adults is relatively low and less than half of the minimum amount recommended by the USDA (22). In contrast, refined-grain intake remains high, both in our subset from NHANES and in larger analyses (79). Replacing habitually consumed refined-grain foods with whole-grain foods is a practical dietary strategy, even in older adults (80), because it does not require major modifications to dietary habits. Therefore, the elucidation of adjuvant nutritional approaches to increase protein balance could offer valuable flexibility to optimize dietary recommendations for certain populations. However, protein and energy intake were greater effectors on muscle function than whole-grain intake in our report. It remains possible that in settings of more robust anabolic stimuli, like optimal protein and energy intake (81), resistance exercise training (82), or anabolic steroids (83), whole-grain intake could have less potential.
The premise behind our clinical-translational conclusions is that skeletal muscle protein turnover is represented in our WBPT assessments. It should be noted that WBPT is a weighted average of protein turnover from all body tissues and organs; it has roughly equal contribution from 3 major sources: gut, liver, and skeletal muscle (28, 84). Skeletal muscle protein turnover comprises 25–30% of WBPT, thus differences in WBPT cannot fully be attributed to skeletal muscle as detailed by Deutz et al. (85). Still, skeletal muscle accounts for ∼40% of total body mass (86) and remains the body's largest malleable reservoir for protein mobilization or storage. This is evident in negative net protein balance (catabolic) conditions, where increased amino acid needs contribute to a net amino acid extraction from muscle (87), leading to muscle loss (88); and in positive net protein balance (anabolic) conditions, where anabolic stimuli in combination with increased amino acid availability contribute to a net amino acid assimilation into muscle (42), leading to muscle growth (89). Therefore, using our in vitro data as rationale that whole-grains can stimulate skeletal muscle protein synthesis, we conclude that the positive net balance observed with the whole-grain diet in our clinical trial is likely to be realized, in part, within skeletal muscle. Nevertheless, direct skeletal muscle protein turnover assessments using arteriovenous difference techniques with skeletal muscle biopsies are needed to confirm this assumption.
In conclusion, our data suggest that when whole-grain intake aligns with USDA recommendations (50 g/1000 kcal, equivalent to 5–6 servings), WBPT is enhanced in adults with overweight/obesity. We provide proof-of-concept that whole-grain components augment global protein synthesis in skeletal muscle, and that greater whole-grain intake might preserve muscle function in older adults. Taken together, these data implicate a novel role for whole-grains in human protein metabolism and related health outcomes.
Supplementary Material
ACKNOWLEDGEMENTS
The authors’ responsibilities were as follows—JTM: designed research, conducted research, analyzed data, and wrote the manuscript; ARS, ELK, SKM: designed research, conducted research, and analyzed data; SY, AP: conducted research, analyzed data, and reviewed and edited the manuscript; ZEF, RAF: analyzed data and reviewed and edited the manuscript; J-PG, ABR: designed research, conducted research, analyzed data, and reviewed and edited the manuscript; JPK: designed research, conducted research, analyzed data, reviewed and edited the manuscript, provided supervision, and had primary responsibility for final content; and all authors: read and approved the final manuscript.
Notes
This research was supported by an investigator-initiated grant from Nestlé (JPK), a National Center for Research Resources grant (UL1RR024989), a National Center for Complementary and Integrative Health training grant (T32AT004094—JTM trainee), a National Center for Complementary and Integrative Health (5P50AT002776-14) grant supporting the Botanical Dietary Supplement Research Center, and a National Institute of General Medical Sciences grant (U54GM104940), which funds the Louisiana Clinical and Translational Science Center. Nestlé Product Technology Center and Cereal Partners Worldwide provided the study meals and foods.
Author disclosures: J-PG is an employee of Société des Produits Nestlé SA. ABR is a former employee of Société des Produits Nestlé SA. All other authors report no conflicts of interest.
Supplemental Table 1 is available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/cdn/.
Abbreviations used: ACS, American Chemical Society; Akt, protein kinase B; FFM, fat-free mass; mTOR, mammalian target of rapamycin; OBB, Odyssey Blocking Buffer; SUnSET, surface sensing of translation; TBST, Tris-buffered saline with 0.1% Tween 20; UPLC, ultra performance liquid chromatography; WBPT, whole-body protein turnover.
Contributor Information
Jacob T Mey, Pennington Biomedical Research Center, Baton Rouge, LA, USA.
Jean-Philippe Godin, Nestlé Research, Institute of Food Safety and Analytical Sciences, Lausanne, Switzerland.
Amanda R Scelsi, Lerner Research Institute, Cleveland Clinic, Cleveland, OH, USA.
Emily L Kullman, Health and Human Performance, Cleveland State University, Cleveland, OH, USA.
Steven K Malin, Department of Kinesiology and Health, Rutgers University, New Brunswick, NJ, USA.
Shengping Yang, Pennington Biomedical Research Center, Baton Rouge, LA, USA.
Z Elizabeth Floyd, Pennington Biomedical Research Center, Baton Rouge, LA, USA.
Alexander Poulev, Department of Kinesiology and Health, Rutgers University, New Brunswick, NJ, USA.
Roger A Fielding, Jean Mayer USDA Human Nutrition Research Center on Aging, Boston, MA, USA.
Alastair B Ross, AgResearch, Lincoln, New Zealand.
John P Kirwan, Email: John.Kirwan@pbrc.edu, Pennington Biomedical Research Center, Baton Rouge, LA, USA.
Data Availability
Data described in the manuscript, code book, and analytic code will be made available upon request pending application, approval, and generation of appropriate legal agreements.
References
- 1. Ferruzzi MG, Jonnalagadda SS, Liu S, Marquart L, McKeown N, Reicks M, Riccardi G, Seal C, Slavin J, Thielecke Fet al. Developing a standard definition of whole-grain foods for dietary recommendations: summary report of a multidisciplinary expert roundtable discussion. Adv Nutr. 2014;5(2):164–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Jacobs DR Jr, Gallaher DD. Whole grain intake and cardiovascular disease: a review. Curr Atheroscler Rep. 2004;6(6):415–23. [DOI] [PubMed] [Google Scholar]
- 3. McKeown NM, Troy LM, Jacques PF, Hoffmann U, O'Donnell CJ, Fox CS. Whole- and refined-grain intakes are differentially associated with abdominal visceral and subcutaneous adiposity in healthy adults: the Framingham Heart Study. Am J Clin Nutr. 2010;92(5):1165–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Maki KC, Palacios OM, Koecher K, Sawicki CM, Livingston KA, Bell M, Nelson Cortes H, McKeown NM. The relationship between whole grain intake and body weight: results of meta-analyses of observational studies and randomized controlled trials. Nutrients. 2019;11(6):1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dutta C. Significance of sarcopenia in the elderly. J Nutr. 1997;127(5 Suppl):992S–3S. [DOI] [PubMed] [Google Scholar]
- 6. Fielding RA. Effects of exercise training in the elderly: impact of progressive-resistance training on skeletal muscle and whole-body protein metabolism. Proc Nutr Soc. 1995;54(3):665–75. [DOI] [PubMed] [Google Scholar]
- 7. Ross AB, Pere-Trepat E, Montoliu I, Martin FP, Collino S, Moco S, Godin JP, Cleroux M, Guy PA, Breton Iet al. A whole-grain-rich diet reduces urinary excretion of markers of protein catabolism and gut microbiota metabolism in healthy men after one week. J Nutr. 2013;143(6):766–73. [DOI] [PubMed] [Google Scholar]
- 8. Pereira MA, Jacobs DR Jr, Slattery ML, Ruth KJ, Van Horn L, Hilner JE, Kushi LH. The association of whole grain intake and fasting insulin in a biracial cohort of young adults: the CARDIA study. CVD Prev. 1998;1(3):231–42. [PMC free article] [PubMed] [Google Scholar]
- 9. Malin SK, Kullman EL, Scelsi AR, Godin JP, Ross AB, Kirwan JP. A whole-grain diet increases glucose-stimulated insulin secretion independent of gut hormones in adults at risk for type 2 diabetes. Mol Nutr Food Res. 2019;63(7):1800967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fukagawa NK, Minaker KL, Rowe JW, Goodman MN, Matthews DE, Bier DM, Young VR. Insulin-mediated reduction of whole body protein breakdown. Dose-response effects on leucine metabolism in postabsorptive men. J Clin Invest. 1985;76(6):2306–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kristensen M, Toubro S, Jensen MG, Ross AB, Riboldi G, Petronio M, Bugel S, Tetens I, Astrup A. Whole grain compared with refined wheat decreases the percentage of body fat following a 12-week, energy-restricted dietary intervention in postmenopausal women. J Nutr. 2012;142(4):710–16. [DOI] [PubMed] [Google Scholar]
- 12. Harris Jackson K, West SG, Vanden Heuvel JP, Jonnalagadda SS, Ross AB, Hill AM, Grieger JA, Lemieux SK, Kris-Etherton PM. Effects of whole and refined grains in a weight-loss diet on markers of metabolic syndrome in individuals with increased waist circumference: a randomized controlled-feeding trial. Am J Clin Nutr. 2014;100(2):577–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Malin SK, Kullman EL, Scelsi AR, Haus JM, Filion J, Pagadala MR, Godin JP, Kochhar S, Ross AB, Kirwan JP. A whole-grain diet reduces peripheral insulin resistance and improves glucose kinetics in obese adults: a randomized-controlled trial. Metabolism. 2018;82:111–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Katcher HI, Legro RS, Kunselman AR, Gillies PJ, Demers LM, Bagshaw DM, Kris-Etherton PM. The effects of a whole grain-enriched hypocaloric diet on cardiovascular disease risk factors in men and women with metabolic syndrome. Am J Clin Nutr. 2008;87(1):79–90. [DOI] [PubMed] [Google Scholar]
- 15. Landi F, Liperoti R, Russo A, Giovannini S, Tosato M, Capoluongo E, Bernabei R, Onder G. Sarcopenia as a risk factor for falls in elderly individuals: results from the ilSIRENTE study. Clin Nutr. 2012;31(5):652–8. [DOI] [PubMed] [Google Scholar]
- 16. Bortone I, Sardone R, Lampignano L, Castellana F, Zupo R, Lozupone M, Moretti B, Giannelli G, Panza F. How gait influences frailty models and health-related outcomes in clinical-based and population-based studies: a systematic review. J Cachexia Sarcopenia Muscle. 2021;12(2):274–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cesari M, Pahor M, Lauretani F, Zamboni V, Bandinelli S, Bernabei R, Guralnik JM, Ferrucci L. Skeletal muscle and mortality results from the InCHIANTI Study. J Gerontol A Biol Sci Med Sci. 2009;64(3):377–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Evans W. Functional and metabolic consequences of sarcopenia. J Nutr. 1997;127(5 Suppl):998S–1003S. [DOI] [PubMed] [Google Scholar]
- 19. Milne AC, Potter J, Vivanti A, Avenell A. Protein and energy supplementation in elderly people at risk from malnutrition. Cochrane Database Syst Rev. 2009(2):CD003288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. von Berens A, Fielding RA, Gustafsson T, Kirn D, Laussen J, Nydahl M, Reid K, Travison TG, Zhu H, Cederholm Tet al. Effect of exercise and nutritional supplementation on health-related quality of life and mood in older adults: the VIVE2 randomized controlled trial. BMC Geriatr. 2018;18(1):286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fielding RA, Travison TG, Kirn DR, Koochek A, Reid KF, von Berens A, Zhu H, Folta SC, Sacheck JM, Nelson MEet al. Effect of structured physical activity and nutritional supplementation on physical function in mobility-limited older adults: results from the VIVE2 randomized trial. J Nutr Health Aging. 2017;21(9):936–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. US Department of Agriculture, US Department of Health and Human Services . Dietary guidelines for Americans, 2020–2025. 9th ed[Internet]. 2020; [cited April 20, 2021]. Available from: dietaryguidelines.gov. [Google Scholar]
- 23. Kirwan JP, Malin SK, Scelsi AR, Kullman EL, Navaneethan SD, Pagadala MR, Haus JM, Filion J, Godin JP, Kochhar Set al. A whole-grain diet reduces cardiovascular risk factors in overweight and obese adults: a randomized controlled trial. J Nutr. 2016;146(11):2244–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Schmidt EK, Clavarino G, Ceppi M, Pierre P. SUnSET, a nonradioactive method to monitor protein synthesis. Nat Methods. 2009;6(4):275–7. [DOI] [PubMed] [Google Scholar]
- 25. US Department of Agriculture, US Department of Health and Human Services . Dietary guidelines for Americans, 2010–2015. [Internet]. 2010; [cited April 20, 2021]. Available from: dietaryguidelines.gov. [Google Scholar]
- 26. Schisterman EF, Mumford SL, Sjaarda LA. Failure to consider the menstrual cycle phase may cause misinterpretation of clinical and research findings of cardiometabolic biomarkers in premenopausal women. Epidemiol Rev. 2014;36(1):71–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Matthews DE, Motil KJ, Rohrbaugh DK, Burke JF, Young VR, Bier DM. Measurement of leucine metabolism in man from a primed, continuous infusion of L-[1-3C]leucine. Am J Physiol. 1980;238(5):E473–9. [DOI] [PubMed] [Google Scholar]
- 28. Duggleby SL, Waterlow JC. The end-product method of measuring whole-body protein turnover: a review of published results and a comparison with those obtained by leucine infusion. Br J Nutr. 2005;94(2):141–53. [DOI] [PubMed] [Google Scholar]
- 29. Boveia V, Schutz-Geschwender A. Quantitative analysis of signal transduction with in-cell western immunofluorescence assays. Methods Mol Biol. 2015;1314:115–30. [DOI] [PubMed] [Google Scholar]
- 30. Davuluri G, Allawy A, Thapaliya S, Rennison JH, Singh D, Kumar A, Sandlers Y, Van Wagoner DR, Flask CA, Hoppel Cet al. Hyperammonaemia-induced skeletal muscle mitochondrial dysfunction results in cataplerosis and oxidative stress. J Physiol. 2016;594(24):7341–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Davuluri G, Krokowski D, Guan BJ, Kumar A, Thapaliya S, Singh D, Hatzoglou M, Dasarathy S. Metabolic adaptation of skeletal muscle to hyperammonemia drives the beneficial effects of L-leucine in cirrhosis. J Hepatol. 2016;65(5):929–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gran P, Cameron-Smith D. The actions of exogenous leucine on mTOR signalling and amino acid transporters in human myotubes. BMC Physiol. 2011;11:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Talvas J, Obled A, Fafournoux P, Mordier S. Regulation of protein synthesis by leucine starvation involves distinct mechanisms in mouse C2C12 myoblasts and myotubes. J Nutr. 2006;136(6):1466–71. [DOI] [PubMed] [Google Scholar]
- 34. DeBoer ML, Martinson KM, Pampusch MS, Hansen AM, Wells SM, Ward C, Hathaway M. Cultured equine satellite cells as a model system to assess leucine stimulated protein synthesis in horse muscle. J Anim Sci. 2018;96(1):143–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Atherton PJ, Smith K, Etheridge T, Rankin D, Rennie MJ. Distinct anabolic signalling responses to amino acids in C2C12 skeletal muscle cells. Amino Acids. 2010;38(5):1533–9. [DOI] [PubMed] [Google Scholar]
- 36. Boudreau A, Poulev A, Ribnicky DM, Raskin I, Rathinasabapathy T, Richard AJ, Stephens JM. Distinct fractions of an Artemisia scoparia extract contain compounds with novel adipogenic bioactivity. Front Nutr. 2019;6:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Health Statistics . National Health and Nutrition Examination Survey (NHANES), 1999–2000. [Internet]. Inter-university Consortium for Political and Social Research [distributor]; 2012. Available from: 10.3886/ICPSR25501.v4. [DOI] [Google Scholar]
- 38. Abellan van Kan G, Rolland Y, Andrieu S, Bauer J, Beauchet O, Bonnefoy M, Cesari M, Donini LM, Gillette Guyonnet S, Inzitari Met al. Gait speed at usual pace as a predictor of adverse outcomes in community-dwelling older people an International Academy on Nutrition and Aging (IANA) Task Force. J Nutr Health Aging. 2009;13(10):881–9. [DOI] [PubMed] [Google Scholar]
- 39. Cruz-Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyere O, Cederholm T, Cooper C, Landi F, Rolland Y, Sayer AAet al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019;48(4):601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Fukagawa NK, Minaker KL, Young VR, Matthews DE, Bier DM, Rowe JW. Leucine metabolism in aging humans: effect of insulin and substrate availability. Am J Physiol. 1989;256(2 Pt 1):E288–94. [DOI] [PubMed] [Google Scholar]
- 41. Phillips SM, Paddon-Jones D, Layman DK. Optimizing adult protein intake during catabolic health conditions. Adv Nutr. 2020;11(4):S1058–S69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Biolo G, Maggi SP, Williams BD, Tipton KD, Wolfe RR. Increased rates of muscle protein turnover and amino acid transport after resistance exercise in humans. Am J Physiol. 1995;268(3 Pt 1):E514–20. [DOI] [PubMed] [Google Scholar]
- 43. Drummond MJ, Marcus RL, Lastayo PC. Targeting anabolic impairment in response to resistance exercise in older adults with mobility impairments: potential mechanisms and rehabilitation approaches. J Aging Res. 2012;2012:486930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Moore DR, Robinson MJ, Fry JL, Tang JE, Glover EI, Wilkinson SB, Prior T, Tarnopolsky MA, Phillips SM. Ingested protein dose response of muscle and albumin protein synthesis after resistance exercise in young men. Am J Clin Nutr. 2009;89(1):161–8. [DOI] [PubMed] [Google Scholar]
- 45. Fry CS, Drummond MJ, Glynn EL, Dickinson JM, Gundermann DM, Timmerman KL, Walker DK, Dhanani S, Volpi E, Rasmussen BB. Aging impairs contraction-induced human skeletal muscle mTORC1 signaling and protein synthesis. Skelet Muscle. 2011;1(1):11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Drummond MJ, Dreyer HC, Pennings B, Fry CS, Dhanani S, Dillon EL, Sheffield-Moore M, Volpi E, Rasmussen BB. Skeletal muscle protein anabolic response to resistance exercise and essential amino acids is delayed with aging. J Appl Physiol. 2008;104(5):1452–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Pacy PJ, Price GM, Halliday D, Quevedo MR, Millward DJ. Nitrogen homeostasis in man: the diurnal responses of protein synthesis and degradation and amino acid oxidation to diets with increasing protein intakes. Clin Sci. 1994;86(1):103–18. [DOI] [PubMed] [Google Scholar]
- 48. Pol K, Christensen R, Bartels EM, Raben A, Tetens I, Kristensen M. Whole grain and body weight changes in apparently healthy adults: a systematic review and meta-analysis of randomized controlled studies. Am J Clin Nutr. 2013;98(4):872–84. [DOI] [PubMed] [Google Scholar]
- 49. Fujita S, Rasmussen BB, Bell JA, Cadenas JG, Volpi E. Basal muscle intracellular amino acid kinetics in women and men. Am J Physiol Endocrinol Metab. 2007;292(1):E77–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Smith GI, Atherton P, Reeds DN, Mohammed BS, Jaffery H, Rankin D, Rennie MJ, Mittendorfer B. No major sex differences in muscle protein synthesis rates in the postabsorptive state and during hyperinsulinemia-hyperaminoacidemia in middle-aged adults. J Appl Physiol. 2009;107(4):1308–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Pannemans DL, Wagenmakers AJ, Westerterp KR, Schaafsma G, Halliday D. The effect of an increase of protein intake on whole-body protein turnover in elderly women is tracer dependent. J Nutr. 1997;127(9):1788–94. [DOI] [PubMed] [Google Scholar]
- 52. Price GM, Halliday D, Pacy PJ, Quevedo MR, Millward DJ. Nitrogen homeostasis in man: influence of protein intake on the amplitude of diurnal cycling of body nitrogen. Clin Sci. 1994;86(1):91–102. [DOI] [PubMed] [Google Scholar]
- 53. Abdelmoez AM, Sardon Puig L, Smith JAB, Gabriel BM, Savikj M, Dollet L, Chibalin AV, Krook A, Zierath JR, Pillon NJ. Comparative profiling of skeletal muscle models reveals heterogeneity of transcriptome and metabolism. Am J Physiol Cell Physiol. 2020;318(3):C615–C26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Gwin JA, Church DD, Hatch-McChesney A, Howard EE, Carrigan CT, Murphy NE, Wilson MA, Margolis LM, Carbone JW, Wolfe RRet al. Effects of high versus standard essential amino acid intakes on whole-body protein turnover and mixed muscle protein synthesis during energy deficit: a randomized, crossover study. Clin Nutr. 2021;40:767–77. [DOI] [PubMed] [Google Scholar]
- 55. Churchward-Venne TA, Pinckaers PJM, Smeets JSJ, Betz MW, Senden JM, Goessens JPB, Gijsen AP, Rollo I, Verdijk LB, van Loon LJC. Dose-response effects of dietary protein on muscle protein synthesis during recovery from endurance exercise in young men: a double-blind randomized trial. Am J Clin Nutr. 2020;112(2):303–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Michelsen CB, Askanazi J, Kinney JM, Gump FE, Elwyn DH. Effect of an anabolic steroid on nitrogen balance and amino acid patterns after total hip replacement. J Trauma. 1982;22(5):410–13. [DOI] [PubMed] [Google Scholar]
- 57. Stevenson L, Phillips F, O'Sullivan K, Walton J. Wheat bran: its composition and benefits to health, a European perspective. Int J Food Sci Nutr. 2012;63(8):1001–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Tighe P, Duthie G, Vaughan N, Brittenden J, Simpson WG, Duthie S, Mutch W, Wahle K, Horgan G, Thies F. Effect of increased consumption of whole-grain foods on blood pressure and other cardiovascular risk markers in healthy middle-aged persons: a randomized controlled trial. Am J Clin Nutr. 2010;92(4):733–40. [DOI] [PubMed] [Google Scholar]
- 59. Rebello CJ, Johnson WD, Martin CK, Xie W, O'Shea M, Kurilich A, Bordenave N, Andler S, van Klinken BJ, Chu YFet al. Acute effect of oatmeal on subjective measures of appetite and satiety compared to a ready-to-eat breakfast cereal: a randomized crossover trial. J Am Coll Nutr. 2013;32(4):272–9. [DOI] [PubMed] [Google Scholar]
- 60. Kim J, Hoang T, Bu SY, Kim JM, Choi JH, Park E, Lee SM, Park E, Min JY, Lee ISet al. Associations of dietary intake with cardiovascular disease, blood pressure, and lipid profile in the Korean population: a systematic review and meta-analysis. J Lipid Atheroscler. 2020;9(1):205–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Zhu Y, Sang S. Phytochemicals in whole grain wheat and their health-promoting effects. Mol Nutr Food Res. 2017;61(7). doi:10.1002/mnfr.201600852. [DOI] [PubMed] [Google Scholar]
- 62. Hiramoto S, Yahata N, Saitoh K, Yoshimura T, Wang Y, Taniyama S, Nikawa T, Tachibana K, Hirasaka K. Dietary supplementation with alkylresorcinols prevents muscle atrophy through a shift of energy supply. J Nutr Biochem. 2018;61:147–54. [DOI] [PubMed] [Google Scholar]
- 63. Cholewa JM, Hudson A, Cicholski T, Cervenka A, Barreno K, Broom K, Barch M, Craig SAS. The effects of chronic betaine supplementation on body composition and performance in collegiate females: a double-blind, randomized, placebo controlled trial. J Int Soc Sports Nutr. 2018;15(1):37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Liu J, Rehm CD, Shi P, McKeown NM, Mozaffarian D, Micha R. A comparison of different practical indices for assessing carbohydrate quality among carbohydrate-rich processed products in the US. PLoS One. 2020;15(5):e0231572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Downer S, Berkowitz SA, Harlan TS, Olstad DL, Mozaffarian D. Food is medicine: actions to integrate food and nutrition into healthcare. BMJ. 2020;369:m2482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Mozaffarian D. Dairy foods, obesity, and metabolic health: the role of the food matrix compared with single nutrients. Adv Nutr. 2019;10(5):917S–23S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Rimm EB, Appel LJ, Chiuve SE, Djousse L, Engler MB, Kris-Etherton PM, Mozaffarian D, Siscovick DS, Lichtenstein AH, American Heart Association Nutrition Committee of the Council on Lifestyle and Cardiometabolic Health, et al. Seafood long-chain n-3 polyunsaturated fatty acids and cardiovascular disease: a science advisory from the American Heart Association. Circulation. 2018;138(1):e35–e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Mozaffarian D, Wu JHY. Flavonoids, dairy foods, and cardiovascular and metabolic health: a review of emerging biologic pathways. Circ Res. 2018;122(2):369–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Cawthon PM, Travison TG, Manini TM, Patel S, Pencina KM, Fielding RA, Magaziner JM, Newman AB, Brown T, Kiel DPet al. Establishing the link between lean mass and grip strength cut points with mobility disability and other health outcomes: Proceedings of the Sarcopenia Definition and Outcomes Consortium Conference. J Gerontol A Biol Sci Med Sci. 2020;75(7):1317–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Porter MM, Vandervoort AA, Lexell J. Aging of human muscle: structure, function and adaptability. Scand J Med Sci Sports. 1995;5(3):129–42. [DOI] [PubMed] [Google Scholar]
- 71. Yarasheski KE. Exercise, aging, and muscle protein metabolism. J Gerontol A Biol Sci Med Sci. 2003;58(10):M918–22. [DOI] [PubMed] [Google Scholar]
- 72. Dickinson JM, Volpi E, Rasmussen BB. Exercise and nutrition to target protein synthesis impairments in aging skeletal muscle. Exerc Sport Sci Rev. 2013;41(4):216–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Volpi E, Sheffield-Moore M, Rasmussen BB, Wolfe RR. Basal muscle amino acid kinetics and protein synthesis in healthy young and older men. JAMA. 2001;286(10):1206–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Granic A, Jagger C, Davies K, Adamson A, Kirkwood T, Hill TR, Siervo M, Mathers JC, Sayer AA. Effect of dietary patterns on muscle strength and physical performance in the very old: findings from the Newcastle 85+ study. PLoS One. 2016;11(3):e0149699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Leon-Munoz LM, Garcia-Esquinas E, Lopez-Garcia E, Banegas JR, Rodriguez-Artalejo F. Major dietary patterns and risk of frailty in older adults: a prospective cohort study. BMC Med. 2015;13:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Zbeida M, Goldsmith R, Shimony T, Vardi H, Naggan L, Shahar DR. Mediterranean diet and functional indicators among older adults in non-Mediterranean and Mediterranean countries. J Nutr Health Aging. 2014;18(4):411–18. [DOI] [PubMed] [Google Scholar]
- 77. Mahan LK, Escott-Stump S. Krause's food & nutrition therapy. 12th ed. St Louis (MO): Saunders/Elsevier; 2008. [Google Scholar]
- 78. Mendonca N, Granic A, Mathers JC, Hill TR, Siervo M, Adamson AJ, Jagger C. Prevalence and determinants of low protein intake in very old adults: insights from the Newcastle 85+ Study. Eur J Nutr. 2018;57(8):2713–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. McGuire S. Scientific Report of the 2015 Dietary Guidelines Advisory Committee. Washington, DC: US Departments of Agriculture and Health and Human Services, 2015. Adv Nutr. 2016;7(1):202–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Windhauser MM, Ernst DB, Karanja NM, Crawford SW, Redican SE, Swain JF, Karimbakas JM, Champagne CM, Hoben KP, Evans MA. Translating the Dietary Approaches to Stop Hypertension diet from research to practice: dietary and behavior change techniques. DASH Collaborative Research Group. J Am Diet Assoc. 1999;99(8):S90–S95. [DOI] [PubMed] [Google Scholar]
- 81. Tarnopolsky M. Protein requirements for endurance athletes. Nutrition. 2004;20(7-8):662–8. [DOI] [PubMed] [Google Scholar]
- 82. Yarasheski KE, Pak-Loduca J, Hasten DL, Obert KA, Brown MB, Sinacore DR. Resistance exercise training increases mixed muscle protein synthesis rate in frail women and men >/= 76 yr old. Am J Physiol. 1999;277(1):E118–25. [DOI] [PubMed] [Google Scholar]
- 83. Sheffield-Moore M, Urban RJ, Wolf SE, Jiang J, Catlin DH, Herndon DN, Wolfe RR, Ferrando AA. Short-term oxandrolone administration stimulates net muscle protein synthesis in young men. J Clin Endocrinol Metab. 1999;84(8):2705–11. [DOI] [PubMed] [Google Scholar]
- 84. Morais JA, Ross R, Gougeon R, Pencharz PB, Jones PJ, Marliss EB. Distribution of protein turnover changes with age in humans as assessed by whole-body magnetic resonance image analysis to quantify tissue volumes. J Nutr. 2000;130(4):784–91. [DOI] [PubMed] [Google Scholar]
- 85. Deutz NE, Wagenmakers AJ, Soeters PB. Discrepancy between muscle and whole body protein turnover. Curr Opin Clin Nutr Metab Care. 1999;2(1):29–32. [DOI] [PubMed] [Google Scholar]
- 86. Kim J, Wang Z, Heymsfield SB, Baumgartner RN, Gallagher D. Total-body skeletal muscle mass: estimation by a new dual-energy X-ray absorptiometry method. Am J Clin Nutr. 2002;76(2):378–83. [DOI] [PubMed] [Google Scholar]
- 87. Morrison WL, Bouchier IA, Gibson JN, Rennie MJ. Skeletal muscle and whole-body protein turnover in cirrhosis. Clin Sci. 1990;78(6):613–19. [DOI] [PubMed] [Google Scholar]
- 88. Ebadi M, Bhanji RA, Mazurak VC, Montano-Loza AJ. Sarcopenia in cirrhosis: from pathogenesis to interventions. J Gastroenterol. 2019;54(10):845–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Morton RW, Murphy KT, McKellar SR, Schoenfeld BJ, Henselmans M, Helms E, Aragon AA, Devries MC, Banfield L, Krieger JWet al. A systematic review, meta-analysis and meta-regression of the effect of protein supplementation on resistance training-induced gains in muscle mass and strength in healthy adults. Br J Sports Med. 2018;52(6):376–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data described in the manuscript, code book, and analytic code will be made available upon request pending application, approval, and generation of appropriate legal agreements.