Abstract
The cellular response to DNA damage includes activation of the nuclear Lyn protein tyrosine kinase. Using cells deficient in Lyn expression, the present studies demonstrate that Lyn is required in part for induction of the stress-activated protein kinase (SAPK) in the response to 1-β-d-arabinofuranosylcytosine (ara-C) and other genotoxic agents. By contrast, exposure of Lyn-deficient cells to ara-C, ionizing radiation, or cisplatin had no effect on activation of extracellular signal-regulated protein kinase or p38 mitogen-activated protein kinase. Similar findings were obtained in cells stably expressing a kinase-inactive, dominant-negative Lyn(K-R) mutant. Coexpression studies demonstrate that Lyn, but not Lyn(K-R), induces SAPK activity. In addition, the results demonstrate that Lyn activates SAPK by an MKK7-dependent, SEK1-independent mechanism. As MEKK1 functions upstream to MKK7 and SAPK, the finding that a dominant-negative MEKK1(K-M) mutant blocks Lyn-induced SAPK activity supports involvement of the MEKK1→MKK7 pathway. The results also demonstrate that inhibition of Lyn-induced SAPK activity abrogates the apoptotic response of cells to genotoxic stress. These findings indicate that activation of SAPK by DNA damage is mediated in part by Lyn and that the Lyn→MEKK1→MKK7→SAPK pathway is functional in the induction of apoptosis by genotoxic agents.
The cellular response to genotoxic agents includes cell cycle arrest, activation of DNA repair, and, in the event of irreparable damage, induction of apoptosis. While the signaling mechanisms responsible for the regulation of the DNA damage response are largely unknown, exposure of cells to agents that arrest DNA replication or damage DNA is associated with activation of early-response genes that code for transcription factors (7, 8, 25, 30, 52). Certain insights have also been derived from the finding that DNA damage is associated with activation of the stress-activated protein kinase/c-Jun N-terminal kinase (SAPK/JNK) (5, 6, 35, 50, 59, 75). SAPK phosphorylates Ser-63 and -73 of the c-Jun amino terminus and thereby activates the c-Jun transcription function (10, 38). The ATF2 and Elk1 transcription factors are also phosphorylated by SAPK (15, 46, 60). These findings have indicated that SAPK-mediated activation of c-Jun, ATF2, and Elk1, and thereby transcription of early response genes, is associated with the response of cells to arrest of DNA replication or DNA damage.
Other studies have demonstrated that genotoxic agents activate a nuclear complex that consists in part of the c-Abl and Lyn protein tyrosine kinases. c-Abl associates with the DNA-dependent protein kinase (DNA-PK) consisting of the catalytic subunit (DNA-PKcs) and Ku DNA-binding components (20, 27–29). DNA-PK phosphorylates and activates c-Abl, while phosphorylation of DNA-PK by c-Abl inhibits the association of DNA-PK with DNA (27). The finding that c-Abl binds to the p53 tumor suppressor, induces the transactivation function of p53, and activates p21 expression has supported involvement of c-Abl in the G1 growth arrest response (13, 70, 74). Other studies have demonstrated that c-Abl interacts with the p73 homolog of p53 in the apoptotic response to DNA damage (1, 14, 73). The demonstration that cells deficient in c-Abl exhibit a defective SAPK response to DNA-damaging agents has also supported a role for c-Abl as an upstream effector of the SAPK pathway (29). Activation of SAPK by c-Abl is dependent on the SAPK/extracellular signal-regulated kinase 1 (SEK1) (28). In addition, activated forms of Abl confer induction of SAPK activity and early response gene expression (28, 47, 48, 52). These findings have supported a model in which activation of c-Abl in response to DNA damage contributes to the regulation of gene transcription.
The Lyn tyrosine kinase, like c-Abl, is activated by agents that arrest DNA replication or damage DNA (33, 34, 69). Cell fractionation studies and confocal microscopy have demonstrated that Lyn is expressed in the nucleus and that nuclear Lyn is activated by DNA damage (32). In addition, Lyn, like c-Abl, interacts with the DNA-PK complex (37). The interaction between Lyn and DNA-PK induces the release of DNA-PKcs from Ku-DNA complexes (37). The activation of nuclear Lyn by DNA damage is also associated with binding of Lyn to Cdc2 (32–34, 69). The finding that Lyn phosphorylates Cdc2 on Tyr-15, and thereby inactivates Cdc2, has supported a potential role for Lyn in regulation of a DNA damage-dependent premitotic checkpoint (32, 34). Other studies have demonstrated that arrest of DNA replication by exposure to 1-β-d-arabinofuranosylcytosine (ara-C) is associated with binding of activated Lyn to Cdk2 (72). These findings have collectively supported a role for nuclear Lyn in the response to DNA damage.
The present studies have addressed the involvement of Lyn in stress signals activated in response to genotoxic agents. The results demonstrate that Lyn is required in part for activation of SAPK, but not the extracellular signal-regulated protein kinase (ERK) or the p38 mitogen-activated protein kinase (MAPK), in response to arrest of DNA replication and to DNA damage. The results also demonstrate that the Lyn→SAPK pathway is involved in the apoptotic response to genotoxic stress.
MATERIALS AND METHODS
Cell culture.
Chicken DT40 B cells and the Lyn- or Syk-deficient variants (56) were cultured in RPMI 1640 medium supplemented with 10% heat-inactivated fetal bovine serum (FBS), 100 U of penicillin per ml, 100 μg of streptomycin per ml, 2 mM l-glutamine, 50 μM 2-mercaptoethanol, and 1% chicken serum. Human U-937 myeloid leukemia cells (American Type Culture Collection [ATCC], Manassas, Va.) were grown in RPMI 1640 medium supplemented with 10% FBS, 100 U of penicillin per ml, 100 μg of streptomycin per ml, and 2 mM l-glutamine. HeLa cells (ATCC) and 293T embryonal kidney cells (ATCC) were grown in Dulbecco modified Eagle medium containing 10% FBS and antibiotics. Cells were treated with ara-C (Sigma), etoposide (VP-16; Sigma), adriamycin (ADR; Sigma), or cisplatin (CDDP; Sigma). Irradiation was performed at room temperature using a Gammacell 1000 (Atomic Energy of Canada, Otawa, Ontario, Canada) under aerobic conditions with a 137Cs source emitting at a fixed dose rate of 0.76 Gy/min as determined by dosimetry.
Immunoprecipitation and immune complex kinase assays.
Cells were washed with ice-cold phosphate-buffered saline (PBS) and lysed on ice for 30 min in lysis buffer (50 mM Tris-HCl, pH 7.6; 150 mM NaCl; 1% Nonidet P-40; 0.1% sodium dodecyl sulfate [SDS]; 1 mM sodium vanadate; 1 mM phenylmethylsulfonyl fluoride [PMSF]; 1 mM dithiothreitol [DTT]; 10 mM sodium fluoride; and 10 μg of aprotinin, leupeptin, and pepstatin A per ml). Soluble proteins were incubated with anti-SAPK antibody (sc-474; Santa Cruz Biotechnology [SCBT]), anti-ERK antibody (sc-93; SCBT), or anti-p38 MAPK antibody (sc-535; SCBT) for 2 h at 4°C followed by 1 h of incubation with protein A-Sepharose beads (Pharmacia-Biotech). The immune complexes were washed three times with washing buffer (50 mM Tris-HCl, pH 7.6; 150 mM NaCl; 0.1% Nonidet P-40; 0.1% SDS; 1 mM sodium vanadate; 1 mM PMSF; 1 mM DTT; 10 mM sodium fluoride; and 10 μg of aprotinin, leupeptin, and pepstatin A per ml), and once with kinase buffer (50 mM HEPES, pH 7.4; 10 mM MgCl2; 2 mM DTT; 0.1 mM sodium vanadate). The immunoprecipitates were resuspended in kinase buffer containing 20 μM ATP, 2 to 5 μCi of [γ-32P]ATP (3,000 Ci/mmol; DuPont/NEN), and 2 μg of glutathione S-transferase (GST)–c-Jun (amino acids 1 to 102) (51), 5 μg of myelin basic protein (MBP), or 2 μg of GST-ATF2 (amino acids 1 to 109) (42) for SAPK, ERK, or p38 MAPK kinase assays, respectively. After incubation for 20 min at 30°C, the reactions were terminated by adding 5× SDS sample buffer and boiling the mixtures for 5 min. Samples were resolved by SDS-polyacrylamide gel electrophoresis (PAGE) and autoradiography. Equal loading of the lanes was determined by Coomassie blue staining of the gel. Autoradiograms were scanned by laser densitometry, and the intensity of the signals was quantitated with the ImageQuant program (Molecular Dynamics, Sunnyvale, Calif.).
Tyrosine kinase assays.
Lyn tyrosine kinase assays were performed as described earlier (34) with minor modifications. Cells were washed with PBS and lysed in lysis buffer. Cell lysates were subjected to immunoprecipitation with anti-Lyn antibody (sc-15; SCBT). The immunoprecipitates were washed three times with washing buffer, once with tyrosine kinase buffer (50 mM HEPES, pH 7.4; 10 mM MnCl2; 10 mM MgCl2; 2 mM DTT; 0.1 mM sodium vanadate), and then resuspended in 30 μl of kinase buffer containing 2 to 5 μCi of [γ-32P]ATP and 2.5 μg of acid-treated enolase. The reaction was incubated for 15 min at 30°C. The proteins were resolved by SDS–10% PAGE and analyzed by autoradiography.
Immunoblot analysis.
Soluble cell lysates were separated by SDS–7.5 or 10% PAGE and then transferred to nitrocellulose filters by the dry transfer method (Bio-Rad). The residual binding sites were blocked by incubating the filters in 5% dry milk in PBS–0.05% Tween 20 (PBST) overnight at 4°C. The blots were then incubated with anti-SAPK, anti-ERK, anti-p38 MAPK, anti-Lyn (Transduction Laboratories), anti-Syk (sc-1077; SCBT), anti-phospho-SAPK (New England Biolabs, Inc.), anti-GST (Upstate Biotechnology), anti-c-Abl (sc-23; SCBT), anti-Flag (Sigma), anti-MEKK1 (22), anti-MKK7 (sc-7103; SCBT), or anti-SEK1 (sc-837; SCBT) antibodies in PBST at room temperature. After two washes with PBST, the filters were incubated with anti-rabbit (SCBT), anti-goat (SCBT), or anti-mouse (Amersham) immunoglobulin G peroxidase conjugate in 5% milk–PBST for 1 h at room temperature. Antigen-antibody complexes were visualized by chemiluminescence (NEN Life Science Products).
Plasmid construction.
A kinase-inactive Lyn mutant generated by site-directed mutagenesis was subcloned into pSRα-neo and pEF2-neo vectors as described elsewhere (68). The 1.1-kb fragment from the kinase-inactive MKK7(K-R) mutant (65) was subcloned into a pCMV-GST vector (65) and a pGEX4T vector (Pharmacia-Biotech). The 2.2-kb EcoRI fragments from wild-type MEKK1 and the kinase-inactive MEKK1(K-M) mutant (18, 22) were subcloned into the Flag tag containing pcDNA3 vector (pcDNA3-Flag) (12, 71).
Cell transfections and SAPK kinase assays.
pEF2-neo, pEF2-neo/Lyn(K-R), pSRα-neo, or pSRα-neo/Lyn(K-R) vectors were stably introduced into cells by electroporation (Gene Pulser; Bio-Rad, 0.25 V, 960 μF) and selection in G418 (GIBCO) as described earlier (68). The chicken Lyn cDNA cloned into pApuro vector was stably expressed in Lyn-deficient DT40 cells by transfection and selection in the presence of 0.5 μg of puromycin per ml (56). 293T cells were transiently transfected with pEBG, pEBG-SAPK (52), pSRα-neo, pSRα-neo/Lyn, pSRα-neo/Lyn(K-R), pSRαMSV/c-Abl (53), pEBG-SEK1, pEBG-SEK1(K-R) (52), pCMV-Flag-SEK1(K-R) (65), pCMV-GST-MKK7 (65), pCMV-GST-MKK7(K-R), pCMV-Flag-MKK7(K-R) (65), pcDNA3-Flag-MEKK1, or pcDNA3-Flag-MEKK1(K-M) by the calcium phosphate method. At 48 h posttransfection, cells were harvested for preparation of lysates. The lysates were incubated with glutathione-Sepharose beads (Pharmacia-Biotech) for 1 h, and the complexes were suspended in kinase buffer containing [γ-32P]ATP and GST–c-Jun. The reaction mixtures were incubated for 15 min at 30°C and analyzed by SDS–12% PAGE and autoradiography.
Kinase assays for MEKK1 and MKK7.
Lysates from transfected 293T cells were incubated with anti-MEKK1 or anti-MKK7 antibodies and then with protein G-Sepharose beads (SCBT). After washing, the immune complexes were resuspended in kinase buffer containing [γ-32P]ATP and 5 μg of GST-MKK7(K-R) or GST-SAPK(K-R). The reaction mixtures were incubated for 15 min at 30°C and analyzed by SDS–10% PAGE and autoradiography.
Subcellular fractionation.
Subcellular fractionation was performed as described earlier (32, 72).
Flow cytometry.
DNA content was assessed by staining ethanol-fixed cells with propidium iodide and monitoring by FACScan (Becton Dickinson). Numbers of cells with sub-G1 DNA content were determined with a MODFIT LT program (Verity Software House, Topsham, Maine).
DNA fragmentation assays.
293T cells were cotransfected with pSRα-neo/Lyn and pCMV-Flag or pCMV-Flag-MKK7(K-R). At 36 h posttransfection, cells were left untreated or treated with 10 μM ara-C for 12 h. Cells were also treated with 20 Gy ionizing radiation (IR) and then harvested at 12 h. Cells were harvested by gentle scraping and collected by centrifugation for 5 min at 4°C. After washing with PBS, the pellets were resuspended in lysis buffer (10 mM Tris-HCl, pH 8.0; 10 mM EDTA; 0.5% [vol/vol] Triton X-100) and incubated for 10 min at 4°C. The samples were then centrifuged at 14,000 rpm for 20 min at 4°C. The supernatants were incubated with 0.5% SDS and 25 μg of RNase (Boehringer Mannheim) per ml for 1 h, followed by incubation of 100 μg of proteinase K (Sigma) per ml overnight at 37°C. The samples were then extracted with equal volumes of a mixture of phenol:chloroform:isoamyl alcohol (25:24:1) and chloroform. The DNA was precipitated with 0.05 volume of 5 M NaCl and 2.5 volumes of ethanol and then resuspended with TE buffer (10 mM Tris-HCl, pH 7.8; 1 mM EDTA). Samples were run in 1.5% agarose gels and DNA was visualized under UV light.
RESULTS
Lyn functions in induction of SAPK, but not ERK or p38 MAPK, in response to DNA damage.
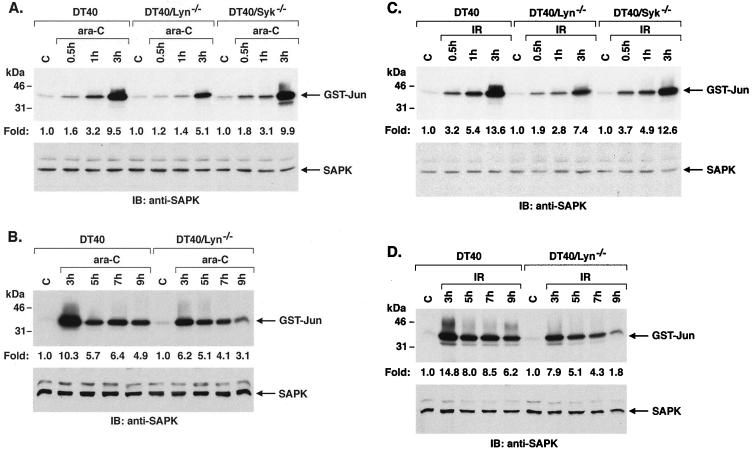
To determine whether Lyn functions as an upstream effector of the SAPK response to DNA damage, we exposed wild-type DT40 cells to ara-C and analyzed anti-SAPK immunoprecipitates for phosphorylation of GST–c-Jun. ara-C treatment was associated with induction of SAPK activity that was detectable at 15 min and maximal at 3 h (Fig. 1A and data not shown). By contrast, similar studies performed on Lyn-deficient DT40 (DT40/Lyn−/−) cells demonstrated attenuation of the ara-C-induced SAPK response (Fig. 1A). Analysis of three separate experiments confirmed that the induction of SAPK activity by ara-C is abrogated in part by deficiency of Lyn expression (Fig. 1A). To assess specificity, other studies were performed on DT40 cells deficient in the Syk tyrosine kinase (DT40/Syk−/−). There was no apparent difference in ara-C-induced SAPK activation in the DT40/Syk−/− cells compared to wild-type DT40 cells (Fig. 1A). Since ara-C-induced activation of SAPK is maximal at 3 h, cells were analyzed after longer periods of drug exposure. The results demonstrate that induction of SAPK activity by ara-C remains attenuated in Lyn-deficient cells over an extended time course (Fig. 1B). ara-C is incorporated into DNA and causes arrest of DNA replication by functioning as a relative chain terminator (36, 41), while IR induces single and double-stranded DNA breaks (23, 45). Treatment of DT40 cells with IR also resulted in induction of SAPK activity (Fig. 1C). Moreover, as shown for ara-C, the SAPK response to IR treatment was attenuated in the DT40/Lyn−/− cells (Fig. 1C). In addition, IR-induced SAPK activation was similar in the DT40/Syk−/− cells compared to wild-type DT40 cells (Fig. 1C). The finding that IR-induced SAPK activation is also attenuated in DT40/Lyn−/− cells at longer intervals provided further support for involvement of Lyn in the IR response (Fig. 1D). These findings indicated that Lyn is involved, at least in part, in the induction of SAPK by arrest of DNA replication and by DNA damage.
FIG. 1.
Activation of SAPK is attenuated in Lyn-deficient but not Syk-deficient cells by DNA-damaging agents. (A and B) Wild-type, Lyn-deficient, and Syk-deficient DT40 cells were treated with 10 μM ara-C for the indicated times. (C and D) Cells were treated with 20 Gy of IR and harvested at the indicated times. The cells were then lysed and subjected to immunoprecipitation with anti-SAPK antibody. The immunoprecipitates were incubated with GST–c-Jun and [γ-32P]ATP. GST–c-Jun phosphorylation was assessed by SDS-PAGE and autoradiography (upper panels). Lysates were also subjected to immunoblot analysis with anti-SAPK antibody (lower panels). Levels of GST–c-Jun phosphorylation were quantitated by intensity of the signals. The results are expressed as the mean (standard error, <10%) of three independent experiments.
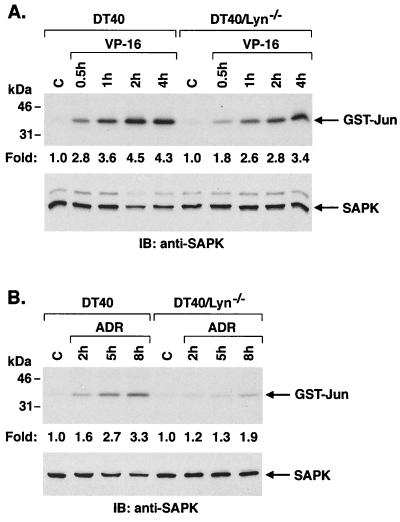
Previous studies have indicated that Lyn is involved in the induction of apoptosis, but not SAPK activation, in the response of DT40 cells to treatment with the genotoxic agents VP-16 and ADR (39). Given the findings with ara-C and IR, we assessed the effects of VP-16 and ADR on activation of SAPK in wild-type and Lyn-deficient DT40 cells. The results demonstrate that exposure of DT40 cells to VP-16 is associated with induction of SAPK activity (Fig. 2A). Similar findings were obtained with ADR treatment (Fig. 2B). Moreover, the SAPK response to VP-16 and ADR was attenuated in part in DT40/Lyn−/− cells (Fig. 2). These results indicate that Lyn is involved in SAPK activation in the cellular response to diverse genotoxic agents.
FIG. 2.
Attenuation of SAPK activity in Lyn-deficient DT40 cells in response to topoisomerase II inhibitors. Cells were treated with 5 μM VP-16 (A) or 500 ng of ADR (B) per ml for the indicated times. Lysates were subjected to immunoprecipitation with anti-SAPK. SAPK activity was assessed by the phosphorylation of GST–c-Jun (upper panel). Lysates were also subjected to immunoblot analysis with anti-SAPK activity (lower panels).
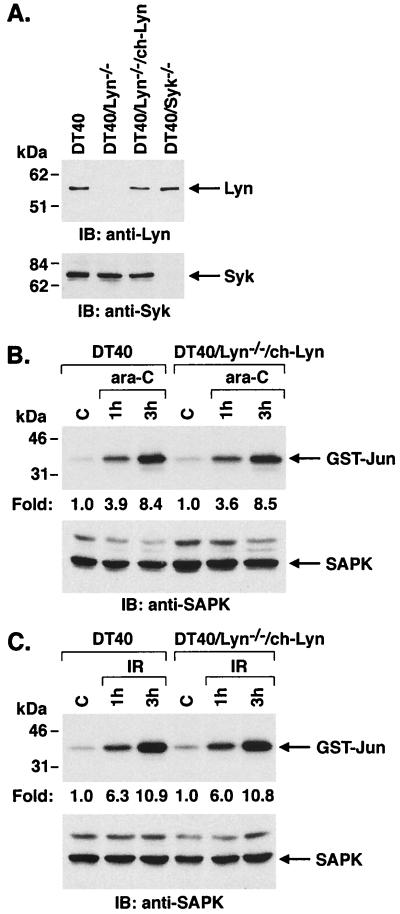
To confirm that Lyn functions in DNA damage-induced activation of SAPK, we stably transfected DT40/Lyn−/− cells to express the chicken Lyn cDNA. The stable transfectants, designated DT40/Lyn−/−/ch-Lyn, expressed Lyn at levels comparable to those found in DT40 and DT40/Syk−/− cells (Fig. 3A). Treatment of DT40/Lyn−/−/ch-Lyn cells with ara-C was associated with induction of SAPK activity which was similar to that obtained in wild-type DT40 cells (Fig. 3B). The activation of SAPK in response to IR treatment was also restored by stable expression of ch-Lyn in the DT40/Lyn−/− cells (Fig. 3C). These findings provided support for a model in which Lyn functions as a upstream effector to SAPK activation in the response of cells to DNA damage.
FIG. 3.
Restoration of SAPK activation by DNA-damaging agents in Lyn-deficient cells reconstituted to express chicken Lyn. (A) Cell lysates were subjected to immunoblot analysis with anti-Lyn (upper panel) or anti-Syk (lower panel) antibodies. (B and C) Wild-type and DT40/Lyn−/−/ch-Lyn cells were treated with ara-C (B) or IR (C) for the indicated times. Cell lysates were immunoprecipitated with anti-SAPK antibody, and in vitro immune complex kinase assays containing GST–c-Jun were analyzed by SDS-PAGE and autoradiography (upper panels). Lysates were also subjected to immunoblot analysis with anti-SAPK antibody (lower panels).
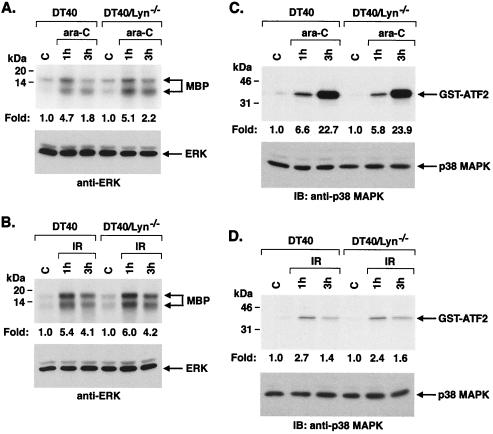
The family of MAPKs includes SAPK, ERK, and p38 MAPK (9). To determine if Lyn is required for the induction of ERK, anti-ERK immunoprecipitates from ara-C-treated DT40 and DT40/Lyn−/− cells were analyzed for phosphorylation of MBP. The results demonstrate that ERK activation is similar in ara-C-treated DT40 and DT40/Lyn−/− cells (Fig. 4A). Activation of ERK in response to IR treatment was also comparable in wild-type and Lyn-deficient DT40 cells (Fig. 4B). To assess involvement of Lyn in activation of p38 MAPK, anti-p38 MAPK immunoprecipitates from ara-C- and IR-treated cells were analyzed for phosphorylation of GST-ATF2. There was little if any difference in p38 MAPK activation in DT40 compared to DT40/Lyn−/− cells (Fig. 4C and D). CDDP is another genotoxic agent that forms DNA intrastrand cross-links (55) and induces both SAPK and p38 MAPK activation (29, 42). Analysis of CDDP-induced SAPK and p38 MAPK activity in DT40 and DT40/Lyn−/− cells demonstrated that Lyn is required for activation of SAPK but not p38 MAPK (data not shown). These findings indicate that activation of SAPK, and not ERK or p38 MAPK, is dependent on Lyn in the response to diverse DNA damaging agents.
FIG. 4.
Activation of ERK and p38 MAPK in wild-type and Lyn-deficient DT40 cells by DNA-damaging agents. (A and B) Cells were treated with 10 μM ara-C (A) or 20 Gy of IR (B) for the indicated times, lysed, and then subjected to immunoprecipitation with anti-ERK antibody. The immunoprecipitates were incubated with MBP and [γ-32P]ATP. MBP phosphorylation was assessed by SDS-PAGE and autoradiography (upper panels). Lysates were also subjected to immunoblot analysis with anti-ERK antibody (lower panels). (C and D) Cells were treated with 10 μM ara-C (C) or 20 Gy of IR (D), lysed, and then subjected to immunoprecipitation with anti-p38 MAPK antibody. The immunoprecipitates were incubated with GST-ATF2 and [γ-32P]ATP. GST-ATF2 phosphorylation was assessed by SDS-PAGE and autoradiography (upper panels). Lysates were also subjected to immunoblot analysis with anti-p38 MAPK antibody (lower panels).
Defective activation of SAPK in cells expressing a kinase-inactive Lyn mutant.
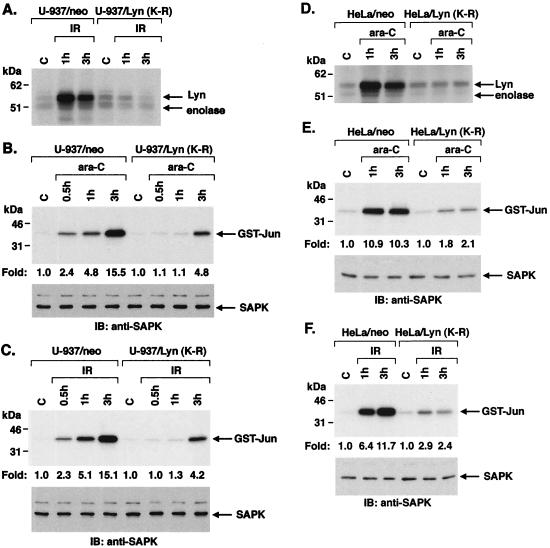
To confirm a role for Lyn in DNA damage-induced SAPK activity, we stably expressed a kinase inactive Lyn(K-R) mutant in U-937 cells. The finding that IR induces activity in U-937/neo but not U-937/Lyn(K-R) cells supported abrogation of the Lyn kinase function by expression of Lyn(K-R) (Fig. 5A). Anti-SAPK immunoprecipitates from the ara-C-treated U-937/neo and U-937/Lyn(K-R) cells were analyzed for phosphorylation of GST–c-Jun. In contrast to the induction of SAPK activity in ara-C-treated U-937/neo cells, SAPK activation was attenuated in the U-937/Lyn(K-R) cells (Fig. 5B). Studies with IR-treated cells also demonstrated that activation of SAPK is attenuated in U-937/Lyn(K-R) compared to U-937/neo cells (Fig. 5C). Similar results were obtained following treatment with CDDP (data not shown). There was no apparent difference, however, in ara-C-induced ERK or p38 MAPK activity in U-937/neo compared to U-937/Lyn(K-R) cells (data not shown). IR-induced ERK and p38 MAPK activity was also unaffected in U-937/Lyn(K-R) compared to U-937/neo cells (data not shown). To ensure that the findings obtained with Lyn(K-R) are applicable to other cell types, we prepared HeLa cells that stably express the empty vector or Lyn(K-R). Expression of Lyn(K-R) abrogated the activation of Lyn in response to DNA damage (Fig. 5D). In addition, ara-C-induced SAPK activity was attenuated in HeLa/Lyn(K-R) cells compared to that obtained with HeLa/neo cells (Fig. 5E). IR treatment of HeLa/Lyn(K-R) cells was also associated with an attenuated SAPK response (Fig. 5F). In contrast, expression of Lyn(K-R) had no effect on activation of ERK or p38 MAPK by these agents (data not shown). These findings, like those in DT40 cells, support the involvement of Lyn as an effector of SAPK, and not ERK or p38 MAPK, activation in the response to genotoxic agents.
FIG. 5.
Defective activation of SAPK in U-937 and HeLa cells expressing the kinase-inactive Lyn(K-R) mutant in response to DNA damage. (A) U-937/neo and U-937/Lyn(K-R) cells were exposed to 20 Gy of IR and harvested at the indicated times. Cell lysates were subjected to immunoprecipitation with anti-Lyn. The immunoprecipitates were analyzed for the phosphorylation of Lyn and enolase. (B and C) U-937/neo and U-937/Lyn(K-R) cells were treated with 10 μM ara-C (B) or 20 Gy of IR (C). Anti-SAPK immunoprecipitates were assayed for the phosphorylation of GST–c-Jun (upper panels). Lysates were also subjected to immunoblot analysis with anti-SAPK antibody (lower panels). (D) HeLa/neo and HeLa/Lyn(K-R) cells were treated with 10 μM ara-C. Anti-Lyn immunoprecipitates were assayed for the phosphorylation of Lyn and enolase. (E and F) HeLa/neo and HeLa/Lyn(K-R) cells were treated with 10 μM ara-C (E) or 20 Gy of IR (F). Anti-SAPK immunoprecipitates were assayed for the phosphorylation of GST–c-Jun (upper panels). Lysates were also subjected to immunoblot analysis with anti-SAPK (lower panels).
Overexpression of Lyn, but not Lyn(K-R), induces SAPK activity.
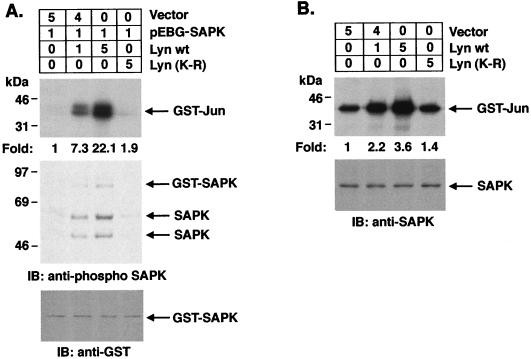
To establish a role for Lyn in SAPK activation, we performed transient-cotransfection studies with GST-SAPK and pSRα-neo/Lyn or pSRα-neo/Lyn(K-R) in 293T cells. Activation of SAPK was assessed by assaying precipitates from glutathione-Sepharose beads for phosphorylation of GST–c-Jun. Cotransfections with the wild-type Lyn vector demonstrated a dose-dependent induction of SAPK activity (Fig. 6A). As a control, there was no detectable activation of SAPK upon cotransfection with the kinase-inactive Lyn(K-R) mutant (Fig. 6A). To assess the effects of Lyn on activation of endogenous SAPK, anti-SAPK immunoprecipitates were analyzed from cells transfected with Lyn or Lyn(K-R). The results demonstrate that Lyn, but not Lyn(K-R), activates endogenous SAPK (Fig. 6B).
FIG. 6.
Overexpression of wild-type but not kinase-inactive Lyn induces SAPK activity. (A) 293T cells were cotransfected with the indicated amounts (in micrograms) of pEBG-SAPK and pSRα-neo (vector), pSRα-neo/Lyn, or pSRα-neo/Lyn(K-R) (top panel). At 48 h posttransfection, cells were lysed and incubated with glutathione-Sepharose beads for 1 h. In vitro kinase assays were performed on the resulting protein complexes by using GST–c-Jun as a substrate. Proteins were separated by SDS-PAGE and analyzed by autoradiography (second panel). Lysates were also subjected to immunoblot analysis with anti-phospho-SAPK (third panel) and anti-GST (bottom panel) antibodies. (B) 293T cells were transfected with pSRα-neo (vector), pSRα-neo/Lyn, or pSRα-neo/Lyn(K-R) as indicated (upper panel). After 48 h, anti-SAPK antibody immunoprecipitates were assayed for the phosphorylation of GST–c-Jun (middle panel). Lysates were also subjected to immunoblot analysis with anti-SAPK antibody (lower panel).
Lyn induces SAPK by a MKK7- and MEKK1-dependent mechanism.
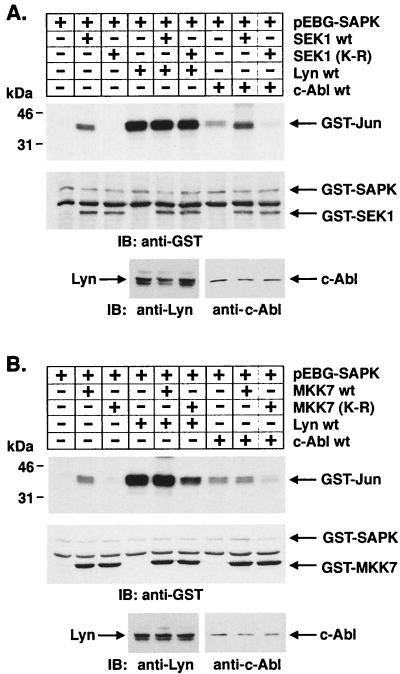
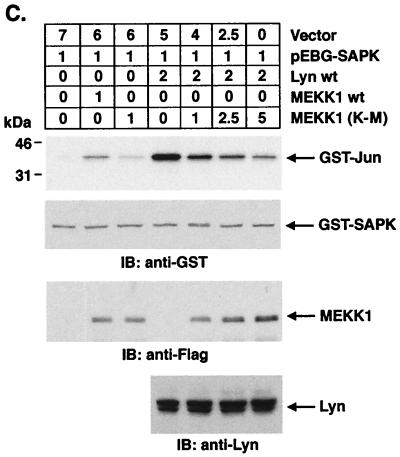
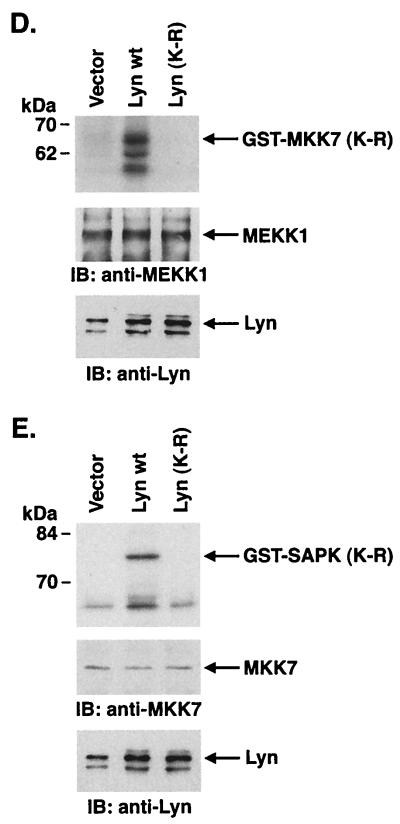
To define potential effectors downstream to Lyn in the SAPK pathway, cells were cotransfected with Lyn and wild-type SEK1 or an SEK1(K-R) mutant. Analysis of precipitates from glutathione-Sepharose beads demonstrated no detectable effect of SEK1(K-R) on Lyn-induced SAPK activity (Fig. 7A). By contrast, and as shown previously (28), activation of SAPK by c-Abl expression was blocked by SEK1(K-R) (Fig. 7A). Recent studies have demonstrated that SAPK can be activated by a SEK1-independent pathway involving MKK7 (19). To determine whether Lyn activates SAPK by a MKK7-dependent mechanism, lysates from cells cotransfected with Lyn and wild-type MKK7 or a kinase-inactive MKK7(K-R) mutant were subjected to precipitation with glutathione-Sepharose beads. Analysis of the precipitates for SAPK activity demonstrated that Lyn-induced activation of SAPK is inhibited by MKK7(K-R) (Fig. 7B). These findings demonstrate that Lyn activates SAPK by an SEK1-independent, MKK7-dependent mechanism. Since MEKK1 has been identified as another upstream effector of SAPK, we performed cotransfection experiments with Lyn and wild-type MEKK1 or a dominant-negative MEKK1(K-M) mutant. Analysis of precipitates from glutathione-Sepharose beads demonstrated that Lyn-induced activation of SAPK is inhibited by MEKK1(K-M) expression in a dose-dependent manner (Fig. 7C). To confirm these findings, 293T cells were transfected with empty vector, wild-type Lyn, or Lyn(K-R). Anti-MEKK1 immunoprecipitates were analyzed for phosphorylation of GST-MKK7(K-R). The results demonstrate activation of MEKK1 in cells transfected with wild-type Lyn but not in cells transfected with empty vector or Lyn(K-R) (Fig. 7D). Analysis of anti-MKK7 immunoprecipitates for phosphorylation of GST-SAPK(K-R) similarly demonstrated activation of MKK-7 only in cells transfected with wild-type Lyn (Fig. 7E). These results support a model in which Lyn-dependent activation of SAPK is mediated by MEKK1 and MKK7 in the response to DNA damage.
FIG. 7.
Lyn induces SAPK activation by an MKK7- and MEKK1-dependent, SEK1-independent mechanism. (A and B) 293T cells were cotransfected with pEBG-SAPK and pEBG-SEK1, pEBG-SEK1(K-R) (A), pCMV-GST-MKK7, pCMV-GST-MKK7(K-R) (B), pSRα-neo/Lyn, or pSRαMSV/c-Abl (top panel). The total DNA concentration was kept constant by including empty vector. At 48 h posttransfection, cells were lysed and incubated with glutathione-Sepharose beads for 1 h. In vitro kinase assays were performed by using GST–c-Jun as a substrate. Proteins were separated by SDS-PAGE and analyzed by autoradiography (second panel). Lysates were also subjected to immunoblot analysis with anti-GST (third panel), anti-Lyn, and anti-c-Abl (bottom panel) antibodies. (C) 293T cells were cotransfected with the indicated amounts (in micrograms) of pEBG-SAPK and pcDNA3-Flag/MEKK1, pcDNA3-Flag/MEKK1(K-M), or pSRα-neo/Lyn (top panel). At 48 h posttransfection, precipitates by glutathione-Sepharose beads were assayed for the phosphorylation of GST–c-Jun (second panel). Lysates were also subjected to immunoblot analysis with anti-GST (third panel), anti-Flag (fourth panel), and anti-Lyn (bottom panel) antibodies. (D and E) 293T cells were transfected with 3 μg of pSRα-neo (vector), pSRα-neo/Lyn, or pSRα-neo/Lyn(K-R). At 48 h posttransfection, cells were lysed and incubated with anti-MEKK1 (D) or anti-MKK7 (E) followed by incubation with protein G-Sepharose beads. The immunoprecipitates were assayed for the phosphorylation of 5 μg of GST-MKK7(K-R) (D) or GST-SAPK(K-R) (E) (upper panels). Lysates were also subjected to immunoblot analysis with anti-MEKK1 (D), anti-MKK7 (E) (middle panels), or anti-Lyn (lower panels) antibodies.
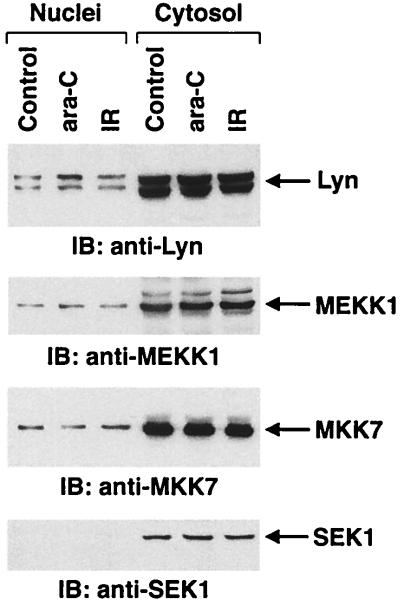
Previous studies have demonstrated that nuclear Lyn is activated by DNA-damaging agents (33, 34, 69). To determine whether Lyn associates with MEKK1 and/or MKK7, cells were treated with ara-C or IR, and then nuclear and cytoplasmic fractions were subjected to immunoprecipitation with anti-Lyn. Analysis of the immunoprecipitates with anti-MEKK1 and anti-MKK7 demonstrated no evidence for Lyn-MEKK or Lyn-MKK7 complexes (data not shown). In addition, there was little, if any, effect of genotoxic stress on nuclear levels of Lyn (Fig. 8). The results also demonstrate that MEKK-1 and MKK7, but not SEK1, are detectable in the nucleus and that their nuclear expression is unaffected by genotoxic stress (Fig. 8). These findings indicate that Lyn is not translocated to the cytosol in the response to DNA damage and that other members of the Lyn→MEKK1→MKK7 cascade are also localized to the nucleus.
FIG. 8.
Localization of Lyn, MEKK1, MKK7, and SEK1 in U-937 cells. U-937 cells were treated with 10 μM ara-C or 20 Gy of IR and harvested at 1 h. Equal amounts of protein from nuclei and cytosol were subjected to immunoblot analysis with the indicated antibodies.
Role for the Lyn→SAPK pathway in the apoptotic response to DNA damage.
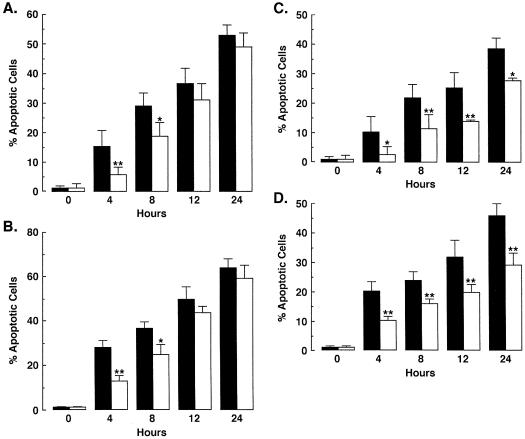
SAPK is activated by diverse DNA-damaging agents and has been implicated as an effector of apoptosis (6, 21, 59, 61, 75). The demonstration that Lyn regulates SAPK in the DNA damage response thus suggested that the Lyn→SAPK pathway may contribute to the induction of apoptosis. To determine whether Lyn is involved in the apoptotic response, we treated wild-type and Lyn-deficient DT40 cells with ara-C and assayed for the percentage of cells with sub-G1 DNA. The results demonstrate that, compared to wild-type cells, there was significantly less apoptosis of the DT40/Lyn−/− cells at 4 and 8 h of ara-C exposure (Fig. 9A). The finding that the percentage of apoptotic wild-type and Lyn-deficient cells was similar at 12 and 24 h indicated that this response is delayed in the absence of Lyn expression (Fig. 9A). Similar findings were obtained after IR exposure (Fig. 9B). To extend the analysis, ara-C-treated U-937/neo and U-937/Lyn(K-R) cells were also assessed for the percentage of cells with sub-G1 DNA content. The induction of apoptosis by ara-C was decreased in U-937/Lyn(K-R) cells compared to U-937/neo cells (Fig. 9C). Similar results were obtained after IR treatment (Fig. 9D). These findings provide support for the involvement of Lyn in the apoptotic response to DNA damage.
FIG. 9.
Lyn is involved in the apoptotic response to DNA damage. (A and B) DT40 (■) and Lyn-deficient (□) cells were treated with 10 μM ara-C (A) or 20 Gy of IR (B) and harvested at the indicated times. (C and D) U-937/neo (■) and U-937/Lyn(K-R) (□) cells were also treated with 10 μM ara-C (A) or 20 Gy of IR (B). The DNA content was analyzed by flow cytometry with propidium iodide staining. The results (mean ± the standard deviation [SD] of three independent experiments) are presented as the percentage of apoptotic cells with sub-G1 DNA. As determined by t test, the significance of the differences is indicated (∗, P < 0.05; ∗∗, P < 0.01).
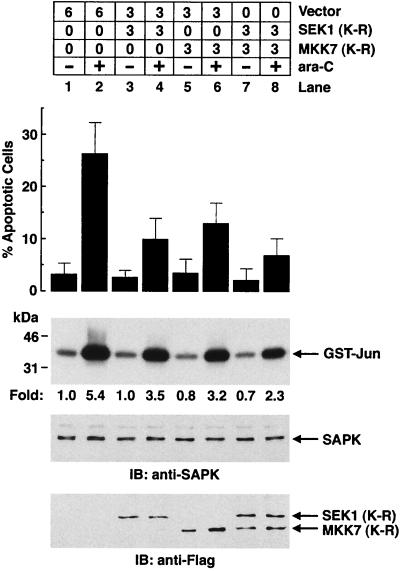
To determine whether activation of SAPK is associated with ara-C-induced apoptosis, cells were transfected with SEK1(K-R) to block the c-Abl→SAPK cascade. Expression of SEK1(K-R) resulted in inhibition of both ara-C-induced apoptosis and SAPK activation (Fig. 10). Similar results were obtained by expressing the MKK7(K-R) mutant (Fig. 10). Moreover, expression of both SEK1(K-R) and MKK7(K-R) was associated with further decreases in the induction of apoptosis and SAPK activity by ara-C-treatment to block Lyn→SAPK signaling (Fig. 10). These results demonstrate that inhibition of DNA damage-induced SAPK activation by both the c-Abl→SAPK and Lyn→SAPK pathways is associated with abrogation of the apoptotic response.
FIG. 10.
Expression of the dominant-negative form of SEK1 and MKK7 synergistically inhibits SAPK activity and ara-C-induced apoptosis. 293T cells were transfected with the indicated amounts (in micrograms) of pCMV-Flag (vector), pCMV-Flag/SEK1(K-R), or pCMV-Flag/MKK7(K-R) (top panel). At 36 h posttransfection, cells were left untreated or were treated with 10 μM ara-C and harvested at 24 h after treatment. DNA content was analyzed by flow cytometry with propidium iodide staining. The results (mean ± the SD of three independent experiments) are presented as the percentage of apoptotic cells with sub-G1 DNA (second panel). The transfected 293T cells were also harvested at 3 h after ara-C treatment. Anti-SAPK immunoprecipitates were assayed for the phosphorylation of GST–c-Jun as a substrate (third panel). Lysates were also subjected to immunoblot analysis with anti-SAPK (fourth panel) and anti-Flag (bottom panel) antibodies.
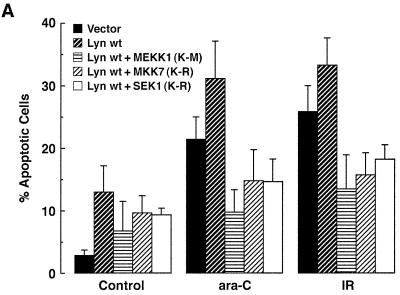
To extend these findings and to determine whether Lyn→SAPK signaling affects the apoptotic response, we transiently transfected 293T cells with Lyn and then treated them with ara-C or IR. Overexpression of Lyn was associated with the induction of apoptosis in control cells and an increase in the apoptotic response to ara-C and IR (Fig. 11A). Cotransfection of Lyn and MEKK1(K-M) resulted in the inhibition of ara-C- and IR-induced apoptosis (Fig. 11A). Similar effects were observed when Lyn was cotransfected with MKK7(K-R) or SEK1(K-R) (Fig. 11A). These results indicate that inhibition of the Lyn→SAPK pathway with MKK7(K-R) results in an abrogation of the apoptotic response similar to that observed when blocking the c-Abl→SAPK cascade with SEK1(K-R). As another measure of apoptosis, internucleosomal DNA fragmentation was decreased in ara-C- and IR-treated cells expressing the MKK7(K-R) mutant (Fig. 11B). These findings indicated that the Lyn→MEKK1→MKK7→SAPK pathway is functional in the apoptotic response to DNA damage.
FIG. 11.
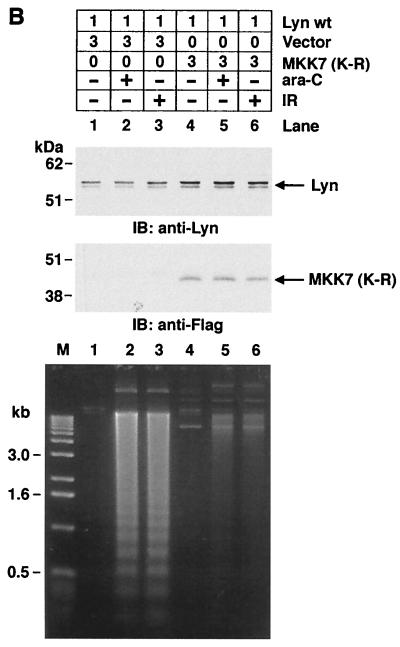
SAPK regulates the Lyn-dependent apoptotic response to DNA damage. (A) 293T cells were cotransfected with 1 μg of pSRα-neo/Lyn and 3 μg of pcDNA3-Flag/MEKK1(K-M), pCMV-Flag/MKK7(K-R), or pCMV-Flag/SEK1(K-R). As a control, transfections of vector alone were also included. At 36 h posttransfection, cells were left untreated or treated with 10 μM ara-C or 20 Gy of IR for 24 h. The results (mean ± the SD of three independent experiments) are presented as the percentage of apoptotic cells with sub-G1 DNA. (B) 293T cells were cotransfected with the indicated amounts of pSRα-neo/Lyn and pCMV-Flag (vector; lanes 1, 2, and 3) or pCMV-Flag-MKK7(K-R) (lanes 4, 5, and 6) (top panel). Transfection efficiency as determined with pcDNA3-LacZ and β-gel staining was 78.4% ± 7.5%. At 36 h posttransfection, cells were left untreated (lanes 1 and 4) or were treated with 10 μM ara-C (lanes 2 and 5) or 20 Gy of IR (lanes 3 and 6) and collected at 12 h after treatment. Cells were lysed and subjected to immunoblot analysis with anti-Lyn (second panel) and anti-Flag antibodies (third panel) to assess the levels of expression of transfected Lyn and Flag-MKK7(K-R). Cellular DNA was extracted and subjected to 1.5% agarose gel electrophoresis for analysis of DNA fragmentation (bottom panel).
DISCUSSION
The mechanisms by which genotoxic stress is converted into intracellular signals that control cell behavior are for the most part unknown. Certain insights into signals involved in the genotoxic stress response have been derived from the finding that the Lyn tyrosine kinase is activated by agents that arrest DNA replication or induce DNA lesions (26, 69). Lyn is a member of the Src family of tyrosine kinases and, as a result of alternate splicing mechanisms, is expressed as p56lyn and p53lyn forms (63, 64). The Src-like kinases are often associated with the inner surface of the plasma membrane and transduce signals from cell surface receptors (4). In this context, Lyn associates with the B-cell antigen receptor complex and participates in signaling to phosphatidylinositol 3-kinase and protein kinase C (43, 62). Other studies have demonstrated that Lyn is expressed in the nucleus and that nuclear, but not cytoplasmic, Lyn is activated by genotoxic agents (24, 69). The functional significance of nuclear Lyn activation has been attributed to the downregulation of Cdc2 and Cdk2 and thereby cell cycle arrest in the DNA damage response (2, 26, 32, 69). These findings have been extended in the present studies with the demonstration that Lyn also functions in the activation of SAPK in the response to genotoxic stress. A selective role for Lyn in the regulation of the SAPK pathway is supported by studies in Syk-deficient cells. In addition, selectivity of Lyn→SAPK signaling in the response to DNA damage is supported by the findings that osmotic-stress-induced SAPK activation is independent of Lyn expression (44). The results also demonstrate that Lyn has little, if any, effect on regulation of ERK or p38 MAPK activation in response to treatment with genotoxic agents.
SAPK is activated in diverse cell types by agents, such as ara-C, that block DNA replication and by those, such as IR, that induce DNA strand breaks (5, 6, 28, 29, 31, 59). In eukaryotic cells, checkpoints monitor for the presence of unreplicated or damaged DNA and either block cell cycle progression or induce apoptosis (16). Thus, the findings that SAPK is activated by ara-C and IR have supported induction of this pathway by both DNA replication and DNA damage checkpoints. Previous studies have shown that the nuclear c-Abl tyrosine kinase is an upstream effector of the SAPK response to both replication arrest and DNA damage (29, 31). In this context, activation of SAPK in the response of proliferating cells to genotoxic agents is in part dependent on a c-Abl-mediated mechanism (29, 31). Also, activated forms of Abl confer induction of SAPK activity (47, 49, 52). The present results similarly demonstrate that expression of kinase-active Lyn is associated with SAPK activation. The demonstration that overexpression of Lyn(K-R) has no effect on activation of SAPK provides further support for dependence on the Lyn kinase function. In addition, stable expression of Lyn(K-R) in different cell types blocked both ara-C and IR-induced SAPK activity. These findings thus support a model in which activation of Lyn by DNA replication and DNA damage checkpoints transduces signals that function upstream to the SAPK pathway.
Lyn and c-Abl form a nuclear complex (35) and both tyrosine kinases interact with the DNA-PK catalytic subunit (3, 57, 58). Nuclear c-Abl regulates activation of SAPK in the response to genotoxic stress by a mechanism dependent on SEK1 (29). Other studies have demonstrated that induction of SAPK activity by activated forms of Abl is blocked by the kinase-inactive SEK1(K-R) mutant (52). The present studies demonstrate that, while SEK1(K-R) blocks c-Abl-induced SAPK activation, the SEK1(K-R) mutant has no detectable effect on Lyn-mediated induction of SAPK activity. These findings supported an alternate pathway for Lyn→SAPK signaling. Another member of the MAPK kinase group, designated MKK7, has been identified as an activator of SAPK and not p38 MAPK (11, 57, 67). Expression of MKK7 causes activation of the SAPK pathway (57). In addition, a kinase-negative MKK7(K-R) mutant inhibits interleukin-1β-, lipopolysaccharide-, and MEKK1-induced SAPK activation (67). Other studies have indicated that, while MKK7 is activated by diverse stimuli (11, 17, 40), this kinase has not been implicated in the DNA damage response. Our results demonstrate that expression of MKK7(K-R) blocks Lyn-induced SAPK activation. The results also demonstrate that the kinase-inactive MEKK1(K-M) mutant blocks Lyn-induced SAPK activation. MEKK1(K-M) also blocks activation of SAPK by c-Abl expression (unpublished data). In this context, MEKK1 functions as an upstream effector of both SEK1 (66) and MKK7 (17, 67). Our findings thus support a model in which Lyn activates the MEKK1→MKK7→SAPK pathway.
Recent studies have demonstrated that Lyn interacts with the SHPTP1 protein tyrosine phosphatase in cells treated with genotoxic agents (68). In addition, expression of SHPTP1 attenuates the apoptotic response to DNA damage (68). More recent studies have demonstrated that expression of SHPTP1 inhibits Lyn-mediated induction of SAPK activity (unpublished data). These findings and the association of SAPK activation with induction of apoptosis suggest that the functional significance of Lyn→SAPK signaling resides in the apoptotic response to genotoxic stress. In this regard, stable expression of Lyn(K-R) in U-937 cells partially abrogated the induction of apoptosis in response to ara-C and IR treatment. To address whether Lyn-dependent induction of apoptosis by genotoxic agents is mediated by the SAPK pathway, we treated cells that express the MKK7(K-R) mutant. The results demonstrate that the apoptotic response to both ara-C and IR is attenuated by inhibition of the MKK7→SAPK pathway. These findings support a model in which induction of apoptosis by arrest of DNA replication and DNA damage is mediated at least in part by a Lyn→SAPK signaling cascade.
ACKNOWLEDGMENTS
We thank T. Kurosaki for DT40 B cells and chicken Lyn cDNA, L. Zon and J. Kyriakis for SAPK and SEK1 cDNAs, H. Itoh for SEK1 and MKK7 cDNAs, S. Ohno for MEKK1 cDNA, and T. Yamamoto for Lyn cDNA.
This investigation was supported by PHS grants CA29431, CA55241 (D.K.) and CA75216 (S.K.) awarded by the National Cancer Institute, Department of Health and Human Services.
REFERENCES
- 1.Agami R, Blandino G, Oren M, Shaul Y. Interaction of c-Abl and p73α and their collaboration to induce apoptosis. Nature. 1999;399:809–813. doi: 10.1038/21697. [DOI] [PubMed] [Google Scholar]
- 2.Al-Khodairy F, Carr A M. DNA repair mutants defining G2 checkpoint pathways in Schizosaccharomyces pombe. EMBO J. 1992;11:1343–1350. doi: 10.1002/j.1460-2075.1992.tb05179.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aruoma O I, Halliwell B, Hoey B M, Butler J. The antioxidant action of N-acetylcysteine: its reaction with hydrogen peroxide, hydroxyl radical, superoxide, and hypochlorous acid. Free Radic Biol Med. 1989;6:593–597. doi: 10.1016/0891-5849(89)90066-x. [DOI] [PubMed] [Google Scholar]
- 4.Cantley L, Auger K R, Carpenter C, Duckworth B, Graziani A, Kapeller R, Soltoff S. Oncogenes and signal transduction. Cell. 1991;64:281–302. doi: 10.1016/0092-8674(91)90639-g. [DOI] [PubMed] [Google Scholar]
- 5.Chen Y-R, Meyer C F, Tan T-H. Persistent activation of c-Jun N-terminal kinase 1 (JNK1) in γ radiation-induced apoptosis. J Biol Chem. 1996;271:631–634. doi: 10.1074/jbc.271.2.631. [DOI] [PubMed] [Google Scholar]
- 6.Chen Y-R, Wang X, Templeton D, Davis R J, Tan T-H. The role of c-Jun N-terminal kinase (JNK) in apoptosis induced by ultraviolet C and γ radiation. J Biol Chem. 1996;271:31929–31936. doi: 10.1074/jbc.271.50.31929. [DOI] [PubMed] [Google Scholar]
- 7.Datta R, Kharbanda S, Kufe D. Regulation of jun-B gene expression by 1-β-d-arabinofuranosylcytosine in human myeloid leukemia cells. Mol Pharmacol. 1990;38:435–439. [PubMed] [Google Scholar]
- 8.Datta R, Rubin E, Sukhatme V, Qureshi S, Weichselbaum R, Kufe D. Ionizing radiation activates transcription of the EGR-1 gene via CArG elements. Proc Natl Acad Sci USA. 1992;89:10149–10153. doi: 10.1073/pnas.89.21.10149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davis R J. MAPKs: new JNK expands the group. Trends Biochem Sci. 1994;19:470–473. doi: 10.1016/0968-0004(94)90132-5. [DOI] [PubMed] [Google Scholar]
- 10.Derijard B, Hibi M, Wu I H, Barrett T, Su B, Deng T, Karin M, Davis R J. JNK1: A protein kinase stimulated by UV light and Ha-Ras that binds and phosphorylates the c-Jun activation domain. Cell. 1994;76:1025–1037. doi: 10.1016/0092-8674(94)90380-8. [DOI] [PubMed] [Google Scholar]
- 11.Foltz I N, Gerl R E, Wieler J S, Luckach M, Salmon R A, Schrader J W. Human mitogen-activated protein kinase kinase 7 (MKK7) is a highly conserved c-Jun N-terminal kinase/stress-activated protein kinase (JNK/SAPK) activated by environmental stresses and physiological stimuli. J Biol Chem. 1998;273:9344–9351. doi: 10.1074/jbc.273.15.9344. [DOI] [PubMed] [Google Scholar]
- 12.Georgiev O, Bourquin J P, Gstaiger M, Knoepfel L, Schaffner W, Hovens C. Two versatile eukaryotic vectors permitting epitope tagging, radiolabelling and nuclear localisation of expressed proteins. Gene. 1996;168:165–167. doi: 10.1016/0378-1119(95)00764-4. [DOI] [PubMed] [Google Scholar]
- 13.Goga A, Liu X, Hambuch T M, Senechal K, Major E, Berk A J, Witte O N, Sawyers C L. p53 dependent growth suppression by the c-Abl nuclear tyrosine kinase. Oncogene. 1995;11:791–799. [PubMed] [Google Scholar]
- 14.Gong J, Costanzo A, Yang H-Q, Melino G, Kaelin W G, Jr, Levrero M, Wang J Y J. The tyrosine kinase c-Abl regulates p73 in response to cisplatin-induced DNA damage. Nature. 1999;399:806–809. doi: 10.1038/21690. [DOI] [PubMed] [Google Scholar]
- 15.Gupta S, Campbell D, Dérijard B, Davis R J. Transcription factor ATF2 regulation by the JNK signal transduction pathway. Science. 1995;267:389–393. doi: 10.1126/science.7824938. [DOI] [PubMed] [Google Scholar]
- 16.Hartwell L H, Weinert T A. Checkpoints: controls that ensure the order of cell cycle events. Science. 1989;246:629–634. doi: 10.1126/science.2683079. [DOI] [PubMed] [Google Scholar]
- 17.Hirai S, Noda K, Moriguchi T, Nishida E, Yamashita A, Deyama T, Fukuyama K, Ohno S. Differential activation of two JNK activators, MKK7 and SEK1, by MKN28-derived nonreceptor serine/threonine kinase/mixed lineage kinase 2. J Biol Chem. 1998;273:7406–7412. doi: 10.1074/jbc.273.13.7406. [DOI] [PubMed] [Google Scholar]
- 18.Hirano M, Osada S, Aoki T, Hirai S, Hosaka M, Inoue J, Ohno S. MEK kinase is involved in tumor necrosis factor α-induced NF-κB activation and degradation of IκB-α. J Biol Chem. 1996;271:13234–13238. doi: 10.1074/jbc.271.22.13234. [DOI] [PubMed] [Google Scholar]
- 19.Ip Y T, Davis R J. Signal transduction by the c-Jun N-terminal kinase (JNK)—from inflammation to development. Curr Opin Cell Biol. 1998;10:205–219. doi: 10.1016/s0955-0674(98)80143-9. [DOI] [PubMed] [Google Scholar]
- 20.Jin S, Kharbanda S, Mayer B, Kufe D, Weaver D T. Binding of Ku and c-Abl at the kinase homology region of DNA-dependent protein kinase catalytic subunit. J Biol Chem. 1997;272:24763–24766. doi: 10.1074/jbc.272.40.24763. [DOI] [PubMed] [Google Scholar]
- 21.Johnson N L, Gardner A M, Diener K M, Lange-Carter C A, Gleavy J, Jarpe M B, Minden A, Karin M, Zon L I, Johnson G L. Signal transduction pathways regulated by mitogen-activated/extracellular response kinase kinase kinase induce cell death. J Biol Chem. 1996;271:3229–3237. doi: 10.1074/jbc.271.6.3229. [DOI] [PubMed] [Google Scholar]
- 22.Kaneki M, Kharbanda S, Pandey P, Yoshida K, Takekawa M, Liou J-R, Stone R, Kufe D. Functional role for protein kinase Cβ as a regulator of stress-activated protein kinase activation and monocytic differentiation of myeloid leukemia cells. Mol Cell Biol. 1999;19:461–470. doi: 10.1128/mcb.19.1.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kemp L M, Sedgwick S G, Jeggo P A. X-ray sensitive mutants of Chinese hamster ovary cells defective in double-strand break rejoining. Mutat Res. 1984;132:189–196. doi: 10.1016/0167-8817(84)90037-3. [DOI] [PubMed] [Google Scholar]
- 24.Kharbanda S, Bharti A, Pei D, Wang J, Pandey P, Ren R, Weichselbaum R, Walsh C T, Kufe D. The stress response to ionizing radiation involves c-Abl-dependent phosphorylation of SHPTP1. Proc Natl Acad Sci USA. 1996;93:6898–6901. doi: 10.1073/pnas.93.14.6898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kharbanda S, Datta R, Kufe D. Regulation of c-jun gene expression in HL-60 leukemia cells by 1-β-d-arabinofuranosylcytosine. Potential involvement of a protein kinase C dependent mechanism. Biochemistry. 1991;30:7947–7952. doi: 10.1021/bi00246a011. [DOI] [PubMed] [Google Scholar]
- 26.Kharbanda S, Huberman E, Kufe D. Activation of the jun-D gene in human myeloid cells by 1-β-d-arabinofuranosylcytosine. Biochem Pharmacol. 1993;45:2055–2061. doi: 10.1016/0006-2952(93)90016-p. [DOI] [PubMed] [Google Scholar]
- 27.Kharbanda S, Pandey P, Jin S, Inoue S, Bharti A, Yuan Z-M, Weichselbaum R, Weaver D, Kufe D. Functional interaction of DNA-PK and c-Abl in response to DNA damage. Nature. 1997;386:732–735. doi: 10.1038/386732a0. [DOI] [PubMed] [Google Scholar]
- 28.Kharbanda S, Pandey P, Ren R, Feller S, Mayer B, Zon L, Kufe D. c-Abl activation regulates induction of the SEK1/stress activated protein kinase pathway in the cellular response to 1-β-d-arabinofuranosylcytosine. J Biol Chem. 1995;270:30278–30281. doi: 10.1074/jbc.270.51.30278. [DOI] [PubMed] [Google Scholar]
- 29.Kharbanda S, Ren R, Pandey P, Shafman T D, Feller S M, Weichselbaum R R, Kufe D W. Activation of the c-Abl tyrosine kinase in the stress response to DNA-damaging agents. Nature. 1995;376:785–788. doi: 10.1038/376785a0. [DOI] [PubMed] [Google Scholar]
- 30.Kharbanda S, Rubin E, Gunji H, Hinz H, Giovanella B, Pantazis P, Kufe D. Camptothecin and its derivatives induce expression of the c-jun protooncogene in human myeloid leukemia cells. Cancer Res. 1991;51:6636–6642. [PubMed] [Google Scholar]
- 31.Kharbanda S, Saleem A, Shafman T, Emoto Y, Weichselbaum R, Woodgett J, Avruch J, Kyriakis J, Kufe D. Ionizing radiation stimulates a Grb2-mediated association of the stress activated protein kinase with phosphatidylinositol 3-kinase. J Biol Chem. 1995;270:18871–18874. doi: 10.1074/jbc.270.32.18871. [DOI] [PubMed] [Google Scholar]
- 32.Kharbanda S, Saleem A, Yuan Z-M, Kraeft S, Weichselbaum R, Chen L B, Kufe D. Nuclear signaling induced by ionizing radiation involves colocalization of the activated p56/p53lyn tyrosine kinase with p34cdc2. Cancer Res. 1996;56:3617–3621. [PubMed] [Google Scholar]
- 33.Kharbanda S, Yuan Z, Taneja N, Weichselbaum R, Kufe D. p56/p53lyn tyrosine kinase activation in mammalian cells treated with mitomycin C. Oncogene. 1994;9:3005–3011. [PubMed] [Google Scholar]
- 34.Kharbanda S, Yuan Z-M, Weichselbaum R, Kufe D. Activation of the Src-like p56/p53lyn tyrosine kinase by ionizing radiation. J Biol Chem. 1994;269:20739–20743. [PubMed] [Google Scholar]
- 35.Kharbanda S, Yuan Z-M, Weichselbaum R, Kufe D. Determination of cell fate by c-Abl activation in the response to DNA damage. Oncogene. 1998;17:3309–3318. doi: 10.1038/sj.onc.1202571. [DOI] [PubMed] [Google Scholar]
- 36.Kufe D, Munroe D, Herrick D, Spriggs D. Effects of ara-C incorporation on eukaryotic DNA template function. Mol Pharmacol. 1984;26:128–134. [PubMed] [Google Scholar]
- 37.Kumar S, Pandey P, Bharti A, Jin S, Weichselbaum R, Weaver D, Kufe D, Kharbanda S. Regulation of DNA-dependent protein kinase by the Lyn tyrosine kinase. J Biol Chem. 1998;273:25654–25658. doi: 10.1074/jbc.273.40.25654. [DOI] [PubMed] [Google Scholar]
- 38.Kyriakis J M, Banerjee P, Nikolakaki E, Dai T, Rubie E A, Ahmad M F, Avruch J, Woodgett J R. The stress-activated protein kinase subfamily of c-Jun kinases. Nature. 1994;369:156–160. doi: 10.1038/369156a0. [DOI] [PubMed] [Google Scholar]
- 39.Maruo A, Oishi I, Sada K, Nomi M, Kurosaki T, Minami Y, Yamamura H. Protein tyrosine kinase Lyn mediates apoptosis induced by topoisomerase II inhibitors in DT40 cells. Int Immunol. 1999;11:1371–1380. doi: 10.1093/intimm/11.9.1371. [DOI] [PubMed] [Google Scholar]
- 40.Moriguchi T, Toyoshima F, Masuyama N, Hanafusa H, Gotoh Y, Nishida E. A novel SAPK/JNK kinase, MKK7, stimulated by TNFα and cellular stresses. EMBO J. 1997;16:7045–7053. doi: 10.1093/emboj/16.23.7045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ohno Y, Spriggs D, Matsukage A, Ohno T, Kufe D. Effects of 1-β-d-arabinofuranosylcytosine incorporation on elongation of specific DNA sequences by DNA polymerase β. Cancer Res. 1988;48:1494–1498. [PubMed] [Google Scholar]
- 42.Pandey P, Raingeaud J, Kaneki M, Weichselbaum R, Davis R, Kufe D, Kharbanda S. Activation of p38 mitogen-active protein kinase by c-Abl-dependent and -independent mechanisms. J Biol Chem. 1996;271:23775–23779. doi: 10.1074/jbc.271.39.23775. [DOI] [PubMed] [Google Scholar]
- 43.Pleiman C M, Clark M R, Timson Gauen L K, Winitz S, Coggeshall K M, Johnson G L, Shaw A S, Cambier J C. Mapping of sites on the Src family protein tyrosine kinases p55blk, p59fyn, and p56lyn which interact with the effector molecules phospholipase C-γ2, microtubule-associated protein kinase, GTPase-activating protein, and phosphatidylinositol 3-kinase. Mol Cell Biol. 1993;13:5877–5887. doi: 10.1128/mcb.13.9.5877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Qin S, Minami Y, Kurosaki T, Yamamura H. Distinctive functions of Syk and Lyn in mediating osmotic stress- and ultraviolet C irradiation-induced apoptosis in chicken B cells. J Biol Chem. 1997;272:17994–17999. doi: 10.1074/jbc.272.29.17994. [DOI] [PubMed] [Google Scholar]
- 45.Radford I R. The level of induced DNA double-strand breakage correlates with cell killing after X-irradiation. International J Rad Biol. 1985;48:45–54. doi: 10.1080/09553008514551051. [DOI] [PubMed] [Google Scholar]
- 46.Raingeaud J, Whitmarsh A J, Barrett T, Derijard B, Davis R J. MKK3- and MKK6-regulated gene expression is mediated by the p38 mitogen-activated signal transduction pathway. Mol Cell Biol. 1996;16:1247–1255. doi: 10.1128/mcb.16.3.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Raitano A B, Halpern J R, Hambuch T M, Sawyers C L. The Bcr-Abl leukemia oncogene activates Jun kinase and requires Jun for transformation. Proc Natl Acad Sci USA. 1995;92:11746–11750. doi: 10.1073/pnas.92.25.11746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Renshaw M W, Lea-Chou E, Wang J Y J. Rac is required for v-Abl tyrosine kinase to activate mitogenesis. Curr Biol. 1996;6:76–83. doi: 10.1016/s0960-9822(02)00424-4. [DOI] [PubMed] [Google Scholar]
- 49.Renshaw M W, McWhirter J R, Wang J Y J. The human leukemia oncogene bcr-abl abrogates the anchorage requirement but not the growth factor requirement for proliferation. Mol Cell Biol. 1995;15:1286–1293. doi: 10.1128/mcb.15.3.1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Saleem A, Datta R, Kharbanda S, Kufe D. Involvement of stress activated protein kinase in the cellular response to 1-β-d-arabinofuranosylcytosine and other DNA-damaging agents. Cell Growth Differ. 1995;6:1651–1658. [PubMed] [Google Scholar]
- 51.Saleem A, Yuan Z-M, Kufe D W, Kharbanda S M. Activation of serine/threonine protein kinases and early growth response 1 gene expression by tumor necrosis factor in human myeloid leukemia cells. J Immunol. 1995;154:4150–4156. [PubMed] [Google Scholar]
- 52.Sanchez I, Hughes R T, Mayer B J, Yee K, Woodgett J R, Avruch J, Kyriakis J M, Zon L I. Role of SAPK/ERK kinase-1 in the stress-activated pathway regulating transcription factor c-Jun. Nature. 1994;372:794–798. doi: 10.1038/372794a0. [DOI] [PubMed] [Google Scholar]
- 53.Sawyers C L, McLaughlin J, Goga A, Havilik M, Witte O. The nuclear tyrosine kinase c-Abl negatively regulates cell growth. Cell. 1994;77:121–131. doi: 10.1016/0092-8674(94)90240-2. [DOI] [PubMed] [Google Scholar]
- 54.Sherman M, Datta R, Hallahan D, Weichselbaum R, Kufe D. Ionizing radiation regulates expression of the c-jun protooncogene. Proc Natl Acad Sci USA. 1990;87:5663–5666. doi: 10.1073/pnas.87.15.5663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sherman S E, Lippard S J. Structural aspects of platinum anticancer drug interaction with DNA. J Chem Rev. 1987;87:1153–1181. [Google Scholar]
- 56.Takata M, Sabe H, Hata A, Inazu T, Homma Y, Nukada T, Yamamura H, Kurosaki T. Tyrosine kinases Lyn and Syk regulate B cell receptor-coupled Ca2+ mobilization through distinct pathways. EMBO J. 1994;13:1341–1349. doi: 10.1002/j.1460-2075.1994.tb06387.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tournier C, Whitmarsh A J, Cavanagh J, Barrett T, Davis R J. Mitogen-activated protein kinase kinase 7 is an activator of the c-Jun NH2-terminal kinase. Proc Natl Acad Sci USA. 1997;94:7337–7342. doi: 10.1073/pnas.94.14.7337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Valius M, Kozlauskas A. Phospholipase C-γ1 and phosphatidylinositol 3 kinase are the downstream mediators of the PKGF receptor's mitogenic signal. Cell. 1993;73:321–334. doi: 10.1016/0092-8674(93)90232-f. [DOI] [PubMed] [Google Scholar]
- 59.Verheij M, Bose R, Lin X H, Yhao B, Jarvis W D, Grant S, Birrer M J, Szabo E, Zon L I, Kyriakis J M, Haimovitz-Friedman A, Fuks Z, Kolesnick R N. Requirement for ceramide-initiated SAPK/JNK signalling in stress-induced apoptosis. Nature. 1996;380:75–79. doi: 10.1038/380075a0. [DOI] [PubMed] [Google Scholar]
- 60.Whitmarsh A J, Shore P, Sharrocks A D, Davis R J. Integration of MAP kinase signal transduction pathways at the serum response element. Science. 1995;269:403–407. doi: 10.1126/science.7618106. [DOI] [PubMed] [Google Scholar]
- 61.Xia Z, Dickens M, Raingeaud J, Davis R J, Greenberg M E. Opposing effects of ERK and JNK-p38 MAP kinases on apoptosis. Science. 1995;270:1326–1331. doi: 10.1126/science.270.5240.1326. [DOI] [PubMed] [Google Scholar]
- 62.Yamanashi Y, Fukui Y, Wongsasant B, Kinoshita Y, Ichimori Y, Toyoshima K, Yamamoto T. Activation of Src-like protein-tyrosine kinase Lyn and its association with phosphatidylinositol 3-kinase upon B-cell antigen receptor-mediated signaling. Proc Natl Acad Sci USA. 1992;89:1118–1122. doi: 10.1073/pnas.89.3.1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yamanashi Y, Fukushige S, Semba K, Sukegawa J, Miyajima N, Matsubara K-I, Yamamoto T, Toyoshima K. The yes-related cellular gene lyn encodes a possible tyrosine kinase similar to p56lck. Mol Cell Biol. 1987;7:237–243. doi: 10.1128/mcb.7.1.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yamanashi Y, Mori S, Yoshida M, Kishimoto T, Inoue K, Yamamoto T, Toyoshima K. Selective expression of a protein-tyrosine kinase, p56lyn, in hematopoietic cells and association with production of human T-cell lymphotrophic virus type I. Proc Natl Acad Sci USA. 1989;86:6538–6542. doi: 10.1073/pnas.86.17.6538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yamauchi J, Kaziro Y, Itoh H. Differential regulation of mitogen-activated protein kinase kinase 4 (MKK4) and 7 (MKK7) by signaling from G protein βγ subunit in human embryonal kidney 293 cells. J Biol Chem. 1999;274:1957–1965. doi: 10.1074/jbc.274.4.1957. [DOI] [PubMed] [Google Scholar]
- 66.Yan M, Dai T, Deak J C, Kyriakis J M, Zon L I, Woodgett J R, Templeton D J. Activation of stress-activated protein kinase by MEKK1 phosphorylation of its activator SEK1. Nature. 1994;372:798–800. doi: 10.1038/372798a0. [DOI] [PubMed] [Google Scholar]
- 67.Yao Z, Diener K, Wang X S, Zukowski M, Matsumoto G, Zhou G, Mo, T R, Penninger J M. Activation of stress-activated protein kinases/c-Jun N-terminal protein kinases (SAPKs/JNKs) by novel a mitogen-activated protein kinase kinase (MKK7) J Biol Chem. 1997;272:32378–32383. doi: 10.1074/jbc.272.51.32378. [DOI] [PubMed] [Google Scholar]
- 68.Yoshida K, Kharbanda S, Kufe D. Functional interaction between SHPTP1 and the Lyn tyrosine kinase in the apoptotic response to DNA damage. J Biol Chem. 1999;274:34663–34668. doi: 10.1074/jbc.274.49.34663. [DOI] [PubMed] [Google Scholar]
- 69.Yuan Z, Kharbanda S, Kufe D. 1-β-d-arabinofuranosylcytosine activates the Src-like p56/p53lyn tyrosine kinase in human myeloid leukemia cells. Biochemistry. 1995;34:1058–1063. doi: 10.1021/bi00003a041. [DOI] [PubMed] [Google Scholar]
- 70.Yuan Z-M, Huang Y, Fan M M, Sawyers C, Kharbanda S, Kufe D. Genotoxic drugs induce interaction of the c-Abl tyrosine kinase and the tumor suppressor protein p53. J Biol Chem. 1996;271:26457–26460. doi: 10.1074/jbc.271.43.26457. [DOI] [PubMed] [Google Scholar]
- 71.Yuan Z-M, Huang Y, Ishiko T, Nakada S, Utsugisawa T, Kharbanda S, Sung P, Shinohara A, Weichselbaum R, Kufe D. Regulation of Rad51 function by c-Abl in response to DNA damage. J Biol Chem. 1998;273:3799–3802. doi: 10.1074/jbc.273.7.3799. [DOI] [PubMed] [Google Scholar]
- 72.Yuan Z-M, Huang Y, Kraeft S-K, Chen L B, Kharbanda S, Kufe D. Interaction of cyclin-dependent kinase 2 and the Lyn tyrosine kinase in cells treated with 1-β-d-arabinofuranosylcytosine. Oncogene. 1996;13:939–946. [PubMed] [Google Scholar]
- 73.Yuan Z-M, Shioya H, Ishiko T, Sun X, Gu J, Huang Y, Lu H, Kharbanda S, Weichselbaum R, Kufe D. p73 is regulated by tyrosine kinase c-Abl in the apoptotic response to DNA damage. Nature. 1999;399:814–817. doi: 10.1038/21704. [DOI] [PubMed] [Google Scholar]
- 74.Yuan Z M, Huang Y, Whang Y, Sawyers C, Weichselbaum R, Kharbanda S, Kufe D. Role for the c-Abl tyrosine kinase in the growth arrest response to DNA damage. Nature. 1996;382:272–274. doi: 10.1038/382272a0. [DOI] [PubMed] [Google Scholar]
- 75.Zanke B W, Boudreau K, Rubie E, Winnett E, Tibbles L A, Zon L, Kyriakis J, Liu F-F, Woodgett J R. The stress-activated protein kinase pathway mediates cell death following injury induced by cis-platinum, UV irradiation or heat. Curr Biol. 1996;6:606–613. doi: 10.1016/s0960-9822(02)00547-x. [DOI] [PubMed] [Google Scholar]