Abstract
Background
Corona virus disease 2019 (COVID‐19) patients are at increased risk for thromboembolic events. It is unclear whether the risk for gastrointestinal (GI) bleeding is also increased.
Methods
We considered 4128 COVID‐19 patients enrolled in the Lean European Open Survey on SARS‐CoV‐2 (LEOSS) registry. The association between occurrence of GI bleeding and comorbidities as well as medication were examined. In addition, 1216 patients from COKA registry were analyzed focusing on endoscopy diagnostic findings.
Results
A cumulative number of 97 patients (1.8%) with GI bleeding were identified in the LEOSS registry and COKA registry. Of 4128 patients from the LEOSS registry, 66 patients (1.6%) had a GI bleeding. The rate of GI bleeding in patients with intensive care unit (ICU) admission was 4.5%. The use of therapeutic dose of anticoagulants showed a significant association with the increased incidence of bleeding in the critical phase of disease. The Charlson comorbidity index and the COVID‐19 severity index were significantly higher in the group of patients with GI bleeding than in the group of patients without GI bleeding (5.83 (SD = 2.93) vs. 3.66 (SD = 3.06), p < 0.01 and 3.26 (SD = 1.69) vs. 2.33 (SD = 1.53), p < 0.01, respectively). In the COKA registry 31 patients (2.5%) developed a GI bleeding. Of these, the source of bleeding was identified in upper GI tract in 21 patients (67.7%) with ulcer as the most frequent bleeding source (25.8%, n = 8) followed by gastroesophageal reflux (16.1%, n = 5). In three patients (9.7%) GI bleeding source was located in lower GI tract caused mainly by diverticular bleeding (6.5%, n = 2). In seven patients (22.6%) the bleeding localization remained unknown.
Conclusion
Consistent with previous research, comorbidities and disease severity correlate with the incidence of GI bleeding. Also, therapeutic anticoagulation seems to be associated with a higher risk of GI bleeding. Overall, the risk of GI bleeding seems not to be increased in COVID‐19 patients.
Keywords: COVID‐19, critically ill, GI bleeding, LEOSS, SARS‐CoV‐2
INTRODUCTION
The outbreak of “severe acute respiratory syndrome corona virus 2” (SARS‐CoV‐2) in Wuhan, China has developed into a pandemic within a remarkably short time. By now, more than 180 million people worldwide suffered from "corona virus disease 2019" (COVID‐19) and more than 3.9 million people died. 1
Key summary.
Therapeutic dose of anticoagulants was significantly associated with the increased incidence of bleeding in the critical phase of disease
Charlson Comorbidity Index (CCI) and COVID‐19 Severity Index (CSI) were significantly higher in corona virus disease 2019 (COVID‐19) patients developing gastrointestinal (GI) bleeding compared to COVID‐19 patients without GI bleeding
67.7% of the GI bleedings occurred in the upper GI tract and 9.7% in the lower GI tract
The risk of GI bleeding does not seem to be increased in COVID‐19 patients, in comparison to current literature reports of non‐COVID‐19 patients
Transmission of the virus occurs predominantly via droplet infection. 2 Therefore, strict isolation measures must be implemented in SARS‐CoV‐2 positive patients. 3 Not only the strict isolation measures and the high number of cases challenge the health systems of the affected countries, but also the occasionally severe courses with numerous complications. 4 Complications comprise pulmonary, neurological, cardiovascular as well as gastrointestinal (GI) and thromboembolic events such as mesenterial ischemia, deep vein thrombosis or pulmonary artery embolism. 3 , 5 In order to prevent these events numerous guidelines for both, in‐ and outpatient therapy of COVID‐19 patients recommend prophylaxis of thromboembolism. 6 , 7 , 8 Notably, oral anticoagulation as well as prophylaxis of thromboembolism leads to an increased risk of GI bleeding in hospitalized patients. 9 , 10 , 11 It seems therefore reasonable to assume that anticoagulation leads to gastrointestinal bleeding in COVID‐19 patients also. Furthermore, other possible additional factors influencing the occurrence and frequency of GI bleeding in COVID‐19 patients are neither fully recognized nor understood.
However, the current evidence on SARS‐CoV‐2 infection as a risk factor for a GI bleeding and the rate of GI bleeding in COVID‐19 patients is limited. For example, Trinidade et al. described a GI bleeding rate of approximately 3% in COVID‐19 patients, while factors influencing GI bleeding risk could not be identified. 12 This bleeding rate falls in the range of 1.5% to 5.5% reported in critically ill patients and thus does not appear to be increased. 13 However, several recent publications describe severe GI bleeding events associated with COVID‐19. 14 , 15 , 16
In the present study, we provide an analysis of COVID‐19 patients with GI bleeding from the Lean European Open Survey on SARS‐CoV‐2 infected patients (LEOSS) registry and the COVID‐19 registry of Augsburg University Hospital (COKA). Our aim was to assess the GI bleeding rate of COVID‐19 patients as well as to determine risk factors associated with higher incidence of GI bleeding. In addition, endoscopic findings will be described and analyzed.
MATERIAL AND METHODS
Data and study design
A total of 6457 patients included in the LEOSS registry between March 2020 and February 2021 were analyzed in this study. The LEOSS registry is a prospective, multi‐center cohort registry enclosing data on hospitalized COVID‐19 patients having a laboratory‐confirmed COVID‐19 diagnosis. The LEOSS registry aims at addressing the lack of in‐depth knowledge on epidemiology and clinical course of this disease. Besides socio‐demographic data, the registry collects baseline characteristics, laboratory parameters and important comorbidities of the patients. The clinical course of the disease is divided into four phases related to the health state of the patient such as uncomplicated (oligo‐/asymptomatic), complicated (predominantly characterized by the need for oxygen supplementation), critical (need for life supporting therapy) and recovery phase (clinical improvement, discharge). Data available in the LEOSS registry are collected in a rough form as categorical values only. In order to ensure anonymity in all steps of the analysis process, an individual LEOSS Scientific Use File (SUF) was created, which is based on the LEOSS Public Use File (PUF) principles described in Jakob et al. 17
In addition, a total of 1216 patients included in the COKA registry between February 2020 and December 2020 were analyzed. The COKA registry is a prospective, monocentric cohort registry that collects data on patients hospitalized at University Hospital Augsburg with a COVID‐19 diagnosis confirmed by a positive result of a polymerase chain reaction test. The registry collects baseline characteristics, laboratory parameters and important comorbidities as well as endoscopic findings of the patients.
The primary endpoint of the study was occurrence of gastroenterological‐relevant bleeding in any phase of disease. Among the 6457 patients included in the LEOSS registry, only those having explicit information on GI bleeding in any phase of the clinical course of COVID‐19 were included in the data analysis. Accordingly, a total of 2329 (36.1%) cases were excluded due to missing information on primary outcome yielding a sample of 4128 (63.9%) cases. Besides demographics, such as age and sex, information on intensive care unit (ICU) admission, comorbidities according to Charlson Comorbidity Index (CCI), baseline characteristics according to COVID‐19 Severity Index (CSI) and several coagulopathic laboratory parameters were analyzed. Comorbidities were dichotomized expressing either presence or absence of each disease. Laboratory parameters were dichotomized indicating either normal or abnormal values. Characteristics documented as unknown were considered as missing.
Of the 1216 patients included in the COKA registry all patients with GI bleeding were included in the analysis. Besides demographics such as age and sex, information on ICU admission, bleedings symptoms, endoscopic findings, information about anticoagulation as well as other bleeding relevant data for example blood transfusion and hemoglobin drop were analyzed.
Statistical analysis
The patients' characteristics are reported as absolute numbers and percentages. CCI was calculated in line with Charlson et al. 18 and CSI was calculated according to Altschul et al. 19 Both indices are reported as mean and standard deviation. The inference‐statistical comparison of percentages between two and more groups was conducted using ꭓ2‐test or Fisher exact test when appropriate. The odds ratios of demographics, comorbidities and baseline characteristics were assessed in univariate and multivariate logistic regression models. Parsimonious logistic regression was calculated applying backwards elimination technique. The comparison of both examined indexes between the group of bleeders and non‐bleeders was conducted using Mann‐Whitney‐U test due to missing normal distribution of the data and inequality of both groups in terms of the case numbers. The significance level was defined at p < 0.05. Data management, descriptive and inference‐statistical analysis were conducted using IBM SPSS Version 27.
RESULTS
Characteristics of the study population
Among 4128 cases from LEOSS registry included in the data analyses, 42.9% (n = 1771) were female (Table 1). The most common age group was 76–85 (19.9%, n = 823). The biggest share of patients 20.7% (n = 854) with available information on BMI were overweight. The clinical manifestations of COVID‐19 at baseline were distributed as follows: uncomplicated phase 70.5% (n = 2912), complicated phase 22.8% (n = 941), critical phase 5.7% (n = 234), recovery phase 0.1% (n = 6) and 0.9% (n = 35) missing information. Progression from uncomplicated to complicated or critical phases was observed in 29.5% (n = 856) and 7.9% (n = 231) patients, respectively. A total of 21.1% patients (n = 871) were administered to ICU with 10.4% (n = 91) of ICU‐patients receiving extracorporeal membrane oxygenation (ECMO). Among 4128 patients examined, 1.6% (n = 66) developed a GI bleeding in one of clinical phases. The proportion of GI bleeding on admission accounted for 0.9% (n = 35). The highest rate of GI bleeding was observed in the critical phase with 4.5% (n = 32).
TABLE 1.
Characteristics of the Lean European Open Survey on SARS‐CoV‐2 (LEOSS) study population
| Characteristics | Category | n/N | % |
|---|---|---|---|
| Age | 1–14 years | 30/4128 | 0.7 |
| 15–25 years | 172/4128 | 4.2 | |
| 26–35 years | 346/4128 | 8.4 | |
| 36–45 years | 394/4128 | 9.5 | |
| 46–55 years | 642/4128 | 15.6 | |
| 56–65 years | 727/4128 | 17.7 | |
| 66–75 years | 640/4128 | 15.6 | |
| 76–85 years | 823/4128 | 19.9 | |
| >85 years | 334/4128 | 8.1 | |
| Sex | Male | 2348/4128 | 57.0 |
| Female | 1771/4128 | 42.9 | |
| BMI | <18.5 kg/m2 | 70/4128 | 1.7 |
| 18.5–24.9 kg/m2 | 742/4128 | 18.0 | |
| 25–29.9 kg/m2 | 854/4128 | 20.7 | |
| 30–34.9 kg/m2 | 464/4128 | 11.2 | |
| >34.9 kg/m2 | 286/4128 | 6.9 | |
| Unknown | 1712/4128 | 41.5 | |
| Phase | Baseline | 4128/4128 | |
| Uncomplicated | 3452/4128 | 83.6 | |
| Complicated | 1849/4128 | 44.8 | |
| Critical | 706/4128 | 17.1 | |
| Recovery | 2082/4128 | 50.4 | |
| Phase at baseline | Uncomplicated | 2912/4128 | 70.5 |
| Complicated | 941/4128 | 22.8 | |
| Critical | 234/4128 | 5.7 | |
| Recovery | 6/4128 | 0.1 | |
| Unknown | 35/4128 | 0.9 | |
| ICU admission | Yes | 871/4128 | 21.1 |
| No | 1686/4128 | 40.8 | |
| Not available | 1571/4128 | 38.1 | |
| ECMO | 91/871 | 10.4 | |
| Bleeding | Total | 66/4128 | 1.6 |
| ICU admission | 39/871 | 4.5 | |
| No ICU admission | 13/1686 | 0.8 | |
| Not available | 14/1571 | 0.9 | |
| Baseline | 35/4128 | 0.9 | |
| Complicated | 19/1849 | 1.0 | |
| Critical | 32/706 | 4.5 |
Abbreviations: BMI, Body mass index; ECMO, Extracorporeal membrane oxygenation; ICU, intensive care unit; n, Number.
Univariable and multivariable relationships of patient characteristics with gastrointestinal bleeding
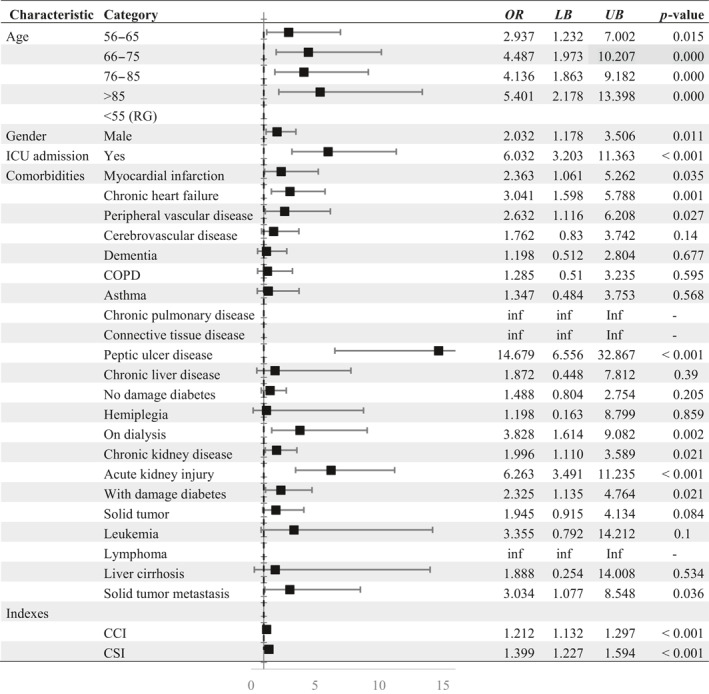
In order to compare clinical course of COVID‐19 in terms of GI complications, the group of bleeders was compared to a total of patients without GI bleeding during their inpatient stay. The data of both examined patients groups are shown in Table 2. Univariate analyses revealed higher age, male sex OR = 2.032 (CI: 1.178–3.506), admission to ICU OR = 6.032 (CI: 3.203–11.363) and several comorbidities, such as myocardial infarction OR = 2.363 (CI: 1.061–5.262), chronic heart failure OR = 3.041 (CI: 1.598–5.788), peripheral vascular disease OR = 2.632 (CI: 1.116–6.208), peptic ulcer disease OR = 14.679 (CI: 6.556–32.867), dialysis OR = 3.828 (CI: 1.614–9.082), acute kidney injury OR = 6.263 (CI: 3.491– 11.235), diabetes with damage OR = 2.325 (CI: 1.135–4.764) and solid tumor with metastasis OR = 3.034 (CI: 1.077–8.548) to be significantly associated with increased risk of GI bleeding.
TABLE 2.
Univariate analysis of patient and clinical characteristics of gastrointestinal (GI) bleeding in Lean European Open Survey on SARS‐CoV‐2 (LEOSS) study population
|
Abbreviations: CCI, Charlson comorbidity Index; CSI, COVID‐19 severity index; ICU, Intensive care unit; n, Number; inf, Infinity due to small number of cases; LB, Lower bound; OR, Odds ratio; UB, Upper bound; RG, Reference group.
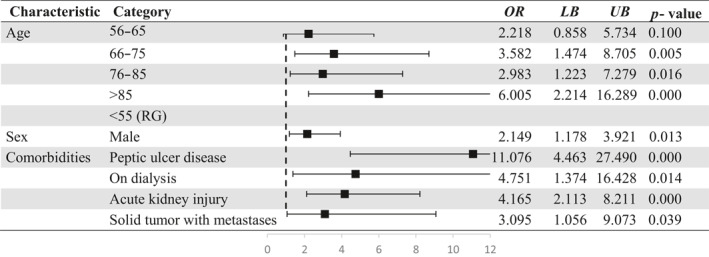
In a parsimonious multivariate regression model, higher age, male sex OR = 2.149 (CI: 1.178–3.921) as well as four comorbidities such as peptic ulcer disease OR = 11.076 (CI: 4.463–27.490), acute kidney injury OR = 4.165 (CI: 2.113–8.211), dialysis OR = 4.751 (CI: 1.374–16.428) and solid tumor with metastasis OR = 3.095 (CI: 1.056–9.073) were significantly associated with a higher risk of GI bleeding (Table 3).
TABLE 3.
Parsimonious multivariate regression model of gastrointestinal (GI) bleeding risk factors in Lean European Open Survey on SARS‐CoV‐2 (LEOSS) study population
|
Abbreviations: LB, Lower bound; OR, Odds ratio; UB, Upper bound; RG, Reference group.
Comparison of the group of non‐bleeders and bleeders revealed a significant difference in terms of CCI and CSI (Table S1). Thus, mean CCI in patients developing GI bleeding was 5.83 (SD = 3.111) whereas in patients without this GI complication it was 3.66 (SD = 3.067; p < 0.001). Similarly, there was a significant difference in the CSI between two examined groups, with a higher value in patients with GI bleeding (3.26 (SD = 1.694) vs. 2.33 (SD = 1.527), p < 0.001).
Coagulopathic laboratory parameters and its influence on the bleeding
At baseline C‐reactive protein (CRP) levels above 3 mg/L were observed in 56.5% (n = 2333) of patients with available laboratory values (Table S2 and S3). Over one fifth, 22.2% (n = 916) of the study population had anemia (<12 g/dL) and further 57.3% (n = 2367) platelet disorder (<119,999/μL). Prolongation of the activated partial thromboplastin time (aPTT) was a seldom condition observed only in 5.2% (n = 214) of the examined patients, the International Normalized Ratio (INR) was increased in 8.6% (n = 357) of cases. 2.4% of the patients having increased INR level (>1.25) developed GI bleeding whereas this proportion in the control group was significantly lower (1.0%; Table 4). Anemia was significantly associated with occurrence of GI bleeding. 2.3% of the patients with this coagulopathy developed a bleeding, among patients without anemia it was observed only in 0.6% of cases. Furthermore, patients with prolongation of the aPTT showed a noticeably higher rate of GI bleedings in comparison to the control group. However, this difference was marginally not significant (2.5% vs. 1.2%, p = 0.77).
TABLE 4.
Coagulopathic laboratory parameters and occurrence of gastrointestinal (GI) bleeding in Lean European Open Survey on SARS‐CoV‐2 (LEOSS) study population
| Phase | Normal | Abnormal | p‐value | |||
|---|---|---|---|---|---|---|
| n/N | % | n/N | % | |||
| Baseline | INR | 21/2036 | 1.0 | 8/333 | 2.4 | 0.035 |
| GPT (ALT) | 18/1741 | 1.0 | 8/562 | 1.4 | 0.447 | |
| GOT (AST) | 14/1324 | 1.1 | 11/766 | 2.5 | 0.443 | |
| CRP | 4/354 | 1.1 | 26/2208 | 1.2 | 0.938 | |
| Fibrinogen | 5/431 | 1.2 | 9/468 | 1.9 | 0.356 | |
| Platelets | 4/269 | 1.5 | 25/2248 | 1.1 | 0.543 | |
| Hemoglobin | 11/1731 | 0.6 | 20/866 | 2.3 | 0.001 | |
| aPTT | 23/2138 | 1.1 | 5/200 | 2.5 | 0.077 | |
| AT III | 2/169 | 1.2 | 2/118 | 1.7 | 0.716 | |
| Complicated | INR | 10/1079 | 0.9 | 6/359 | 1.7 | 0.244 |
| GPT (ALT) | 5/607 | 1.2 | 11/890 | 0.8 | 0.446 | |
| GOT (AST) | 9/611 | 1.5 | 6/798 | 0.8 | 0.191 | |
| CRP | 0/47 | 0.0 | 18/1577 | 1.1 | 1.000 | |
| Fibrinogen | 4/477 | 0.6 | 1/155 | 0.8 | 1.000 | |
| Platelets | 16/1338 | 1.2 | 1/221 | 0.5 | 0.494 | |
| Hemoglobin | 3/795 | 0.4 | 15/796 | 1.9 | 0.007 | |
| aPTT | 10/1079 | 0.7 | 6/359 | 2.5 | 0.009 | |
| AT III | 0/106 | 0.0 | 1/92 | 1.1 | 0.465 | |
| Critical | INR | 9/277 | 3.2 | 20/309 | 6.5 | 0.072 |
| GPT (ALT) | 5/177 | 2.8 | 23/412 | 5.6 | 0.149 | |
| GOT (AST) | 4/98 | 4.1 | 25/478 | 5.2 | 0.802 | |
| CRP | 0/10 | 0.0 | 29/607 | 4.8 | 0.072 | |
| Fibrinogen | 1/63 | 1.6 | 18/330 | 5.5 | 0.333 | |
| Platelets | 16/445 | 3.6 | 11/139 | 7.9 | 0.034 | |
| Hemoglobin | 5/134 | 3.7 | 22/467 | 4.7 | 0.629 | |
| aPTT | 6/235 | 2.6 | 23/339 | 6.8 | 0.023 | |
| AT III | 6/85 | 7.1 | 14/215 | 6.5 | 0.864 | |
Abbreviations: ALT, Alanine transaminase; aPTT, Activated partial thromboplastin time; AST, Aspartate transaminase; AT III, Antithrombin III; CRP, C‐reactive protein; GOT, Glutamic oxaloacetic transaminase; GPT, Glutamic pyruvic transaminase INR, International Normalized Ratio; n/N, Number.
In the complicated phase of disease, the share of patients with abnormal laboratory blood values was increased (Table 3). Thus, the proportion of patients with CRP levels above 3 mg/L accounted for 89.2% (n = 1649), and with anemia for 45.1% (n = 833). Prolongation of aPTT was observed in 15.8% (n = 292) of the cases and abnormal INR in 20.1% (n = 372) of the patients.
In the patient group with anemia the proportion of GI bleeding was significantly higher in comparison to those with normal hemoglobin levels (0.4% vs. 1.9%, p < 0.01; Table 4). This tendency applies also to aPTT levels: Patients with longer aPTT developed GI bleeding more frequently. Patients in the critical phase exhibit higher proportions of the abnormal blood values with a notably higher rate of patients with anemia 71.8% (n = 507; Table S3). In the critical phase increased intestinal bleeding rates were associated with platelets disorders (p = 0.034) and abnormal aPTT levels (p = 0.023; Table 4). Patients having increased INR levels also showed a higher rate of GI bleedings (6.5% vs. 3.2%), however, this difference was not significant (p = 0.072).
Treatment in the complicated and critical phase
In the complicated phase the majority of patients received anticoagulation with low‐molecular‐weight heparin (LMH) 51.5% (n = 953) or heparin 13.7% (n = 254), however, mostly prophylactic doses. 17.5% (n = 324) of the patients in the complicated stage had no anticoagulation at all (Table S4). In the complicated phase of the disease, anticoagulation had no significant impact on the bleeding rate (p = 0.361).
In the critical stage LMH remained the most used coagulation method 44.6% (n = 315) followed by heparin 41.5% (n = 293). Similar to the complicated phase both medications were administered in prophylactic doses. 15.4% (n = 109) of the patients received no anticoagulation despite the critical course of the disease. Due to statistical analysis, the anticoagulation method had a significant effect on the bleeding rate (p = 0.029; Table 5). The highest rate was observed in the patient group receiving Marcumar (16.7%, n = 1) and the lowest proportion in patients receiving subtherapeutic dose of heparin (0%, n = 0). However, post‐hoc analysis revealed a significant deviation in the group administered with therapeutic doses of heparin or LMH (8.7%, n = 14) and prophylactic doses of heparin or LMH (2.6%, n = 7).
TABLE 5.
Anticoagulation and occurrence of gastrointestinal (GI) bleeding in Lean European Open Survey on SARS‐CoV‐2 (LEOSS) study population
| Anticoagulation | Complicated | Critical | ||||
|---|---|---|---|---|---|---|
| n/N | % | p‐value | n/N | % | p‐value | |
| Prophylactic heparin/LMH | 10/870 | 1.1 | 0.92 | 7/270 | 2.6 | 0.02 |
| Therapeutic heparin/LMH | 1/194 | 0.5 | 0.42 | 14/161 | 8.7 | 0.01 |
| Subtherapeutic heparin/LMH | 1/90 | 1.1 | 1.00 | 0/36 | 0.0 | 0.16 |
| Marcumar | 1/18 | 5.6 | 0.07 | 1/6 | 16.7 | 0.19 |
| NOAC | 0/128 | 0.0 | 0.19 | 3/35 | 8.6 | 0.32 |
| Unknown | 1/76 | 1.3 | 0.84 | 3/25 | 12.0 | 0.11 |
| No anticoagulation | 5/321 | 1.6 | 0.42 | 4/103 | 3.9 | 0.55 |
| Total | 0.361 | 0.03 | ||||
Abbreviations: LMH, low‐molecular‐weight heparin; NOAC, New oral anticoagulants; n/N, Number.
Endoscopic findings from COKA registry
Thirty‐one (2.5%) COVID‐19 patients in the COKA registry developed a GI bleeding (Table 6). Females were 38.7% and the mean age was 75.45 (SD = 11.145). 51.6% (n = 16) of the patients showed GI bleeding upon hospitalization, 25.8% (n = 8) of bleeders were managed in the ICU. 29.0% (n = 9) of patients with Hb‐relevant bleedings developed hematemesis and further 32.3% (n = 10) melena as symptoms. The majority of bleeders 93.6% (n = 29) underwent endoscopic procedure. The location of the GI bleeding was distributed as follows: 67.7% (n = 21) in the upper GI tract, 9.7% (n = 3) in the colon and rectum, and in 22.6% (n = 7) of cases the origin of bleeding remained unknown. Twenty‐five (80.6%) patients were on anticoagulants, of which 25.8% (n = 8) were administrated heparin or LMH and 16.1% received NOAC (n = 5). An equivalent share received acetylsalicylic acid (ASA) 16.1% (n = 5), and seven patients (22.6%) were administrated both, anticoagulation, and anti‐platelet agents. Eighteen (58.1%) patients received blood transfusion and 5 (16.1%) patients required a re‐intervention after their initial bleeding. In 23 patients (74.2%), a reduction of hemoglobin by at least 2 mg/dl was observed.
TABLE 6.
Characteristics and outcomes of the Augsburg cohort with corona virus disease 2019 (COVID‐19) and gastrointestinal (GI) bleeding in COKA study population
| Characteristic | n/N | % | |
|---|---|---|---|
| Demographics | |||
| Sex | Male | 19/31 | 61.3 |
| Female | 12/31 | 38.7 | |
| Age, years, mean (SD) | 75.45 (11.145) | ||
| BMI, mean (SD) | 25.58 (4.256) | ||
| CCI, mean (SD) | 3.65 (2.43) | ||
| CSI, mean (SD) | 4.35 (1.603) | ||
| GI bleeding characteristics | |||
| ICU | 8/31 | 25.8 | |
| Upon hospitalization | 16/31 | 51.6 | |
| Symptoms | |||
| Hematemesis | 9/31 | 29.0 | |
| Melena | 10/31 | 32.3 | |
| Endoscopic findings | |||
| Endoscopy | 29/31 | 93.6 | |
| Upper | 21/31 | 67.7 | |
| Gastroesophageal reflux | 5/31 | 16.1 | |
| Stomach ulcer | 5/31 | 16.1 | |
| Forrest III | 3/5 | ||
| Forrest IIa | 2/5 | ||
| Duodenal ulcer | 3/31 | 9.7 | |
| Forrest III | 1/3 | ||
| Forrest IIa | 1/3 | ||
| Forrest IIb | 1/3 | ||
| Axial hiatus hernia | 3/31 | 9.7 | |
| Angiectasia of inferior part of duodenum | 1/31 | 3.2 | |
| Erosive gastritis | 1/31 | 3.2 | |
| Esophageal varices | 2/31 | 6.5 | |
| Bleeding of foot point anastomosis | 1/31 | 3.2 | |
| Middle | 0/31 | 0.0 | |
| Lower | Ulcer in the sigmoid colon | 1/31 | 3.2 |
| Diverticular bleeding | 2/31 | 6.5 | |
| Unknown | 7/31 | 22.6 | |
| Anticoagulation upon bleeding | |||
| ASA | 5/31 | 16.1 | |
| ASA + Heparin/LMH | 6/31 | 19.4 | |
| Heparin/LMH | 8/31 | 25.8 | |
| NOAC | 5/31 | 16.1 | |
| Clopidogrel, ASA, LMH | 1/31 | 3.2 | |
| No anticoagulation | 6/31 | 19.4 | |
| Other bleeding relevant data | |||
| Steroid intake at baseline | 2/31 | 6.5 | |
| Steroid intake at time of bleeding | 5/31 | 16.1 | |
| Blood transfusion | 18/31 | 58.0 | |
| CT | 2/31 | 6.5 | |
| Re‐intervention | 5/31 | 16.1 | |
| Hemoglobin reduction by 2 mg/dl | 23/31 | 74.2 | |
Abbreviations: ASA, Acetylsalicylic acid; BMI, Body mass index; CCI, Charlson comorbidity Index; CSI, COVID‐19 severity index; ICU, intensive care unit; LMH, low‐molecular‐weight heparin; NOAC, New oral anticoagulants; n, Number.
DISCUSSION
This study presents data on GI bleeding and endoscopic findings in COVID‐19 patients based on LEOSS and COKA registries. This multicenter cohort study provides information on epidemiological and clinical characteristics of COVID‐19 patients developing GI bleeding. Our LEOSS‐cohort consisted of 4128 patients with confirmed COVID‐19 diagnosis and available information on GI bleeding hospitalized at one of LEOSS study sites. Socio‐demographics and comorbidities of the examined cohort were comparable with previously published scientific manuscripts. 20 Analysis of data from the COKA registry focused primarily on endoscopic findings and gives an overview on demographics, information on ICU admission, and information about anticoagulation as well as other bleeding relevant data.
In the analysis of LEOSS registry, GI bleeding occurring on admission accounted for 0.9% which is equal to the GI bleeding rate shown in Trindade et al. 12 Nevertheless, the rate of GI bleeders across all considered LEOSS‐phases accounted for 1.6%. In contrast, in the American cohort of COVID‐19 patients, the point prevalence of bleeding was 3%. 12 Notably, the difference in bleeding rate could be explained by different source of information: The analysis of Trinidade et al. is based on billing data while the present analysis is based on LEOSS registry data which might be more accurate. However, both data sources did not consider endoscopic findings. In the COKA registry, the GI bleeding rate verified by endoscopic examination was 2.5% lying between values reported by Trinidade et al. and LEOSS. However, with only 1216 monocentric included patients, data from the COKA registry is smaller than the other mentioned study populations. Considering patients in the critical phase of the disease, GI bleedings appeared in 4.5% of cases which is similar to the reported prevalence rate of 4.7% in non‐COVID‐19 patients by Cook & Guyatt 2018. 13 Furthermore, ICU admission was significantly more often associated with increased risk of GI bleeding in both COVID‐19 patients and non‐COVID‐19 patients.
Several factors are currently recognized as risk factors for severe course of COVID‐19 and occurrence of complications of many kinds. The COVID‐19 Severity Index is a predictive score for hospitalized patients aiming at identification of high‐risk patients based on metabolic parameters, 19 while the Charlson comorbidity index is a measure developed and validated to predict mortality risk and disease burden for use in longitudinal studies. 18 However, several recent publications prove a significant association of the CCI and severe COVID‐19 course and its poor outcome. 21 , 22 In the present study both examined indexes were significantly higher in the group of GI bleeders in comparison to non‐bleeders referring to an association between this GI complication and severe course of disease. A detailed analysis of the comorbidities used to calculate the CCI revealed a significant relationship with advanced age and chronic illnesses related to the GI tract, kidney disease and malignant tumors to be risk factors for GI bleeding in COVID‐19 patients. These findings are in line with previous reports showing age above 75 years and history of chronic kidney disease or peptic ulcer disease to be associated with an increased risk of GI bleeding. 23 , 24 , 25
It is well‐known that COVID‐19 leads to an increased risk of thromboembolism in infected patients. Therefore, guidelines for inpatient therapy of COVID‐19 patients frequently recommend medication‐based prophylaxis of thromboembolism with anticoagulants which might lead to an increased risk of GI bleeding. However, the relative importance of this risk has not been well studied yet. In the present study, full dose of heparin/or low weight heparin in the critical phase of disease was significantly associated with higher rate of GI bleeding, whereas patients with prophylactic dose developed GI bleeding significantly less frequent. This insight is in line with Qadeer et al. reporting coagulation to predispose to this complication. 26
The present study has notable strengths. Firstly, the data used for analysis are from the LEOSS registry comprising patients with confirmed COVID‐19 diagnosis from different medical centers across Europe. Secondly, the data includes relatively large number of GI bleeding events, which makes the findings more generalizable. However, the study also has several limitations worth mentioning. Firstly, as the data were taken from a registry, some variables show a high proportion of missing data; a total of 2329 (36.1%) out of 6457 cases had to be excluded from the analysis. This is a major limitation of our study. Secondly, the information of an endoscopic examination of the GI bleeders has not been obtained in the LEOSS registry. However, this shortcoming could be partially compensated by detail examination of the Augsburg cohort of the COVID‐19 patients (COKA registry). Thirdly, the definition of GI bleeding might vary across health care centers included in the LEOSS registry. Fourthly, the COKA data was collected only at one study site.
In conclusion, the data presented in this study suggest that the incidence of GI bleeding in COVID‐19 patients does not differ compared to non‐COVID‐19 patients in similar settings or in other clinical scenarios. In critical stage of disease, being on therapeutic dose of anticoagulation was associated with a significant increase the risk of GI bleeding.
CONFLICTS OF INTEREST
All authors declare that they have no conflict of interest.
ETHICAL APPROVAL
The study was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines. LEOSS was approved by the applicable local ethics committees of all participating centers and registered at the German Clinical Trials Register (DRKS, No. S00021145). COKA was approved by Ethics committee of the Ludwig‐Maximilians‐Universität (EK‐No. 20‐426). Ethics approval was additionally granted by the Ethics committee of the Ludwig‐Maximilians‐Universität München (EK‐No. 21‐0101).
Supporting information
Table S1
Table S2
Table S3
Table S4
ACKNOWLEDGEMENTS
The authors express our deep gratitude to all study teams supporting the LEOSS study. The LEOSS study group contributed at least 5 per mille to the analyses of this study: University Hospital Regensburg (Frank Hanses), Hospital Bremen‐Center (Christiane Piepel), Technical University of Munich (Christoph Spinner), University Hospital Augsburg (Christoph Roemmele), Hospital St. Joseph‐Stift Dresden (Lorenz Walter), University Hospital Tuebingen (Siri Goepel), Johannes Wesling Hospital Minden Ruhr University Bochum (Kai Wille), Hospital Dortmund gGmbH (Martin Hower), University Hospital Jena (Maria Madeleine Ruethrich), University Hospital Schleswig‐Holstein Luebeck (Jan Rupp), Municipal Hospital Karlsruhe (Christian Degenhardt), Elisabeth Hospital Essen (Ingo Voigt), Hospital Ingolstadt (Stefan Borgmann), Hospital Passau (Martina Haselberger), Tropical Clinic Paul‐Lechler Hospital Tuebingen (Claudia Raichle), University Hospital Duesseldorf (Bjoern‐Erik Jensen), University Hospital Frankfurt (Maria Vehreschild), Evangelisches Hospital Saarbruecken (Mark Neufang), Hospitals of Cologne gGmbH (Ana Harth), Hospital Braunschweig (Jan Kielstein), Medical School Hannover (Gernot Beutel), Oberlausitz Hospital (Maximilian Worm), Robert‐Bosch‐Hospital Stuttgart (Katja Rothfuss), University Hospital Essen (Sebastian Dolff).
The LEOSS study infrastructure group: Jörg Janne Vehreschild (Goethe University Frankfurt), Carolin E. M. Jakob (University Hospital of Cologne), Lisa Pilgram (Goethe University Frankfurt), Melanie Stecher (University Hospital of Cologne), Max Schons (University Hospital of Cologne), Susana Nunes de Miranda (University Hospital of Cologne), Clara Bruenn (University Hospital of Cologne), Nick Schulze (University Hospital of Cologne), Sandra Fuhrmann (University Hospital of Cologne), Annika Claßen (University Hospital of Cologne), Bernd Franke (University Hospital of Cologne), Fabian Praßer (Charité, Universitätsmedizin Berlin) und Martin Lablans (University Medical Center Mannheim).
Open access funding enabled and organized by Projekt DEAL.
Zellmer S, Hanses F, Muzalyova A, Classen J, Braun G, Piepel C, et al. Gastrointestinal bleeding and endoscopic findings in critically and non‐critically ill patients with corona virus disease 2019 (COVID‐19): results from Lean European Open Survey on SARS‐CoV‐2 (LEOSS) and COKA registries. United European Gastroenterol J. 2021;9(9):1081–90. 10.1002/ueg2.12165
Stephan Zellmer, Frank Hanses, Alanna Ebigbo and Christoph Römmele contributing equally.
DATA AVAILABILITY STATEMENT
Data available on request from the authors.
REFERENCES
- 1. Dong E, Du H, Gardner L. An interactive web‐based dashboard to track COVID‐19 in real time. Lancet Infect Dis. 2020;20(5):533–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Repici A, Maselli R, Colombo M, Gabbiadini R, Spadaccini M, Anderloni A, et al. Coronavirus (COVID‐19) outbreak: what the department of endoscopy should know. Gastrointest Endosc. 2020;92(1):192–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Robert Koch Institut . Empfehlungen des RKI zu Hygienemaßnahmen im Rahmen der Behandlung und Pflege von Patienten mit einer Infektion durch SARS‐CoV‐2; 2021. [8 December, 2020]; ww.rki.de [Google Scholar]
- 4. Blumenthal D, Fowler EJ, Abrams M, Collins SR. Covid‐19—implications for the health care system. N Engl J Med. 2020;383(15):1483–8. [DOI] [PubMed] [Google Scholar]
- 5. Keshavarz P, Rafiee F, Kavandi H, Goudarzi S, Heidari F, Gholamrezanezhad A. Ischemic gastrointestinal complications of COVID‐19: a systematic review on imaging presentation. Clin Imag. 2021;73:86–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cuker A, Tseng EK, Nieuwlaat R, Angchaisuksiri P, Blair C, Dane K, et al. American Society of Hematology 2021 guidelines on the use of anticoagulation for thromboprophylaxis in patients with COVID‐19. Blood Adv. 2021;5(3):872–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kluge S, Janssens U, Spinner CD, Pfeifer M, Marx G, Karagiannidis C. Clinical Practice guideline: recommendations on inpatient treatment of patients with COVID‐19. Dtsch Arztebl Int. 2021;118. (Forthcoming). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Antithrombotic therapy in patients with COVID‐19. NIH COVID‐19 treatment guidelines. https://www.covid19treatmentguidelines.nih.gov/antithrombotic‐therapy/. Accessed 10 May 2021. [Google Scholar]
- 9. Gotz M, Anders, M , Biecker, E , Bojarski, C , Braun, G , Brechmann, T , et al. [S2k guideline gastrointestinal bleeding—guideline of the German society of gastroenterology DGVS]. Z Gastroenterol. 2017;55(9):883–936. [DOI] [PubMed] [Google Scholar]
- 10. Schunemann HJ, Cushman M, Burnett AE, Kahn SR, Beyer‐Westendorf J, Spencer FA, et al. American Society of Hematology 2018 guidelines for management of venous thromboembolism: prophylaxis for hospitalized and nonhospitalized medical patients. Blood Adv. 2018;2(22):3198–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Radaelli F, Dentali F, Repici A, Amato A, Paggi S, Rondonotti E, et al. Management of anticoagulation in patients with acute gastrointestinal bleeding. Dig Liver Dis. 2015;47(8):621–7. [DOI] [PubMed] [Google Scholar]
- 12. Trindade AJ, Izard S, Coppa K, Hirsch JS, Lee C, Satapathy SK, et al. Gastrointestinal bleeding in hospitalized COVID‐19 patients: a propensity score matched cohort study. J Intern Med. 2021;289(6):887–94. [DOI] [PubMed] [Google Scholar]
- 13. Cook D, Guyatt G. Prophylaxis against upper gastrointestinal bleeding in hospitalized patients. N Engl J Med. 2018;378(26):2506–16. [DOI] [PubMed] [Google Scholar]
- 14. Wang YL, Mu J‐S, Qi X‐B, Zhang W‐H. A case of COVID‐19 complicated by massive gastrointestinal bleeding. Gastroenterol Rep (Oxf). 2021;9(1):85–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chen T, Yang Q, Duan H. A severe coronavirus disease 2019 patient with high‐risk predisposing factors died from massive gastrointestinal bleeding: a case report. BMC Gastroenterol. 2020;20(1):318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Melazzini F, Lenti MV, Mauro A, De Grazia F, Di Sabatino A. Peptic ulcer disease as a common cause of bleeding in patients with coronavirus disease 2019. Am J Gastroenterol. 2020;115(7):1139–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jakob CEM, Kohlmayer F, Meurers T, Vehreschild JJ, Prasser F. Design and evaluation of a data anonymization pipeline to promote Open Science on COVID‐19. Sci Data. 2020;435(7):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chron Dis. 1987;40(5):373–83. [DOI] [PubMed] [Google Scholar]
- 19. Altschul DJ, Unda SR, Benton J, de la Garza Ramos R, Cezayirli P, Mehler M, et al. A novel severity score to predict inpatient mortality in COVID‐19 patients. Sci Rep. 2020;10(1):16726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ruthrich MM, au fnm, Giessen‐Jung C, Borgmann S, Classen AY, Dolff S, et al. COVID‐19 in cancer patients: clinical characteristics and outcome—an analysis of the LEOSS registry. Ann Hematol. 2021;100(2):383–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Christensen DM, Strange JE, Gislason G, Torp‐Pedersen C, Gerds T, Fosbøl E, et al. Charlson comorbidity index score and risk of severe outcome and death in Danish COVID‐19 patients. J Gen Intern Med. 2020;35(9):2801–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tuty Kuswardhani RA, Henrina J, Pranata R, Anthonius Lim M, Lawrensia S, Suastika K. Charlson Comorbidity Index and a composite of poor outcomes in COVID‐19 patients: a systematic review and meta‐analysis. Diabetes Metab Syndr. 2020;14(6):2103–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mahady SE, Margolis KL, Chan A, Polekhina G, Woods RL, Wolfe R, et al. Major GI bleeding in older persons using aspirin: incidence and risk factors in the ASPREE randomised controlled trial. Gut. 2021;70(4):717–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Laporte JR, Ibanez L, Vidal X, Vendrell L, Leone R. Upper gastrointestinal bleeding associated with the use of NSAIDs: newer versus older agents. Drug Saf. 2004;27(6):411–20. [DOI] [PubMed] [Google Scholar]
- 25. Ishigami J, Grams ME, Naik RP, Coresh J, Matsushita K. Chronic kidney disease and risk for gastrointestinal bleeding in the community: the atherosclerosis risk in communities (ARIC) study. Clin J Am Soc Nephrol. 2016;11(10):1735–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Qadeer MA, Richter JE, Brotman DJ. Hospital‐acquired gastrointestinal bleeding outside the critical care unit: risk factors, role of acid suppression, and endoscopy findings. J Hosp Med. 2006;1(1):13–20. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1
Table S2
Table S3
Table S4
Data Availability Statement
Data available on request from the authors.


