Dear Editor,
The clinical spectrum of SARS-CoV-2 infection varies widely ranging from asymptomatic infection to severe viral pneumonia with respiratory failure.1 Some patients of COVID, who develop respiratory failure have hypoxemia but without signs of respiratory distress also termed as “silent hypoxemia”. This silent hypoxemia may be responsible for the quick deterioration because it gives a false sense of well-being even when the oxygen debt is actually increasing.2, 3 This mandates regular monitoring of oxygen levels in these patients. SpO2/FiO2 (S/F) ratio has been found to have good correlation with PaO2/FiO2 (P/F) ratio in adult and pediatric patients with pneumonia, acute respiratory distress syndrome (ARDS) and acute lung injury in various studies.4, 5, 6 However, in COVID patients, there can be discordance between S/F ratio and P/F ratio due to multiple reasons like shift of oxyhemoglobin dissociation curve to left or right, inaccuracy of SpO2 at lower levels of saturation and during critical illness.7 Moreover, the linear correlation between SpO2 and FiO2 is lost when SpO2 is 100% and even the PaO2 cannot be estimated when SpO2 is 100%.
The aim of this study was to assess the correlation between S/F and the P/F ratios in patients with COVID pneumonia requiring oxygen therapy and to find whether initial S/F ratio on admission can indicate the requirement of invasive mechanical ventilation (IMV) later in the course of the disease.
This was a prospective observational study conducted in tertiary care COVID center, AIIMS, India after ethical committee approval (IEC-856,4.9.20 dated 14.10.20). Adult patients of ≥18 years of age suffering from moderate to severe COVID (RT-PCR positive) requiring oxygen support or IMV admitted in the intensive care unit (ICU) were included after consent. The patients were administered oxygen with different interfaces as per their baseline SpO2 and clinical condition to target SpO2 92–94% (88–92% in patients with COPD) (life scope bedside monitor from nihonkohden BSM-37630 series). The first arterial blood gas (ABG) analysis (werfen diagnostic corporation, Gem premiere-3000) was done at the time of admission to ICU, and subsequent analysis were done according to clinical condition of patients at the physicians discretion. No specific time points was selected, however, ABG s were done at different FiO2 in the same patient were recorded. The FiO2 and SpO2 were noted at the time of ABG analysis. The FiO2 delivered with standard facemask was calculated as 0.4 with 5–6 l of oxygen, 0.5 with 6–7 l and 0.6 with >7 l of oxygen flow and with non rebreathing mask (NRBM) as 0.9 with 12–15 l of flow. The exact FiO2 was set on the high flow nasal cannula (HFNC) machine and non invasive ventilation (NIV) machine according to the patient requirements. The demographic data, vitals, FiO2, S/F ratio and P/F ratio and outcomes were noted.
Assuming significant correlation with r = 0.65, the calculated sample size was 80 for 80% power with 5% level of significance at two sided test. A total of 80 patients were enrolled in this study and 249 observations were noted. Data was analyzed using Statistical software packages IBM SPSS, version 21.0. The correlation between S/F ratio and P/F ratio was established using spearmann correlation coefficient and linear regression test was used to develop the equation for S/F and 95% confidence interval were reported.
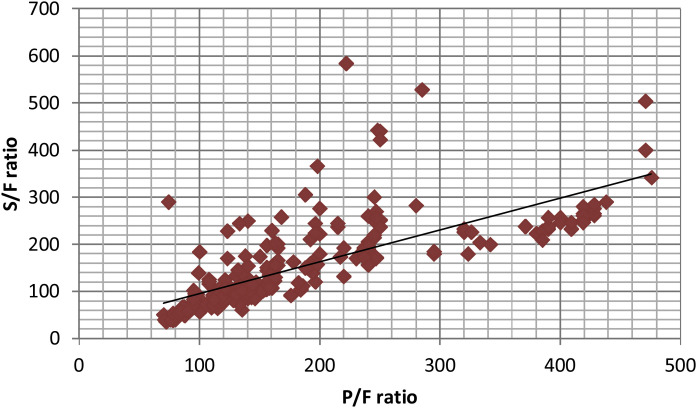
The mean age of study population was 52 ± 13 years and 65% were males. Out of 80 patients, 60 had comorbidities, diabetes mellitus being the most common. The initial respiratory support varied with 37.5% on facemask, 28.7% on NRBM, 8.7% on HFNC/NIV and 25% on IMV (Supplementary file 1). The mean initial S/F ratio of the patients was 159.77 ± 72.14 and mean P/F ratio was 147.86 ± 103.26. A scatter plot of S/F and P/F ratios [249 observations] demonstrated a linear correlation (Fig. 1 ). The value of r was 0.86, almost similar as in study by Rice et al.9 (r = 0.89) indicating a positive correlation.
Figure 1.
S/F ratio vs P/F ratio scatter plot. S/F ratio – SpO2/FiO2; P/F ratio – PaO2/FiO2. The line represents the best fit linear relationship SpO2/FiO2 = 0.80(PaO2/FiO2) + 59.8 (p < 0.001).
The SF ratio could be predicted well from PF ratio, described by the linear regression equation SpO2/FiO2 = 0.80 (PaO2/FiO2) + 59.8 [95% CI for regression coefficient 0.71–0.89]. Based on this equation, SF ratio of 219 and 299 corresponds to PF ratio of 200 and 300 [p < 0.001] which is similar to results by Rice et al.9 (S/F ratio of 235 and 315 surrogates for P/F ratio of 200 and 300). Rice et al.9 had included patients with ALI/ARDS due to various causes like sepsis, trauma, pneumonia and aspiration and those who were on IMV as per ARDS net trial protocol, whereas we included patients on oxygen as well as patients on IMV.
Recently, Fukuda et al.8 reported that S/F was useful for predicting the clinical outcomes in mechanically ventilated patients with acute hypoxemic respiratory failure with bilateral opacities. Similarly, Choi et al.9 reported S/F ratio on admission as a strong predictor of occurrence of ARDS in COVID patients requiring oxygen therapy.
We examined whether initial S/F ratio can indicate the requirement of IMV. In our study, 19 out of 60 patients required IMV later in the course of the disease (ventilated group) and 41 did not (non ventilated). We compared these two groups (Table 1 ). There were no differences in the demographic characteristics, initial S/F ratio and P/F ratio, in the two groups, however, the ventilated group patients were significantly more tachycardiac and tachypneic on admission pointing to the fact that patients were able to maintain oxygenation in the initial phase of the disease at the expense of tachypnea and use of accessory muscles.
Table 1.
Comparison between patients who required invasive ventilation (ventilated group) and who did not require invasive ventilation (non ventilated group).
| Variables | Invasive ventilation group (n = 19) Number (%)/Mean ± SD/Median [Range] |
Without invasive ventilation group (n = 41) Number (%)/Mean ± SD/Median [Range] |
P-value |
|---|---|---|---|
| Age | 49.52 ± 17.79 | 52.56 ± 12.99 | 0.457 |
| Male/female | 12/7 | 26/15 | 0.985 |
| HR (beats/min) | 101.93 ± 24.19 | 88.26 ± 16.37 | 0.0132* |
| SBP (mm/Hg) | 124.7 ± 22.5 | 128.8 ± 21.8 | 0.54 |
| DBP (mm/Hg) | 74.8 ± 11.6 | 72.1 ± 10.6 | 0.37 |
| RR (breaths/min) | 33.47 ± 6.08 | 27.23 ± 3.55 | 0.0001* |
| Accessory muscles use | 12 (63) | 5 (12.1) | 0.0001* |
| Initial respiratory support | 0.012* | ||
| Facemask | 5 (26.3) | 25 (60.9) | |
| NRBM | 9 (47.3) | 14 (34.1) | |
| HFNC/NIV | 5 (26.3) | 2 (4.88) | |
| S/F ratio | 116 (80–250) | 160 (71–333) | 0.14 |
| P/F ratio | 100 (41–442) | 145 (36–528) | 0.739 |
HR – heart rate; RR – respiratory rate; SBP – systolic blood pressure; DBP – diastolic blood pressure; S/F ratio – SpO2/FiO2; P/F ratio – PaO2/FiO2; NRBM – non rebreathing mask; HFNC – high flow nasal cannula; NIV – non invasive ventilation.
Significant.
The median initial S/F ratio [147.5 (71–333)] in our cohort was much lower than in the study by Choi et al.,9 (287.5 and 452.4) indicating patients were more hypoxemic and in advanced disease in our study probably owing to the delayed presentation to hospital in our cohort. Moreover, factors other than oxygenation e.g. secondary infections, altered sensorium could be reasons for deterioration and mechanical ventilation.
Furthermore, it is imperative to note that some patients with COVID may not have dyspnea despite being hypoxemic, and therefore clinical monitoring of vitals gains paramount importance in these patients. They require aggressive management in order to halt further deterioration.
In conclusion, S/F ratio can be used as surrogate of P/F ratio in patients with COVID pneumonia and can be highly useful in resource limited settings during this pandemic. However, initial S/F ratio on admission cannot indicate the need of invasive ventilation later in the course of the disease.
Authors’ contribution
Ashutosh Kumar – acquisition of data, or analysis and interpretation of data.
Richa Aggarwal – conception and design of the study, analysis and interpretation of data, drafting the article.
Puneet Khanna – conception and design of the study.
Rakesh kumar – drafting the article or revising it critically for important intellectual content.
Kapil Dev Soni – drafting the article or revising it critically for important intellectual.
AnjanTrikha – revising it critically for important intellectual content and final approval of the version to be submitted.
Funding source
None.
Conflict of interest
The authors have no competing interests to declare.
Footnotes
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.medin.2021.10.005.
Appendix A. Supplementary data
The following are the supplementary data to this article:
References
- 1.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tobin M.J., Jubran A., Laghi F. Misconceptions of pathophysiology of happy hypoxemia and implications for management of COVID-19. Respir Res. 2020;21:249. doi: 10.1186/s12931-020-01520-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dhont S., Derom E., Van Braeckel E., Depuydt P., Lambrecht B.N. The pathophysiology of ‘happy’ hypoxemia in COVID-19. Respir Res. 2020;21:198. doi: 10.1186/s12931-020-01462-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rice T.W., Wheeler A.P., Bernard G.R., Hayden D.L., Schoenfeld D.A., Ware L.B., et al. Comparison of the SpO2/FiO2 ratio and the PaO2/FiO2 ratio in patients with acute lung injury or ARDS. Chest. 2007;132:410–417. doi: 10.1378/chest.07-0617. [DOI] [PubMed] [Google Scholar]
- 5.Bilan N., Dastranji A., Behbahani A.G. Comparison of the SpO2/FiO2 ratio and the PaO2/FiO2 ratio in patients with acute lung injury or acute respiratory distress syndrome. J Cardiovasc Thorac Res. 2015;7:28–31. doi: 10.15171/jcvtr.2014.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Riviello E.D., Kiviri W., Twagirumugabe T., Mueller A., Banner-Goodspeed V.M., Officer L., et al. Hospital incidence and outcomes of the acute respiratory distress syndrome using the Kigali modification of the Berlin definition. Am J Respir Crit Care Med. 2016;193:52–59. doi: 10.1164/rccm.201503-0584OC. [DOI] [PubMed] [Google Scholar]
- 7.Tobin M.J., Laghi F., Jubran A. Why COVID-19 silent hypoxemia is baffling to physicians. Am J Respir Crit Care Med. 2020;202:356–360. doi: 10.1164/rccm.202006-2157CP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fukuda Y., Tanaka A., Homma T., Kaneko K., Uno T., Fujiwara A., et al. Utility of SpO2/FiO2 ratio for acute hypoxemic respiratory failure with bilateral opacities in the ICU. PLOS ONE. 2021;16:e0245927. doi: 10.1371/journal.pone.0245927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Choi K.J., Hong H.L., Kim E.J. The association between mortality and the oxygen saturation and fraction of inhaled oxygen in patients requiring oxygen therapy due to COVID-19-associated pneumonia. Tuberc Respir Dis (Seoul) 2021;84:125–133. doi: 10.4046/trd.2020.0126. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.