Abstract
The regression and salinization of the Aral Sea, largely caused by water diversion for irrigation, is among the most severe ecological disasters of the 20th century, and has had severe health and economic consequences for the local population. Introductions of alien species to enhance commercial fisheries before the regression had already impacted the ecology of this system. Crustaceans made up about one-quarter of the original metazoan species and constituted the principal food for native and introduced fish. From 1960 on, crustaceans were recorded at numerous fixed sampling stations, including thanatocoenoses (dead animals from sediment cores). We use this previously unpublished information to document changes in species abundance and discuss their causes in the context of species interactions and changes to physical and chemical parameters. Competition from alien crustaceans led to declines in or even extinction of some native species, but eventually severe salinization became the main detriment, and resulted in the complete collapse of commercial fisheries. This seriously hurt a critical trade, which provided the principal protein source for the local population. We document how comparatively modest conservation efforts enabled the northern Small Aral Sea to partially recover and commercial fishing to resume.
Keywords: Conservation, Fisheries, Long term monitoring, Saline lake, Introduced species
BACKGROUND
The regression of the former Aral Sea is one of the most serious human caused environmental disasters in recent times, affecting both the aquatic fauna and the surrounding human population (Micklin 2007 2016; Micklin et al. 2020). Until the middle of the 20th century, the Aral Sea was a semistable, brackish water lake with a diverse fauna. Fisheries played a central part in the area’s economy and food supply, yielding 44,000 tons of fish per year before 1960. The Aral Sea regressed in area during the latter part of the 20th century due to water diversion for irrigation; this led the water to become increasingly salinized, had catastrophic consequences for the biota (Figs. 1–3), and led to the complete collapse of commercial fisheries (Aladin et al. 2004; Chen 2018 2020). The virtual desiccation of the lake system also caused multiple health problems for the local human population. One example is dust particles, sometimes contaminated with pesticides or other chemicals, sent into the air from the large salt pans (Jensen et al. 1997; Small et al. 2001; Erdinger et al. 2004; Crighton et al. 2003 2011; Indoitu et al. 2015; O’Hara et al. 2000; Whish-Wilson 2002). The break up of the former USSR exacerbated the problems, which went largely unmanaged until very recently.
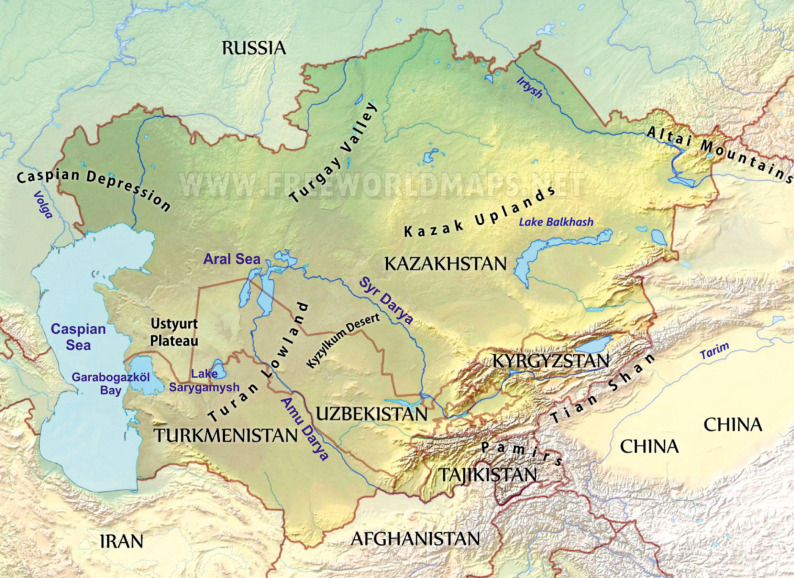
Geography and climate combined to make the Aral Sea an ecologically complex system. The original Aral Sea was an endorheic saline lake, and the fourth largest lake in the world. The history of the area prior to 1960 was described in the Encyclopedia Britannica (2020), and by Krinovogov (2014), Krivonogov et al. (2014), Micklin et al. (2014) and Zonn et al. (2009). The Aral receives two rivers, the Syr Darya in the northeast and the Amu Darya in the south (Figs. 1, 2A). The average depth is 16 m, but reaches 69 m in places along the western coast. The water has an average salinity at 10.3 ppt, but a much higher proportion of divalent ions than in the ocean. Salinity and temperature range was mostly stable, but variable conditions existed due to freshwater inflow near the river deltas or where high temperatures could cause local fluctuations (Bortnik and Cistayevaya 1990; Kosarev 1975; Zenkevich 1963). In some shallow regions and bays salinity could increase to > 50 ppt (Dengina 1959; Husainova 1960). A similar locally increased salinity is also known in the nearly isolated Garabogazköl Bay in the Caspian Sea. Finally, small, adjacent isolated and hypersaline water bodies interacted faunistically with the Aral itself through wind or animal mediated dispersal of organisms.
Fig. 1.
Map of Central Asia with the Aral Sea and its affluent rivers (modified from www.freeworldmaps.net).
Unlike the Caspian Sea, the Aral Sea is faunistically almost completely isolated, because no natural or artificial waterways connect it to other water bodies (Micklin et al. 2020). Good scientific studies of the Aral Sea started with the monograph by Berg (1908). The fauna was virtually undisturbed until the middle of the 20th century, when the first planned and accidental species introductions occurred. Since 1960, physical, chemical and biological changes were all monitored by scientists from the former USSR and the later CIS countries (Aladin and Potts 1992; Aladin et al. 2019). These records provide a chronology of the chemical, physical and biological events during the ecological disaster, but major parts remain unpublished. The records are particularly on Crustacea, which are a primary food source for both native and introduced fish.
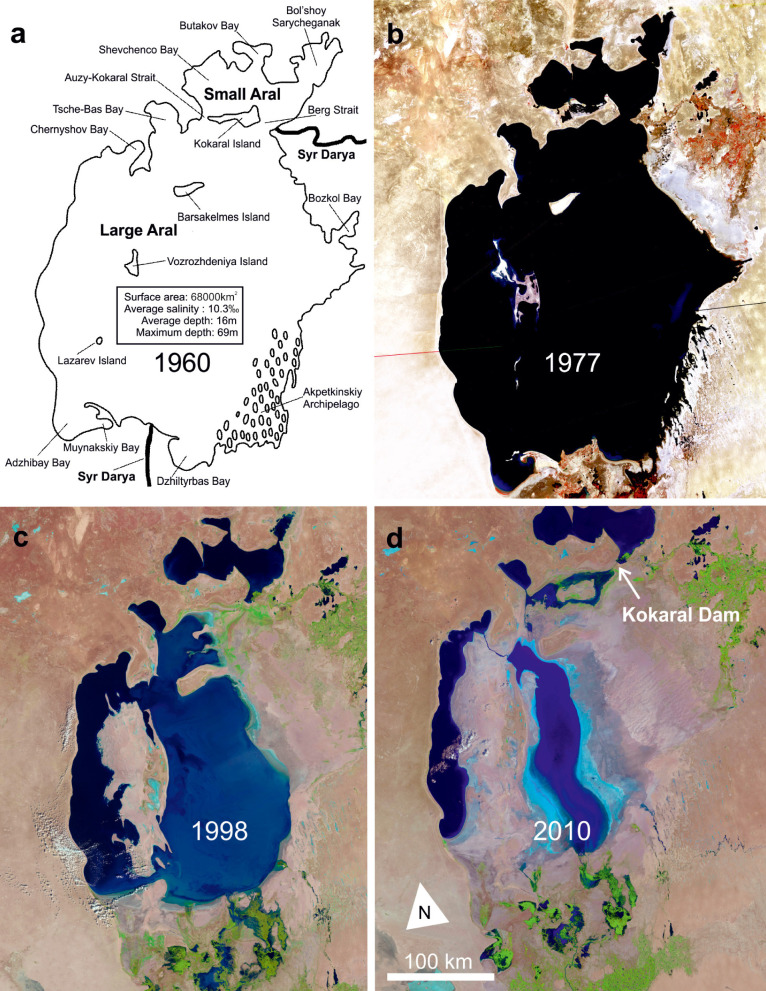
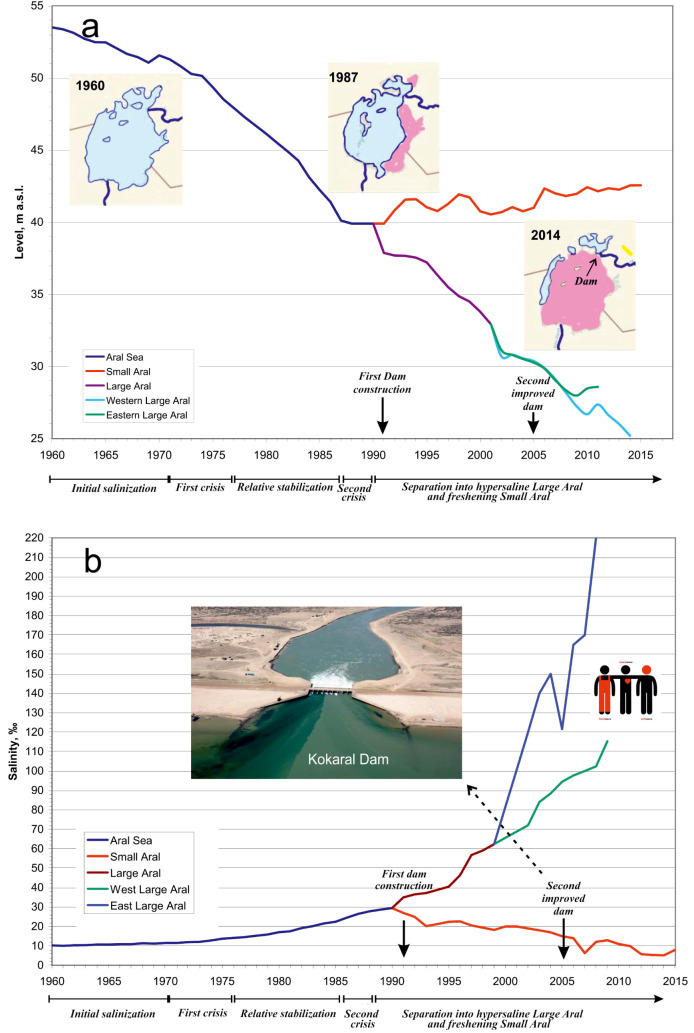
The modern anthropogenic regression began in 1961 (Fig. 2). Due to large scale irrigation projects and a natural low flow period, the water supply in the two rivers began to decline, causing a decrease in area and an increase in salinity (Bortnik and Chistyaeva 1990). By the late 1980s, both the Berg Strait and the Auzy-Kokaral Strait in the north had dried up, creating two separate water bodies with very different hydrologies (Fig. 3). The northern Small Aral (3200 km2) was then receiving water only from the Syr Darya, but it retained a positive water balance. Surplus water began to spill into the southern Large Aral, but this and inflow from the Amu Darya were insufficient to stem the ongoing desiccation and salinization (Aladin et al. 1995). The Large Aral is now fragmented into a western part, the former Tschebas Bay, and an extensive eastern part (Bortnik and Chistyaeva 1990). The western part is shrinking. The eastern part still receives irregular inflow from the Amu Darya and oscillates in size, but may also be facing complete desiccation.
Fig. 2.
The original Aral Sea around 1960 (a) and NASA Landsat images (b–d) documenting the modern regression (source NASA); separation of the northern Small Aral and the southern Large Aral had occurred by 1998; in 2010 the continued regression resulted in fragmentation of the Large Aral into several hypersaline water bodies; in contrast, construction of the Kokaral Dam (d) now preserves the water level in the Small Aral and allows it to gradually increase.
In 1992 a primitive dam was built across the Berg Strait. This caused the water level to increase and salinity to decline in the Small Aral (Fig. 3), enabling fisheries to resume (Chen 2018 2020). In 2004–2005 the World Bank financed a new and improved structure, the Kokaral Dam (Fig. 3). This supports the continued preservation of the Small Aral and the gradual restoration of its lost biodiversity (Aladin 1991 2014; World Bank 2014), but also raises the question of whether the reconstituted water body should remain a single, ecologically homogenous entity, or if future restoration projects should aim to diversify habitats that facilitate biodiversity. Such decisions require a detailed knowledge of the faunal history.
Fig. 3.
Changes to water level (a) and salinity (b) in the Aral Sea during the modern regression; m.a.s.l, metres above sea level; salinity given in parts per thousand; inserted images show the concomitant decrease in surface area and the Kokaral Dam (Redrawn from data in Micklin 2014).
Here we report on changes to the crustacean fauna in the Aral Sea from 1960 to the present. We put special emphasis on crustacean-fish relationships, including planned species introductions with the purpose of enhancing commercial fisheries. We document that even before the modern regression, species introductions (of both crustaceans and fish) caused displacement of native forms and dramatic disturbance of food webs. Eventually, all faunistic changes depended entirely on the progressing salinization. We end by using our data to predict how different restoration projects could affect the Aral Sea crustacean fauna to the benefit of fisheries and the human population in the area.
MATERIALS AND METHODS
We summarize investigations into the aquabiological and hydrological conditions of the Aral Sea over an approximately 60-year period. Due to the long time span and the multiple research teams involved, the sampling methodology vary. Hydrochemical data used and illustrated here derive from previously published studies. Water samples were obtained as described by Chechko (2015). For further details see Micklin (2007), Micklin et al. (2014) and references therein.
Zooplankton and benthos sampling
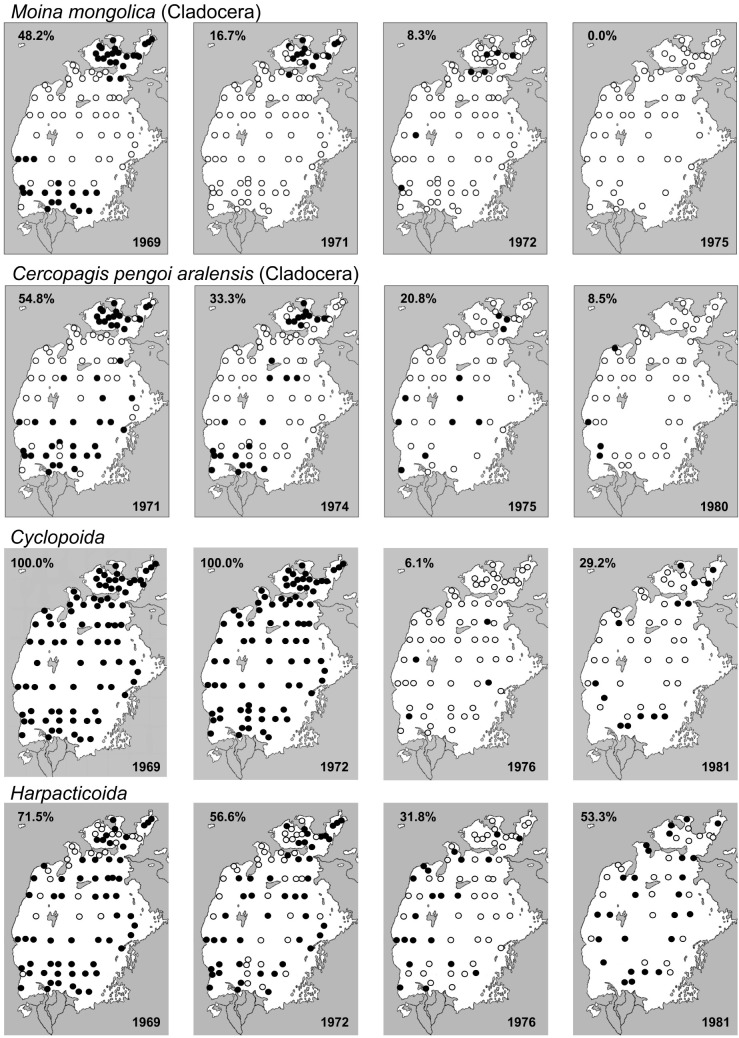
Sampling took place at a large number of fixed stations covering the entire Aral Sea system (Figs. 4, 5). Plankton was most commonly collected using Juday and Apstein nets while a Petersen bottom sampler was used for benthic sampling (Baker et al. 1977; Skjoldal et al. 2019; RECO 2020; WRiMS 2020) Plankton was sampled across the entire water column, from the bottom to the surface. For the benthos, the number of sample repeats varied based on substrate hardness, with one or two samples taken on the soft bottom and five on solid ground. A piston bottom sampler was used in the shallower areas to collect plankton and benthos. In addition, plankton and benthos samples were sometimes collected by divers using a Borok plankton net (Dyjachenko 1963) and a shark mouth (“closing jaw” type) bottom sampler, both constructs of the I. D. Papanin Institute for Biology of Inland Waters, Russian Academy of Sciences, Borok, Russia (Mathnet.ru 2020). All collected zooplankton and zoobenthos samples were fixed in formalin before being analyzed. We present the faunistic results for all native and introduced crustacean species within the period of investigation, organized as a time series that illustrates faunistic changes. We depict the occurrence through time of selected crustacean species at the fixed sampling stations.
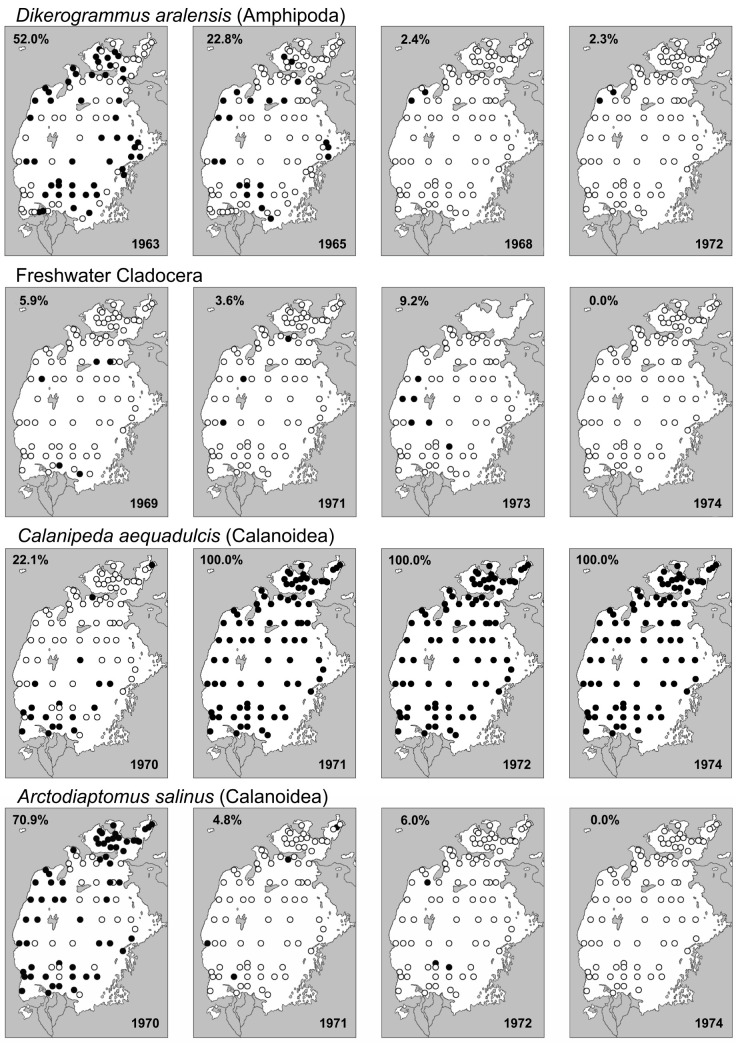
Fig. 4.
Occurrence of selected crustacean species at fixed sampling stations during the study period; filled circles = species present; percentages are of the number of stations where the species was found.
Fig. 5.
Occurrence of selected crustacean species at fixed sampling stations during the study period; filled circles = species present; percentages are of the number of stations where the species was found.
Thanatocenoses
The invertebrate composition from the recent past was analyzed using samples of thanatocoenoses (dead organisms) found in the dried bottom substrate and dried layers of sea grass. These samples provided data on Ostracoda, Branchiopoda and Malacostraca. The samples were collected from shallow holes dug in the hardened muddy ground of the former bottom. Data analysis was conducted using samples dated to 1960, 1965, 1970, 1975, 1980, 1985 and 1990–2011. Dating of these dried samples was based on known coastline positions. Physiological data and salinity tolerances used to discuss crustacean faunistic changes are based on Vinogradov and Bobovich (1970) and Aladin (1996).
Data analysis and storage
After the collapse of the USSR, notes and journals concerning sampling and sample processing that survived with the Aral branch of the Kazakh Research Institute of Fishery (KazNIIRKH) were transferred to and safeguarded at the ZIN (Zoological Institute Russian Academy of Sciences). The information was since returned and is stored in the Republic of Kazakhstan office of the IPA CIS (Inter-Parliamentary Assembly of the Member States of the Commonwealth of Independent States). The database is not yet available on the Internet, but is free for on site personal use.
Literature data
Mordukhai-Boltovskoi (1972 1974) provided important results for the period before the modern crisis. Some additional information was also published concerning the early part of the crisis, but mostly in Russian, often in hard to access sources not electronically available, and mostly without an English abstract (Andreev 1989; Andreeva 1989; Andreev and Andreeva 1988; Kortunova 1968 1975; Kortunova et al. 1972; Husainova 1958 1960; Karpevich 1975; Kazakhbaev 1974; Lukonina 1960a). Our account is also based on these valuable sources.
RESULTS
As with most water bodies, crustaceans of all kinds constituted a key component of the fauna in the original Aral Sea, both as primary consumers of photosynthetic organisms and as other parts of the food web in both the plankton and the bottom fauna. Table 1 (native species), table 2 (introduced species) and table 3 (parasites) document the faunistic changes from 1960 onwards. Tables 1–3 also provide the taxonomic data. Changes in selected crustacean species at the fixed sampling stations are detailed in figures 4 and 5. Table 4 lists major events and their implied causes.
Table 1.
Free-living crustaceans of the Aral Sea (native and naturally introduced)
| Taxa | species | native | before 1961 | 1961–1970 | 1971–1980 | 1981–1990 | Small Aral | Large Aral | ||
| (#) = species in found in family | 1991–2000 | After 2000 | 1991–2000 | after 2000 | ||||||
| BRANCHIOPODA | ||||||||||
| Anostraca | ||||||||||
| Artemiidae (1) | Artemia parthenogenetica Bowen et Sterling, 1978 | no | – | – | – | – | – | – | ↑ | + |
| Cladocera | ||||||||||
| Sididae (1) | Diaphanosoma brachyurum Lievin, 1848 | yes | + | + | ↓ | – | ↑ | + | – | – |
| Chydoridae (2) | Chydorus sphaericus s.l. (O.F. Müller, 1785) | yes | + | ↓ | – | – | ↑ | + | – | – |
| Coronatella rectangula s.l. (Sars, 1861) | yes | + | ↓ | – | – | ↑ | + | – | – | |
| Bosminidae (1) | Bosmina longirostris (O.F. Müller, 1785) | yes | + | ↓ | – | – | ↑ | + | – | – |
| Ilyocryptidae (1) | Ilyocryptus agilis Kurz, 1878 | ? | ? | ? | ? | ? | ↑ | + | – | – |
| Daphniidae (5) | Daphnia longispina (O.F. Müller, 1776) | yes | + | + | ↓ | – | ↑ | + | – | – |
| D. galeata G.O. Sars, 1864 | ? | ? | ? | ? | ? | ↑ | + | – | – | |
| Ceriodaphnia reticulate (Jurine, 1820) | yes | + | + | ↓ | – | ↑ | – | – | ||
| C. cornuta G. Sars, 1885 | yes | + | ↓ | – | – | ↑ | + | – | – | |
| C. pulchella G. Sars, 1862 | yes | + | ↓ | – | – | ↑ | ? | – | – | |
| Moinidae (2) | Moina mongolica Daday, 1888 | yes | + | + | ↓ | ↑ | + | + | ↓ | |
| M. micrura Kurz, 1874 | yes | + | ? | ? | – | ↑? | +? | – | – | |
| Polyphemidae (1) | Polyphemus pediculus s.l. (Linnaeus, 1761) | ? | ? | ? | ± | – | – | ? | – | – |
| Podonidae (4) | Evadne anonyx G. Sars, 1897 | yes | + | + | + | ↓ | ↑ | + | – | – |
| Podonevadne camptonyx (G. Sars, 1897) | yes | + | + | + | ↓ | ↑ | + | – | – | |
| P. angusta (G. Sars, 1902) | yes | + | + | + | ↓ | ↑ | + | – | – | |
| P. trigona(G. Sars, 1897) | ? | ? | ? | ± | ? | ? | ? | – | – | |
| Cercopagididae (1) | Cercopagis pengoi aralensisM.-Boltovskoi, 1971 | yes | + | + | + | – | – | – | – | – |
| COPEPODA | ||||||||||
| Calanoida | ||||||||||
| Diaptomidae (2) | Arctodiaptomus salinus (Daday, 1885) | yes | + | + | ↓ | – | – | – | – | – |
| Phyllodiaptomus blanci (Guerne et Richard, 1896) | yes | + | + | ? | ? | ? | ↑ | – | – | |
| Pseudodiaptomidae (1) | Calanipeda aquaedulcis Kritchagin, 1873 | no | – | + | + | + | + | + | ↓ | – |
| Cyclopoida | ||||||||||
| Cyclopidae (14) | Halicyclops rotundipes aralensis Borutzky, 1971 | yes | + | + | + | + | + | ↓ | + | – |
| Diacyclops bisetosus (Rehberg, 1880) | yes | + | + | ↓? | –? | – | ↑ | – | – | |
| Megacyclops viridis (Jurine, 1820) | yes | + | + | + | + | + | ? | ? | – | |
| Cyclops strenuus s.l. Fischer, 1851 | yes | + | + | ↓ | – | – | ↑? | – | – | |
| C. vicinus Uljanin, 1875 | yes | + | + | ↓ | – | – | ↑ | – | – | |
| Eucyclops macrurus (G.O. Sars, 1863) | yes | + | + | ↓ | – | – | ↑? | – | – | |
| E. serrulatus (Fischer, 1851) | yes | + | + | ↓ | – | – | ↑? | – | – | |
| Macrocyclops albidus (Jurine, 1820) | yes | + | + | ↓ | – | – | ↑? | – | – | |
| M. fuscus (Jurine, 1820) | yes | + | + | ↓ | – | – | ↑? | – | – | |
| Mesocyclops leuckarti s.l. (Claus, 1857) | yes | + | + | ↓ | – | ↑ | + | – | – | |
| Microcyclops bicolor (G.O. Sars, 1863) | yes | + | + | ↓ | – | – | ↑? | – | – | |
| Thermocyclops crassus (Fischer, 1853) | yes | + | + | ↓ | – | – | ↑? | – | – | |
| Th. dybowski (Lande, 1890) | yes | + | + | ↓ | – | – | ↑? | – | – | |
| Tropocyclops prasinus (Fischer, 1860) | yes | + | + | ↓ | – | – | ↑? | – | – | |
| Apocyclops dengizicus (Lepeshkin, 1900) | no | – | – | – | – | – | – | – | ↑ | |
| Harpacticoida | ||||||||||
| Ectinosomatidae (1) | Halectinosoma abrau (Kritchagin, 1873) | yes | + | + | + | ? | + | ? | – | – |
| Diosaccidae (3) | Schizopera aralensis Borutzky, 1971 | yes | + | + | + | ↓? | ? | ? | + | – |
| S. jugurtha (Blanchard et Richard, 1891) | yes | + | + | + | ↓? | ? | +? | – | – | |
| S. reducta Borutzky, 1971 | yes | + | + | + | ↓? | ? | +? | – | – | |
| Ameiridae (2) | Nitocra lacustris (Schmankewitsch, 1875) | yes | + | + | + | + | + | +? | +? | +? |
| N. hibernica (Brady, 1880) | yes | + | + | + | +? | + | ? | – | – | |
| Canthocamptidae (1) | Mesochra aestuarii Gurney, 1921 | yes | + | + | + | ? | + | ? | – | – |
| Laophontidae (1) | Onychocamptus mohammed (Blanchard et Richard, 1891) | yes | + | + | + | ? | + | ? | – | – |
| Cletodidae (4) | Cletocamptus retrogressus Schmankewitsch, 1875 | yes | + | + | + | + | + | +? | + | + |
| C. confluens (Schmeil, 1894) | yes | + | + | + | + | + | +? | +? | +? | |
| Limnocletodes behningi Borutzky, 1926 | yes | + | + | ↓ | – | – | +? | – | – | |
| Enhydrosoma birsteini Borutzky, 1971 | yes | + | + | + | ? | ? | ? | –? | – | |
| Nannopodidae (1) | Nannopus palustris Brady, 1880 | yes | + | + | ? | ? | + | +? | – | – |
| Darcythompsoniidae (1) | Leptocaris brevicornis (Van Douwe, 1905) | yes | + | + | + | ? | ? | ? | – | – |
| Leptastacidae (1) | Paraleptastacus spinicauda trisetosus Noodt, 1954 | yes | + | + | + | + | ? | ? | ? | – |
| OSTRACODA | ||||||||||
| Darwinulidae (1) | Darwinula stevensoni (Brady et Robertson, 1870) | yes | + | + | ↓ | – | ? | ↑? | – | – |
| Candonidae (1) | Typhlocypris (Typhlocypris) marchica (Hartwig, 1899) | yes | + | + | ↓ | – | ? | ↑? | – | – |
| Cyprididae (4) | Cyclocypris laevis (O.F. Müller, 1776) | yes | + | + | ↓ | – | ? | ↑? | – | – |
| Plesiocypridopsis newtoni (Brady et Robertson, 1870) | yes | + | + | ↓ | – | ? | ↑? | – | – | |
| Cyprinotus salinus (Brady, 1868) | no | ? | ? | ? | ? | + | +? | ? | – | |
| Eucypris mareotica (Fischer, 1855) | no | – | – | – | – | + | +? | + | + | |
| Cytherideidae (1) | Cyprideis torosa (Jones, 1850) | yes | + | + | + | + | + | + | + | ↓? |
| Leptocytheridae (1) | Amnicythere cymbula (Livental, 1929) | yes | + | + | ↓ | – | ? | ↑? | – | – |
| Hemicytheridae (1) | Tyrrhenocythere amnicola donetziensis (Dubowsky, 1926) | yes | + | + | ↓ | – | ? | ↑? | – | – |
| Limnocytheridae (3) | Limnocythere dubiosa Daday, 1903 | yes | +? | – | – | – | – | – | – | – |
| Limnocythere inopinata (Baird, 1850) | yes | + | + | ↓ | – | ? | ↑? | – | – | |
| Galolimnocythere aralensis Schornikov, 1973 | yes | + | + | ↓ | – | ? | ↑? | – | – | |
| Loxoconchidae (1) | Loxoconchissa (Loxocaspia) immodulata (Stepanaitys, 1958) | yes | + | + | ↓ | – | ? | ↑? | – | – |
| MALACOSTRACA | ||||||||||
| Amphipoda | ||||||||||
| Gammaridae (1) | Dikerogammarus aralensis (Uljanin, 1875) | yes | + | + | ↓ | – | – | – | – | – |
+, present; -, absent; ↓, disappeared; ↑, appeared. Occurrence based sampling at standard stations and shown for selected species and species groups in Figs. 1 and 2; s.l., species sensu lato.
Table 2.
Free-living crustacean introduced by man
| Taxa | Species | Before 1961 | 1961–1970 | 1971–1980 | 1981–1990 | Small Aral | Large Aral | ||
| 1991–2000 | after 2000 | 1991–2000 | after 2000 | ||||||
| COPEPODA | |||||||||
| Calanoida | |||||||||
| Pseudodiaptomidae | Calanipeda aquaedulcis Kritchagin, 1873 | – | ↑ | + | + | + | + | ↓ | – |
| MALACOSTRACA | |||||||||
| Mysida | |||||||||
| Mysidae | Paramysis (Mesomysis) intermedia (Czerniavsky, 1882) | + | + | + | – | – | ↑ | – | – |
| Paramysis (Serrapalpisis) lacustris (Czerniavsky, 1882) | + | + | + | – | – | ↑? | – | – | |
| Paramysis (Metamysis) ullskyi Czerniavsky, 1882 | – | + | + | – | – | ↑? | – | – | |
| Decapoda | |||||||||
| Palaemonidae | Palaemon elegans Rathke, 1837 | + | + | + | + | + | + | ↓ | – |
| Panopeidae | Rhithropanopeus harrisii tridentata Maitland, 1874 Only in Large Aral | – | ↑ | + | + | – | – | ↓ | – |
+, present; -, absent; ↓, disappeared; ↑, appeared. Occurrence based on sampling at standard stations and shown for selected species and species groups in Figs. 1 and 2; s.l., species sensu lato. Taxonomic naming issues are discussed in the text.
Table 3.
Parasitic crustaceans of the Aral Sea (native and naturally introduced)
| Taxa | Species | Native | before 1961 | 1961–1970 | 1971–1980 | 1981–1990 | Small Ara after 2000 | Large Aral after 2000 | ||
| Copepoda | ||||||||||
| Cyclopoida | ||||||||||
| Ergasilidae | Ergasilus sieboldi Nordmann, 1832 | yes | + | + | ? | – | – | +? | – | – |
| Lernaeidae | Lernaea cyprinacea Linnaeus, 1758 | yes | + | +? | ||||||
| Lamproglena pulchella Nordmann, 1832 | yes | + | + | ? | ? | – | +? | – | – | |
| Caligoida | ||||||||||
| Caligidae | Caligus lacustris Steenstrup et Lütken, 1861 | yes | + | + | ? | – | – | +? | – | – |
| Lernaeopodidae | Achtheres percarum Nordmann, 1832 | no | – | ↑ | + | – | – | +? | – | – |
| Branchiura | ||||||||||
| Argulidae | Argulus foliaceus (Linnaeus, 1758) | yes | + | + | ? | ? | – | + | – | – |
+, present; -, absent; Occurrence based regular sampling at standard stations
Table 4.
Major changes to Crustacea and fish in the Aral Sea during the modern regression
| Time | Period | Physical and chemical factors | Main changes to Crustacea and fish |
| Pre 1961 | Before regression | Native semi-stable state |
|
| 1961–1971 | Initial salinization | Slow salinity increased to 11.5 ppt |
|
| 1971–1976 | First crisis | Salinity passed 12-13 ppt | All freshwater crustaceans disappeared |
| 1976–1987 | Relative stabilization |
|
|
| 1987–1990 | Second crisis | Salinity passed 27-32 ppt |
|
| Post 1990 | Separation of Large and Small Aral |
|
|
Native crustacean fauna
The native, free-living crustacean fauna (Table 1) comprised 56 species and subspecies: Anostraca (1), Cladocera (18), Copepoda (30), Ostracoda (11) and Malacostraca (1) (Fig. 6a, d, e). This fauna comprised freshwater species, Ponto-Caspian brackish-water species, marine species, and representatives of the fauna from saline continental water bodies of the arid zone.
Fig. 6.
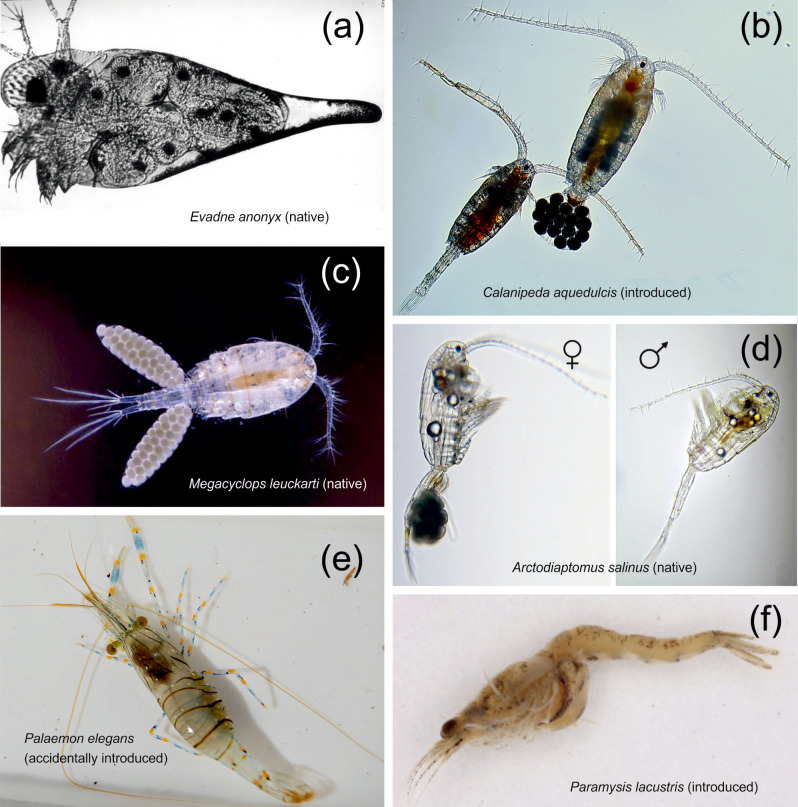
Selected native and invasive crustaceans from the Aral Sea: (a) Evadne anonyx (native), (b) Calanipeda aquedulcis (planned introduction), (c) Megacyclops leuckarti (native), (d) Arctodiaptomus salinus (native), (e) Palaemon elegans (accidentally introduced), (f) Paramysis lacustris (planned introduction). Calanipeda aquedulcis and P. lacustris were both introduced to become a food resource for commercially important fish. Further explanation in text (photo credits: a, NV Aladin, b, AN Khanaychenko Russian Academy of Sciences with permission; c, Dr. Ulrich Hopp, Germany, with permission; d, LS Svetlichny, National Academy of Sciences of Ukraine with permission; e, from Wikimedia Commons; f, T Lipinskya, National Academy of Sciences of Belarus with permission)
Halobiontic cladocerans
These comprised species from three orders and nine families. The open sea contained Ponto-Caspian species belonging to the Onychopoda: Cercopagis pengoi aralensis, Evadne anonyx, Podonevadne camptonyx, P. angusta and P. trigona. Of these, only E. anonyx and P. camptonyx occurred consistently all over the sea, except for the portions diluted from freshwater inflow. Being extremely rare, P. trigona was not reported before 1981. Three anomopod species occurred throughout the Aral Sea, but none commonly: Ceriodaphnia reticulata, Coronatella rectangula sensu lato and Moina mongolica. Population explosions occasionally took place, primarily in M. mongolica, but this species eventually declined and was gone by 1973.
Freshwater cladocerans
Some nine species were always restricted to areas with low salinity. Eight were ctenopodes: Diaphanosoma brachyurum, Moina micrura, Chydorus sphaericus, Bosmina longirostris, and Ceriodaphnia cornuta, C. pulchella, Daphnia longispina and Daphnia galeata. Diaphanosoma galeata, was first found in the 2nd half of the 1990s in the diluted zone of the Small Aral (Stuge 2001; Stuge and Saduakasova 2005). The only onychopod was Polyphemus pediculus.
Anostraca
The only representative was the brine shrimp Artemia, which inhabits hypersaline continental water bodies. In the past, parthenogenetic Artemia clones were sometimes locally encountered in the Aral, but only in its most saline regions such as small shallow bays and lagoons with 52 ppt salinity on the eastern coast of the Large Aral (Aladin and Filippov 1993). Artemia was first found in the Large Aral, when the salinity in its open part reached ~58 ppt (Musaev et al. 2012). By the 2000s it became the dominant planktonic crustacean (Marden et al. 2012) in the residual and fragmented water bodies, and it now seems to be the only crustacean in the shallow eastern part (Aladin and Plotnikov 2008).
Free-living copepods
The original fauna comprised 31 species, representing three orders and 10 families (Calanoida 2 spp; Cyclopoida 14 spp; Haparcticoida 15 spp). These represented both euryhaline, brackish water and freshwater species with some being widely distributed and a few being endemic to the Aral Sea.
Two species of Calanoida (Diaptomidae) occurred. The large phyto-detritophagous Arctodiaptomus salinus (Fig. 6d) was found throughout the Aral (Behning 1934 1935), but by the early 1960s it declined catastrophically in numbers due to predation by introduced planktophagous fish (smelt and herring). The freshwater Phyllodiaptomus blanci occurred only in highly diluted areas near the deltas of the Amu Darya and Syr Darya.
Cyclopoida
Of the 14 free-living species, the widely distributed and euryhaline Halicyclops rotundipes aralensis belongs to the marine fauna, while the remaining 13 species are widespread freshwater forms. Nine inhabit only areas with low salinity: Cyclops strenuus, Diacyclops bisetosus, Eucyclops macrurus, E. serrulatus, Macrocyclops albidus, M. fuscus, Microcyclops bicolor, Thermocyclops dybowskii and Tropocyclops prasinus. Mesocyclops leuckarti and, less often, Cyclops vicinus and Thermocyclops crassus were found not only in the diluted areas, but also at “normal” salinity. The widely distributed and euryhaline Megacyclops viridis occurred at even up to 50 ppt salinity. Throughout the Aral Sea, except for strongly salinized areas, the most numerous cyclopid was Mesocyclops leuckarti (Fig. 6c). Halicyclops rotundipes aralensis was also present throughout the Aral, but always in low numbers.
Harpacticoida occurred with 15 euryhaline, brackish and marine species from eight families: Halectinosoma abrau, Schizopera aralensis, S. jugurtha, S. reducta, Nitocra lacustris, N. hibernica, Leptocaris brevicornis, Mesochra aestuarii, Laophonte mohammed, Cletocamptus retrogressus, C. confluens, Limnocletodes behningi, Nannopus palustris, Enhydrosoma birsteini and Paraleptastacus spinicauda trisetosus). Three of these were endemic to the Aral Sea: Schizopera aralensis, S. reducta, and Enhydrosoma birsteini. Another three (Nitocra lacustris, Cletocamptus retrogressus, C. confluens) are euryhaline and found in continental hypersaline waters > 100 ppt (Loffler 1961; Mirabdullayev et al. 2004; Mokievsky and Miljutina 2011; Shadrin 2012; Carrasco and Perisinotto 2012).
Ostracoda
The native fauna comprised 11 species from eight families of the Podocopida: Five are freshwater forms: Darwinula stevensoni, Typhlocypris marchica, Cyclocypris laevis, Limnocythere inopinata and Loxoconchissa (Loxocaspia) immodulata. The remaining species are brackish and marine: Plesiocypridopsis newtoni, Cyprideis torosa, Amnicythere cymbula, Tyrrhenocythere amnicola donetziensis, Limnocythere dubiosa and Galolimnocythere aralensis. The most common ostracod, Cyprideis torosa, is found from fresh to hyperhaline waters and occurs in the Aral as the “amphiosmotica” form of freshwater origin, which is a robust osmoregulator with salinity tolerance up to 104 ppt (Aladin 1989a).
Two additional ostracods in Cyprididae, Cyprinotus salinus and Eucypris mareotica, are not listed in Mordukhai-Boltovskoi (1974), and were apparently only recently autointroduced to the Aral Sea. These euryhaline and widespread marine species were first found in 1995 in the Gulf Bolshoy Syrycheganak of the Small Aral (Aladin et al. 2004).
Malacostraca
The only original malacostracan was the euryhaline gammarid amphipod Dikerogammarus aralensis, widespread in the Ponto-Caspian fauna province (Mordukhai-Boltovskoi 1974). In the salinized gulfs of the Caspian Sea, it is numerous at a salinity of 50 ppt (Behning 1937). In the Aral Sea it was found throughout the entire salinity gradient.
Parasitic crustaceans
The original fauna contained only seven parasitic crustaceans (Table 3), all infecting fish by means of their free-swimming larvae: from Cyclopoida Ergasilus sieboldi, Lernaea cyprinacea, L. esocina and Lamproglena pulchella; from Caligoida Caligus lacustris and Achtheres percarum; and from Branchiura Argulus foliaceus.
Introduced crustaceans
Six alien species of free-living crustaceans were introduced either intentionally or accidentally during initiatives to enhance commercial fisheries (Fig. 6; Table 2). Several of these crustaceans and some of the introduced fish caused ecological disturbance even before the area regression became serious: the introduction of the shrimp Palaemon elegans led to the disappearance of the native gammarid Dikerogammarus aralensis; the introduced calanoid Calanipeda aquaedulcis largely replaced the native Arctodiaptomus salinus although the latter was already seriously declining, while the introduction of herring caused a serious decline in all crustacean zooplankton.
Palaemon elegans, a caridean shrimp, was the first crustacean to become naturalized in the Aral Sea (Fig. 6e). It was accidentally introduced during the unsuccessful attempt to acclimatize two species of mullets intended for commercial fisheries. First found in 1957, the shrimp spread throughout the entire water body.
Mysids
In 1958–1960 relict Ponto-Caspian species of mysids were introduced from the Don River delta as a possible new food source for commercially harvested fish (Fig. 6f). About 90% of the specimens consisted of Paramysis (Serrapalpisis) lacustris, the remaining part being P. (Mesomysis) intermedia and few P. (Paramysis) baeri (Karpevich 1960b). The first batches perished when they were transported from fresh water to the small Aral to a salinity of ~10 ppt, but in 1959–1960 new introductions were successful in the diluted bay near the mouth of the Syr Darya. The mysids spread throughout the Aral, but only P. lacustris and P. intermedia became established. To accelerate the naturalization, mysids were also transported in 1964 into the Large Aral near the Amu Darya delta, from where expansion continued. By the second half of the 1960s, P. intermedia had invaded all nonsalinized littoral areas, and replaced P. lacustris. The latter remained only near the Syr Darya mouth and eventually disappeared altogether. In 1965, Paramysis (Metamysis) ullskyi appeared independently in the mouth of the Syr Darya, having penetrated from reservoirs on the river, where it was introduced in 1963.
Calanoida
The native calanoid Arctodiaptomus salinus had dwindled by the early 1960s. To remediate this, the Mediterranean-Atlantic Calanipeda aquaedulcis (Pseudodiaptomidae) was introduced in 1965–1970 as an alternative food source for introduced plantivorous fish (Fig. 6b). The specimens came from the Kuban estuaries and the Taganrog Bay of the Sea of Azov and were released in the southern part of the Large Aral, in diluted bays and at the mouth of the Amu Darya. By 1970 C. aquaedulcis had spread into the open waters, and in 1971 it even began to dominate the zooplankton throughout the entire Aral Sea, including areas with salinity up to 15–18 ppt. Within a single year, the native A. salinus decreased even more and has not been seen in the Aral since 1974.
Mud crabs and parasites
In 1970, the mud crab Rhithropanopeus harrisii tridentata (Panopeidae) and the parasitic copepod Achtheres percarum (Lernaeopodidae) were accidentally introduced into the Large Aral. The source larvae in water bags containing Calanipeda aquaedulcis. In 1976, R. harrisii had become established in the Large Aral, but it never penetrated to the Small Aral. Achtheres percarum appeared on pike perch, Sander lucioperca (L., 1758), in the late 1970s.
Modern Regression
Eventually salinity increase became the main cause for changes in the Aral Sea crustacean fauna (Fig. 2). Based on physical and biological changes, we divide the modern regression into four periods (Fig. 3, Table 4). The initial period (1961–1971) was dominated by changes due to introduced species. The first crisis period (1971–1976) ended with the disappearance of all brackish and fresh water species due to salinization. The following (1976–1987) period had relatively stable conditions for the surviving, halophilic organisms. Finally, the second crisis period (1987–1990) saw the most dramatic salinity increase, accompanied by the disintegration of the Aral Sea into several separate water bodies and the extinction of the majority of metazoan species, including most crustaceans. After 1990, the development must be described separately for northern Small Aral and the southern Large Aral. Main changes to crustaceans and ichthyofauna are summarized in Table 4 and details for selected species appear in figures 4 and 5.
Initial salinization (1961–1971): During this decade the desiccation was slow and average salinity increased only 1.5 ppt, reaching 11.5 ppt in 1971 (Fig. 3). The crustacean faunal changes were occasionally drastic, but primarily associated with species introductions.
First crisis (1971–1976): Desiccation and salinization accelerated, and from 1974 the inflow from the rivers was dramatically reduced, further deteriorating the situation. Salinity initially increased only slowly with few if any faunistic changes, but a dramatic change happened when it reached 12–13 ppt and all freshwater cladocerans disappeared completely (Plotnikov and Aladin 2011, Plotnikov et al. 1991). Ceriodaphnia reticulata disappeared before 1971, followed by Coronatella rectangula by 1974. By 1975 only four cladoceran species remained (Evadne anonyx, Podonevadne camptonyx, P. angusta and Cercopagis pengoi aralensis), all belonging to the saline Ponto-Caspian fauna province.
Relative stabilization (1976–1987): The salinization caused the fauna of free-living cyclopid copepods to decrease from 22 to 16 species. After the freshwater Mesocyclops leuckarti disappeared, the most numerous cyclopoid was the euryhaline and more salinity halotolerant Halicyclops rotundipes aralensis. The number of harpacticoid species also declined, but the more euryhaline ones survived.
Of the cladocerans that survived the first crisis, Cercopagis pengoi aralensis was the most salinity sensitive, becoming rare and then, from 1981, absent. Podonevadne camptonyx was now the most numerous cladoceran, with Evadne anonyx being less widely distributed and present in smaller numbers. Since 1971, the recent invading planktonic copepod Calanipeda aquaedulcis had become numerous and replaced Arctodiaptomus salinus. The euryhaline cyclopoid Halicyclops rotundipes aralensis, although present all over the sea, decreased in numbers everywhere. Diacyclops bisetosus was only occasionally observed.
Second crisis (1987–1990): By 1987 salinity had reached 27 ppt. Many remaining species could not survive beyond the 27–32 ppt range (Plotnikov and Aladin 2011), and with continued salinity increase the crustacean fauna entered a “second crisis period”, entailing another fast decrease in diversity (Plotnikov et al. 1991).
The last remaining Ponto-Caspian crustaceans disappeared; by this time they were represented only by the Podonidae. Evadne anonyx disappeared by 1988, when salinity reached 28 ppt, and by 1990, Podonevadne was gone (Aladin 1989b). The thanatocenoses from 1985 and 1990 (Aladin 1991) showed changes in the ostracod fauna. The carapaces of Amnicythere cymbula, Tyrrhenocythere amnicola donetziensis and Galolimnocythere aralensis could no longer be found. Of the aboriginal and benthic ostracods, only Cyprideis torosa survived the crisis. After this crisis, the now much depleted crustacean fauna entered a period of relative stabilization.
Post 1990: The cladoceran Podonevadne camptonyx reappeared in 1991 in the Small Aral, apparently from dormant eggs (Plotnikov 2016). It was only absent in Butakov Bay, where the salinity remained higher. From 1991–1996 no new changes were observed in the composition of the crustacean fauna. The copepod Calanipeda aquaedulcis was still the most widespread species in both the Small and the Large Aral, while the cyclopid Halicyclops rotundipes aralensis was uncommon. Benthic crustaceans were represented only by the shrimp Palaemon elegans and (only in the Large Aral) the mud crab Rhithropanopeus harrisii tridentata (see Filippov 1995).
The small Aral Sea: Following separation of the two water bodies, water from the Syr Darya flowed only to the Small Aral. This, and especially the restrained outflow due to the new dam, combined to raise the water level, causing a significant salinity decrease, and the formation of a large freshwater zone. The effect on both crustacean and fish fauna was almost immediate. In 2016, the planktonic crustacean fauna had increased, including recolonizing fresh-brackish water species (Plotnikov et al. 2016), including the cladocerans Bosmina longirostris, Chydorus sphaericus, Diaphanosoma brachyurum, Ceriodaphnia reticulata and Podonevadne angusta, Evadne anonyx and the copepods Phyllodiaptomus blanci, Cyclops vicinus, Mesocyclops leuckarti and Megacyclops viridis. The intentionally introduced Calanipeda aquedulcis remained present, and is now again common after a decline in the 1990s. Mysids immigrated from the lower Syr Darya, with Paramysis intermedia now found in Shevchenko Bay. Other species are known or suspected to be returning (Tables 1, 2).
Post 1990 -The hypersaline Large Aral: After the separation, the salinity in the Large Aral accelerated and, upon reaching 47–52 ppt in the second half of the 1990s, the fauna reflected this condition. In 1998, the dominant zooplankton species had disappeared, including Calanipeda aquaedulcis and Halicyclops rotundipes aralensis. Most truly marine harpacticoids also disappeared, and only three aboriginal and high salinity (> 100 ppt tolerant species may now remain: the presence of Cletocamptus retrogressus is documented, but there is no information on C. confluens and Nitocra lacustris (Mirabdullayev et al. 2004; Mokievsky and Miljutina 2011). The ostracod Cyprinotus salinus disappeared before 2002, while the very salinity tolerant Cyprideis torosa remained in the western part in 2005 (Zavialov 2012).
During hypersalinization there were temporary species invasions. The cladoceran Moina mongolica reappeared in 1996, but was gone again by 2002 (Mirabdullayev et al. 2004; Aladin and Plotnikov 2008). The halophilic copepod Apocyclops dengizicus appeared in the western Great Aral in 2004 and seems to have become established (Mirabdullayev et al. 2007). Carapaces of the invasive and euryhaline ostracod Eucypris mareotica were frequently found in thanatocenoses in 2005 (Aladin and Plotnikov 2008). Artemia appeared in 1998, when salinity in the open parts reached ~58 ppt (Musaev et al. 2012). The shallow eastern Large Aral salinized more strongly than the deeper western part. In some years, it dries out completely, and its crustacean fauna now comprises only Artemia (Aladin and Plotnikov 2008).
DISCUSSION
Native crustacean fauna
Compared to the similarly saline Caspian Sea, the native Aral Sea had a much poorer fauna, with both fewer species and the absence of several major taxa. Absent were species of the Centropagidae and Temoridae (Copepoda) and the cladocerans Apagis (Cercopagidae), Cornigerius, Caspievadne and Pleopis (Podonidae). One species of Cercopagis (Cercopagididae) occurred. The only native malacostracan was the amphipod Dikerogrammus aralensis, contrasting with a rich and diverse malacostracan fauna in the Caspian (Birstein et al. 1968; Mordukhai-Boltovskoi 1974).
Natural and accidental introductions
The isolation of the Aral Sea basin limited the number of species invasions to a few from the local area and anthropogenic causes, whether accidental or planned. This differs from the Caspian Sea, where many alien species were brought in by ships in ballast or as biofouling after navigable canals connected the inflowing Volga to the Black and Baltic Seas (Aladin et al. 2004).
Branchiopoda
The halobiontic cladoceran P. trigona was not recorded until 1981. It most likely invaded from the Ponto-Caspian Sea with introduced fish or invertebrates (Aladin and Andreev 1984). The halobiontic cladoceran Ilyocryptus agilis was previously (Nikolsky and Pankratova 1934) found in the nearby Lake Sudochye system. Although this lake is separated from the Amu Darya delta the species spread, probably naturally, to the Aral Sea. Artemia was always present in the general area, but occurred in the Aral Sea itself only after salinization reached sufficient levels (Arashkevich et al. 2009).
Palaemon elegans
This caridean is naturally distributed in coastal areas of the Atlantic, Baltic, Mediterranean, Black and Azov Seas (Borisov 2012). It has a salinity tolerance of 5 to > 62 ppt (Plotnikov 2016) and is a good osmoregulator, and therefore also invaded the Caspian Sea (Karpevich 1975). Its main food is detritus, aquatic plants and benthic invertebrates (Mordukhai-Boltovskoi 1974). The frequency increase of P. elegans correlates with the decline of the native amphipod D. aralensis (Malinovskaya 1961; Mordukhai-Boltovskoi 1972), which disappeared in 1973 before salinization became significant. We therefore suggest that P. elegans predation was a primary cause of this decline.
Mud crabs
The accidentally introduced Rhithropanopeus harrisii occurs naturally in the Gulf of Mexico and along the Atlantic coast of North America. It is a good osmoregulator (Bayly 1972); tolerates salinities > 56 ppt; and occurs in freshwater, rivers mouths and true marine habitats (Borisov 2012). It has invaded the Mediterranean Sea, Black Sea, Azov Sea, parts of the Baltic Sea and the Caspian Sea. The effect of this invader on the Aral Sea fauna has been negligible, although it may have provided a new food resource for wading birds. In contrast, Kotta et al. (2018) recently demonstrated how introduction of Rhithropanopeus harrisii to the Baltic caused a surprising ecological change from a bottom-up to a strong top-down controlled ecosystem, with a decline in benthic invertebrate biomass but an increase in pelagic nutrients and phytoplankton. This emphasizes how the effect of an alien invader can vary critically with the ecology of the receiving ecosystem.
Planned introductions of fish and crustaceans
Aladin et al. (2019) treated the Aral Sea ichthyofauna, so here we discuss only events closely associated with the crustacean fauna. The native ichthyofauna consisted of freshwater fish that migrated into the rivers for reproduction, since eggs and fry could not develop in saline conditions (Ermakhanov et al. 2012). In the 20th century, non-native fish (herring, plaice, and cyprinids) and crustaceans (mysids and calanoids intended as fish food) were artificially introduced to enhance fisheries. In addition, the accidentally introduced Palaemon elegans also became a food source for bottom foraging fish. The introduction of cyprinids and plaice, Platichthys flesus (L., 1758), were a success, while the other attempts had either no effect or caused ecological disruption such as P. elegans displacing D. aralensis.
Effect of introduced fish
The big sand smelt, Atherina boyeri caspia (Eichwald, 1838), became accidentally established in the Aral during unsuccessful attempts to introduce mullets from the Caspian Sea. The Baltic herring (Clupea harengus membras Valenciennes, 1847) was intentionally introduced in 1954–1956, with catastrophic results. The herrings increased rapidly in number, causing heavy predation on native zooplankton, which was previously consumed mainly by sticklebacks (Pungitius platygaster Kessler, 1859) and juvenile fish. All large sized crustacean zooplankton (Arctodiaptomus salinus, Moina mongolica, Cercopagis pengoi aralensis, Ceriodaphnia reticulata) declined and virtually disappeared, and during the winter of 1960–1961 this caused massive deaths among the introduced fish due to starvation (Pankratova 1935, Osmanov 1961; Karpevich 1975; Kortunova and Lukonina 1970; Kortunova 1975). In the original Aral Sea, sticklebacks seem to have had a relatively minor ecological role, since they were not numerous and do not forage for zooplankton in deeper waters, but if introduced to small, shallow waters they will normally almost completely eradicate planktonic crustaceans (JM personal observation). Another, unexpected change was recently described from the Baltic coast of Sweden, where a decreased predation by larger fish caused a 50-fold increase in stickleback numbers. As a result, the former predator dominated system changed to prey dominance, because the small, but very numerous sticklebacks now prey upon the juveniles of larger species (Eklöf et al. 2020).
Introduction of mysids and calanoids
The three introduced mysids species are all detritophageous from brackish and marine waters in the Ponto-Caspian region. They were selected as potential fish food due to their fertility and better salinity tolerance (up to 17–20 ppt) than most of the native crustaceans (Daribaev 1967; Karpevich 1958a b 1960a b 1975; Bokova 1960). We conclude that Paramysis intermedia became established because it tolerates the relatively cold temperatures that can prevail in the Aral Sea (Karpevich and Bokova 1970). The quick demise of P. baeri was either due to being introduced in insufficient numbers or to an intolerance of low temperatures. Unfavourable thermal conditions may explain the eventual disappearance of P. lacustris, but fish predation may also have been a contributing factor for this large and less mobile mysid (Karpevich and Bokova 1970; Kortunova 1968 1970). The native calanoid, Arctodiaptomus salinus, is widely distributed in saline continental waters of the Palaearctic. It is monocyclic (one generation per year) and has low fertility (4–12 eggs) (Lukonina 1960b; Kortunova 1975). Calanipeda aquaedulcis was introduced to compensate for the decline of A. salinus, because it is much more fertile (six generations per year) and tolerates a wider salinity range (0.5–56 ppt) (Gubareva and Svetlichny 2011). Eventually the alien calanoid replaced both A. salinus and Moina mongolica in the plankton (Plotnikov 2016). Salinity increase cannot explain the disappearance of M. mongolica, which can tolerate up to > 80 ppt (Aladin 1996).
Plaice: a saviour for fisheries
Plaice (flounder gloss) was successfully introduced after other commercially important fish had seriously declined because of increasing salinity (Ermakhanov et al. 2012; Lim 1986). Fishermen from Denmark, where plaice fishing is important, gave valuable technical advice as part of the Joint Danish-Kazakhstani environmental project “From Kattegat to the Aral Sea”, 1996–2008 (KSC 2020). The biologically and technically well founded introduction meant that plaice became the only asset for commercial fisheries in the Aral during the 1990s.
Introduced crustaceans and ichthyofauna
The planned introduction of alien mysids for fish food seems not to have had the desired effect. The accidental introduction of the caridean shrimp negatively affected the native fauna of gammarids, but there is no record that it affected the fish fauna. The planned introduction of the calanoid Calanipeda aquaedulcis displaced local planktonic crustaceans, but had no effect on the planktivorous fish, which just changed to the new food source. The native fish were unaffected by the decline in zooplankton, since they utilized other food sources, but eventually almost all fishery, whether on native or introduced species, ceased in the Aral itself due to salinization (Ermakhanov et al. 2012). Most initiatives to improve fisheries occurred without any coordination with the diversion of water for agricultural irrigation (see chapters in Micklin et al. 2014). Without the regression, the species introductions might have led to a stable but much changed fauna..
Parasitic crustaceans
Many of the parasitic crustaceans in the Caspian Sea were absent from the native Aral Sea fauna. This includes the ergasilid copepod Thersitina gasterostei, whence the sticklebacks were free from this parasite. It is remarkable that no parasitic crustaceans were introduced along with alien fish hosts. The only introduced parasite, Achtheres percarum, entered as larvae in transport bags. This and the similar pathway for mud crab larvae emphasizes the danger of transporting any alien species to a new habitat.
Among the parasitic crustaceans, Ergasilus sieboldi, Argulus foliaceus, Caligus lacustris and Achtheres percarum are euryhaline, but infestation by Lernaea cyprinacea, L. esocina and Lamproglena pulchella occurs only in fresh water (Osmanov 1967 1971; Osmanov and Yusupov 1985), and during salinization these parasites began to disappear. Increased consumption of their larvae by introduced planktophagous fish may also have been a factor (Osmanov et al. 1976; Osmanov and Yusupov 1985). From the mid-1970s salinity increase had caused the disappearence of all native fish along with their crustacean parasites from the Aral itself (Osmanov and Yusupov 1985), but both groups persisted in the inflowing rivers and the diluted floodplains and delta lakes (Ermakhanov et al. 2012). The Syr Darya crustacean parasites may therefore help repopulate the reconstituted Small Aral, but there are no recent parasitological studies investigating this.
In its native range the introduced mud crab P. harrisii is frequently infested by the parasitically sterilizing rhizocephalan Loxothylacus panopei (Gissler, 1884). Rhizocephalans are not commonly introduced alongside with their hosts (Thresher et al. 2000), but a notable exception is a P. harrisii population infested with this parasite in the eastern Mediterranean (Øksnebjerg 2000). For the Aral Sea, P. harrisii entered as larvae, and this prevented the simultaneous introduction of the parasite due to the nature of the rhizocephalan life cycle (Høeg 1995; Glenner 2001).
Crisis and partial recovery
Recovery of the Small Aral fauna
No introductions took place after the dam construction, so most species must have entered naturally from the Syr Darya and lakes in its lower reaches. Some crustaceans perhaps also reappeared by hatching from dormant eggs. We explain the even earlier reappearance of Podanevadne to a somewhat decreasing salinization when the Small and Large Aral became separated. The decreased salinity and the abundance of crustaceans for food explain why commercial fisheries are now rebounding, even though they have not reached the catches obtained in the original Aral Sea (Chen 2018 2020). With timely action, the Aral system has therefore proven to be rather resilient. The list of crustaceans now recorded in the Small Aral (Plotnikov et al. 2016) is probably incomplete, because some infrequent species may well have escaped notice, but the marine copepod Halicyclops rotundipes aralensis was never numerous and may have disappeared completely due to decreasing salinity.
Demise of the Large Aral
The extreme salinization during the 1990s caused the loss of many species, but also the appearance of crustaceans, which must have derived from resting stages from other hyperhaline waters in the general region. The result is a now much poorer fauna with new dominant species such as Eucypris mareotica, Apocyclops dengizicus and especially Artemia.
Artemia in the Large Aral
The complexity of the Aral Sea system makes it an excellent example of the zoogeography and dispersal of the halobiontic Artemia. The introduction occurred from latent eggs, presumably by aeolian means or by bird transfer from other nearby high-salinity waters (Aladin and Filippov 1993; Aladin et al. 2004; Reynolds et al. 2015; Rogers 2014). Such transfer may be critical to the long-term survival of this species in arid regions, where any locality may be short lived (Green and Figuerola 2005; Figuerola and Green 2002; Figuerola et al. 2005). Charalambidou and Santamaria (2005) suggested from field evidence that the fast digestion of wading birds allows for the transport of ingested resting eggs. This was verified for anostracans in general by the experimental study of Rogers (2014), which also demonstrated that this method, unlike spreading by wind, favours directed transport to habitable new locations. Rogers (2015) furthermore discussed how patchy distribution and bird transfer to virgin habitats might together forge the evolution of Artemia clones. Undoubtedly, Artemia dispersal by resting eggs has always occurred in the Aral area, but before the 20th century, salinity in the sea itself remained too low for development. The phyto-detritophagous copepod Calanipeda aquaedulcis would also have been a fierce competitor for food, and planktivorous fish would quickly have exterminated any emerging Artemia (Musaev et al. 2012). Disappearance of fish and high salinity created the conditions for the establishment of Artemia (Arashkevicha et al. 2009). The genus is now harvested commercially for export, and this industry could be increasingly important in this otherwise economically depressed region. Nonetheless, problems remain for this industry in terms of both reliable water supply to the Artemia sites, logistics and economical factors (Marden et al. 2012). In many arid areas of the World, brine shrimps are also an important food source for birds, and the heavily increased Artemia populations could be such a novel resource. This underlines the often neglected, but important connection between conservation efforts in terrestrial and aquatic systems (Abell and Harrison 2020). Unfortunately, we are unaware of any recent studies on the avian ecology in the Aral Sea area.
Future of the Aral Sea
The Small Aral
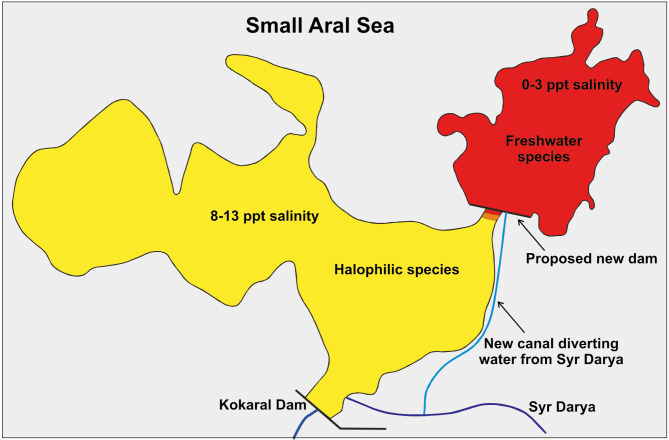
Further perspectives on the Aral Sea crustacean fauna depend primarily on salinity, and this in turn depends on future changes to existing dams or construction of new ones to retain inflowing water. A further salinity decrease in the Small Aral may negatively affect marine species and the fauna of saline continental water bodies. Salinity decrease could threaten Palaemon elegans but would also favour a return of the amphipod Dikerogammarus aralensis. Extinction is also likely for cladocerans of the Podonidae, the copepods Calanipeda aquaedulcis, Halicyclops rotundipes aralensis and all freshwater intolerant harpacticoids. Lower salinity would on the other hand allow for the re-establishment of all ostracods, except Limnocythere aralensis, and all freshwater tolerating copepods (Plotnikov et al. 2016). A project has been proposed for an additional dam in the neck of Bolshoy Sarycheganak Bay (Fig. 7). The dam would have a spillway into the main Small Aral and be fed by a channel from the Aklak control structure, diverting part of the Syr Darya flow (Micklin et al. 2020). If realized, the Small Aral will become a cascade of two water bodies. The novel dammed in bay would have a very low salinity (< 2 ppt) and be inhabited by freshwater species immigrating from the Syr Darya, while marine and brackish organisms would disappear. The main part would remain brackish, allowing at least some halophilic species to remain. An alternative project suggests to elevate the existing Kokaral Dam, causing the entire Small Aral to increase in area and remain a single brackish water body, with a diluted zone only in the vicinity of the Syr Darya delta (Micklin 2016; Micklin et al. 2020; Plotnikov et al. 2016). Clearly, any new project must take into serious consideration the predicted changes to biodiversity and make a choice that also best benefits fisheries and thereby the surrounding human population.
Fig. 7.
Future of the Small Aral Sea. A new dam would divide the Small Aral Sea into two basins with different salinities and resulting faunas. The canal feeding the new, northern basin could have several different courses with only one shown here. See details in text and Micklin et al. (2020).
The Large Aral
The absence of any permanent water inflow from the Amu Darya means that stabilization of the water level or decrease in salinity is impossible. With no action taken to arrest salinization in the western parts, the decline in biodiversity will continue and soon cause the extinction of the ostracod Cyprideis torosa and eventually also Eucypris mareotica. The harpacticoids Cletocamptus confluens, Nitocra lacustris, C. retrogressus and the cyclopid Apocyclops dengizicus are more salinity tolerant and will remain for some time. But eventually Artemia will be the only crustacean present (Plotnikov et al. 2016), and when its salinity tolerance (350 ppt) is exceeded, the western Large Aral will resemble the Dead Sea, with no metazoan life at all (Oren et al. 2010). The crustacean fauna of the eastern Large Aral might be restored, but only if it again receives a reliable inflow from the Amu Darya (Fig. 2). At present, such discharge happens only irregularly and not every year, and the outflow from the Small Aral presently evaporates in vain on the salt pan without ever reaching Large Aral waters. Another concern may be opposition to any re-flooding after the discovery of rich natural gas fields under the former seabed in Uzbekistan.
CONCLUSIONS
The isolated situation of the Aral makes it a rare site for studying how species originally invaded naturally and interacted to become and a unique ecosystem. Anthropogenic species introductions, whether accidental or planned, and the virtual breakdown of the ecology happened within the last half of the 20th century and were therefore well documented (Micklin 2007 2016; Micklin et al. 2014 2020). We have described changes to the important crustacean fauna and shown that they were caused by changes in physical and chemical factors, interactions among native and introduced crustaceans, and predation by introduced fish. The changes did not occur gradually following the salinity increase, but occurred stepwise and often catastrophically, when species met an introduced predator or a physiological barrier. Such a nonlinear pattern is now also expected for species extinctions in general due to climate change (Sunday 2020; Trisos et al. 2020). This implies that an observed, slow extinction rate does not by itself guard against sudden, catastrophic changes. On the other hand, the restoration projects for the Aral Sea have demonstrated that a relatively small effort can very rapidly result in a naturally occurring, partial restoration of the fauna, especially with respect to crustaceans and fish (Micklin and Aladin 2008; Micklin et al. 2014). We have also highlighted the consequences of future restoration projects on crustacean fauna. This should inform attempts to protect other threatened salines lakes such as Lake Balkhash in Kazakhstan, Lake Urmia in Iran and the Great Salt Lake in the U.S.A. (Aladin et al. 2020; Stone 2021). The Aral Sea is ecologically unique, and its affluent rivers economically crucial for the human population in the two adjacent countries for both fisheries and agriculture. Cotton and rice are now the prevalent crops and require copious amounts of water. Unfortunately, most of the diverted water never reaches the crops; they could, however, be saved by implementing properly designed and maintained irrigation systems (Dukhovny et al. 2008). This calls for internationally coordinated restoration and management projects in the area (Cosens et al. 2018) coupled with full cooperation with the local population, as emphasized by Kim (2018).
Acknowledgments
This work was supported by the theme of the State assignment for 2019–2021 “Systematization of the biodiversity of salt lakes and not full-saline inland seas in the critical salinity zone, study of the role of brackish water species in ecosystems” AAAA-A19-119020690091-0. JTH was financed by a grant (7014-00058B) from Danish Agency for Independent Research (FNU). We also are infinitely grateful to the numerous colleagues, too numerous to mention, from both the Commonwealth of Independent States and elsewhere that contributed to this study. Colleagues that kindly allowed reproduction of their photos are listed in the figure legends. A very helpful referee improved substantially on the text. We dedicate the paper to all astronauts, cosmonauts, and taikonauts past and present, who have pioneered the monitoring the Aral Sea disaster from space. Conceived and designed the study: ISP, NVA. Performed the research: ISP, NVA. Analyzed the data: ISP, JTH, NVA, JM. Wrote the paper: ISP, JTH, NVA, JM.
Footnotes
Authors’ contributions: Problem defined by Aladin and Plotnikov; Data sampling by ladin and Plotnikov. Data Analysis by ALL authors; Write the paper ALL authors.
Competing interests: The authors declare that they have no competing of interests.
Availability of data and materials: The information has now been returned and is stored in the office of the IPA CIS in the Republic of Kazakhstan (IPA CIS, Inter-Parliamentary Assembly of the Member States of the Commonwealth of Independent States). The database is not yet Internet available but free for on site personal use.
Consent for publication: We declare that All authors hereby consent for publication. This letter is sent CC to all authors.
Ethics approval consent to participate: All authors hereby consent to participate. This letter is sent CC to all authors.
References
- Abell R, Harrison IJ. 2020. A boost for freshwater conservation. Science 370(6512):38–39. doi:10.1126/science.abe3887. [DOI] [PubMed]
- Aladin NV. 1989a. Osmoregulation in Cyprideis torosa from various seas of the USSR. Zool Zhur 68:40–50. (in Russian)
- Aladin NV. 1989b. Zooplankton and zoobenthos in coastal waters near the Isle of Barsakelmes, the Aral Sea. Proc Zool Inst Acad of Sci USSR 199:110–114. (in Russian)
- Aladin NV. 1991. Thanatocenoses of separating bays and gulfs of the Aral Sea. Proc Zool Inst Acad of Sci USSR 237:60–63. (in Russian)
- Aladin NV. 1996. Salinity adaptations in Ostracoda and Branchiopoda. Proc Zool Inst RAS 265:1–206. (in Russian)
- Aladin N. 2014. The dam of life or dam lifelong. The Aral Sea and the construction of the dam in Berg Strait. Part one (1988–1992). El Alfoli 15:3–17.
- Aladin NV, Andreev NI. 1984. Influence of salinity of the Aral Sea on changes in composition of fauna of Cladocera. Gidrobiol Zhur 20:23–28. (in Russian)
- Aladin NV, Filippov AA. 1993. On the viability of the resting eggs of Artemia salina and Moina mongolica from the bottom sediments from dryed up bays of the Aral Sea. Proc Zool Inst RAS 250:114–120. (in Russian)
- Aladin NV, Gontar VI, Zhakova LV, Plotnikov IS, Smurov AO, Rzymski P, Klimaszyk P. 2019. The zoocenosis of the Aral Sea: six decades of fast-paced change. Environ Sci Pollut Res 26:2228–2237. doi:10.1007/s11356-018-3807-z. [DOI] [PMC free article] [PubMed]
- Aladin NV, Høeg JT, Plotnikov I. 2020. Small Aral Sea brings hope for Lake Balkhash. Science 370:1283. doi:10.1126/science. abf6682. [DOI] [PubMed]
- Aladin NV, Plotnikov IS. 2008. Modern fauna of residual water bodies formed on the place of the former Aral Sea. Proc Zool Inst RAS 312:145–154. (in Russian)
- Aladin NV, Plotnikov IS, Potts WTW. 1995. The Aral Sea desiccation and possible ways of rehabilitating and conserving its Northern part. Int J Environmetrics 6:17–29.
- Aladin NV, Plotnikov IS, Smurov AO, Gontar VI. 2004. The role of introduced animal species in the ecosystem of the Aral Sea. In: Alimov AF, Bogutskaya NG (eds) Biological invasions in aquatic and terrestrial ecosystems. KMK, Moscow/St. Petersburg, pp. 275–296. (in Russian)
- Aladin NV, Potts WTW. 1992. Changes in the Aral Sea ecosystem during the period 1960–1990. Hydrobiologia 237:67–79.
- Andreev NI. 1989. Zooplankton of the Aral Sea in the initial period of its salinization. Proc Zool Inst Acad Sci USSR 199:26–52. (in Russian)
- Andreev NI, Andreeva SI. 1988. The crab Rhithropanopeus harrisii tridentatus (Decapoda, Xanthidae) in the Aral Sea. Zool Zhur 67(1):135–136. (in Russian)
- Andreeva SI. 1989. Zoobenthos of the Aral Sea in the initial period of its salinization. Proc Zool Inst Acad Sci USSR 199:53–82. (in Russian)
- Arashkevich EG, Sapozhnikov PV, Soloviov KA, Kudyshkin TV, Zavialov PO. 2009. Artemia parthenogenetica (Branchiopoda: Anostraca) from the Large Aral Sea: Abundance, distribution, population structure and cyst production. J Mar Syst 76:359–366. doi:10.1016/j.jmarsys.2008.03.015.
- Baker JH, Kimball KT, Bedinger Jr CA. 1977. Comparison of benthic sampling procedures: Petersen Grab vs. Mackin Corer. Water Res 11:597–601. doi:10.1016/0043-1354(77)90171-3.
- Bayly IAE. 1972. Salinity Tolerance and Osmotic Behavior of Animals in Athalassic Saline and Marine Hypersaline Waters. Ann Review Ecol Syst 3:233–268.
- Behning AL. 1934. Hydrological and hydrobiological materials for fishery map of the Aral Sea. Rep Aral-Sea div Inst mar fish 3:183–205. (in Russian)
- Behning AL. 1935. Materials for fishery map of the Aral Sea. Rep Aral-Sea div Inst mar fish 4:139–195. (in Russian)
- Behning AL. 1937. About the benthos of bays Komsomolets (Meretvy Kultuk) and Kaidak. Trudy Caspiyskoy komissii 1:155–182. (in Russian)
- Berg LS. 1908. The Aral Sea. Attempt at a physical-geographical monograph. Stasyulevich Publisher, St. Petersburg. (in Russian)
- Birstein YaA, Vinogradov LG, Kondakov NN, Kun MS, Astahova TV, Romanova NN (eds). 1968. Atlas of invertebrates of the Caspian Sea. Pischevaya Pronyshlennost, Moscow. (in Russian)
- Bokova EN. 1960. Materials for the biological basis of acclimatizaton of some benthic invertebrates in the Aral Sea. Trudy VNIRO 43:225–234. (in Russian)
- Borisov RR. 2012. Decapod crustaceans (Decapoda) of continental waters of northern Eurasia. Actual problems of studying continental crustacean waters. In: Krylov AV (ed) Collection of lectures and reports of the International School-Conference (Borok, November 5–9, 2012). Kostromskoy pechatnyy dom, Kostroma. (in Russian), pp. 7–20.
- Bortnik VN, Chistyaevaya SP (eds). 1990. Hydrometeorology and hydrochemistry of the seas of the USSR, VII: Aral Sea. USSR. Gidrometeoizdat, Leningrad. (in Russian)
- Carrasco NK, Perissinotto R. 2012. Development of a halotolerant community in the St. Lucia estuary (South Africa) during a hypersaline phase. PLoS ONE 7:e29927. doi:10.1371/journal. pone.0029927. [DOI] [PMC free article] [PubMed]
- Charalambidou I, Santamaria L. 2005. Field evidence for the potential of waterbirds as dispersers of aquatic organisms. Wetlands 25:252–258. doi:10.1672/2.
- Chechko VA. 2015. The piston horizontal bathometer for the point water sampling in shallow reservoirs. Russ Meteorol Hydrol 40:707–710. doi:10.3103/S106837391510009X.
- Chen DH. 2018. Once written off for dead, the Aral Sea is now full of life. National Geographic. 16 Mar. 2018.
- Chen DH. 2020. The country that brought a sea back to life. In: BBC future. Available via BBC Future. 28 July 2018.
- Cosens BA, Gunderson L, Chaffin BC. 2018. Introduction to the special feature practicing panarchy: assessing legal flexibility, ecological resilience, and adaptive governance in regional water systems experiencing rapid environmental change. Ecol Soc 23:4. doi:10.5751/ES-09524-230104.
- Crighton EJ, Barwin L, Small I, Upshur RE. 2011. What have we learned? A review of the literature on children’s health and the environment in the Aral Sea area. Int J Public Health 56:125–38. doi:10.1007/s00038-010-0201-0. [DOI] [PMC free article] [PubMed]
- Crighton EJ, Elliott SJ, van der Meer J, Small I, Upshur RE. 2003. Impacts of an environmental disaster on psychosocial health and well-being in Karakalpakstan. Social Sci Med 56:551–567. doi:10.1016/s0277-9536(02)00054-0. [DOI] [PubMed]
- Daribaev AK. 1967. Experience of acclimatization of mysids and Calanipeda in the southern part of the Aral Sea. Gidrobiol Zhur 3:69–70. (in Russian)
- Dengina RS. 1959. Benthos of Karabaili Archipelago of the Aral Sea. Trudy laboratorii ozerovedeniya 8:234–255. (in Russian)
- Dukhovny V, Mirzaev N, Sokolov V. 2008. IWRM implementation: experiences with water sector reforms in Central Asia. In: Rahaman MM, Varis O (eds) Central Asian Waters. Social, economic, environmental and governance puzzle. Water & Development Publications, Helsinki University of Technology, Helsinki, pp. 19–31.
- Dyjachenko IP. 1963. Comparative efficiency of various devices for collecting plankton. Bull Inst Biol Vnutrennih Vod Acad Nauk USSR 8–9:79–83. (in Russian)
- Eklöf JS, Sundblad G, Erlandsson M, Donadi S, Hansen JP, Eriksson BK, Bergström U. 2020. A spatial regime shift from predator to prey dominance in a large coastal ecosystem. Commun Biol 3:459. doi:10.1038/s42003-020-01180-0. [DOI] [PMC free article] [PubMed]
- Encyclopedia Britannica. 2020. Aral Sea. In: Encyclopedia Britannica. Available at www.britannica.com/place/Aral-Sea. Accessed 30 Oct. 2020.
- Erdinger L, Eckl P, Ingel F, Khussainova S, Utegenova E, Mann V, Gabrio T. 2004. The Aral Sea disaster-human biomonitoring of Hg, As, HCB, DDE, and PCBs in children living in Aralsk-and Akchi, Kazakhstan. Internat J Hygiene Environ Health 207:541–547. doi:10.1078/1438-4639-00325. [DOI] [PubMed]
- Ermakhanov ZK, Plotnikov IS, Aladin NV, Micklin P. 2012. Changes in the Aral Sea ichthyocenosis and fishery in the period of ecological crisis. Lakes & Reserv 17:3–9. doi:10.1111/j.1440-1770.2012.00492.x.
- Figuerola J, Green AJ. 2002. Dispersal of aquatic organisms by waterbirds: a review of past research and priorities for future studies. Freshw Biol 47:483–494. doi:10.1046/j.1365-2427.2002.00829.x.
- Figuerola J, Green AJ, Michot TC. 2005. Invertebrate eggs can fly: evidence of waterfowl-mediated gene flow in aquatic invertebrates. Amer Nat 165:274–280. doi:10.1086/427092. [DOI] [PubMed]
- Filippov AA. 1995. Macrozoobenthos of inshore zone of the Aral Sea North in modern polyhaline conditions: quantity, biomass and spatial distribution. Proc Zool Inst RAS 262:103–166. (in Russian)
- Glenner H. 2001. Cypris metamorphosis, injection and earliest internal development of the kentrogonid rhizocephalan Loxothylacus panopaei (Gissler). Crustacea: Cirripedia: Rhizocephala: Sacculinidae. J Morphol 249:43–75. doi:10.1002/jmor.1040. [DOI] [PubMed]
- Green AJ, Figuerola J. 2005. Recent advances in the study of long-distance dispersal of aquatic invertebrates via birds. Divers Distrib 11:149–156. doi:10.1111/j.1366-9516.2005.00147.x.
- Gubareva ES, Svetlichny LS. 2011. Salinity tolerance of copepods Calanipeda aquaedulcis and Arctodiaptomus salinus (Calanoida, Copepoda). Mar Ecol J 10:32–39. (in Russian)
- Høeg JT. 1995. The biology and life cycle of the Rhizocephala (Cirripedia). J mar biol Ass UK 75:517–550. doi:10.1017/S0025315400038996.
- Husainova NZ. 1958. Biological features of some mass bottom feeding invertebrates of the Aral Sea. KGU, Alma-Ata. (in Russian)
- Husainova NZ. 1960. Kultuks of the Aral Sea eastern coast and their life. Vestnik Akademii Nauk SSSR 6:34–42. (in Russian)
- Indoitu R, Kozhoridze K, Batyrbaeva M, Vitkovskaya I, Orlovsky N, Blumberg D, Orlovsky I. 2015. Dust emission and environmental changes on the dried bottom of the Aral Sea. Aeolian Res 17:101–115. doi:10.1016/j.aeolia.2015.02.004.
- Jensen S, Mozhitova Z, Zetterstrom R. 1997. Environmental pollution and child health in the Aral Sea region in Kazakhstan. Science Total Environ 206:187–193. [PubMed]
- Karpevich AF. 1958a. Biological basis of acclimatization of mysids in the Aral Sea and Lake Balkhash. In: Annotatsii rabot, vypolnennykh VNIRO v 1956 g, Vol 3. VNIRO, Moscow, pp. 45–48. (in Russian)
- Karpevich AF. 1958b. Survival, reproduction and respiration mysid Mesomysis kowalevskyi (Paramysis lacustris kowalevskyi Czern.) in the water of brackish water bodies of the USSR. Zool Zhur 37(8):1121–1135. (in Russian)
- Karpevich AF. 1960a. Biological Basing of aquatic organisms acclimatization in the Aral Sea. Trudy VNIRO 43:76–115. (in Russian)
- Karpevich AF. 1960b. Biological basis for acclimatization of mysids in the Aral Sea and some other brackish water bodies. Trudy VNIRO 43:198–218. (in Russian)
- Karpevich AF. 1975. Theory and practice of aquatic organisms acclimatization. Pischevaya pronyshlennost, Moscow. (in Russian)
- Karpevich AF, Bokova EN. 1970. Effect of climate and biotechniques on acclimatization of Caspian Sea Mysida. Trudy VNIRO 76:163–178. (in Russian)
- Kazakhbaev SK. 1974. Calanipeda in the southern part of the Aral Sea. Gidrobiol Zhur 10:89–91. (in Russian)
- Kim E. 2018. Sustainability of irrigation in Uzbekistan: Implications for women farmers, water and sustainability. PT Chandrasekaran (ed) IntechOpen. Available at https://www.intechopen.com/books/water-and-sustainability/sustainability-of-irrigation-in-uzbekistan-implications-for-women-farmers. Accessed 5 Feb. 2021.
- Kortunova TA. 1968. On distribution of mysids and Nereis acclimatized the Aral Sea. In: Karpevich AF (ed) Acclimatization of fishes and invertebrates in water bodies of the USSR. Nauka, Moscow, pp. 115–119. (in Russian)
- Kortunova TA. 1970. Some data on food organisms introduced to the Aral Sea. Proc VNIRO 76:178–184. (in Russian)
- Kortunova TA. 1975. On the changes in the Aral Sea zooplankton in 1959–1968. Zool Zhur 54:567–669. (in Russian)
- Kortunova TA, Burlayeva AF, Yarygina LN. 1972. Crustacean Calanipeda in the Aral Sea. Rybnoe Hozyaistvo 7:32–32 (in Russian)
- Kortunova TA, Lukonina NK. 1970. Quantitative characteristic of the Aral Sea zooplankton. Rybnye resursy vodoemov Kazakhstana i ikh ispolzovanie, Vol 6. Nauka, Alma-Ata, pp. 52–60. (in Russian)
- Kosarev AN. 1975. Hydrology of the Caspian and Aral seas. Moscow University, Moscow. (in Russian)
- Kotta J, Wernberg T, Jänes H, Kotta I, Nurkse K, Pärnoja M, Orav-Kotta H. 2018. Novel crab predator causes marine ecosystem regime shift. Sci Rep 8:4956. doi:10.1038/s41598-018-23282-w. [DOI] [PMC free article] [PubMed]
- Krivonogov S. 2014. Changes of the Aral Sea level. In: Micklin PN. Aladin NV, Plotnikov I (eds) The Aral Sea: the devastation and partial rehabilitation of a great lake. Springer, Heidelberg, pp. 77–111.
- Krivonogov SK, Burr GS, Kuzmin YV, Gusskov SA, Kurmanbaev RK, Kenshinbay TI, Voyakin DA. 2014. The fluctuating Aral Sea: a multidisciplinary-based history of the last two thousand years. Gondwana Res 26:284–300. doi:10.1016/j.gr.2014.02.004.
- KSC. 2020. Kasakhstan. “From Kattegat to the Aral Sea” 1996–2008. Living Sea/Aral Tenizi. In: KFC Fishing Advice. Available via KFC. kscfa.dk/aralsea/. Accessed 30 Oct. 2020.
- Lim RM. 1986. About acclimatization of flounder–gloss in the Aral Sea. In: Biologicheskie osnovy rybnogo khozyaistva vodoemov Sredney Azii i Kazakhstana. Ashhabad, pp. 249–250. (in Russian)
- Loffler H. 1961. Beitrage zur Kenntnis der Iranischen Binnengewässer. II. Regional limnologische Studien mit besonderer Berücksichtigung der Crustaceenfauna. Int Rev Gesamten Hydrobiol Hydrogeo 46:309–407.
- Lukonina NK. 1960a. Zooplankton of the Aral Sea. Proc VNIRO 43(1):177–197. (in Russian)
- Lukonina NK. 1960b. Dynamics of Diaptomus salinus Daday population in the Aral Sea. Zool Zhur 39:167–187. (in Russian)
- Malinovskaya AS. 1961. On the biology of shrimps acclimatized in the Aral Sea. Sbornik pabot po ihtiologii i gidrobiologii, Vol 3. Alma-Ata, pp. 113–124. (in Russian)
- Marden B, van Stappen G, Musaev A, Mirabdullayev I, Joldasova I, Sorgeloos P. 2012. Assessment of the production potential of an emerging Artemia population in the Aral Sea. J Mar Syst 92:42–52. doi:10.1016/j.jmarsys.2011.10.004.
- Mathnet.ru. 2020. I. D. Papanin Institute for biology of inland waters, Russian Academy of Sciences, Borok, Russia. Available via Mathnet.ru. www.mathnet.ru/php/organisation.phtml?option_ lang=eng&orgid=8431. Accessed 14 Oct. 2020.
- Micklin P. 2007. The Aral Sea Disaster. Ann Rev Earth Pl Sc 35:47–72. doi:10.1146/annurev.earth.35.031306.140120.
- Micklin P. 2016. The future Aral Sea: hope and despair. Environ Earth Sci 75:844. doi:10.1007/s12665-016-5614-5.
- Micklin P, Aladin N. 2008. Reclaiming the Aral Sea. Sci Amer 98:64–71. [DOI] [PubMed]
- Micklin P, Aladin N, Plotnikov I (eds). 2014. The Aral Sea: the devastation and partial rehabilitation of a great lake. Springer Earth System Sciences, Springer, Heidelberg.
- Micklin P, Aladin NV, Chida T, Boroffka N, Plotnikov IS, Krivonogov S, White K. 2020. The Aral Sea: A Story of Devastation and Partial Recovery of a Large Lake. In: Mischke S (ed) Large Asian Lakes in a Changing World. Natural State and Human Impact. Springer, Heidelberg, pp. 109–141. doi:10.1007/978-3-030-42254-7.
- Mirabdullayev I, Abdullayeva L, Musaev A, Zholdasova I, Mustafaeva Z, Jumaniezova N. 2007. Sharp fluctuations in ecosystem parameters of the East Big Aral. Geophys Res Abstracts 9:772.
- Mokievsky VO, Miljutina MA. 2011. Nematodes in meiofauna of the Large Aral Sea during the desiccation phase: taxonomic composition and redescription of common species. Russian J Nematology 19:31–43.
- Mirabdullayev IM, Joldasova IM, Mustafaeva ZA, Kazakhbaev S, Lyubimova SA, Tashmukhamedov BA. 2004. Succession of the ecosystems of the Aral Sea during its transition from oligohaline to polyhaline water body. J Mar Syst 47:101–107. doi:10.1016/j.jmarsys.2003.12.012.
- Mordukhai-Boltovskoi FD. 1972. The current state of the Aral Sea fauna. Gidrobiol Zhur 3:14–20. (in Russian)
- Mordukhai-Boltovskoi FD (ed). 1974. Atlas of the Aral Sea invertebrates. Pischevaya Promyshlennost, Moscow. (in Russian)
- Musaev AK, Zholdasova IM, Mirabdullaev IM, Temirbekov RO. 2012. Development of the resources of the Aral Sea Artemia. In: Materials of the International Conference “Wildlife of Kazakhstan and adjacent areas”, devoted to the 80th anniversary of the Institute of Zoology 22–23 November 2012. Institut Zoologii, Almaty, pp. 144–146. (in Russian)
- Nikolsky GV, Pankratova Vya. 1934. Some data on hydrology, hydrobiology and ichthyology of the Aibugir hollow (Sudochie lake). Rep Aral-Sea Div Inst mar Fish 3:155–181. (in Russian)
- O’Hara SL, Wiggs GF, Mamedov B, Davidson G, Hubbard H. 2000. Exposure to airborne dust contaminated with pesticide in the Aral Sea region. Lancet 355:627–628. doi:10.1016/S0140-6736(99)04753-4. [DOI] [PubMed]
- Øksnebjerg B. 2000. The Rhizocephala (Crustacea: Cirripedia) of the Mediterranean and Black Seas: Taxonomy, biogeography, and ecology. Israel J Zool 46:1–102.
- Oren A, Plotnikov IS, Sokolov SB, Aladin NV. 2010. The Aral Sea and the Dead Sea: disparate lakes with similar histories. Lakes Reserv 15:223–236. doi:10.1111/j.1440-1770.2010.00436.x.
- Osmanov SO. 1961. On the death of atherine in the Aral Sea. Vestnik Karakalpakskogo filiala AN UzSSR 3:95–96. (in Russian)
- Osmanov SO. 1967. About composition and origin of the Aral Sea fishes parasitofauna. Vestnik Karakalpakskogo filiala AN UzSSR 3–4:57–63. (in Russian)
- Osmanov SO. 1971. Parasites of fishes of Uzbekistan. Fan, Tashkent. (in Russian)
- Osmanov SO, Arystanov EA, Ubaidullayev K, Yusupov OY, Teremuratov TA. 1976. Problems of the Aral Sea parasitology. Fan, Tashkent. (in Russian)
- Osmanov SO, Yusupov O. 1985. Impact of the Aral Sea salinization on the parasitofauna of fishes. Parisitologicheskiy sbornik 33:14–43. (in Russian)
- Pankratova VY. 1935. Materials on the feeding of the Aral Sea fishes. Rep Aral-Sea div Inst marine Fish 4:199–220. (in Russian)
- Plotnikov IS. 2016. Long-term changes of the free-living aquatic invertebrate fauna of the Aral Sea. Zoological Institute Russian Academy of Sciences, St. Petersburg. (in Russian)
- Plotnikov IS, Aladin NV. 2011. An overview of hybrid marine and lacustrine seas and saline lakes of the world. Lakes Reserv 16:97–108. doi:10.1111/j.1440-1770.2011.00452.x.
- Plotnikov IS, Aladin NV, Filippov AA. 1991. The past and present of the Aral Sea fauna. Zool Zhur 70:5–15. (in Russian)
- Plotnikov IS, Ermakhanov ZK, Aladin NV, Micklin P. 2016. Modern state of the Small (Northern) Aral Sea fauna. Lakes Reserv 21:315–328. doi:10.1111/lre.12149.
- RECO. 2020. Plankton Nets Apstein. In: RECO Scientific Instrument and Technology Development. Available at RECO. https://reecotech.com.vn/product/hydrographic-and-enviromental-monitoring/sampling-equipment/plankton-nets/plankton-nets-apstein?lang=en. Accessed 14 Oct. 2020.
- Reynolds C, Miranda AFN, Cumming GS. 2015. The role of waterbirds in the dispersal of aquatic alien and invasive species. Divers Distrib 21:744–754. doi:10.1111/ddi.12334.
- Rogers DC. 2014. Larger hatching fractions in avian dispersed anostracan eggs (Branchiopoda). J Crustacean Biol 34:135–143. doi:10.1163/1937240X-00002220.
- Rogers DC. 2015. A conceptual model for anostracan biogeography. J Crustacean Biol 35:686–699. doi:10.1163/1937240X-00002369.
- Shadrin NV. 2012. Crustaceans in hypersaline water bodies: specificity of existence and adaptation. Actual problems of studying crustacean continental waters. In: Krylov AV (ed) Collection of lectures and reports of the International School-Conference (Borok, November 5–9, 2012). Kostromskoy pechatnyy dom, Kostroma, pp. 316–318. (in Russian)
- Skjoldal HR, Prokopchuk I, Bagøien E, Dalpadado P, Nesterova V, Rønning J, Knutsen T. 2019. Comparison of Juday and WP2 nets used in joint Norwegian-Russian monitoring of zooplankton in the Barents Sea. J Plankton Res 41:759–769. doi:10.1093/plankt/fbz054.
- Small I, van der Meer J, Upshur RE. 2001. Acting on an environ-mental health disaster: the case of the Aral Sea. Environ Health Perspect 109:547–549. doi:10.1289/ehp.01109547. [DOI] [PMC free article] [PubMed]
- Stone R. 2021. After revival, Iran’s great salt lake faces peril. Science 372:444–445. doi:10.1126/science.372.6541.444. [DOI] [PubMed]
- Stuge TS. 2001. On the spring zooplankton of the north-eastern part of the Small Aral Sea. Selevinia 1–4:197–198. (in Russian)
- Stuge TS, Saduakasova PE. 2005. The changes of zooplankton in east part of the Small Aral Sea after building of the dam across Berg strait. Trudy Instituta Zoologii-Almaty 49:145–151.
- Sunday JM. 2020. The pace of biodiversity change in a warming world. Science 580:480–481. doi:10.1038/d41586-020-00975-9.
- Thresher RE, Werner M, Høeg JT, Svane I, Glenner H, Murphy N, Wittwer C. 2000. Developing the options for managing marine pests: specificity trials on the parasitic castrator, Sacculina carcini, against the European crab, Carcinus maenas, and related species. J Exp Mar Biol Ecol 254:37–51. doi:10.1016/s0022-0981(00)00260-4. [DOI] [PubMed]
- Trisos CH, Merow C, Pigot AL. 2020. The projected timing of abrupt ecological disruption from climate change. Nature 580:496–501. doi:10.1038/s41586-020-2189-9. [DOI] [PubMed]
- Vinogradov GA, Bobovich MA. 1970. A new modification of the microcryoscope and the experience of its application for studying the osmotic regulation of invertebrates. Gidrobiol Zhur 6:136–141. (in Russian)
- World Bank. 2014. Project Information Document (PID), Concept Stage, Syr Dar’ya Control and Northern Aral Sea Project, Phase 2 (P15200). In: Report No.: PIDC12697, September 24, 2014:1–9. Available at World Bank. https://documents. worldbank.org/en/publication/documents-reports/documentdeta il/963791468044357907/project-information-document-concept-stage-syr-darya-control-and-northern-aral-sea-project-phase-2-p152001.
- Whish-Wilson P. 2002. The Aral Sea environmental health crisis. J Rural Remote Environ Health 1:29–34.
- WRiMS. 2020. Sampling tools for the marine environment. In: World Register of Introduced Marine Species.
- Zavialov PO (ed). 2012. The Large Aral Sea in the beginning of century 21. Nauka, Moscow. (in Russian)
- Zenkevich LA. 1963. Biology of the seas of the U.S.S.R. New York, Interscience. doi:10.5962/bhl.title.6447.
- Zonn IS, Glantz M, Kostianoy AG, Kosarev AN. 2009. The Aral Sea Encyclopedia. Springer, Berlin, Germany.