Abstract
Introduction
While the etiopathogenesis of functional gastrointestinal disorders (FGIDs) is not completely understood, alterations of the intestinal microbiome have been observed. Antibiotics can induce dysbiosis, but whether antibiotics are a risk factor for the onset of FGIDs is uncertain. Antibiotics have been reported as both a risk factor for new onset FGID but also as a therapy for existing FGID. This study aimed to estimate the fraction of cases where antibiotics provoked the onset of FGID.
Method
Electronic medical records were obtained from general practices (primary care) in the United Kingdom. Dates of antibiotic prescription (AP) were compared with first date of FGID diagnosis and contrasted across three prevalent FGIDs and controls without gastrointestinal disorders.
Results
There were 10,926 GI healthy controls, 4326 IBS alone, 3477 FD alone, 340 chronic constipation and 4402 with overlap of multiple conditions. Both the prevalence of AP and rate were higher in FGID patients and increased with diagnosis of multiple FGIDs. 7%–14% of FGID patients were prescribed their first recorded antibiotic in the 12 months prior to their first FGID diagnosis and 20%–33% were prescribed an antibiotic in the same period. Differences between FGID groups were not accounted for by social deprivation and only rate of AP was moderated by social deprivation. In contrast, only 5%–10% of patients ever had a gastrointestinal infection recorded and only 1.5%–3.5% prior to their first FGID diagnosis.
Conclusion
These data indicate that antibiotics are prescribed prior to FGID diagnosis in a significant minority of care‐seeking FGID patients, opening the potential for this medication to contribute to the pathophysiology. APs appears to mostly be for non‐gastrointestinal conditions.
Keywords: antibiotics, dysbiosis, functional gastrointestinal disorders, gastrointestinal infection, social deprivation
Key summary.
Established knowledge on this subject
Antibiotics alter the gastrointestinal microbiome which is thought to be relevant to functional gastrointestinal disorders (FGIDs)
Whether antibiotics are helpful or provocative of symptom is unclear
What are the significant and/or new findings of this study?
Antibiotics are prescribed more often in patients with FGIDs and the rate increases with multiple disorders
A significant minority of of FGID individuals were prescribed antibiotics within the 12 months prior to first FGID diagnosis
INTRODUCTION
The functional gastrointestinal disorders (FGIDs), including irritable bowel syndrome (IBS), functional dyspepsia (FD) and functional constipation (FC), are often described as disorders of the brain‐gut axis. 1 The data to support this assertion is compelling with elevated levels of anxiety and depression 2 among individuals meeting Rome criteria 3 for FGIDs when compared to healthy controls other patient cohorts. Similarly, individuals meeting criteria for IBS have been shown to have greater involvement of emotion‐processing brain regions in response to experimentally induced afferent nociceptive signals. 4 , 5
On the other hand, the term functional in FGIDs has been challenged for some time now as in some cases an organic explanation for patient‐reported symptoms are now identified. 6 In addition to the brain‐gut axis, it is possible to conceive of a brain‐gut‐immune system triangle. Immune dysregulation has been demonstrated in both IBS 7 , 8 and FD 8 with elevated intestinal mast cells in IBS and elevated levels of circulating tumour necrosis factor‐alpha (TNF‐α) also in IBS. 9 The finding of mast cells in close proximity to pain receptors in the intestinal epithelium 10 leads to the hypothesis that a subtle influx of pro‐inflammatory cells might explain a fraction of patient‐reported pain that occurs in the absence of visible pathology in the gastrointestinal tract. To complete the triangle, elevated levels of TNF‐α have been shown to be associated with elevated levels of anxiety in an unselected community sample. 9 Immune dysregulation has been associated with both functional and organic gastrointestinal disorders. 8 While these data are inconclusive as to specific causal pathways, they do suggest a complex interaction between the gut, the immune system and the brain.
Additionally, the gastrointestinal immune system is known to be influenced by the gastrointestinal microbiome, and some microbes are identified as being more likely to trigger an immune response than others. 11 When in a state of eubyosis 12 there is a high level of both species density and diversity, however when dysbiosis occurs the density, diversity and the metabolic functions of the intestinal microbes are altered maladaptively. 13 This has been linked to both organic gastrointestinal disease such as cancer 14 but also to functional gastrointestinal disease. 12 , 15 , 16 Hence the maintenance of eubyosis is likely important for human health.
One well‐documented cause of dysbiosis is diet, 17 with some dietary habits being associated with altered species density and diversity and the potential loss of beneficial bacteria. Another potential cause of dysbiosis is antibiotic use. Depending on the specific antibiotic and the therapeutic regimen, antibiotics can result in short or long‐term loss of microbial diversity in the gut. 18 However the negative effects of antibiotics can be buffered by an individual's lifestyle (including diet) and age. 19 The situation is further complicated by antibiotics being used to treat some gastrointestinal conditions, 20 hence suggesting that antibiotics can be beneficial or harmful, depending maybe on how and when they are used, with evidence that selective use of antibiotics may improve FGID symptoms once they occur. 21
In 2002, Maxwell and colleagues 22 identified antibiotic use as the strongest predictor of new functional symptom onset, having a considerably stronger effect than demographics or psychological traits, which have also been strongly associated with a number of FGIDs. More recently, antibiotic use in response to enteric infection has been demonstrated to be positively associated with the onset of post‐infectious IBS 23 although whether the antibiotics were causally associated cannot be determined from that study. In a chart review study Paula and colleagues 24 identified antibiotic treatment for a non‐enteric infection was an independent risk factor differentiating new onset FGID individuals from GI‐healthy controls, adjusting for age and gender, suggesting a potential causal role in FGID pathogenesis. Further, in a questionnaire study Koloski and colleagues 25 showed that antibiotic use in the 3 months prior to FGID symptom onset was a risk factor over and above early life factors. Baldassare and colleagues 26 showed that use of antibiotics in infants was the strongest predictor of new onset FGID symptoms (particularly regurgitation and colic) compared to breast feeding, mode of delivery, gender and gestational age. Antibiotic treatment has also been shown to impair epithelial barrier function 27 which has, in turn, been shown to be a feature of some FGIDs, 28 , 29 further suggesting a potential role for antibiotics in the pathogenesis of FGIDs.
While previous work has provided evidence, that is, suggestive of a predictive role for antibiotic use in the pathogenesis of FGIDs, it is also plagued by the methodological limitations of generally small sample sizes and potential recall bias in questionnaire studies. We therefore sought to determine in what fraction of individuals with FGID had evidence of antibiotic use prior to being diagnosed with an FGID and how proximal in time was antibiotic use was to diagnosis from a substantial sample of general practice records in the United Kingdom. Using medical records from UK general practitioners is particularly appropriate for this study given the tendency for patients to remain with a given practice for prolonged periods. While it is unlikely that antibiotic use explains all incident cases of FGIDs, understanding in what fraction of FGID cases the use of antibiotics precedes diagnosis will yield an estimate of how large a role antibiotic use might play in FGID onset. Further, given evidence that antibiotic prescription (AP) is affected by socioeconomic factors 30 we sought to confirm this finding in FGID patients and evaluated whether differences between patient groups in antibiotic prescribing were moderated by a measure of socioeconomic deprivation.
METHOD
The methodology of this study has been reported earlier. 31 To summarize, data were obtained from electronic medical records via The Health Improvement Network (THIN). 32 The THIN system collects elements of the electronic medical record of general practices across the United Kingdom and its patient base has been shown to be broadly representative of the UK population. 32 Further, the diagnostic validity of the medical records in THIN has been demonstrated. 33 Ethics approval for this process was obtained via the National Health Service (UK) South‐East Multi‐Centre Research Ethics Committee and funding provided by the Psychology Department of Macquarie University (Sydney, Australia).
Patients were included in this study if they had at least 5 years of medical record available, a GP diagnosis of IBS, FD or FC and an acceptable patient record quality flag in the THIN system.
An IBS diagnosis was identified via the Read codes 34 14CF.00, J521000, J521.00, J521.11, J521.13, FD via a single occurrence of Read codes 1958.00, 8Hg5.00, 8Hl0.00, 9NNK.00, E264400, Eu45318, J16y400 or multiple occurrences of codes J16y412, 195.00, 1954.00 and constipation via 7728100, 8138.00, J503.00, J503100, J503y00, J503z00, Jyu5100, J520000, J520300, E264500, J520.00, J520100, J520200, J520400. It is important to note that general practitioners will often only record diagnoses where there is some clinical purpose in doing so, and this may lead to under‐reporting of some conditions, such as constipation, where there is no specific clinical requirement to report the condition.
Antibiotic prescription was identified via the British National Formulary 35 (BNF) code 5.1 (antibacterials). We note that THIN records prescriptions rather than actual consumption of medications. Gastrointestinal infections were identified through Read codes A07*, A08*, A0Y* and A0z*. These codes include a wide range of bacterial and viral infections of both the upper and lower gastrointestinal tract. Each diagnosis and prescription record has an associated date from which order of first diagnoses and first prescriptions can be identified. It is noted however that these are the first dates available from records of the medical practice from which these data were extracted and does not preclude earlier diagnoses or prescriptions at other medical practices or health facilities.
Comparisons across patient groups with respect to dichotomous outcomes, such as percentage of patients with a GI infection recorded, were made via unconditional logistic regression while quantitative outcomes, such as number of APs per 5‐year period, were made using multiple regression with nonparametric bootstrap inference (2000 bootstrap samples) due to the non‐normal distribution. Contrasts across FGID groups was made unadjusted and after adjusting for age, gender and socioeconomic deprivation (Townsend score).
Analysis of moderation of trends observed used the Townsend scale of socioeconomic deprivation 36 on a five‐point scale of increasing deprivation. Moderation was evaluated via the statistical interaction of linear Townsend score and categorical disease group in unconditional logistic regression models for AP ever and prior to FGID diagnosis (both yes/no) and multiple regression for rate of AP per 5‐year period. In multiple regression models formal statistical inference employed the nonparametric bootstrap with 2000 bootstrap iterations We note that the large sample used in this study yields high statistical power for even quite subtle effect sizes and hence an emphasis on effect size is recommended. Further, Townsend score was available for 22,375 of the 23,471 patients in this study and for all 12,545 patients diagnosed with one or more FGIDs.
RESULTS
The sample has been described previously 31 but patients were classified as gastrointestinal controls (10,926), IBS alone (4326), FD alone (3477), FC (340) or overlap of multiple conditions (4402). The sample was predominantly female in all groups, 55% in controls, 73% in IBS alone, 59% in FD alone, 66% in constipation alone and 71% in the multiple FGID group. Meeting the inclusion criteria of prolonged period of medical record resulted in an older group of patients. The sample was generally older and observed well into adulthood with the age at first FGID diagnosis mean 55 (SD = 13) for IBS alone, 66 (SD = 10) for FD alone, 74 (SD = 10) for constipation alone and 59 (SD = 14) for those with multiple FGIDs. In contrast, controls were on average born approximately 10 years earlier than FGID patients and were at an average age 71 years (SD = 10) when data collection ceased.
Use of antibiotics by patient group
Overall, 85% (19,969) of patients had been prescribed an antibiotic at an average rate of 0.67 (SD = 1.16) per 5 years of medical record. All FGID patient groups were prescribed antibiotics at a higher rate than GI controls (all p < 0.001; Figure 1) and patients with multiple FGID diagnoses were prescribed antibiotics at a higher rate than those with a single FGID (all p < 0.001; Figure 1).
FIGURE 1.
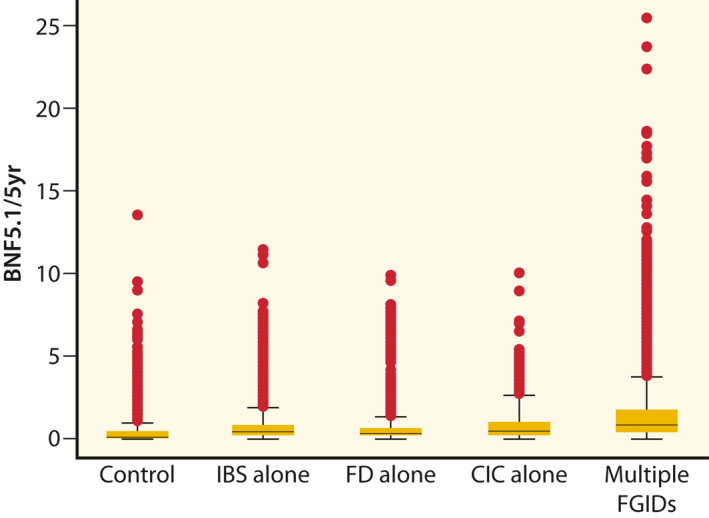
Distribution of number of antibiotic prescriptions per 5 years. The distribution of number of prescriptions via box plots in which the lower and upper boundaries of each box represent the 25th and 75th percentiles of the distribution of each patient group is described. The line in the middle of the box represents the 50th percentile (median) and values above the 75th percentile are represented by solid dots
The first record of AP was found in the medical record of 95% of FGID patients prior to their first record of FGID diagnosis. In 10% (95% confidence interval 9.8–10.9) of FGID patients the first AP occurred within 12 months of first FGID diagnosis. Patients with multiple FGIDs (14.4%) were more likely to have their first AP within 12 months of first FGID diagnosis than those with a single FGID (6.6%–8.3%; all p ≤ 0.004; Table 1).
TABLE 1.
Relationship between antibiotic use and FGID status
| Group | Ever antibiotic a | Antibiotic rate b | 1st antibiotic c | OR (95% confidence interval) p value | Any antibiotic d | OR (95% confidence interval) p value |
|---|---|---|---|---|---|---|
| % (n) | Mean (SD) | % (n) | % (n) | |||
| GI‐controls | 76.1 (8315) | 0.36 (0.61) | n/a | n/a | n/a | n/a |
| IBS only | 91.2 (3957) | 0.70 (0.90) | 8.3 (319) | 0.53 (0.46, 0.62) <0.001 | 25.2 (1089) | 0.70 (0.64, 0.77) <0.001 |
| FD only | 90.5 (3148) | 0.54 (0.79) | 7.8 (239) | 0.50 (0.43, 0.58) <0.001 | 24.4 (849) | 0.67 (0.61, 0.74) <0.001 |
| Constipation only | 92.1 (313) | 0.97 (1.35) | 6.6 (12) | 0.42 (0.23, 0.76) 0.004 | 20.0 (68) | 0.52 (0.39, 0.68) <0.001 |
| Multiple FGIDs | 96.2 (4236) | 1.49 (1.97) | 14.1 (583) | Reference | 32.6 (1433) | Reference |
Abbreviations: n/a, not applicable; Reference, reference category for odds ratios.
Percent (count) ever had antibiotic prescription in medical record.
Mean (SD) number of antibiotic prescriptions per 5 years.
First antibiotic prescription occurs within 12 months of first recorded FGID diagnosis.
Any antibiotic prescription occurs within 12 months of first recorded FGID diagnosis.
In contrast, 27% of FGID patients had a record of an antibiotic in the 12 months prior to their first FGID diagnosis, regardless of whether it was their first antibiotic or not (Table 1). This was more likely to occur in patients with multiple FGIDs (33%) than single (20%–25%; Table 1). Controlling for age, gender and social deprivation (Townsend score) does not remove the statistical significance of the effect of FGID status on ever having been prescribed an antibiotic (p < 0.001), first antibiotic prescribed within 12 months prior to FGID diagnosis (p = 0.004) or any antibiotic prescribed within 12 months prior to FGID diagnosis (p < 0.001).
There was an increasing trend in whether antibiotics had ever been prescribed with increasing deprivation score (Table 2; p < 0.001) which was not moderated by patient group (p = 0.3). However the trend was relatively subtle with odds ratio 1.07 (95% confidence interval 1.04, 1.10). There was no evidence of a trend in first antibiotics being prescribed in the 12 months before first diagnosis of FGID (Table 2; OR = 0.96; 95% confidence interval 0.91, 1.01; p = 0.1) but there was a subtle trend for any antibiotics being prescribed in the 12 months before first diagnosis of FGID (Table 2; OR = 1.09; 95% confidence interval 1.06, 1.12; p < 0.001). Neither of these latter trends was moderated by social deprivation There was positive trend in rate of APs per 5 years with increasing deprivation (Table 2; slope = 0.06; 95% confidence interval 0.05, 0.07; p < 0.001) which was moderated by patient group (p < 0.001). The trend with deprivation was strongest among patients with multiple FGIDs (slope = 0.15; SE = 0.02) and weakest in GI controls (slope = 0.02; SE = 0.005).
TABLE 2.
Trends in antibiotic prescription measures according to increasing socioeconomic deprivation (Townsend score)
| Townsend | Ever antibiotic a | Antibiotic before FGID b | Antibiotics per 5 years a |
|---|---|---|---|
| (n) | % (n) | % (n) | Mean (SD) |
| 1 (4384) | 84.9 (3722) | 93.2 (2276) | 0.61 (0.97) |
| 2 (5075) | 84.4 (4285) | 92.8 (2514) | 0.60 (1.05) |
| 3 (5259) | 85.9 (4519) | 92.6 (2569) | 0.65 (1.07) |
| 4 (4717) | 86.1 (4063) | 91.3 (2299) | 0.73 (1.29) |
| 5 (2940) | 88.1 (2591) | 93.0 (1532) | 0.87 (1.47) |
| p value for trend | <0.001 | <0.001 | <0.001 |
Applies to all patient groups.
Only applies to FGID groups.
An exploratory analysis of antibiotic classes prescribed to different FGID groups and controls failed to suggest any differential patterns. For example, penicillin was the single most commonly prescribed class and ranged from 49% of prescriptions in the multiple FGID group to 55% in both controls and the FD alone group. The difference between lowest and highest rate of prescription was <5% for any class examined.
Gastrointestinal infection by patient group
Overall, 6% (1373) of patients had a record of a gastrointestinal infection. A higher percentage of FGID patients (8.1%) had a recorded diagnosis of GI infection than GI controls (3.3%; p < 0.001; Table 3). Of FGID patients 3.1% had a GI infection prior to their first recorded diagnosis of FGID, but only 0.7% were within 12 months prior to that diagnosis, making the causal connection generally unclear (Table 3). While there was some variation across patient groups, it was by subtle degree (0.3% in FC to 0.9% in the multiple FGID group) and failed to reach statistical significance (p = 0.2) and as also unaffected by controlling for age, gender and social deprivation (p = 0.4).
TABLE 3.
Relationship between recorded gastrointestinal infection and FGID status
| Group | Ever GI infection a | OR (95% confidence interval) p value | GI infection prior b | OR (95% confidence interval) p value | GI infection within 12 m c | OR (95% confidence interval) p value |
|---|---|---|---|---|---|---|
| GI‐controls | 3.3 (360) | Reference | n/a | n/a | n/a | n/a |
| IBS only | 7.7 (334) | 2.46 (2.11, 2.86) <0.001 | 3.4 (149) | 1.16 (0.92, 1.48) 0.2 | 0.8 (34) | 0.91 (0.57, 1.45) 0.7 |
| FD only | 6.1 (213) | 1.92 (1.61, 2.28) <0.001 | 2.6 (91) | 0.88 (0.67, 1.15) 0.3 | 0.5 (18) | 0.60 (0.34, 1.05) 0.07 |
| Constipation only | 5.0 (17) | 1.54 (0.94, 2.54) 0.09 | 4.1 (14) | 1.40 (0.80, 2.46) 0.2 | 0.3 (1) | 0.34 (0.05, 2.47) 0.3 |
| Multiple FGIDs | 10.2 (449) | 3.33 (2.89, 3.85) <0.001 | 3.0 (131) | Reference | 0.9 (38) | Reference |
Abbreviations: n/a, not applicable; Reference: reference category for odds ratios.
Percent (count) ever had gastrointestinal (GI) infection in medical record.
Gastrointestinal (GI) infection recorded prior to first recorded FGID diagnosis.
Gastrointestinal (GI) infection recorded <12 months prior to first recorded FGID diagnosis.
DISCUSSION
The literature on the role of antibiotics in FGIDs is somewhat conflicting, with some studies suggesting them as risk factors that maybe causally involved in FGID pathogenesis 18 via induction of dysbiosis in the GI microbiome but other studies suggesting that antibiotics can be helpful in correcting dysbiosis in FGID patients. 20 This study sought to interrogate general practice medical records solely to identify in what proportion of patients use of antibiotics precedes the first recorded diagnosis of an FGID. The correct temporal order (antibiotic use precedes FGID diagnosis) is a necessary but not sufficient condition 37 to determine causality but at least puts an upper boundary on the percentage of FGID patients where antibiotic use might be causally involved in disorder onset.
The incidence of AP was high in this patient sample (Table 1) but higher in all FGID groups (>90%) than GI‐controls (76%). The rate of AP per 5‐year period was higher in all FGID groups than GI controls but was also higher in FGID patients with multiple conditions diagnosed than those groups where a single condition was diagnosed (Table 1). The percentage of patients with a recorded GI infection was lower in absolute terms than had been prescribed an antibiotic but was also higher in FGID patients than GI controls and higher in those with multiple FGIDs diagnosed than single conditions (Table 2). These parallel patterns open the possibility that a small fraction of APs in GI patients are for GI infections.
More importantly, 25%–30% of FGID patients had been prescribed an antibiotic in the 12 months prior to their first recorded FGID diagnosis (Table 1), with the higher figure occurring in the group with multiple FGIDs diagnosed. In approximately one‐third of these patients it was their first ever recorded antibiotic (Table 1).
Whether antibiotic use harms or benefits patients' microbiome, and therefore their gut health, is likely to depend on whether at the time of ingestion they are in a state of eubiosis or dysbiosis. If a eubiotic patient takes an antibiotic for a non‐gastrointestinal indication the medication cannot improve and may harm their gut health while in a setting of dysbiosis there might be beneficial effects. 38 Our data suggest that the upper boundary of this percentage is 25%–30%. Further, our data are consistent with recently published data in other (non‐gastrointestinal) chronic diseases suggesting an association between antibiotic use in the 12 months prior to heart disease, kidney disease and obesity. 39 Unfortunately we have no data on the state of the microbiome of the patients recruited to this study and establishing what constitutes eubiosis objectively remains controversial. 40
We did however find evidence that AP in FGID patients is affected by an increasing deprivation score. Whether this is clinically appropriate or not cannot be determined by our data but warrants further investigation.
Our data also suggest that the incidence of AP outstrips recorded diagnoses of gastrointestinal infection by 10–20 fold, further, although unsurprisingly, suggesting that a majority of antibiotic use in general practice is for non‐gastrointestinal purposes. This suggests that, that a significant fraction of incident FGID cases where antibiotics had been prescribed in the previous 12 months the medication may have been causally involved. An alternative explanation is that patients with FGID are more likely to receive antibiotic therapy when they present with non‐GI diseases, potentially infection related symptoms and they are subsequently more likely to receive an antibiotic.
Nevertheless, our data support the possibility that antibiotic use may be a factor in the aetiology of a subgroup of cases with functional gastrointestinal disease. This tenet is based on records from general practices, where most FGIDs are first diagnosed and lends clinical credibility to our data, underscoring the clinical significance of these findings.
While our data have considerable strengths, there are also limitations that need to be understood. Important among these is that we were not using a database designed primarily for research purposes, but medical records intended primarily for clinical patient management. This may affect the likelihood of conditions being noted in the record, or not, and therefore there could be some under ascertainment of gastrointestinal conditions not immediately relevant to patient care. Further, diagnoses are made according to clinical judgement, not necessarily using consensus criteria such as the Rome criteria. 3 Our core conclusions are based on interpretation of the indirect evidence provided by the data although we have carefully stressed this limitation when making our conclusions. In addition, while a patient inclusion criterion was a prolonged period with a given general practice the possibility of an earlier FGID diagnosis or AP cannot be completely ruled out. Finally, the medical records used in this study go back some decades and patterns of AP may have changed over time. However, this will always be a challenge in studies with prolonged periods of observation.
Our findings have important consequences for clinical practice given the strengthening data suggesting antibiotic use may be involved in up to one‐fifth of all incident cases of common FGIDs. Clinicians outside of gastroenterology need to be aware of the potential harm that unnecessary use of antibiotics can do to gut health and the consequential harm that may cause to patient health in a broader sense.
CONFLICT OF INTEREST
The authors declare no conflict of interests.
AUTHOR CONTRIBUTIONS
Michael P. Jones: Co‐conceived of project, acquired funding, data analysis, lead manuscript preparation. Ayesha Shah: Co‐conceived of project, contributed to manuscript preparation. Marjorie M. Walker: Co‐conceived of project, contributed to manuscript preparation. Natasha Koloski: Co‐conceived of project, contributed to manuscript preparation. Gerald Holtmann: Co‐conceived of project, contributed to empirical approach, contributed to manuscript preparation. Nicholas J. Talley: Co‐conceived of project, contributed to empirical approach, contributed to manuscript preparation.
ACKNOWLEDGEMENTS
The data for this project were acquired with funding from the Psychology Department at Macquarie University (NSW, Australia). The department played no other role in the project.
Jones MP, Shah A, Walker MM, Koloski NA, Holtmann G, Talley NJ. Antibiotic use but not gastrointestinal infection frequently precedes first diagnosis of functional gastrointestinal disorders. United European Gastroenterol J. 2021;9(9):1074–80. 10.1002/ueg2.12164
DATA AVAILABILITY STATEMENT
Data available upon reasonable request.
REFERENCES
- 1. Koloski NA, Jones M, Talley NJ. Evidence that independent gut‐to‐brain and brain‐to‐gut pathways operate in the irritable bowel syndrome and functional dyspepsia: a 1‐year population‐based prospective study. Aliment Pharmacol Therapeut. 2016;44(6):592–600. [DOI] [PubMed] [Google Scholar]
- 2. Lee C, Doo E, Choi JM, Jang S‐h, Ryu H‐S, Lee JY, et al. The increased level of depression and anxiety in irritable bowel syndrome patients compared with healthy controls: systematic review and meta‐analysis. J Neurogastroenterol Motil. 2017;23(3):349–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lacy BE, Mearin F, Chang L, Chey WD, Lembo AJ, Simren M, et al. Bowel disorders. Gastroenterology. 2016;150(6):1393–407. [DOI] [PubMed] [Google Scholar]
- 4. Guleria A, Karyampudi A, Singh R, Khetrapal CL, Verma A, Ghoshal UC, et al. Mapping of brain activations to rectal balloon distension stimuli in male patients with irritable bowel syndrome using functional magnetic resonance imaging. J Neurogastroenterol Motil. 2017;23(3):415–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yuan YZ, Tao RJ, Xu B, et al. Functional brain imaging in irritable bowel syndrome with rectal balloon‐distention by using fMRI. World J Gastroenterol. 2003;9(6):1356–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Talley NJ. Functional gastrointestinal disorders as a public health problem. Neuro Gastroenterol Motil. 2008;20(Suppl 1):121–9. [DOI] [PubMed] [Google Scholar]
- 7. Barbara G, Cremon C, Carini G, Bellacosa L, Zecchi L, Giorgio RD, et al. The immune system in irritable bowel syndrome. J Neurogastroenterol Motil. 2011;17(4):349–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Keely S, Walker MM, Marks E, Talley NJ. Immune dysregulation in the functional gastrointestinal disorders. Eur J Clin Invest. 2015;45(12):1350–9. [DOI] [PubMed] [Google Scholar]
- 9. Liebregts T, Adam B, Bredack C, Röth A, Heinzel S, Lester S, et al. Immune activation in patients with irritable bowel syndrome. Gastroenterology. 2007;132(3):913–20. [DOI] [PubMed] [Google Scholar]
- 10. Barbara G, Wang B, Stanghellini V, de Giorgio R, Cremon C, Di Nardo G, et al. Mast cell‐dependent excitation of visceral‐nociceptive sensory neurons in irritable bowel syndrome. Gastroenterology. 2007;132(1):26–37. [DOI] [PubMed] [Google Scholar]
- 11. Cheng H‐Y, Ning M‐X, Chen D‐K, Ma W‐T. Interactions between the gut microbiota and the host innate immune response against pathogens. Front Immunol. 2019;10(607). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sundin J, Öhman L, Simrén M. Understanding the gut microbiota in inflammatory and functional gastrointestinal diseases. Psychosom Med. 2017;79(8):857–67. [DOI] [PubMed] [Google Scholar]
- 13. Lynch SV, Pedersen O. The human intestinal microbiome in health and disease. N Engl J Med. 2016;375(24):2369–79. [DOI] [PubMed] [Google Scholar]
- 14. Sommer F, Bäckhed F. The gut microbiota—masters of host development and physiology. Nat Rev Microbiol. 2013;11(4):227–38. [DOI] [PubMed] [Google Scholar]
- 15. Saffouri GB, Shields‐Cutler RR, Chen J, Yang Y, Lekatz HR, Hale VL, et al. Small intestinal microbial dysbiosis underlies symptoms associated with functional gastrointestinal disorders. Nat Commun. 2019;10(1):2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Shah A, Talley NJ, Koloski N, Macdonald GA, Kendall BJ, Shanahan ER, et al. Duodenal bacterial load as determined by quantitative polymerase chain reaction in asymptomatic controls, functional gastrointestinal disorders and inflammatory bowel disease. Aliment Pharmacol Ther. 2020;52(1):155–67. [DOI] [PubMed] [Google Scholar]
- 17. Jiang Z, Jacob JA, Li J, Wu X, Wei G, Vimalanathan A, et al. Influence of diet and dietary nanoparticles on gut dysbiosis. Microb Pathog. 2018;118:61–5. [DOI] [PubMed] [Google Scholar]
- 18. Taggart H, Bergstrom L. An overview of the microbiome and the effects of antibiotics. J Nurse Pract. 2014;10(7):445–50. [Google Scholar]
- 19. Ianiro G, Tilg H, Gasbarrini A. Antibiotics as deep modulators of gut microbiota: between good and evil. Gut. 2016;65(11):1906–15. [DOI] [PubMed] [Google Scholar]
- 20. Vickers RJD, Tillotson GSP, Nathan RMD, Hazan S, Pullman J, Lucasti C, et al. Efficacy and safety of ridinilazole compared with vancomycin for the treatment of Clostridium difficile infection: a phase 2, randomised, double‐blind, active‐controlled, non‐inferiority study. Lancet Infect Dis. 2017;17(7):735–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ahmad OF, Akbar A. Microbiome, antibiotics and irritable bowel syndrome. Br Med Bull. 2016;120(1):91–9. [DOI] [PubMed] [Google Scholar]
- 22. Maxwell PR, Rink E, Kumar D, Mendall MA. Antibiotics increase functional abdominal symptoms. Am J Gastroenterol. 2002;97(1):104–8. [DOI] [PubMed] [Google Scholar]
- 23. Klem F, Wadhwa A, Prokop LJ, Sundt WJ, Farrugia G, Camilleri M, et al. Prevalence, risk factors, and outcomes of irritable bowel syndrome after infectious enteritis: a systematic review and meta‐analysis. Gastroenterology. 2017;152(5):1042–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Paula H, Grover M, Halder SL, Locke GR, Schleck CD, Zinsmeister AR, et al. Non‐enteric infections, antibiotic use, and risk of development of functional gastrointestinal disorders. Neuro Gastroenterol Motil. 2015;27(11):1580–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Koloski NA, Jones M, Weltman M, Kalantar J, Bone C, Gowryshankar A, et al. Identification of early environmental risk factors for irritable bowel syndrome and dyspepsia. Neuro Gastroenterol Motil. 2015;27(9):1317–25. [DOI] [PubMed] [Google Scholar]
- 26. Baldassarre ME, Di Mauro A, Salvatore S, Tafuri S, Bianchi FP, Dattoli E, et al. Birth weight and the development of functional gastrointestinal disorders in infants. Pediatr Gastroenterol Hepatol Nutr. 2020;23(4):366–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Yoon H, Schaubeck M, Lagkouvardos I, Blesl A, Heinzlmeir S, Hahne H, et al. Increased pancreatic protease activity in response to antibiotics impairs gut barrier and triggers colitis. Cell Mol Gastroenterol Hepatol. 2018;6(3):370–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Camilleri M, Madsen K, Spiller R, Van Meerveld BG, Verne GN. Intestinal barrier function in health and gastrointestinal disease. Neuro Gastroenterol Motil. 2012;24(6):503–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Farré R, Vicario M. Abnormal barrier function in gastrointestinal disorders. Handb Exp Pharmacol. 2017;239:193–217. [DOI] [PubMed] [Google Scholar]
- 30. Saliba‐Gustafsson EA, Dunberger Hampton A, Zarb P, Orsini N, Borg MA, Stålsby Lundborg C. Factors associated with antibiotic prescribing in patients with acute respiratory tract complaints in Malta: a 1‐year repeated cross‐sectional surveillance study. BMJ Open. 2019;9(12):e032704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Jones MP, Walker MM, Ford AC, Talley NJ. The overlap of atopy and functional gastrointestinal disorders among 23 471 patients in primary care. Aliment Pharmacol Ther. 2014;40(4):382–91. [DOI] [PubMed] [Google Scholar]
- 32. Blak BT, Thompson M, Dattani H, Bourke A. Generalisability of the Health Improvement Network (THIN) database: demographics, chronic disease prevalence and mortality rates. Inform Prim Care. 2011;19(4):251–5. [DOI] [PubMed] [Google Scholar]
- 33. Lewis JD, Schinnar R, Bilker WB, Wang X, Strom BL. Validation studies of the health improvement network (THIN) database for pharmacoepidemiology research. Pharmacoepidemiol Drug Saf. 2007;16(4):393–401. [DOI] [PubMed] [Google Scholar]
- 34. Chisholm J. The Read clinical classification. BMJ. 1990;300(6732):1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. British Medical Association and Royal Pharmaceutical Society of Great Britain . British National Formulary. London: BMJ Publishing Group Ltd and Royal Pharmaceutical Society; 2013. [Google Scholar]
- 36. Mackenbach JP. Health and deprivation. Inequality and the north: by P. Townsend, P. Phillimore and A. Beattie (eds.) Croom Helm Ltd, London, 1987 221 pp., ISBN 0‐7099‐4352‐0, £8.95. Health Pol. 1988;10(2):207–6. [Google Scholar]
- 37. Doll R, Hill AB. Smoking and carcinoma of the lung. Br Med J. 1950;2(4682):739–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Shah A, Gurusamy SR, Hansen T, Callaghan G, Talley NJ, Koloski N, et al. Concomitant irritable bowel syndrome does not influence response to antimicrobial therapy in patient with functional dyspepsia Digest Dis Sci. 2021. [DOI] [PubMed] [Google Scholar]
- 39. Wilkins LJ, Monga M, Miller AW. Defining dysbiosis for a cluster of chronic diseases. Sci Rep. 2019;9(1):12918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. McBurney MI, Davis C, Fraser CM, Schneeman BO, Huttenhower C, Verbeke K, et al. Establishing what constitutes a healthy human gut microbiome: state of the science, regulatory considerations, and future directions. J Nutr. 2019;149(11):1882–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data available upon reasonable request.


