Abstract
Background
Pyogenic liver abscesses (PLAs) represent potentially life‐threatening abdominal conditions that require immediate diagnosis and therapy. European and American incidence figures vary between one and 15 per 100,000 per year. Structured epidemiological data for European countries are not available.
Objective
To systematically characterize the epidemiology and clinical outcome of PLA in Germany.
Methods
In representative statutory health insurance data from four million people in 2013–2019, the prevalence and incidence with clinical coding of International Statistical Classification of Diseases and Related Health Problems (ICD)‐10 code K75.0 were selected (n = 1118). Furthermore, demographics, relevant comorbidities, hospitalizations, mortality and complications were determined within one year.
Results
The incidence of PLA was approximately seven per 100,000. The average age at diagnosis was 66 years; 65% were male. Of these, biliary disease was documented in over 60% and infectious intestinal diseases were found in 21% within the same or previous calendar year. PLA patients had high comorbidity indices. Liver transplant status, malignancies of the liver and biliary system, liver cirrhosis and pancreatitis were strongly associated. Intensive care was documented in 27% of PLA cases. Nine percent died within 12 months, most with an underlying malignant disease.
Conclusion
Pyogenic liver abscess is a rare disease with high morbidity. Predisposing and risk factors include intestinal and biliary diseases as well as hepatic malignancies. Further research should focus on PLA therapy within prospective surveys and controlled clinical trials.
Keywords: clinical coding, comorbidity, electronic health records, epidemiology, Germany, incidence, liver abscess, mortality rate, prevalence, pyogenic, risk factors
INTRODUCTION
Pyogenic liver abscesses (PLAs) represent potentially life‐threatening abdominal conditions that require immediate diagnosis and therapy. PLA often occur at an advanced stage of an underlying disease; it used to be fatal in most cases, 1 until the introduction of antibiotics and interventional therapy. Treatment management recommendations have significantly improved the prognosis, 2 with a reported mortality rate of 6%–10%. 3 , 4
Key summary.
Summarize the established knowledge on this subject
-
•
Pyogenic liver abscesses (PLA) are potentially life‐threatening medical conditions that significantly contribute to the mortality of patients with chronic diseases, especially, malignancies.
-
•
Due to the worldwide spread of multidrug‐resistant and/or hypervirulent bacterial strains, a significant dynamic in the number of PLA cases is to be expected. However, there are insufficient surveys on the epidemiology and prognosis of PLA in Europe.
-
•
PLA require a fast and structured interdisciplinary approach, but the available evidence on treatment strategies is largely based on case reports and expert opinion.
What are the significant and/or new findings of this study?
-
•
This is the first systematic analysis of health insurance data on the epidemiology and mortality of patients with PLA in Germany revealing an incidence of approximately seven per 100,000.
-
•
Liver transplant status, malignancies, liver cirrhosis and pancreatitis were strongly associated with PLA.
-
•
A severe course requiring intensive care treatment was documented in 27% of the cases.
-
•
The median survival time of PLA patients was 5.3 years. Mortality was strongly associated with underlying malignancies.
Pyogenic liver abscesses often represent a clinical challenge because of unspecific symptoms and comorbidities. 5 For the clinical management, it is particularly important to consider the aetiology. Therapeutic strategies are always based on anti‐infective treatment and elimination of the causal focus. Optimal treatment results are achieved through intensive interdisciplinary cooperation. 6 The incidence varies between one and 15 per 100,000 in Europe, North America and New Zealand and significantly differs by region. 2 , 3 , 4 , 5 , 6 , 7 , 8 , 9 Structured data on the epidemiology, diagnosis, treatment strategies and outcome of PLA are not yet available for Europe. Published studies comprise older administrative register figures from Denmark 3 and monocentric studies from the UK 8 and Italy. 10 Hence, a systematic analysis of representative routine data from the statutory health insurance (SHI) system can support the development of future evidence‐based treatment recommendations. SHI data increasingly contribute to health services research as they include patient‐related and cross‐sectional demographic data, outpatient and inpatient services and diagnoses, as well as information on drug prescriptions and costs. 11
The available evidence on the therapeutic management of PLA is largely based on case reports and retrospective studies, mainly from Asian‐Pacific countries, and only comprises few randomised controlled trials. 12 , 13 , 14 Consequently, treatment recommendations are mainly based on expert opinions. National guidelines are not yet available. Therefore, our aim was to systematically characterize the epidemiology and clinical outcome of PLA using a longitudinal routine data cohort study.
MATERIALS AND METHODS
For this study, accounting data of approximately four million SHI‐insured persons were provided by the German Analysis Database for Evaluation and Health Services Research (DADB). The database includes approximately 5% of the total individuals covered by SHI in Germany and has been proven to constitute a representative sample of the total German population. It comprises clinical diagnoses according to the International Statistical Classification of Diseases and Related Health Problems (ICD)‐10 from electronic health records and procedural coding, which are the basis for reimbursement of in‐hospital services. An epidemiological evaluation and adjustment to the SHI‐wide prevalence and incidence of PLA (ICD‐10 code K75.0) was conducted.
In addition to demographic and morbidity‐related characteristics, the cross‐sectoral and longitudinal patient journey was considered. For this purpose, the coded diagnoses (ICD‐10‐GM), procedures ('Operationen‐ und Prozeduren‐Schlüssel', OPS), outpatient accounting codes ('Einheitlicher Bewertungsmaßstab'), and prescribed drugs (Anatomical Therapeutic Chemical classification, code) were extracted from the accounting data. 15 , 16 , 17
Inclusion and exclusion criteria
To determine the prevalence, adult individuals with a complete data‐set were selected per calendar year from 2013 to 2019. From this population, PLA patients were extracted by an in‐patient main or secondary diagnosis according to ICD‐10 code K75.0. Patients with simultaneous coding of echinococcosis (ICD‐10 code B67) were excluded, as medical miscoding could be assumed there. We initially intended an investigation of amoebic liver abscesses (ICD‐10 code A06.4). Nevertheless, we refrained from this analysis, because over 97% of these cases had only outpatient documentation and, therefore, insufficient coding quality was supposed. However, patients with both ICD‐10 K75.0 and A06.4 coding were not excluded from the analysis.
Primary endpoints
Prevalence and incidence (per 100,000 inhabitants) were determined. For the incidence, patients also had to be observable in the previous calendar year and were not allowed to have a PLA diagnosis in this time interval. For incident cases, the following endpoints were evaluated 30 days after initial diagnosis and 365 days thereafter: Hospitalisation, surgery and death. In addition, diagnoses in the previous or reporting year before the PLA were examined, which could serve as indicators for PLA aetiology (ICD‐10 codes see Table S1).
Secondary endpoints
In addition, complications during hospitalisations were evaluated on the basis of secondary diagnoses and procedures. For these additional endpoints, a minimum follow‐up period of one year was required. To determine the possible cause of PLA, comorbidities such as the presence of immunosuppression (see criteria in Table S2), the previous use of proton pump inhibitors (PPIs) and the occurrence of typical symptoms (e.g., abdominal discomfort, nausea and fever) verifiable through ICD‐10 coding were analysed in the previous year.
Adjustment and control cohort
The age and gender distribution of all individuals covered by SHI based on 40 age and gender groups was used to apply weighting factors to the DADB population to ensure a maximal comparability of prevalence and incidence. 18 For the assessment of comorbidities, a control group without PLA, with corresponding age and gender profiles in the DADB, was compiled to adjust for the frequency of comorbidities in the remaining database. To estimate the degree of morbidity, the Charlson Comorbidity Index (CCI) was determined in the previous year based on age and comorbidities. 19 , 20
Statistics
For descriptive key figures, location and dispersion parameters were given (standard deviation [SD], 95% confidence interval [CI]). The sex ratio was determined using an exact binomial test. For age difference by sex, a two‐sample t‐test was used. Mann–Kendall tests were used to identify trends in the development of prevalence and incidence. Chi‐square tests were applied to the investigated comorbidities, and odds ratios (ORs) were determined. The probability of survival after PLA was estimated using the method of Kaplan and Meier. It was compared for patients with and without malignant disease using a log‐rank test. All calculations were done using SQL Server Management Studio v17.7 and the statistical software RStudio, version 1.3.1093‐1.
Ethical considerations
This study was performed according to the guidelines of ‘Good Practice Secondary Data Analysis’ (GPS) 21 and in accordance with the current Declaration of Helsinki (2013), reviewed and approved in advance by the local ethics committee (University of Leipzig, vote number 170/20‐ek, 6 May 2020) and registered in the German Register of Clinical Studies (DRKS, register number DRKS00021793). For this retrospective approach, informed consent was waived.
RESULTS
From a total of 3,668,262 adult persons, 1,341 prevalent cases were selected (Figure 1). The prevalence of PLA, adjusted by age and gender to the total of SHI‐insured persons, was eight per 100,000, without significant increase over the years 2013–2019 (p = 0.133). For further analysis, 1,118 PLA cases were observable and diagnosis‐free in the previous year. The SHI‐adjusted incidence of PLA was approximately seven per 100,000, with no significant increase over the study period (p = 0.130). Sixty‐five percent were men (n = 725, p < 0.001). PLA rarely occurred before 40 years of age (Figure 2). The mean age was 66 (SD ± 14) years for both genders (p = 0.542). Two patients were coded with both pyogenic and amoebic liver abscess diagnosis in the same year.
FIGURE 1.
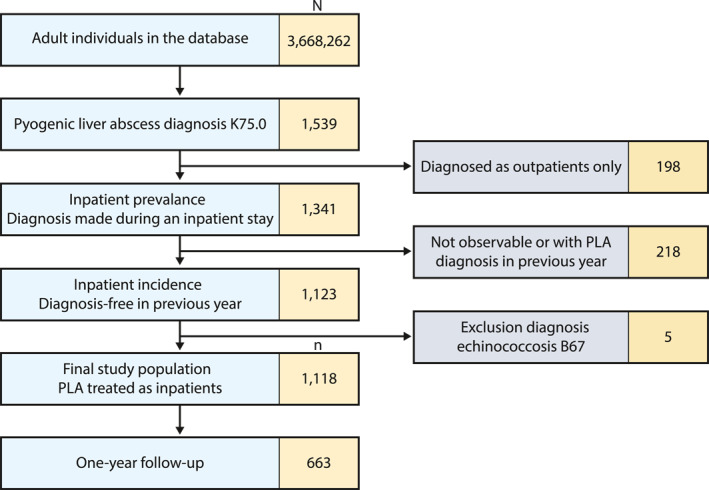
Flowchart of the study population
FIGURE 2.
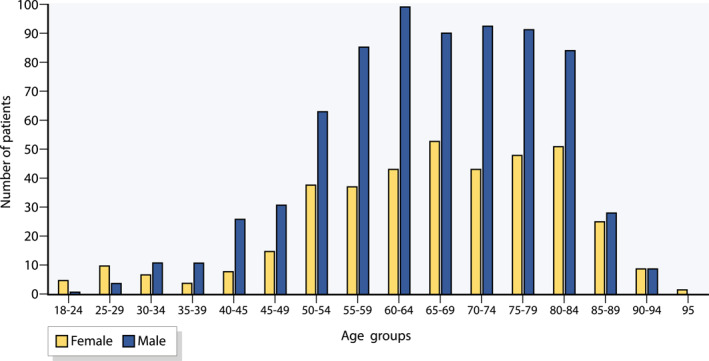
Age and gender distribution of the study population
Clinical symptoms
Typical symptoms were diagnosed in 73% (n = 487) of patients prior to in‐patient treatment. Nausea or vomiting was coded in 19% (n = 123), pleural effusion in 17% (n = 114), malaise/fatigue and fever in 13% each (n = 88 and 84, respectively).
Comorbidity and predisposing diseases
Patients had a high degree of comorbidity in the calendar year of PLA diagnosis (Table 1); the mean CCI was 4.65 (CI [4.47; 4.83]). Only 1.5% and 8% (n = 84) of the patients were affected by a previous liver transplantation or biliary obstruction, respectively. However, these were statistically noticeable, as they occurred about 40 times more frequently than in the overall SHI (p < 0.001 in each case). Malignancies of the liver and biliary tract system, as well as liver cirrhosis and pancreatitis, were also significantly more often coded as comorbidities (p < 0.001 in each case; Figure 3).
TABLE 1.
Predisposing conditions prior to the occurrence of pyogenic liver abscess
| Pre‐existing conditions (ICD‐10‐GM) | Number | Proportion |
|---|---|---|
| Biliary abnormalities a | 677 | 60.6% |
| Thereof cholangitis | 179 | 16.0% |
| Malignancies | 517 | 46.2% |
| Other intestinal disorders b | 302 | 27.0% |
| Infectious intestinal diseases | 234 | 20.9% |
| Ileus/diverticulitis/gastrointestinal abscesses | 176 | 15.7% |
| Non‐infectious intestinal inflammation c | 155 | 13.9% |
| Infection after surgery | 137 | 12.3% |
| Appendicitis | 25 | 2.2% |
| Vascular diseases of the intestine | 18 | 1.6% |
| Liver transplant status | 17 | 1.5% |
| Endocarditis | 15 | 1.3% |
| Renal abscess | 5 | 0.4% |
| Without any predisposition d | 100 | 8.9% |
Abbreviations: ICD, International Statistical Classification of Diseases and Related Health Problems.
Cholecystitis, cholelithiasis, acute and chronic pancreatitis, other diseases of the biliary tract.
Irritable bowel syndrome, other diseases of the intestine, anus and rectum, functional bowel disorders.
Crohn's disease, ulcerative colitis, other non‐infectious gastroenteritis and colitis.
None of the listed predisposing diagnoses.
FIGURE 3.
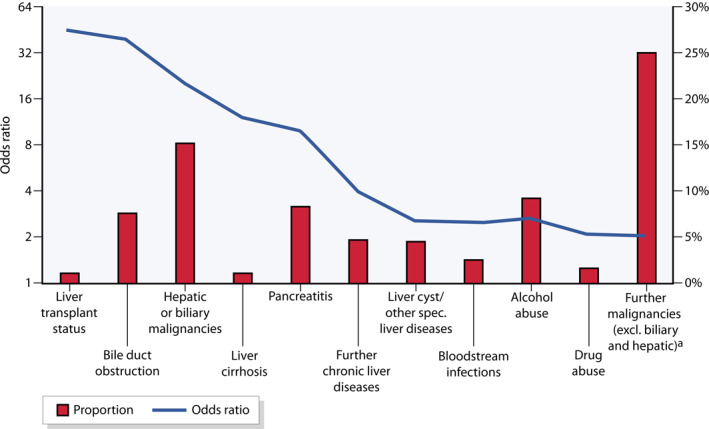
Comorbidities according to ICD‐10 codes compared to the general population in the data‐set of statutory health insurance (SHI, proportions and odds ratios [OR]), weighting of the comparison group according to age and gender analogous to the PLA population; (a) Without basal cell carcinoma (ICD‐10 C44). ICD, International Statistical Classification of Diseases and Related Health Problems
In the previous year, most PLA cases had already been diagnosed with predisposing diseases. More than 60% (n = 677) had biliary disorders, of which 26% had cholangitis (n = 179). Malignancies were coded in 46% (n = 517). Furthermore, 21% (n = 234) had infectious intestinal diseases and 12% (n = 137) had an infection after a surgical intervention (Table 2). 16% (n = 176) had a diagnosis of either ileus, diverticulitis or gastrointestinal abscess, and 9% (n = 97) were considered immunosuppressed. On average, 50% of patients (n = 564) were on a PPI treatment before PLA diagnosis, compared with 26% in the age‐ and gender‐weighted comparison group.
TABLE 2.
Treatment intensity indicators and complications during the hospital stay
| Numbers | Proportion | |
|---|---|---|
| Treatment intensity indicators (according to ICD‐10‐GM) | ||
| Complex intensive care treatment | 180 | 27.1% |
| Blood transfusion | 158 | 23.8% |
| Mechanical ventilation | 66 | 10.0% |
| Complex treatment for multidrug‐resistant pathogens | 28 | 4.2% |
| Complications (according to OPS codes) | ||
| SIRS/sepsis | 162 | 24.4% |
| Organ failure | 157 | 23.7% |
| Complication during surgery | 112 | 16.9% |
| Pleural effusion | 101 | 15.2% |
| Nosocomial pneumonia | 37 | 5.6% |
Abbreviations: ICD, International Statistical Classification of Diseases and Related Health Problem; OPS, Operationen‐ und Prozeduren‐Schlüssel.
Nine percent (n = 100) of all PLA patients had no predisposing diseases. The incidence of this subgroup was thus less than one per 100,000 persons.
Mortality
For the mortality analysis, follow‐up data for at least one year was required. This subpopulation (n = 663, Figure 1) could be followed up for 2.5 years (median, maximum 6.8 years). Only two PLA patients (0.3%) died within 30 days, and 9% (n = 57) in the first year after diagnosis. Of these, 74% (n = 42) had an underlying malignant disease. The Kaplan–Meier analysis showed a median survival time of 5.3 years (Figure 4). Mortality was strongly associated with underlying malignancies (4.2 years estimated median survival with vs. 6.1 years without diagnosis of concomitant malignancy, p < 0.001). After 5 years, the estimated mortality was 15% (CI [10%; 19%]) for PLA patients without and 49% (CI [40%; 57%]) with malignancy. In the groups of PLA patients without malignancies (n = 407) and those without any predisposing diseases, only 4% in each group (n = 15 and n = 3, respectively) died within the first year.
FIGURE 4.
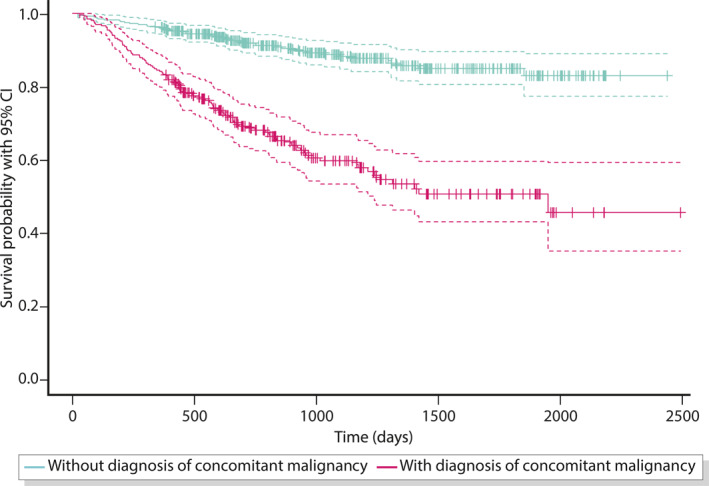
Kaplan–Meier survival analysis
Treatment modalities and complications
The mean length of hospital stay was 22 (CI [20; 23]) days. A severe course with intensive care was coded in 27% of cases (n = 180). Organ failure, SIRS/sepsis, blood transfusion or mechanical ventilation occurred with a comparable frequency (Table 2), confirming critical illness in these cases.
OPS codes for imaging methods and interventional therapies were only documented in a small number of cases in the present cohort, so that the foreseen analysis could not be performed. Also, microbiological diagnostics and types of anti‐infective therapy are not evaluable within the ICD‐10 and the Diagnosis Related Groups (DRG) reimbursement system.
Our accounting data show that in 25% of cases (n = 164) surgical treatment was applied, although it is not clear whether the PLA was an indication for or a consequence of surgery. In 2% (n = 11) of cases, the PLA diagnosis was already present at the time of admission to the hospital and followed by surgery. 8% (n = 54) underwent surgery within one day.
vThe rehospitalisation rate in the first year was 21% (n = 142) with 42% (n = 60) of these patients having a concomitant malignancy.
DISCUSSION
This is the first systematic analysis on epidemiology, comorbidity and clinical outcome of PLAs in Germany, which provides important prevalence estimations for large European countries.
The in‐patient diagnostic incidence is approx. seven per 100,000 per year and was constant in the observation period from 2013 to 2019. The incidence of PLA is lower in Germany than in Asia 22 , 23 and lies in the same range as malignancies of the bile ducts and oesophagus. 24 This corresponds to previous monocentric cohort studies from other European countries. 8 , 10 There is a striking age and gender dependency: the disease very rarely occurs before the age of 40, and the sex ratio is almost 2:1 (males to females), which can be explained by the association with underlying diseases.
In at least a quarter of the cases, the course of disease is severe and requires intensive care, which underlines its clinical and economic importance. The high individual comorbidity is decisive, as reflected in a mean CCI of 4.65. 25
There are strong associations with malignancies of the liver and biliary tract (p < 0.001) as well as other causes of biliary obstruction of different origin, including advanced extrahepatic malignancies. Therefore, after the diagnosis of PLA, an appropriate exclusion of malignancy should always be ensured, with colonoscopy being of particular importance. 13 The most important aetiology appears to be cholangitis, with a share of 16%. A comparison with a large single‐centre case series from the Indian subcontinent confirms that the main cause of PLA is biliary disease and/or obstruction. Also, indicators of disease severity, that is, complication rates and mortality, are comparable in both cohorts. In contrast to Germany, malignant abscesses play a subordinate role in South Asia, while amoebic liver abscesses tend to dominate and affect younger patients there. 26
Liver transplant status appears infrequently, but compared with the adjusted control population, the risk in PLA patients is significantly increased, with an OR of 46 (Figure 3 ). Therefore, PLA should be considered as a serious consequence and thus potential risk factor after liver transplantation. This finding may contribute to the transplantation decision itself and demands to focus on modifiable preventive factors of liver transplant patients.
PLA without coded predisposition or risk factors affected only 9% of the cohort, corresponding to an incidence of well below one per 100,000. In this subgroup, the cases of Klebsiella liver abscess syndrome, which have been increasingly reported in recent years, are to be suspected. 27 , 28 , 29 However, a stratification according to pathogen type is not possible on the basis of diagnosis data, as these are not represented in the ICD‐10 system. Analysis of other potential risk factors confirmed the frequent presence of diabetes mellitus with an OR of 1.3 compared with the weighted healthy population (p < 0.001), but the correlation was unexpectedly low. 30 A recently published retrospective single‐centre study showed an increased mortality in PLA patients receiving PPI treatment. 31 In our study, we observed almost twice the frequency of PPI prescriptions in the year prior to PLA diagnosis compared with the adjusted comparison group, which could indicate a possible predisposition to infectious complications with PPI therapy. However, the analysis of upper abdominal symptom codes prior to the PLA diagnosis suggests that a relevant proportion of patients may have had an empirical PPI indication so that causality cannot be confirmed.
Coding of diagnostic and interventional procedures (e.g., abdominal sonography and needle aspiration) did not provide meaningful results in the data‐set. In 20%, a PLA was coded during the hospital stay without the expected diagnostic measures, especially, without ultrasound as additional imaging during the intervention, which indicates deficient coding quality. This reflects possible bias of the German hospital accounting system (G‐DRG), which is unique in Europe and may not provide sufficient revenue incentives for a complete diagnosis and documentation of symptoms. 32 Therefore, a systematic analysis of diagnostic and therapeutic procedures was not feasible. In contrast, a plausible analysis could be conducted for surgical interventions with obligatory coding, which were documented in 25% of patients. In 10% of all cases, a primary surgical therapy of the PLA could be suspected on the basis of the admission diagnosis and date of surgery within 2 days (n = 65 of 663). Insufficient procedure documentation is an inherent problem of SHI databases. To avoid the influence of documentation bias, the authors believe that there is a need for prospective surveys on the efficiency and safety of the different treatment approaches.
In the defined subgroup with sufficient follow‐up, a survival time analysis of patients with PLA could be completed for the first time and shows a 1‐year mortality rate of 9%. The prognosis importantly depends on the presence of an underlying malignant disease. Even in PLA patients without malignancy, the 5‐year mortality rate of 15% is relevantly increased. Nevertheless, the mortality does not appear to be high enough for purely palliative care and even in highly multimorbid and acutely debilitated patients, the start and continuation of active PLA treatment can be worthwhile. There possibly will be a demand for a structured treatment process that has not yet been adequately addressed.
Limitations
Any analysis of SHI routine data has intrinsic limitations. Although almost four million insured people were examined in this study, the sample is relatively small in relation to the total German population (5%). Nevertheless, this study represents the largest corresponding analysis for Europe to date. It is limited to in‐patient diagnoses, because the modalities of outpatient coding do not allow a similar approach for this indication. 33 , 34 The primary purpose for collecting SHI routine data is the billing of services, which means that a bias due to upcoding and rightcoding, as a specific issue of the German SHI accounting system, 35 is to be expected. Essential clinical aspects of care are not naturally recorded in the medical coding and therefore cannot be evaluated with the applied methodology. The gold standard for a correspondingly in‐depth analysis is a prospective clinical registry, the establishment of which would represent an important step toward quality assurance of PLA therapy. In order to obtain all necessary data on the clinical care of these patients, it is crucial that a registry needs to be multicentric and includes different European countries. As another step, European or national treatment guidelines for PLA would be welcome.
CONCLUSIONS
For the first time, a representative incidence and prevalence calculation for pyogenic (bacterial) liver abscesses was carried out in Germany. With an in‐patient coded prevalence of approx. eight per 100,000 persons (incidence: seven per 100,000), it represents a rare disease, with less than 1 case per 100,000 persons attributable to PLA without predisposition. The 1‐year mortality rate is 9% and is mainly associated with underlying malignancies. Our analysis also confirms predisposing factors, especially, diseases of the biliary system and malignancies. Prospective surveys and controlled clinical trials are needed for further health care research.
CONFLICT OF INTEREST STATEMENT
The authors declare that there is no conflict of interests.
AUTHOR CONTRIBUTIONS
Lisa Zimmermann, Christoph Lübbert and Thomas Karlas participated in the study conception and design. Lisa Zimmermann and Sebastian Wendt contributed to the data collection. Lisa Zimmermann, Sebastian Wendt, Christoph Lübbert and Thomas Karlas were involved in the analysis of the results. Lisa Zimmermann, Christoph Lübbert and Thomas Karlas draughted the manuscript and Sebastian Wendt contributed to the manuscript with important intellectual content. All authors read and approved the final version to be published.
Supporting information
Supplementary Material 1
ACKNOWLEDGEMENT
The authors would like to thank the Gesundheitsforen Leipzig GmbH for providing the German analysis database. This research received no specific grant from any funding agency in the public, commercial, or not‐for‐profit sectors.
Open Access funding enabled and organized by Projekt DEAL.
Zimmermann L, Wendt S, Lübbert C, Karlas T. Epidemiology of pyogenic liver abscesses in Germany: analysis of incidence, risk factors and mortality rate based on routine data from statutory health insurance. United European Gastroenterol J. 2021;9(9):1039–47. 10.1002/ueg2.12132
Thomas Karlas and Christoph Lübbert should be considered joint senior author.
German Register of Clinical Studies (DRKS): register number DRKS00021793.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author, upon reasonable request.
REFERENCES
- 1. Annesley J. On the treatment of abscess in the liver. Med Chir Rev. 1829;10:435–44. [PMC free article] [PubMed] [Google Scholar]
- 2. Lübbert C, Wiegand J, Karlas T. Therapy of liver abscesses. Viszeralmedizin. 2014;30:334–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jepsen P, Vilstrup H, Schønheyder HC, Sorensen HT. A nationwide study of the incidence and 30‐day mortality rate of pyogenic liver abscess in Denmark, 1977‐2002. Aliment Pharmacol Ther. 2005;21:1185–8. [DOI] [PubMed] [Google Scholar]
- 4. Meddings L, Myers RP, Hubbard J, Shaheen AA, Laupland KB, Dixon E, et al. A population‐based study of pyogenic liver abscesses in the United States: incidence, mortality, and temporal trends. Am J Gastroenterol. 2010;105:117–24. [DOI] [PubMed] [Google Scholar]
- 5. Pang TCY, Fung T, Samra J, Hugh TJ, Smith RC. Pyogenic liver abscess: an audit of 10 years' experience. World J Gastroenterol. 2011;17:1622–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Angelescu M, Pusch B, Feyerherd P, Rambach W, Schmitt S, Foitzik T. KF‐1.5 Der pyogene Leberabszess: eine interdisziplinäre Herausforderung (B). Augsburg;22.
- 7. Kaplan GG, Gregson DB, Laupland KB. Population‐based study of the epidemiology of and the risk factors for pyogenic liver abscess. Clin Gastroenterol Hepatol. 2004;2:1032–8. [DOI] [PubMed] [Google Scholar]
- 8. Mohsen AH, Green ST, Read RC, McKendrick MW. Liver abscess in adults: ten years experience in a UK centre. QJM. 2002;95:797–802. [DOI] [PubMed] [Google Scholar]
- 9. Kubovy J, Karim S, Ding S. Pyogenic liver abscess: incidence, causality, management and clinical outcomes in a New Zealand cohort. N Z Med J. 2019;132:30–5. [PubMed] [Google Scholar]
- 10. Serraino C, Elia C, Bracco C, Rinaldi G, Pomero F, Silvestri A, et al. Characteristics and management of pyogenic liver abscess: a European experience. Medicine (Baltim). 2018;97:e0628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Schubert I, Köster I, Küpper‐Nybelen J, Ihle P. Versorgungsforschung mit GKV‐Routinedaten. Nutzungsmöglichkeiten versichertenbezogener Krankenkassendaten für Fragestellungen der Versorgungsforschung. Bundesgesundheitsblatt ‐ Gesundheitsforsch ‐ Gesundheitsschutz. 2008;51:1095–105. 10.1007/s00103-008-0644-0 [DOI] [PubMed] [Google Scholar]
- 12. González‐Alcaide G, Peris J, Ramos JM. Areas of research and clinical approaches to the study of liver abscess. World J Gastroenterol. 2017;23:357–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mohan BP, Meyyur Aravamudan V, Khan SR, Chandan S, Ponnada S, Asokkumar R, et al. Prevalence of colorectal cancer in cryptogenic pyogenic liver abscess patients. Do they need screening colonoscopy? A systematic review and meta‐analysis. Dig Liver Dis. 2019;51:1641–5. 10.1016/j.dld.2019.08.016 [DOI] [PubMed] [Google Scholar]
- 14. Wichmann D, Königsrainer A, Schweizer U, Archid R, Nadalin S, Manncke S. Pyogenic liver abscesses caused by acute appendicitis: frequency and diagnostic and therapeutic recommendations surgical infections. 2020. https://pubmed.ncbi.nlm.nih.gov/32552531/ [DOI] [PubMed]
- 15. DIMDI . ICD‐10‐GM | OPS Version 2019: Internationale statistische Klassifikation der Krankheiten und verwandter Gesundheitsprobleme 10; Revision German Modification Version 2019 | Operationen‐ und Prozedurenschlüssel Version 2019. Available from: https://www.dimdi.de/dynamic/de/klassifikationen/
- 16. Kassenärztliche Bundesvereinigung (KBV) . Einheitlicher Bewertungsmaßstab (EBM): Stand: 4. Quartal 2019; 2019. Available from: https://www.kbv.de/media/sp/EBM_Gesamt_‐_Stand_4._Quartal_2019.pdf
- 17. WIdO/DIMDI . Amtlicher ATC‐Index mit DDD‐Angaben für Deutschland im Jahr; 2019. Available from: https://www.dimdi.de/dynamic/.downloads/arzneimittel/atcddd/atc‐ddd‐amtlich‐2019.pdf
- 18. Bundesamt für Soziale Sicherung Info‐Dateien auf Kassenartenebene; 2021. Accessed 8 March 2021.
- 19. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chron Dis. 1987;40:373–83. [DOI] [PubMed] [Google Scholar]
- 20. Quan H, Sundararajan V, Halfon P, Fong A, Burnand B, Luthi J‐C, et al. Coding algorithms for defining comorbidities in ICD‐9‐CM and ICD‐10 administrative data. Med Care. 2005;43:1130–9. [DOI] [PubMed] [Google Scholar]
- 21. Swart E, Gothe H, Geyer S, Jaunzeme J, Maier B, Grobe T, et al. Gute Praxis Sekundärdatenanalyse (GPS): Leitlinien und Empfehlungen. Gesundheitswesen. 2015;77:120–6. [DOI] [PubMed] [Google Scholar]
- 22. Tsai F‐C, Huang Y‐T, Chang L‐Y, Wang J‐T. Pyogenic liver abscess as endemic disease, Taiwan. Emerg Infect Dis. 2008;14:1592–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ko M‐C, Lin W‐H, Martini S, Chang Y‐H, Chiu C‐T, Li C‐Y. A cohort study of age and sex specific risk of pyogenic liver abscess incidence in patients with type 2 diabetes mellitus. Medicine (Baltim). 2019;98:e15366–e15366. 10.1097/MD.0000000000015366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zentrum für Krebsregisterdaten im Robert Koch‐Institut . Datenbankabfrage mit Schätzung der Inzidenz, Prävalenz und des Überlebens von Krebs in Deutschland auf Basis der epidemiologischen Landeskrebsregisterdaten. Mortalitätsdaten bereitgestellt vom Statistischen Bundesamt; 2019. Available from: www.krebsdaten.de/abfrage
- 25. Ofori‐Asenso R, Zomer E, Chin KL, Si S, Markey P, Tacey M, et al. Effect of comorbidity assessed by the Charlson comorbidity Index on the length of stay, costs and mortality among older adults hospitalised for acute stroke. Int J Environ Res Publ Health. 2018;15:2532. 10.3390/ijerph15112532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Jindal A, Pandey A, Sharma MK, Mukund A, Vijayaraghavan R, Arora V, et al. Management practices and predictors of outcome of liver abscess in adults: a series of 1630 patients from a liver unit. J Clin Exp Hepatol. 2021;11:312–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pichler C, Büchsel M, Rossen JW, Vavra M, Reuter S, Kern WV, et al. First report of invasive liver abscess syndrome with endophthalmitis caused by a K2 serotype ST2398 hypervirulent Klebsiella pneumoniae in Germany. New Microbes New Infect. 2016;17:77–80. 10.1016/j.nmni.2017.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wendt S, Lübbert C, Karlas T. An emerging cause for liver abscesses with poor prognosis. Gastroenterology. 2018;155:e18–e19. [DOI] [PubMed] [Google Scholar]
- 29. Siu LK, Yeh K‐M, Lin J‐C, Fung C‐P, Chang F‐Y. Klebsiella pneumoniae liver abscess: a new invasive syndrome. Lancet Infect Dis. 2012;12:881–7. 10.1016/S1473-3099(12)70205-0 [DOI] [PubMed] [Google Scholar]
- 30. Khim G, Em S, Mo S, Townell N. Liver abscess: diagnostic and management issues found in the low resource setting. Br Med Bull. 2019;132:45–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bettinger D, Martin D, Rieg S, Schultheiss M, Buettner N, Thimme R, et al. Treatment with proton pump inhibitors is associated with increased mortality in patients with pyogenic liver abscess. Aliment Pharmacol Ther. 2018;47:801–8. [DOI] [PubMed] [Google Scholar]
- 32. Schreyögg J, Milstein R. Bedarfsgerechte Gestaltung der Krankenhausvergütung –Reformvorschläge unter der Berücksichtigung von Ansätzen anderer Staaten: im Auftrag der Techniker Krankenkasse (TK); 2020. Available from: https://www.tk.de/resource/blob/2090886/90a4ec1624cb79d28da08e0edab46328/gutachten‐der‐krankenhausfinanzierung‐2020‐data.pdf
- 33. Zimmermann T, Kaduszkiewicz H, vd Bussche H, Schön G, Wegscheider K, Werle J, et al. Reliabilität ärztlicher Morbiditätsangaben zu chronischen Krankheiten. Ergebnisse einer Längsschnittstudie im hausärztlichen Bereich. Bundesgesundheitsblatt. 2012;55:260–9. [DOI] [PubMed] [Google Scholar]
- 34. Czwikla J, Domhoff D, Giersiepen K. ICD‐Codierqualität ambulanter Krebsdiagnosen in GKV‐Routinedaten. Z Evid Fortbild Qual Gesundhwes. 2016;118–119:48–55. https://zefq‐journal.com/article/S1865‐9217(16)30200‐8/fulltext [DOI] [PubMed] [Google Scholar]
- 35. Swart E, Schmitt J. Standardized reporting of secondary data Analyses (STROSA)—Vorschlag für ein Berichtsformat für Sekundärdatenanalysen. Z Evid Fortbild Qual Gesundhwes. 2014;108:511–6. https://pubmed.ncbi.nlm.nih.gov/25523850/ [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material 1
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, upon reasonable request.


