Abstract
Aim
To quantify lymphovascular invasion (LVI) and to assess the prognostic value in patients with pT1b esophageal adenocarcinoma.
Methods
In this nationwide, retrospective cohort study, patients were included if they were treated with surgery or endoscopic resection for pT1b esophageal adenocarcinoma. Primary endpoint was the presence of metastases, lymph node metastases, or distant metastases, in surgical resection specimens or during follow‐up. A prediction model to identify risk factors for metastases was developed and internally validated.
Results
248 patients were included. LVI was distributed as follows: no LVI (n = 196; 79.0%), 1 LVI focus (n = 16; 6.5%), 2–3 LVI foci (n = 21; 8.5%) and ≥4 LVI foci (n = 15; 6.0%). Seventy‐eight patients had metastases. The risk of metastases was increased for tumors with 2–3 LVI foci [subdistribution hazard ratio (SHR) 3.39, 95% confidence interval (CI) 2.10–5.47] and ≥4 LVI foci (SHR 3.81, 95% CI 2.37–6.10). The prediction model demonstrated a good discriminative ability (c‐statistic 0.81).
Conclusion
The risk of metastases is higher when more LVI foci are present. Quantification of LVI could be useful for a more precise risk estimation of metastases. This model needs to be externally validated before implementation into clinical practice.
Keywords: endoscopic mucosal resection, esophagectomy lLymphovascular invasion, LVI, lymph node metastases, prediction, quantification, risk assessment, submucosal esophageal adenocarcinoma, T1b adenocarcinoma
Key summary.
Summarise the established knowledge on this subject
Lymphovascular invasion (LVI) is an important risk factor for lymph node metastasis in patients with submucosal esophageal adenocarcinoma (EAC)
What are the significant and/or new findings of this study
The risk of metastases in patients with pT1b EAC is higher when more LVI foci are present
Quantification of LVI may further refine risk estimation in pT1b EAC
External validation of the model and more research regarding LVI quantification are required to confirm our findings
INTRODUCTION
The presence of lymph node metastases (LNM) is an important prognostic factor in submucosal esophageal adenocarcinoma (pT1b EAC). 1 Various risk factors for LNM have been identified including depth of infiltration, grade of differentiation, and lymphovascular invasion (LVI). 2 , 3 , 4 , 5 , 6
We recently developed a prediction model based on histopathological risk factors to estimate the risk for metastases in patients with pT1b EAC. 6 We showed that LVI was the strongest predictor for metastases with an estimated 5‐year risk of developing metastases ranging between 15.7% and 70.1% for pT1b EAC with LVI as compared to 5.9%–35.2% for those without LVI. 6 Other studies reported a worse 5‐year survival in patients with pT1b EAC with LVI compared to pT1b EAC without LVI (27% vs. 77%, p < 0.01). 7 , 8
LVI is often reported as a dichotomous parameter, either absent or present. 6 , 7 , 8 , 9 , 10 , 11 , 12 , 13 , 14 Intuitively, there may be a differential metastatic risk in case of only a single LVI focus versus a case with extensive LVI. This is supported by a study on gastric cancer in which a higher number of lymphatic tumor emboli was an independent predictor for LNM. 15 We hypothesized that the number of LVI foci in pT1b EAC is of influence on patient outcome. Quantification of LVI may possibly further classify these patients into low and high risk for the development of metastases.
The aim of this study was to quantify LVI and to assess the prognostic value in patients with pT1b EAC.
MATERIALS AND METHODS
This was a nationwide retrospective cohort study. Patients diagnosed with pT1b EAC between 1989 and 2016 were included. This study was approved by all Medical Ethical Review Committees in the participating centers (MEC‐2016‐050). A detailed description of in‐ and exclusion criteria, data collection, patient selection, reassessment of histopathological variables, and the statistical analyses can be found in our previous study, as the present study is an extension to our previous study. 6 Histopathological reassessment was performed by three gastrointestinal pathologists for 84 patients to calculate the inter‐observer agreement as explained in our previous study. 16 For the other 164 patients, histopathological reassessment was performed by one pathologist for LVI (к = 0.88) and differentiation grade (к = 0.77) because of the good and excellent interobserver agreement. 16
A model was developed to predict the risk of metastases in patients with pT1b EAC. 6 In the prediction model of the present study, quantification of LVI on a 4‐tier assessment scale is incorporated instead of interpreting LVI as a dichotomous parameter. The same patient cohort is used, and the same statistical analyses are performed as in our previous study. 6 The added value of LVI quantification to the model was assessed using the likelihood ratio test and quantified by the increase in Harrell's concordance index (c‐statistic).
Histopathological reassessment of LVI and LVI quantification
Whole case hematoxylin and eosin stained (H&E) slide sets were used for LVI reassessment. Immunohistochemical staining was not performed because formalin‐fixed paraffin embedded tissue blocks were not available. Initially, LVI was defined as being present or absent as in standard clinical practice. All slides were assessed with high power up to and including the submucosa. When LVI was present, all LVI foci were counted in every H&E slide that was available. In general, resection specimens of 2‐mm thickness were assessed when endoscopic resection was performed in contrast to 5‐mm thickness when surgical resection was performed. Figure 1 shows an example of an H&E slide with three LVI foci. The LVI focus number was the sum of all counted LVI foci in all H&E slides from one patient. In patients in whom LVI was analyzed by three pathologists, the highest LVI focus number was included for analysis. Patients were categorized into four groups based on the number of LVI foci: no LVI, 1 LVI focus, 2–3 LVI foci, or ≥4 LVI foci. This 4‐tier assessment scale was used to create different LVI foci groups with less LVI (1 LVI focus), moderate LVI (2–3 LVI foci), or extensive LVI (≥4 LVI foci).
FIGURE 1.
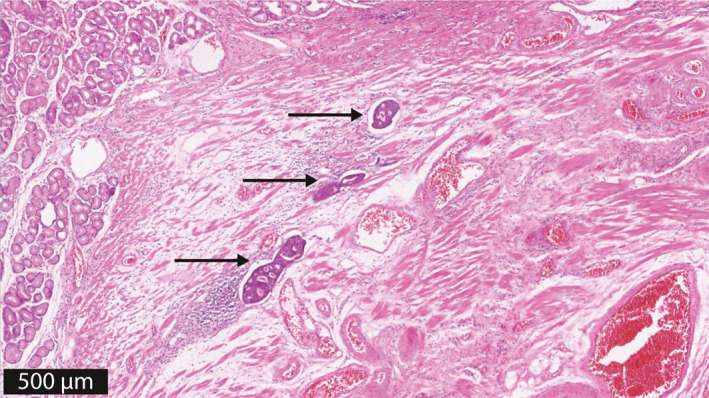
Quantification of LVI; three LVI foci, indicated by an arrow. This case nicely illustrated an example of three foci of LVI, which may very well all be located in the same lymph vessel
Endpoints
The primary endpoint was the presence metastases defined as LNM in surgical resection specimens or the development of metastases (LNM or distant metastases) during follow‐up. In case surgical resection was performed, at least 12 lymph nodes were required for adequate assessment of LNM. 17 The development of metastases during follow‐up was used as a surrogate endpoint in case no surgery was performed after endoscopic resection (ER), or if surgical resection specimen contained <12 lymph nodes without LNM. In these cases, a minimum follow‐up period of 2 years was required. If patients died within these 2 years, they were only included if cause of death was known.
RESULTS
In total, 248 patients with pT1b EAC were included. Patient characteristics are presented in Table 1. Primary endoscopic resection was performed in 82 of 248 (33.1%) patients and primary surgery was performed in 166 of 248 (66.9%) patients. Of patients who were treated with endoscopic resection, additional surgery was performed in 49/82 (59.8%) patients. Local recurrence was detected during follow‐up in 12 of 248 (4.8%) patients, of whom 11 also developed metastasis.
TABLE 1.
Patient characteristics and number of LVI foci (n = 248)
| Parameter | Total cohort (n = 248) |
|---|---|
| Gender, n (%) | |
| Male | 217 (87.5%) |
| Female | 31 (12.5%) |
| Median age, years (IQR) | 65.6 (57.8–72.5) |
| Presence of LVI, n (%) | 52 (21.0%) |
| LVI foci | |
| 1 | 16 |
| 2 | 10 |
| 3 | 11 |
| 4 | 6 |
| 5–10 | 9 |
| Metastases, n (%) | |
| LNM | 49 (19.8%) |
| Distant metastasis | 6 (2.4%) |
| LNM + distant metastasis | 23 (9.3%) |
Abbreviations: IQR, interquartile range; LNM, lymph node metastases; LVI, lymphovascular invasion.
Quantification of LVI
There were 52 patients with LVI positive tumors. The LVI focus number was distributed from 1 to 10 foci (Table 1). LVI quantification was determined by three gastrointestinal pathologist in 17/52 patients (F.tK., M.D., K.B.). In the remaining 35 patients, LVI quantification was determined by one pathologist (F.tK.) because the inter‐observer variability was excellent for LVI (к = 0.88). 16 The median LVI focus number was 2.5 [interquartile range (IQR) 1–4]. One, two, or three LVI foci were most often present (37/52%; 71.2%). LVI was categorized as follows: no LVI (n = 196; 79.0%), 1 LVI focus (n = 16; 6.5%), 2–3 LVI foci (n = 21; 8.5%), and ≥4 LVI foci (n = 15, 6.0%).
Metastases
Some 78 of 248 patients had metastases (Table 1), with a 5‐year cumulative incidence of 30.9% (95% CI 25.1–36.8%) (Figure 2). In patients treated with primary surgery (≥12 lymph node dissections), the median follow‐up time was 3.3 years (IQR 1.8–5.3). In patients treated with surgery (<12 lymph node dissections) or endoscopic resection only, the median follow‐up period was 5.5 years (IQR 4.9–7.7) The majority of patients developed LNM only (49/78). These patients were all treated with surgery; LNM were detected during surgery in 47 patients and during follow‐up in 2 patients. Six patients developed distant metastases only, and all were treated with surgery for EAC (without LNM). Adequate lymph nodes (>12) were resected in only 2 of these 6 patients. Twenty‐three patients developed both LNM and distant metastases. Surgery was performed in 22/23 patients, LNM was found in surgical resection specimen in 19 patients and during follow‐up in 3 patients. One patient was treated with endoscopic resection only due to comorbidity, metastases were detected during follow‐up. Of all patients who developed metastases during follow‐up, the median interval between EAC diagnosis and detection of metastases was 1.9 years (IQR: 1.2–4.9).
FIGURE 2.
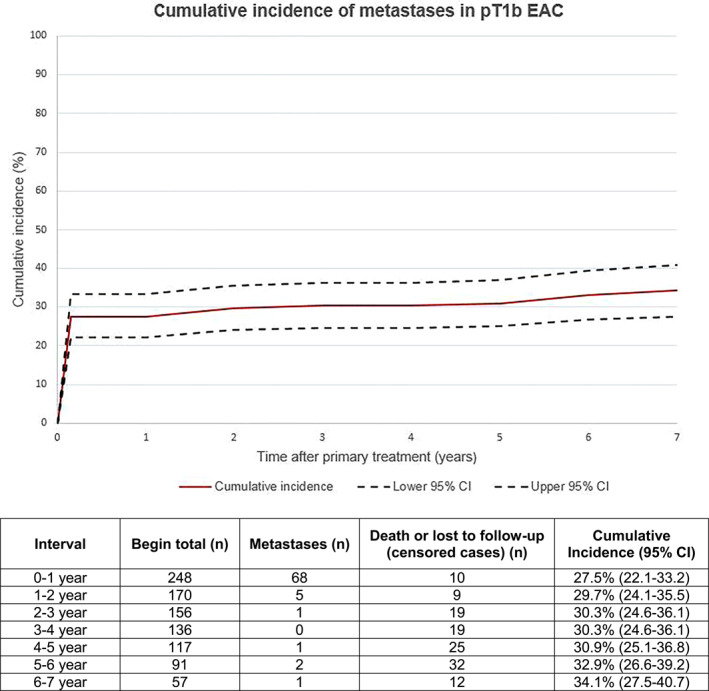
Cumulative incidence of developing metastases, with death (not related to esophageal adenocarcinoma or metastases) as competing risk. CI, confidence interval; EAC, esophageal adenocarcinoma
Risk factor analysis
Univariable analysis
The number of LVI foci was associated with the presence of metastases. Tumors with 1 LVI focus had metastases in 37.5%, tumors with 2–3 LVI foci had metastases in 71.4%, and tumors with ≥4 LVI foci had metastases in 93.3% (Table 2). In contrast, 22% of patients without LVI developed metastases.
TABLE 2.
Univariable and multivariable subdistributional hazard regression analyses of risk factors associated with metastases (no metastases; n = 170, metastasis; n = 78)
| Variable | No metastases (n = 170) | Metastases (n = 78) | Univariable subdistributional hazard regression | Multivariable subdistributional hazard regression | ||
|---|---|---|---|---|---|---|
| SHR (95% CI) | p‐value | SHR (95% CI) | p‐value | |||
| Sex, n (%) | ||||||
| Female | 24 (77%) | 7 (23%) | Reference | – | – | |
| Male | 146 (67%) | 71 (33%) | 1.51 (0.74–3.10) | 0.26 | ||
| Differentiation grade, n (%) | ||||||
| G1/G2 (good/moderate) | 121 (75%) | 41 (25%) | Reference | Reference | ||
| G3/4 (poor/undifferentiated) | 49 (57%) | 37 (43%) | 1.78 (1.20–2.65) | <0.01 | 0.98 (0.65–1.50) | 0.94 |
| Median tumor length (mm) (IQR) | 20 (13–29) | 30 (25–44) | 1.39 (1.25–1.53)* | <0.01 | 1.23 (1.09–1.38)* | <0.01 |
| Median submucosal invasion (μm) (IQR) | 958 (409–2100) | 2165 (1173–3500) | 1.13 (1.08–1.18)** | <0.01 | 1.08 (1.02–1.14)** | <0.01 |
| LVI, n (%) | ||||||
| No LVI | 153 (78%) | 43 (22%) | Reference | – | Reference | |
| LVI 1 | 10 (62.5%) | 6 (37.5%) | 1.81 (0.84–3.90) | 0.13 | 1.72 (0.89–3.32) | 0.11 |
| LVI 2–3 | 6 (28.6%) | 15 (71.4%) | 3.90 (2.47–6.15) | <0.01 | 3.39 (2.10–5.47) | <0.01 |
| LVI ≥4 | 1 (6.7%) | 14 (93.3% | 5.54 (3.82–8.04) | <0.01 | 3.81 (2.37–6.10) | <0.01 |
Abbreviations: CI, confidence interval; IQR, interquartile range; LVI, lymphovascular invasion; SHR, subdistribution hazard ratio.
*For every increase of 10 mm.
**For every 500 μm increase of sm invasion.
Multivariable analysis
For every increase of tumor invasion with 500 μm, the subdistribution hazard of developing metastases increased with 1.08 (95% CI 1.02–1.14) (Table 2). The presence of 2–3 LVI foci (SHR 3.39, 95% CI 2.10–5.47) and the presence of ≥4 LVI foci (SHR 3.81, 95% CI 2.37–6.10) were independent predictors for metastases compared to patients without LVI. In contrast, the presence of only one LVI focus was not significantly correlated with metastases after multivariable analysis. For every increase of tumor size with 10 mm, the subdistribution hazard of developing metastases increased with 1.23 (95% CI 1.09–1.38). Poor differentiation grade was not an independent risk factor for metastases (SHR 0.98, 95% CI 0.65–1.50), and this variable was therefore not incorporated into the prediction model.
Prediction model development and validation
The 5‐year risk of developing metastases for different combinations of histopathological tumor characteristics is illustrated in Table 4. The prediction model with LVI incorporated as a dichotomous variable is presented in Table 3. 6 In Table 4, the predicted score was 16.1% (95% CI 6.2–29.2) for patients with pT1b EAC ≥20 mm, sm1 invasion depth without LVI, compared to 22.2% (95% CI 6.2–45.3) when one LVI focus is present, 37.0% (95% CI 16.0–62.3) when 2–3 LVI foci are present, and 47.1% (95% CI 21.1–72.9) when ≥4 LVI foci are present. Internal validation of the prediction model showed a good discriminative ability with a c‐statistic of 0.81 (95% CI 0.74–0.87), which did not increase compared to the c‐statistic of the previous model (p = 0.71).
TABLE 4.
Score chart; 5‐year risk (%) of developing metastases for different combinations of histopathological characteristics in pT1b esophageal adenocarcinoma; LVI incorporated as absent, 1 LVI focus, 2–3 LVI foci, or ≥4 LVI foci
| Tumor size | Submucosal invasion | LVI− | LVI 1× | LVI 2–3 | LVI ≥4 |
|---|---|---|---|---|---|
| % (95% CI) | % (95% CI) | % (95% CI) | % (95% CI) | ||
| <20 mm | sm1 | 5.9 (2.3–11.2) | 10.9 (3.0–24.4) | 19.5 (7.4–37.7) | 25.7 (9.7–48.3) |
| sm2 | 7.3 (2.6–13.8) | 13.4 (3.8–30.1) | 24.1 (8.6–44.2) | 31.6 (11.0–55.5) | |
| sm3 | 14.1 (7.9–21.9) | 26.3 (10.6–45.3) | 43.5 (26.6–61.5) | 54.4 (33.7–72.8) | |
| ≥20 mm | sm1 | 16.1 (6.2–29.2) | 22.2 (6.2–45.3) | 37.0 (16.0–62.3) | 47.1 (21.1–72.9) |
| sm2 | 19.4 (8.6–32.2) | 26.3 (8.4–51.0) | 44.4 (20.2–66.2) | 55.2 (26.4–77.9) | |
| sm3 | 35.2 (25.8–44.7) | 48.4 (21.5–69.3) | 70.2 (56.6–81.4) | 80.6 (72.3–88.5) |
Abbreviations: CI, confidence interval; LVI, lymphovascular invasion; sm, submucosal.
TABLE 3.
Score chart; 5‐year risk (%) of developing metastases for different combinations of histopathological characteristics in pT1b esophageal adenocarcinoma; LVI incorporated as present or absent
| Tumor size | Submucosal invasion | LVI− | LVI+ |
|---|---|---|---|
| % (95% CI) | % (95% CI) | ||
| <20 mm | sm1 | 5.9 (2.3–11.2) | 15.7 (6.0–29.3) |
| sm2 | 7.3 (2.6–13.8) | 19.3 (6.3–36.8) | |
| sm3 | 14.1 (7.9–21.9) | 34.7 (19.7–50.8) | |
| ≥20 mm | sm1 | 16.1 (6.2–29.2) | 38.8 (17.0–61.4) |
| sm2 | 19.4 (8.6–32.2) | 45.6 (20.8–67.9) | |
| sm3 | 35.2 (25.8–44.7) | 70.1 (60.5–78.7) |
Abbreviations: CI, confidence interval; LVI, lymphovascular invasion; sm, submucosal.
DISCUSSION
LVI has so far only been evaluated as a dichotomous parameter: either absent or present. 6 , 7 , 8 , 9 In this study, we aimed to determine whether quantification of LVI provides additional prognostic information in patients with pT1b EAC. The results of our study show that the presence of more LVI foci was correlated with a higher risk for metastases. We believe that quantification of LVI may further classify pT1b EAC for a more accurate risk estimation of metastases.
Accurate risk estimation is important for shared decision‐making, whether or not to undergo adjuvant therapy after ER of pT1b EAC. Compared to the risk in LVI+ patients in the previous model, the metastases risk in the current model is lower in case of 1 LVI focus and higher in case of 2–3 LVI foci and ≥4 LVI foci. 6 As a consequence, a lower metastases risk can result in the decision to perform endoscopic surveillance instead of esophagectomy and a high metastases risk may result in an advice to offer neo‐adjuvant therapy before esophagectomy. The more accurate this prediction is, the better a patient can be informed and the better the decision about (neo‐)adjuvant therapy can be made.
Remarkable is that the presence of only 1 LVI focus was not an independent predictor for metastases. The predicted 5‐year metastases risk in patients with only 1 LVI focus is more in line with the risk in patients without LVI. One could argue that the presence of 1 LVI focus is negligible.
To our knowledge, this is the first study that evaluates the quantification of LVI in pT1b EAC. We could therefore not compare our results with previous studies. LVI has also been identified as a strong predictor for LNM in other cancer types. 15 , 18 The number of lymphatic tumor emboli was an independent predictor for LNM in early gastric cancer with LVI. 15 The more lymphatic tumor emboli were present, the higher the LNM risk. 15
Despite the promising results of our study, it is unlikely that the application of the current model will change clinical practice soon. The difference in predicted metastasis risk between the LVI foci groups is relatively small and still high in all scenarios. Although the metastases risk in patients with only 1 LVI focus is much lower than the risk in patients with 2–3 or ≥4 LVI foci, the advice for adjuvant treatment or active surveillance after ER will probably not change based on the number of LVI foci. We are looking for a better risk assessment for metastasis to make active surveillance possible on the one hand. On the other hand, the presence of extensive LVI could be a reason to combine surgery with neo‐adjuvant therapy. The better this risk assessment, the better we can apply tailored therapy for patients with pT1b EAC. Therefore, further research on LVI quantification is necessary to assess whether it is possible to identify a subgroup of patients with LVI‐positive tumors with a low or high risk of metastases.
Our study is subject to certain limitations. First, the retrospective design of our study could result in information and selection bias. There was no standard follow‐up regime, which could have resulted in a different number of reported and actual numbers of metastases. Second, although the inter‐observer agreement for LVI was excellent as presented in our previous study, differences in LVI foci between pathologists were not discussed in a consensus meeting. 16 Moreover, when multiple LVI foci were detected, it was uncertain whether these foci represented LVI in different vascular structures. It is very well possible that multiple foci in the H&E section actually represented tumor localization in the same vascular structure. Third, additional immunohistochemical staining was not performed in the assessment of LVI and this may improve LVI detection. 19 Moreover, retraction artifacts related to tissue specimen preparation are difficult to distinguish from LVI in H&E slides. 20 Fourth, endoscopic and surgical resection specimens were combined in the results of our study, which introduces heterogeneity. Sampling and processing of endoscopic and surgical resection specimens are different (2 vs. 5 mm) and this might introduce bias in histological interpretation. In addition, the number of LVI foci was counted in every available H&E slide. It might be that the number of LVI foci is higher when more slides are available in one patient. However, studies about this subject are lacking. The last limitation is that external validation of our prediction model was not performed. Although our sample size was large, it was still not large enough to perform external validation. As a consequence, we do not know whether our model has adequate predictive value to be used in clinical practice. External validation is desirable when implementing it into daily clinical practice.
A major strength of our study is that all histopathological slides and tumor characteristics were reassessed by gastrointestinal pathologists. The incorporated tumor characteristics were valid without missing data. Another strength of the study is the cooperation with the Netherlands Cancer Registration, which enabled us to incorporate all eligible patients.
To conclude, this prediction model suggests that quantification of LVI may further refine risk estimation in pT1b EAC. External validation of the model and more research regarding LVI quantification are required to confirm our findings.
CONFLICT OF INTEREST
The authors have no conflicts of interest to declare.
van de Ven SEM, Suzuki L, Gotink AW, ten Kate FJC, Nieboer D, Weusten BLAM, et al. Lymphovascular invasion quantification could improve risk prediction of lymph node metastases in patients with submucosal (T1b) esophageal adenocarcinoma. United European Gastroenterol J. 2021;9(9):1066–73. 10.1002/ueg2.12151
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
REFERENCES
- 1. van der Schaaf M, Johar A, Wijnhoven B, Lagergren P, Lagergren J. Extent of lymph node removal during esophageal cancer surgery and survival. J Natl Cancer Inst. 2015;107. [DOI] [PubMed] [Google Scholar]
- 2. Barbour AP, Jones M, Brown I, Gotley DC, Martin I, Thomas J, et al. Risk stratification for early esophageal adenocarcinoma: analysis of lymphatic spread and prognostic factors. Ann Surg Oncol. 2010;17:2494–502. [DOI] [PubMed] [Google Scholar]
- 3. Liu L, Hofstetter WL, Rashid A, Swisher SG, Correa AM, Ajani JA, et al. Significance of the depth of tumor invasion and lymph node metastasis in superficially invasive (T1) esophageal adenocarcinoma. Am J Surg Pathol. 2005;29:1079–85. [PubMed] [Google Scholar]
- 4. Leers JM, DeMeester SR, Oezcelik A, Klipfel N, Ayazi S, Abate E, et al. The prevalence of lymph node metastases in patients with T1 esophageal adenocarcinoma a retrospective review of esophagectomy specimens. Ann Surg. 2011;253:271–8. [DOI] [PubMed] [Google Scholar]
- 5. Shimada H, Nabeya Y, Matsubara H, Okazumi S‐i, Shiratori T, Shimizu T, et al. Prediction of lymph node status in patients with superficial esophageal carcinoma: analysis of 160 surgically resected cancers. Am J Surg. 2006;191:250–4. [DOI] [PubMed] [Google Scholar]
- 6. Gotink AW, van de Ven SEM, Ten Kate FJ, Nieboer D, Suzuki L, Weusten BLAM, et al. Individual risk calculator to predict lymph node metastases in patients with submucosal (T1b) esophageal adenocarcinoma: a multicenter cohort study. Endoscopy. 2021. [DOI] [PubMed] [Google Scholar]
- 7. Watanabe M, Kuwano H, Araki K, Kawaguchi H, Saeki H, Kitamura K, et al. Prognostic factors in patients with submucosal carcinoma of the oesophagus. Br J Cancer. 2000;83:609–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cen P, Hofstetter WL, Correa AM, Wu T‐T, Lee JH, Ross WA, et al. Lymphovascular invasion as a tool to further subclassify T1b esophageal adenocarcinoma. Cancer. 2008;112:1020–7. [DOI] [PubMed] [Google Scholar]
- 9. Manner H, Wetzka J, May A, Pauthner M, Pech O, Fisseler‐Eckhoff A, et al. Early‐stage adenocarcinoma of the esophagus with mid to deep submucosal invasion (pT1b sm2‐3): the frequency of lymph‐node metastasis depends on macroscopic and histological risk patterns. Dis Esophagus. 2017;30:1–11. [DOI] [PubMed] [Google Scholar]
- 10. Torres CM, Wang HH, Turner JR, Richards W, Sugarbaker D, Shahsafaei A, et al. Pathologic prognostic factors in esophageal squamous cell carcinoma: a follow‐up study of 74 patients with or without preoperative chemoradiation therapy. Mod Pathol. 1999;12:961–8. [PubMed] [Google Scholar]
- 11. Tomita N, Matsumoto T, Hayashi T, Arakawa A, Sonoue H, Kajiyama Y , et al. Lymphatic invasion according to D2‐40 immunostaining is a strong predictor of nodal metastasis in superficial squamous cell carcinoma of the esophagus: algorithm for risk of nodal metastasis based on lymphatic invasion. Pathol Int. 2008;58:282–7. [DOI] [PubMed] [Google Scholar]
- 12. Amano T, Matsumoto T, Hayashi T, Arakawa A, Sonoue H, Kajiyama Y, et al. Subepithelial extension of squamous cell carcinoma in the esophagus: histopathological study using D2‐40 immunostaining for 108 superficial carcinomas. Pathol Int. 2007;57:759–64. [DOI] [PubMed] [Google Scholar]
- 13. Mori D, Yamasaki F, Shibaki M, Tokunaga O. Lateral peritumoral lymphatic vessel invasion can predict lymph node metastasis in esophageal squamous cell carcinoma. Mod Pathol. 2007;20:694–700. [DOI] [PubMed] [Google Scholar]
- 14. Inoue A, Moriya H, Katada N, Tanabe S, Kobayashi N, Watanabe M, et al. Intratumoral lymphangiogenesis of esophageal squamous cell carcinoma and relationship with regulatory factors and prognosis. Pathol Int. 2008;58:611–19. [DOI] [PubMed] [Google Scholar]
- 15. Park JW, Ahn S, Lee H, Min B‐H, Lee JH, Rhee P‐L, et al. Predictive factors for lymph node metastasis in early gastric cancer with lymphatic invasion after endoscopic resection. Surg Endosc. 2017;31:4419–24. [DOI] [PubMed] [Google Scholar]
- 16. Gotink AW, Ten Kate FJ, Doukas M, Wijnhoven BP, Bruno MJ, Looijenga LH, et al. Do pathologists agree with each other on the histological assessment of pT1b oesophageal adenocarcinoma? United Eur Gastroenterol J. 2019;7:261–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dutkowski P, Hommel G, Bottger T, Schlick T, Junginger T. How many lymph nodes are needed for an accurate pN classification in esophageal cancer? Evidence for a new threshold value. Hepato‐Gastroenterology. 2002;49:176–80. [PubMed] [Google Scholar]
- 18. Blok JM, Pluim I, Daugaard G, Wagner T, Jóźwiak K, Wilthagen EA, et al. Lymphovascular invasion and presence of embryonal carcinoma as risk factors for occult metastatic disease in clinical stage I nonseminomatous germ cell tumour: a systematic review and meta‐analysis. BJU Int. 2020;125:355–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hosono I, Miyahara R, Furukawa K, Funasaka K, Sawada T, Maeda K, et al. Use of Immunostaining for the diagnosis of Lymphovascular invasion in superficial Barrett's esophageal adenocarcinoma. BMC Gastroenterol. 2020;20:175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kojima M, Shimazaki H, Iwaya K, Kage M, Akiba J, Ohkura Y, et al. Pathological diagnostic criterion of blood and lymphatic vessel invasion in colorectal cancer: a framework for developing an objective pathological diagnostic system using the Delphi method, from the Pathology Working Group of the Japanese Society for Cancer of the Colon and Rectum. J Clin Pathol. 2013;66:551–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.


