Abstract
Background
As COVID-19 became a pandemic, the urgent need to find an effective treatment vaccine has been a major objective. Vaccines contain adjuvants which are not exempt from adverse effects and can trigger the autoimmune/inflammatory syndrome induced by adjuvants (ASIA). There is very little information about autoimmune endocrine disease and the ASIA after the use of mRNA-based SARS-CoV2 vaccination.
Case series
We report three cases and also review the literature showing that the thyroid gland can be involved in the ASIA induced by the mRNA-based SARS-CoV2 vaccination. We present the first case to date of silent thyroiditis described in the context of SARS-CoV2 vaccination with Pfizer/BioNTech. Also, we discuss the first subacute thyroiditis in the context of SARS-CoV2 vaccination with the Moderna’s vaccine. Finally, we provide another case to be added to existing evidence on Graves’ disease occurring post-vaccination with the Pfizer/BioNTech vaccine.
Discussion
Adjuvants play an important role in vaccines. Their ability to increase the immunogenicity of the active ingredient is necessary to achieve the desired immune response. Both the Moderna and the Pfizer/BioNTech vaccines use mRNA coding for the SARS-CoV2 S protein enhanced by adjuvants. In addition, the cross-reactivity between SARS-CoV2 and thyroid antigens has been reported. This would explain, at least, some of the autoimmune/inflammatory reactions produced during and after SARS-CoV2 infection and vaccination.
Conclusion
The autoimmune/inflammatory syndrome induced by adjuvants involving the thyroid could be an adverse effect of SARS-CoV2 vaccination and could be underdiagnosed.
Keywords: Autoimmune/inflammatory syndrome induced by adjuvants (ASIA), SARS-CoV2 vaccination, Thyroiditis, COVID-19, Graves’ disease
Background
Since the pandemic of coronavirus disease (COVID-19) was declared, finding an effective prevention method has been a major objective. After enormous efforts, vaccines offering proven effectiveness with a safety profile that outweighs the risks have emerged.
Vaccines contain adjuvants which activate the innate immune system through the pattern recognition receptor (PRR) system [1]. Some of the adjuvants approved by regulatory agencies are: aluminium salts, oil-in-water emulsions type (MF-59-squalene, polysorbate 80 and sodium citrate-AS03…), based on TLR (toll-like receptors) agonists, CpG-ODN (a synthetic DNA molecule) and AS04 (aluminium salt and monophosphoryl-lipid A-MPL) [2, 3].
The autoimmune/inflammatory syndrome induced by adjuvants (ASIA), described in 2011, covers a wide range of diseases like macrophagic myofasciitis syndrome, post-vaccination phenomena, Gulf War syndrome and Silicosis, among others [1, 4, 5]. It has been hypothesized that it is the consequence of dysregulation of both innate and adaptive immune systems, following exposure to an adjuvant [5]. Since the ASIA was described, more than 4000 cases have been reported. Diagnostic criteria for the ASIA are shown in footnotes of Table 1.
Table 1.
ASIA cases involving the thyroid gland reported after SARS-CoV2 vaccination
| Gender | Age | Vaccine | Dose | Medical history | Symptoms | TSH (0.350–4.950 µUI/mL) | fT3 (1.58–3.91 pg/mL) | fT4 (0.70–1.48 ng/dL) | Anti-thyroglobulin (0.00–4.11 UI/mL) | Anti-TPO (0.00–5.60 UI/mL) | Anti-TSH (0–1.75 UI/mL) | Diagnosis | Reference | Days until symptoms |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Women | 40 | Pfizer/BioNtech | 1st | HTA, previous COVID infection | Distal tremor, hyperreflexia, arrythmia | < 0.001 | 10.5 | 3.57 | 210 | 3405 | 16.56 | Graves’ disease | 20 | 2 |
| Women | 28 | Pfizer/BioNtech | 1st | No medical history | Anxiety, insomnia, palpitations and distal tremor | < 0.001 | 9.2 | 1.82 | 33 | 833 | 5.85 | Graves’ disease | 20 | 3 |
| Women | 35 | CoronaVac® | 2nd | No medical history | Anterior cervical pain, palpitations and fatigue | 0.473 | 6.15 | 14.1 | 0.9 | 1.2 | 1.5 | Subacute thyroidits | 14 | 4 |
| Women | 34 | CoronaVac® | 1st | Previous COVID infection | Anterior cervical pain, fever, palpitations and fatigue | 0.01 | 11.8 | 5.2 | 0.9 | 1.2 | 1.5 | Subacute thyroidits | 14 | 4 |
| Women | 37 | CoronaVac® | 1st | No medical history | Anterior cervical pain | 0.9 | 6.05 | 3.85 | 0.9 | 4.1 | 1.5 | Subacute thyroidits | 14 | 7 |
| Women | 42 | Pfizer/BioNtech | 1st | No medical history | Anterior cervical pain and palpitations | 0.01 | 11.8 | 4.58 | – | – | – | Subacute thyroidits | 13 | 5 |
| Women | 38 | Moderna | 1st | Asma and Gilbert disease | Anterior cervical pain, palpitations, distal tremor, axillar and inguinal bilateral ganglionic reaction | 0.008 | 5.44 | 1.86 | 7.40 | – | 0.69 | Subacute thyroidits | Own case report | 8 |
| Man | 32 | Pfizer/BioNTech | 1st | Type 1 diabetes | Weight loss, nervousness and insomnia | 0.001 | – | 2.37 | 42 | 186 | – | Silent thyroidits | Own case report | 10 |
| Woman | 38 | Pfizer/BioNTech | 1st | Mental retardation and esquizofrenia | Nervousness, increase sweating and insomnia | < 0.001 | 7.46 | 2.01 | 36.57 | 3303.71 | 12.54 | Graves’ disease | Own case report | 12 |
| Woman | 38 | CoronaVac® | 2nd | No prior medical history | Neck swelling, pain, fatigue, loss of appetite and sweating | 0.008 | 12.88 | 4.65 | – | 9.49 | – | Subacute thyroidits | 15 | 14 |
| Hombre | 67 | CoronaVac® | 2nd | Hypertension and frequent atrial extra beats | Fever, weight loss and neck and ear pain | < 0.005 | 8.06 | 2.87 | – | – | – | Subacute thyroidits | 16 | 18 |
To meet the diagnostic criteria of Asia defined by Shoenfeld and Agmon-Levi [4, 5] it is mandatory to comply two major criteria or one major criteria with two minor criteria for the ASIA diagnosis. Major criteria are the following: exposure to external stimuli as it is vaccination and immunization procedures (among others) before the initiation of the symptoms; presentation of one of the following symptoms (no excluding other symptoms): muscle pain, weakness, myalgia, myositis, arthralgia or arthritis, chronic fatigue, malaise and sleep disturbances, neurological manifestations, cognitive deficits, fever, dry mouth other sicca syndrome like symptoms; the removal of the adjuvants leads to a full or partial recovery; biopsy of involved organs. Minor criteria are the following: positivity for auto-antibodies or antibodies targeting the suspected adjuvant; other clinical manifestations such as fibromyalgia, irritable bowel syndrome…; genetic predisposition (i.e. HLA DQB1, HLA DRB1…); development of some autoimmune disease or familiar history of autoimmune disease
We describe three patients with thyroid autoimmune/inflammatory disease developed shortly after receiving an mRNA-based vaccine against SARS-CoV2. In addition we review the other cases reported in the literature.
Case series
Patient 1: COVID vaccination and subacute or De Quervain’s thyroiditis
A 38-year-old female was studied at the Endocrinology Service of Son Llàtzer University Hospital because intense anterior cervical pain and mild distal tremor and palpitations. She had received a first dose of Moderna vaccine 8 days before the onset of the symptoms. On physical examination, the right thyroid lobe was increased in size and painful on superficial palpation. Urgent blood test was requested with the following results: TSH < 0.008 µUIU/mL (0.350–4.950 µUIU/mL), FT3 5.44 pg/mL (1.58–3.91 pg/mL), FT4 1.86 ng/dL (0.70–1.48 ng/dL) and antibodies (abs.) anti-thyroglobulin 7.40 IU/mL (0.00–4.11 IU/mL). Abs anti-thyroid peroxidase (TPO) and anti-TSH-receptor (TSI) were negative.
Because the patient developed fever and intense local symptoms a fine needle aspiration (FNA) was performed in order to distinguish the etiology. Giant cells with other inflammatory cells were observed in the fine needle aspiration (FNA) cytology suggesting granulomatous thyroiditis (De Quervain’s thyroiditis). The aspirate was negative for molecular detection of the following respiratory pathogens: Adenovirus, Coronavirus (229E, HKU1, NL63, OC43, MERS-CoV, SARS-CoV-2), Metapneumovirus, Rhinovirus/Enterovirus, Influenza A and B, Parainfluenza, Respiratory syncytial virus, Bordetella parapertussis, Bordetella pertussis, Chlamydia pneumoniae and Mycoplasma pneumoniae. It’s remarkable that FNA results ruled out SARS-CoV2 infection, strengthening the hypothesis that the overall picture we were seeing could be more related to vaccination than to the direct effect of the virus.
Furthermore, IgG anti-N, which detects natural immunity, in serum was undetectable. On the other hand, the assay for quantitative IgG anti-S antibody 2, reflecting both natural immunity and acquired immunity after vaccination, was 133.00 UA/mL (positive > 15 UA/mL). The Nucelocapsid Protein (N Protein) of SARS-CoV2 is located in the viral core. Thus, the IgG anti-N it is produced due to SARS-CoV2 infection but not due to vaccination. If we bear in mind that the detection of IgG anti-N is negative, these results suggest that the positive result for quantitative IgG anti-S is due to vaccination.
Thyroid ultrasound showed an enlarged right lobe with diffuse hypoechogenicity (Fig. 1) and thyroid scintigraphy revealed very low uptake compatible with subacute thyroiditis (Fig. 2).
Fig. 1.
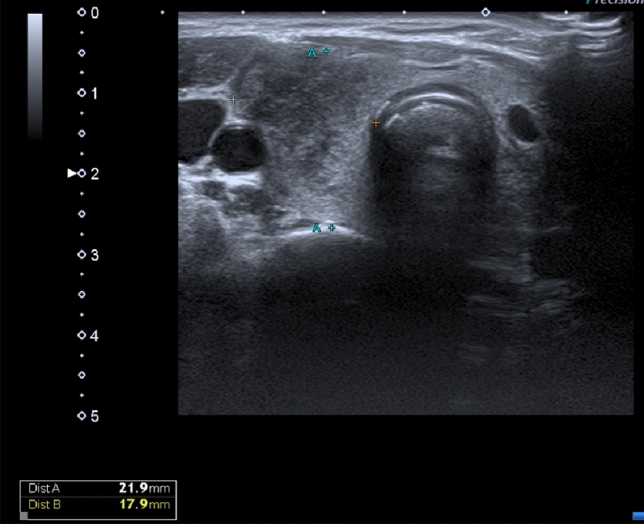
Thyroid ultrasound of case 1 with showing an enlarged right lobe with diffuse hypoechogenicity suggestive of thyroiditis
Fig. 2.
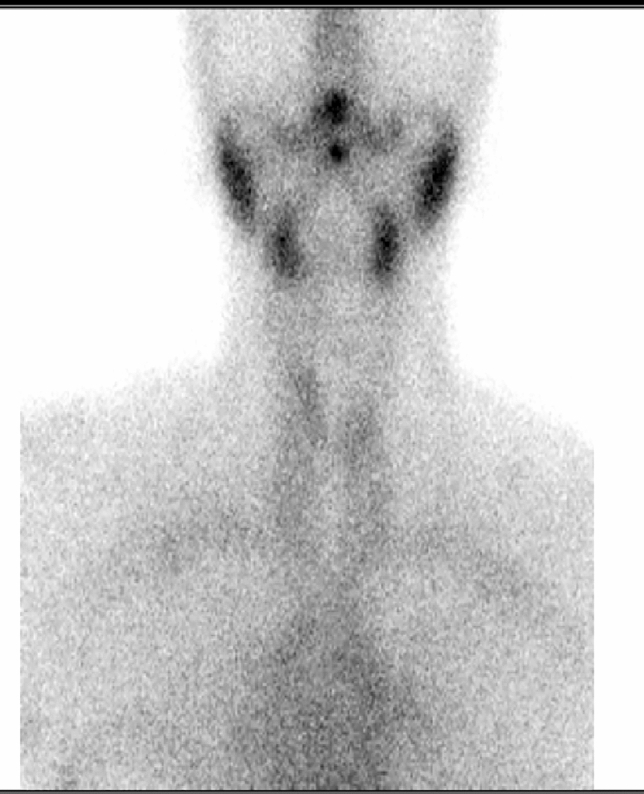
Thyroid scintigraphy of case 1 with very low uptake of the thyroid gland compatible with subacute thyroiditis
Treatment was started with prednisone, propranolol and Ibuprofen. One week later, improvement of the symptomatology allowed tapering the dose of prednisone. Currently the patient is asymptomatic and her thyroid function is normal without medication.
Patient 2: COVID vaccination and silent thyroiditis
A 32-year-old male with type 1 diabetes was evaluated because of palpitations and insomnia. He had been vaccinated with a first dose of Pfizer/BioNTech vaccine 10 days prior to the onset of these symptoms.
Lab tests were requested with the following results: FT4 2.37 ng/dL (0.70–1.48 ng/dL), TSH 0.01 µIU/mL (0.350–4.950 µUI/mL), TSI 0.4 UI/L (< 0.7 UI/L), abs. anti-thyroglobulin 42 IU/mL (0.00–4.11 IU/mL) and anti-TPO 186 IU/mL (0–5.60 IU/mL).
A thyroid ultrasound was carried out showing parenchymal changes compatible with an inflammatory process. A scintigraphy found a complete absence of activity at the thyroid parenchyma compatible with thyroiditis. This case was classified as a silent subacute thyroiditis given that the patient had never presented previous cervical pain, and also fulfilled the ASIA criteria. Because the symptoms were mild, no treatment was given.
Eight weeks later, the patient presented overt hypothyroidism with TSH levels of 116.5 µIU/mL (0.350–4.950 µUIU/mL), FT4 of 0.15 ng/dL (0.70–1.48 ng/dL), and anti-TPO levels of 247 IU/mL (0–5.60 IU/mL). TSI and abs anti-thyroglobulin were normal. Treatment with levothyroxine was started.
Patient 3: COVID vaccination and Graves’ disease
A 38-year-old woman without prior history of thyroid disease but with history of schizophrenia was evaluated because of behavioural disturbance with nervousness, insomnia and sweating. She had received a first dose of the Pfizer/BioNTech vaccine 12 days before the onset of the symptoms. On physical examination, a slight increase in the size of the thyroid, predominantly in the right lobe, was found.
The thyroid profile was: TSH < 0.008 µIU/mL (0.350–4.950 µUIU/mL), FT3 7.46 pg/mL (0.70–1.48 ng/dL), FT4 2.01 ng/dL (0.70–1.48 ng/dL), anti-thyroid peroxidase (TPO) 3303.71 IU/mL (0–5.60 IU/mL), abs anti-thyroglobulin 36.57 IU/mL (0–5.60 IU/mL) and TSI 12.54 UI/mL (< 0.7 UI/mL). Thyroid scintigraphy concluded a hyperfunctioning diffuse goitre compatible with Graves’ disease. A thyroid ultrasound showed a diffuse decrease in echogenicity with some echogenic septum and increased vascularity. Methimazole was started.
Discussion
These cases meet criteria for ASIA diagnosis: temporal correlation, compatible symptomatology, and presence of transient autoimmunity.
Silent thyroiditis is considered a variant of chronic autoimmune thyroiditis, or Hashimoto’s Disease, forming part of the spectrum of autoimmune pathology with thyroid involvement [6]. In fact, a significant percentage of patients with silent thyroiditis present elevated anti-TPO and antithyroglobulin antibodies [6].
To our knowledge, only a case with silent thyroiditis has been described in the scientific literature in the context of SARS-CoV2 infection [7]. Therefore, the case described above would be the first case of silent thyroiditis described in the context of Sars-CoV2 vaccination with Pfizer/BioNtech to date.
Subacute or De Quervain’s has been associated with various viral infections such as adenovirus, Coxsackievirus and, among others, SARS-CoV2 [8–11]. Subacute thyroiditis is not an autoimmune disorder per se, but one possible explanation could be that viral infection or virus-induced tissue damage might cause activation of the antigen-HLA B35 complex, leading to immune-mediated destruction of the thyroid follicular cell [8–11].
In a 2020 systematic review by Bragazzi et al. [12], 52 cases of subacute thyroiditis following exposure to adjuvants that met the criteria for the ASIA syndrome were described. Of these 52 cases, 41 were subsequent to papillomavirus vaccine administration, eight cases after the influenza vaccination, one case after the Hepatitis B virus vaccination, one case after a breast prosthesis implantation and one case after exposure to mineral oil. Symptoms appeared 2–20 days after exposure. All cases fully recovered.
Franquemont et al. [13] reported a 42-year-old woman who began developing anterior cervical pain and palpitations 5 days after receiving the SARS-CoV2 vaccine from Pfizer/BioNTech. She underwent a negative PCR for COVID. Thyroid function tests were TSH < 0.01 µIU/mL, FT4 4.58 ng/dL and FT3 of 11.8 pg/mL. Anti-TPO antibodies were negative and erythrocyte sedimentation rate was elevated. The patient was treated with prednisone and propranolol, leading to a rapid improvement of symptoms.
İremli et al. [14] described three cases of subacute thyroiditis probably caused by the CoronaVac® SARS-CoV2 vaccine. Three female healthcare workers began developing symptoms compatible with thyrotoxicosis and anterior cervical pain within 4–7 days after receiving the CoronaVac® vaccine. In the analysis, the profile of one of the three patients was compatible with hyperthyroidism. All three had a normal immune profile, the ultrasound showed a heterogeneous parenchyma with decreased blood flow and the physical exam found anterior cervical pain on palpation [16].
Saygili et al. [15] explained the case of a 38-year-old female physician with no prior illness, vaccination or drug use who develops neck swelling, pain, fatigue, loss of appetite and sweating 2 weeks after the administration of the second dose of Coronavac® vaccine. Physical examination pointed out a stage 2 goitre and pain in the right lobe when it was touched. Blood test revealed: TSH 0.008 µIU/mL (0.350–4.950 µUIU/mL), FT3 12.88 pg/mL (0.70–1.48 ng/dL), FT4 4.65 ng/dL (0.70–1.48 ng/dL), anti-thyroid peroxidase (TPO) 9.49 IU/mL (0–5.60 IU/mL). Thyroid ultrasonography was compatible with subacute thyroiditis. Naproxen sodium and propranolol was initiated.
Tekin et al. [16] presented a case of a 67-year-old male with history of hypertension and frequent atrial extra beats was admitted to the hospital due to fever, weight loss and neck and ear pain 18 days after the administration of the second dose of Coronavac® vaccine. The laboratory results showed TSH < 0.005 µIU/mL (0.350–4.950 µUIU/mL), FT3 8.06 pg/mL (0.70–1.48 ng/dL), FT4 2.87 ng/dL (0.70–1.48 ng/dL).Thyroid ultrasonography showed increased dimensions of the gland and heterogeneous echotexture compatible with subacute thyroiditis. Ibuprofen was started.
As far as we know, our patient would be the first case of subacute thyroiditis in the context of SARS-CoV2 vaccination with the Moderna’s vaccine.
The most common cause of hyperthyroidism globally is Graves’ disease. Viral infections have been described as a risk factors for developing Graves’ disease [17, 18]. Several cases of Graves’ disease have been reported after SARS-CoV2 infection [19]. Regarding its pathophysiology, in case of genetic predisposition and the accumulation of environmental insults, T cells will become excessively sensitized to the TSH receptor antigen, activating B cells to produce and secrete autoantibodies against the TSH receptor [17, 18].
After mass vaccination with SARS-CoV2 vaccines, some cases of Graves’ disease have been reported. Vera-Lastra et al. described two cases of Graves’ disease in healthcare personnel who had received the Pfizer/BioNTech SARS-CoV2 vaccine [20]. Both women began to show clinical symptoms compatible with hyperthyroidism within 2 or 3 days. Elevated T3 and T4 levels with suppressed TSH, elevated anti-TPO, anti-thyroglobulin and anti-TSH antibodies were observed. Both cases fulfilled ASIA criteria.
Our patient also meets the ASIA criteria. It therefore provides another case of Graves’ disease occurring post-vaccination with Pfizer/BioNTech vaccine.
Thyroid disease after SARS-CoV2 vaccination is summarized in Table 1.
Adjuvants play an important role in vaccines and drugs. Their ability to increase the immunogenicity of the active ingredient is necessary to achieve the desired immune response with vaccination and/or the use of a drug [2]. However, these components are not exempt from adverse effects and can trigger the ASIA syndrome [5, 21].
Both the Pfizer/BioNTech and the Moderna vaccines do not specify the use of adjuvants in their vaccines [22]. The RNA molecules would already exert immunostimulatory effects and would also stimulate the pathogen recognition patterns of innate immunity [1, 2]. It should be noted that these vaccines are transported by lipid nanoparticles to preserve and maintain RNA stability. It is a matter of debate whether they have adjuvant capacity [22].
On the other hand, the SARS-CoV2 virus enters into the host cells via the ACE2 receptor. This receptor is distributed heterogeneously throughout the body and is not specific to the airway. Lazatigues et al. have observed that multiple endocrine organs have receptors for ACE2 and this would explain some of the endocrinological alterations seen in patients infected by SARS-CoV [23]. Soldevilla et al. mentioned that virtually all endocrine tissue could be affected by the SARS-CoV2 virus [24]. Cellular entry of the virus, cellular dysfunction caused by systemic inflammation or immune/antibody-mediated reaction, would be responsible at the pathophysiological level for the endocrinological consequences of SARS-CoV2 infection [14, 23, 24].
Both the Moderna and Pfizer/BioNTech vaccines use mRNA coding for the SARS-CoV2 S protein [22]. It is this virus protein that binds to the ACE2 receptor and allows the virus to enter the cell. Therefore, blocking this protein could slow down viral entry into the cell. Protein S has two subfractions: S1 contains the RBD subdomain that allows initial anchoring to the host cell, while S2 allows fusion of the viral cell to the host, thereby initiating infection.
Rotondi et al. investigated whether the mRNA encoding for ACE2 it is expressed by the thyroid. Evaluating fifteen different thyroid tissue specimens and two primary thyroid cell cultures obtained from surgical patients they provide the first evidence that mRNA of ACE2, the SARS-CoV2 receptor, it is expressed in the thyroid cells [25]. Moreover, compared with the expression of other reported genes, ACE2 receptor is abundantly expressed by the thyroid. Further studies will be required to confirm this results [24].
Coperchini et al. investigated about the proinflammatory effects of IFN-γ and TNF-α on the expression of ACE2 mRNA levels in primary cultures of human thyroid cel [26]. Both cytokines are highly upregulated in patients with SARS-CoV2 infection [26]. The authors found that elevated levels of proinflammatory cytokines could increase the expression of ACE2 on the thyroid gland [26]. The hypothesis needs specific studies to be confirmed.
In addition, in 2021, Vodjani et al. demonstrated cross-reactivity to human antigens of antibodies against protein S and the nucleocapsid of SARS-CoV2 [27] (Fig. 3). This would explain, at least in part, some of the autoimmune/inflammatory reactions produced during SARS-CoV2 infection and also by the vaccine. Thus it is possible that proteins generated due to the vaccine cross-react with thyroid target proteins due to the molecular mimicry triggering autoimmunity together with an inflammatory reaction in genetically predisposed individuals [20, 27]. These phenomena could be enhanced by adjuvants in the context of post-vaccination ASIA. The observation that most cases occurs few days after vaccination, in a time when viral proteins reach its peak would support this hypothesis [14–16, 20].
Fig. 3.
The natural or acquired immune response against protein S of SARS-CoV2 due to the structural similarity with some human antigens can generate a cross reactivity immune reaction against the thyroid gland
Finally, it is obvious that the relationship between SARS-CoV2 vaccination and thyroid disease is far from being proved and further studies are needed to demonstrate its causal relationship. Furthermore we have to remark that ASIA is unfrequently reported and the risks of COVID clearly outweigh the risks of the vaccination. Thus vaccination should be still highly recommended.
Conclusions
We reviewed the cases of thyroid disease subsequent to SARS-CoV2 vaccination, those described in the scientific literature and the three cases from our centre. They constitute new evidence of subacute thyroiditis (first case described after vaccination with Moderna) and the first case of silent thyroiditis reported so far. Moreover, we report a patient to be added to the existing evidence on Graves’ disease occurring post-vaccination with the Pfizer/BioNTech vaccine. All of them meet ASIA criteria.
The notification of adverse effects of vaccination is an important part of pharmacovigilance and an approach that we should take towards any drug.
Thyroid disease secondary to SARS-CoV2 vaccination is probably being underdiagnosed due to the novelty of the facts, the absence of previous experience and the lack of solid scientific evidence on the subject. We believe that our work can add relevant information on something little known, but perhaps not so uncommon: the consequences of COVID and the ASIA syndrome post-vaccination affecting the thyroid. Surely, the hypothesis that thyroid dysfunction can be caused by mRNA-based SARS-CoV2 vaccination will require more investigation.
Author contributions
AP, LM and L-AG were responsible for conducting the search, interpreting the results, writing the manuscript and approving the final version of the manuscript. CG coordinated and supervised data and reviewed and revised the final manuscript. JN contributed to the conception of the manuscript, reviewed the text and approved the final version. PS coordinated and supervised data and reviewed and revised the final manuscript. MG-F coordinated and supervised data and reviewed and revised the final manuscript. AAL-G reviewed the text and approved the final version. KD contributed to the conception of the manuscript, reviewed the text and approved the final version.
Funding
This research did not receive any specific grant from any funding agency in the public, commercial or not-for-profit sector.
Declarations
Conflict of interest
The authors declare no conflict of interest.
Research involving human participants and/or animals
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
Informed consent has been obtained from the patients for publication of the case reports.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
A. Pujol, A. Gómez and L. Masmiquel have contributed equally to this work.
Contributor Information
A. Pujol, Email: antelm.pujol@hsll.es
L.-A. Gómez, Email: lgomez@hsll.es
C. Gallegos, Email: mcgallegos@hsll.es
J. Nicolau, Email: jnicoalu1@hsll.es
P. Sanchís, Email: pilar.sanchis@uib.es
M. González-Freire, Email: martagonzalezfreire@gmail.com
Á. A. López-González, Email: angarturo@gmail.com
K. Dotres, Email: kdotres@gmail.com
L. Masmiquel, Email: lmasmiquel@hsll.es
References
- 1.Watad A, Bragazzi NL, McGonagle D, Adawi M, Bridgewood C, Damiani G, Alijotas-Reig J, Esteve-Valverde E, Quaresma M, Amital H, Shoenfeld Y. Autoimmune/inflammatory syndrome induced by adjuvants (ASIA) demonstrates distinct autoimmune and autoinflammatory disease associations according to the adjuvant subtype: insights from an analysis of 500 cases. Clin Immunol. 2019;203:1–8. doi: 10.1016/j.clim.2019.03.007. [DOI] [PubMed] [Google Scholar]
- 2.Shi S, Zhu H, Xia X, Liang Z, Ma X, Sun B. Vaccine adjuvants: understanding the structure and mechanism of adjuvanticity. Vaccine. 2019;37(24):3167–3178. doi: 10.1016/j.vaccine.2019.04.055. [DOI] [PubMed] [Google Scholar]
- 3.Gupta T, Gupta SK. Potential adjuvants for the development of a SARS-CoV-2 vaccine based on experimental results from similar coronaviruses. Int Immunopharmacol. 2020;86:106717. doi: 10.1016/j.intimp.2020.106717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bragazzi NL, Hejly A, Watad A, Adawi M, Amital H, Shoenfeld Y. ASIA syndrome and endocrine autoimmune disorders. Best Pract Res Clin Endocrinol Metab. 2020;34(1):101412. doi: 10.1016/j.beem.2020.101412. [DOI] [PubMed] [Google Scholar]
- 5.Shoenfeld Y, Agmon-Levin N. ‘ASIA’ - autoimmune/inflammatory syndrome induced by adjuvants. J Autoimmun. 2011;36:4–8. doi: 10.1016/j.jaut.2010.07.003. [DOI] [PubMed] [Google Scholar]
- 6.Pearce EN, Farwell AP, Braverman LE. Thyroiditis. N Engl J Med. 2003;348:2646. doi: 10.1056/NEJMra021194. [DOI] [PubMed] [Google Scholar]
- 7.Barahona San Millán R, Tantinyà Daura M, Hurtado Ganoza A, Recasens Sala M. Painless thyroiditis in SARS-CoV-2 infection. Endocrinol Diabetes Nutr. 2020 doi: 10.1016/j.endinu.2020.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Domin R, Szczepanek-Parulska E, Dadej D, Ruchała M. Subacute thyroiditis – literature overview and COVID-19. J Med Sci. 2020;89(4):e472. doi: 10.20883/medical.e472. [DOI] [Google Scholar]
- 9.Fatourechi V, Aniszewski JP, Fatourechi GZ, Atkinson EJ, Jacobsen SJ. Clinical features and outcome of subacute thyroiditis in an incidence cohort: Olmsted County, Minnesota, study. J Clin Endocrinol Metab. 2003;88(5):2100–2105. doi: 10.1210/jc.2002-021799. [DOI] [PubMed] [Google Scholar]
- 10.Desailloud R, Hober D. Viruses and thyroiditis: an update. Virol J. 2009;6:5. doi: 10.1186/1743-422X-6-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brancatella A, Ricci D, Viola N, Sgrò D, Santini F, Latrofa F. Subacute thyroiditis after Sars-COV-2 infection. J Clin Endocrinol Metab. 2020;105(7):dgaa276. doi: 10.1210/clinem/dgaa276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bragazzi NL, Ashraf Hejly A, Watad A, Adawi M, Amital H, Shoenfeld Y. ASIA syndrome and endocrine autoimmune disorders. Best Pract Res Clin Endocrinol Metab. 2020;34(1):101412. doi: 10.1016/j.beem.2020.101412. [DOI] [PubMed] [Google Scholar]
- 13.Franquemont S, Galvez J. Subacute thyroiditis after mRNA vaccine for Covid-19. J Endocr Soc. 2021;5(S1):956–957. doi: 10.1210/jendso/bvab048.1954. [DOI] [Google Scholar]
- 14.İremli BG, Şendur SN, Ünlütürk U. Three cases of subacute thyroiditis following SARS-CoV-2 vaccine: postvaccination ASIA syndrome. J Clin Endocrinol Metab. 2021;106(9):2600–2605. doi: 10.1210/clinem/dgab373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saygılı ES, Karakilic E. Subacute thyroiditis after inactive SARS-CoV-2 vaccine. BMJ Case Rep. 2021;14(10):e244711. doi: 10.1136/bcr-2021-244711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Şahin Tekin M, Şaylısoy S, Yorulmaz G. Subacute thyroiditis following COVID-19 vaccination in a 67-year-old male patient: a case report. Hum Vaccines Immunother. 2021;1:1–3. doi: 10.1080/21645515.2021.1947102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Davies TF, Andersen S, Latif R, Nagayama Y, Barbesino G, Brito M, Eckstein AK, Stagnaro-Green A, Kahaly GJ. Graves' disease. Nat Rev Dis Primers. 2020;6(1):52. doi: 10.1038/s41572-020-0184-y. [DOI] [PubMed] [Google Scholar]
- 18.Smith TJ, Hegedüs L. Graves’ disease. N Engl J Med. 2016;375(16):1552–1565. doi: 10.1056/NEJMra1510030. [DOI] [PubMed] [Google Scholar]
- 19.Mateu-Salat M, Urgell E, Chico A. SARS-COV-2 as a trigger for autoimmune disease: report of two cases of Graves’ disease after COVID-19. J Endocrinol Investig. 2020;43(10):1527–1528. doi: 10.1007/s40618-020-01366-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Domiguez MP, Medina G, Sánchez Valadez TI, Jara LJ. Two cases of graves' disease following SARS-CoV-2 vaccination: an autoimmune/inflammatory syndrome induced by adjuvants. Thyroid. 2021;31(9):1436–1439. doi: 10.1089/thy.2021.0142. [DOI] [PubMed] [Google Scholar]
- 21.Watad A, Sharif K, Shoenfeld Y. The ASIA syndrome: basic concepts. Mediterr J Rheumatol. 2017;28(2):64–69. doi: 10.31138/mjr.28.2.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chung YH, Beiss V, Fiering SN, Steinmetz NF. COVID-19 vaccine frontrunners and their nanotechnology design. ACS Nano. 2020;14(10):12522–12537. doi: 10.1021/acsnano.0c07197. [DOI] [PubMed] [Google Scholar]
- 23.Lazartigues E, Qadir MMF, Mauvais-Jarvis F. Endocrine significance of SARS-CoV-2's reliance on ACE2. Endocrinology. 2020;161(9):bqaa108. doi: 10.1210/endocr/bqaa108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Soldevila B, Puig-Domingo M, Marazuela M. Basic mechanisms of SARS-CoV-2 infection. What endocrine systems could be implicated? Rev Endocr Metab Disord. 2021 doi: 10.1007/s11154-021-09678-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rotondi, et al. Detection of SARS-COV-2 receptor ACE-2 mRNA in thyroid cells: a clue for COVID-19-related subacute thyroiditis. J Endocrinol Investig. 2021;44(5):1085–1090. doi: 10.1007/s40618-020-01436-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Coperchini F, et al. Modulation of ACE-2 mRNA by inflammatory cytokines in human thyroid cells: a pilot study. Endocrine. 2021;5:1–8. doi: 10.1007/s12020-021-02807-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vojdani A, Vojdani E, Kharrazian D. Reaction of human monoclonal antibodies to SARS-CoV-2 proteins with tissue antigens: implications for autoimmune diseases. Front Immunol. 2021;11:617089. doi: 10.3389/fimmu.2020.617089. [DOI] [PMC free article] [PubMed] [Google Scholar]



