Abstract
Summary
The objective of this consensus statement is to inform the clinical practice communities, research centres and policymakers across Africa of the results of the recommendations for osteoporosis prevention, diagnosis and management. The developed guideline provides state-of-the-art information and presents the conclusions and recommendations of the consensus panel regarding these issues.
Purpose
To reach an African expert consensus on a treat-to-target strategy, based on current evidence for best practice, for the management of osteoporosis and prevention of fractures.
Method
A 3-round Delphi process was conducted with 17 osteoporosis experts from different African countries. All rounds were conducted online. In round 1, experts reviewed a list of 21 key clinical questions. In rounds 2 and 3, they rated the statements stratified under each domain for its fit (on a scale of 1–9). After each round, statements were retired, modified or added in view of the experts’ suggestions and the percent agreement was calculated. Statements receiving rates of 7–9 by more than 75% of experts’ votes were considered as achieving consensus.
Results
The developed guidelines adopted a fracture risk-centric approach. Results of round 1 revealed that of the 21 proposed domains, 10 were accepted whereas 11 were amended. In round 2, 32 statements were presented: 2 statements were retired for similarity, 9 statements reached consensus, whereas modifications were suggested for 21 statements. After the 3rd round of rating, the experts came to consensus on the 32 statements. Frequency of high-rate recommendation ranged from 83.33 to 100%. The response rate of the experts was 100%. An algorithm for the osteoporosis management osteoporosis was suggested.
Conclusion
This study is an important step in setting up a standardised osteoporosis service across the continent. Building a single model that can be applied in standard practice across Africa will enable the clinicians to face the key challenges of managing osteoporosis; furthermore, it highlights the unmet needs for the policymakers responsible for providing bone health care together with and positive outcomes of patients’ care.
Keywords: Osteoporosis, Guidelines, Africa, Bisphosphonates, Denosumab, Parathyroid hormone, Romosozumab, FRAX, Falls, African osteoporosis guidelines
Introduction
Osteoporosis is a public health epidemic that has negative impacts on health outcomes together with an enormous economic burden. The clinical relevance of osteoporosis lies in its associated fragility fractures, especially hip fractures, and usually it is a silent disease until such an event occurs [1]. Worldwide, osteoporosis causes more than 9 million fractures a year, meaning that there is a fragility fracture every 3 s [2]. In the Western World, it is estimated that about 1 in 3 women and 1 in 5 men above the age of 50 years old will sustain a fracture during their remaining lifetime [3]. After the age of 50 years, most sites of fracture can be considered characteristic locations of osteoporosis. Hip and vertebral fractures are the most common and serious osteoporotic fractures. Other fragility fractures, particularly in women, such as those of the humerus, forearm, ribs, pelvis and tibia (but not including ankle fractures), after the age of 50 years, have been reported to be associated with low bone mineral density (BMD) [4]. The direct annual cost of treating osteoporotic fractures of people on average is reported to be between 5000 and 6500 billion US Dollars in Canada, Europe and the USA alone, not considering indirect costs such as disability and loss of productivity [5]. Therefore, prevention of this disease can significantly reduce the morbidity, mortality and costs incurred by the health system.
Across Africa, the issue of bone health continues to be a major challenge. Osteoporosis has been overlooked as a health care priority in Africa, particularly in the sub-Saharan region [6]. This has been linked to several reasons. Firstly, health authorities have been overwhelmed by the burden of communicable diseases such as tuberculosis and human immunodeficiency virus (HIV) [7]. Secondly, tools to assess the BMD and consequently diagnose osteoporosis such as dual X-ray absorptiometry (DXA) are not widely available, hindering early diagnosis and treatment of the disease. Furthermore, concerns regarding inadequate calcium intake during adolescent years, low body mass index, prolonged duration of lactation amongst women during childbearing years, low levels of vitamin D and physical inactivity amongst Africans are all risk factors for osteoporosis [8].
On another front, the demographic changes taking place in Africa have fuelled both the diagnostic and therapeutic osteoporosis inertia in Africa. Census reports showed that both the total population size and life expectancy in Africa have increased significantly in the last two decades. An overview of demographic ageing in Africa published by the United Nations [9] found that Africa will have the fastest growth rate of older adults compared to any other region in the world. Between 2020 and 2050, the older African population is projected to triple from 74.4 million to 235.1 million and will outpace that of any other region of the world [10]. This has been supported by the new Census report on ageing trends in Africa [11] which revealed that between 2017 and 2050, 50% of the world’s population is expected to be in 9 countries (India, Nigeria, Democratic Republic of the Congo, Pakistan, Ethiopia, United Republic of Tanzania, United States of America (USA), Uganda and Indonesia), i.e. 5 of them are African nations.
African older adults play critical economic, family and community roles. Studies show that majority of the adults aged 60 to 64 years and around half of those aged 65 years and older in Africa remain in the labour force. Many older Africans, particularly women, contribute substantial amounts to providing unpaid home and care work [9]. Therefore, caring for this sector of the population become a priori. So far, there have not been any guidelines or treatment recommendations published for the management of osteoporosis in Africa. This guideline has been developed as an initiative by the African Society of Bone Health and Metabolic Diseases (ASBoM) with the intention of reducing the risk of osteoporosis-related fractures and thereby maintaining the quality of life for African people living with osteoporosis. It has been based on the outcomes of systematic reviews carried out in Africa on epidemiology of osteoporotic fractures as well as risk factors of osteoporosis in Africa. The objective is to provide a consensus, evidence-based information about the diagnosis, evaluation and treat-to-target management of osteoporosis in both men and postmenopausal women for the African health care professionals managing osteoporosis patients in general, regulatory bodies, health-related organizations and interested patients’ groups/laypersons. Although framed for Africa, it is hoped that these guidelines will be valuable for bone health specialists across the globe.
Methods
A qualitative synthesis of scientific evidence and consensus, based on clinical experience and existing scientific evidence, was used to formulate the study design and the following procedures. This work conforms to the preferred reporting items for systematic reviews and meta-analyses guidelines for reporting systematic reviews [12].
Study teams
Core team
Core team was composed of four experts in bone metabolism who were selected by the African Society of Bone Health and Metabolic Bone Diseases. Their task was to supervise, coordinate and assist with developing the scope of the project and initial Patient/Population, Intervention, Comparison, and Outcomes (PICO) questions. The core team prespecified outcomes as critical for each PICO question for the systematic literature review. The team also nominated the expert panel and drafting the manuscript.
Literature review team
Led by an experienced literature review consultant and based on specific research questions identified to focus on the treat-to-Target management of osteoporosis, the literature review was conducted with the assistance of an expert in methodology. The team completed the literature search (the PubMed/ MEDLINE, EMBASE and Cochrane databases), data abstraction and the quality of evidence rating [13]. Following the revision, each of the experts responsible for the literature review provided recommendations regarding each section based on evidence, when that was available or on their own experience. The level of evidence was determined for each section using the Oxford Centre for Evidence-based Medicine (CEBM) system (Table 1) [14].
Table 1.
Levels of evidence and grades of recommendation
| Level of evidence | |
|---|---|
| 1 | Systematic review of all relevant randomised clinical trials or n-of-1 trials |
| 2 | Randomised trial or observational study with dramatic effect |
| 3 | Non-randomised controlled cohort/follow-up study (observational) |
| 4 | Case series, case–control study or historically controlled study |
| 5 | Mechanism-based reasoning (expert opinion, based on physiology, animal or laboratory studies) |
| Grades of recommendation | |
| A | Consistent level 1 studies |
| B | Consistent level 2 or 3 studies, or extrapolations from level 1 studies |
| C | Level 4 studies, or extrapolations from level 2 or 3 studies |
| D | Level 5 evidence or troubling, inconsistent or inconclusive studies of any level |
Data sources and search strategies
The PICO questions (Table 2) were used to conduct the literature search in PubMed, Embase and Cochrane Library databases. Literature search strategies were carried out to locate randomised clinical trials evaluating the efficacy of osteoporosis quality improvement strategies published from 1990 to April 2021. The language was limited to English and French for pragmatic reasons. The search strategies were designed to be broad to have high sensitivity for identifying relevant literature. We used the following Medical Subject Headings (MeSH) terms: osteoporosis, postmenopausal osteoporosis, osteop?nia, T-score, bone resorption, fracture, osteoporosis treatment, osteoporosis management, calcium, vitamin D, alendronate, risedronate, etidronate, ibandronate, zoledronic acid, raloxifene, calcitonin, teriparatide, hormone replacement therapy, teriparatide, abaloparatide, romosozumab, denosumab, glucocorticoids, treatment induced osteoporosis, hip protectors, meta-analysis, systematic reviews, randomized controlled trials, bone density, FRAX, fracture liaison service, falls, Covid-19, treat to target. Keywords used were dependent on the PICO elements used in different combinations. Literature searches on 23rd April 2021 for PubMed and Cochrane Library databases, and on 28th April 2021 for Embase. Duplicate screening of literature search results was performed electronically. Additional relevant studies were retrieved by reviewing the reference lists of studies identified with the database search strategies that met the inclusion criteria.
Table 2.
Key questions used to develop the guideline
| 1 | Who are the targeted people for these guidelines? |
| 2 | What are the fracture risk factors? |
| 3 | How to assess for fracture risk and what are the cut-off points? |
| 4 | How is osteoporosis diagnosed? |
| 5 | When osteoporosis is diagnosed, what is the approach for an appropriate evaluation? |
| 6 | What are the fundamental non-pharmacologic measures recommended for optimum bone health? |
| 7 | Who is in need for pharmacologic therapy? |
| 8 | What medication should be used to treat osteoporosis? |
| 9 | What is the approach for osteoporosis pharmacological management? |
| 10 | What are the recommendations for calcium and vitamin D supplement therapy? |
| 11 | How is treatment monitored? |
| 12 | Treat-to-target: What are the targets that reflect successful osteoporosis management? |
| 13 | How long should patients be treated? |
| 14 | Is there an opportunity for a drug holiday? |
| 15 | What is the role of concomitant use of therapeutic agents? |
| 16 | What is the role of sequential use of therapeutic agents? |
| 17 | What is the role of vertebral augmentation for compression fractures? |
| 18 | What is the importance of falls assessment? |
| 19 | How important is the implementation of fracture liaison service (FLS)? |
| 20 | How osteoporosis in men is managed? |
| 21 | How to manage the patients on glucocorticoids therapy? |
| 22 | What is the advice given to osteoporosis patients during the COVID-19 pandemic? |
Study selection
We selected relevant studies by applying inclusion and exclusion criteria to the literature retrieved with the search strategies.
Inclusion criteria
Articles included were systematic reviews, randomised controlled trials (RCTs), uncontrolled trials, observational studies including cohort, case–control and cross-sectional studies, or those where economic evaluation was made.
Exclusion criteria
Editorials, commentaries, conference abstracts and non-evidence-based narrative/personal reviews were excluded.
Expert panel
Given the fact that the developed guideline will be adopted across the continent of Africa, it was vital that the participating expert panel involved in developing the guideline would include experts from all the African continent regions (Central, North, West, East and South Africa). Expert panel members were appointed by the core team. The core leadership team nominated 17 participants. The criteria for their selection included existence of professional knowledge and experience (at least 8 years of experience) in the field of bone health, management of osteoporosis and active participation in scientific research on bone health disorders. The expert panel assisted with developing the scope of the project and refining the PICO questions. PICO questions were drafted into recommendation statements and were sent to the expert panel with the evidence report who voted on the recommendations.
Key questions used to develop the guideline
This guideline was based on a series of structured key questions that define the targeted population, fracture assessment, diagnostic tools, investigation, the comparison(s) utilised and the outcomes used to measure efficacy, effectiveness or risk. The evidence to answer the clinical questions was collected according to the following steps: formulation of clinical questions, structuring of questions, search for evidence, critical evaluation and selection of evidence, presentation of results and recommendations. These questions, shown in Table 2, formed the basis of the systematic literature search and consequently the clinical care standards.
Developing the standards of clinical care framework
Based on the answers to the structured key questions and the literature review, a structured template was developed to facilitate standardised identification of guideline components. For each guideline component, the format in which the recommendations/information was provided and extracted has been identified.
Delphi process
The Delphi technique is a structured method widely used to gather important information on a specific topic. It relies on the key assumption that forecasts from a group are generally more accurate than those from individuals. Therefore, the aim of the Delphi method is to construct consensus forecasts from a group of experts in a structured iterative manner. Its methodology is based on a series of questionnaires or ‘rounds’ addressed to experts. The key features of this method are the anonymity of participants and controlled feedback [15].
Delphi rounds
This was based on a three-stage on-line survey.
The first round: the participants were asked to consider the items identified by the systematic literature review, to suggest new items that might have been missed and to clarify items that might be unclear.
The second round was based on the results of the first round, and participants were asked to rate each item from 1 (not appropriate) to 9 (completely appropriate) and give their comments.
The third round, the participants reviewed the responses to each item obtained in the second round, after amendments wherever appropriate, and to rate the items after alterations (from 1 to 9)
Voting process
Live online-delivered voting was carried out in repeated rounds that were strictly time limited. All members of the task force were invited to participate and pre-informed of the time of opening and closure of each round of votes. Unique access links were sent out, and anonymous votes were gathered and processed. Comments on re-phrasing, potential ambiguity and unidentified overlaps were gathered regarding each statement at the same time in the voting process. Only the members of the task force had the right to vote on the statements.
Rating
Each statement is rated between 1 and 9 where 1 being ‘complete disagreement’ and 9 being ‘complete agreement’. Generally, 1–3, 4–6 and 7–9 represent disagreement, uncertainty and agreement, respectively. The ‘uncertainty’ vote represents ‘inconvenience about the accuracy of the recommendation’. There was no requirement to vote on all statements, and the members were encouraged to abstain if they felt that a statement fell outside their area of expertise. All statements were allowed for the entry of comments, which were reviewed by the scientific committee after each round of voting. The same scenario was adopted in each round of votes; the members were further urged to leave comments wherever they vote a disagreement. This enabled the panel to identify an instance of misinterpretation of statement and invalidate the vote on that statement.
Definition of consensus
Definition of consensus was established before data analyses. It was determined that consensus would be achieved if at least 75% of participants reached agreement (score 7–9) or disagreement (score 1–3) [15–18]. A statement was retired if it had a mean vote below 3 or a ‘low’ level of agreement. Statements whose rate come in the uncertainty score (4–6) were revised in view of the comments. The levels of agreement on each statement of recommendation were defined as ‘high’ if after the second round of votes, all votes on a statement fell into the agreement bracket (7–9) [18].
Data management and analysis
Survey data were combined as a total sample, included 17 individual responses, were analysed using SPSS Statistical Software (Statistical Package of Social Science, Armonk, New York), and results reported as means and standard deviation. Analysis by geography was not possible due to the anonymity of survey responses.
Ethical aspects
This study was performed in accordance with the Helsinki Declaration. Ethics approval was deemed unnecessary. Verbal informed consent was required from all the participants included in the study. All the participants were kept anonymous, in compliance with data protection regulations. The research questions, project oversight and resulting consensus-based practice guidelines were created by the core team and expert panel. The resulting guidelines do not reference or recommend any specific product; rather, they focus on how to assess and utilise the evidence to put a frame that is best suited for the patients’ management.
Results
Literature research and evidence selection
Evidence was obtained through literature searches and 3443 potentially relevant studies were initially identified. A total of 3339 studies were excluded for duplication (1266) or by screening of title and abstracts (2073). These are the studies which did not examine population or intervention of interest, did not match study design of interest or did not report outcome measures of interest. Therefore, relevant 104 studies were included for full article review. In total, 78 studies were excluded as citations did not provide evidence matching a PICO. Therefore, 26 studies were included in this work (Fig. 1).
Fig. 1.

Flow chart for the study selection process
Expert panel characteristics
The Delphi form was sent to the expert panel (n = 17), who participated in the three rounds. Respondents were drawn from several countries covering the different geographical regions of Africa: Egypt: 8 (47%), Morocco: 1 (5.8%), South Africa: 3 (17.64%) and 1 (5.8%), from each of: Cameron, Nigeria, Ghana, Tunisia and Sudan. The participants were rheumatologists who are specialised in the management of osteoporosis with mean experience of 20.3 ± 11.24 years.
Delphi round 1
The key clinical question comprised of 22 questions. In this round, the participants were asked to rate the overall principles considered in the decision-making for treat-to-target management of patients living with osteoporosis. The response rate for round 1 was 100% (17/17). The 17 experts accepted 10 of the proposed domains, suggested amendments for the remaining 12. Of the suggested changes, comments were raised regarding the domain ‘who are targeted in this guideline’, particularly regarding definite discrimination of both male and female osteoporosis; hence, it was considered when formulating the statements. There was a diversity of comments whether to merge the statements on treatment duration and the drug holiday, but it was decided to keep them separate. Other comments suggested the stratification of osteoporosis management into pharmacological and non-pharmacological with recommendation to clarify the concomitant use of therapeutic agents and adjunctive therapy; hence, a new subheading was added. Some of the modifications consisted of minor wording changes. Table 3 shows a list of the major as well as minor osteoporosis risk factors.
Table 3.
Modifiable and non-modifiable osteoporosis risk factors
| Non-modifiable risk factors |
Previous fracture Parental history of osteoporosis History of early menopause (below age of 45 years) |
| Modifiable risk factors |
Low BMI (< 20 kg/m2) Smoking Low bone mineral density Alcohol intake |
| Co-existing diseases |
Diabetes Inflammatory rheumatic diseases (RA or SLE) Inflammatory bowel disease and malabsorption Institutionalised patients with epilepsy Human immunodeficiency virus Primary hyperparathyroidism and endocrine diseases Chronic liver disease Neurological diseases (including Alzheimer’s disease, Parkinson’s disease, multiple sclerosis, stroke) Moderate to severe chronic kidney disease Asthma |
| Drug therapy |
Long-term antidepressants Antiepileptics Aromatase inhibitors Long-term DMPA GnRH agonists (in men with prostate cancer) PPIs Oral glucocorticoids TZDs |
RA, rheumatoid arthritis; SLE, systemic lupus erythematosus; DMPA, depot medroxyprogesterone acetate; GnRH, gonadotropin-releasing hormone; PPIs, proton pump inhibitors; TZD, thiazolidinediones
Delphi round 2
Based on input from round 1, the 17 experts were presented with 32 statements stratified under 22 domains. The response rate for round 2 was 100% (17/17). Of the statements presented, two statements were retired for similarity with another statement, 9 statements reached consensus (i.e. ≥ 75% of respondents strongly agreed or agreed) and were retained, whereas modifications were suggested for 21 statements. Comments (excluding minor editing suggestions) were more frequent for 4 domains: osteoporosis fracture risk, non-pharmacologic measures for bone health, male osteoporosis and steroid-associated osteoporosis. The statements were revised and amended. In addition, one statement was suggested to be added to the daily supplementation of calcium and vitamin D therapy domain and another one was added to the non-pharmacologic measures of bone health domain.
Delphi round 3
Based on input from round 2, the 17 experts were presented with 32 statements stratified under 22 domains. The response rate for round 3 was 100% (17/17). The experts came to consensus on the 32 statement to retain in the treat-to-target management guideline. The core team reviewed and made minor revisions to one of the retained statements that reached consensus. Consensus was reached (i.e. ≥ 75% of respondents strongly agreed or agreed) on all the clinical standards. Frequency of high-rate recommendation (rank 7–9) ranged from 83.33 to 100%. The experts were comfortable with the final list of the statements and with the Delphi process overall. Table 4 shows the level of evidence and grade of recommendation assigned to each statement, in accordance with the Oxford Centre for Evidence-Based Medicine (CEBM) criteria as well as mean ± standard deviation and level of agreement [14]. Agreement was unanimous (> 80% agreement) for the wording of the statements.
Table 4.
Breakdown of statements of recommendations, its individual rank by expert opinion and level of agreement
| No | Domain | Statements | LE | GoR | Mean rate | SD | % of agreement | Level of agreement |
|---|---|---|---|---|---|---|---|---|
| Who are targeted in these guidelines? |
Women *Postmenopausal women aged ≥ 50 years (2a) Postmenopausal women at high risk of fractures (2a) Postmenopausal women who have experienced a recent fracture (2a) Women with secondary causes of osteoporosis (2a) Men with a T score in the osteopenic range (T score − 1 to − 2.5) if they have been identified to have a high or very high fracture risk (2b) Men: - Men at the age of 70 years or older (2a) - Men whose age is less than 70 years old, at high risk of fracture (2a) - Men Over 60 years old who have experienced a recent fracture (2a) - Men with secondary causes of osteoporosis (2a) - Men with a T score in the osteopenic range (T score − 1 to − 2.5) if they have been identified to have a high or very high fracture risk (2b) |
2 2 2 2 2 2 2 2 2 2 |
A A A A B A A A A B |
8.9 | 0.5 | 94.1% | H | |
| What are the osteoporosis fracture risk factors? | See Table 3 | 2 | A | 8.67 | 1.25 | 81.66 | H | |
|
How to assess for fracture risk? and what are the cut-off points? |
• Screening for fracture risk: - FRAX, without BMD, is an important web-based tool in the assessment of fragility fracture risk in osteoporosis and should be used to screen the patients and stratify them according to their fracture risk - Consider bone mineral density testing based on clinical fracture risk profile - It is advisable to calculate the FRAX score according to the validated national measures - If no national measures are available, the FRAX can be calculated according to regional validated measures - Adjustment of the conventional FRAX estimates of probabilities of hip fracture and a major osteoporotic fracture should be carried out to modulate the risk assessment whenever appropriate - Wherever possible, FRAX should be adjusted for TBS (trabecular bone score) - Patients should be stratified according to their risk of fracture, low, moderate, high and very high risk Low risk includes no prior hip or spine fractures, a BMD T score at the hip and spine both above − 1.0 and 10-year hip fracture risk < 1% and 10-year risk of major osteoporotic fractures < 10% Moderate risk includes no prior hip or spine fractures, a BMD T-score at the hip and spine both between – 1 and − 2.5 or 10-year hip fracture risk < 3% or risk of major osteoporotic fractures < 20% High risk includes a prior spine or hip fracture, or a BMD T score at the hip or spine of − 2.5 or below or 10-year hip fracture risk > 3% or risk of major osteoporotic fracture risk > 20% Very high fracture risk includes recent fracture (e.g. within preceding 12 months), fracture whilst on anti-osteoporosis medication, multiple fractures, fractures whilst taking drugs that affect bone adversely (e.g. long-term glucocorticoid therapy), a BMD T-score ≤ − 3, high risk of falls or previous history of injurious falls and a very high fracture probability (the example given is a FRAX score > 30% for major osteoporotic fracture and > 4.6% for hip fracture) Adjustment of FRAX 10-year probability of fracture should be carried out for all patients taking glucocorticoid therapy. The individual patient’s risk should be adjusted for the glucocorticoid dose [19] |
1 2 |
A A |
8.9 | 0.5 | 94.1 | ||
| How is osteoporosis diagnosed? |
- BMD testing is the gold standard in diagnosing osteoporosis. The WHO recommended the diagnosis of osteoporosis based on the T-scores at either hip or spine - The 1/3 radius may be considered as an alternate site when the lumbar spine/hip is not evaluable or as an additional site in patients with primary hyperparathyroidism: Cut-off points of T-score: - T-score ≥ − 1 indicates normal BMD - T-score between − 1 and − 2.5 indicates osteopenia or low bone mass - T-score ≤ − 2.5 indicates osteoporosis - T-score ≤ − 2.5 accompanied by a fragility fracture denotes severe osteoporosis - When the initial diagnosis of osteoporosis is made based on a T-score of − 2.5 or below, the diagnosis of osteoporosis remains even when a subsequent DXA assessment shows a T-score better than − 2.5 (grade 2a) - Osteoporosis can be diagnosed in patients with a T-score between − 1.0 and − 2.5 and increased fracture risk using FRAX® or sustain a fragility fracture - The diagnostic DXA criteria established by the WHO apply only to the axial measurements (i.e. lumbar spine, femoral neck and total hip) and distal 1/3 of the radius. Thus, other technologies should not be used to diagnose osteoporosis but may be used to assess fracture risk |
2 | A | 8.58 | 0.82 | 100 | H | |
| When osteoporosis is diagnosed, what is the approach for an appropriate evaluation? |
Height should be measured every 1–2 years in adults ≥ 50 years of age - Assess for causes of secondary osteoporosis Biochemical tests: Bone profile: calcium, phosphorous, alkaline phosphatase, eGFR, creatinine Whenever indicated: - 25-hydroxyvitamin D: symptoms of vitamin D deficiency - Parathyroid hormone (PTH): persistent hypercalcaemia - Serum testosterone, LH, FSH and SHBG, PSA (men) - 24-h urinary cortisol/dexamethasone suppression test - Endomysial and/or tissue transglutaminase antibodies (coeliac disease) Radiological: Assessment for presence of vertebral fracture(s) either by: - X-ray, - DXA-based Vertebral fracture assessment (VFA) or - Other radiological investigations such as CT or MRI are of value particularly for vertebral fracture assessment |
2 | A | 8.83 | 1 | 100 | H | |
| What are the fundamental non-pharmacologic measures for bone health? |
- Patient education/group therapy can be of value in osteoporosis management - Shared decision-making tools are a good and preferable option to ensure patient adherence to therapy and positive treatment outcomes - Lifestyle measures such as increasing levels of physical activity and perform weight-bearing exercise, stop smoking and alcohol intake, care for other relevant comorbidities as renal or ischaemic cardiovascular diseases are very important in improving bone health - Exercise is important for managing osteoporosis, with appropriate safety precautions - Counsel patients to limit alcohol intake to no more than 2 units per day - Pre-treatment dental check-up is advised particularly in the presence of risk factors such as diabetes mellitus or history of poor dental health status - Prevention of falls and consider hip protectors |
2 | A | 8.9 | 0.14 | 100 | H | |
| Who needs pharmacologic therapy? |
- Patients with high (10-year probability for major osteoporotic fracture is ≥ 20% or the 10-year probability of hip fracture is ≥ 3%) or very high (10-year probability for major osteoporosis fracture > 30%, hip fracture > 4.5%) fracture risk as assessed by FRAX - Patients with T-score of − 2.5 or lower in the spine, femoral neck or total hip - Patients with T-score between − 1.0 and − 2.5 if the FRAX® (after adjustment or recalculated using TBS if available) 10-year probability for major osteoporotic fracture is ≥ 20% or that of hip fracture is ≥ 3%, wherever applicable using the country-specific thresholds - Patients with imminent fracture risk (history of low trauma fracture within the past 12 months) or sustaining multiple fractures - Patients who sustain fractures whilst on osteoporosis pharmacological therapy - All patient who are on long-term therapy (> 3 months), or sustaining fractures, whilst on medication that may impact negatively on the bone health such as long-term glucocorticoids, androgen depletion therapy, hormone antagonist therapy - Patients with osteopaenia (T score from − 1 to − 2.5) who have moderate risk of fracture FRAX fracture risk probability 1–3% at the hip and 10–20% at spine may be good candidates for prophylactic zoledronic acid every 18 months for 4 infusions [20] |
1 | A | 8.92 | 0.5 | 100 | H | |
| What medication should be used to treat OP? |
Anti-resorptives: - Bisphosphonates: alendronate, risedronate and zoledronate are appropriate as initial therapy for most osteoporotic patients with high fracture risk (grade 1) - Denosumab: appropriate as initial therapy (if there is contraindication to or intolerability to oral bisphosphonates) for osteoporotic patients with high fracture risk (grade 1) - HRT/raloxifene: may be appropriate initial therapy in some cases who are intolerable or have contraindications to bisphosphonate therapy Anabolics: - Abaloparatide, teriparatide - Romosozumab can be considered for patients who did not respond positively (increase in the BMD) or sustain a fracture whilst on bisphosphonate therapy; or as initial therapy for patients at very high fracture risk |
1 1 2 1 2 |
A A B A B |
8.8 | 0.18 | 100 | H | |
| Osteoporosis pharmacological management: |
- Oral bisphosphonates (alendronate, risedronate) are first-line treatments in the majority of osteoporosis cases. Ibandronate is not recommended to reduce non-vertebral or hip fracture risk (grade 1) - Patients aged ≥ 65 years with osteopaenia (T score from − 1 to − 2.5 at either the total hip or the femoral neck on either side) who have moderate risk of fracture (10-year fracture probability at the hip in the range of 1–3% and 10–20% at the spine) can be eligible to receive prophylactic treatment zoledronic acid 5 mg IV every 18 months for 4 doses (grade 2b) - In osteoporotic women who are intolerant of oral bisphosphonates or in whom they are contraindicated; intravenous bisphosphonates or denosumab provide the most appropriate alternatives as initial therapy (with raloxifene or hormone replacement therapy as additional options); however, this should be decided and prescribed by osteoporosis specialist (grade 2a) - Oral and intravenous bisphosphonates are contraindicated in patients with hypocalcaemia, hypersensitivity to bisphosphonates and severe renal impairment (eGFR ≤ 35 mL/min for alendronate and zoledronic acid and ≤ 30 mL/min for other bisphosphonates). Pregnancy and lactation are also contraindications. Oral bisphosphonates are contraindicated in people with abnormalities of the oesophagus that delay oesophageal emptying such as stricture or achalasia, and inability to stand or sit upright for at least 30–60 min. They should be used with caution in patients with other upper gastrointestinal disorders. Pre-existing hypocalcaemia must be investigated and, where due to vitamin D deficiency, treated with vitamin D before treatment is initiated (grade 2a) - IV zoledronate should be prescribed and administered only by osteoporosis specialist when used for osteoporosis management (grade 2b) - Denosumab is contraindicated in women with hypocalcaemia or with hypersensitivity to any of the constituents of the formulation. Its use is not recommended in pregnancy or in the paediatric population (age ≤ 18 years) (grade 2a) - Monitoring of calcium levels should be conducted prior to each dose of denosumab and within 2 weeks after the initial dose in patients predisposed to hypocalcaemia (e.g. patients with severe renal impairment, creatinine clearance ≤ 30 mL/min) or if suspected symptoms of hypocalcaemia occur or if otherwise indicated. Patients should be advised to report symptoms of hypocalcaemia (grade 2a) - Osteoporotic women age < 60 years old and less than 10 years past menopause and low thrombosis risk, who are intolerant to bisphosphonates and denosumab can be considered for HRT or SERM: * If with vasomotor symptoms and low cancer breast risk, HRT can be used * If no uterus: oestrogen * If uterus is present: oestrogen + progesterone * If without vasomotor symptoms and high cancer breast risk, SERM should be used |
1 2 2 2 2 2 2 |
A B A A B A A |
7.33 | 1 | 83.33 | H | |
| Calcium and vitamin D daily supplemental therapy |
Every patient should be taking calcium (1 g/day) and vitamin D (1000 IU/day) supplement therapy in addition to the osteoporosis medication. The dose can be adjusted to the patient-associated comorbidities - Vitamin D supplement therapy with a daily dose of 1000 to 2000 international units (IU) is typically required to maintain an optimal serum 25(OH)D level. However, higher doses of vitamin D3 may be necessary in patients with present factors such as obesity, malabsorption and older age - Serum 25-hydroxyvitamin D (25[OH]D) should be maintained in the range of 30 to 50 ng/mL in patients with osteoporosis |
2 | A | 8.92 | 0.5 | 100 | H | |
| How is treatment monitored? |
- BMD testing can be used to monitor response to therapy (grade 2b) - FRAX can be used to monitor response to therapy (grade 5) - Check adherence within 3 months and yearly thereafter, including tolerability, new cautions and contraindications, calcium/vitamin D intake, change in fracture and fall risks (grade 2b) - Monitor BMD serial changes in lumbar spine, total hip; if lumbar spine, hip or both are not evaluable, monitoring with 1/3 radius site may be acceptable but is limited by a small area and a very large least significant change (LSC) (grade 2b) - Patients monitoring, ideally, should be carried out in the same facility with the same DXA scanning system, provided that the acquisition, analysis and interpretation adhere to International Society for Clinical Densitometry DXA best practices (grade 3) - In the case of oral bisphosphonate or denosumab, repeat BMD measurement should be carried out after initial 2 years of osteoporosis therapy to assess the response to treatment and then at 5 years when the patient completes the treatment course. In the case of IV zoledronate, repeat DXA scan should be carried out after 3 years of therapy (grade 2a) - At the repeat BMD assessment carried out 2 years after starting osteoporosis therapy, good response to treatment is identified if there is increase (increase of the BMD above the precision error) or stability of BMD without the occurrence of low trauma fracture (grade 1) - Treatment failure is considered when the BMD falls significantly from baseline (by more than the precision error) or if further fractures took place despite an adequate trial and adherence to drug treatment - However, it is important to realise that even the best treatments will only decrease the fracture rate (grade 2a) - Patients should continue to receive the same treatment for osteoporosis during the initial 2 years of treatment even if they experience a fragility fracture (grade 2a) - If a patient remains at high fracture risk or develop a fragility fracture, or more, after 2 years of being on the same treatment, in spite of good adherence to therapy and after exclusion of secondary causes, then consider switching to another therapy (grade 2a) - If a patient has a new fracture, during their treatment break, they should be reassessed immediately (grade 2a) - During treatment, all patients should be encouraged to maintain good oral hygiene, receive routine dental check-ups and report any oral symptoms such as dental mobility, pain or swelling (grade 2b) - During treatment, patients should be advised to report any thigh, hip or groin pain and any patient presenting with such symptoms should be evaluated for an atypical femur fracture (grade 2b) - During treatment with bisphosphonate or denosumab, patients should be advised to report jaw pain, swelling or gum infections; development of exposed bone in the mouth along either the top or bottom jaws; loosening of teeth; poor healing of the gums especially after dental work or numbness or a feeling of heaviness in the jaw (grade 2b) |
2 3 2 1 2 2 2 2 2 2 2 |
B A A A A A B B B |
8.9 | 0.1 | 86.76 | H | |
| Treat-to-target: What is successful treatment of osteoporosis? |
- Treatment target: T score > − 1.5 – ‘low fracture risk’ (from clinical and/or screening tests) should be established particularly for postfracture patients - Fracture-free interval of 3 to 5 years |
2 | B | 8.58 | 1.7 | 81.66 | H | |
| How long should patients be treated? |
- Oral bisphosphonate treatment should last for 5 years, whereas for IV zoledronate therapy should last for 3 years - Continuation of oral bisphosphonate (alendronate and risedronate) treatment beyond 5 years can generally be recommended in the following situations: - Fracture risk remains high - T-score − 2.5 or less - Previous history of a hip or vertebral fracture - Current treatment with oral glucocorticoids ≥ 7.5 mg prednisolone/day or equivalent - Occurrence of one or more low trauma fractures whilst on therapy, after exclusion of poor adherence to treatment (e.g. less than 80% of treatment has been taken) and after causes of secondary osteoporosis has been excluded. In such cases, class switching may be considered (grade 2a) - Denosumab therapy should initially last for 5 years (grade 2a). If denosumab therapy is discontinued, patients should be transitioned to another antiresorptive - Whenever indicated, the following therapies can be continued for: •10 years—alendronic acid and denosumab •7 years—risendronic acid •3 years—zolendronic acid - Parathyroid hormone therapy 20 μg daily for a maximum duration of treatment of 24 months (grade 2a). The medication should be followed by a drug intended for long-term use, such as a bisphosphonate or denosumab) (grade 1) - Romosozumab therapy should last for 12 months. The medication should be followed by a drug intended for long-term use, such as a bisphosphonate or denosumab (grade 2) |
2 | a | 8.58 | 0.82 | 91.76 | H | |
| Drug holiday |
- Drug holiday can be considered after completing 5 years of oral bisphosphonate/denosumab therapy or 3 years of zoledronate IV therapy if the target of treatment has been achieved (grade 2a) - Patients with low to moderate fracture risk: consider giving bisphosphonate then stopping for a drug holiday (grade 2a) - Once a holiday has begun, fracture risk and BMD should be re-evaluated every 1 to 3 years after discontinuation - The ending of a bisphosphonate holiday should be tailored to the patient’s bone health status, such as a significant drop in BMD (by more than a precision error) or increase in the fracture risk may lead to re-initiation of osteoporosis therapy, depending on the individual’s fracture risk before the 5-year maximum holiday is completed - Patients on glucocorticoids (≥ 7.5 mg/day) or patients who have had a vertebral fracture should not usually be considered for a treatment break |
2 | A | 8.67 | 1.25 | 91.66 | H | |
| What is the role of concomitant use of therapeutic agents? | Combination therapy of parathyroid hormone and denosumab may be considered in very high fracture risk patients. This should be considered on an individual basis; patients should be assessed and managed by osteoporosis specialist (grade 2b) | 2 | B | 8.58 | 0.82 | 82.33 | H | |
| What is the role of sequential use of therapeutic agents? |
- In postmenopausal women with osteoporosis at very high risk of fracture, particularly those with history of osteoporotic vertebral fracture, sequential therapy can be adopted with an anabolic agent (e.g. abaloparatide, romosozumab, teriparatide) followed with a bisphosphonate or denosumab to prevent bone density decline and loss of fracture efficacy - Sequential therapy starting with parathyroid hormone is an option for treatment of osteoporosis in postmenopausal women who are at very high risk for fracture particularly those who have past history of multiple vertebral fractures. This should be decided and prescribed by osteoporosis specialist - Sequential therapy starting with romosozumab is an option for treatment of osteoporosis in postmenopausal women who are at very high risk for fracture particularly those who have past history of hip or vertebral fractures. This should be decided and prescribed by osteoporosis specialist |
2 | B | 8.67 | 0.82 | 91.67 | H | |
| The role of vertebral augmentation for compression fractures? | Kyphoplasty/vertebroplasty are not recommended as first-line treatment of vertebral fractures, given an unclear benefit on overall pain and a potential increased risk of vertebral fractures in adjacent vertebrae (grade 1) | 1 | A | 8.67 | 0.5 | 84.1 | H | |
| Implementation of FLS |
Fracture liaison services (FLS) should be provided for all patients sustaining a fragility fracture: - Ensure treatment initiation within 8–12 weeks of fracture - FLS should be patient-centred and integrated between orthopaedic surgery, orthogeriatrics, rheumatology and osteoporosis centres of care - Physicians should follow up patients at 4 and 12 months to review the use of medications that increase the risk of falls and/or fracture, to ensure co-prescription of calcium and vitamin D with bone protective interventions and to monitor adherence to therapy |
2 | A | 8.83 | 0.5 | 100 | H | |
| The importance of fall assessment | Falls risk should be assessed for every patient evaluated for fracture risk (grade 2a) | 2 | A | 9 | 91.4 | H | ||
| Osteoporosis in men |
Osteoporosis screening in men should be carried out in the age of 70 years or older - Men at age less than 70 years old can be assessed for osteoporosis if they develop risk factors - Men with osteopaenia (T score from − 1 to − 2.5) who have moderate risk of fracture FRAX fracture risk probability 1–3% at the hip and 10–20% at spine may be good candidates for prophylactic zoledronic acid every 18 months for 4 infusions - For the purposes of FRAX calculations, the BMD T-scores in men are calculated based on the female reference database - Secondary causes of osteoporosis are commonly found amongst men, so this population requires thorough investigation - Intervention thresholds for men are similar to those recommended for women - All men starting on androgen deprivation therapy should have their fracture risk assessed - Consider referring men with osteoporosis to specialist centres, particularly younger men or those with severe disease - Men are assessed and treated following the same management protocol suggested above for postmenopausal women, excluding the HRT |
2 | A | 8.75 | 0.5 | 94.1 | H | |
| Patients on glucocorticoids therapy |
- Women and men age ≥ 70 years, with a previous fragility fracture or taking high doses of glucocorticoids (≥ 7.5 mg/day prednisolone) should be considered for bone protective therapy, after BMD baseline assessment - In other individuals, fracture probability should be estimated using FRAX with adjustment for glucocorticoid dose. Baseline BMD assessment is advised - Bone-protective treatment should be started at the onset of glucocorticoid therapy in individuals at moderate/high risk of fracture - Alendronate and risedronate are first-line treatment options. Where these are contraindicated or not tolerated, zoledronic acid, teriparatide or denosumab (in order) are alternative options - Bone-protective therapy may be appropriate in some premenopausal women and younger men, particularly in individuals with a previous history of fracture or receiving high doses of glucocorticoids - For women in the childbearing period: the first-line therapy is an oral bisphosphonate; second-line therapy is a parathyroid hormone |
2 | A | 8.58 | 1.25 | 91.67 | H | |
| COVID-19 and osteoporosis |
- Patients do not warrant higher prioritization for vaccination against COVID-19 due to their osteoporosis - Standard non-pharmacologic approaches for optimization of bone health include maintenance of vitamin D supplementation, maintenance of adequate physical activity and adherence to a balanced diet; these strategies should be continued because of their musculoskeletal benefits and their potential roles as facilitators of immunocompetence - Osteoporosis therapies do not increase the risk or severity of COVID-19 infection and do not interfere with the efficacy or side effect profile of COVID-19 vaccines - Oral bisphosphonates, as well as the self-administered skeletal anabolic agents, teriparatide and abaloparatide, should not be discontinued during vaccination It’s recommended an interval of 1 week between intravenous bisphosphonate infusion and COVID-19 vaccination because of the possibility of treated patients developing an acute phase reaction as a result of administration of either agent - In terms of denosumab and romosozumab, it seems prudent to allow for an interval of 4 to 7 days between these drugs and vaccination because of putative injection site reactions |
2 | B | 8.63 | 0.82 | 94.1 | H |
LE, level of evidence according to the Oxford Centre for Evidence-Based Medicine (CEBM) criteria; H, high level of agreement; GoR, grade of recommendations; COVID, coronavirus disease
Recommendations for management of osteoporosis
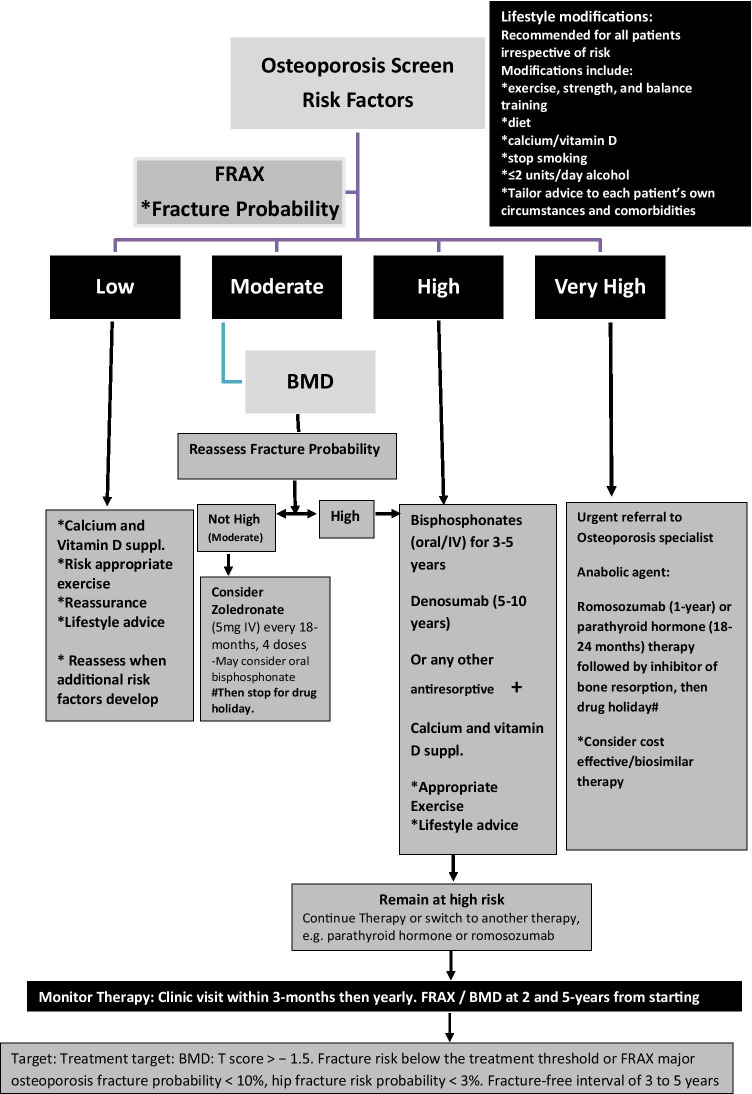
At the end of round 3, a total of twenty-two domains were obtained and a consensus was reached on all the statements developed. As health care professionals need information that is clear and readily accessible as well as applicable in standard clinical practice, it was important to articulate the developed osteoporosis guideline for the day-to-day practice. This is summarised in Fig. 2 which shows an algorithm for the treat-to-target management of postmenopausal osteoporosis.
Fig. 2.
An algorithm summarising the fracture-centric approach and the group’s consensus recommendations for the management of osteoporosis patients stratified according to their fracture risk. Case finding and management approach were set up according to the fracture risk category. The determination of fracture risk was carried out based on fracture risk score calculation (e.g. FRAX) and the measurement of lumbar spine and hip BMD
Discussion
The prevalence of osteoporosis is increasing steadily in developing countries secondary to increased longevity, with osteoporosis and its consequent fractures are becoming a major public health issue [21]. Epidemiological studies showed that in sub-Saharan Africa, the prevalence of osteoporosis was 20reported to range from 18.2 to 65.8% across a heterogenous at-risk population. Similar figures were reported in North Africa. The prevalence of osteoporosis in Egypt was 21.9% in men and 28.4% in women; whilst 26% of men and 53.9% of women having osteopenia [22]. The prevalence of osteoporosis in Moroccan postmenopausal women ranged between 21 and 31% [23, 24], which is similar to Tunisia, where 25% of postmenopausal women have osteoporosis [25], whereas the rate was slightly higher (35.8%) amongst Algerian women [26]. These rates were in concordance with the outcomes of studies evaluating the major clinical consequences of osteoporosis, i.e. fractures. A recent study investigating the incidence of hip fractures amongst Black South Africans found an age-adjusted hip fracture rate of 69.2 per 100,000 per annum and 73.1 per 100,000 per annum for women and men, respectively [27]. This evidence challenges the long-held view that osteoporosis-related fractures are rare in Blacks [28, 29] and highlight the importance of addressing the issue of osteoporosis in Africa.
The developed guideline agrees with the published guidelines from individual African nations for the management of osteoporosis including Egypt [30] and South Africa [31] as well as the international guidelines [32–34]. The guideline recommends very early intervention with osteoanabolic agents, for patients who are at very high risk of fracture. This agrees with the most recently published treatment recommendations, which endorsed the sequential as well as switch approaches of therapy [35, 36]. It also advocated a preventive approach for osteopenia patients at moderate to high risk of fractures, which agree with the recent available evidence [20]. The statements on osteoporosis management for patients with COVID-19 agree with those recently published by the National Osteoporosis Foundation [37]. This highlights the importance of having updated recommendations for osteoporosis management based on up-to-date evidence for best clinical practice. This guideline not only provides solutions for early identification of the cases, which will consequently help to will reduce fracture rates, but also enables health care professionals with special interest in bone health to face the key challenges of managing osteoporosis in Africa. The guidelines will assist the policymakers responsible for providing care for populations in relation to bone health to formulate appropriate policies.
Either a BMD-centric or a fracture risk-centric approach could be adopted to identify patients for whom pharmacological intervention should be considered. This guideline adopted the fracture risk-centric approach, though the BMD measurement was integrated, mainly for those with moderate fracture risk. This was based on the finding that DXA studies are seldomly used in Africa to assess for osteoporosis risk. This has been attributed in several cases to the lack of DXA machines. In other cases, it was linked to its prohibitive cost, even where the DXA machines are available [38]. Furthermore, the limitations of BMD for risk assessment have been one of the motives for the development of fracture risk prediction algorithms that integrate clinical risk factors for fracture. There are several fracture risk assessment tools available. Of these, the FRAX tool. (https://www.sheffield.ac.uk/FRAX/tool.aspx) has been the most extensively used. FRAX calculates the 10-year probability of a major fracture (clinical spine, humerus or wrist fracture) and 10-year probability of a hip fracture. A unique feature of FRAX is that it is based on a countries’ epidemiological data and considers competing mortality in the fracture risk estimation procedure [19].
Unfortunately, in Africa, there is a huge treatment gap between those at risk of fracture and those receiving treatment for the prevention of fragility fractures. Both the economic and societal burden of osteoporotic fragility fractures is enormous and is expected to rise owing to an increasing skew towards an older population [9, 10]. However, over the last two decades, there has been a significant shift towards the better ability to predict those at risk, using fracture prediction tools and an increasing understanding of scanning modalities, such as DXA or qualitative ultrasound scans [38]. This will allow appropriate earlier identification of patients with osteopenia or osteoporosis, who are at high or very high risk of fracturing, to benefit from osteoporosis therapy. Furthermore, a variety of generic therapeutic options are now available, at economically feasible prices. The developed guidelines endorse this armamentarium and provide a solid background to making appropriate treatment decisions, which is a step forward towards closing this gap in Africa.
The paradigm of treat-to-target aims at enhanced and individualised care of osteoporotic patients. Such strategy enables the treating clinicians to select the most appropriate initial osteoporosis therapy and guides subsequent decisions to continue, change or stop treatment [19, 33, 39]. Though some publications revealed that FRAX can be used to predict new posttreatment fracture probability and assess the reduction in the fracture risk in women currently on osteoporosis therapy [40, 41], the predominant trend is that FRAX cannot be used to monitor response to therapy. On the other hand, repeat DXA informs on the long-term treatment effect on BMD. The new concept of very high fracture risk and the development of new intervention thresholds [30, 42] provide a new manifesto based on which this guideline has been developed. The very high fracture risk and the consequent further utility loss immediately after a subsequent fracture (imminent risk) suggest that preventive treatment given as soon as possible after fracture would avoid a higher number of new fractures and reduce the attendant morbidity, compared with the treatment given later [30, 43].
Monitoring of patients on osteoporosis therapy should include regular communication with a health care professional to make sure that (1) the osteoporosis therapy is taken correctly and regularly as well as to ensure that treatment has been initiated within 16 weeks of non-traumatic fracture; (2) calcium and vitamin D supplement therapy are taken regularly and in appropriate dose (check adherence at 3 months and at 12 months); (3) address any concerns or adverse effects the patient might have; and (4) there are no comorbidities or other medications that might impact the expected treatment outcome. Whilst BMD is considered a surrogate marker for bone strength and fracture risk, stability or a significant increase in BMD is considered an acceptable treatment outcome and is associated with a reduction in fracture risk [44]. The time interval when treatment effect can be detected may vary depending on treatment modality, risk factors and current medications. A DXA scan should only be repeated if the results will impact the clinical management, or if changes in BMD are expected to exceed the least significant change (LSC) for the DXA equipment used. The annual rates of loss during these intervals are approximately 1.8–2.3% in the spine and 1.0–1.4% in the hip. This is well below the least significant change (LSC), averaging 2–3% for most DXA machines at the total hip. Therefore, repeat DXA scan should be considered after 2–3 years of the former scan, which has been recommended in this guideline. The target of bone mineral density (BMD) (in the range of − 1.5 to − 2) as suggested in this guideline agrees with those reported in other studies [19, 30, 39].
This guideline includes health care professionals from the entire African continent, ensuring that all the regions have been covered and represented; therefore, it is expected to be implemented across the whole of Africa. The aim is to streamline the osteoporosis service provided to the patients across the continent and ensure that osteoporosis therapy is determined or escalated according to the patient’s risk factors and fracture risk within an approved framework. The Delphi technique has proven to be a reliable measurement instrument in developing new concepts and setting the direction of future-orientated research [16]. In Delphi methodology, consensus usually arises when agreement or disagreement ranges from 50 to 80% [17]. In our work, the agreement ranged between 83.3 and 100%, indicating a strong trend amongst the African health care professionals to have a treat-to-target approach for osteoporosis management. These findings agree with the results of the Spanish consensus on osteoporosis management [45] as well as the Egyptian guidelines for osteoporosis management [31], which revealed similar scores on the treat to target policy.
Limitations of the guideline
This guideline reflects the best data available at the time the report was prepared. Caution should be exercised in interpreting the data; the results of future studies may require alteration of the conclusions or recommendations in this report. It may be necessary or even desirable to depart from the guidelines in the interests of specific patients and special circumstances. Just as adherence to guidelines may not constitute defence against a claim of negligence, so deviation from them should not necessarily be deemed negligent.
Plans for guideline revision
This field of osteoporosis therapeutics is in a rapid phase of development, and revision of the scope and content of the guideline will therefore occur on regular basis. Where necessary, the guideline will be updated.
In conclusion, with changing demography, the cost of treating osteoporosis is expected to increase considerably in Africa by the year 2030. Understanding of the impact of clinical risk factors can influence prevention and treatment of osteoporotic fractures. Therefore, it is vital to screen the patients and stratify them according to their identified fracture risk. This was a wide and representative panel of experts who established consensus regarding the management of postmenopausal osteoporosis in Africa. It also expanded to give guidance for the management of osteoporosis in men and the potential role of fracture liaison service in standard practice. The algorithm developed in this study facilitates the incorporation of several recent developments into the standard patient management protocols.
Declarations
Conflicts of interest
None.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Y El Miedany, Email: yasserelmiedany@gmail.com.
Farhanah Paruk, Email: paruk@ukzn.ac.za.
Asgar Kalla, Email: kallaa@iafrica.com.
A. Adebajo, Email: a.o.adebajo@sheffield.ac.uk
Maha El Gaafary, Email: maha_elgaafary@med.asu.edu.eg.
Abdellah El Maghraoui, Email: a.elmaghraoui@um5.net.ma.
Madeleine Ngandeu, Email: ngandeum@yahoo.fr.
Dzifa Dey, Email: dzifakay@gmail.com.
Naglaa Gadallah, Email: naglagadallah@gmail.com.
Mohamed Elwy, Email: elwy.mohamed@yahoo.com.
Farzana Moosajee, Email: farzana@sun.ac.za.
Mohammed Hassan Abu-Zaid, Email: drmhassan113@yahoo.com.
Salwa Galal, Email: dr_salwa07@yahoo.com.
Soussen Miladi, Email: Saoussenmiladi@gmail.com.
Waleed Hassan, Email: waleed22101979@yahoo.com.
Abubaker Fadlelmola, Email: Abubakerfadlelmola@gmail.com.
Sally Saber, Email: Sally_saber@med.asu.edu.eg.
References
- 1.International Osteoporosis Foundation (2018): what is osteoporosis? 2018. https://www.iofbonehealth.org/what-is-osteoporosis Accessed 31 July 2021
- 2.Johnell O, Kanis JA. An estimate of the worldwide prevalence and disability associated with osteoporotic fractures. Osteoporos Int. 2006;17:1726–1733. doi: 10.1007/s00198-006-0172-4. [DOI] [PubMed] [Google Scholar]
- 3.Kanis J, Johnell O, Oden A, Sernbo I, Redlund-Johnell I, Dawson A, De Laet C, Jonsson B. Long-term risk of osteoporotic fracture in Malmö. Osteoporos Int. 2000;11:669–674. doi: 10.1007/s001980070064. [DOI] [PubMed] [Google Scholar]
- 4.Kanis JA, Oden A, Johnell O, Jonsson B, de Laet C, Dawson A. The burden of osteoporotic fractures: a method for setting intervention thresholds. Osteoporos Int. 2001;12:417–427. doi: 10.1007/s001980170112. [DOI] [PubMed] [Google Scholar]
- 5.Rashki Kemmak A, Rezapour A, Jahangiri R, Nikjoo S, Farabi H, Soleimanpour S. Economic burden of osteoporosis in the world: a systematic review. Med J Islam Repub Iran. 2020;34:154. doi: 10.34171/mjiri.34.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Handa R, Kalla AA, Maalouf G. Osteoporosis in developing countries. Best Pract Res Clin Rheumatol. 2008;22:693–708. doi: 10.1016/j.berh.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 7.Paruk F, Tsabasvi M, Kalla AA. Osteoporosis in Africa—where are we now. Clin Rheumatol. 2021;40(9):3419–3428. doi: 10.1007/s10067-020-05335-6. [DOI] [PubMed] [Google Scholar]
- 8.Billek-Sawhney B. Osteoporosis risk – think twice about bone density in Ethiopians African Americans. Int Phys Med Rehab J. 2019;4(3):91–94. [Google Scholar]
- 9.Yumiko Kamiya, DESA, United Nations: department of economic and social affairs. Population Division United Nations Regional Workshop, Lilongwe, July 2016. https://www.un.org/development/desa/pd/sites/www.un.org.development.desa.pd/files/unpd_ws201607_demographic_trends_in_africa_yk.pdf
- 10.Census Bureau Releases New Report on Aging in Africa, SEPTEMBER 02, 2020. RELEASE NUMBER CB20-TPS.53. https://www.census.gov/newsroom/press-releases/2020/aging-in-africa.html. Accessed 11 Aug 2021
- 11.National Institute of Aging. New census report on aging trends in Africa. https://www.nia.nih.gov/news/new-census-report-aging-trends-africa. Accessed 2 Aug 2021
- 12.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Intern Med. 2009;151:W65–94. doi: 10.7326/0003-4819-151-4-200908180-00136. [DOI] [PubMed] [Google Scholar]
- 13.Leclercq E, Leeflang MM, van Dalen EC, Kremer LC. Validation of search filters for identifying pediatric studies. J Pediatr. 2013;162:629–634. doi: 10.1016/j.jpeds.2012.09.012. [DOI] [PubMed] [Google Scholar]
- 14.OCEBM Levels of Evidence Working Group . The Oxford levels of evidence 2. Oxford: Oxford Centre for Evidence-Based Medicine; 2011. [Google Scholar]
- 15.Von der Gracht H. Consensus measurement in Delphi studies: review and implications for future quality assurance. Technol Forecast Soc. 2012;79(8):1525–1536. doi: 10.1016/j.techfore.2012.04.013. [DOI] [Google Scholar]
- 16.Rowe G, Wright G. The Delphi technique as a forecasting tool: issues and analysis. Int J Forecast. 1999;15:353–375. doi: 10.1016/S0169-2070(99)00018-7. [DOI] [Google Scholar]
- 17.Rayens MK, Hahn EJ. Building consensus using the policy Delphi method. Policy Polit Nurs Pract. 2000;1(4):308–315. doi: 10.1177/152715440000100409. [DOI] [Google Scholar]
- 18.Diamond IR, Grant RC, Feldman BM, Pencharz PB, Ling SC, Moore AM, Wales PW. Defining consensus: a systematic review recommends methodologic criteria for reporting of Delphi studies. J Clin Epidemiol. 2014;67(4):401–409. doi: 10.1016/j.jclinepi.2013.12.002. [DOI] [PubMed] [Google Scholar]
- 19.Kanis JA, Johansson H, Oden A, McCloskey EV. Guidance for the adjustment of FRAX according to the dose of glucocorticoids. Osteoporos Int. 2011;22:809–816. doi: 10.1007/s00198-010-1524-7. [DOI] [PubMed] [Google Scholar]
- 20.Reid IR, Horne AM, Mihov B, Stewart A, Garratt E, Wong S, Wiessing KR, Bolland MJ, Bastin S, Gamble GD. Fracture prevention with zoledronate in older women with osteopenia. N Engl J Med. 2018;379(25):2407–2416. doi: 10.1056/NEJMoa1808082. [DOI] [PubMed] [Google Scholar]
- 21.Kinsella K, Wan H. An aging world: 2008. U.S. Census Bureau. International Population Reports. 2009; 9(1): 9
- 22.Gheita T, Hammam N. Epidemiology and awareness of osteoporosis: a viewpoint from the Middle East and North Africa. Int J Clin Rheumatol. 2018;13(3):134–147. [Google Scholar]
- 23.Ouzzif Z, Oumghar K, Sbai K, et al. Relation of plasma total homocysteine, folate and vitamin B12 levels to bone mineral density in Moroccan healthy postmenopausal women. Rheumatol Int. 2012;32(1):123–8. doi: 10.1007/s00296-010-1551-x. [DOI] [PubMed] [Google Scholar]
- 24.El Maghraoui A, Sadni S, El Maataoui A, Majjad A, Rezqi A, Ouzzif Z, Mounach A. Influence of obesity on vertebral fracture prevalence and vitamin D status in postmenopausal women. Nutr Metab (Lond) 2015;14(12):44. doi: 10.1186/s12986-015-0041-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ben Aissa R, Laatar A, Kerkeni S et al (2006) Prévalence de Et, l’ostéoporose chez les femmes ménopausées des gouvernorats de l’Ariana 119, de la Manouba-Tunis. Tun. Méd. 84(Suppl 10)
- 26.Haouichat C, Hammoumraoui N, Lehtihet S, et al. SAT0461 Prevalence of postmenopausal osteoporosis in Algerian women. Ann Rheum Dis. 2014;73:760. doi: 10.1136/annrheumdis-2014-eular.2980. [DOI] [Google Scholar]
- 27.Paruk F, Matthews G, Cassim B. Osteoporotic hip fractures in Black South Africans: a regional study. Arch Osteoporos. 2017;12(1):107. doi: 10.1007/s11657-017-0409-1. [DOI] [PubMed] [Google Scholar]
- 28.Cauley JA, Wu L, Wampler NS, et al. Clinical risk factors for fractures in multi-ethnic women. the women’s health initiative. J Bone Miner Res. 2007;22:1816–1826. doi: 10.1359/jbmr.070713. [DOI] [PubMed] [Google Scholar]
- 29.Peled R, Dahan D, Endevelt R, et al. Osteoporosis among Ethiopian immigrant women: a risk analysis. Arch Osteoporos. 2007;2:45–52. doi: 10.1007/s11657-007-0013-x. [DOI] [Google Scholar]
- 30.El Miedany Y, Abu-Zaid MH, El Gaafary M, et al. Egyptian consensus on treat-to-target approach for osteoporosis: a clinical practice guideline from the Egyptian Academy of bone health and metabolic bone diseases. Egypt Rheumatol Rehabil. 2021;48:5. doi: 10.1186/s43166-020-00056-9. [DOI] [Google Scholar]
- 31.Amod A, Ascott-Evans B, Brown S, Cassim B, Davey M, de Lange W, de Villiers T et al (2017) South African clinical guideline for the diagnosis and management of osteoporosis: JEMDSA 2017; 22(1) (Supplement 1)
- 32.Camacho PM, Petak SM, Binkley N, Diab DL, Eldeiry LS, Farooki A, Harris ST, Hurley DL, Kelly J, Lewiecki EM, Pessah-Pollack R, McClung M, Wimalawansa SJ, Watts NB. American Association of Clinical Endocrinologists/American College of Endocrinology clinical practice guidelines for the diagnosis and treatment of postmenopausal osteoporosis-2020 update. Endocr Pract. 2020;26(Suppl 1):1–46. doi: 10.4158/GL-2020-0524SUPPL. [DOI] [PubMed] [Google Scholar]
- 33.Kanis J, Cooper C, Rizzoli R, et al. European guidance for the diagnosis and management of osteoporosis in postmenopausal women. Osteoporos Int. 2019;30:3–44. doi: 10.1007/s00198-018-4704-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Meeta M, Harinarayan CV, Marwah R, Sahay R, Kalra S, Babhulkar S. Clinical practice guidelines on postmenopausal osteoporosis: *an executive summary and recommendations - update 2019–2020. J Midlife Health. 2020;11(2):96–112. doi: 10.4103/jmh.JMH_143_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Eastell R, Rosen CJ, Black DM, Cheung AM, Murad MH, Shoback D. Pharmacological management of osteoporosis in postmenopausal women: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2019;104(5):1595–1622. doi: 10.1210/jc.2019-00221. [DOI] [PubMed] [Google Scholar]
- 36.UK consensus guideline on the management of patients at low, high, and very high risk of osteoporotic fracture. Cyrus Cooper, Kassim Javaid, Mary Elliott, David Stephens, Nuttan Tanna. www.Guidelines.co.uk 2020 [https://www.guidelines.co.uk/musculoskeletal-and-joints-/osteoporotic-fracture-guideline/455546.article ] accessed on 15th July 2021
- 37.National Osteoporosis Foundation. Joint guidance on COVID-19 vaccination and osteoporosis management from the American Society for Bone and Mineral Research (ASBMR), American Association of Clinical Endocrinology (AACE), Endocrine Society, European Calcified Tissue Society (ECTS), the International Osteoporosis Foundation (IOF), and the National Osteoporosis Foundation (NOF). Published online March 9, 2021. Accessed July 15, 2021. https://www.nof.org/news/statement-joint-guidance-on-covid-19-vaccination-and-osteoporosis-management-from-the-asbmr-aace-endocrine-society-ects-iof-and-nof/
- 38.Atiase Y, Quarde A. A call to action for osteoporosis research in sub-Saharan Africa. Ghana Med J. 2020;54(1):58–67. doi: 10.4314/gmj.v54i1.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lewiecki EM. Osteoporosis: Treat-to-Target. Curr Osteoporos Rep. 2017;15(2):103–109. doi: 10.1007/s11914-017-0350-7. [DOI] [PubMed] [Google Scholar]
- 40.Leslie WD, Lix LM, Johansson H, Oden A, McCloskey E, Kanis JA. Does osteoporosis therapy invalidate FRAX for fracture prediction? J Bone Miner Res. 2012;27:1243–1251. doi: 10.1002/jbmr.1582. [DOI] [PubMed] [Google Scholar]
- 41.El Miedany YE, Gaafary ME, Yassaki AE, Youssef S, Nasr A, Ahmed I. Monitoring osteoporosis therapy: can FRAX help assessing success or failure in achieving treatment goals? World J Rheumatol. 2014;4(2):14–21. doi: 10.5499/wjr.v4.i2.14. [DOI] [Google Scholar]
- 42.Kanis JA, Harvey NC, McCloskey E, et al. Algorithm for the management of patients at low, high and very high risk of osteoporotic fractures. Osteoporos Int. 2020;31:1–12. doi: 10.1007/s00198-019-05176-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lewiecki EM. Bone density testing to monitor osteoporosis therapy in clinical practice. Am Fam Physician. 2010;82(7):749–754. [PubMed] [Google Scholar]
- 44.Lewiecki EM, Watts NB. Assessing response to osteoporosis therapy. Osteoporos Int. 2008;19(10):1363–1368. doi: 10.1007/s00198-008-0661-8. [DOI] [PubMed] [Google Scholar]
- 45.Nogués X, Nolla JM, Casado E, Jódar E, Muñoz-Torres M, Quesada-Gómez JM, Canals L, Balcells M, Lizán L. Spanish consensus on treat to target for osteoporosis. Osteoporos Int. 2018;29(2):489–499. doi: 10.1007/s00198-017-4310-y. [DOI] [PMC free article] [PubMed] [Google Scholar]