Abstract
Coronavirus disease 2019 (COVID-19) is caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which leads to critical pneumonia, although the clinical courses vary. In some cases, COVID-19 pneumonia causes secondary pulmonary fibrosis, which can retain radiological changes and prolong respiratory symptoms. Interstitial lung disease (ILD) secondary to COVID-19 is thought to be caused by multiple pathologies, such as excessive cytokines and abnormal repair processes elaborated by lung cells (epithelium, mesenchyme, and alveolar macrophages) after lung injury rather than viral invasion itself. Immunosuppression therapy may improve chronic respiratory symptoms and radiological changes in post-COVID-19 ILD, although the treatment is not yet established. Herein, we report three patients with post-COVID-19 ILD who presented with profound hypoxemia that had a good response to high-dose corticosteroid therapy. Further and larger studies are needed to establish post-COVID-19 ILD.
Keywords: COVID-19, Post-COVID-19, SARS-CoV-2, Interstitial lung disease, Corticosteroid therapy
1. Introduction
Coronavirus disease 2019 (COVID-19) is caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and is a global problem. COVID-19 leads to acute and critical pneumonia, although the clinical courses vary [[1], [2], [3]]. Pulmonary fibrosis secondary to COVID-19 is an essential problem because it might prolong or even deteriorate respiratory symptoms in patients recovering from COVID-19, and pulmonary fibrosis in COVID-19 is thought to be due to an abnormal repair process following lung injury caused by an excessive inflammatory response, abnormality of lung epithelium, mesenchyme, and macrophages, and mechanical injuries in alveoli [[4], [5], [6]]. Since cytokine storms are crucial in the pathogenesis of COVID-19 pneumonia, immunosuppressive agents may also improve interstitial lung disease (ILD) secondary to COVID-19 [7,8], although proper therapeutic strategies have not yet been established.
Here, we report three cases of post-COVID-19 ILD presenting with profound hypoxemia, which demonstrated a good response to high-dose corticosteroid therapy.
2. Case reports
2.1. Case 1: male, 63 years old
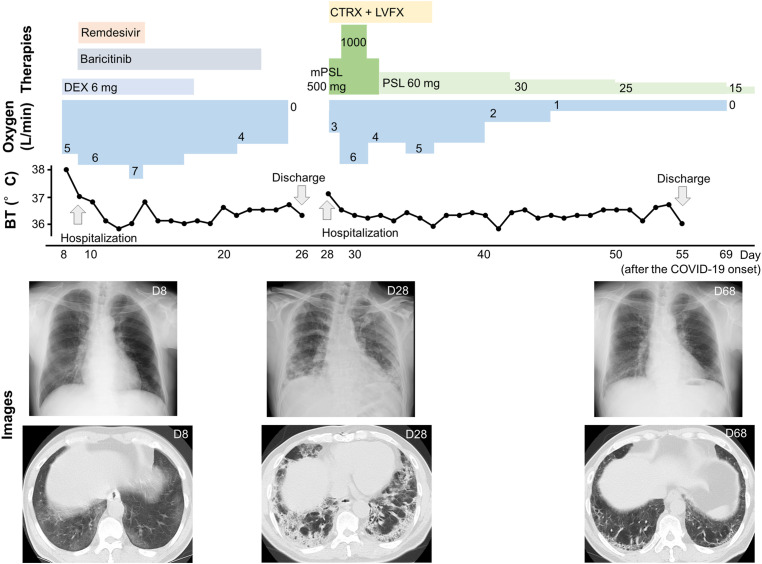
The patient underwent coronary artery bypass graft (CABG) for angina pectoris at 61 years old and was also diagnosed with type 2 diabetes mellitus; in addition, he was a current smoker (82 pack-years). He noticed fever, and eight days after the onset of this symptom, he experienced dyspnea and was diagnosed with COVID-19 (the SARS-CoV-2 PCR test result was positive). At that time, the patient exhibited hypoxemia and mild pneumonia and was transferred to our hospital. He was treated with dexamethasone (6 mg/day, for ten days), baricitinib (4.0 mg/day, for 14 days), and remdesivir (200 mg/day on the first day, 100 mg/day from the second to the fifth day). His condition gradually improved, and he was discharged on the 26th day after the onset of COVID-19. However, on the 28th day, he was readmitted to our hospital because of recurrence of dyspnea. Physical examination revealed clear consciousness, low-grade fever (37.1 °C), and hypoxemia (SpO2 84% on room air). The laboratory test indicated high levels of ILD markers (KL-6: 1462 U/L, SP-D: 161 U/L) (also see Table 1 ). The computed tomography (CT) image revealed bilateral consolidation with subpleural distribution, traction bronchiectasis, and irregular reticulation (Fig. 1 and E1). From the findings, we diagnosed post-COVID-19 ILD and started corticosteroid pulse therapy (at a dose of 500 mg of methylprednisolone every 12 hours, six times in total) with antibiotics. Following the initial therapy, corticosteroid therapy was continued (at a dose of 1.0 mg/kg/d of prednisolone, also see in Fig. 1 ). Such therapy could prevent deterioration of respiratory failure and improve hypoxemia. The patient was discharged on the 55th day after the onset of COVID-19. He continued steroid tapering in an outpatient setting and was weaned from oxygen therapy on the 69th day.
Table 1.
Patient's laboratory data at the point of post-COVID-19 interstitial lung disease onset.
| Hematology | Case 1 | Case 2 | Case 3 | Biochemistry | Case 1 | Case 2 | Case 3 | Immunology | Case 1 | Case 2 | Case 3 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| WBC (/μL) | 11930 | 14670 | 8800 | AST (IU/L) | 110 | 18 | 24 | CRP (mg/dl) | 14.09 | 20.73 | 4.84 |
| Neut | 89.3% | 80.6% | 70.3% | ALT (IU/L) | 154 | 35 | 23 | KL-6 (U/L) | 1462 | 595 | 4680 |
| Lym | 5.6% | 7.4% | 12.1% | ALP (IU/L) | 481 | 216 | 280 | SP-D (ng/ml) | 161 | N/M | 482 |
| Mo | 4.1% | 11.2% | 11.2% | LDH (IU/L) | 336 | 239 | 343 | IgA (mg/dl) | 314 | 444 | 566 |
| Eo | 0.5% | 0.5% | 5.9% | BUN (mg/dl) | 10 | 14 | 12 | IgG (mg/dl) | 1260 | 940 | 1208 |
| Baso | 0.5% | 0.3% | 0.5% | Cr (mg/dl) | 0.65 | 0.77 | 0.64 | IgM (mg/dl) | 73 | 91 | 77 |
| RBC (X106/μL) | 398 | 405 | 463 | CK (IU/L) | 66 | 23 | 44 | C3 (mg/dl) | 185 | 163 | 141 |
| Hb (g/dL) | 11.7 | 12.3 | 14.7 | TP (g/dl) | 6.5 | 6.4 | 6.7 | C4 (mg/dl) | 41 | 34 | 20 |
| Ht | 36.0% | 38.0% | 43.6% | Alb (g/dl) | 2.6 | 2.7 | 2.8 | Complement, Total (CH50/mL) | 29.0 | 48.8 | 48.4 |
| Plt (X103/μL) | 131 | 232 | 257 | Ferritin (ng/ml) | 721 | N/M | 1285 | RF (U/mL) | 3 | 6 | 4 |
| Na (mEq/l) | 137 | 127 | 137 | C-ANCA (U/mL) | 1.0 | 2.1 | 1.0 | ||||
|
Coagulation |
K (mEq/l) | 3.7 | 4.3 | 3.9 | MPO-ANCA (U/mL) | 1.0 | 1.0 | 1.0 | |||
| PT (sec) | 11.9 | N/M | 11.7 | Cl (mEq/l) | 100 | 94 | 99 | Antinuclear antibodies | Neg | Neg | Neg |
| APTT (sec) | 36.4 | N/M | 29.3 | Ca (mg/dl) | 8.8 | 8.3 | 9.3 | ||||
| D-Dimer (ng/ml) | 2.6 | N/M | 1.3 | FBS (mg/dl) | 126 | 238 | 106 |
Auto-Antibody, Names |
|||
| HbA1c (%, NGSP) | 6.8 | 8.2 | 7.6 | ARS | Neg | Neg | Neg | ||||
| NT-pro BNP (pg/ml) | 517 | N/M | 397 | Centromere | Neg | Neg | Neg | ||||
| Scl-70 | Neg | Neg | Neg | ||||||||
| Sm | Neg | Neg | Neg | ||||||||
| RNP | Neg | Neg | Neg | ||||||||
| CCP (U/ml) | 0.6 | 0.6 | 0.6 |
N/M; not measured, Neg; Negative, ARS; aminoacyl tRNA synthetase, RNP; ribonucleoprotein, CCP; cyclic citrullinated peptide.
Fig. 1.
The figure shows the clinical course in Case 1. Oxygen at 1–4 L/min was administered using a nasal cannula, and 5–7 L/min was administered using a simple face mask. DEX; Dexamethasone, mPSL; Methylprednisolone, PSL; Prednisolone, CTRX; Ceftriaxone, LVFX; Levofloxacin. The images are indicated as representative chest X-rays and CT images.
2.2. Case 2: male, 75 years old
The patient was being treated for hypertension and was a past smoker (40 pack-years). He experienced cough and dyspnea, and four days after the onset of these symptoms, he noticed fever and was diagnosed with COVID-19 (the SARS-CoV-2 antigen test result was positive). The patient exhibited hypoxemia and was hospitalized. He was treated with dexamethasone (6 mg/day, for ten days) and remdesivir (200 mg/day on the first day, 100 mg/day from the second to the fifth day). The fever improved, although the hypoxemia worsened from the 10th day to the 12th day after the onset of COVID-19. The hypoxemia was improved on the 13th day. However, on the 17th day, the fever recurred (38.7 °C), and the hypoxemia (SpO2 94% on nasal cannula O2 2 L/min) also deteriorated. The laboratory test indicated high C-reactive protein (CRP, 20.73 mg/dl) and KL-6 (595 U/L) values (Table 1). The high HbA1c (8.2%, NGSP) level indicated that the patient had diabetes mellitus. Chest X-ray showed new bilateral peripheral infiltrates, and CT imaging revealed bilateral consolidation with subpleural distribution, suggesting organizing pneumonia (Fig. 2 and E2). We started corticosteroid pulse therapy (at a dose of 500 mg of methylprednisolone every 12 hours, six times in total) based on acute exacerbation of ILD. Following the pulse therapy, we continued corticosteroid therapy using betamethasone (at the dose of 4.0 mg per day, also see in Fig. 2 ). The hypoxemia was resolved immediately after high-dose corticosteroid treatment. The corticosteroid was tapered, and the patient was discharged on the 28th day. On the 47th day, he complained of dyspnea, although hypoxemia was not observed (SpO2 93% on room air). The laboratory test indicated an increase in CRP (5.99 mg/dl) and KL-6 (723 U/L) values, and the corticosteroid dose was increased. However, the patient's dyspnea worsened, and he was hospitalized on the 54th day. The radiological changes were improved over those on the 19th day, but hypoxemia was observed (SpO2 89% on room air). There was no evidence of pulmonary embolism or heart failure, and he was introduced to long-term oxygen therapy and discharged on the 62nd day. In outpatient settings, the patient's symptoms improved gradually, and he restarted the steroid tapering and was weaned from oxygen therapy on the 102nd day.
Fig. 2.
The figure shows the clinical course in Case 2. Oxygen at 1–3 L/min was administered using a nasal cannula. DEX; Dexamethasone, mPSL; Methylprednisolone, BM; Beclomethasone, PSL; Prednisolone. The images are indicated as representative chest X-rays and CT images.
2.3. Case 3: male, 59 years old
The patient had undergone CABG for myocardial infarction at 47 years of age and was also treated for hypertension, hyperlipidemia, and type 2 diabetic mellitus; moreover, he was a past smoker (27 pack-years). He noticed fever and dyspnea, was admitted to an emergency hospital and was diagnosed with COVID-19 (the SARS-CoV-2 PCR test result was positive). The patient had severe hypoxemia and was intubated, and intermittent positive-pressure ventilation (IPPV) was started. He was treated with dexamethasone (6 mg/day, for ten days) and remdesivir (200 mg/day on the first day, 100 mg/day from the second to the fifth day). Additionally, he was administered sulbactam/ampicillin because of aspiration pneumonia (from the sixth to the 12th day). The patient's condition improved, and he was weaned from IPPV on the 9th day after the onset of COVID-19. He was managed using high-flow nasal cannula oxygen therapy (HFNC) from the 10th day to the 14th day, and his radiological changes and respiratory failure improved (Fig. 3 and E3). He was transferred to our hospital on the 17th day. Physical examination revealed clear consciousness, low-grade fever (37.2 °C), and hypoxemia (SpO2 91% on nasal cannula O2 4 L/min). However, after transfer, the hypoxemia deteriorated, and we performed further examinations. The laboratory test indicated CRP elevation (4.84 mg/dl) and extremely high levels of ILD markers (KL-6: 4680 U/L, SP-D: 482 U/L) (Table 1). On CT imaging, compared to that on the 11th day, bilateral consolidation had respread, and traction bronchiectasis and irregular reticulation were observed (Fig. 3 and E3). From these findings, we diagnosed post-COVID-19 ILD and started corticosteroid pulse therapy (at a dose of 500 mg of methylprednisolone every 12 hours, six times in total). Following the initial therapy, corticosteroid therapy was continued (at a dose of 1.0 mg/kg/d of prednisolone, also see in Fig. 3 ). The patient was treated using HFNC from the 21st day to the 38th day. Although severe hypoxemia was prolonged, he was weaned from HFNC and continued rehabilitation. The hypoxemia and radiological changes gradually improved, and the corticosteroid dose was tapered. The patient was discharged on the 61st day after the onset of COVID-19.
Fig. 3.
The figure shows the clinical course in Case 3. Oxygen at 1–4 L/min was administered using a nasal cannula, 5–7 L/min was administered using a simple face mask, and 7–10 L/min was administered using a nonrebreather face mask. IPPV; Intermittent positive pressure ventilation, HFNC; High-flow nasal cannula, DEX; Dexamethasone, mPSL; Methylprednisolone, PSL; Prednisolone, S/A; Sulbactam/ampicillin (the dose was not confirmed), AZM; Azithromycin. The images are indicated as representative chest X-rays and CT images.
3. Discussion
These three patients showed profound hypoxemia after COVID-19; at that time, CT images indicated pulmonary fibrosis, and laboratory tests revealed high levels of KL-6, suggesting ILD secondary to COVID-19. It is known that the presence of ILD confers an increased risk of COVID-19 mortality and that COVID-19 may also cause acute exacerbation of ILD [9]. The present cases had no evidence of ILD before the incidence of COVID-19, and no upregulation of autoantibodies associated with collagen disease or vasculitis was observed (Table 1). We excluded drug-induced lung injury by the drug history.
In general, CT images in patients recovering from COVID-19 infection are known to be divided into four stages: the early stage (0–4 days), progressive stage (5–8 days), peak stage (9–13 days), and absorption stage (>14 days), although in cases with a poor prognosis, the radiological changes are not resolved and even spread [10] and, in severe COVID-19 cases, the improvement of CT images needs more time than those in nonsevere cases do [11]. Compared with the patients in these previous reports, in our patients, the severity of hypoxemia was diphasic, and the peak stage was also more delayed and prolonged.
Pulmonary fibrosis secondary to COVID-19 is thought to be caused by an abnormal repair process after lung cell injury caused by SARS-CoV-2. The abnormal repair process in pulmonary fibrosis might be caused by mechanical stress, prolonged exposure to hypoxemia or hyperoxia, thromboembolism, immunological dysregulation (ex. dysregulation of EGFR, IL-6, and TGF-β), etc. [5,12,13]. According to these reports, preventing ventilator-associated lung injury, inappropriate and excessive supplemental oxygen, and thromboembolism by using anticoagulants might decrease the risk of post-COVID-19 ILD.
The respiratory symptoms and imaging abnormalities caused by COVID-19 may be prolonged [4,14,15]. Imaging abnormalities are improved without specific treatments in some cases [14], and corticosteroid therapy has the potential to improve respiratory symptoms and radiological changes in persistent post-COVID-19 ILD [7,16]. Although the regimen of corticosteroid therapy for post-COVID-19 ILD has not been established yet, in some case reports, a systemic corticosteroid therapy with prednisolone at a dose of 0.5–1.0 mg/kg/day with or without corticosteroid pulse therapy was performed as an initial therapy [17,18]. Recently, baricitinib and tocilizumab monotherapy or combined therapy was also found to have the potential to improve ILD secondary to COVID-19 [8].
In our patients, high-dose corticosteroid therapy prevented the worsening of post-COVID-19 ILD and improved respiratory failure, and the steroid dose was reduced steadily. We plan to taper off the steroid if there are no signs of worsening of ILD.
We administered corticosteroid pulse therapy in all three cases. Corticosteroid pulse therapy has strong potential to suppress the inflammatory response and promote faster clinical recovery from symptoms than oral therapy through not only genomic effects but also nongenomic glucocorticoid activities [19,20]. When comparing our three patients, patients 1 and 3 had similar clinical features: acute and prolonged hypoxemia, high KL-6 levels, and CT images indicating traction bronchiectasis and irregular reticulation without macrocystic honeycombing (Figure E1 and E3). The radiological features were consistent with nonspecific interstitial pneumonia patterns with acute exacerbation of ILD, so we administered corticosteroid pulse therapy based on the treatment of acute exacerbation of ILD [21]. On the other hand, patient 2 had relatively short-term and mild hypoxemia and a lower KL-6 level. CT imaging showed mainly organizing pneumonia patterns (Figure E2) [22]. However, the patient had high-grade fever, dyspnea, and a high CRP level; therefore, we administered corticosteroid pulse therapy with the expectation of early systemic inflammatory improvement. An adaption of corticosteroid pulse therapy needs to be further discussed to avoid complications associated with exposure to a large amount of corticosteroids. Serological markers and radiological interstitial lung patterns might be treatment strategies for pulmonary fibrosis secondary to COVID-19.
Interestingly, all three patients had diabetes mellitus (DM). DM has the potential to be associated with ILD [23], and impaired glucose tolerance might be one of the risk factors for pulmonary fibrosis secondary to COVID-19. To develop treatments for fibrosis secondary to COVID-19, we need further studies, especially regarding the molecular mechanisms.
Authorship statement
Kiyoshi Uemasu: Writing- Original draft, Review and editing, Obtaining the informed consent of the patients described in the case report, Review of patient's medical file.
Yuto Yasuda: Writing - Review & Editing & Obtaining the informed consent of the patient described in the case report.
Yutaka Hirayama: Writing - Review & Editing.
Soichi Arasawa: Writing - Review & Editing.
Daisuke Iwashima: Writing - Review & Editing.
Ken-ichi Takahashi: Writing - Review & Editing, Supervision.
Declaration of competing interest
All the authors have no conflict of interest to declare.
Patient consent statement
This work was performed following local law and ethical considerations. The patients described in this article have provided informed consent for the publication of their cases.
Acknowledgments
We are grateful to all Kishiwada city hospital staff associated with COVID-19 treatment.
Also, we would like to thank the patients described in this article.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jiac.2021.11.010.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
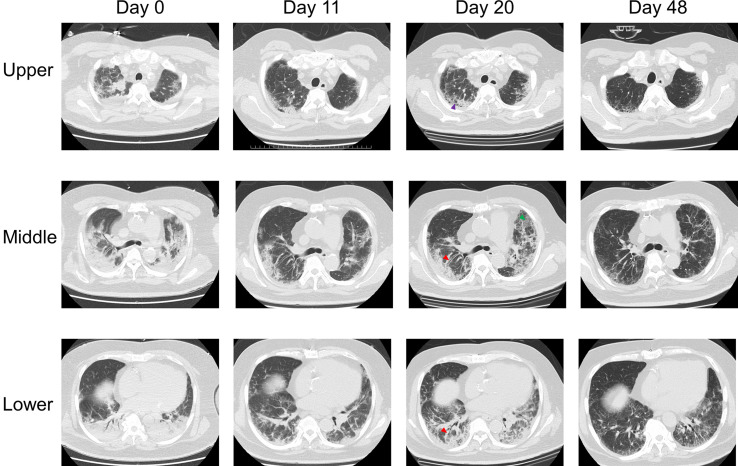
Figure E1.
The figure shows the CT changes in Case 1. Eight days after COVID-19 onset, bilateral ground-glass attenuation was observed, consistent with the characteristics of the images in the progressive stage of COVID-19 pneumonia. On the 28th day, bilateral consolidation, traction bronchiectasis (red arrow), irregular reticulation (purple arrow), and subpleural lines (orange arrow) without macrocystic honeycombing were observed, suggesting fibrotic nonspecific interstitial pneumonia. On the 68th day, both the consolidation and interstitial changes were improved.
Figure E2.
The figure shows the CT changes in Case 2. Nineteen days after COVID-19 onset, bilateral and patchy consolidations (yellow arrows) and some consolidations with sparing of subpleural space (gray arrows), such as those associated with postinfectious organizing pneumonia, were observed. On the 54th day, the consolidation and ground-glass attenuation were shrinking.
Figure E3.
The figure shows the CT changes in Case 3. On the day of COVID-19 onset, bilateral consolidation was observed to be broadly consistent with severe COVID-19 pneumonia. On the 11th day, the consolidation had shrunk. On the 20th day, consolidation and broad fibrotic changes, traction bronchiectasis (red arrow), and irregular reticulation (purple arrow) without macrocystic honeycombing were observed, suggesting fibrotic nonspecific interstitial pneumonia. On the 48th day, both the consolidation and interstitial changes were improved, although fibrotic changes remained.
References
- 1.Jamil S., Mark N., Carlos G., Cruz C.S.D., Gross J.E., Pasnick S. Diagnosis and management of COVID-19 disease. Am J Respir Crit Care Med. 2020;201(10):P19–P20. doi: 10.1164/rccm.2020C1. [DOI] [PubMed] [Google Scholar]
- 2.Okuhama A., Ishikane M., Hotta M., Sato L., Akiyama Y., Morioka S., et al. Clinical and radiological findings of silent hypoxia among COVID-19 patients. J Infect Chemother. 2021;27(10):1536–1538. doi: 10.1016/j.jiac.2021.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu X., Liu X., Zhou Y., Yu H., Li R., Zhan Q., et al. 3-month, 6-month, 9-month, and 12-month respiratory outcomes in patients following COVID-19-related hospitalisation: a prospective study. The Lancet Respiratory medicine. 2021;9(7):747–754. doi: 10.1016/S2213-2600(21)00174-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ojo A.S., Balogun S.A., Williams O.T., Ojo O.S. Pulmonary fibrosis in COVID-19 survivors: predictive factors and risk reduction strategies. Pulm Med. 2020;2020:6175964. doi: 10.1155/2020/6175964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McDonald L.T. Healing after COVID-19: are survivors at risk for pulmonary fibrosis? Am J Physiol Lung Cell Mol Physiol. 2021;320(2):L257–l265. doi: 10.1152/ajplung.00238.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Doglioni C., Ravaglia C., Chilosi M., Rossi G., Dubini A., Pedica F., et al. Covid-19 interstitial pneumonia: histological and immunohistochemical features on cryobiopsies. Respiration. 2021;100:488–498. doi: 10.1159/000514822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Myall K.J., Mukherjee B., Castanheira A.M., Lam J.L., Benedetti G., Mak S.M., et al. Persistent post-COVID-19 interstitial lung disease. An observational study of corticosteroid treatment. Ann Am Thorac Soc. 2021;18(5):799–806. doi: 10.1513/AnnalsATS.202008-1002OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rosas J., Liaño F.P., Cantó M.L., Barea J.M.C., Beser A.R., JTA Rabasa, et al. Experience with the use of baricitinib and tocilizumab monotherapy or combined, in patients with interstitial pneumonia secondary to coronavirus COVID19: a real-world study. Reumatol Clínica. 2020;S1699–1258X(1620) doi: 10.1016/j.reuma.2020.10.009. 30271-30270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Drake T.M., Docherty A.B., Harrison E.M., Quint J.K., Adamali H., Agnew S., et al. Outcome of hospitalization for COVID-19 in patients with interstitial lung disease. An international multicenter study. Am J Respir Crit Care Med. 2020;202(12):1656–1665. doi: 10.1164/rccm.202007-2794OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pan F., Ye T., Sun P., Gui S., Liang B., Li L., et al. Time course of lung changes at chest CT during recovery from coronavirus disease 2019 (COVID-19) Radiology. 2020;295(3):715–721. doi: 10.1148/radiol.2020200370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yun Y., Wang Y., Hao Y., Xu L., Cai Q. The time course of chest CT lung changes in COVID-19 patients from onset to discharge. Eur J Radiol Open. 2021:8. doi: 10.1016/j.ejro.2020.100305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ferrara F., Granata G., Pelliccia C., La Porta R., Vitiello A. The added value of pirfenidone to fight inflammation and fibrotic state induced by SARS-CoV-2 : anti-inflammatory and anti-fibrotic therapy could solve the lung complications of the infection? Eur J Clin Pharmacol. 2020;76(11):1615–1618. doi: 10.1007/s00228-020-02947-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ambardar S.R., Hightower S.L., Huprikar N.A., Chung K.K., Singhal A., Collen J.F. Post-COVID-19 pulmonary fibrosis: novel sequelae of the current pandemic. J Clin Med. 2021;10(11):2452. doi: 10.3390/jcm10112452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mandal S., Barnett J., Brill S.E., Brown J.S., Denneny E.K., Hare S.S., et al. Long-COVID': a cross-sectional study of persisting symptoms, biomarker and imaging abnormalities following hospitalisation for COVID-19. Thorax. 2021;76(4):396–398. doi: 10.1136/thoraxjnl-2020-215818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miyazato Y., Morioka S., Tsuzuki S., Akashi M., Osanai Y., Tanaka K., et al. Prolonged and late-onset symptoms of coronavirus disease 2019. Open Forum Infect Dis. 2020;7(11):ofaa507. doi: 10.1093/ofid/ofaa507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.George P.M., Barratt S.L., Condliffe R., Desai S.R., Devaraj A., Forrest I., et al. Respiratory follow-up of patients with COVID-19 pneumonia. Thorax. 2020;75(11):1009–1016. doi: 10.1136/thoraxjnl-2020-215314. [DOI] [PubMed] [Google Scholar]
- 17.Vadász I., Husain-Syed F., Dorfmüller P., Roller F.C., Tello K., Hecker M., et al. Severe organising pneumonia following COVID-19. Thorax. 2021;76(2):201. doi: 10.1136/thoraxjnl-2020-216088. [DOI] [PubMed] [Google Scholar]
- 18.Kostorz-Nosal S., Jastrzębski D., Chyra M., Kubicki P., Zieliński M., Ziora D. A prolonged steroid therapy may be beneficial in some patients after the COVID-19 pneumonia. Eur Clin Respir J. 2021;8(1) doi: 10.1080/20018525.2021.1945186. 1945186-1945186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sinha A., Bagga A. Pulse steroid therapy. Indian J Pediatr. 2008;75(10):1057–1066. doi: 10.1007/s12098-008-0210-7. [DOI] [PubMed] [Google Scholar]
- 20.Beck I.M.E., Vanden Berghe W., Vermeulen L., Yamamoto K.R., Haegeman G., De Bosscher K. Crosstalk in inflammation: the interplay of glucocorticoid receptor-based mechanisms and kinases and phosphatases. Endocr Rev. 2009;30(7):830–882. doi: 10.1210/er.2009-0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Song J.W., Hong S.B., Lim C.M., Koh Y., Kim D.S. Acute exacerbation of idiopathic pulmonary fibrosis: incidence, risk factors and outcome. Eur Respir J. 2011;37(2):356–363. doi: 10.1183/09031936.00159709. [DOI] [PubMed] [Google Scholar]
- 22.Mueller-Mang C., Grosse C., Schmid K., Stiebellehner L., Bankier A.A. What every radiologist should know about idiopathic interstitial pneumonias. Radiographics. 2007;27(3):595–615. doi: 10.1148/rg.273065130. [DOI] [PubMed] [Google Scholar]
- 23.Rajasurya V., Gunasekaran K., Surani S. Interstitial lung disease and diabetes. World J Diabetes. 2020;11(8):351–357. doi: 10.4239/wjd.v11.i8.351. [DOI] [PMC free article] [PubMed] [Google Scholar]