Abstract
Background
With increasing number of clinical trials relating to fecal microbiota transplantation (FMT), it is crucial to identify and recruit long‐term, healthy, and regular fecal donors.
Objective
We aimed to report the outcomes of screening and recruitment of fecal donors for FMT.
Methods
Potential donors were recruited via advertisement through internal mass emails at a university. They were required to undergo a pre‐screening telephone interview, a detailed questionnaire, followed by blood and stool investigations.
Results
From January 2017 to December 2020, 119 potential donors were assessed with 75 failed pre‐screening. Reasons for failure included: inability to come back for regular and long‐term donation (n = 19), high body mass index (n = 17), underlying chronic illness or on long‐term medications (n = 11), being healthcare professionals (n = 10), use of antibiotics within 3 months (n = 5) and others (n = 13). Forty‐four donors completed questionnaires and 11 did not fulfill the clinical criteria. Of the remaining 33 potential donors who had stool and blood tests, 21 failed stool investigations (19 extended‐spectrum beta‐lactamase [ESBL] organisms, one Clostridioides difficile, one C. difficile plus Methicillin Resistant Staphylococcus aureus), one failed blood tests (high serum alkaline phosphatase level), one required long‐term medication and nine withdrew consent and/or lost to follow‐up. In total, only one out of 119 (0.8%) potential donors was successfully recruited as a regular donor.
Conclusion
There was a high failure rate in donor screening for FMT. Main reasons for screening failure included high prevalence of positive ESBL organisms in stool and failed commitment to regular stool donation.
Keywords: COVID‐19 pandemic, donor recruitment, ESBL organisms, extended‐spectrum beta‐lactamase, fecal donor, fecal microbiota transplantation
Key summary.
Summarize the established knowledge on this subject
With the increasing number of clinical trials and practice of fecal microbiota transplantation (FMT), there is a huge demand of healthy fecal donors
What are the significant and/or new findings of this study?
The failure rate in donor screening for FMT is extremely high
High prevalence of positive extended‐spectrum beta‐lactamase organisms in stool and failed commitment to regular stool donation contribute to the high failure rate in donor recruitment for FMT
INTRODUCTION
Fecal microbiota transplantation (FMT) is defined as infusion of stool from healthy donors to rectify the recipient's intestinal microbial community by introducing micro‐organisms associated with a “healthy” state to normalize microbiota composition and function. Currently, FMT is recognized as an effective and approved therapy for recurrent or refractory Clostridioides difficile infection (CDI) with a cure rate of around 90% in patients who did not respond to antibiotic therapy. 1 , 2 , 3 There is a growing body of literature exploring the efficacy of FMT in treating other gastrointestinal diseases, including inflammatory bowel disease (IBD), 4 irritable bowel syndrome, 5 chronic constipation 6 and pouchitis, 7 etc. Moreover, FMT has been used for treatment of diseases beyond the gastrointestinal tract, including metabolic diseases like diabetes mellitus (DM), obesity, neurological disorders like Parkinson's disease and autism etc. 8 , 9 , 10 , 11 Thus, there is a huge demand for FMT donors.
With the increasing demand for FMT in daily practice and clinical trials, a recent international consensus on stool banking for FMT has been developed. 12 Previous systematic review and meta‐analysis of 168 studies showed that there was heterogeneity in FMT donor selection and less than 50% of studies screened donors for transmittable pathogens, including Giardia, Isospora, Cyclospora, Yersinia, Escherichia coli O157, Aeromonas, vancomycin resistant Enterococcus, meticillin resistant Staphylococcus aureus (MRSA), Helicobacter pylori, rotavirus, norovirus, hepatitis E virus, cytomegalovirus (CMV), human T‐lymphotropic virus and Epstein–Barr virus. 13 In June 2019, the Food and Drug Administration (FDA) of the United States has issued a safety alert regarding the use of FMT in immunocompromised patients, as two immunocompromised adults who received investigational FMT developed invasive infections caused by extended‐spectrum beta‐lactamase (ESBL)‐producing E. coli, with one case of fatality. 14 Therefore, it is crucial to develop a standardized screening process for FMT donors to ensure safety.
Since the Coronavirus Disease 2019 (COVID‐19) pandemic from December 2019, there has been concerns about the risk of fecal transmission of SARS‐CoV‐2 virus. 15 In view of this potential risk, the FDA has issued another alert on additional screening procedures for COVID‐19 symptoms and stool for SARS‐CoV‐2 RNA before donation in April 2020. 16 We aimed to report the screening procedure, characteristics, and outcomes of our FMT donors in Hong Kong.
MATERIALS AND METHODS
Potential donors were identified mainly by personal referral from staff or from advertisement through the internal mass email system of the Chinese University of Hong Kong, Hong Kong. There were three parts of the screening process. Part 1 is a prescreening by phone interview for potential donors to assess for preliminary eligibility. During pre‐screening, questions asked included body mass index (BMI), chronic illnesses, any regular usage of medication, any usage of antibiotics within three months, family history of colon cancer or IBD in first‐degree relatives, any regular contact with patients or clinical specimen and the availability for regular stool donation in the long‐term (Table S1). Part 2 is a questionnaire‐based assessment. All potential donors needed to complete two questionnaires after they passed the prescreening procedures. Potential donors' information on risk of infectious disease, bowel habits and history of gastrointestinal disease, past medical history and medications used, family history, travel history and social history were collected via questionnaires (Table 1). All procedures were conducted in the Center for Gut Microbiota Research located in the Prince of Wales Hospital, The Chinese University of Hong Kong.
TABLE 1.
Screening criteria of potential fecal donors
| Potential donors will be excluded from donation if they: | |
|---|---|
| Basic information |
|
| |
| |
| Risk of infectious diseases |
|
| |
| |
| |
| |
| |
| |
| |
| |
| |
| Bowel habits and bowel diseases |
|
| |
| |
| |
| |
| |
| Medical history and medications |
|
| |
| |
| |
| |
| |
| |
| Travel history |
|
| |
| |
| |
| Others |
|
| |
| |
| |
| |
Abbreviations: HTLV, human T‐cell lymphotropic virus; IBD, inflammatory bowel disease.
Donor inclusion criteria were adults between 18 and 50 years old, with normal body weight (18 < BMI < 23), and without any chronic diseases, which is defined as any condition that last 1 year or more and require ongoing medical attention and/or limit activities of daily living or both 17 or significant past medical history. Donors should not take any long‐term medications for the treatment of underlying chronic medical illnesses. These include history of malaria, trypanosomiasis, intestinal infestation (worms, parasites), systemic autoimmunity diseases, atopic diseases, cardiovascular or metabolic syndrome, neurological diseases, chronic pain syndromes, congenital, chronic liver disease or any malignancy; history of depression, bipolar disorder, schizophrenia or delusional disorder, eating disorder or other psychiatric illnesses; took antibiotics or probiotics within 3 months; took proton pump inhibitor or drugs for gastric problems regularly; took experimental medicine or experimental vaccine within 6 months; received live vaccine within 6 months and took immunosuppressive agents or drugs including growth hormone. A 3‐month washout period from last antibiotic use was chosen as most healthy individuals can achieve gut microbiome recovery 3 months after antibiotic treatment. A study in healthy individuals receiving antibiotics showed that the gut microbiome of majority of the individuals would recover to the baseline abundance level at 90 days. 18 The upper age limit was chosen as such due to the increasing probability of chronic diseases or malignancy over 50 years. In addition, both underweight (BMI < 18) and overweight (BMI > 23) have been reported to affect the composition of the gut microbiota. 19 , 20 Applicants were excluded if they had any risk factors for infectious diseases which might be transmitted to recipients. Potential donors who had irregular bowel habits, gastrointestinal diseases, past or concurrent medical history and medication, family history of IBD or colorectal cancer, and recent travel history were excluded. The details of screening criteria of potential fecal donors are shown in Table 1. There was no dietary restriction for potential donors except they were reminded to avoid raw foods and raw eggs to reduce the risk of food‐borne infections such as parasitic infestations. Strict vegetarians were excluded from FMT donation, as it is possible that a specific diet might lead to nutrition deficiency 21 and lower bacteria diversity or altered microbiome profile 22 which might impact the efficacy of FMT. Dietary supplements such as vitamins were allowed.
Stool and blood screening tests (Table 2) would then be arranged for the eligible potential donors to screen for any infectious agents, including norovirus, rotavirus, bacterial infection, H. pylori, C. difficile, drug‐resistant organisms and parasites. The potential donors would be invited to come to Prince of Wales Hospital for stool donation 5 days per week for 3 weeks consecutively if they passed all the above screening tests. Donors were required to fill in additional health declaration form (symptoms of cough, fever, diarrhea, any newly diagnosed diseases and hospitalization in the last 3 weeks) before each donation. All stool samples would then be processed and stored at −80°C freezer. All stool samples were then quarantined for 3 weeks and donors would be contacted at 3 weeks' interval to ensure they do not have any clinical symptoms in the past weeks. The quarantined stool samples from each donor would be pooled together to repeat another round of infectious agents screening. The batch of donor stools would only be released for clinical use if the donor stool passed the repeated testing and the donor passed the health declaration assessment. If a batch of donor stool failed the above tests, all samples stored from the same donor in the corresponding batch would be discarded and further stool donation would be suspended for several months according to physicians' discretion. The same stool testing procedures would then be done before the applicant can be reinstated as a healthy donor. All recruited donors are required to have blood and stool tests every year if they continue to be donors after 1 year. This is to ensure the health of the donors and the safety of the FMT stool bank. If the donor develops a new medical condition during follow‐up, he/she will be suspended from stool donation according to physicians' discretion and the samples will be withdrawn from the stool bank. The whole donor screening procedure is shown in Figure 1a.
TABLE 2.
Blood and stool screening tests for potential FMT donors
| Blood tests | Stool tests |
|---|---|
|
|
|
|
|
|
| |
|
|
| |
|
|
| |
| |
| |
|
|
| |
|
|
|
Abbreviations: Anti HBc, hepatitis B core antibody; CRE, carbapenem‐resistant Enterobacteriaceae; ESBL, extended‐spectrum beta‐lactamases; FMT, fecal microbiota transplantation; GDH, glutamate dehydrogenase; HBs Ag, Hepatitis B surface antigen; HTLV, human T‐cell lymphotropic virus; IgM, immunoglobulin M; MDRA, multidrug‐resistant Acinetobacter; MRSA, meticillin resistant Staphylococcus aureus; PCR, polymerase chain reaction; RNA, ribonucleic acid; VDRL, Venereal Disease Research Laboratory; VRE, vancomycin resistant Enterococcus.
FIGURE 1.
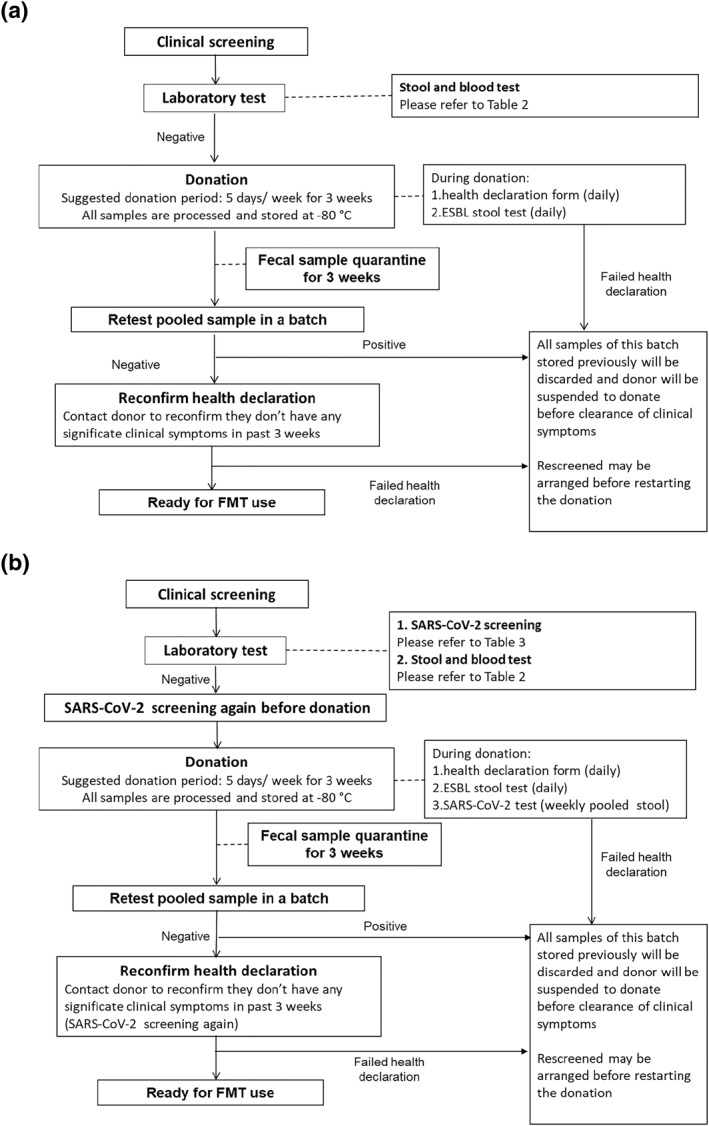
Screening procedure flow chart (a: before COVID‐19 era; b: after COVID‐19 era)
Since the FDA alert on ESBL E. coli septicemia after FMT in June 2019, we have added routine stool test for ESBL‐producing Enterobacteriaceae in our screening protocol. All potential donors would have stool screened for ESBL‐producing organisms after passing the questionnaires in part 1 and 2. Those who were screened positive for ESBL would be excluded. For those who were negative for ESBL at screening, they would have an additional ESBL screening on each day of stool donation. Only those with consecutive negative ESBL results would be used for FMT procedure (Figure 1a).
Besides, additional screening measures for SARS‐CoV‐2 (Table 3) were implemented in July 2020 to further enhance the safety. All potential donors were assessed for COVID‐19 symptoms (upper respiratory tract symptoms and fever), travel history and close contact with confirmed or suspected COVID‐19 cases within 30 days by phone in addition to the routine pre‐screening in part 1. After passing the pre‐screening phone interview and the questionnaires in part 2, they were invited to undergo deep throat saliva (DTS) and stool for SARS‐CoV‐2 testing. 23 Before the start of donation sessions, all potential donors were required to repeat DTS and stool for SARS‐CoV‐2 testing. The donated fecal samples would then be stored at −80°C freezer and quarantined for 3 weeks. A weekly pooled stool tests for SARS‐CoV‐2 would be performed. After the 3‐week quarantine period, potential donors were required to complete a post‐donation health record and screened for any COVID‐19 symptoms or close contact with COVID‐19 patients during the 3‐week donation period. DTS and stool for SARS‐CoV‐2 testing were also repeated. If any of the above tests failed, the whole batch of donor stool from the same donor would be discarded (Figure 1b).
TABLE 3.
Evaluation criteria for COVID‐19
| Symptoms | No cough, chills, sore throat, and fever within 30 days |
| Travel history | Stayed in Hong Kong for past 30 days |
| Contact with cases | No contact with confirmed or suspected cases within 30 days |
| Deep throat saliva test for SARS‐CoV‐2 (RT‐PCR) | |
| Stool test for SARS‐CoV‐2 (RT‐PCR) | |
Abbreviation: RT‐PCR, reverse transcription‐polymerase chain reaction.
RESULTS
A total of 119 potential donors were assessed for eligibility from January 2017 to December 2020 (Figure 2). There were 49 males (41%) with an average age of 29 years old (range 17–60). A total of 75 subjects failed prescreening. The commonest reasons for failing pre‐screening included: 19 (16.0%) were not available to come to hospital for long‐term regular stool donation, 17 (14.3%) failed to meet the BMI criteria (Four had BMI < 18, 13 had BMI > 23), 11 (9.2%) had long‐term medications or chronic diseases and 10 (8.4%) had regular close contact with patients or clinical specimens (Figure 2).
FIGURE 2.
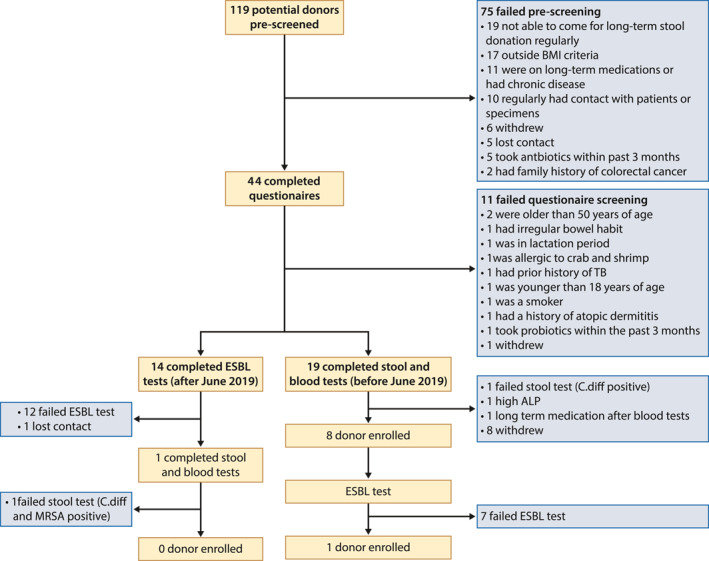
Donor screening outcomes
A total of 44 eligible potential donors completed the questionnaires, with 11 subjects further excluded from donation, leaving only 33 potential FMT donors. Reasons for exclusion were listed in Figure 2.
Since the FDA alert on post‐FMT ESBL E. coli septicemia in June 2019, we have added routine stool test for ESBL in our screening protocol. The remaining 33 subjects were divided into two cohorts, namely those before routine ESBL screening (n = 19) and those after (n = 14). Before June 2019, 19 potential donors who passed questionnaires were arranged to have stool and blood tests: one subject failed due to testing positive for C. difficile, one subject failed due to high serum alkaline phosphatase level, and one subject was started on long‐term medications after blood and stool tests. Sixteen potential donors passed the stool and blood screening procedures, but half of them failed to return to donate stool regularly. Finally, the remaining eight donors (Table S2) were recruited with regular donation of fecal samples before June 2019.
After June 2019, 14 potential donors who passed questionnaires were arranged to have stool test for ESBL‐organisms: 12 subjects (85.7%) failed due to positive results for ESBL organisms; one subject lost contact with the research team. The only one remaining donor who passed the stool for ESBL test failed to become an FMT donor eventually due to positive results for C. difficile and MRSA upon repeated testing. ESBL testing was also arranged to all the previously recruited eight donors (D1, D4, D8, D9, D15, D16, D18, D19). Seven out of eight donors (87.5%) were found to be positive for ESBL organisms. We retrospectively performed random testing of ESBL‐producing organisms in any one of the previously donated stool samples received during the first and third week of each month from each donor. The overall ESBL positivity rate in the donated stool samples was 55.4% and ranged from 0% to 100% in each donor (Figure 3a).
FIGURE 3.
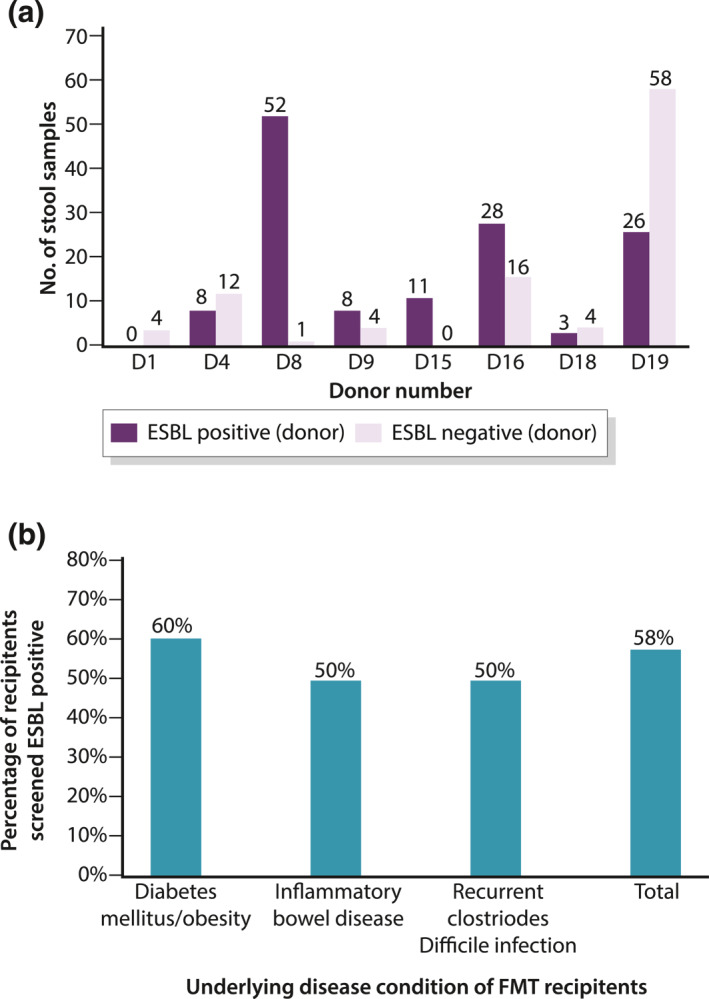
Extended‐spectrum beta‐lactamase (ESBL) status of fecal microbiota transplantation (FMT) donors and recipients. (a) ESBL test results of eight recruited donors after June 2019. (b) Percentage of FMT recipients screened ESBL positive
We also arranged ESBL‐organism testing for recipients, who were selected for FMT according to three indications that is a randomized controlled trial for DM or obesity, a pilot study for IBD and recurrent C. difficile. The percentage of recipients screened to be ESBL positive was 60%, 50% and 50% in the three groups respectively (Figure 3b).
Since the COVID‐19 pandemic in December 2019, we further implemented screening measures for SARS‐CoV‐2 to ensure the safety of recipients starting from July 2020. After COVID‐19 pandemic, we screened 36 subjects and 35 of them failed in prescreening and questionnaires. The reasons of failure in these 35 potential donors included: inability to come back for regular and long‐term donation (n = 9), BMI below 18 or over 23 (n = 6), lost contact (n = 5), withdrawal from study (n = 5), intake of antibiotics within 3 months (n = 4), age over 50 (n = 2), on long‐term medication (n = 2), being healthcare professionals (n = 1) and use of probiotics within 3 months (n = 1). The remaining one potential donor was tested negative for SARS‐CoV‐2 in both DTS and stool, but failed stool screening for MRSA.
After the stringent screening process, only one (0.8%) out of the initial 119 applicants was deemed suitable for donating healthy stool samples. This donor was a Chinese man aged 21 with a BMI of 22.8.
DISCUSSION
Our study has shown that recruitment of FMT donors is extremely challenging during the time of high prevalence of ESBL positivity and the COVID‐19 pandemic. With the addition of stringent screening for ESBL‐producing Enterobacteriaceae and SARS‐CoV‐2 amongst FMT donors, the successful FMT donor recruitment rate dropped from 6.7% to 0.8%. Our screening success rate was lower than those previously reported, even before the addition of ESBL‐producing Enterobacteriaceae testing. 24 , 25 A large proportion (86%) of healthy population failed stool testing due to positivity for ESBL‐producing Enterobacteriaceae. Besides, a high commitment of long‐term donation is also one of the major barriers for FMT donations in Hong Kong. Most of potential donors failed in the pre‐screening phase as they are unable to commit to regular donation over a prolonged period of time, accounting for 16% of screen failure in the pre‐screening stage. This was also reported by Sudarshan Paramsothy et al. in which their recruitment rate of fecal donors was only 10% as most of the subjects failed to meet the high commitment requirement of long‐term stool donation. 24
The donor selection criteria in Hong Kong are largely similar to those reported by international societies 12 , 26 , 27 , 28 on risk assessment for infectious diseases, donors' medical history and medications used. However, there are some differences in the practice in Hong Kong. We also excluded subjects who had gastrointestinal surgery, those with a history of cardiovascular diseases, chronic pain syndromes, congenital or chronic liver disease, pregnant or lactating women, current/past smoker or current heavy drinkers, strict vegetarians, and subjects who had regular contact with patients or clinical specimens or animals; the above was not mentioned in the previous consensus by Cammarota et al. While some stool banks 12 , 26 , 28 only excluded subjects with a BMI of >30 kg/m2, we had a tighter limit and would exclude those with a BMI < 18 or >23 kg/m2 according to the definition of overweight in Asia. 29 , 30 In terms of blood and stool screening, given the high prevalence of H. pylori in Hong Kong, we performed both serological assessment of H. pylori and stool testing for HP antigen. We also checked for human T‐Lymphotropic virus during donor screening while the previous consensus by Cammarota et al. did not.
Before the FDA report on post‐FMT ESBL E. coli septicemia in June 2019, majority of the stool banks did not check for ESBL‐producing organisms in stool. 31 However, fecal ESBL‐producing Enterobacteriaceae (ESBL‐E) carriage is currently a global problem, which can potentially lead to widespread infections with limited therapeutic options. A recent systematic review revealed that the global pooled prevalence of ESBL E. coli intestinal carriage in community was 16.5%, with the highest reported prevalence rate in South‐East Asia (27.5%), followed by Western Pacific (24.5%). 32 , 33 A recent community study in Hong Kong showed that the prevalence of ESBL‐E carriage was 52.8%. 34 Thus, with the additional ESBL‐producing organisms screening, 12 out of 13 potential donors (92.3%) were excluded due to positive results. In the eight previously recruited donors, seven (87.5%) were tested positive for ESBL organisms for at least once, indicating a very high positivity rate amongst our potential donors. This is on the contrary to what have been reported in another large stool bank in North America, where none of the 571 potential stool donors were tested positive for ESBL‐producing organisms in stool. 28 The high rate of ESBL‐E fecal colonization in Hong Kong can be explained by several factors, including a densely packed population, high turnover of travelers, geographical close proximity with mainland China and Southeast Asia, and the consumption of raw vegetables, poultry and retail chicken meat. 35 , 36 , 37
As Hong Kong is in the area with high ESBL‐E carriage, this is a significant barrier for donor recruitment. To overcome this, we now provide dietary suggestions to potential donors who are due for screening and donation to reduce the rate of exposure to ESBL‐producing organisms via specific food consumption. 38 We also exclude travelers from certain high‐risk areas, including India, Egypt and Thailand. 39 Donors with consumption of two or more courses of antibiotics within 6 months were also excluded from stool donation so as to lower the risk of ESBL‐E carriage. 34 Besides, another potential solution to the high failure rate due to ESBL‐E positivity is to apply the concept of patient‐donor CMV matching in the context of hematopoietic stem cell transplantation (Table 4). There are three main ESBL gene families, namely bla TEM, bla SHV, and bla CTX−M. The bla CTX−M type can be further divided into five subgroups based on their amino acid sequence: groups 1, 2, 8, 9 and 25. The commonest ESBL gene isolated in Hong Kong was bla CTX−M with group 9 (68.1%) and group 1 (25.8%). 34 Further studies should be conducted to explore the feasibility and safety to transfer FMT materials from donor to recipient who carry the same group of ESBL‐producing Enterobacteriaceae.
TABLE 4.
Key actions to improve recruitment rate for FMT donors
|
|
|
|
|
Abbreviations: ESBL, extended‐spectrum beta‐lactamases; FMT, fecal microbiota transplantation.
The emergence of the COVID‐19 pandemic created another barrier for FMT donor recruitment. Fecal microbiota transplantation is a potentially life‐saving procedure for patients with recurrent or refractory C. difficile infection. Recruitment of FMT donors should not be interrupted despite COVID‐19. However, with more than 190 million people worldwide being infected, 40 enhanced screening of FMT donors for SARS‐CoV‐2 infection has been advocated by various international expert groups. 41 , 42 , 43 A specific validated molecular testing for SARS‐CoV‐2 should be done before FMT donation. We have previously validated the protocol for stool SARS‐CoV‐2 viral quantification using reverse transcription quantitative polymerase chain reaction technique. 23 However, there are case reports of asymptomatic COVID‐19 subjects with positive SARS‐CoV‐2 RNA in stool. 44 Prolonged shedding of SARS‐CoV‐2 virus in feces after negative respiratory samples have also been reported 45 , 46 , 47 , 48 and the level of viral RNA presented in stool can fluctuate around the margin of laboratory detection. Thus, in our screening protocol, all potential donors were required to undergo stool and DTS tests for SARS‐CoV‐2 at multiple timepoints (before start of the 3‐week donation cycle, once a week during the 3‐week donation cycle, once after completion of the 3‐week donation cycle). This is to ensure the safety of the FMT recipients. So far, there has been no donor diagnosed with confirmed SARS‐CoV‐2 infection by this vigorous screening method.
With the development of the SARS‐CoV‐2 vaccination, this might bring changes to the fecal donation for the FMT program. In general, taking live or experimental vaccine within 6 months is one of the exclusion criteria of potential fecal donors. It was suggested that potential donors should wait 7–10 days after vaccination before screening as subjects might be suffering from effects of COVID‐19 vaccines after vaccination, like fatigue, fever, headache, etc. 49 The currently available SARS‐CoV‐2 vaccine in Hong Kong are messenger RNA vaccine (BNT162b2 by BioNtech) and inactivated vaccine by Sinovac Biotech Ltd, which are not live vaccines. It is still possible that the subjects may get COVID‐19 after vaccination even though the risk is very low. Thus, for the safety of recipients, donors who have completed SARS‐CoV‐2 vaccination are still required to undergo SARS‐CoV‐2 screening until we have more data or evidence for exemption.
Another major barrier for FMT donations is the high commitment required for the whole process. In fact, 16% of subjects failed pre‐screening as they were not able to come for donation every weekday for a prolonged duration due to time constraints and inconvenience. This has also been reported in other stool bank centers. 12 , 50 , 51 One possible solution is to recruit donors working in/around the same hospital via mass media and posters (Table 4). This may increase the donor uptake rate due to the shorter distance between the donors' workplace and the donation site. There might be concerns that healthcare workers have a higher rate of colonization by multidrug‐resistant organisms (MDRO). However, a recent study suggests that healthcare workers have a similar prevalence rate of MDRO as other staff who do not contact patients and/or specimens. 52 Besides, stool tests of MDRO can screen out MDRO intestinal carriers. As the status of MDRO carriage can change if the donors keep donation for a prolonged duration, screening tests should be repeated in a higher frequency of every 8–12 weeks. 51
Other possible solutions to increase the number of recruited donors include loosening the BMI criteria as BMI > 23 is the second commonest failing reason during prescreening part in our cohort. According to World Health Organization, obesity is defined as BMI over or equal to 25 in Asian population. 30 Therefore, it is reasonable to increase the upper limit of BMI to 25 to avoid the inappropriate exclusion of potential donors. Furthermore, only 119 potential donors were pre‐screened for stool donation in our center. To reach a wider population, promotion of stool donation via social media is another possible solution. Lastly, a recent research on the prevalence of ESBL‐producing E. coli in food in Germany demonstrated that chicken meat (71.9%) had a significantly higher proportion of ESBL positive samples than pork (12.1%) and beef (4.2%). 38 The Consumer Council in Hong Kong also found that over 60% of chicken meat in market were found to be containing ESBL‐producing bacteria. 53 Therefore, in the future, we could advise all potential donors to consume less chicken meat and to cook the meat thoroughly and be careful when handling raw meat and maintain proper hygiene at all times to reduce risks of getting infected and remember to wash hands in order to minimize the chances of becoming infected. The key points of possible solutions are summarized in Table 4.
One of the strengths of this study is that the data was collected from an institution which has offered FMT for the past 3 years, and provides real‐world data on donor screening and recruitment. In addition, all the screening procedures were performed in house, allowing us to access the data with regard to all facets of FMT screening. There are also some limitations in this study: first, the number of applicants was quite limited. Additionally, the inter‐individual stool heterogeneity should not be neglected even though we have used the same criteria to select eligible donors. Gut microbiota in each individual is complex and consists of different species of bacteria and non‐bacterial microbes like virus, fungi, Archaea and parasites. For example, one donor may have the components efficacious in treating CDI but not IBD. 54 However, the link between specific components of stools from healthy donors and the treatment efficacy has yet to be fully elucidated. 55
In conclusion, it is hard to recruit regular and healthy donors for FMT with a high failure rate during the screening process. With the expanding indications and clinical trials of FMT, it is pressing to standardize and improve stool donor screening procedures to ensure safety for patients, and to achieve a higher donor recruitment rate so as to ensure the sustainability of such services.
CONFLICT OF INTEREST
The authors have no conflict of interest to declare.
AUTHOR CONTRIBUTIONS
Guarantor of article: Siew Chien Ng and Kay Sheung Paul Chan. Study concept and design: Kay Sheung Paul Chan, Ka Leung Francis Chan, and Siew Chien Ng. Acquisition of data: Yuk Kam Yau, Wai Yin Rita Ng, Choi Yan Kitty Cheung, Ying Lee Amy Li, and Miu Ling Chin. Analysis and interpretation of data: Yuk Kam Yau. Drafting of the manuscript: Yuk Kam Yau. Critical revision of the manuscript for important intellectual content: Wing Yan Joyce Mak, Nok Shun Rashid Lui, and Ho Shing Louis Lau.
ETHICS APPROVAL
The study was approved by the Joint The Chinese University of Hong Kong—New Territories East Cluster Clinical Research Ethics Committee.
Supporting information
Supporting Information S1
Yau YK, Mak WYJ, Lui NSR, Ng WYR, Cheung CYK, Li YLA, et al. High prevalence of extended‐spectrum beta‐lactamase organisms and the COVID‐19 pandemic impact on donor recruitment for fecal microbiota transplantation in Hong Kong. United European Gastroenterol J. 2021;9(9):1027–38. 10.1002/ueg2.12160
Contributor Information
Kay Sheung Paul Chan, Email: Paulkschan@cuhk.edu.hk.
Siew Chien Ng, Email: siewchienng@cuhk.edu.hk.
DATA AVAILABILITY STATEMENT
The data underlying this article will be shared on reasonable request to the corresponding author.
REFERENCES
- 1. Gupta S, Allen‐Vercoe E, Petrof EO. Fecal microbiota transplantation: in perspective. Ther Adv Gastroenterol. 2016;9:229–39. 10.1177/1756283x15607414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lui RN, Wong SH, Lau LH, Chan TT, Cheung KC, Li AY, et al. Faecal microbiota transplantation for treatment of recurrent or refractory Clostridioides difficile infection in Hong Kong. Hong Kong Med J. 2019;25:178–82. [DOI] [PubMed] [Google Scholar]
- 3. Van Nood E, Vrieze A, Nieuwdorp M, Fuentes S, Zoetendal EG, de Vos WM, et al. Duodenal infusion of donor feces for recurrent Clostridium difficile. N Engl J Med. 2013;368:407–15. 10.1056/NEJMoa1205037 [DOI] [PubMed] [Google Scholar]
- 4. Moayyedi P, Surette MG, Kim PT, Libertucci J, Wolfe M, Onischi C, et al. Fecal microbiota transplantation induces remission in patients with active ulcerative colitis in a randomized controlled trial. Gastroenterology. 2015;149:102–9. 10.1053/j.gastro.2015.04.001 [DOI] [PubMed] [Google Scholar]
- 5. El‐Salhy M, Hatlebakk JG, Gilja OH, Bråthen Kristoffersen A, Hausken T. Efficacy of faecal microbiota transplantation for patients with irritable bowel syndrome in a randomised, double‐blind, placebo‐controlled study. Gut. 2020;69:859–67. 10.1136/gutjnl-2019-319630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ohkusa T, Koido S, Nishikawa Y, Sato N. Gut microbiota and chronic constipation: a review and update. Front Med. 2019;6:9. 10.3389/fmed.2019.00019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Landy J, Al‐Hassi HO, Mann ER, Peake ST, McLaughlin SD, Perry‐Woodford ZL, et al. PWE‐078 A prospective controlled pilot study of fecal microbiota transplantation for chronic refractory pouchitis. Gut. 2013;62:A162. 10.1136/gutjnl-2013-304907.367 [DOI] [Google Scholar]
- 8. Hartstra AV, Bouter KEC, Backhed F, Nieuwdorp M. Insights into the role of the microbiome in obesity and type 2 diabetes. Diabetes Care. 2015;38:159–65. 10.2337/dc14-0769 [DOI] [PubMed] [Google Scholar]
- 9. Kootte RS, Levin E, Salojarvi J, Smits LP, Hartstra AV, Udayappan SD, et al. Improvement of insulin sensitivity after lean donor feces in metabolic syndrome is driven by baseline intestinal microbiota composition. Cell Metab. 2017;26:611–9. 10.1016/j.cmet.2017.09.008 [DOI] [PubMed] [Google Scholar]
- 10. Li Q, Han Y, Dy ABC, Hagerman RJ. The gut microbiota and autism spectrum disorders. Front Cell Neurosci. 2017;11:120. 10.3389/fncel.2017.00120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Vendrik KEW, Ooijevaar RE, de Jong PRC, Laman JD, van Oosten BW, van Hilten JJ, et al. Fecal microbiota transplantation in neurological disorders. Front Cell Infect Microbiol. 2020;10:98. 10.3389/fcimb.2020.00098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cammarota G, Ianiro G, Kelly CR, Mullish BH, Allegretti JR, Kassam Z, et al. International consensus conference on stool banking for faecal microbiota transplantation in clinical practice. Gut. 2019;68:2111–21. 10.1136/gutjnl-2019-319548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lai CY, Sung J, Cheng F, Tang W, Wong SH, Chan PKS, et al. Systematic review with meta‐analysis: review of donor features, procedures and outcomes in 168 clinical studies of faecal microbiota transplantation. Aliment Pharmacol Ther. 2019;49:354–63. 10.1111/apt.15116 [DOI] [PubMed] [Google Scholar]
- 14. FDA TFaDA. Important safety alert regarding use of fecal microbiota for transplantation and risk of serious adverse reactions due to transmission of multi‐drug resistant organisms; 2019 [cited 2021 Jul 26]. Available from: https://www.fda.gov/vaccines‐blood‐biologics/safety‐availability‐biologics/important‐safety‐alert‐regarding‐use‐fecal‐microbiota‐transplantation‐and‐risk‐serious‐adverse [Google Scholar]
- 15. Wong SH, Lui RN, Sung JJ. Covid‐19 and the digestive system. J Gastroenterol Hepatol. 2020;35:744–8. 10.1111/jgh.15047 [DOI] [PubMed] [Google Scholar]
- 16. FDA TFaDA . Fecal microbiota for transplantation: new safety information—regarding additional protections for screening donors for COVID‐19 and exposure to SARS‐CoV‐2 and testing for SARS‐CoV‐2; 2020 [cited 2021 Jul 26]. Available from: https://www.fda.gov/safety/medical‐product‐safety‐information/fecal‐microbiota‐transplantation‐new‐safety‐information‐regarding‐additional‐protections‐screening [Google Scholar]
- 17. Prevention CfDCa . About chronic diseases; 2021. [cited 2021 Jul 26]. Available from: https://www.cdc.gov/chronicdisease/about/index.htm [Google Scholar]
- 18. Raymond F, Ouameur AA, Déraspe M, Iqbal N, Gingras H, Dridi B, et al. The initial state of the human gut microbiome determines its reshaping by antibiotics. ISME J. 2016;10:707–20. 10.1038/ismej.2015.148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gao XF, Zhang MR, Xue JM, Huang J, Zhuang R, Zhou X, et al. Body mass index differences in the gut microbiota are gender specific. Front Microbiol. 2018;9:10. 10.3389/fmicb.2018.01250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yun Y, Kim HN, Kim SE, Heo SG, Chang Y, Ryu S, et al. Comparative analysis of gut microbiota associated with body mass index in a large Korean cohort. BMC Microbiol. 2017;17:9. 10.1186/s12866-017-1052-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Craig WJ. Nutrition concerns and health effects of vegetarian diets. Nutr Clin Pract. 2010;25:613–20. 10.1177/0884533610385707 [DOI] [PubMed] [Google Scholar]
- 22. Barnes D, Park K. Donor considerations in fecal microbiota transplantation. Curr Gastroenterol Rep. 2017;19:10. [DOI] [PubMed] [Google Scholar]
- 23. Ng SC, Chan FKL, Chan PKS. Screening FMT donors during the COVID‐19 pandemic: a protocol for stool SARS‐CoV‐2 viral quantification. Lancet Gastroenterol Hepatol. 2020;5:642–3. 10.1016/S2468-1253(20)30124-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Paramsothy S, Borody TJ, Lin E, Finlayson S, Walsh AJ, Samuel D, et al. Donor recruitment for fecal microbiota transplantation. Inflamm Bowel Dis. 2015;21:1600–6. 10.1097/mib.0000000000000405 [DOI] [PubMed] [Google Scholar]
- 25. Tariq R, Weatherly R, Kammer P, Pardi DS, Khanna S. Donor screening experience for fecal microbiota transplantation in patients with recurrent C. difficile infection. J Clin Gastroenterol. 2018;52:146–50. 10.1097/mcg.0000000000000768 [DOI] [PubMed] [Google Scholar]
- 26. Mullish BH, Quraishi MN, Segal JP, McCune VL, Baxter M, Marsden GL, et al. The use of faecal microbiota transplant as treatment for recurrent or refractory Clostridium difficile infection and other potential indications: joint British Society of Gastroenterology (BSG) and Healthcare Infection Society (HIS) guidelines. Gut. 2018;67:1920–41. 10.1136/gutjnl-2018-316818 [DOI] [PubMed] [Google Scholar]
- 27. Chen J, Zaman A, Ramakrishna B, Olesen SW. Stool banking for fecal microbiota transplantation: methods and operations at a large stool bank. Front Cell Infect Microbiol. 2021;11:622949. 10.3389/fcimb.2021.622949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kassam Z, Dubois N, Ramakrishna B, Ling K, Qazi T, Smith M, et al. Donor screening for fecal microbiota transplantation. N Engl J Med. 2019;381:2070–2. 10.1056/NEJMc1913670 [DOI] [PubMed] [Google Scholar]
- 29. WHO Expert Consultation . Appropriate body‐mass index for Asian populations and its implications for policy and intervention strategies. Lancet 2004;363:157–63. 10.1016/S0140-6736(03)15268-3 [DOI] [PubMed] [Google Scholar]
- 30. World Health Organization. Regional Office for the Western Pacific . The Asia‐Pacific perspective: redefining obesity and its treatment. Sydney: Health Communications Australia; 2000. [Google Scholar]
- 31. Woodworth MH, Neish EM, Miller NS, Dhere T, Burd EM, Carpentieri C, et al. Laboratory testing of donors and stool samples for fecal microbiota transplantation for recurrent Clostridium difficile infection. J Clin Microbiol. 2017;55:1002–10. 10.1128/jcm.02327-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bezabih YM, Sabiiti W, Alamneh E, Bezabih A, Peterson GM, Bezabhe WM, et al. The global prevalence and trend of human intestinal carriage of ESBL‐producing Escherichia coli in the community. J Antimicrob Chemother. 2020;76:22–9. 10.1093/jac/dkaa399 [DOI] [PubMed] [Google Scholar]
- 33. Turbett SE, Mansour MK. Editorial commentary: fecal ESBL screening: are we ready for this information? Clin Infect Dis. 2016;63:319–21. 10.1093/cid/ciw288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kwok KO, Chan E, Chung PH, Tang A, Wei W‐I, Zhu C, et al. Prevalence and associated factors for carriage of Enterobacteriaceae producing ESBLs or carbapenemase and methicillin‐resistant Staphylococcus aureus in Hong Kong community. J Infect. 2020;81:242–7. 10.1016/j.jinf.2020.05.033 [DOI] [PubMed] [Google Scholar]
- 35. Ben Said L, Jouini A, Klibi N, Dziri R, Alonso CA, Boudabous A, et al. Detection of extended‐spectrum beta‐lactamase (ESBL)‐producing Enterobacteriaceae in vegetables, soil and water of the farm environment in Tunisia. Int J Food Microbiol. 2015;203:86–92. 10.1016/j.ijfoodmicro.2015.02.023 [DOI] [PubMed] [Google Scholar]
- 36. Van Hoek A, Veenman C, van Overbeek WM, Lynch G, de Roda Husman AM, Blaak H. Prevalence and characterization of ESBL‐ and AmpC‐producing Enterobacteriaceae on retail vegetables. Int J Food Microbiol. 2015;204:1–8. 10.1016/j.ijfoodmicro.2015.03.014 [DOI] [PubMed] [Google Scholar]
- 37. Karanika S, Karantanos T, Arvanitis M, Grigoras C, Mylonakis E. Fecal colonization with extended‐spectrum beta‐lactamase‐producing Enterobacteriaceae and risk factors among healthy individuals: a systematic review and metaanalysis. Clin Infect Dis. 2016;63:310–8. 10.1093/cid/ciw283 [DOI] [PubMed] [Google Scholar]
- 38. Kaesbohrer A, Bakran‐Lebl K, Irrgang A, Fischer J, Kämpf P, Schiffmann A, et al. Diversity in prevalence and characteristics of ESBL/pAmpC producing E. coli in food in Germany. Vet Microbiol. 2019;233:52–60. 10.1016/j.vetmic.2019.03.025 [DOI] [PubMed] [Google Scholar]
- 39. Tham J, Odenholt I, Walder M, Brolund A, Ahl J, Melander E. Extended‐spectrum beta‐lactamase‐producing Escherichia coli in patients with travellers' diarrhoea. Scand J Infect Dis. 2010;42:275–80. 10.3109/00365540903493715 [DOI] [PubMed] [Google Scholar]
- 40. World Health Organization . Weekly epidemiological update on COVID‐19—20 July 2021; 2021. [Google Scholar]
- 41. Green CA, Quraishi MN, Shabir S, Sharma N, Hansen R, Gaya DR, et al. Screening faecal microbiota transplant donors for SARS‐CoV‐2 by molecular testing of stool is the safest way forward. Lancet Gastroenterol Hepatol. 2020;5:531. 10.1016/s2468-1253(20)30089-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ianiro G, Mullish BH, Kelly CR, Kassam Z, Kuijper EJ, Ng SC, et al. Reorganisation of faecal microbiota transplant services during the COVID‐19 pandemic. Gut. 2020;69:1555–63. 10.1136/gutjnl-2020-321829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ianiro G, Mullish BH, Kelly CR, Sokol H, Kassam Z, Ng SC, et al. Screening of faecal microbiota transplant donors during the COVID‐19 outbreak: suggestions for urgent updates from an international expert panel. Lancet Gastroenterol Hepatol. 2020;5:430–2. 10.1016/s2468-1253(20)30082-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Xie J, Long X, Ren C, He R, Yan X, Li W, et al. Follow‐up study of long‐time positive RT‐PCR in stool specimens from asymptomatic children infected with SARS‐CoV‐2. Pediatr Infect Dis J. 2020;39:e315–7. [DOI] [PubMed] [Google Scholar]
- 45. Chen Y, Chen L, Deng Q, Zhang G, Wu K, Ni L, et al. The presence of SARS‐CoV‐2 RNA in the feces of COVID‐19 patients. J Med Virol. 2020;92:833–40. 10.1002/jmv.25825 [DOI] [PubMed] [Google Scholar]
- 46. Xu Y, Li X, Zhu B, Liang H, Fang C, Gong Y, et al. Characteristics of pediatric SARS‐CoV‐2 infection and potential evidence for persistent fecal viral shedding. Nat Med. 2020;26:502–5. 10.1038/s41591-020-0817-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Xing Y‐H, Ni W, Wu Q, Li W‐J, Li G‐J, Wang W‐D, et al. Prolonged viral shedding in feces of pediatric patients with coronavirus disease 2019. J Microbiol Immunol Infect. 2020;53:473–80. 10.1016/j.jmii.2020.03.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Zhao F, Yang Y, Wang Z, Li L, Liu L, Liu Y. The time sequences of respiratory and rectal viral shedding in patients with coronavirus disease 2019. Gastroenterology. 2020;159(3):1158–1160.e1152. 10.1053/j.gastro.2020.05.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ianiro G, Mullish BH, Hvas CL, Segal JP, Kuijper EJ, Costello SP, et al. SARS‐CoV‐2 vaccines and donor recruitment for FMT. Lancet Gastroenterol Hepatol. 2021;6:264–6. 10.1016/S2468-1253(21)00032-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kassam Z, Dubois NE, Ling K, Ramakrishna B, Qazi T, Allegretti JR, et al. Donor health screening for fecal microbiota transplantation: prospective evaluation of 15,317 candidate donors. Gastroenterology. 2019;156:S100–1. [Google Scholar]
- 51. Bibbo S, Settanni CR, Porcari S, Bocchino E, Ianiro G, Cammarota G, et al. Fecal microbiota transplantation: screening and selection to choose the optimal donor. J Clin Med. 2020;9:14. 10.3390/jcm9061757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Decker BK, Lau AF, Dekker JP, Spalding CD, Sinaii N, Conlan S, et al. Healthcare personnel intestinal colonization with multidrug‐resistant organisms. Clin Microbiol Infect. 2018;24(1):82E1–82E4. 10.1016/j.cmi.2017.05.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Council TC. Over 60% of chicken models found to contain ESBL‐producing bacteria in the first ever chicken test; 2016. [cited 2021 Aug 21]. Available from: https://www.consumer.org.hk/ws_en/news/press/482/chicken‐and‐antibiotics.html [Google Scholar]
- 54. Olesen SW, Leier MM, Alm EJ, Kahn SA. Searching for superstool: maximizing the therapeutic potential of FMT. Nat Rev Gastroenterol Hepatol. 2018;15:387–8. 10.1038/s41575-018-0019-4 [DOI] [PubMed] [Google Scholar]
- 55. Shankar V, Hamilton MJ, Khoruts A, Kilburn A, Unno T, Paliy O, et al. Species and genus level resolution analysis of gut microbiota in Clostridium difficile patients following fecal microbiota transplantation. Microbiome. 2014;2:10. 10.1186/2049-2618-2-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information S1
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.


