Abstract
The objective of this study was to determine the interactive effects of dietary fiber solubility and lipid source on growth performance, visceral organ weights, gut histology, and gut microbiota composition of weaned pigs. A total of 280 nursery pigs [initial body weight (BW) = 6.84 kg] weaned at 21 d were housed in 40 pens (7 pigs/pen). The pigs were fed four diets (10 pens/diet) in a randomized complete block design in two phases: Phase 1 from 0 to 2 wk and Phase 2 from 2 to 5 wk. The diets were corn-soybean meal-based with either sugar beet pulp (SBP) or soybean hulls (SBH) as a fiber source and either soybean oil (SBO) or choice white grease (CWG) as a lipid source in a 2 × 2 factorial arrangement. The BW and feed intake were determined by phase, whereas visceral organ weights, intestinal histology, and gut microbial composition were determined at the end of the trial. Dietary fiber solubility and lipid source did not interact (P > 0.05) on average daily feed intake and average daily gain across all phases. However, the gain to feed ratio (G:F) for CWG-containing diets was lower (P < 0.05) than that for SBO-containing diets for Phase 1. Also, G:F for SBP-containing diets was lower (P < 0.05) than that for SBH-containing diets for Phase 1 and for the entire study period. Pigs fed SBP-containing diets had greater (P < 0.05) stomach weight, and tended to have greater (P < 0.10) small and large intestine weights relative to BW than those fed SBH-containing diets. Duodenal villous height to crypt depth ratio for CWG-based diets tended to be greater (P = 0.09) than that for SBO-based diets. Fiber solubility and lipid source interacted (P < 0.05) on relative abundance of Bacteroides in the colon such that the relative abundance of the Bacteroides for CWG was greater (P < 0.05) than that for the SBO in SBP-based diet, but not in SBH-based diet. Relative abundance of Butyricicoccus in the colon for SBH-based diet was greater (P < 0.05) than that for SBP-based diet. In conclusion, inclusion of SBH instead of SBP in corn-soybean meal-based diets for weaned pigs can result in increased feed efficiency and relative abundance of Butyricicoccus in the colon, which is associated with improved gut health. Also, inclusion of SBO instead of CWG in the diets for weaned pigs can result in improved feed efficiency during Phase 1 feeding; however, the pigs may recover from the low feed efficiency induced by dietary inclusion of CWG instead of SBO after Phase 1 feeding.
Keywords: fiber solubility, growth performance, gut health, lipid source, weaned pig
Introduction
Moderate amounts of fibrous feedstuffs can be added in diets for weaned pigs to improve growth performance and gut health. Insoluble dietary fiber (IDF) can increase performance of weaned pigs by stimulating feed intake (Gerritsen et al., 2012) and by reducing gut infections through increasing the rate of passage of digesta in gastrointestinal tract (GIT) that result in reduced attachment of pathogens to GIT mucosa (Molist et al., 2014). Soluble dietary fiber (SDF), which is more fermentable than IDF (Jaworski and Stein, 2017), can improve performance by generating volatile fatty acid (VFA) during its fermentation in the GIT; VFAs are a source of energy for intestinal epithelial cells, and hence, they promote intestinal mucosal growth and integrity (Wang et al., 2004; Tao et al., 2019). Conversely, SDF may reduce small intestinal nutrient digestibility if it increases digesta viscosity (Owusu-Asiedu et al., 2006).
Fibrous feedstuffs have relatively low energy value (Hansen et al., 2006), and hence, lipids are often added in high-fiber diets to improve the dietary energy level. Dietary lipids reduce fiber fermentation in the rumen (Maia et al., 2007). Thus, dietary lipids that escape digestion in the small intestine can reduce the dietary fiber fermentation in the hindgut of pigs (Yan et al., 2013). Unsaturated fatty acids are more digestible than saturated fatty acids (Powles et al., 1994), implying that replacement of dietary unsaturated fatty acids with saturated fatty acids may result in reduced fiber fermentation in hindgut. Since “viscous” SDF reduces small intestinal nutrient digestibility, it can negatively interact with dietary fatty acids as it can increase the flow of the fatty acids to the hindgut, leading to reduced hindgut fermentation of organic matter. Thus, the effects of adding a combination of fibrous feedstuffs and fat in diets for weaned pigs on growth performance and hindgut fermentation can vary depending on fiber type and fat type.
Some of the insoluble and soluble fiber-rich feedstuffs that can be added in weaned pig diets include soybean hulls (SBH) and sugar beet pulp (SBP), respectively. The unsaturated fatty acids-rich feedstuffs that can be added in the swine diets include corn oil and soybean oil (SBO), whereas the saturated fatty acids-rich feedstuffs that can be added in the swine diets include beef tallow and choice white grease (CWG). Ndou et al. (2019) determined the effects of dietary inclusion of cellulose (IDF) or pectin (SDF) each with either corn oil or beef tallow on nutrient digestibility of pigs. In their (Ndou et al., 2019) study, they observed an interaction between fiber source and fat source such that the addition of beef tallow to pectin-containing diet, but not to cellulose-containing diet, reduced ileal digestibility of total fatty acids. However, information is lacking on the effects of dietary inclusion of SBH or SBP each with either SBO or CWG on growth performance, nutrient digestibility, and indicators of gut health such as intestinal morphology and gut microbial composition of weaned pigs. Soluble fiber in SBP, unlike that in pectin, has limited effects on digesta viscosity (Flis et al., 2017). Furthermore, effects of purified fibers (such as cellulose and pectin) on growth performance, nutrient digestibility, and the indicators of gut health of pigs may differ from the effects of fibers in the matrix of fibrous feedstuffs such as SBH and SBH. Thus, there was a need to fill this gap in knowledge.
Because of the limited effects of SDF in SBP on digesta viscosity (Flis et al., 2017), both SBH and SBP may have limited effects on fatty acid digestibility in the small intestine, and hence fatty acids flow to the hindgut. Because of the lower content of unsaturated fatty acids in CWG than in SBO, fatty acids in CWG can be less digestible than fatty acids in SBO, and hence, CWG may more negatively affect fiber fermentation in the hindgut of pigs than SBO. It was hypothesized that replacement of SBO with CWG in SBH-containing diets has limited effects on growth performance and gut microbial composition of weaned pigs, whereas replacement of SBO with CWG in SBP-containing diets negatively affects growth performance and gut microbial composition of weaned pigs. This is because SDF in SBP can improve growth performance and gut mucosal growth and integrity of weaned pigs mainly via its fermentation in the hindgut, and CWG can negatively affect fiber fermentation in the hindgut. The objective of this study was to determine the interactive effects of dietary fiber solubility and lipid source on growth performance, visceral organ weights, gut histomorphology, and gut microbial composition of weaned pigs fed SBP- or SBH-containing diets with either SBO or CWG supplementation.
Materials and Methods
The experimental animal procedures were reviewed and approved by the Institutional Animal Care and Use Committee at South Dakota State University (#18-088E).
Animals and housing
A total of 280 pigs [initial body weight (BW) of 6.84 ± 0.98 kg; Large White-Landrace female × Large White-Hampshire male; Pig Improvement Company] weaned at 21 d of age were obtained from Swine Education and Research Facility, South Dakota State University (Brookings, SD, USA). The pigs were fed an antibiotic-free commercial starter diet during the first 10 d post-weaning. Pigs were then individually weighed and housed in 40 pens (7 pigs/pen). Pens (1.8 × 2.4 m) had fully slatted-concrete floors, metal spindle walls (1.0 m high), and solid polyvinyl chloride gates. Each pen was equipped with a cup drinker, a double-spaced dry feeder, and a heat lamp. Room temperature was maintained at 28 ± 1 °C during the first week. Thereafter, the room temperature was maintained at 24 ± 2 °C throughout the experiment.
Experimental diets
Four experimental diets fed included a corn-soybean meal-based diet with SBP or SBH as fibrous feedstuff and SBO or CWG as a lipid source in a 2 × 2 factorial arrangement (Table 1). The experimental diets were formulated to contain similar total dietary fiber, crude fat, Ca, standardized total tract digestible P, and standardized ileal digestible Lys, Met, and Thr contents. The diets were fed as mash and were formulated to meet or exceed NRC (2012) nutrient recommendations for nursery pigs. The four experimental diets were fed in 2 phases: Phase 1 from days 0 to 14 and Phase 2 from days 14 to 35 of the trial. The SBP and SBH were included at 10% in Phase 1 diets and at 12% in Phase 2 diets. Lipid sources were added in diets at 4.5%.
Table 1.
Ingredient and analyzed compositions of the experimental diets1
| Item | Phase 1 | Phase 2 | ||||||
|---|---|---|---|---|---|---|---|---|
| SBP | SBH | SBP | SBH | |||||
| SBO | CWG | SBO | CWG | SBO | CWG | SBO | CWG | |
| Ingredient, % | ||||||||
| Corn | 42.31 | 42.28 | 43.28 | 43.27 | 52.53 | 52.49 | 51.57 | 51.56 |
| Soybean meal | 30.00 | 30.00 | 28.50 | 28.50 | 28.00 | 28.00 | 28.50 | 28.50 |
| Whey powder | 10.00 | 10.00 | 10.00 | 10.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| SBP | 10.00 | 10.00 | 0.00 | 0.00 | 12.00 | 12.00 | 0.00 | 0.00 |
| SBH | 0.00 | 0.00 | 10.00 | 10.00 | 0.00 | 0.00 | 12.00 | 12.00 |
| SBO | 4.50 | 0.00 | 4.50 | 0.00 | 4.50 | 0.00 | 4.50 | 0.00 |
| CWG | 0.00 | 4.50 | 0.00 | 4.50 | 0.00 | 4.50 | 0.00 | 4.50 |
| Limestone | 0.75 | 0.73 | 1.02 | 1.02 | 0.64 | 0.64 | 0.95 | 0.96 |
| Monocalcium phosphate | 1.02 | 1.05 | 1.06 | 1.06 | 0.96 | 1.00 | 0.98 | 0.98 |
| Salt | 0.59 | 0.60 | 0.65 | 0.66 | 0.57 | 0.57 | 0.63 | 0.63 |
| l-Lysine·HCl | 0.43 | 0.44 | 0.50 | 0.50 | 0.42 | 0.42 | 0.43 | 0.43 |
| l-Threonine | 0.13 | 0.13 | 0.16 | 0.16 | 0.14 | 0.14 | 0.14 | 0.14 |
| dl-Methionine | 0.07 | 0.07 | 0.13 | 0.13 | 0.04 | 0.04 | 0.10 | 0.10 |
| l-Tryptophan | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Vitamin premix2 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 |
| Mineral premix3 | 0.15 | 0.15 | 0.15 | 0.15 | 0.15 | 0.15 | 0.15 | 0.15 |
| Calculated composition, as fed | ||||||||
| NE, kcal/kg | 2,561 | 2,531 | 2,486 | 2,457 | 2,555 | 2,525 | 2,453 | 2,424 |
| SID AA4, % | ||||||||
| Lys | 1.35 | 1.35 | 1.35 | 1.35 | 1.23 | 1.23 | 1.23 | 1.23 |
| Met | 0.39 | 0.39 | 0.39 | 0.39 | 0.36 | 0.36 | 0.36 | 0.36 |
| Thr | 0.79 | 0.79 | 0.79 | 0.79 | 0.73 | 0.73 | 0.73 | 0.73 |
| Trp | 0.27 | 0.27 | 0.22 | 0.22 | 0.25 | 0.25 | 0.20 | 0.20 |
| Ca, % | 0.80 | 0.80 | 0.80 | 0.80 | 0.70 | 0.70 | 0.70 | 0.70 |
| STTD5 P, % | 0.40 | 0.40 | 0.40 | 0.40 | 0.33 | 0.33 | 0.33 | 0.33 |
| Total dietary fiber, % | 24.31 | 24.31 | 24.35 | 24.35 | 22.19 | 22.18 | 21.86 | 21.85 |
| Analyzed composition, % as fed | ||||||||
| Dry matter | 88.5 | 88.3 | 88.1 | 88.0 | 87.6 | 88.0 | 87.8 | 88.4 |
| Crude protein | 18.8 | 19.3 | 19.6 | 18.9 | 17.0 | 17.8 | 18.4 | 17.9 |
| Crude ash | 6.55 | 6.59 | 5.59 | 6.05 | 5.38 | 5.42 | 5.55 | 5.21 |
| Ether extract | 4.90 | 4.59 | 4.52 | 4.37 | 3.79 | 5.30 | 4.19 | 4.82 |
| Total dietary fiber | 14.12 | 14.20 | 16.21 | 15.52 | 16.13 | 15.36 | 17.52 | 17.51 |
| Soluble dietary fiber | 0.98 | 1.21 | 0.40 | 0.36 | 1.48 | 1.47 | 0.57 | 0.50 |
| Insoluble dietary fiber | 13.14 | 12.99 | 15.81 | 15.16 | 14.65 | 13.89 | 16.95 | 17.01 |
| Neutral detergent fiber | 14.74 | 14.81 | 16.81 | 16.72 | 15.49 | 15.59 | 17.01 | 17.32 |
| Acid detergent fiber | 6.19 | 6.24 | 7.67 | 7.41 | 6.48 | 6.56 | 10.24 | 10.29 |
1SBP, sugar beet pulp; SBH, soybean hulls; SBO, soybean oil; and CWG, choice white grease.
2Provided the following per kilogram of diet: 11,011 IU vitamin A, 1,652 IU vitamin D3, 55 IU vitamin E, 0.04 mg vitamin B12, 4.4 mg menadione, 9.9 mg riboflavin, 61 mg pantothenic acid, 55 mg niacin, 1.1 mg folic acid, 3.3 mg pyridoxine, 3.3 mg thiamine, and 0.2 mg biotin.
3Provided the following per kilogram of diet: 165 mg Zn as ZnSO4, 23 mg Fe as FeSO4, 17 mg Cu as CuSO4, 44 mg Mn as MnSO4, and 0.30 mg Se as Na2SeO3.
4SID AA = standardized ileal digestible amino acid.
5STTD = standardized total tract digestible.
Experimental design and procedure
The four diets were allotted to the 40 pens (10 pens per diet) in a randomized complete block design with sex as block. Pigs had an ad libitum access to diets and fresh water during the entire period. Individual pig BW and feed intake per pen were measured by phase to calculate average daily gain (ADG), average daily feed intake (ADFI), and gain to feed ratio (G:F).
At the end of the Phase 2, one pig from each pen with BW that was closest to the pen average BW was selected and then euthanized by captive bolt penetration. Visceral organs including heart, liver, kidneys, and spleen were isolated and collected from the euthanized pigs, blot dried, and weighed. The stomach, small intestine, cecum, and large intestine were also collected from the eviscerated pig carcasses, digesta emptied, blot dried, and weighed. Digesta samples were collected from the proximal colon and immediately stored in a −80 °C freezer for further analysis of microbial composition.
For gut histomorphology, the 5 cm of segments of duodenum (at 70 cm below the pylorus), ileum (at 70 cm cranial to ileal–cecal junction), and jejunum (at the middle of the rest of small intestine) were cleaved off from the small intestine. The segments were gently flushed with saline and placed in a 50-mL conical tube filled with 10% formalin, and stored for later analysis.
Sample preparation and analyses
The fibrous feedstuffs (SBP and SBH) and experimental diets were ground to pass through a 0.75-mm screen using a centrifugal mill (model Zm200; Retsh GmbH, Haan, Germany). The samples were analyzed for dry matter (DM) by oven drying at 135 °C for 2 h (method 930.15), crude protein (CP) by a combustion procedure (method 990.03), ether extract (EE; method 2003.06), and crude ash (method 942.05) as per AOAC (2007). The samples were analyzed for acid detergent fiber (ADF) and neutral detergent fiber (NDF) as described by Van Soest et al. (1991) on an Ankom 200 Fiber Analyzer (Ankom Technology, Fairport, NY), and for insoluble dietary fiber (IDF) and soluble dietary fiber (SDF) by using a Megazyme Total Dietary Fiber kit (Megazyme International Ireland Ltd, Wicklow, Ireland) according to AOAC-991.43 and AACC-33-07.01 methods (AOAC, 2012; McCleary et al., 2012). The total dietary fiber (TDF) was calculated as sum of IDF and SDF. The fat sources were analyzed for fatty acid profile (method 996.06) and peroxidation (method 965.33) as per AOAC (2007).
Intestinal tissues for histology analysis were sent to the Animal Disease Research and Diagnostic Laboratory at South Dakota State University for staining with hematoxylin and eosin. The villous height (VH; from the top of the villi to the villous-crypt junction) and crypt depth (CD; from the villous-crypt junction to the base) were measured at 4× magnification using a microscope (Micromaster, Fisher Scientific, Waltham, MA, USA) equipped with a 0.55× wifi camera eyepiece (MC500-W 3rd Gen., Meiji Techno Co. LTD., Saitama, Japan) and Micro-Capture software (Meiji Techno Co. LTD., Saitama, Japan) in 20 well-oriented villi and crypt columns. The villous height-to-crypt depth (VH:CD) ratio was calculated by dividing VH by CD.
For analysis of gut microbial composition, the extracted DNA samples from colonic digesta samples were analyzed for sequencing and bioinformatics. The total microbial DNA was extracted using QIAamp PowerFecal Pro DNA kit (QIAGEN, MD, USA) following the manufacturer’s instructions. The quality of the DNA was determined using NanoDrop one (Thermo Fisher Scientific, DE, USA) and quantified using Qubit Fluorometer 3.0 (Invitrogen, CA, USA). The extracted DNA was stored for further analysis. The extracted DNA samples were used for the sequencing of the hypervariable V3-V4 regions of the bacterial 16S rRNA using Illumina MiSeq platform. The library preparation for metagenomic sequencing was performed using 0.3 ng of DNA with a Nextera XT library preparation kit (Illumina, San Diego, CA, USA) and sequenced on the MiSeq Platform. The variations in bacterial communities within the colonic digesta of weaned pigs were analyzed using 16S rRNA microbial community analysis package in Quantitative Insights into Microbial Ecology framework (QIIME, Version 2.0). Briefly, 32 samples were quality filtered, demultiplexed, and denoised using dada2. The outputs were transferred to R for analysis using phyloseq. The Shannon diversity, Simpson diversity, Choa 1 diversity, and ACE diversity indices were used to estimate the α-diversity index and the Bray NMDS dissimilarity index was used to calculate the β-diversity index. The taxonomy was assigned to ASVs using dada2 package to implement the naive Bayesian classifier method against GreenGenes (http://greengenes.lbl.gov). The operational taxonomic units (OTUs) were clustered with 97% similarity cut off using USEARCH and Chimeric sequences, subsequently filtered out to obtain OTUs for species classification. The sequences have been deposited into the NCBI database, accession number PRJNA723299.
Statistical analysis
Data were subjected to ANOVA using the MIXED procedure (SAS Inst. Inc., Cary, NC) in a randomized complete block design with pen as the experimental unit. Phase was the repeated term in models involving time. Initial BW was used as a covariate for growth performance data. Main effects of fiber solubility and fat source and their interactions were determined. Treatment means were separated by the probability of difference when interactions between fiber solubility and fat source were significant. To test the hypotheses, P < 0.05 was considered significant. If pertinent, tendency (0.05 ≤ P < 0.10) was also reported.
Results
As expected, the SBP contained more SDF and less IDF than SBH (Table 2). The SBO contained less saturated fatty acids (SFA) and more polyunsaturated fatty acids (PUFA) than CWG. The peroxide value for CWG was greater than that for SBO.
Table 2.
Fiber and lipid composition of feedstuffs1, as-fed basis
| Item | SBP | SBH | SBO | CWG |
|---|---|---|---|---|
| Total dietary fiber, % | 51.7 | 66.5 | – | – |
| Soluble dietary fiber, % | 14.7 | 6.00 | – | – |
| Insoluble dietary fiber, % | 37.0 | 60.5 | – | – |
| IDF:SDF ratio | 2.52 | 10.08 | – | – |
| Saturated fatty acids (SFA), % | – | – | 15.24 | 35.53 |
| Polyunsaturated fatty acids (PUFA), % | – | – | 80.75 | 62.42 |
| PUFA:SFA ratio | – | – | 5.30 | 1.76 |
| Peroxide value, meq of active O2/kg lipid | – | – | 10.75 | 21.98 |
1SBP, sugar beet pulp; SBH, soybean hulls; SBO, soybean oil; and CWG, choice white grease.
There were no interactions (P > 0.05) between dietary fiber solubility and lipid source on ADG, ADFI, and G:F (Table 3). However, the main effects of dietary fiber solubility were observed for Phase 1 and for entire study period whereby pigs fed SBH-containing diets had greater (P < 0.05) G:F than those that consumed SBP-containing diets. Also, the G:F for pigs fed CWG-containing diets was lower (P < 0.05) than that for pigs fed SBO-containing diet for Phase 1. However, G:F for pigs fed CWG-containing diets tended to be greater (P = 0.06) than that for pigs fed SBO-containing diets for Phase 2. The overall G:F for pigs fed the SBP-containing diets was greater (P < 0.05) than that of pigs fed SBH-containing diets, whereas the overall G:F for pigs fed the SBO-containing diets did not differ from that of pigs fed CWG-containing diets.
Table 3.
Growth performance of nursery pigs fed diets with different fiber solubility and lipid sources1
| Item2 | SBP | SBH | SEM | P-value | ||||
|---|---|---|---|---|---|---|---|---|
| SBO | CWG | SBO | CWG | Fiber | Lipid | F × L3 | ||
| Body weight, kg | ||||||||
| Initial | 6.82 | 6.83 | 6.85 | 6.86 | 0.228 | 0.920 | 0.977 | 0.991 |
| Phase 1 | 11.13 | 10.89 | 11.57 | 11.32 | 0.411 | 0.460 | 0.671 | 0.997 |
| Phase 2 | 23.38 | 23.68 | 24.02 | 23.83 | 0.699 | 0.697 | 0.957 | 0.807 |
| Average daily gain, g | ||||||||
| Phase 1 | 308 | 290 | 337 | 318 | 14.1 | 0.161 | 0.365 | 0.986 |
| Phase 2 | 583 | 609 | 593 | 596 | 15.9 | 0.924 | 0.524 | 0.616 |
| Overall | 447 | 450 | 464 | 455 | 8.8 | 0.200 | 0.785 | 0.495 |
| Average daily feed intake, g | ||||||||
| Phase 1 | 487 | 506 | 501 | 504 | 17.1 | 0.795 | 0.651 | 0.754 |
| Phase 2 | 1,004 | 1,012 | 1,018 | 991 | 26.4 | 0.923 | 0.802 | 0.634 |
| Overall | 747 | 760 | 759 | 745 | 11.3 | 0.903 | 0.999 | 0.251 |
| Gain to feed ratio | ||||||||
| Phase 1 | 0.630 | 0.568 | 0.671 | 0.629 | 0.015 | 0.003 | 0.003 | 0.530 |
| Phase 2 | 0.581 | 0.604 | 0.581 | 0.603 | 0.012 | 0.972 | 0.058 | 0.945 |
| Overall | 0.605 | 0.586 | 0.626 | 0.616 | 0.011 | 0.023 | 0.182 | 0.670 |
1SBP, sugar beet pulp; SBH, soybean hulls; SBO, soybean oil; and CWG, choice white grease.
2Experimental diets were fed in 2 phases; Phase 1 from days 0 to 14 and Phase 2 from days 14 to 35.
3F × L, fiber by lipid interaction.
There were no (P > 0·10) interactions between dietary fiber solubility and lipid source on visceral organ weights (Table 4) and gut histomorphology (Table 5). Pigs fed SBP-containing diets had greater (P < 0.05) relative weight of stomach, and tended to have greater relative weight of the small intestine (P = 0.079) and large intestine (P = 0.057) than pigs fed SBH-containing diets. Pigs fed CWG-containing diets tended to have greater (P = 0.09) VH:CD in duodenum than pigs fed SBO-containing diets.
Table 4.
Relative visceral organ weights of nursery pigs fed diets with different fiber solubility and lipid sources1
| Item, g/ kg body weight | SBP | SBH | SEM | P-value | ||||
|---|---|---|---|---|---|---|---|---|
| SBO | CWG | SBO | CWG | Fiber | Lipid | F × L2 | ||
| Heart | 5.85 | 5.75 | 5.56 | 5.45 | 0.108 | 0.070 | 0.511 | 0.984 |
| Liver | 27.10 | 26.37 | 24.62 | 26.64 | 0.609 | 0.211 | 0.463 | 0.121 |
| Spleen | 2.10 | 2.14 | 1.99 | 2.20 | 0.085 | 0.857 | 0.335 | 0.476 |
| Stomach | 9.03 | 9.37 | 8.22 | 8.72 | 0.185 | 0.010 | 0.126 | 0.779 |
| Small intestine | 47.92 | 52.51 | 44.82 | 47.30 | 1.613 | 0.079 | 0.133 | 0.649 |
| Cecum | 2.89 | 3.00 | 3.00 | 2.91 | 0.115 | 0.959 | 0.935 | 0.572 |
| Large intestine | 16.96 | 17.26 | 15.30 | 15.85 | 0.546 | 0.057 | 0.582 | 0.873 |
1SBP, sugar beet pulp; SBH, soybean hulls; SBO, soybean oil; and CWG, choice white grease.
2F × L, fiber by lipid interaction.
Table 5.
Gut histomorphology of nursery pigs fed diets with different fiber solubility and lipid sources1
| Item2 | SBP | SBH | SEM | P-value | ||||
|---|---|---|---|---|---|---|---|---|
| SBO | CWG | SBO | CWG | Fiber | Lipid | F × L3 | ||
| Duodenum | ||||||||
| VH, um | 526.4 | 567.9 | 541.5 | 528.1 | 29.42 | 0.661 | 0.621 | 0.350 |
| CD, um | 357.2 | 311.3 | 338.0 | 333.6 | 22.91 | 0.962 | 0.292 | 0.384 |
| VH:CD | 1.53 | 1.84 | 1.61 | 1.63 | 0.098 | 0.489 | 0.093 | 0.140 |
| Jejunum | ||||||||
| VH, um | 487.2 | 440.2 | 472.9 | 476.9 | 20.39 | 0.601 | 0.311 | 0.218 |
| CD, um | 250.5 | 262.9 | 254.3 | 269.4 | 17.08 | 0.759 | 0.432 | 0.943 |
| VH:CD | 2.01 | 1.71 | 1.86 | 1.82 | 0.116 | 0.882 | 0.157 | 0.262 |
| Ileum | ||||||||
| VH, um | 412.2 | 397.1 | 413.8 | 402.9 | 22.62 | 0.864 | 0.577 | 0.917 |
| CD, um | 231.4 | 250.8 | 229.3 | 218.2 | 13.19 | 0.222 | 0.759 | 0.282 |
| VH:CD | 1.79 | 1.61 | 1.83 | 1.89 | 0.113 | 0.170 | 0.609 | 0.293 |
1SBP, sugar beet pulp; SBH, soybean hulls; SBO, soybean oil; and CWG, choice white grease.
2VH, villous height and CD, crypt depth.
3F × L, fiber by lipid interaction.
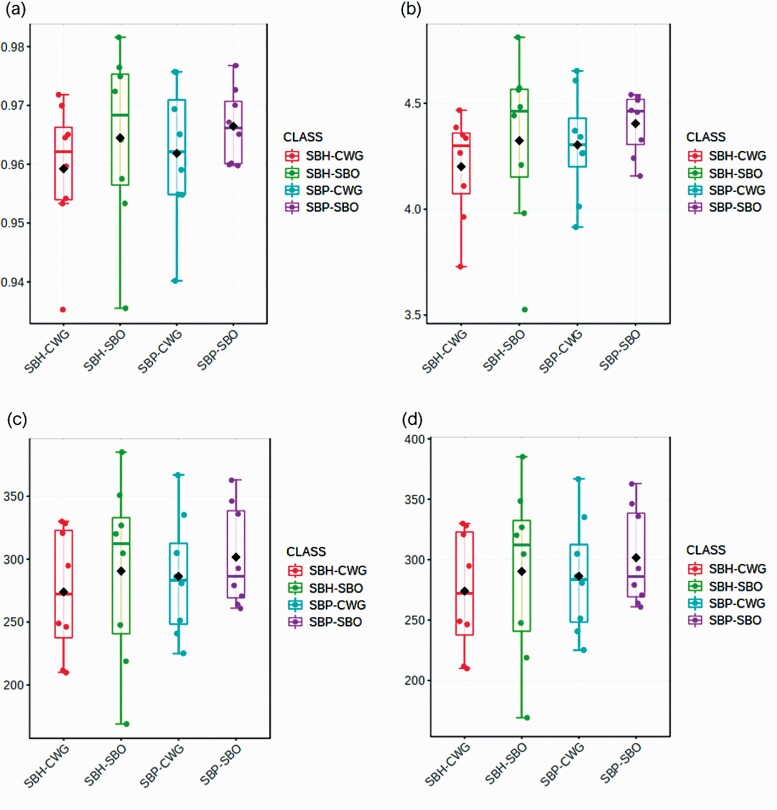
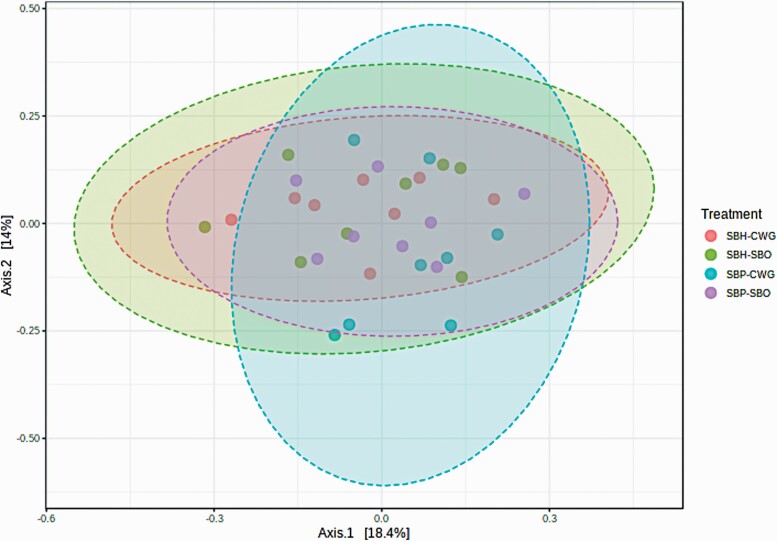
Fiber solubility and lipid source did not affect the alpha diversity of colonic microbiota of pigs measured using Simpson’s index, Shannon index, Chao 1 index, and ACE index (Figure 1). A separation of the samples was shown by a principal coordinates analysis (PCoA) for dietary treatments with PCoA1 and PCoA2, respectively, accounting for 29% and 20% of the total variability among communities (Figure 2). The effects of diets on colonic microbiota at phylum and genus levels are presented in Tables 6 and 7, respectively. At the phylum level, pigs fed SBH-containing diets had greater (P < 0.05) relative abundancy of Proteobacteria than pigs fed SBO-containing diets. At the genus level, there were interactions (P < 0.05) between dietary fiber solubility and lipid source on relative abundance of Lachnospira, Peptococcus and Bacteroides in the colon. For Lachnospira, its relative abundance for the CWG was greater (P < 0.05) than that for the SBO in the SBH-based diet, but not in the SBP-based diet. With regard to Peptococcus, its relative abundance for the CWG was lower (P < 0.05) than for the SBO in SBH-based diet, but not in the SBP-based diet. Concerning the Bacteroides, its relative abundance for the CWG was greater (P < 0.05) than for the SBO in the SBP-based diet, but not in the SBH-based diet. Dietary fiber solubility and lipid source did not interact on relative abundance of other microorganisms at genus level. Pigs fed SBH-containing diets had greater (P < 0.05) relative abundance of Butyricicoccus and Campylobacter and lower (P < 0.05) relative abundance of Prevotella.1 than pigs fed SBP-containing diets. Pigs fed CWG-containing diets had lower (P < 0.05) relative abundance of Coprococcus and greater (P < 0.05) relative abundance of Anaerovibrio than pigs fed SBO-containing diets.
Figure 1.
Box plot analysis of alpha diversity of colonic microbiota using Simpson, Shannon, Chao1, and ACE indices in pigs fed the experimental diets; (a) Simpson index (P-value: 0.641; ANOVA), (b) Shannon index (P-value: 0.554; ANOVA), (c) Chao1 index (P-value: 0.786; ANOVA), and (d) ACE index (P-value: 0.786; ANOVA).
Figure 2.
Principal coordinates analysis (PCoA) plots of Bray–Curtis computed distances in colonic microbiota between pigs fed the experimental diets (P-value: <0.223; PERMANOVA).
Table 6.
Relative abundance of colonic microbiota at Phylum level in nursery pigs fed diets with different fiber solubility and lipid sources1
| Relative abundance, % | SBP | SBH | SEM | P-value | ||||
|---|---|---|---|---|---|---|---|---|
| SBO | CWG | SBO | CWG | Fiber | Lipid | F × L2 | ||
| Firmicutes | 61.10 | 59.49 | 60.75 | 61.74 | 2.660 | 0.723 | 0.907 | 0.628 |
| Bacteroidetes | 23.64 | 25.45 | 20.74 | 20.58 | 2.338 | 0.107 | 0.727 | 0.677 |
| Proteobacteria | 3.13 | 3.30 | 6.46 | 5.76 | 1.246 | 0.027 | 0.833 | 0.730 |
| Actinobacteria | 2.50 | 2.09 | 2.05 | 2.75 | 0.352 | 0.759 | 0.688 | 0.126 |
| Tenericutes | 1.71 | 2.42 | 2.34 | 1.64 | 0.476 | 0.876 | 0.986 | 0.151 |
| Spirochaetes | 0.57 | 1.73 | 2.08 | 1.10 | 0.569 | 0.448 | 0.875 | 0.069 |
| TM7 | 2.46 | 1.68 | 1.63 | 1.20 | 0.497 | 0.194 | 0.235 | 0.732 |
| Cyanobacteria | 2.03 | 1.56 | 1.41 | 1.92 | 0.484 | 0.788 | 0.964 | 0.321 |
| Euryarchaeota | 1.64 | 0.53 | 0.98 | 1.26 | 0.449 | 0.935 | 0.358 | 0.132 |
| Chlamydiae | 0.92 | 1.20 | 0.72 | 0.80 | 0.395 | 0.453 | 0.650 | 0.789 |
| Deferribacteres | 0.30 | 0.55 | 0.73 | 0.45 | 0.359 | 0.647 | 0.965 | 0.472 |
| WPS2 | 0.00 | 0.00 | 0.11 | 0.80 | 0.405 | 0.271 | 0.398 | 0.398 |
1SBP, sugar beet pulp; SBH, soybean hulls; SBO, soybean oil; and CWG, choice white grease.
2F × L, fiber by lipid interaction.
Table 7.
Relative abundance of colonic microbiota at Genus level in nursery pigs fed diets with different fiber solubility and lipid sources1
| Relative abundance, % | SBP | SBH | SEM | P-value | ||||
|---|---|---|---|---|---|---|---|---|
| SBO | CWG | SBO | CWG | Fiber | Lipid | F × L2 | ||
| Lactobacillus | 11.02 | 11.86 | 9.49 | 10.17 | 1.027 | 0.128 | 0.463 | 0.940 |
| Streptococcus | 10.70 | 8.70 | 10.78 | 11.05 | 0.974 | 0.222 | 0.384 | 0.255 |
| Prevotella.1 | 8.13 | 9.12 | 6.94 | 6.33 | 0.961 | 0.047 | 0.841 | 0.412 |
| Blautia | 5.71 | 5.67 | 6.72 | 5.84 | 0.436 | 0.189 | 0.296 | 0.348 |
| Eubacterium | 1.44 | 1.59 | 1.74 | 1.50 | 0.205 | 0.595 | 0.816 | 0.357 |
| Faecalibacterium | 4.92 | 5.64 | 5.27 | 5.46 | 0.509 | 0.875 | 0.375 | 0.611 |
| Roseburia | 5.25 | 4.64 | 4.61 | 4.69 | 0.415 | 0.478 | 0.535 | 0.409 |
| Coprococcus | 5.06 | 4.31 | 4.46 | 3.86 | 0.273 | 0.064 | 0.020 | 0.773 |
| Dialister | 4.09 | 4.15 | 4.54 | 4.84 | 0.471 | 0.235 | 0.701 | 0.801 |
| Lachnospira | 4.37ab | 4.04ab | 3.49b | 4.85a | 0.402 | 0.934 | 0.215 | 0.045 |
| Prevotella.2 | 3.73 | 4.12 | 3.32 | 3.75 | 0.436 | 0.382 | 0.359 | 0.962 |
| Dorea | 3.79 | 3.47 | 3.59 | 3.11 | 0.223 | 0.207 | 0.084 | 0.721 |
| Ruminococcus.1 | 3.45 | 3.25 | 3.69 | 3.06 | 0.315 | 0.931 | 0.194 | 0.501 |
| Oscillospira | 3.07 | 2.91 | 3.24 | 3.20 | 0.435 | 0.597 | 0.819 | 0.889 |
| Megasphaera | 2.51 | 2.96 | 2.58 | 2.46 | 0.388 | 0.593 | 0.674 | 0.468 |
| Ruminococcus.2 | 2.31 | 2.08 | 2.67 | 2.70 | 0.292 | 0.103 | 0.735 | 0.655 |
| Butyricicoccus | 1.95 | 2.05 | 2.85 | 2.87 | 0.296 | 0.007 | 0.850 | 0.878 |
| Acidaminococcus | 1.33 | 1.47 | 1.56 | 1.56 | 0.284 | 0.576 | 0.797 | 0.801 |
| Bulleidia | 1.23 | 1.28 | 1.18 | 0.85 | 0.145 | 0.109 | 0.356 | 0.193 |
| Campylobacter | 0.80 | 0.36 | 1.02 | 1.77 | 0.357 | 0.031 | 0.680 | 0.107 |
| p75a5 | 1.20 | 0.73 | 0.95 | 0.92 | 0.128 | 0.796 | 0.060 | 0.104 |
| Anaerovibrio | 0.64 | 1.60 | 0.19 | 1.06 | 0.366 | 0.186 | 0.018 | 0.892 |
| Desulfovibrio | 0.24b | 0.62ab | 0.95a | 0.59ab | 0.215 | 0.127 | 0.988 | 0.097 |
| Treponema | 0.18 | 0.73 | 0.85 | 0.46 | 0.247 | 0.417 | 0.745 | 0.066 |
| Turicibacter | 0.24 | 0.56 | 0.20 | 0.68 | 0.250 | 0.869 | 0.123 | 0.762 |
| Bifidobacterium | 0.48 | 0.43 | 0.26 | 0.26 | 0.138 | 0.165 | 0.858 | 0.833 |
| Vestibaculum | 0.26 | 0.24 | 0.34 | 0.55 | 0.262 | 0.465 | 0.718 | 0.682 |
| Flexispira | 0.37 | 0.28 | 0.37 | 0.35 | 0.174 | 0.849 | 0.758 | 0.845 |
| Peptococcus | 0.04b | 0.15b | 0.64a | 0.20b | 0.105 | 0.005 | 0.127 | 0.015 |
| Escherichia | 0.24 | 0.24 | 0.25 | 0.19 | 0.130 | 0.879 | 0.854 | 0.815 |
| Bacteroides | 0.00b | 0.42a | 0.00b | 0.00b | 0.102 | 0.048 | 0.048 | 0.048 |
| Methanobrevibacter | 0.00 | 0.00 | 0.08 | 0.20 | 0.077 | 0.081 | 0.419 | 0.419 |
1SBP, sugar beet pulp; SBH, soybean hulls; SBO, soybean oil; and CWG, choice white grease.
2F × L = fiber by lipid interaction.
a,bWithin a row, means without a common superscript differ (P < 0.05).
Discussion
As expected, SBP had a greater content of SDF and lower content of IDF than SBH. Cellulose is the major non-starch polysaccharide in both SBP and SBH, although its content in SBH is greater than that in SBP. For instance, cellulose constituted 50% (Bach Knudsen, 2014) and 35.5% (Zhou et al., 2018) of total non-starch polysaccharide in SBH and SBP, respectively. Pectin and arabinans are the major non-cellulosic polysaccharides in SBP, whereas pectin, xylans, and galactomannans are the major non-cellulosic polysaccharides in SBH. For instance, uronic acid (which is the major component of pectin) and arabinose (which is the major component of arabinans), respectively, constituted 26% and 24% of total non-starch polysaccharide in SBP (Zhou et al., 2018), whereas uronic acid, xylose (which is the major component of xylans), and mannose (which is the major component of galactomannans), respectively, constituted 17%, 12%, and 8% of total non-starch polysaccharide in SBH (Bach Knudsen, 2014). Pectin is the most soluble non-starch polysaccharide in both SBP and SBH (Bach Knudsen, 2014; Zhou et al., 2018). Thus, the greater content of pectin in SBP than in SBH may partly explain why SBP had greater content of SDF than SBH. Also, as expected, SBO had a higher content of polyunsaturated fatty acids than CWG. Peroxide value is the measure of degree of fat rancidity (DeRouchey et al., 2004). Kerr et al. (2020) observed that growth performance of pigs fed diet containing peroxidized SBO (with peroxide value of 3.05 mg/kg diet) did not differ from that of pigs fed the control diet (with peroxide value of 0.27 mg/kg diet). The calculated peroxide values of the SBO diets and CWG diets fed in the current study were 0.48 and 0.99 mg/kg diet, respectively (data not presented). These peroxide values for diets fed in the current study were within the range of values that were reported by Kerr et al. (2020), implying that the performance of pigs in the current study may not have been affected by the dietary peroxide values.
The IDF has an ability to increase feed intake and digestive capacity of GIT by reducing intestinal stasis and increasing activity of digestive enzyme (Molist et al., 2014). Inclusion of SBP in diets for weaned pigs did not affect activity of digestive enzymes in the small intestine (Lizardo et al., 1997). Thus, the improvement in feed efficiency of weaned pigs due to replacement of SBP with SBH in diets was potentially due to improved digestion and absorption, although the digestive capacity was not measured in the current study. Unsaturated fatty acids compared with saturated fatty acids have a greater ability to increase cholecystokinin section (Beardshall et al., 1989) and hence pancreatic digestive enzyme secretion, and to increase activity of pancreatic lipase in the small intestine (Van Kuiken and Behnke, 1994). Thus, the improvement in feed efficiency of weaned pigs during the first 2 wk post-weaning due to replacement of CWG with SBO in diets could be attributed to improved nutrient digestibility by the replacement. The digestive capacity and feed intake of weaned pigs are low, especially during the first 2 wk after weaning (Molist et al., 2014). Thus, the lack of the significant effect of fiber solubility on feed efficiency during Phase 2 feeding could be attributed to the fact that digestive capacity and feed intake of weaned pigs had improved after 2 wk post-weaning. Also, the improvement in feed efficiency due to replacement of CWG with SBO in diets for weaned pigs was expected to be more pronounced during Phase 1 of feeding than during Phase 2 of feeding. However, it is not clear why replacement of CWG with SBO in diets for weaned pigs tended to reduce the feed efficiency for Phase 2.
The SDF swells and retains more water than IDF (Knudsen and Hansen, 1991; Wenk, 2001). The swelling of fiber in the GIT can result in stretching of the GIT walls and hence increase in weight of the GIT (Jiménez-Moreno et al., 2010; Rezaei et al., 2018). Previous studies showed that the SBP had a greater water holding capacity than SBH (Giger-Reverdin, 2000; Kim et al., 2015). Thus, the increase in relative weight of the stomach and small intestine of pigs due to replacement of SBH with SBP in diets could be attributed to the greater content of SDF in the SBP than in SBH. Lizardo et al. (1997) similarly reported increased stomach weight of weaned pigs due to inclusion of SBP in wheat-soybean meal-based diets for weaned pigs. The SDF is more fermentable in GIT of pigs than insoluble fiber (Jha and Berrocoso, 2016). Volatile fatty acids, which are some of the end products of carbohydrate (including fiber) and protein fermentation in GIT, stimulate proliferation of cells in GIT (Pell et al., 1995). Pectin, which constitutes the bulk of SDF in SBP, is fermented in lower part of the small intestine and in the hindgut (Drochner et al., 2004). Thus, the greater fermentability of fiber in the SBP than in SBH could partly explain the increase in relative weight of the small intestine, and explain the increase in relative weight of the large intestine of pigs due to replacement of SBH with SBP in diets.
There were no effects of fiber solubility and fat source on small intestinal histomorphology. Villous height in small intestine of weaned pigs is positively associated with luminal availability of nutrients, especially energy-yielding nutrients (Pluske et al., 1996). As previously mentioned, dietary insoluble fiber and unsaturated fatty acids can increase nutrient digestibility in the small intestine. Thus, we had hypothesized that replacement of SBP with SBH would result in increased villous height because of the higher content of insoluble fiber in latter than in the former. Also, we had hypothesized that replacement of CWG with SBO would result in increased villous height because of the higher content of unsaturated fatty acids in SBO than in CWG. Thus, the reason for the lack of effect of fiber solubility and fat source on small intestinal histomorphology is not clear. However, it should be noted that the negative effects of SDF on intestinal histomorphology of pigs is dependent on the extent to which the SDF increases digesta viscosity (Molist et al., 2014), which may explain the lack of effect of SBP on the small intestinal histomorphology. Also, Zhou et al. (2017) reported a linear increase in ileal digestibility of amino acids due to a linear increase in dietary level of canola oil, which is rich in unsaturated fatty acids, from 0 to 6%, implying that the effect of unsaturated fatty acids on luminal nutrient availability and hence intestinal histomorphology is dependent on dietary level of unsaturated fatty acids. Finally, relatively high amount of IDF in SBH may have caused abrasive damage to villi, leading to reduced villous height.
Microorganisms under the Bacteroidetes phylum produce several fiber-degrading enzymes that enable them to ferment fiber (Flint et al., 2012). Since SDF is more fermentable than IDF, the increase in relative abundance of Prevotella and Bacteroides genera that are under Bacteroidetes phylum and hence numerical increase in overall relative abundance of Bacteroidetes phylum in colonic digesta of the pigs due to replacement of SBH with SBP in diets is attributed to the higher content of SDF in the SBP than in SBH. The abundance of Prevotella genus (that is under Bacteroidetes phylum) in the GIT of pigs was positively correlated with uronic acid (that is a component of pectin) intake (Ivarsson et al., 2014), which may explain why the relative abundance of this genus was particularly greater in pigs fed SBP-containing diet than in those fed SBH-containing diets. Other studies have also reported increased abundance of Bacteroidetes in GIT of pigs (Ndou et al., 2018) or humans (Scott et al., 2014) due to consumption of soluble fiber.
The abundance of Proteobacteria phylum in GIT is partly affected by GIT pH; it decreases with a decrease in pH (Isaacson and Kim, 2012; Xu et al., 2020). Indeed, the abundance of proteobacteria decreased with an increase in fermentable fiber intake and increased with an increase in protein intake (Liu et al., 2017). Although not measured in the current study, the pH in the hindgut of pigs fed SBP-containing diets was expected to lower than that in pigs fed SBH-containing diets due to the higher fermentability of fiber in SBP than in SBH. Thus, the decrease in the relative abundance of Campylobacter genus that is under the Proteobacteria phylum and hence the decrease in relative abundance of Proteobacteria phylum in colon due to replacement of SBH with SBP in diets for pigs may have been due to lower pH in the hindgut of pig fed SBP-containing diets than in pigs fed SBH-containing diets.
Inclusion of wheat bran-derived arabinoxylan oligosaccharides in diets for mice resulted in increased abundance of Butyricicoccus genera in GIT (Suriano et al., 2017). Also, Nielsen et al. (2014) reported that arabinoxylans were a better substrate for butyric acid production by microorganisms in pigs than resistant starch. Ivarsson et al. (2014) observed a positive correlation between xylose intake and butyric acid production in GIT of pigs, implying that it is a xylose component of arabinoxylans that promote the growth of butyric acid-producing microorganisms in the GIT. The SBH has a higher content of xylose than SBP because, as previously mentioned, xylans are one of the major non-starch polysaccharides in SBH. For instance, the xylose content (on DM basis) in SBH was 8.8%, whereas that in SBP was 3.1% (Miron et al., 2001). Thus, in the current study, the greater in relative abundance of Butyricicoccus in colon of pigs fed SBH-containing diets than that in the colon of pigs fed SBP-containing diets could be attributed to the greater content of xylose in SBH than in SBP. Because the increase in relative abundance of butyric acid-producing microorganisms such as Butyricicoccus that is associated with improved gut health because butyric acid improves gut barrier function and has anti-inflammatory affects in the gut (Eeckhaut et al., 2016; Boesmans et al., 2018; He et al., 2018), the replacement of SBP with SBH in weaned pig diets can result in improved gut health.
The abundance of Peptococcus in feces of mice was reduced by inclusion of resistant starch (a highly fermentable dietary fiber) in high-fat diets (Zhang et al., 2020), whereas consumption of a low-fat diet resulted in increased abundance of Peptococcus in feces of men (Cuevas-Sierra et al., 2021). Cao et al. (2003) reported that inclusion of cellulose in diets for chickens at 10% increased the count of Peptococcaceae in the cecal digesta. Results from these studies of Cao et al. (2003), Zhang et al. (2020) and Cuevas-Sierra et al. (2021) indicate that the abundance of Peptococcus in hindgut is negatively correlated with the availability of fermentable fiber in the hindgut and positively correlated with availability of fat and IDF in the hindgut. Thus, the increase in relative abundance of Peptococcus in colon of pigs due to replacement of SBP with SBH in SBO-containing diets could have been due to the greater IDF in SBH than in SBP. However, it is not clear why the relative abundance of Peptococcus in colon of pigs was unaffected by the replacement of SBP with SBH in CWG-containing diets.
As previously mentioned, SBH has a greater content of cellulose than SBP. Fermentation of cellulose results in production of VFA with high molar ratio of acetic acid (Sunvold et al., 1995). Fermentation of cellulose by cellulolytic microorganisms (such as Ruminococcus) into acetic acid results in generation of H2, which is then utilized by methane-producing microorganisms such as Methanobrevibacter to synthesize methane (Pavlostathis et al., 1990). Thus, the increase in relative abundance of Methanobrevibacter in colon of pigs due to replacement of SBP with SBH could be attributed to the greater content of cellulose in the SBH than in the SBP. Zhang et al. (2018) similarly reported that replacement of wheat bran with SBH in diets for pigs increased abundance of Methanobrevibacter in feces. In the current study, the relative abundance of Ruminococcus in colon was increased numerically by the replacement of SBP with SBH.
The small intestinal digestibility of unsaturated fatty acids is greater than that of saturated fatty acids (Powles et al., 1994). Thus, the small intestinal digestibility of fatty acids in SBO is expected to be generally greater than that of fatty acids in CWG. Fat inhibits microbial fermentation of carbohydrates (Palmquist, 1994), implying that fat that escape small intestinal fermentation can inhibit fiber fermentation in the hindgut of pigs. The abundance of Bacteroidetes phylum compared with that of Firmicutes phylum is more negatively affected by ingestion of fat (Hildebrandt et al., 2009). Thus, it had been hypothesized that replacement of CWG with SBO in the SBP-containing diet would result in increased relative abundance of Bacteroides in colon of the pigs. This is because fiber in SBP (compared to that in SBH) is expected to be more fermentable and hence to be more affected by fat-induced reduction in microbial fermentation. It is not clear why the relative abundance of Bacteroides was increased by the replacement of SBO with CWG in SBP-containing diet.
Inclusion of corn oil (at the expense of olive oil or milk fat) in diets for mice resulted in increased abundance of Coprococcus in GIT (Abulizi et al., 2019). In the current study, pigs fed SBO-containing diets had greater relative abundance of Coprococcus in colon than pigs fed CWG-containing diets. Corn oil, like SBO, has a higher content of polyunsaturated fatty acids than olive oil, milk fat, or CWG (Rodrigues and Gioielli, 2003; Liu et al., 2018; Okazaki and Katayama, 2021). Thus, it appears that consumption of fat that is rich in polyunsaturated fatty acids can result in increased relative abundance of Coprococcus in GIT; however, the mechanisms by which this is achieved are not clear. The increase in relative abundance of Anaerovibrio due to replacement of SBO with CWG in diets for pigs could have been due to greater availability of fat in the hindgut of pigs fed the CWG-containing diets. This is because pigs have lower small intestinal digestibility of fat in CWG than in SBO (Powles et al., 1994). Anaerovibrio produce enzymes that hydrolyze triglycerides to liberate glycerol, which is then used as a source of energy by the same microorganisms (Liu et al., 2017; Yang et al., 2020), and hence, their relative abundance in hindgut is likely to increase with an increase in availability of fat in the hindgut. Generally, fat source compared with fiber solubility had limited effects on fecal microbial composition. A previous study with mice (Morrison et al., 2020) also demonstrated lower effect of fat than of fiber on gut microbial composition.
Fiber solubility and fat source did not interact on most of the response criteria measured in the current study. This is contrary to results from a previous study (Ndou et al. 2019) in which fiber source (cellulose vs. pectin) and fat source (corn oil vs. beef tallow) interacted on apparent ileal digestibility of fatty acids in growing pigs. In this study of Ndou et al. (2019), dietary replacement of cellulose with pectin reduced the apparent ileal digestibility of total fatty acids for both corn oil- and beef tallow-containing diets, but the magnitude of reduction in the digestibility for beef tallow-containing diet was greater than that for corn oil-containing diet. They (Ndou et al., 2019) attributed this interaction between fiber source and fat source on apparent ileal digestibility of total fatty acids to the fact that pectin increases digesta viscosity, and that the digestibility of fat in beef tallow is lower than that in corn oil due to the greater content of saturated fatty acids in the former than in the latter. As previously mentioned, SDF in SBP has limited effect on digesta viscosity (Flis et al., 2017). Also, the beef tallow has higher content of saturated fatty acids than CWG (Liu et al., 2018). Finally, fats were included in diets at 4.5% in the current study and at 6% in the study of Ndou et al. (2019). Thus, the difference between the current study and that of Ndou et al. (2019) with regard to interactions between fiber source and fat source could be attributed to differences in SDF source, saturated fatty acid source, and level of inclusion of fat in diets among the studies.
In conclusion, inclusion of SBH instead of SBP in corn-soybean meal-based diets for weaned pigs can result in improved feed efficiency. Also, inclusion of SBH instead of SBP in corn-soybean meal-based diets for weaned pigs can result in improved gut microbial composition of weaned pigs through increased relative abundance of Butyricicoccus genus in colon. Inclusion of SBO instead of CWG in corn-soybean meal-based diets for weaned pigs can result in improved feed efficiency during Phase 1 feeding; however, the pigs may recover from the low feed efficiency induced by dietary inclusion of CWG instead of SBO after Phase 1 feeding. Dietary lipid sources have limited effects on colonic microbial composition. Also, fiber sources (SBH and SBP) and lipid sources (SBO and CWG) fed in the current study may not interact on growth performance, digestive organ weights, small intestinal histomorphology, and composition of most of bacterial genera in colon of weaned pigs when the fiber sources and lipid sources are included in diets at 10% to 12%, and at 4.5%, respectively.
Acknowledgment
This research was financed by South Dakota State University Agricultural Experiment Station.
Glossary
Abbreviations
- ADF
acid detergent fiber
- ADFI
average daily feed intake
- ADG
average daily gain
- BW
body weight
- CD
crypt depth
- CP
crude protein
- DM
dry matter
- EE
ether extract
- G:F
gain to feed ratio
- GIT
gastrointestinal tract
- IDF
insoluble dietary fiber
- NDF
neutral detergent fiber
- OTU
operational taxonomic unit
- PCoA
principal coordinates analysis
- PUFA
polyunsaturated fatty acids
- SDF
soluble dietary fiber
- SFA
saturated fatty acids
- TDF
total dietary fiber
- VFA
volatile fatty acid
- VH
villous height
- VH:CD
villous height to crypt depth.
Conflict of interest statement
The authors declare no real or perceived conflicts of interest.
Literature Cited
- Abulizi, N., Quin C., Brown K., Chan Y. K., Gill S. K., and Gibson D. L.. . 2019. Gut mucosal proteins and bacteriome are shaped by the saturation index of dietary lipids. Nutrients. 11:418. doi: 10.3390/nu11020418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AOAC . 2007. Official methods of analysis. 18th ed. Gaithersburg (MD): Association of Official Analytical Chemists. [Google Scholar]
- AOAC . 2012. Official methods of analysis. 18th ed. Gaithersburg (MD): Association of Official Analytical Chemists. [Google Scholar]
- Bach Knudsen, K. E. 2014. Fiber and nonstarch polysaccharide content and variation in common crops used in broiler diets. Poult. Sci. 93:2380–2393. doi: 10.3382/ps.2014-03902 [DOI] [PubMed] [Google Scholar]
- Beardshall, K., Frost G., Morarji Y., Domin J., Bloom S. R., and Calam J.. . 1989. Saturation of fat and cholecystokinin release: implications for pancreatic carcinogenesis. Lancet 2:1008–1010. doi: 10.1016/s0140-6736(89)91017-9. [DOI] [PubMed] [Google Scholar]
- Boesmans, L., Valles-Colomer M., Wang J., Eeckhaut V., Falony G., Ducatelle R., Van Immerseel F., Raes J., and Verbeke K.. . 2018. Butyrate producers as potential next-generation probiotics: safety assessment of the administration of Butyricicoccus pullicaecorum to healthy volunteers. Msystems. 3:e00094–18. doi: 10.1128/mSystems.00094-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao, B. H., Zhang X. P., Guo Y. M., Karasawa Y., and Kumao T.. . 2003. Effects of dietary cellulose levels on growth, nitrogen utilization, retention time of diets in digestive tract and caecal microflora of chickens. Asian-Australas. J. Anim. Sci. 16: 863–866. doi: 10.5713/ajas.2003.863. [DOI] [Google Scholar]
- Cuevas-Sierra, A., Romo-Hualde A., Aranaz P., Goni L., Cuervo M., Martínez J. A., Milagro F. I., and Riezu-Boj J. I.. . 2021. Diet-and sex-related changes of gut microbiota composition and functional profiles after 4 months of weight loss intervention. Eur. J. Nutr. 16:1–23. doi: 10.1007/s00394-021-02508-0. [DOI] [PubMed] [Google Scholar]
- DeRouchey, J. M., Hancock J. D., Hines R. H., Maloney C. A., Lee D. J., Cao H., Dean D. W., and Park J. S.. . 2004. Effects of rancidity and free fatty acids in choice white grease on growth performance and nutrient digestibility in weanling pigs. J. Anim. Sci. 82:2937–2944. doi: 10.2527/2004.82102937x. [DOI] [PubMed] [Google Scholar]
- Drochner, W., Kerler A., and Zacharias B.. . 2004. Pectin in pig nutrition, a comparative review. J. Anim. Physiol. Anim. Nutr. (Berl). 88:367–380. doi: 10.1111/j.1439-0396.2004.00490.x. [DOI] [PubMed] [Google Scholar]
- Eeckhaut, V., Wang J., Van Parys A., Haesebrouck F., Joossens M., Falony G., Raes J., Ducatelle R., and Van Immerseel F.. . 2016. The Probiotic Butyricicoccus pullicaecorum reduces feed conversion and protects from potentially harmful intestinal microorganisms and necrotic enteritis in broilers. Front. Microbiol. 7:1416. doi: 10.3389/fmicb.2016.01416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flint, H. J., Scott K. P., Duncan S. H., Louis P., and Forano E.. . 2012. Microbial degradation of complex carbohydrates in the gut. Gut Microbes 3:289–306. doi: 10.4161/gmic.19897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flis, M., Sobotka W., and Antoszkiewicz Z.. . 2017. Fiber substrates in the nutrition of weaned piglets – a review. Ann. Anim. Sci. 17:627–643. doi: 10.1515/aoas-2016-0077. [DOI] [Google Scholar]
- Gerritsen, R., van der Aar P., and Molist F.. . 2012. Insoluble nonstarch polysaccharides in diets for weaned piglets. J. Anim. Sci. 90(Suppl 4):318–320. doi: 10.2527/jas.53770. [DOI] [PubMed] [Google Scholar]
- Giger-Reverdin, S. 2000. Characterisation of feedstuffs for ruminants using some physical parameters. Anim. Feed Sci. Technol. 86:53–69. doi: 10.1016/S0377-8401(00)00159-0. [DOI] [Google Scholar]
- Hansen, M. J., Chwalibog A., Tauson A. H., and Sawosz E.. . 2006. Influence of different fibre sources on digestibility and nitrogen and energy balances in growing pigs. Arch. Anim. Nutr. 60:390–401. doi: 10.1080/17450390600884385. [DOI] [PubMed] [Google Scholar]
- He, B., Bai Y., Jiang L., Wang W., Li T., Liu P., Tao S., Zhao J., Han D., and Wang J.. . 2018. Effects of oat bran on nutrient digestibility, intestinal microbiota, and inflammatory responses in the hindgut of growing pigs. Int. J. Mol. Sci. 19:2407. doi: 10.3390/ijms19082407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildebrandt, M. A., Hoffmann C., Sherrill-Mix S. A., Keilbaugh S. A., Hamady M., Chen Y. Y., Knight R., Ahima R. S., Bushman F., and Wu G. D.. . 2009. High-fat diet determines the composition of the murine gut microbiome independently of obesity. Gastroenterology 137:1716–24.e1. doi: 10.1053/j.gastro.2009.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaacson, R., and Kim H. B.. . 2012. The intestinal microbiome of the pig. Anim. Health Res. Rev. 13:100–109. doi: 10.1017/S1466252312000084. [DOI] [PubMed] [Google Scholar]
- Ivarsson, E., Roos S., Liu H. Y., and Lindberg J. E.. . 2014. Fermentable non-starch polysaccharides increases the abundance of Bacteroides-Prevotella-Porphyromonas in ileal microbial community of growing pigs. Animal 8:1777–1787. doi: 10.1017/S1751731114001827. [DOI] [PubMed] [Google Scholar]
- Jaworski, N. W., and Stein H. H.. . 2017. Disappearance of nutrients and energy in the stomach and small intestine, cecum, and colon of pigs fed corn-soybean meal diets containing distillers dried grains with solubles, wheat middlings, or soybean hulls. J. Anim. Sci. 95:727–739. doi: 10.2527/jas.2016.0752. [DOI] [PubMed] [Google Scholar]
- Jha, R., and Berrocoso J. F.. . 2016. Dietary fiber and protein fermentation in the intestine of swine and their interactive effects on gut health and on the environment: A review. Anim. Feed Sci. Technol. 212:18–26. doi: 10.1016/j.anifeedsci.2015.12.002. [DOI] [Google Scholar]
- Jiménez-Moreno, E., González-Alvarado J. M., González-Sánchez D., Lázaro R., and Mateos G. G.. . 2010. Effects of type and particle size of dietary fiber on growth performance and digestive traits of broilers from 1 to 21 days of age. Poult. Sci. 89:2197–2212. doi: 10.3382/ps.2010-00771. [DOI] [PubMed] [Google Scholar]
- Kerr, B. J., Lindblom S. C., Zhao J., and Faris R. J.. . 2020. Influence of feeding thermally peroxidized lipids on growth performance, lipid digestibility, and oxidative status in nursery pigs. J. Anim. Sci. 98:skaa392. doi: 10.1093/jas/skaa392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, H. W., Lee Y. J., and Kim Y. H. B.. . 2015. Efficacy of pectin and insoluble fiber extracted from soy hulls as a functional non-meat ingredient. LWT-Food Sci. Technol. 64:1071–1077. doi: 10.1016/j.lwt.2015.07.030. [DOI] [Google Scholar]
- Knudsen, K. B., and Hansen I.. . 1991. Gastrointestinal implications in pigs of wheat and oat fractions: 1. Digestibility and bulking properties of polysaccharides and other major constituents. Br. J. Nutr. 65:217–232. doi: 10.1079/BJN19910082. [DOI] [PubMed] [Google Scholar]
- Liu, Y., Kil D. Y., Perez-Mendoza V. G., Song M., and Pettigrew J. E.. . 2018. Supplementation of different fat sources affects growth performance and carcass composition of finishing pigs. J. Anim. Sci. Biotechnol. 9:56. doi: 10.1186/s40104-018-0274-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, K., Xu Q., Wang L., Wang J., Guo W., and Zhou M.. . 2017. The impact of diet on the composition and relative abundance of rumen microbes in goat. Asian-Australas. J. Anim. Sci. 30:531–537. doi: 10.5713/ajas.16.0353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lizardo, R., Peiniau J., and Aumaitre A.. . 1997. Inclusion of sugar-beet pulp and change of protein source in the diet of the weaned piglet and their effects on digestive performance and enzymatic activities. Anim. Feed Sci. Technol. 66:1–14. doi: 10.1016/S0377-8401(96)01144-3. [DOI] [Google Scholar]
- Maia, M. R., Chaudhary L. C., Figueres L., and Wallace R. J.. . 2007. Metabolism of polyunsaturated fatty acids and their toxicity to the microflora of the rumen. Antonie Van Leeuwenhoek 91:303–314. doi: 10.1007/s10482-006-9118-2. [DOI] [PubMed] [Google Scholar]
- McCleary, B. V., DeVries J. W., Rader J. I., Cohen G., Prosky L., Mugford D. C., and Okuma K.. . 2012. Determination of insoluble, soluble, and total dietary fiber (CODEX definition) by enzymatic-gravimetric method and liquid chromatography: collaborative study. J. AOAC Int. 95:824–844. doi: 10.5740/jaoacint.cs2011_25. [DOI] [PubMed] [Google Scholar]
- Miron, J., Yosef E., and Ben-Ghedalia D.. . 2001. Composition and in vitro digestibility of monosaccharide constituents of selected byproduct feeds. J. Agric. Food Chem. 49:2322–2326. doi: 10.1021/jf0008700. [DOI] [PubMed] [Google Scholar]
- Molist, F., Van Oostrum M., Pérez J. F., Mateos G. G., Nyachoti C. M., and Van Der Aar P. J.. . 2014. Relevance of functional properties of dietary fibre in diets for weanling pigs. Anim. Feed Sci. Technol. 189:1–10. doi: 10.1016/j.anifeedsci.2013.12.013. [DOI] [Google Scholar]
- Morrison, K. E., Jašarević E., Howard C. D., and Bale T. L.. . 2020. It’s the fiber, not the fat: significant effects of dietary challenge on the gut microbiome. Microbiome 8:15. doi: 10.1186/s40168-020-0791-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ndou, S. P., Kiarie E., Walsh M. C., Ames N., de Lange C. F. M., and Nyachoti C. M.. . 2019. Interactive effects of dietary fibre and lipid types modulate gastrointestinal flows and apparent digestibility of fatty acids in growing pigs. Br. J. Nutr. 121:469–480. doi: 10.1017/S0007114518003434. [DOI] [PubMed] [Google Scholar]
- Ndou, S. P., Tun H. M., Kiarie E., Walsh M. C., Khafipour E., and Nyachoti C. M.. . 2018. Dietary supplementation with flaxseed meal and oat hulls modulates intestinal histomorphometric characteristics, digesta-and mucosa-associated microbiota in pigs. Sci. Rep. 8:1–15. doi: 10.1038/s41598-018-24043-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen, T. S., Lærke H. N., Theil P. K., Sørensen J. F., Saarinen M., Forssten S., and Knudsen K. E.. . 2014. Diets high in resistant starch and arabinoxylan modulate digestion processes and SCFA pool size in the large intestine and faecal microbial composition in pigs. Br. J. Nutr. 112:1837–1849. doi: 10.1017/S000711451400302X. [DOI] [PubMed] [Google Scholar]
- NRC . 2012. Nutrient requirements of swine. 11th rev. ed. Natl. Acad. Press, Washington, DC. [Google Scholar]
- Okazaki, Y., and Katayama T.. . 2021. The effects of different high-fat (lard, soybean oil, corn oil or olive oil) diets supplemented with fructo-oligosaccharides on colonic alkaline phosphatase activity in rats. Eur. J. Nutr. 60:89–99. doi: 10.1007/s00394-020-02219-y. [DOI] [PubMed] [Google Scholar]
- Owusu-Asiedu, A., Patience J. F., Laarveld B., Van Kessel A. G., Simmins P. H., and Zijlstra R. T.. . 2006. Effects of guar gum and cellulose on digesta passage rate, ileal microbial populations, energy and protein digestibility, and performance of grower pigs. J. Anim. Sci. 84:843–852. doi: 10.2527/2006.844843x. [DOI] [PubMed] [Google Scholar]
- Palmquist, D. L. 1994. The role of dietary fats in efficiency of ruminants. J. Nutr. 124(8 Suppl):1377S–1382S. doi: 10.1093/jn/124.suppl_8.1377S. [DOI] [PubMed] [Google Scholar]
- Pavlostathis, S. G., Miller T. L., and Wolin M. J.. . 1990. Cellulose fermentation by continuous cultures of Ruminococcus albus and Methanobrevibacter smithii. Appl. Microbiol. Biotechnol. 33:109–116. doi: 10.1007/BF00170581. [DOI] [Google Scholar]
- Pell, J. D., Johnson I. T., and Goodlad R. A.. . 1995. The effects of and interactions between fermentable dietary fiber and lipid in germfree and conventional mice. Gastroenterology 108:1745–1752. doi: 10.1016/0016-5085(95)90136-1. [DOI] [PubMed] [Google Scholar]
- Pluske, J. R., Williams I. H., and Aherne F. X.. . 1996. Villous height and crypt depth in piglets in response to increases in the intake of cows’ milk after weaning. Anim. Sci. 62:145–158. doi: 10.1017/S1357729800014429P. [DOI] [Google Scholar]
- Powles, J., Wiseman J., Cole D. J. A., and Hardy B.. . 1994. Effect of chemical structure of fats upon their apparent digestible energy value when given to young pigs. Anim. Sci. 58:411–417. doi: 10.1017/S0003356100007364. [DOI] [Google Scholar]
- Rezaei, M., Karimi Torshizi M. A., Wall H., and Ivarsson E.. . 2018. Body growth, intestinal morphology and microflora of quail on diets supplemented with micronised wheat fibre. Br. Poult. Sci. 59:422–429. doi: 10.1080/00071668.2018.1460461. [DOI] [PubMed] [Google Scholar]
- Rodrigues, J. N., and Gioielli L. A.. . 2003. Chemical interesterification of milkfat and milkfat-corn oil blends. Food Res. Int. 36:149–159. doi: 10.1016/S0963-9969(02)00130-8. [DOI] [Google Scholar]
- Scott, K. P., Martin J. C., Duncan S. H., and Flint H. J.. . 2014. Prebiotic stimulation of human colonic butyrate-producing bacteria and bifidobacteria, in vitro. FEMS Microbiol. Ecol. 87:30–40. doi: 10.1111/1574-6941.12186. [DOI] [PubMed] [Google Scholar]
- Sunvold, G. D., Hussein H. S., G. C.Fahey, Jr, Merchen N. R., and Reinhart G. A.. . 1995. In vitro fermentation of cellulose, beet pulp, citrus pulp, and citrus pectin using fecal inoculum from cats, dogs, horses, humans, and pigs and ruminal fluid from cattle. J. Anim. Sci. 73:3639–3648. doi: 10.2527/1995.73123639x. [DOI] [PubMed] [Google Scholar]
- Suriano, F., Bindels L. B., Verspreet J., Courtin C. M., Verbeke K., Cani P. D., Neyrinck A. M., and Delzenne N. M.. . 2017. Fat binding capacity and modulation of the gut microbiota both determine the effect of wheat bran fractions on adiposity. Sci. Rep. 7:5621. doi: 10.1038/s41598-017-05698-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao, S., Bai Y., Zhou X., Zhao J., Yang H., Zhang S., and Wang J.. . 2019. In Vitro Fermentation Characteristics for Different Ratios of Soluble to Insoluble Dietary Fiber by Fresh Fecal Microbiota from Growing Pigs. ACS Omega 4:15158–15167. doi: 10.1021/acsomega.9b01849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Kuiken, B. A., and Behnke W. D.. . 1994. The activation of porcine pancreatic lipase by cis-unsaturated fatty acids. Biochim. Biophys. Acta 1214:148–160. doi: 10.1016/0005-2760(94)90039-6. [DOI] [PubMed] [Google Scholar]
- Van Soest, P. J., Robertson J. B., and Lewis B. A.. . 1991. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 74:3583–3597. doi: 10.3168/jds.S0022-0302(91)78551-2. [DOI] [PubMed] [Google Scholar]
- Wang, J. F., Zhu Y. H., Li D. F., Wang Z., and Jensen B. B.. . 2004. In vitro fermentation of various fiber and starch sources by pig fecal inocula. J. Anim. Sci. 82:2615–2622. doi: 10.2527/2004.8292615x. [DOI] [PubMed] [Google Scholar]
- Wenk, C. 2001. The role of dietary fibre in the digestive physiology of the pig. Anim. Feed Sci. Technol. 90:21–33. doi: 10.1016/S0377-8401(01)00194-8. [DOI] [Google Scholar]
- Xu, Y., Curtasu M. V., Bendiks Z., Marco M. L., P Nørskov N., Knudsen K. E. B., Hedemann M. S., and Lærke H. N.. . 2020. Effects of dietary fibre and protein content on intestinal fibre degradation, short-chain fatty acid and microbiota composition in a high-fat fructose-rich diet induced obese Göttingen Minipig model. Food Funct. 11:10758–10773. doi: 10.1039/d0fo02252g. [DOI] [PubMed] [Google Scholar]
- Yan, H., Potu R., Lu H., Vezzoni de Almeida V., Stewart T., Ragland D., Armstrong A., Adeola O., Nakatsu C. H., and Ajuwon K. M.. . 2013. Dietary fat content and fiber type modulate hind gut microbial community and metabolic markers in the pig. PLoS One 8:e59581. doi: 10.1371/journal.pone.0059581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, F., Zhang S., Tian M., Chen J., Chen F., and Guan W.. . 2020. Different Sources of High Fat Diet Induces Marked Changes in Gut Microbiota of Nursery Pigs. Front. Microbiol. 11:859. doi: 10.3389/fmicb.2020.00859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, Y., Chen L., Hu M., Kim J. J., Lin R., Xu J., Fan L., Qi Y., Wang L., Liu W., . et al. 2020. Dietary type 2 resistant starch improves systemic inflammation and intestinal permeability by modulating microbiota and metabolites in aged mice on high-fat diet. Aging (Albany. NY). 12:9173–9187. doi: 10.18632/aging.103187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, Y. J., Liu Q., Zhang W. M., Zhang Z. J., Wang W. L., and Zhuang S.. . 2018. Gastrointestinal microbial diversity and short-chain fatty acid production in pigs fed different fibrous diets with or without cell wall-degrading enzyme supplementation. Livest. Sci. 207:105–116. doi: 10.1016/j.livsci.2017.11.017. [DOI] [Google Scholar]
- Zhou, X., Beltranena E., and Zijlstra R. T.. . 2017. Apparent and true ileal and total tract digestibility of fat in canola press-cake or canola oil and effects of increasing dietary fat on amino acid and energy digestibility in growing pigs. J. Anim. Sci. 95:2593–2604. doi: 10.2527/jas.2016.0757. [DOI] [PubMed] [Google Scholar]
- Zhou, P., Theil P. K., Wu D., and Bach Knudsen K. E.. . 2018. In vitro digestion methods to characterize the physicochemical properties of diets varying in dietary fibre source and content. Anim. Feed Sci. Technol. 235:87–96. Doi: 10.1016/j.anifeedsci.2017.11.012. [DOI] [Google Scholar]