Abstract
The field of infectious diseases currently takes a reactive approach and treats infections as they present in patients. Although certain populations are known to be at greater risk of developing infection (eg, immunocompromised), we lack a systems approach to define the true risk of future infection for a patient. Guided by impressive gains in “omics” technologies, future strategies to infectious diseases should take a precision approach to infection through identification of patients at intermediate and high-risk of infection and deploy targeted preventative measures (ie, prophylaxis). The advances of high-throughput immune profiling by multiomics approaches (ie, transcriptomics, epigenomics, metabolomics, proteomics) hold the promise to identify patients at increased risk of infection and enable risk-stratifying approaches to be applied in the clinic. Integration of patient-specific data using machine learning improves the effectiveness of prediction, providing the necessary technologies needed to propel the field of infectious diseases medicine into the era of personalized medicine.
Keywords: high-throughput technologies, infectious diseases, invasive fungal infections, systems immunology
Translational systems immunology approaches to infectious diseases will enable the switch from reactive to precision treatment of patients, which will improve clinical outcomes while reducing the use of prophylactic antibiotics and incidence of infection in high-risk individuals.
Infectious diseases (ID) physicians currently take a reactive approach in which we provide diagnostic and therapeutic advice on patients with established infection. For example, Staphylococcus aureus bacteremia is commonly encountered in inpatient settings. Although S aureus colonization and unsterile techniques contribute to incidents of infection, there is clearly a role for host factors in modulation of potential infection and disease severity. The most common conditions that portend increased risk for S aureus bacteremia are diabetes, intravenous drug use, presence of central lines in patients requiring dialysis, cancer chemotherapy, and corticosteroid use [1]. The underlying assumption is that bacteremia in this patient population cannot be accurately predicted, and therapeutic approaches are purely reactive—treatment begins once the infection is established and subsequently diagnosed. Unfortunately, this approach permits widespread host damage from metastatic infection. Mortality rates of 20%–40% have not changed in the past several decades, indicating that another approach is required [1]. The goal for the field of ID in the 21st century should be to predict and prevent infections in individual patients before they occur by interrogating the patients’ immune system using multiomics approaches. We have focused this review on the human immunology because the pathogen virulence and its impact has been discussed elsewhere [2–4].
The need for a preemptive, risk-modifying approach to infections in ID is evident by the increasing number of high-risk individuals due to advances in immunosuppression enabling solid organ transplantation, chemotherapy for cancer, and immunomodulatory therapeutics for autoimmune diseases [5–9]. Evidence-based prophylaxis has reduced ID burden in immune compromised patient (eg, Pneumocystis jirovecii pneumonia prophylaxis in human immunodeficiency virus [HIV] patients with CD4 count of <200 cells/µL) [10]. However, total T-cell counts do not account for specific effector T-cell populations of function, rendering this approach less precise. The future of ID medicine should predict and prevent infection to avoid severe outcomes (eg, delay of therapy for underlying processes and death). As a field, we are poised to leverage our understanding of basic mechanisms in microbiology and immunology to learn how to risk-stratify patients and judiciously deploy prophylactic antimicrobials to prevent infections using a personalized medicine approach. In the near term, multiomics studies can nominate specific molecular and cellular biomarkers that can be measure using platforms currently deployed in patient care that will inform clinical decisions.
Medical mycology would greatly benefit from a precision approach to infection. Invasive fungal infections (IFIs) are dreaded complications in immunocompromised populations, often carrying morality rates exceeding 50% [7, 11, 12]. Clinical data and current literature implicate certain components of the immune system as critical for swift clearance of fungal pathogens [13–15]. However, the rules governing an inflammatory response that contributes to the broad spectrum of clinical outcomes in immunocompromised patients are not known. For example, individuals that receive a single- or double-lung transplant due to end-stage pulmonary disease are at high-risk of developing and succumbing to IFIs. The opportunistic fungal pathogen Aspergillus fumigatus is the most commonly diagnosed fungal pathogen in lung transplant recipients (LTRs), with lower incidence of infection by Mucor, Cryptococcus, and endemic fungi (Histoplasma, Blastomyces, Coccidioides) [16, 17]. Furthermore, A fumigatus colonization in these patients is associated with accelerated chronic rejection [18, 19]. Understanding the underlying factors that predict development of fungal infection in LTRs, and consequent rejection, will enable targeted preventative strategies (eg, vaccine, prophylactics, and/or alteration in immunosuppression) in those at the greatest risk of poor outcome.
Application of precision ID approaches benefits not only relatively small, highly defined cohorts (~4000 lung transplants annually), but also larger, more heterogenous at-risk populations. Risk of infection in healthcare settings is amplified by the need for invasive procedures (eg, insertion of central venous catheters) leading to a break in the skin barrier and disruption of commensal fungal populations [20, 21]. Candida spp is the seventh most prominent pathogen in healthcare-associated infection ([HAI] <10% of all pathogens) and is a leading cause of bloodstream infection (~22% of all pathogens) [22]. The observation that only a subset of at-risk patients develop candidemia despite ubiquitous exposure indicates that host factors potently modulate risk for this infection. Precision ID enables identification of patients at the highest risk of developing invasive candidiasis so that a targeted prophylaxis approach will lead to less morbidity and improved outcomes. It is not hard to imagine the benefits of a screen to identify hospitalized patients at-risk of not only Candida infection, but also common bacterial pathogens contributing to HAIs (eg, Clostridium difficile, S aureus, Pseudomonas aeruginosa) [22]. Although the idea of personalized medicine is not new [23–26], technologies and bioinformatic approaches required to advance precision ID are becoming available, and they are broadly applied to other fields of medicine (eg, oncology).
POTENTIAL APPLICATION OF NEXT-GENERATION TECHNOLOGIES IN THE CLINICAL SETTING
The immune system, one of the most complex and dynamic biological systems in mammals, comprises diverse cell types with varying functional states. Advances in high-throughput profiling technologies, particularly single-cell omics platforms, enable comprehensive characterization of immune components at multiple scales. However, immunity is not merely a sum of its components, and its behavior cannot be explained or predicted solely by examining individual components. Therefore, systems biology approaches are essential for decoding the cellular complexity, plasticity, and functional diversity of the immune system. The emerging field of systems immunology enables physician-scientists to better understand how the immune system works in health and disease. Evaluation of clinical samples from known high-risk populations will empower future risk-stratification of these populations and improve our ability to deliver precision ID care. In addition, there is increasing evidence that local immunity provides a better window into immune responses than interrogating peripheral blood alone [27]. Interrogation of tissue resident memory cells has provided significant insight into host responses and autoimmune diseases [28, 29]. Specifically, group 3 innate lymphoid cells and T helper type 17 (Th17) cells may serve as key components of tissue-resident memory cells acquired over time or elicited by mucosal immunization that provides the host with enhanced immunity against specific pathogens [30]. For example, LTRs are often diagnosed with pulmonary infection, and therefore it is critical to interrogate both systemic (ie, peripheral blood) and local (ie, bronchoalveolar lavage fluid and lung biopsies) patient samples to provide a fuller picture of immunity in response to infection. Understanding local and systemic immune response to infection at such a large-scale will enable refinement of future clinical assays and identification of relevant biomarkers in susceptible patient populations. We envision an even closer relationship between the ID/transplant physicians and oncologists/rheumatologists whereby ID physicians assists the treating physician to select appropriate preventative strategies to avoid life-threatening infections in at-risk patients.
Transcriptional Genomics
Development of systematic transcriptomic profiling and computational analyses have provided meaningful translational insights into various disease states and immunological response in infections [31–37]. In the immune system, cell populations may appear homogeneous, but analysis by single-cell sequencing of ribonucleic acid (scRNA-seq) or examination of epigenetic modifications can reveal cell-to-cell variability that may help subpopulations of cells to rapidly adapt to evolving environments. Single-cell omics approaches, which result in quantitative and high-resolution snapshots of thousands to millions of cells, interrogate human systems and underlying phenotypes that may contribute to disease. Although it is less expensive, bulk RNA-sequencing remains a lower powered approach because results generated reflect an average of all cells, which may lead to critical changes in rare cell populations being overlooked. The scRNA-seq analysis provides an unbiased, data-driven way to systematically detect cellular states to reveal diverse simultaneous facets of cellular identity, from discrete cell types to continuous dynamic transitions, which cannot be defined by a handful of predefined markers or for which markers are not yet known (Figure 1A). For example, scRNA-seq identified rare epithelial cells, ionocytes, as the major source of cystic fibrosis transmembrane conductance regulator, which reshaped the cystic fibrosis field [38]. In addition, a recent study demonstrated a monocyte phenotype, termed MS1, associated with bacterial sepsis, which appears to be a hallmark of severe infections [39]. In previous studies, after the MS1 marker genes were defined from scRNA-seq, there was evidence of its presence in dozens of bulk transcriptional studies of sepsis with diverse anatomic sources and microbiological causes, underscoring the power of this approach.
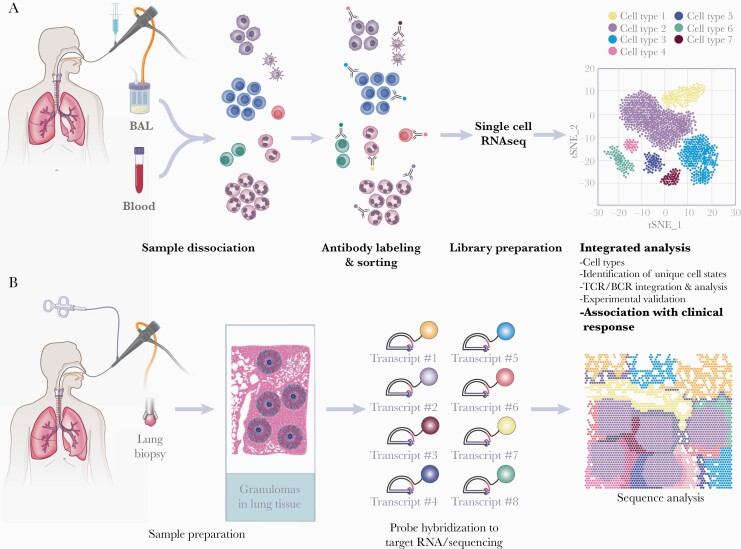
Figure 1.
Workflow of transcriptional genomics from patient samples through single-cell ribonucleic acid sequencing (scRNA-seq) (A) and spatially resolved transcriptomics (B). (A) Samples for scRNA-seq require dissociation of cells to ensure cells are not clumped together. Cells are sorted via antibody labeling to sort immune cells and nonimmune cells. Samples undergo reverse transcription and complementary deoxyribonucleic acid amplification and profiled by sequencing through selected sequencing technologies. These libraries often achieve 50000 reads, providing detailed readouts of cell populations and substates, T-cell receptor (TCR) and B-cell receptor (BCR) profiling, and underlying immune pathways in disease correlating with disease. (B) Tissue samples from infected regions, in this example lung tissue from lung transplant recipients infected with Aspergillus, are snap frozen and sectioned into thin slices for spatial transcriptomics. After permeabilization, tissues are exposed to probes designed to target specific RNA sequences followed by amplification that enables visualization of transcripts. Although methodology differs depending on the approach used, the current example of these probes requires a ligation and crosslinking with a fluorescent tag. The resulting data allow analysis of transcripts in their spatial location. BAL, bronchoalveolar lavage. (Printed with permission from Wolf N: 2021).
Methodological adaptations such as T-cell receptor (TCR) or B-cell receptor (BCR) repertoire profiling and cellular indexing of transcriptomes and epitopes by sequencing (CITE-seq) further increase the power of scRNA-seq. T-cell responses are essential to adaptive immunity to pathogens, including in IFIs. For example, investigations in patients with HIV demonstrate that a loss of T-cell immunity is closely tied to incidence of cryptococcal meningitis [40–42]. Impaired T-cell responsiveness in other immunologically vulnerable populations have also been observed in invasive Aspergillus and Candida infections [43, 44]. In addition, the importance of B cells in ID has been well documented in viral and bacterial infections, including severe acute respiratory syndrome coronavirus 2, Klebsiella, and Haemophilus influenzae [45–48]. The RNA-seq paired with TCR or BCR repertoire analyses enables investigators to interrogate expansion of T- and B-cell populations in disease. In addition to these paired receptor analyses, CITE-seq, which has been implemented in large-scale translational human immunology projects, enables simultaneous single-cell measurements of a predefined array of surface proteins and unbiased gene expression [49]. The CITE-seq utilizes oligonucleotide barcoded antibodies to quantitate protein expression through flow cytometry measurements in tandem with messenger RNA information provided my RNA-seq. Coupled with scRNA-seq, these paired analyses provide a comprehensive look at immune cells and tissues in disease.
Although scRNA-seq provides ample information about the transcriptome in different cell populations, there is a loss of spatial data due to the required step of tissue dissociation. Through advances in sequencing and imaging technologies, understanding expression of transcripts at the single-cell level in their spatial layout provides better understanding within the distinct microenvironment in infected regions and tissues (Figure 1B). Numerous novel spatial transcriptomics approaches have been reviewed previously [37, 50]. Additional complementary approaches such as multiplexed cytometric imaging (CODEX) and in situ hybridization (RNAscope) may be used to improve power of spatial transcriptomics [37, 51, 52]. A key advantage to spatial transcriptomics is the ability to determine the impact of local immune response in tissue, with temporal resolution. In infection, development of a granuloma occurs during infection to contain pathogens. Use of spatial transcriptomics of these granulomas may provide insight into the molecular and cellular mechanisms that govern pathogen containment. Thus, complementary scRNA-seq and spatial transcriptomics approaches may provide ample information on host-pathogen responses during infections and insights into prognosis.
Epigenomics
Although studies investigating genetic susceptibilities have identified polymorphisms associated with infection (eg, CARD9 mutations in IFIs), translation to the clinic does not provide the whole story for risk and development of infection [53]. Common genetic traits do not produce consistent phenotype, but epigenetic modifications may bridge the gap between phenotype and genes. Epigenetic modifications alter gene expression and function through deoxyribonucleic acid methylation and histone modification (eg, acetylation, methylation, phosphorylation) [54]. Methodology to interrogate epigenomic changes utilize chromatin conformation studies (eg, assay for transposase-accessible chromatin using sequencing [ATAC-seq]) and histone modification profiling (eg, chromatin immunoprecipitation) of clinical samples (Figure 2) [55, 56]. Longitudinal studies that examine epigenetic modifications paired with scRNA-seq in high-risk patients may provide a roadmap to infection susceptibility and ability to effectively clear pathogens. Epigenetic-based treatments are being explored in the context of HIV infections, leading to preclinical and clinical trials for combination antiretroviral therapy [57, 58].
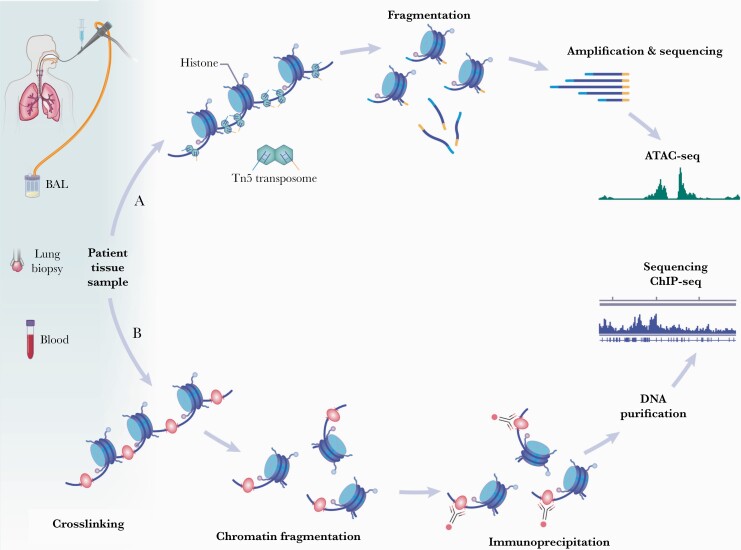
Figure 2.
Epigenomic approaches utilizing clinical local (eg, lung tissue and bronchoalveolar lavage [BAL]) and systemic (eg, blood) samples. (A) Assay for transposase-accessible chromatin using sequencing (ATAC-seq) measures chromatin conformation differences. The hyperactive transposase Tn5 loaded with a next-generation sequencing library enables fragmentation of open chromatin regions. These fragments are amplified and sequenced to provide physician-scientists with accessible chromatin regions at the single-cell level. (B) Histone modifications profiling through chromatin immunoprecipitation (ChIP) is an antibody-based technology that selectively enriches deoxyribonucleic acid (DNA)-binding proteins and their respective DNA targets (eg, histone modifications by methylation or acetylation). The DNA and its associated proteins on the chromatin are first crosslinked followed by fragmentation by sonication or a nuclease digestion. These fragments are then immunoprecipitated via antibody selection, which removes remaining cellular debris. Although there are multiple downstream analyses that can be run on ChIP precipitates, sequencing after DNA purification provides information on genome-wide binding in health and disease. (Printed with permission from Wolf N: 2021).
Historically, immune memory has been considered a function of the adaptive immune system (eg, T cells and B cells), providing highly specific, long-lasting protection against invaders. Trained immunity is the concept of long-term functional reprogramming (namely, through epigenetic processes) in early immune responders, also known as innate immune cells (eg, neutrophils, monocytes, dendritic cells) [59]. Interaction with these innate cells and a pathogen leads to altered response during a second challenge with the same pathogen, contributing to innate-mediated short-term (ranging from 3 months to 1 year) protection against foreign invaders [59]. Histone methylation or acetylation are hallmarks of trained immunity in innate immune cells after stimulation with the fungal cell wall carbohydrate β-1,3-glucan, a major component of Candida spp [60–62]. Expansion of these studies into individuals with an elevated risk of infection may provide insights into novel preventative and therapeutic strategies to preemptively treat infections.
Metabolite Profiling
Metabolomics systematically quantifies metabolites in biological samples. These metabolites are derived from metabolic processes as well as complex biological interactions within an organism. Clinical metabolomics detects the direct result generated by a biochemical response to a variety of factors, including invading pathogens. The benefit of metabolite profiling is well demonstrated in the fields of cardiovascular disease, kidney disease, cancer metabolism, and emerging in the field of ID [63–71]. Identification of circulating metabolites offers biomarker profiles that precede disease and track severity of disease. Technology platforms for metabolic profiling typically utilize mass spectrometry (MS) coupled with chromatographic separation (including liquid chromatography and gas chromatography) and/or nuclear magnetic resonance spectroscopy (NMR) [72, 73]. Because there is ample diversity of metabolites, priority should be made to process through complementary detection methods. Liquid chromatography-MS, which has emerged as the workhorse for large-scale metabolomics, enables quantification of a broad range of metabolites including lipids, sugars, organic acids, amino acids, amines, nucleotides, bile acids, and acylcarnitines based on the detection method used (Figure 3). Although there are hundreds of known compounds through reference libraries, follow-up protocols (including NMR and other MS approaches) are necessary to identify thousands of unknown peaks. One barrier to keep in mind with respect to comprehensive metabolomic profiling is the dynamic nature of metabolism and metabolite signatures, which requires immediate processing to accurately dissect patient profiles. Furthermore, an advantage of metabolomics is the ability to identify metabolites from small sample volumes (as little as 10 µL), contributing to the potential to provide early clinical measurements leading to preventative strategies and treatments of disease.
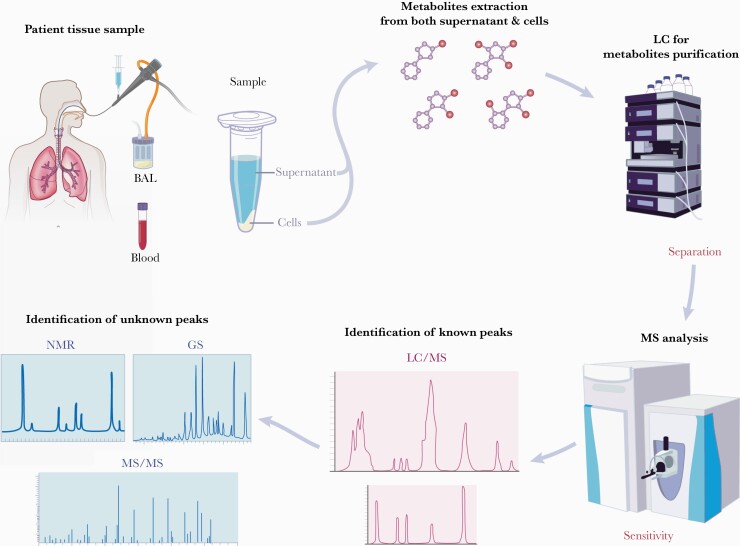
Figure 3.
Metabolite profiling through nontargeted approaches of known and unknown peaks. Patient samples for metabolomics require quick processing to extract metabolites prior to them being changed by biological mechanisms. Because the metabolome consists of molecules with very different physical properties, for example, both cationic and anionic compounds ranging from very polar to very nonpolar, it is necessary to devise distinct sample preparation and liquid chromatography-mass spectrometry (LC/MS) procedures to optimize metabolite coverage. These methods utilize different settings for separation via gas (gas chromatography [GC]) or LC step. Mass spectrometry analysis in the positive (C8-pos or hydrophilic interaction chromatography [HILIC]-pos) or negative (C18-neg or HILIC-neg) ion mode provides a wide array of metabolic peaks. These peaks can be compared with known metabolite library for identification. In addition to matches to known metabolites, there are often thousands of unknown peaks, which requires rigorous methodology to identify and authenticate metabolites. Identification and authentication approaches rely on tandem mass spectrometry (MS/MS)-based structure prediction as well as compound isolation and subsequent processing through GC or nuclear magnetic resonance spectroscopy (NMR) methodologies. Interrogation of the metabolome loops back to the patient by identification of metabolic biomarkers of disease. (Printed with permission from Wolf N: 2021).
It has become evident that immune cells rely on changes in cellular metabolism to mount effective antimicrobial responses, with glucose metabolism being a central player in immune cell function, although data from actively infected human patients remain limited [74]. In recent studies, researchers have demonstrated the role of metabolism in trained immunity to fungal pathogens. Emerging data suggest that an increase in glycolysis and glutaminolysis used in the tricarboxylic acid cycle and a corresponding decrease in oxidative phosphorylation are important to the host defense against fungal pathogens [60, 75–77]. It is unfortunate that these studies primarily utilize ex vivo stimulation of peripheral blood samples from healthy individuals. Expansion of large-scale, nontargeted metabolomics in high-risk patient populations is warranted. In limited studies into metabolic changes in organ transplantation, researchers identified an increase in glycolysis during transplant rejection, which provides rapid adenosine triphosphate generation and biosynthetic intermediates that support anabolic processes [78, 79]. Expansion of these data in primary clinical samples (both systemic and locally) will provide novel monitoring strategies for LTRs for rejection and development of IFIs.
Considerations for timing of clinical sample collection, particularly in ID medicine, is critical and further studies are needed. In sepsis, metabolism rapidly changes throughout progression of disease. Metabolic profiles differentiated patients who developed sepsis in adults admitted to the intensive care unit due to traumatic injury [80]. Thus, these profiles could be applied to precision medicine approaches to identify patients most likely to develop infection. Understanding longitudinal changes in infection would enable clinicians to better predict the course of disease. Furthermore, examination of the metabolome may be applied to predict drug response. Investigation into novel therapeutics and treatment strategies is essential with the emergence of more multidrug-resistant pathogens. Metabolomics could be applied as an indicator for the pathogen, which currently relies on culture methods. A study of fungal infections in neonates identified elevations of the amino acid, serine, during active infection, which declined after antifungal therapy, when compared with healthy controls [81]. Thus, large-scale metabolomics to identify diagnostic biomarkers, monitor progress of infection, and development of new treatment is warranted.
Proteomic Analyses
The proteome encompasses the overall protein content within a cell, including protein-protein interactions and posttranslational modifications (eg, phosphorylation) at a particular time point. Measurements of global protein expression within cells or tissues encompass a variety of approaches ranging from targeted, high-dimensional panels to large-scale, unbiased techniques. Protein phosphorylation is an essential posttranslational modification that enables regulation of most biological processes [82–86]. In one sense, the recipe for a successful immune response to an invading pathogen is timely activation and inhibition of distinct signaling pathways in immune cells to provide efficient antimicrobial effect without inducing excessive tissue damage. Correlating these changes by monitoring phosphorylation status of key intracellular signaling molecules with clinical outcome may provide critical insights into the mechanism of protective immunity. Mass cytometry-based (ie, cytometry by time of flight [CyTOF]) phosphoproteomics methodology provides a platform to globally study activation or inhibition of multiple signaling pathways across the entire immune system with single-cell resolution [87]. Indeed, novel insights into TLR signaling and T-cell activation have been uncovered by phosphoproteomics analysis (Figure 4A) [88, 89]. Insights into the distribution and activation or inhibition of intracellular signaling pathways by targeting phosphorylation states of known signaling molecules in immune cells provides mechanistic understanding of disease states and infection [85]. Targeted profiling via the CyTOF platform enables investigators to tailor a panel of ~50 markers to dissect specific cell subsets and activation states of different proteins. For example, the CyTOF panel examining differences in innate immunity from invasive candidiasis HAI may include proteins associated with Candida immunity, such as iNOS, Arg1, Ym1, Ym2, IL-4, and Egr2 [90–92].
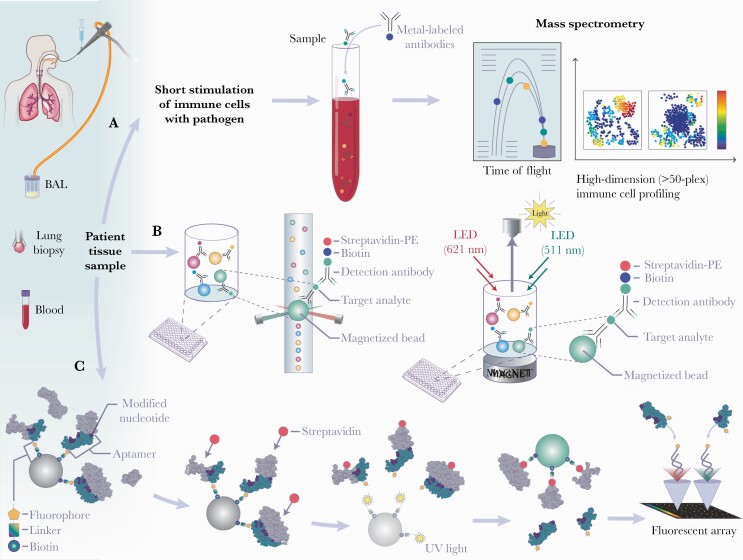
Figure 4.
Workflow for mass cytometry (A), multianalyte array (B), and aptamer-based assay (C) proteomic approaches utilizing human patient samples. (A) To interrogate activated and inhibited pathways through phosphoproteomics, biological samples are briefly (15 minutes to 6 hours) stimulated with pathogen (eg, Aspergillus) of interest as well as with proper controls (eg, unstimulated and lipopolysaccharide). Stimulated samples are then incubated with metal-labeled antibodies targeting immune cells (cell surface antibodies), intracellular signaling proteins (phosphor-specific antibodies), and/or cytokines (intracellular cytokine antibodies). Cytometry by time-of-flight mass spectrometry (CyTOF) merges traditional flow cytometry with inductively coupled mass spectrometry to assess more than 50 simultaneously measured parameters on a cell-by-cell basis. (B) Targeted multianalyte arrays enable measurement of multiple proteins within a 96- or 384-well plate. Cell supernatants are put in individual wells containing color-coded beads precoated with antibodies for multiple analytes of interest. Detection antibodies for each target analyte as well as streptavidin-phycoerythrin (PE) are added for biotinylated detection. Detection and quantification of each analyte can be determined using a flow-based instrument or magnetic beads. Panels can be created to target specific secreted proteins. (C) Aptamer-based proteomics enables aptamers (eg, Slow Off-rate Modified Aptamers [SOMAmers]) labeled with a fluorophore, photocleavable linker, and biotin to be immobilized on streptavidin-coated beads and incubated with patient samples. After a biotin-tagging step, these aptamer-protein complexes are released by ultraviolet (UV) light-mediated photocleavage of the linker. The biotin labeled- aptamer-protein complexes are captured by a second set of streptavidin-coated beads and aptamers are released after incubation with denaturing buffer. A microarray chip is used to quantify fluorescence intensity within to total protein amount of the initial sample. Throughout this process, unbound proteins and nonspecific interactions are removed. BAL, bronchoalveolar lavage; LED, light-emitting diode. (Printed with permission from Wolf N: 2021).
Secretion of proteins to the external environment is critical in maintaining cell-cell communication and recruitment of immune cells in response to pathogens. These secreted proteins include hormones, cytokines, chemokines, and growth factors. Measurement of these secreted proteins (secretome) can be done through a targeted multiplex array approach (eg, multianalyte assay). Multiplex assays allow for simultaneous detection and quantification of multiple proteins (Figure 4B). Stimulation of peripheral blood mononuclear cells (PBMCs) or tissues from patients with a pathogen may provide insights in the variations of the secretome in patients with high-risk of infection compared with healthy controls. Multianalyte assays have been used to investigate numerous infections [93–96].
Although a targeted approach may provide single-cell resolution and insights into known immune factors, application of large-scale, unbiased proteomic approaches contributes to identification of novel proteins associated with disease. Furthermore, this type of approach may identify biomarkers of disease and clinical severity. The SomaScan assay developed by SomaLogic is an aptamer-based approach that measures up to 7000 unique human proteins (Figure 4C). Although it is optimized for a set of core sample types (plasma, serum, urine), the SomaScan assay sample source has the ability to interrogate noncore sample sources (bronchoalveolar lavage, cell-conditioned media, cerebrospinal fluid, exosomes, sputum, tissue homogenates). Aptamer-based proteomics is widely used in neurodegenerative disease, cardiovascular diseases, and infections [97–100]. Indeed, use of Slow Off-rate Modified Aptamers (SOMAmers) in the SomaScan assay and RNA-seq was used to identify tissue-specific clinical markers of heart, kidney, liver, and skeletal muscle damage in patients with coronavirus disease 2019 (COVID-19) [101]. These biomarkers can be used upon presentation to the hospital using blood samples to detect COVID-19-driven organ damage. Taken together, these proteomic approaches identify activated pathways and biomarkers of infection critical for implementation of precision ID medicine into clinical practice.
PRECISION MEDICINE IN INFECTIOUS DISEASES
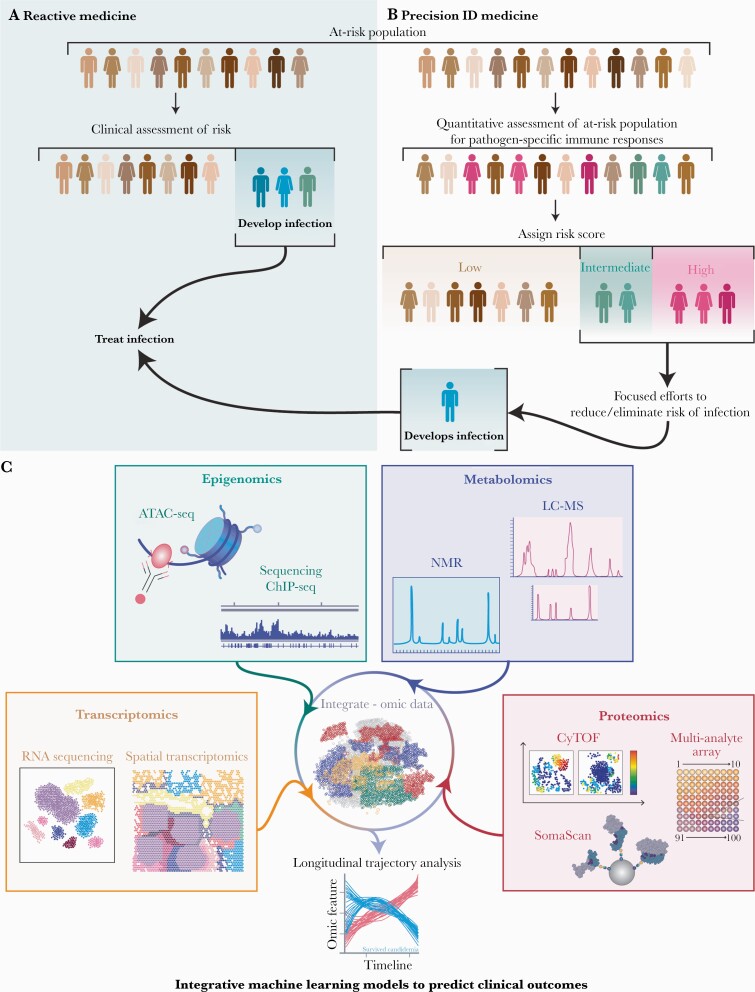
Transcriptional expression, epigenetic forms, metabolites, and protein expression are all interwoven to directly impact immune responses in disease. Individually, high-throughput omics approaches provide a window into immune responses and phenotypes that portend increased risk of infection and may foretell clinical course. Given the complex interplay among different aspects of the immune system, combining multiomics modalities into a computational framework increases predictive power and reveals crosstalk between different layers of biological profiling (Figure 5). However, merging transcriptomic, epigenomic, metabolomic, and proteomic features into predictive models will require continued development of new statistical tools designed to study high-dimensional datasets. Recent advances in bioinformatic processes overcome many analytic challenges that previously prevented the development of models to accurately predict patient outcomes [86, 102, 103]. Regularized regression methods such as the Elastic Net algorithm have proven useful for selection of key predictive features and development of clinical models [104]. A stacked generalization method that combines multiple regularized regression models developed from individual omic datasets has also been recognized as a valuable approach for data integration and can improve overall model performance [86]. This approach has been successfully implemented for the prediction of various clinical outcomes, including development of insulin resistance [105], onset of spontaneous labor [106], survival of persons with pancreatic cancer [107], and severity of COVID-19 infections [85]. Furthermore, development of effective visualization methods for these integrated machine learning models improves understanding of results [108, 109]. Taken together, these analytic approaches provide mechanistic information regarding the crosstalk between various biological systems that could not otherwise be identified from each assay individually.
Figure 5.
Workflow of reactive (A) and precision (B) infectious diseases (ID) medicine. A precision approach would enable clinicians to use preventative strategies, leading to fewer infections and infectious complications, including targeted prophylaxis or therapies that enhance immunity to specific pathogens. (C) Incorporation of multiomics approaches into an integrative machine learning model more accurately predicts clinical outcomes. Multiomics include transcriptomics (bulk or single-cell ribonucleic acid [RNA] sequencing [with or without paired analyses] and spatial transcriptomics), epigenomics (chromatin immunoprecipitation [ChIP] and assay for transposase-accessible chromatin using sequencing [ATAC-seq]), metabolomics (liquid chromatography tandem mass spectrometry [LC-MS] and one-dimensional proton nuclear magnetic resonance spectroscopy [NMR]), and proteomics (cytometry by time-of-flight [CyTOF], aptamer-based methods [SomaScan], and multianalyte array). Combining these multiomics methodologies across longitudinal samples of local and peripheral immune responses provides insight into relevant pathways and predicts clinical outcomes in disease. (Printed with permission from Wolf N: 2021).
Implementation of precision ID medicine requires immune profiling of well phenotyped human cohorts, particularly patients with known risk factors (eg, solid organ transplantation, cancer, invasive procedures). Although recent advances have improved cost effectiveness and availability of these omics technologies, there is still a heavy burden on resources. To realize the full potential of these technologies, there needs to be significant reduction in cost of processing and analyzing each sample to ensure access to all patients. Major funding agencies acknowledge this need as demonstrated by new funding mechanisms through the National Institutes of Health (Human Immunology Project Consortium) and European Research Council (Horizon Program) [110, 111]. Further multiomics studies are warranted to make precision ID a clinical reality.
CONCLUSIONS
Translational systems immunology provides the framework to shift from reactive to proactive precision ID medicine, improving the quality of life and dampening complications in high-risk populations (Figure 5). Although many omics approaches have been used to understand host response to pathogens, barriers remain that need to be addressed. One major barrier to our understanding is the reliance on animal models to study human disease, because these often do not recapitulate the complexity of disease. Furthermore, many reports using these omics approaches utilize samples from healthy patients exposed ex vivo to pathogens. In previous studies, researchers have attempted to understand initial immune responses in C albicans-stimulated PBMCs using RNA-seq, and they unveiled the predominance of interferon responses and activation of major innate cell populations (eg, neutrophils, macrophages, monocytes). Although the assumptions are foundational, the generalizability of these ex vivo stimulations of blood from normal volunteers is limited. In addition, single-cell resolution permits identification of the precise cell types driving observed transcriptional changes, thus suggesting candidate immune pathways/circuits for precision diagnostics and therapeutic targeting. Thus, there is a need for multiomics investigations using well defined clinical cohorts at greater risk of infections. Integration of these data will result in the necessary information to apply risk stratification to high-risk populations, preventive strategies to reduce the burden of infection, and allow for targeted rather than prophylactic antibiotic strategies.
Acknowledgments
We thank Nicole Wolf for assistance with the artwork (illustrations for Figures 1–5).
Financial support. This study was funded by the National Institutes of Health, Grant Numbers R01AI136529 and R01AI150181 (to J. M. V.), K08HL122528 and R01HL157414 (to J. L. H.), R35GM138353 (to N. A.), R35GM137936 and 1P01HD106414 (to B. G.), and R01AI132638 to M. K. M.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1. Souli M, Ruffin F, Choi SH, et al. Changing characteristics of Staphylococcus aureus bacteremia: results from a 21-year, prospective, longitudinal study. Clin Infect Dis 2019; 69:1868–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lee MH, Nuccio SP, Raffatellu M.. Pathogen interference: targeting virulence factors to tackle intracellular microbes. Cell Chem Biol 2020; 27:765–7. [DOI] [PubMed] [Google Scholar]
- 3. Jack RS. Evolution of immunity and pathogens. Results Probl Cell Differ 2015; 57:1–20. [DOI] [PubMed] [Google Scholar]
- 4. Chin VK, Lee TY, Rusliza B, Chong PP.. Dissecting Candida albicans infection from the perspective of C. albicans virulence and omics approaches on host-pathogen interaction: a review. Int J Mol Sci 2016; 17:1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Baddley JW, Cantini F, Goletti D, et al. ESCMID study group for infections in compromised hosts (ESGICH) consensus document on the safety of targeted and biological therapies: an infectious diseases perspective (soluble immune effector molecules [I]: anti-tumor necrosis factor-α agents). Clin Microbiol Infect 2018; 24:10–20. [DOI] [PubMed] [Google Scholar]
- 6. Rawson TM, Moore LSP, Zhu N, et al. Bacterial and fungal coinfection in individuals with coronavirus: a rapid review to support COVID-19 antimicrobial prescribing. Clin Infect Dis 2020; 71:2459–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rayens E, Norris KA, Cordero JF.. Mortality trends in risk conditions and invasive mycotic disease in the United States, 1999-2018. Clin Infect Dis 2021. doi: 10.1093/cid/ciab336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zembower TR. Epidemiology of infections in cancer patients. Cancer Treat Res 2014; 161:43–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Varughese T, Taur Y, Cohen N, et al. Serious infections in patients receiving ibrutinib for treatment of lymphoid cancer. Clin Infect Dis 2018; 67:687–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sidhu VK, Foisy MM, Hughes CA.. Discontinuing Pneumocystis jirovecii pneumonia prophylaxis in HIV-infected patients with a CD4 cell count <200 cells/mm3. Ann Pharmacother 2015; 49:1343–8. [DOI] [PubMed] [Google Scholar]
- 11. Cornely OA, Gachot B, Akan H, et al. ; EORTC Infectious Diseases Group. Epidemiology and outcome of fungemia in a cancer Cohort of the Infectious Diseases Group (IDG) of the European Organization for Research and Treatment of Cancer (EORTC 65031). Clin Infect Dis 2015; 61:324–31. [DOI] [PubMed] [Google Scholar]
- 12. Taccone FS, Van den Abeele AM, Bulpa P, et al. ; AspICU Study Investigators. Epidemiology of invasive aspergillosis in critically ill patients: clinical presentation, underlying conditions, and outcomes. Crit Care 2015; 19:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Romani L. Immunity to fungal infections. Nat Rev Immunol 2011; 11:275–88. [DOI] [PubMed] [Google Scholar]
- 14. Ward RA, Vyas JM.. The first line of defense: effector pathways of anti-fungal innate immunity. Curr Opin Microbiol 2020; 58:160–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Köhler JR, Hube B, Puccia R, et al. Fungi that infect humans. Microbiol Spectr 2017; 5. doi: 10.1128/microbiolspec.FUNK-0014-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Baker AW, Maziarz EK, Arnold CJ, et al. Invasive fungal infection after lung transplantation: epidemiology in the setting of antifungal prophylaxis. Clin Infect Dis 2020; 70:30–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kennedy CC, Razonable RR.. Fungal infections after lung transplantation. Clin Chest Med 2017; 38:511–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pasupneti S, Manouvakhova O, Nicolls MR, Hsu JL.. Aspergillus-related pulmonary diseases in lung transplantation. Med Mycol 2017; 55:96–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Weigt SS, Copeland CAF, Derhovanessian A, et al. Colonization with small conidia Aspergillus species is associated with bronchiolitis obliterans syndrome: a two-center validation study. Am J Transplant 2013; 13:919–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Arias S, Denis O, Montesinos I, et al. Epidemiology and mortality of candidemia both related and unrelated to the central venous catheter: a retrospective cohort study. Eur J Clin Microbiol Infect Dis 2017; 36:501–7. [DOI] [PubMed] [Google Scholar]
- 21. Poissy J, Damonti L, Bignon A, et al. ; FUNGINOS; Allfun French Study Groups. Risk factors for candidemia: a prospective matched case-control study. Crit Care 2020; 24:109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Magill SS, Edwards JR, Bamberg W, et al. ; Emerging Infections Program Healthcare-Associated Infections and Antimicrobial Use Prevalence Survey Team. Multistate point-prevalence survey of health care-associated infections. N Engl J Med 2014; 370:1198–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Carvalho A, Goldman GH.. Editorial: an omics perspective on fungal infection: toward next-generation diagnosis and therapy. Front Microbiol 2017; 8:85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Moser C, Lerche CJ, Thomsen K, et al. Antibiotic therapy as personalized medicine - general considerations and complicating factors. APMIS 2019; 127:361–71. [DOI] [PubMed] [Google Scholar]
- 25. van de Veerdonk FL, Gresnigt MS, Verweij PE, Netea MG.. Personalized medicine in influenza: a bridge too far or the near future? Curr Opin Pulm Med 2017; 23: 237–40. [DOI] [PubMed] [Google Scholar]
- 26. Al-Mozaini MA, Mansour MK.. Personalized medicine. Is it time for infectious diseases? Saudi Med J 2016; 37:1309–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Farber DL. Tissues, not blood, are where immune cells function. Nature 2021; 593:506–9. [DOI] [PubMed] [Google Scholar]
- 28. Quinton LJ, Jones MR, Robson BE, Mizgerd JP.. Mechanisms of the hepatic acute-phase response during bacterial pneumonia. Infect Immun 2009; 77:2417–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Woodruff PG, Modrek B, Choy DF, et al. T-helper type 2-driven inflammation defines major subphenotypes of asthma. Am J Respir Crit Care Med 2009; 180:388–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Iwanaga N, Kolls JK.. Updates on T helper type 17 immunity in respiratory disease. Immunology 2019; 156:3–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sade-Feldman M, Yizhak K, Bjorgaard SL, et al. Defining T cell states associated with response to checkpoint immunotherapy in melanoma. Cell 2018; 175:998–1013.e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Villani AC, Satija R, Reynolds G, et al. et al. Single-cell RNA-seq reveals new types of human blood dendritic cells, monocytes, and progenitors. Science 2017; 356:eaah4573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Dixit A, Parnas O, Li B, et al. Perturb-seq: dissecting molecular circuits with scalable single-cell RNA profiling of pooled genetic screens. Cell 2016; 167:1853–1866.e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Li B, Dewey CN.. RSEM: accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinformatics 2011; 12:323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Li B, Gould J, Yang Y, et al. Cumulus provides cloud-based data analysis for large-scale single-cell and single-nucleus RNA-seq. Nat Methods 2020; 17:793–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sun Z, Chen L, Xin H, et al. A Bayesian mixture model for clustering droplet-based single-cell transcriptomic data from population studies. Nat Commun 2019; 10:1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Asp M, Bergenstråhle J, Lundeberg J.. Spatially resolved transcriptomes-next generation tools for tissue exploration. Bioessays 2020; 42:e1900221. [DOI] [PubMed] [Google Scholar]
- 38. Montoro DT, Haber AL, Biton M, et al. A revised airway epithelial hierarchy includes CFTR-expressing ionocytes. Nature 2018; 560:319–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Reyes M, Filbin MR, Bhattacharyya RP, et al. An immune-cell signature of bacterial sepsis. Nat Med 2020; 26:333–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Jarvis JN, Casazza JP, Stone HH, et al. The phenotype of the cryptococcus-specific CD4+ memory T-cell response is associated with disease severity and outcome in HIV-associated cryptococcal meningitis. J Infect Dis 2013; 207:1817–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Linyu L, Ali Abuderman AW, Muzaheed, et al. Modulation of host immune status by cryptococcus co-infection during HIV-1 pathogenesis and its impact on CD+4 cell and cytokines environment. Microb Pathog 2020; 139:103864. [DOI] [PubMed] [Google Scholar]
- 42. Tugume L, Rhein J, Hullsiek KH, et al. ; COAT and ASTRO-CM teams. HIV-associated cryptococcal meningitis occurring at relatively higher CD4 counts. J Infect Dis 2019; 219:877–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Camargo JF, Bhimji A, Kumar D, et al. Impaired T cell responsiveness to interleukin-6 in hematological patients with invasive aspergillosis. PLoS One 2015; 10:e0123171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Liu F, Fan X, Auclair S, et al. Sequential dysfunction and progressive depletion of candida albicans-specific CD4 T cell response in HIV-1 infection. PLoS Pathog 2016; 12:e1005663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. García-Gil A, Lopez-Bailon LU, Ortiz-Navarrete V.. Beyond the antibody: B cells as a target for bacterial infection. J Leukoc Biol 2019; 105:905–13. [DOI] [PubMed] [Google Scholar]
- 46. Hurwitz JL. B cells, viruses, and the SARS-CoV-2/COVID-19 pandemic of 2020. Viral Immunol 2020; 33:251–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Zargaran FN, Akya A, Rezaeian S, et al. B cell epitopes of four fimbriae antigens of Klebsiella pneumoniae: a comprehensive in silico study for vaccine development. Int J Pept Res Ther 2020: doi: 10.1007/s10989-020-10134-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Perrett KP, John TM, Jin C, et al. Long-term persistence of immunity and B-cell memory following Haemophilus influenzae type B conjugate vaccination in early childhood and response to booster. Clin Infect Dis 2014; 58:949–59. [DOI] [PubMed] [Google Scholar]
- 49. Stoeckius M, Hafemeister C, Stephenson W, et al. Simultaneous epitope and transcriptome measurement in single cells. Nat Methods 2017; 14:865–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ward RA, Thompson GR, Villani A-C, et al. The known unknowns of the immune response to Coccidioides. J Fungi 2021; 7:377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Wang F, Flanagan J, Su N, et al. RNAscope: a novel in situ RNA analysis platform for formalin-fixed, paraffin-embedded tissues. J Mol Diagn 2012; 14:22–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Goltsev Y, Samusik N, Kennedy-Darling J, et al. Deep profiling of mouse splenic architecture with CODEX multiplexed imaging. Cell 2018; 174:968–81.e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Maskarinec SA, Johnson MD, Perfect JR.. Genetic susceptibility to fungal infections: what is in the genes? Curr Clin Microbiol Rep 2016; 3:81–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Berdasco M, Esteller M.. Clinical epigenetics: seizing opportunities for translation. Nat Rev Genet 2019; 20:109–27. [DOI] [PubMed] [Google Scholar]
- 55. Rodríguez-Ubreva J, Ballestar E.. Chromatin immunoprecipitation. Methods Mol Biol 2014; 1094:309–18. [DOI] [PubMed] [Google Scholar]
- 56. Buenrostro JD, Wu B, Chang HY, Greenleaf WJ.. ATAC-seq: a method for assaying chromatin accessibility genome-wide. Curr Protoc Mol Biol 2015; 109:21.29.1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Archin NM, Liberty AL, Kashuba AD, et al. Administration of vorinostat disrupts HIV-1 latency in patients on antiretroviral therapy. Nature 2012; 487:482–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Rasmussen TA, Tolstrup M, Brinkmann CR, et al. Panobinostat, a histone deacetylase inhibitor, for latent-virus reactivation in HIV-infected patients on suppressive antiretroviral therapy: a phase ½, single group, clinical trial. Lancet HIV 2014; 1:e13–21. [DOI] [PubMed] [Google Scholar]
- 59. Netea MG, Domínguez-Andrés J, Barreiro LB, et al. Defining trained immunity and its role in health and disease. Nat Rev Immunol 2020; 20:375–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Arts RJ, Novakovic B, Ter Horst R, et al. Glutaminolysis and fumarate accumulation integrate immunometabolic and epigenetic programs in trained immunity. Cell Metab 2016; 24:807–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Bekkering S, Arts RJW, Novakovic B, et al. Metabolic induction of trained immunity through the mevalonate pathway. Cell 2018; 172:135–46.e9. [DOI] [PubMed] [Google Scholar]
- 62. Cheng SC, Quintin J, Cramer RA, et al. mTOR- and HIF-1α-mediated aerobic glycolysis as metabolic basis for trained immunity. Science 2014; 345:1250684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Zhang E, Chai JC, Deik AA, et al. Plasma lipidomic profiles and risk of diabetes: 2 prospective cohorts of HIV-infected and HIV-uninfected individuals. J Clin Endocrinol Metab 2021; 106:999–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Guasch-Ferre M, Hu FB, Ruiz-Canela M, et al. Plasma metabolites from choline pathway and risk of cardiovascular disease in the PREDIMED (Prevention With Mediterranean Diet) study. J Am Heart Assoc 2017; 6. doi: 10.1161/JAHA.117.006524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Lloyd-Price J, Arze C, Ananthakrishnan AN, et al. ; IBDMDB Investigators. Multi-omics of the gut microbial ecosystem in inflammatory bowel diseases. Nature 2019; 569:655–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Mayers JR, Wu C, Clish CB, et al. Elevation of circulating branched-chain amino acids is an early event in human pancreatic adenocarcinoma development. Nat Med 2014; 20:1193–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Paynter NP, Balasubramanian R, Giulianini F, et al. Metabolic predictors of incident coronary heart disease in women. Circulation 2018; 137:841–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Rhee EP, Waikar SS, Rebholz CM, et al. ; CKD Biomarkers Consortium. Variability of two metabolomic platforms in CKD. Clin J Am Soc Nephrol 2019; 14:40–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Wang TJ, Larson MG, Vasan RS, et al. Metabolite profiles and the risk of developing diabetes. Nat Med 2011; 17:448–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Zeleznik OA, Eliassen AH, Kraft P, et al. A prospective analysis of circulating plasma metabolites associated with ovarian cancer risk. Cancer Res 2020; 80:1357–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Sindelar M, Stancliffe E, Schwaiger-Haber M, et al. Longitudinal metabolomics of human plasma reveals prognostic markers of COVID-19 disease severity. Cell Rep Med 2021; 2:100369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Markley JL, Brüschweiler R, Edison AS, et al. The future of NMR-based metabolomics. Curr Opin Biotechnol 2017; 43:34–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Zhou B, Xiao JF, Tuli L, Ressom HW.. LC-MS-based metabolomics. Mol Biosyst 2012; 8:470–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Pearce EL, Pearce EJ.. Metabolic pathways in immune cell activation and quiescence. Immunity 2013; 38:633–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Pellon A, Sadeghi Nasab SD, Moyes DL.. New insights in Candida albicans innate immunity at the mucosa: toxins, epithelium, metabolism, and beyond. Front Cell Infect Microbiol 2020; 10:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Traven A, Naderer T.. Central metabolic interactions of immune cells and microbes: prospects for defeating infections. EMBO Rep 2019; 20:e47995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Domínguez-Andrés J, Arts RJW, Ter Horst R, et al. Rewiring monocyte glucose metabolism via C-type lectin signaling protects against disseminated candidiasis. PLoS Pathog 2017; 13:e1006632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Ochando J, Fayad ZA, Madsen JC, et al. Trained immunity in organ transplantation. Am J Transplant 2020; 20:10–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Braza MS, van Leent MMT, Lameijer M, et al. Inhibiting inflammation with myeloid cell-specific nanobiologics promotes organ transplant acceptance. Immunity 2018; 49:819–828.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Blaise BJ, Gouel-Chéron A, Floccard B, et al. Metabolic phenotyping of traumatized patients reveals a susceptibility to sepsis. Anal Chem 2013; 85:10850–5. [DOI] [PubMed] [Google Scholar]
- 81. Dessì A, Liori B, Caboni P, et al. Monitoring neonatal fungal infection with metabolomics. J Matern Fetal Neonatal Med 2014; 27(Suppl 2):34–8. [DOI] [PubMed] [Google Scholar]
- 82. Candia J, Cheung F, Kotliarov Y, et al. Assessment of variability in the SOMAscan assay. Sci Rep 2017; 7:14248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. DeBoer EM, Wagner BD, Popler J, et al. Novel application of aptamer proteomic analysis in cystic fibrosis bronchoalveolar lavage fluid. Proteomics Clin Appl 2019; 13:e1800085. [DOI] [PubMed] [Google Scholar]
- 84. Aghaeepour N, Lehallier B, Baca Q, et al. A proteomic clock of human pregnancy. Am J Obstet Gynecol 2018; 218:347.e1–e14. [DOI] [PubMed] [Google Scholar]
- 85. Feyaerts D, Hedou J, Gillard J, et al. Integrated plasma proteomic and single-cell immune signaling network signatures demarcate mild, moderate, and severe COVID-19 [preprint ]. bioRxiv 2021. doi: 10.1101/2021.02.09.430269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Ghaemi MS, DiGiulio DB, Contrepois K, et al. Multiomics modeling of the immunome, transcriptome, microbiome, proteome and metabolome adaptations during human pregnancy. Bioinformatics 2019; 35:95–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Bendall SC, Simonds EF, Qiu P, et al. Single-cell mass cytometry of differential immune and drug responses across a human hematopoietic continuum. Science 2011; 332:687–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Joshi RN, Binai NA, Marabita F, et al. Phosphoproteomics reveals regulatory T cell-mediated DEF6 dephosphorylation that affects cytokine expression in human conventional T cells. Front Immunol 2017; 8:1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Sjoelund V, Smelkinson M, Nita-Lazar A.. Phosphoproteome profiling of the macrophage response to different toll-like receptor ligands identifies differences in global phosphorylation dynamics. J Proteome Res 2014; 13:5185–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Leigh JE, McNulty KM, Fidel PL Jr. Characterization of the immune status of CD8+ T cells in oral lesions of human immunodeficiency virus-infected persons with oropharyngeal Candidiasis. Clin Vaccine Immunol 2006; 13:678–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Suram S, Silveira LJ, Mahaffey S, et al. Cytosolic phospholipase A(2)α and eicosanoids regulate expression of genes in macrophages involved in host defense and inflammation. PLoS One 2013; 8:e69002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Terayama Y, Matsuura T, Ozaki K.. Induction of severe chronic hyperplastic candidiasis in rat by opportunistic infection of C. albicans through combination of diabetes and intermittent prednisolone administration. Toxicol Pathol 2017; 45:745–55. [DOI] [PubMed] [Google Scholar]
- 93. Won EJ, Choi JH, Cho YN, et al. Biomarkers for discrimination between latent tuberculosis infection and active tuberculosis disease. J Infect 2017; 74:281–93. [DOI] [PubMed] [Google Scholar]
- 94. Gómez-Escobar LG, Hoffman KL, Choi JJ, et al. Cytokine signatures of end organ injury in COVID-19. Sci Rep 2021; 11:12606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Beardsley J, Hoang NLT, Kibengo FM, et al. Do intracerebral cytokine responses explain the harmful effects of dexamethasone in human immunodeficiency virus-associated cryptococcal meningitis? Clin Infect Dis 2019; 68:1494–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Akilimali NA, Chang CC, Muema DM, et al. Plasma but not cerebrospinal fluid interleukin 7 and interleukin 5 levels pre-antiretroviral therapy commencement predict cryptococcosis-associated immune reconstitution inflammatory syndrome. Clin Infect Dis 2017; 65:1551–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Ahmad S, Milan MDC, Hansson O, et al. CDH6 and HAGH protein levels in plasma associate with Alzheimer’s disease in APOE ε4 carriers. Sci Rep 2020; 10:8233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Ngo D, Sinha S, Shen D, et al. Aptamer-based proteomic profiling reveals novel candidate biomarkers and pathways in cardiovascular disease. Circulation 2016; 134:270–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Penn-Nicholson A, Hraha T, Thompson EG, et al. ; ACS and GC6–74 cohort study groups. Discovery and validation of a prognostic proteomic signature for tuberculosis progression: a prospective cohort study. PLoS Med 2019; 16:e1002781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Dong L, Watson J, Cao S, et al. Aptamer based proteomic pilot study reveals a urine signature indicative of pediatric urinary tract infections. PLoS One 2020; 15:e0235328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Filbin MR, Mehta A, Schneider AM, et al. Longitudinal proteomic analysis of plasma from patients with severe COVID-19 reveal patient survival-associated signatures, tissue-specific cell death, and cell-cell interactions. Cell Rep Med 2021; 2:100287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Jehan F, Sazawal S, Baqui AH, et al. ; Alliance for Maternal and Newborn Health Improvement , the Global Alliance to Prevent Prematurity and Stillbirth, and the Prematurity Research Center at Stanford University. Multiomics characterization of preterm birth in low- and middle-income countries. JAMA Netw Open 2020; 3:e2029655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Culos A, Tsai AS, Stanley N, et al. Integration of mechanistic immunological knowledge into a machine learning pipeline improves predictions. Nat Mach Intell 2020; 2:619–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Aghaeepour N, Kin C, Ganio EA, et al. Deep immune profiling of an arginine-enriched nutritional intervention in patients undergoing surgery. J Immunol 2017; 199:2171–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Schüssler-Fiorenza Rose SM, Contrepois K, Moneghetti KJ, et al. A longitudinal big data approach for precision health. Nat Med 2019; 25:792–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Stelzer IA, Ghaemi MS, Han X, et al. Integrated trajectories of the maternal metabolome, proteome, and immunome predict labor onset. Sci Transl Med 2021; 13:e1002781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Baek B, Lee H.. Prediction of survival and recurrence in patients with pancreatic cancer by integrating multi-omics data. Sci Rep 2020; 10:18951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Ding H, Sharpnack M, Wang C, et al. Integrative cancer patient stratification via subspace merging. Bioinformatics 2019; 35:1653–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Gallivan KA, Srivastava A, Xiuwen L, Dooren PV.. Efficient algorithms for inferences on Grassmann manifolds. IEEE Workshop on Statistical Signal Processing, 2003 (pp. 315–318). IEEE. Available at https://ieeexplore.ieee.org/document/1289408.
- 110. National Institute of Allergy and Infectious Diseases. Human immunology project consortium (HIPC). Available at: https://www.niaid.nih.gov/research/human-immunology-project-consortium. Accessed 6 July 2021.
- 111. European Commission. Horizon 2020. Available at: https://ec.europa.eu/programmes/horizon2020/en/home. Accessed 6 July 2021.