Abstract
The southwestern Andes of Peru harbor a hidden taxonomic diversity of reptiles. We describe a new species of Liolaemus Wiegmann (Iguania: Liolaemidae) from xerophytic environments of the southwestern slopes of the Andes of Peru, 2,400–2,900 m asl. The new species, previously considered to be a population of L. insolitus Cei, exhibits unique diagnostic characters of morphology, scalation and color pattern, and molecular evidence that suggest that it belongs in the Liolaemus montanus species group and the L. reichei clade. Moreover, the species is endemic to the eastern slopes of La Caldera batholith in the Department of Arequipa, southern Peru. We also provide information on the conservation status of the species and suggest it be included in the IUCN red list of the threatened species as endangered (EN). A key for the species of the L. reichei clade is provided.
Keywords: Lizards, Taxonomy, Phylogeny, L. insolitus, Arequipa
BACKGROUND
The lizard genus Liolaemus Wiegmann is the second most diverse genus of extant tetrapods, surpassed only by Anolis Daudin, and currently includes 269 species distributed from central Peru to southern Argentina and Chile (Tierra del Fuego), occupying different ecosystems along its distribution, including several areas of the Plurinational State of Bolivia (Bolivia), Paraguay, and coastal areas of Brasil and Uruguay (Verrastro et al. 2003; Abdala and Quinteros 2014; Gutiérrez et al. 2018; Valladares et al. 2018; Abdala et al. 2019 2020; Aguilar-Puntriano et al. 2019; Quinteros et al. 2020; Villegas-Paredes et al. 2020).
Liolaemus is divided into two main clades: the subgenus Liolaemus (Liolaemus) or “Chilean group” and the subgenus Liolaemus (Eulaemus) or “Argentinean group” (Laurent 1985; Schulte et al. 2000). Each clade contains numerous monophyletic groups, recently proposed in formal phylogenetic analyses (Abdala and Quinteros 2014; Troncoso-Palacios et al. 2018; Abdala et al. 2020). Liolaemus (Eulaemus) is divided into three monophyletic groups (Schulte et al. 2000; Olave et al. 2015; Breitman et al. 2015): the clade integrated by the L. archeforus-kingii and L. lineomaculatus species groups (Breitman et al. 2013 2015), the L. boulengeri species group (Avila et al. 2006; Abdala 2007), and the L. montanus species group (Aguilar-Puntriano et al. 2018; Abdala et al. 2020). The L. montanus species group is a diverse clade composed of more than 60 valid species, confined mainly to Andean environments from the Peruvian central Andes to southern Mendoza province in Argentina, including Bolivia and Chile (Abdala and Quinteros 2014), with the exception of several populations of the L. reichei clade that inhabit the coastal areas of southern Peru and northern Chile (Abdala et al. 2020; Villegas-Paredes et al. 2020).
The taxonomic history of the L. montanus species group is complex. Several species have been considered synonyms (Langstroth 2011), resurrected (Langstroth 2011; Valladares et al. 2018), and recently described (Abdala et al. 2013 2019; Demangel et al. 2015; Gutiérrez et al. 2018; Aguilar-Puntriano et al. 2019; Chaparro et al. 2020; Villegas-Paredes et al. 2020); however, there are still taxonomic problems to be solved (Abdala et al. 2020). On the other hand, the knowledge of species distribution patterns have been accompanied by the development of recent taxonomic efforts, allowing us to a better understanding the diversity and distribution of this group (Aguilar-Kirigin and Abdala 2016; Aguilar-Kirigin et al. 2016; Gutiérrez et al. 2018).
Recent field work carried out from the coastal desert to the high mountain elevations in Peru, have helped clarify the taxonomy of the L. montanus group, considered to be the most diverse in the country, with 17 known species (Gutiérrez et al. 2013 2018; Aguilar-Puntriano et al. 2019; Villegas-Paredes et al. 2020; Chaparro et al. 2020). The other species groups present in the country are the L. alticolor-bibronii species group (Quinteros 2013; Aguilar et al. 2013) and the L. darwinii species group (Carrillo and Icochea 1995; Abdala 2007; Gutiérrez et al. 2018). According to Abdala et al. (2020), the species of the L. montanus group were grouped into 12 clades; six of which are distributed in Peru (L. dorbignyi, L. forsteri, L. huacahuasicus, L. ortizi, L. reichei, and L. robustus). The L. reichei clade includes nine species, of which three species have been described recently from southern Peru (Aguilar-Puntriano et al. 2019; Villegas-Paredes et al. 2020). Several species of this monophyletic group inhabit coastal areas, such as L. balagueri, L. insolitus, L. nazca, L. poconchiliensis, L. stolzmannii and L. reichei (Zeballos et al. 2002; Valladares et al. 2018; Aguilar-Puntriano et al. 2019; Villegas-Paredes et al. 2020; Troncoso-Palacios and Escobar-Gimpel 2020), and the rest inhabit mid-elevations up to approximately 3,000 m, e.g., L. audituvelatus, L. chiribaya, and L. torresi (Langstroth 2011; Ruiz de Gamboa et al. 2018; Aguilar-Puntriano et al. 2019).
Liolaemus insolitus Cei is restricted to the Department of Arequipa, southern Peru, with its type locality in Alto Inclán (Islay province), at an altitude between 50 and 100 m asl, this species belongs to the L. reichei clade, sensu Abdala et al. (2020). According to the original description, this species included populations that could be found up to an altitude of 2,800 m asl, with populations on high slopes of the La Caldera batholith, near the city of Arequipa (Cei and Péfaur, 1982). Particularly, local researchers have considered that high-altitude populations of L. insolitus represents an undescribed taxon (Zeballos et al. 2002), provisionally named L. aff. insolitus or L. cf. insolitus in scientific collections (Quiroz and Abdala pers. obs.). Recently a total evidence analysis of the L. montanus species group (Abdala et al. 2020) including L. insolitus, considering a population from the coastal zone of Mollendo (type locality), as well as La Caldera batholith population, conclude that both populations are independent lineages, the latter with morphological characters different from the rest of the known species. The purpose of this study is to formally describe this new species.
We use the general or unified concept of species proposed by De Queiroz (1998 2007), which defines species as entities that represent independent historical lineages or divergent lineages of metapopulations. We assessed the independence of these lineages based on a morphological and molecular phylogeny, multivariate statistical analyses, and a description of unique morphological characters, providing decisive evidence that this population is a new species in the L. montanus species group.
MATERIALS AND METHODS
Material examined
We examined specimens of the L. montanus group from the Museo de Historia Natural, Universidad Nacional de San Agustín de Arequipa, Peru (MUSA); Museo de Biodiversidad del Perú, Cusco, Perú (MUBI); and Museo de Historia Natural, Universidad Nacional Mayor de San Marcos de Lima, Peru (MUSM). Appendix 1 details the specimens analyzed for the first time here, as well as those reanalyzed herein but previously examined by Abdala and Quinteros (2008), Abdala et al. (2008 2009 2013 2020), Quinteros et al. (2008), Quinteros and Abdala (2011) and Gutiérrez et al. (2018). Some data were obtained from the literature for Liolaemus stolzmannii without available specimens (Langstroth 2011).
Conservation status
To assess the conservation status of the new species we used the IUCN (2020) criteria and sub-criteria. The presence of extension (EOO) and occupation area (AOO) were obtained using GeoCat tool (http://geocat.kew.org/).
Morphological data
Morphological characters used here were described and cited by Laurent (1985), Etheridge (1995 2000), Abdala (2007), Abdala and Juárez (2013), Gutiérrez et al. (2018), Aguilar-Puntriano et al. (2019) and Abdala et al. (2019 2020). Color in life was described based on field notes and digital photographs of captured specimens. Terminology for color patterns follows Lobo and Espinoza (1999), Abdala (2007) and Abdala et al. (2020). Examination of scalation or pholidosis was performed using a binocular stereoscope (10–40x), and morphometric measurements were taken with a ± 0.01 mm precision Mitutoyo caliper.
Morphometric variables were measured three times on the same individual, and the mean value for each species was used in the multivariate analyses. Only adult individuals were used in the multivariate analysis to avoid confounding effects from intraspecific allometric variation (Losos 1990; Abdala et al. 2019). All bilateral characters were measured on the right side. Measured morphometric traits and meristic characters counted follows Abdala et al. (2019) and are listed in table 1.
Table 1.
Measured morphometric traits and meristic characters
| Morphologial characteristics | L. balagueri | L. chiribaya | L. insolitus | L. nazca | Liolaemus yarabamba sp. nov. |
| n = 12 | n = 10 | n = 15 | n = 7 | n = 13 | |
| SVL | 51.08 –64.96 | 49.28 –68.25 | 47.35 –65.77 | 53.51 –64.34 | 49.86 –68.66 |
| 58.82 ± 4.68 | 59.60 ± 6.59 | 56.79 ± 5.41 | 59.35 ± 4.98 | 59.21 ± 4.94 | |
| DN | 1.03 –2.04 | 1.96 –3.00 | 0.91 –1.96 | 0.63 –1.81 | 1.05 –2.03 |
| 1.31 ± 0.28 | 2.47 ± 0.30 | 1.53 ± 0.36 | 1.47 ± 0.42 | 1.40 ± 0.31 | |
| AH | 3.59 –5.61 | 3.71 –5.67 | 3.21 –5.06 | 1.96 –4.85 | 3.47 –4.78 |
| 4.45 ± 0.54 | 4.73 ± 0.66 | 4.23 ± 0.53 | 3.92 ± 0.93 | 4.24 ± 0.42 | |
| NC | 1.65 –2.91 | 1.07 –2.57 | 1.52 –2.85 | 2.10 –3.14 | 1.97 –2.70 |
| 2.09 ± 0.36 | 2.09 ± 0.52 | 2.09 ± 0.33 | 2.49 ± 0.38 | 2.36 ± 0.25 | |
| EO | 6.11 –8.96 | 7.01 –9.26 | 7.12 –8.88 | 6.16 –8.25 | 6.10 –8.83 |
| 7.49 ± 0.74 | 8.24 ± 0.72 | 7.90 ± 0.49 | 7.11 ± 0.80 | 7.78 ± 0.79 | |
| LEI | 0.89 –1.69 | 0.88 –1.28 | 0.66 –1.58 | 0.47 –2.06 | 1.02 –1.79 |
| 1.28 ± 0.26 | 1.09 ± 0.14 | 1.12 ± 0.26 | 1.31 ± 0.48 | 1.34 ± 0.22 | |
| PA | 0.85 –1.74 | 1.31 –1.72 | 0.90 –1.82 | 0.51 –1.91 | 1.12 –2.32 |
| 1.34 ± 0.26 | 1.43 ± 0.14 | 1.25 ± 0.26 | 1.24 ± 0.47 | 1.58 ± 0.30 | |
| AM | 1.05 –1.76 | 2.00 –2.86 | 1.32 –2.41 | 0.46 –1.31 | 1.19 –1.72 |
| 1.28 ± 0.20 | 2.46 ± 0.28 | 1.94 ± 0.47 | 1.06 ± 0.30 | 1.40 ± 0.15 | |
| LM | 2.05 –3.13 | 0.84 –1.55 | 1.08 –2.92 | 1.23 –2.64 | 1.80 –3.15 |
| 2.53 ± 0.34 | 1.20 ± 0.22 | 1.69 ± 0.66 | 2.16 ± 0.54 | 2.28 ± 0.39 | |
| NB | 1.11 –1.92 | 1.19 –1.63 | 0.96 –1.56 | 1.16 –1.87 | 1.07 –1.55 |
| 1.41 ± 0.23 | 1.42 ± 0.12 | 1.26 ± 0.18 | 1.56 ± 0.28 | 1.23 ± 0.14 | |
| HR | 0.40 –1.04 | 0.64 –1.22 | 0.53 –1.01 | 0.69 –1.54 | 0.55 –0.89 |
| 0.80 ± 0.17 | 0.86 ± 0.19 | 0.77 ± 0.11 | 0.93 ± 0.31 | 0.74 ± 0.11 | |
| ES | 2.83 –4.58 | 3.20 –4.06 | 1.90 –4.16 | 2.93 –6.62 | 2.46 –3.80 |
| 3.72 ± 0.49 | 3.57 ± 0.27 | 3.52 ± 0.53 | 3.93 ± 1.26 | 3.21 ± 0.40 | |
| hTy | 1.69 –2.63 | 1.68 –2.30 | 1.02 –2.09 | 1.72 –2.49 | 1.71 –2.51 |
| 2.16 ± 0.26 | 1.91 ± 0.21 | 1.72 ± 0.25 | 1.95 ± 0.26 | 2.11 ± 0.25 | |
| aTy | 0.47 –1.54 | 1.18 –1.65 | 0.65 –1.22 | 0.67 –1.13 | 0.73 –1.12 |
| 0.97 ± 0.26 | 1.37 ± 0.17 | 0.94 ± 0.20 | 0.94 ± 0.14 | 0.91 ± 0.14 | |
| LPO | 0.91 –1.67 | 0.57 –1.54 | 0.53 –1.49 | 0.75 –2.35 | 0.80 –1.76 |
| 1.20 ± 0.23 | 1.02 ± 0.32 | 1.17 ± 0.24 | 1.43 ± 0.50 | 1.06 ± 0.23 | |
| LPOT | 0.43 –0.85 | 0.48 –0.80 | 0.37 –0.72 | 0.48 –0.92 | 0.36 –0.58 |
| 0.61 ± 0.13 | 0.60 ± 0.11 | 0.52 ± 0.11 | 0.69 ± 0.15 | 0.47 ± 0.07 | |
| LCSP | 1.01 –2.00 | 0.83 –1.42 | 0.54 –1.52 | 1.39 –3.36 | 0.88 –1.69 |
| 1.52 ± 0.34 | 1.14 ± 0.19 | 1.03 ± 0.25 | 2.01 ± 0.67 | 1.14 ± 0.20 | |
| LCLB | 0.68 –1.56 | 0.86 –1.28 | 0.55 –131 | 0.85 –2.14 | 0.52 –1.38 |
| 1.15 ± 0.25 | 0.99 ± 0.12 | 0.97 ± 0.20 | 1.29 ± 0.46 | 0.93 ± 0.22 | |
| DEO | 6.80 –8.83 | 7.31 –9.32 | 7.48 –9.17 | 6.90 –8.67 | 7.03 –9.48 |
| 7.83 ± 0.67 | 8.26 ± 0.68 | 8.36 ± 0.55 | 7.58 ± 0.71 | 8.32 ± 0.82 | |
| 1D | 1.86 –3.21 | 1.84 –3.12 | 1.63 –2.95 | 1.61 –2.82 | 2.06 –3.10 |
| 2.51 ± 0.39 | 2.52 ± 0.44 | 2.32 ± 0.31 | 2.13 ± 0.41 | 2.54 ± 0.35 | |
| G4D | 1.10 –1.59 | 0.74 –1.38 | 1.17 –2.04 | 0.67 –1.35 | 1.02 –2.16 |
| 1.30 ± 0.16 | 1.01 ± 0.21 | 1.53 ± 0.22 | 1.00 ± 0.23 | 1.49 ± 0.30 | |
| 5D | 2.89 –3.84 | 2.41 –4.41 | 2.44 –3.40 | 2.33 –3.93 | 2.27 –4.09 |
| 3.29 ± 0.33 | 3.31 ± 0.56 | 2.84 ± 0.25 | 2.93 ± 0.52 | 2.98 ± 0.52 | |
| AHU | 1.98 –3.63 | 1.99 –4.58 | 2.24 –3.46 | 2.01 –3.93 | 2.32 –4.13 |
| 2.81 ± 0.51 | 3.03 ± 0.78 | 2.77 ± 0.38 | 3.06 ± 0.54 | 3.17 ± 0.53 | |
| LEA1 | 6.94 –11.83 | 8.65 –10.81 | 6.34 –9.45 | 7.01 –8.95 | 7.97 –10.89 |
| 8.89 ± 1.40 | 9.75 ± 0.71 | 8.19 ± 0.86 | 8.17 ± 0.80 | 9.14 ± 0.88 | |
| AMU | 3.76 –5.28 | 3.33 –4.98 | 2.67 –4.68 | 4.82 –7.19 | 3.57 –5.78 |
| 4.54 ± 0.47 | 4.18 ± 0.60 | 3.71 ± 0.73 | 5.96 ± 0.79 | 4.51 ± 0.63 | |
| 1P | 2.87 –3.68 | 1.66 –4.30 | 2.50 –3.78 | 1.73 –4.08 | 2.87 –4.07 |
| 3.19 ± 0.29 | 3.20 ± 0.86 | 3.15 ± 0.37 | 2.92 ± 0.72 | 3.41 ± 0.31 | |
| 4U | 0.93 –2.06 | 0.74 –2.32 | 0.98 –1.77 | 0.75 –1.72 | 1.02 –1.78 |
| 1.45 ± 0.32 | 1.33 ± 0.45 | 1.30 ± 0.22 | 1.33 ± 0.36 | 1.35 ± 0.21 | |
| AL | 16.19 –20.03 | 19.64 –33.02 | 12.12 –19.74 | 19.61 –27.88 | 16.26 –21.04 |
| 17.43 ± 1.06 | 25.76 ± 4.97 | 15.99 ± 2.40 | 24.85 ± 2.70 | 19.00 ± 1.71 | |
| WTB | 6.32 –8.63 | 6.19 –9.15 | 4.91 –8.44 | 6.24 –9.20 | 6.70 –9.03 |
| 7.49 ± 0.76 | 7.76 ± 1.21 | 6.98 ± 1.07 | 7.46 ± 0.88 | 7.95 ± 0.70 | |
| ASPI | 5.39 –6.80 | 4.37 –7.80 | 5.57 –7.84 | 2.70 –7.20 | 4.39 –6.67 |
| 6.08 ± 0.44 | 6.45 ± 1.17 | 6.43 ± 0.69 | 4.55 ± 1.35 | 5.39 ± 0.74 | |
| LPI | 4.01 –6.12 | 4.71 –6.75 | 3.73 –6.40 | 3.23 –6.16 | 3.76 –6.12 |
| 5.07 ± 0.62 | 5.75 ± 0.76 | 5.03 ± 0.82 | 4.90 ± 0.87 | 5.09 ± 0.71 | |
| A11 | 4 –8 | 5 –7 | 5 –9 | 5 –8 | 5 –7 |
| 6.33 ± 0.98 | 6.20 ± 0.63 | 6.27 ± 1.16 | 6.14 ± 1.07 | 6.00 ± 0.91 | |
| A12 | 6 –8 | 7 –9 | 7 –8 | 6 –9 | 7 –9 |
| 7.08 ± 0.79 | 7.60 ± 0.70 | 7.47 ± 0.52 | 7.43 ± 0.98 | 7.54 ± 0.66 | |
| A15 | 6 –8 | 7 –10 | 7 –9 | 6 –8 | 6 –9 |
| 6.67 ± 0.89 | 8.60 ± 0.97 | 7.80 ± 0.56 | 6.57 ± 0.98 | 7.46 ± 0.78 | |
| A13 | 5 –7 | 5 –7 | 5 –8 | 5 –6 | 5 –7 |
| 6.08 ± 0.51 | 6.10 ± 0.57 | 6.40 ± 0.74 | 5.57 ± 0.53 | 5.85 ± 0.55 | |
| A19 | 5 –7 | 5 –7 | 5 –8 | 5 –6 | 5 –7 |
| 5.67 ± 0.65 | 6.10 ± 0.57 | 6.27 ± 0.70 | 5.71 ± 0.49 | 5.69 ± 0.63 | |
| A14 | 4 | 4 –6 | 4 –6 | 4 –5 | 4 –5 |
| 4.00 ± 0.00 | 4.20 ± 0.63 | 4.67 ± 0.82 | 4.14 ± 0.38 | 4.08 ± 0.28 | |
| A16 | 6 –8 | 6 –7 | 6 –8 | 5 –6 | 6 –8 |
| 6.67 ± 0.65 | 6.10 ± 0.32 | 7.07 ± 0.59 | 5.86 ± 0.38 | 7.08 ± 0.49 | |
| A17–1 | 7 –9 | 5 –8 | 7 –8 | 7 –10 | 6 –8 |
| 7.50 ± 0.67 | 6.40 ± 1.07 | 7.20 ± 0.41 | 8.43 ± 0.98 | 7.31 ± 0.63 | |
| A18 | 12 –16 | 14 –18 | 14 –18 | 11 –14 | 13 –18 |
| 13.75 ± 1.29 | 15.90 ± 1.20 | 15.07 ± 1.03 | 12.71 ± 1.11 | 15.38 ± 1.50 | |
| A20–1 | 7 –8 | 7 –8 | 6 –9 | 7 –10 | 6 –8 |
| 7.33 ± 0.49 | 7.30 ± 0.48 | 7.67 ± 1.11 | 8.71 ± 1.11 | 7.08 ± 0.64 | |
| A20–2 | 9 –11 | 11 –13 | 8 –16 | 12 –13 | 9 –12 |
| 10.17 ± 0.83 | 12.60 ± 0.84 | 12.07 ± 2.49 | 12.86 ± 0.38 | 10.69 ± 1.11 | |
| A20–3 | 14 –16 | 14 –16 | 12 –16 | 15 –19 | 12 –16 |
| 14.67 ± 0.65 | 15.30 ± 0.67 | 14.40 ± 1.30 | 15.86 ± 1.57 | 14.08 ± 1.44 | |
| A20–4 | 12 –18 | 17 –19 | 10 –17 | 17 –20 | 14 –20 |
| 15.33 ± 1.67 | 18.20 ± 0.92 | 12.73 ± 2.02 | 18.57 ± 1.13 | 16.23 ± 1.54 | |
| A20–5 | 8 –11 | 8 | 6 –10 | 9 –10 | 7 –10 |
| 9.58 ± 0.79 | 8.00 ± 0.00 | 7.73 ± 1.10 | 9.71 ± 0.49 | 8.69 ± 1.18 | |
| A21–1 | 5 –10 | 9 –10 | 6 –11 | 8 –10 | 8 –10 |
| 8.17 ± 1.53 | 9.20 ± 0.42 | 7.80 ± 1.15 | 8.86 ± 0.90 | 8.69 ± 0.85 | |
| A21–2 | 10 –13 | 11 –12 | 10 –12 | 12 –13 | 10 –13 |
| 11.83 ± 0.94 | 11.20 ± 0.42 | 10.93 ± 0.88 | 12.71 ± 0.49 | 11.77 ± 1.09 | |
| A21–3 | 9 –18 | 14 –16 | 12 –16 | 15 –18 | 14 –19 |
| 15.00 ± 2.37 | 15.40 ± 0.70 | 14.00 ± 1.25 | 16.14 ± 1.21 | 15.85 ± 1.57 | |
| A21–4 | 19 –24 | 18 –21 | 20 –22 | 20 –23 | 18 –22 |
| 20.33 ± 1.50 | 19.50 ± 0.85 | 20.67 ± 0.62 | 21.57 ± 0.98 | 20.46 ± 1.05 | |
| A21–5 | 10 –14 | 11 –13 | 10 –12 | 10 –13 | 10 –15 |
| 11.58 ± 1.16 | 12.50 ± 0.71 | 11.27 ± 0.88 | 11.57 ± 1.51 | 12.08 ± 1.04 | |
| A22 | 52 –56 | 52 –63 | 58 –69 | 53 –56 | 55 –69 |
| 53.50 ± 1.62 | 57.40 ± 3.50 | 63.40 ± 3.48 | 54.14 ± 1.35 | 60.85 ± 3.83 | |
| A26 | 0 –7 | 2 –5 | 0 –8 | 1 –6 | 4 –6 |
| 3.00 ± 2.80 | 3.80 ± 1.03 | 4.20 ± 2.83 | 3.43 ± 1.51 | 4.92 ± 0.76 | |
| M2 | 1 –2 | 2 | 1 | 1 –3 | 1 –2 |
| 1.33 ± 0.49 | 2.00 ± 0.00 | 1.00 ± 0.00 | 1.86 ± 0.69 | 1.92 ± 0.28 | |
| M3 | 6 –9 | 7 –8 | 5 –9 | 6 –9 | 6 –9 |
| 7.50 ± 0.80 | 7.20 ± 0.42 | 7.07 ± 1.28 | 7.57 ± 1.13 | 7.54 ± 0.78 | |
| M5 | 3 –5 | 3 –5 | 4 –8 | 4 –6 | 2 –6 |
| 4.25 ± 0.62 | 4.00 ± 0.47 | 6.73 ± 0.96 | 4.71 ± 0.76 | 4.85 ± 1.14 | |
| M4 | 3 –6 | 3 –5 | 3 –8 | 3 –6 | 4 –8 |
| 4.75 ± 0.87 | 3.80 ± 0.63 | 6.47 ± 1.30 | 4.86 ± 1.07 | 6.08 ± 1.04 | |
| M13 | 1 –6 | 2 –6 | 5 –16 | 4 –11 | 3 –11 |
| 3.92 ± 1.68 | 4.20 ± 1.40 | 10.00 ± 3.21 | 6.57 ± 2.64 | 5.85 ± 2.44 | |
| M14 | 2 –6 | 3 –7 | 2 –8 | 3 –11 | 1 –8 |
| 3.75 ± 1.29 | 4.40 ± 1.07 | 4.27 ± 1.71 | 7.86 ± 2.97 | 4.00 ± 1.83 | |
| M15 | 1 –6 | 1 –8 | 5 –24 | 1 –12 | 1 –8 |
| 3.50 ± 1.51 | 4.60 ± 2.67 | 12.53 ± 5.05 | 5.86 ± 3.72 | 3.62 ± 2.26 | |
| M23 | 26 –30 | 19 –25 | 26 –32 | 21 –25 | 24 –28 |
| 27.17 ± 1.34 | 21.70 ± 1.89 | 28.80 ± 2.48 | 23.86 ± 1.46 | 26.15 ± 0.99 | |
| M26 | 52 –60 | 55 –66 | 52 –60 | 54 –59 | 53 –62 |
| 56.50 ± 2.28 | 61.80 ± 3.68 | 55.80 ± 2.27 | 56.86 ± 1.95 | 56.85 ± 2.88 | |
| M32 | 65 –79 | 67 –77 | 69 –80 | 65 –74 | 67 –77 |
| 73.17 ± 3.69 | 72.70 ± 2.95 | 73.53 ± 3.36 | 70.57 ± 2.88 | 72.23 ± 2.95 | |
| M34 | 1 | 1 | 2 –4 | 1 –2 | 2 –3 |
| 1.00 ± 0.00 | 1.00 ± 0.00 | 2.87 ± 0.52 | 1.86 ± 0.38 | 2.46 ± 0.52 | |
| D6 | 6 –8 | 6 –8 | 6 –8 | 7 –10 | 0 –12 |
| 6.92 ± 0.67 | 7.30 ± 0.67 | 6.47 ± 0.74 | 7.71 ± 1.11 | 8.08 ± 3.77 |
Note: Range in the first line; mean ± standard deviation (mm) for quantitative characters in the second line. Legend: Snout-vent length (SVL); minimum distance between the nasal scales (DN); snout width at the edge of the canthal scale (AH);distance from the nose to the back edge of the canthal scale (NC); distance between the posterior edge of the superciliary series (EO); length of the interparietal (LEI); length of the parietal (PA); mental scale width (AM); length of the mental scale (LM); distance from nostril to mouth (NB); rostral height (HR); length of the subocular scale (ES); auditory meatus height (hTy); auditory meatus width (aTy); length of the preocular scale (LPO); preocular width (LPOT); length of the fourth supralabial scale (LCSP); length of the fourth lorilabial scale (LCLB); length between orbits (DEO); length of the first finger of the forelimb, without claw (1D); length of the claw of the fourth finger of the forelimb (G4D); length of the fifth finger of the forelimb without claw (5D); humerus width (AHU); distance from the insertion of the forelimb in the body toward the elbow (LEA1); thigh width (AMU); length of the first toe of the hind limb without claw (1P); length of the claw of the fourth toe of the hind limb (4U); length of the five dorsal scales in a row in the middle of the body (ED); cloacal opening width, measured distance between the corners of the cloaca (PP); body width (AL); width of the base of the tail (WTB); upper width of the pygal area (ASPI); length of the pygal area (LPI). Number of scales around the interparietal scale (A11); number of supralabials on the right side (A12); number of supralabials on the left side (A15); number of infralabials on the right side (A13); number of infralabials on the left side (A19); number of scales around the mental scale (A14); number of scales around the rostral scale (A16); number of lorilabials (A17–1); Hellmich index (A18); subdigital lamellae of the first finger of the forelimb (A20–1); subdigital lamellae of the second finger of the forelimb (A20–2); subdigital lamellae of the third finger of the forelimb (A20–3); subdigital lamellae of the fourth finger of the forelimb (A20–4); subdigital lamellae of the fifth finger of the forelimb (A20–5); subdigital lamellae of the first toe of the hind limb (A21–1); subdigital lamellae of the second toe of the hind limb (A21–2); subdigital lamellae of the third toe of the hind limb (A21–3); subdigital lamellae of the fourth toe of the hind limb (A21–4); subdigital lamellae of the fifth toe of the hind limb (A21–5); number of dorsal scales between the occiput and the level of the anterior edge of the thigh (A22); number of precloacal pores (A26); number of scales between canthal and nasal scales (M2); number of scales around the nasal scale (M3); number of supraocular enlarged scales in the right side (M5); number of supraocular enlarged scales in the left side (M4); number of organs in the postrostral scales (M13); number of organs in the third lorilabial scale (M14); number of organs in the scale above the row of the lorilabial scales and below the canthal and preocular scales (M15); number of gular scales (M23); number of scales around midbody (M26); number of ventral scales (M32); number of auricular scales, projecting scales on anterior edge of auditory meatus (M34); and number of paravertebral spots in the right side (D6).
Molecular laboratory procedures
Total genomic DNA was extracted from samples of muscle using the GenElute mammalian genomic DNA miniprep kit (Sigma-Aldrich) according to the manufacturer’s instructions. A fragment of the approximately 1174 base pairs of the mitochondrial gene cytochrome b (Cyt-b) gene was amplified through polymerase chain reaction (PCR) using both primers: IguaCytob_ F2 (5’-CCACCGTTGTTATTCAACTAC-3’) and IguaCytob_R2 (5’-GGTTTACAAGACCAATGCTTT-3’), developed by Corl et al. (2010). Each reaction contained 1X PCR buffer (KCl), 2.5 mM MgCl2, 0.25 mM each dNTP, 0.1 μM each primer, 1 unit of Taq DNA polymerase (Thermo Scientific) and 1 μL DNA extract. PCR cycling consisted of 5 minutes initial denaturation at 94°C then 35 cycles of 30 seconds at 94°C; 30 seconds at 55°C; 60 seconds at 72°C and a final elongation step of 2 minutes at 72°C. The PCR product was visualized on 1.5% agarose gel stained with Gel-Red (Biotium Inc.) and subsequently sent to Macrogen (South Korea) for purification and direct sequencing. The nucleotide sequence was visualized and edited using the 4 Peaks software (http://nucleobytes. com/4peaks/), checked manually and nucleotides with ambiguous positions were clarified. The newly sequences obtained in this study are publically available in GenBank (Table S1).
Phylogenetic Analysis
Three matrices were made: (1) morphological data only, (2) molecular characters using mithocondrial gene (Cyt-b), and (3) combining morphological and molecular data.
Total evidence and morphological phylogenetic analyses were performed using the matrix of Abdala et al. (2020). The morphological matrix includes 306 characters and 105 terminals (Ctenoblepharys adspersa and Phymaturus palluma used as outgroups and 96 terminals of the L. montanus species group). The total evidence matrix included 105 terminals and 3390 characters. Parsimony was used as the optimality criterion, and the shortest tree of the one with the fewest homoplasies was selected. We employed TNT 1.5 (Tree Analysis Using New Technology, version 1.5; Goloboff et al. 2003) to generate the phylogenetic hypotheses. The matrix is available in morphobank.org; Project (to be determined).
Continuous characters were analyzed following Goloboff et al. (2006) and were standardized using the function mkstandb.run. considering the value of 2 as the highest transformation cost. Heuristic searches were used to find the shortest trees. The matrix was analyzed using implied weights (Goloboff 1993). We used the value of the constants K = 14 (morphological analysis) and K = 19 (Total Evidence analysis), as in the analysis by Abdala et al. (2020). One thousand replications were performed for each search. Symmetric resampling was used to obtain support values for the results, with 500 replications and a deletion probability of 0.33.
The construction of the molecular phylogenetics tree was based on mitochondrial gen Cyt-b, combining sequences of the Liolaemus montanus group (Aguilar et al. 2016) obtained from GenBank, plus the ones produce for this study (Table S1). A maximum likelihood phylogenetic analysis was carried out with MEGA X (Kumar et al. 2018). Heuristic tree searches were performed with the GTR + G + I substitution model (determined with the Akaike information criterion), and 1,000 bootstrap replications.
Statistical Analysis
Normal distributions for all data were examined using the Kolmogorov-Smirnov test (P < 0.05), and homoscedasticity was evaluated with Levene’s test. To reduce the effect of non-normal distributions of the morphological data, all continuous variables were log10 transformed and meristic variables were square root transformed (Irschick and Losos 1996; Sokal and Rohlf 1998; Peres-Neto and Jackson 2001). All species were analyzed by two distinct treatments.
Principal component analysis (PCA) and discriminant function analyses (DFA) were used to verify morphological variation between and within each Liolaemus species employing a jackknife classification matrix (Manly 2000; McCune and Grace 2002; Quinn and Keough 2002; Zar 2010). Five species belonging to the L. reichei clade and distributed in Peru (L. balagueri, L. chiribaya, L. insolitus, L. nazca, and the new species) were used as comparative groups to build the PCA and DFA.
The PCA was performed to evaluate the distri-bution of individuals of the five species mentioned before in the multivariate space. The PCA was based on the correlation matrices of the morphological variables to reduce dimensionality of the data (Quinn and Keough 2002; Lovett et al. 2000). The PCA and DFA were evaluated separately for continuous and meristic characters, following the recommendations to not combine both matrices in multivariate analyses, although there is no mathematical consensus on this approach (McGarigal et al. 2000). The PCA evaluates relationships within a single group of interdependent variables regardless of any relationships that they may have outside the group of variables.
Once the PCA was performed, and the lineal combinations that explained the highest variation were extracted, whether the new species exhibited similar or different morphological characters was examined independently for continuous and meristic data by means of a DFA, with defines species based on the results of the PCA. The DFA produces a linear combination of variables that maximizes the probability of correctly assigning observations to predetermined groups; and simultaneously, new observations can be classified into one of the groups, providing likelihood values of such classification (McGarigal et al. 2000; Van den Brink et al. 2003). All statistical analyses were performed using the Statistica software 7.0 (Statsoft 2004).
RESULTS
To validate the independent taxonomic status of the species in this study, we integrated morphological, statistical, and molecular evidence. The results of the phylogenetic and statistical analyses performed suggest that the population analyzed can be considered distinct from all other described species of Liolaemus. In accordance with best practices in zoological nomenclature (Kaiser et al. 2013), the results of the statistical, morphological and molecular phylogenetic analyses are provided after the formal presentation of the new species.
TAXONOMY
Liolaemus yarabamba Quiroz, Huamaní-Valderrama, Gutiérrez, Aguilar-Kirigin, Chaparro and Abdala sp. nov.
(Figs. 1–4)
urn:lsid:zoobank.org:act:55F52C1E-0CDD-427D-B185-E1D52C813DD2
Liolaemus insolitus, Cei and Péfaur, 1982, Act. 8th Congr. Latinoam. Zool., 2: 573–586.
Liolaemus insolitus, Zeballos et al., 2002, Dilloniana, 4: 27–34.
Liolaemus aff. insolitus6, Abdala et al., 2020, Zool. J. Linn. Soc., 189: 349–377.
Holotype: MUSA 5570, an adult male (Fig. 1), from Pampa Yarabamba, 1.5 km, District of Yarabamba, Province of Arequipa, Department of Arequipa, Peru (16°33.311'S 71°29.186'W) at 2,516 m above sea level (m asl), collected on 11 November 2013, by R. V. Semhan, C. S. Abdala, R. C. Gutierrez and A. J. Quiroz.
Fig. 1.
Holotype of L. yarabamba sp. nov., dorsal and ventral view. Photographed by A. Quiroz.
Paratypes (Fig. 2): Six adult males: MUBI 17663, same locality and collectors as holotype, from geographic coordinates 16°33.507'S 71°29.026'W, at 2,521 m asl; MUSA 1768, same locality as holotype, collected on 15 April 2010, by J. Cerdeña, L. Arapa and A. J. Quiroz, from geographic coordinates 16°33.200'S 71°29.343'W at 2,509 m asl; MUSA 178–79, same locality as holotype, geographic coordinates 16°33.349'S 71°31.319'W at 2,490 m asl, collected on 11 December 1995, by H. Zeballos; MUSA 5572, MUBI 13528 from Chapi, District of Polobaya, Province of Arequipa, Department of Arequipa, Perú, from geographic coordinates 16°42.706'S 71°20.302'W at 2,925 m asl, collected on 11 de November 2013, same collectors as holotype. Five adult females: MUSA 5571, MUBI 13466, MUBI 17664–66 same locality and collectors as holotype, from geographic coordinates 16°33.507'S 71°29.026'W at 2,521 m asl.
Fig. 2.
Holotype and paratypes of L. yarabamba sp. nov. A–C: Males: MUSA 5570(SVL: 61.06 mm), MUBI 17663 (SVL: 50.87), MUSA1768 (SVL: 68.66 mm), MUSA 5572 (SVL: 60.70), MUBI 13528 (SVL: 63.07 mm); B–D: Females: MUSA 5571(SVL: 57.77 mm), MUBI 17666(SVL: 61.94 mm), MUBI 17665 (SVL: 63.84 mm), MUBI 17664 (SVL: 52.79), MUBI 13466 (SVL: 58.44). Scale bar = 2 cm.
Fig. 3.
Male specimen of L. yarabamba sp. nov., from the type locality. Shows detail of coloration pattern and dorsolateral scales. Photographed by A. J. Quiroz.
Fig. 4.
Female specimen of L. yarabamba sp. nov., from the type locality. Shows detail of coloration pattern and dorsolateral scales. Photographed by A. J. Quiroz.
Diagnosis: Liolaemus yarabamba sp. nov. belongs to the L. montanus group of the subgenus Eulaemus, because it presents a bladelike process on the tibia in association with the presence of a sharp, with the hypertrophy of the tibialis anticus muscle (Etheridgei 1995; Abdala et al. 2006).
The following combination of characters differentiates L. yarabamba sp. nov. within the L. montanus species group (excluded L. reichei clade): medium size (max. SVL = 68.7 mm), with 44–59 midbody scales (mean = 54.88), 60–72 dorsal scales (mean = 66.4), 66–76 (mean = 71.6) ventral scales, has the presence of sub-yuxtaposed dorsal scales, with slight or without keels and without mucron and the females have 4–6 (mean = 4.6) precloacal pores. Here we provide a diagnosis with regards to all species in the L. reichei clade sensu Abdala et al. (2020).
Within this group, L. yarabamba sp. nov. is distinguished from L. audituvelatus by having fewer scales around midbody (44–59 vs. 74–80); fewer dorsal scales (60–72 vs. 78–87); fewer ventral scales (66–76 vs. 86–95); females with precloacal pores and blue scales on the back of the body, both characters absent in L. audituvelatus and males with different dorsal color pattern.
Liolaemus yarabamba sp. nov. differs from L. balagueri by having fewer scales on the neck (22–29 vs. 29–38), presence of a slight keel on the dorsal scales of males, which are absent in L. balagueri; presence of precloacal pores in females (absent in L. balagueri) and different dorsal and ventral color pattern in males and females, highlighting among other characters the presence of conspicuous paravertebral spots or ocelli and green coloration in the scapular region in L. balagueri, absent in L. yarabamba sp. nov, and with a greater quantity of blue scales in the males of L. yarabamba sp. nov (Fig. 5).
Fig. 5.
Plate with photos of male specimens of the different species of L. reichei clade sensu Abdala et al. (2019). (A) L. audituvelatus, (B) L. torresi, (C) L. reichei, (D) L. chiribaya, (E) L. insolitus, (F) L. poconchilensis, (G) L. balagueri, (H) L. nazca, (I) L. yarabamba sp. nov. Photographed by C. S. Abdala (A, B, E, F); P. Valladares: (C); A. J. Quiroz (D, I); L. Huamaní (G); L. Arapa: (H).
Liolaemus yarabamba sp. nov. is distinguished from L. chiribaya by having greater number of dorsal scales (60–72 vs. 52–61); presence of a slight keel on the dorsal scales of males, which are absent in L. chiribaya; absence of keel on the ventral scales of the thigh, which are present in most individuals of L. chiribaya; greater quantity of light blue scales in males of L. yarabamba sp. nov. that are distributed in an irregular way in the back of the body, tail and members, never crowded or associated to the paravertebral and lateral spots as L. chiribaya and the presence of light blue scales in females, reduced or absent in L. chiribaya (Fig. 5).
Liolaemus yarabamba sp. nov. is distinguished from L. insolitus by having fewer scales on the neck (22–29 vs. 35–38); presence of a slight keel on the dorsal scales of males, which are absent in L. insolitus; greater precloacal pores in females (4–6 vs. 0–3) and different color and pattern of dorsal and ventral coloration in males and females, greater quantity of blue dorsal scales, ventral color different, characterized by absence of orange color on the sides of the body in L. yarabamba sp. nov. (Fig. 5).
Liolaemus yarabamba sp. nov. is distinguished from L. nazca by having greater number of dorsal scales (60–72 vs. 53–56); dorsal body scales never imbricated and with less evident keel and not in all individuals of L. yarabamba sp. nov.; greater amount of precloacal pores in females of L. yarabamba sp. nov. and different dorsal and ventral coloration pattern in males and females, highlighting among other characters the presence of conspicuous paravertebral spots or ocelli and green coloration in the scapular region in L. nazca, absent in L. yarabamba sp. nov. and with a greater quantity of blue dorsal scales in the males of L. yarabamba sp. nov. (Fig. 5).
Liolaemus yarabamba sp. nov. is distinguished from L. torresi by having fewer scales around midbody (44–59 vs. 64–72); fewer ventral scales (66–76 vs. 86–97); fewer scales on the neck (22–29 vs. 44–47) and different coloration pattern in males and females where the absence of blue scales in both sexes of L. torresi stands out (Fig. 5).
Liolaemus yarabamba sp. nov. is distinguished from L. poconchilensis by having fewer scales on the neck (22–29 vs. 35–38), presence of a slight keel on the dorsal scales in males; greater precloacal pores in females of L. yarabamba sp. nov. (4–6 vs. 0–2) and different coloration pattern in males and females where the absence of blue scales in females of L. poconchilensis stands out (Fig. 5).
Liolaemus yarabamba sp. nov. is distinguished from L. reichei by having a maximum snout-vent lenght (68.66 vs. 50.8 mm); greater scales around midbody (44–59 vs. 43–47); greater dorsal scales (60–72 vs. 50–54); presence of a slight keel on the dorsal scales of males and different coloration pattern in males and females where the absence of blue scales in both sexes of L. reichei stands out (Fig. 5).
Liolaemus yarabamba sp. nov. differs from L. stolzmannii by having a maximum snout-vent lenght (68.66 vs. 50.8 mm) and a different color pattern, among others, the greater quantity of light blue dorsal scales stands out in L. yarabamba sp. nov., presence of light blue scales in L. yarabamba sp. nov. females, with white ventral scales and absence of yellow on the sides of the body (Fig. 5).
Description of the holotype: Adult male (MUSA 5570). SVL 61.06 mm. Head 1.30 times longer (14.42 mm) than wide (11.11 mm). Head height 9.02 mm. Neck width 10.88 mm. Eye diameter 3.19 mm. Interorbital distance 9.28 mm. Orbit-auditory meatus distance 5.38 mm. Auditory meatus 2.34 mm high, 0.93 wide. Orbit-commissure of mouth distance 5.62 mm. Internasal width 1.36 mm. Subocular undivided scale 3.62 mm. Trunk length 27.31 mm, width 17.06 mm. Tail length 75.92 mm. Femur length 10.39 mm, tibia 10.44 mm, and foot 15.60 mm. Humerus length 9.02 mm. Forearm length 7.6 mm. Hand length 10.09 mm. Pygal region length 4.83 mm, and cloacal region width 5.82 mm (Fig. 1). Dorsal surface of the head wrinkled, with 17scales (Hellmich index), rostral taller than wide. Mental larger than rostral, trapezoidal, bordered by four scales. Nasal not in contact with rostral separated by one scale. Two internasals longer than wide. Nasal surrounded by nine scales, separated from canthal by two scales. Eight scales between frontal and rostral. Frontal divided by four scales. Interparietal smaller than parietals, in contact with five scales. Preocular separated from lorilabial row by two scales. Four superciliaries and thirteen upper ciliaries. One differentiated scale at anterior margin of auditory meatus. Eight temporals. Three lorilabials in contact with subocular. Seven supralabials, not in contact with subocular. Six supraoculars. Six lorilabials. Five infralabials. Five chin shields, four pair separated by four scales. Fifty-five scales around midbody. Sixty-eight rounded, sub-juxtaposed and slightly keeled dorsal scales between occiput and hind limbs; laminate, imbricated, and slightly keeled forelimbs and hind limbs scales; tail with imbricated and strongly keeled dorsal scales. Seventy laminate and imbricated ventral scales from mental to the cloacal region, following the ventral midline of the body. Twenty-six smooth, imbricate gulars. Twenty-eight scales longitudinal fold of the neck, granular and without keels, auricular and antehumeral fold presents, and without gular fold. Ventrally, laminate, sub-yuxtaposed or imbricated forelimbs scales, and without keels; laminate and imbricated hind limbs scales, and without keels. Fourth finger with eighteen subdigital lamellae; fourth toe with 21subdigital lamellae, with three keels. Lamellar ventral tail and imbricated, with slight keels. Four precloacals pores. Supernumerary pores present.
Holotype color in life: Uniform head light brown with slightly darker parietal area. The scales of the temporal region, supralabial, infralabial and lorilabial with similar coloration. Some light gray scales with a light blue hue between frontal and nasal region may be present. Neck, body and tail dorsum with light brown color that is evenly distributed. Numerous celestial scales irregularly distributed throughout the body and tail to the ventral midline of the body. Paravertebral spots rounded of light gray color diffuse, faint and small which are scant over the neck and accentuated towards the tail slightly larger and darker, and with the presence of a subtle white scale bordered on the back. Without, vertebral line, dorsolateral bands, antehumeral arch, and scapular spots. The body sides are light gray combined with light brown shade, becoming lighter towards the belly. Without lateral spots, but with some dark gray scales. Greater celestial scales are grouped over the scapular region. Gray and light brown shades are uniform on the forelimbs and hind limbs with some light blue scales over the dorsum. Hands and feet with light gray scales, almost white dorsally. Larger number of light blue scales lateral and also over the dorsum of the body. From the mental scale to the tail, completely uniform, with white color and shade gray in the center or back of the scale. The gray color is darker in the gular and pectoral scales. A faint yellow color stands out on the sides of the gular scales (Fig 1).
Morphological variation: Based on twenty-one specimens (eight males, ten females, and three juveniles). Dorsal surface of the head wrinkled with 15–18 scales (mean = 15.75; SD = 1). Nasal surrounded by 6–9 scales (mean = 7; SD = 0.89). Supralabials 6–10 (mean = 8.31; SD = 1.19), lorilabials 4–6 (mean = 4.75; SD = 0.86). One row of lorilabials. Supraoculars 5–6 (mean = 5.56; SD = 0.51). Interparietal always smaller than parietals, surrounded by 5–8 scales (mean = 6.38; SD =1.02). Infralabials 5–6 (mean = 5.68; SD = 0.48). Gulars 24–29 (mean = 25.56; SD = 1.50). Temporals 7–11 (mean = 8.5; SD = 0.97) and smooth. Auditory meatus higher 1.07–2.83 mm (mean = 2.26; SD = 0.43) than wide 0.51–1.04 (mean = 0.85; SD = 0.14). Head longer 11.06 to 16.13 (mean = 13.29; SD = 1.35) than wide 8.81 to 11.31 (mean = 10.17; SD = 0.86). Head height 7.72 to 10.42 (mean = 9.21; SD = 0.88). Trunk length 21.02 to 33.43 (mean = 27.41; SD = 3.41). Males SVL 50.87 to 68.66 mm (mean = 60.07 mm; SD = 5.69) and females 52.79 to 63.84 mm (mean = 58.49 mm; SD = 3.65). Femur length 8.42 to 13.45 mm (mean = 10.50 mm; SD = 1.29). Humerus length 6.68 to 11.48 mm (mean = 8.62 mm; SD = 1.25). Forearm length 6.20 to 8.12 mm (mean = 6.96 mm; SD = 0.56). Hand length 7.63 to 10.55 mm (mean = 9.51; SD = 0.90). Scales around midbody 44–62 (mean = 55.25; SD = 3.99). Dorsals 60–72 (mean = 66.19; SD = 3.17) sub-yuxtaposed and slightly keeled and smooth between occiput and limbs. Infradigital lamellae on 4th finger 16–19 (mean = 17.81; SD = 0.75) and 20–24 (mean = 21.81; SD = 1.17) on 4th toe. Ventral scales 66–76 (mean = 71.56; SD = 3.12) larger than dorsals scales. Tail length 50.80 to 78.96 mm (n = 10, mean = 65.46 mm; SD = 7.94). Males with 4–6 (mean = 4.83; SD = 0.98) precloacal pores and females with 4–6 (mean = 4.57; SD = 0.79) precloacal pores.
Color variation in life: Liolaemus yarabamba sp. nov. shows evident sexual dichromatism (Figs. 4–5). In males the head coloration varies dorsally from light brown to gray (Figs. 3, 4). Some individuals have the supralabials and infralabials scales to light gray color from rest of the head. As holotype, greater or fewer celestial scales are present in dorsum of the head. Color on dorsum of the neck, body and tail varies from brown to gray, with celestial scales irregularly distributed, which are grouped both scapular region and sides of the body, and they are also present in forelimbs and hind limbs in smaller quantity. Light blue scales on tail. Presence of circular or sub-quadrangular gray diffuse paravertebral spots with a white scale on the back, these spots can be absent in some individuals. Vertebral line, dorsolateral bands, scapular spots, and antehumeral arch absents. In both sides of the body, it is possible to observe gray spots, with different size and intensity, but never evident. Below the lateral midline of the body with light gray color. Lighter tones in the forelimbs and hind limbs. Prevailing the presence of light gray color on hands and feet. Ventrally, coloration in males is similar, as well as the holotype, however, smaller individuals don’t show the gray color. Gular scales, with intense and variable. In males, light blue scales are present or absent, some males can show bright yellow scales unevenly distributed on vent, and hind limbs. Ventrally, the tail can show a faint yellow with spots and light gray scales (Fig. 4).
Coloration is completely different in females than males (Fig. 5). Head of females varies dorsally from brown to gray, with some dark, and scales. Supralabials, infralabials, and lorilabials scales lighter on dorsum of the head. The dorsum of the body can show light gray or light brown color. Circular or subquadrangular paravertebral spots present, with dark gray or black color, with a white scale on the back and a light blue spot in the center or anterior view of the scale. Small black spots are present in the vertebral section, and beige discontinuous dorsolateral bands with yellow to orange shades which vary in intensity. Faint white spots between paravertebral spots and dorsolateral bands may be present. Sides of the body is lighter than the dorsum. The lateral spots are the same color and shape as well as the paravertebral spots and with greater number of celestial scales associated with them. Numerous small black spots over and below the lateral midline of the body. Same color around the sides of the body as the forelimbs and hind limbs with darker spots. The tail has the same color and design as the body. Ventrally with immaculate white scales.
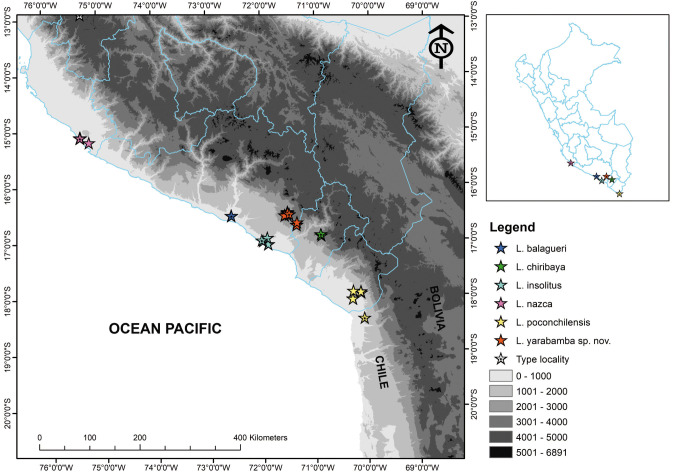
Distribution and natural history: Liolaemus yarabamba sp. nov. is known from two localities from Arequipa Department, Peru: Yarabamba (type locality, 16°33.311'S 71°29.186'W 2,515 m asl) and Chapi (16°42.706'S 71°20.302'W, 2,925 m asl), 24 km SE from the type locality. It is restricted to the eastern slopes of the La Caldera batholith (Fig. 6). The distribution corresponds to the Desert biogeographic province (Morrone 2014).
Fig. 6.
Geographical distribution of all formally described species of the L. reichei clade presents in Peru, including L. yarabamba sp. nov. The symbols with a black dot in the middle represent the type localities of each species.
Liolaemus yarabamba sp. nov. inhabits areas with little slopes of sandy substrates, composed of ash-colored sand of volcanic origin, where exists seasonal herbaceous plants and scattered columnar and prostrate cacti (Fig. 7). Several specimens were observed under rocks and in roots of small to medium bushes, as well as abandoned burrows of rodents. The type locality, Pampa Yarabamba, has some undulations and is crossed by some gorges of the La Caldera batholith. In both localities, L. yarabamba sp. nov. was found in syntopy with other reptile species: Microlophus sp., Phyllodactylus gerrhopygus Wiegmann, Tachymenis peruviana Wiegmann, and in a very narrow strip north of Pampa Yarabamba with Liolaemus etheridgei Laurent.
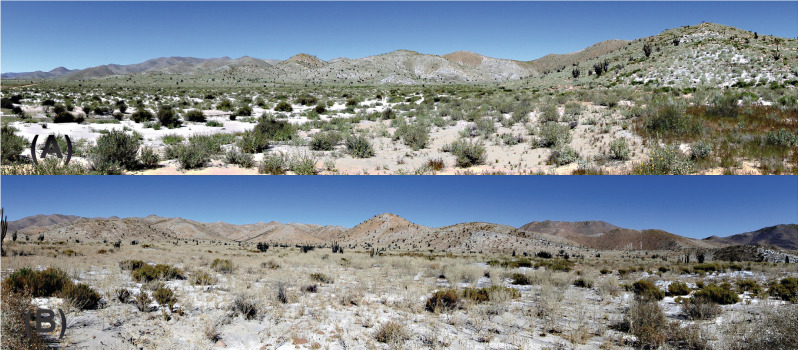
Fig. 7.
Habitat of L. yarabamba sp. nov. in Pampa Yarabamba. In the background is observed the geological formation La Caldera batholith. (A) Humid season. (B) dry season.
Etymology: The specific name refers to the type locality, Pampa Yarabamba. “Yarabamba” is a word in Quechua language (actually spoken along the Andes in Peru, Bolivia, Chile and Argentina) that means “pampa de yaros”. “Yaro” or “yara” refers to a common legume tree in southwestern Peru (Prosopis sp.) and “bamba” means “pampa” or large plains.
Statistical analysis: The summary statistics for all the non-transformed continuous and meristic characters taken from five species of Liolaemus are shown in table 1. The homogeneity of the variance was not supported for either continuous or meristic characters by the Levene’s test in some groups. Therefore, the results of the principal components analyses should be preferred for deriving linear combinations of the variables that summarize the variation in the data set. The results of the PCA for continuous and meristic characters are presented independently in table 2 (Fig. 8) and table 3 (Fig. 9)
Table 2.
Eigenvectors, eigenvalues, and percentage of variance explained for the first three principal components from transformed data in the five putative species of Liolaemus.Principal component axes loadings of continuous characters for L. balagueri (n = 12), L. chiribaya (n = 10), L. insolitus (n = 15), L. nazca (n = 7), and L. yarabamba sp. nov. (n = 13)
| Morphologial characteristics | PC1 | PC2 | PC3 | PC4 | PC5 | PC6 |
| Percentage of variance | 22.8 | 14.44 | 11 | 6.21 | 5.85 | 5.57 |
| Eigenvalues | 7.07 | 4.48 | 3.41 | 1.93 | 1.81 | 1.73 |
| Snout-vent length | –0.87 | –0.04 | –0.04 | 0.3 | 0.02 | –0.02 |
| Minimum distance between the nasal scales | –0.25 | 0.61 | 0.52 | –0.09 | 0.14 | 0.03 |
| Snout width at the edge of the flake canthal | –0.08 | 0.27 | 0.54 | –0.05 | 0.17 | 0.23 |
| Distance from the nose to the back edge of the flake canthal | –0.63 | –0.14 | –0.23 | 0.13 | 0.24 | 0.26 |
| Distance between the posterior edge of the series superciliary | –0.64 | 0.53 | 0 | 0.03 | –0.22 | 0.18 |
| Length of the interparietal | –0.33 | –0.06 | –0.44 | –0.55 | 0.06 | 0.12 |
| Length of the parietal | –0.47 | 0.36 | –0.26 | –0.47 | 0.02 | –0.04 |
| Mental flake width | 0.04 | 0.83 | 0.33 | 0.01 | –0.07 | 0.07 |
| Length of the mental scale | –0.43 | –0.45 | –0.65 | –0.18 | 0.05 | 0 |
| Distance from nostril to the mouth | –0.55 | –0.37 | 0.36 | 0.03 | –0.15 | 0.09 |
| Rostral height | –0.48 | –0.14 | 0.25 | –0.34 | –0.21 | 0.43 |
| Length of the subocular scale | –0.36 | –0.18 | 0.26 | –0.19 | –0.55 | 0.17 |
| Ear height | –0.25 | –0.18 | –0.03 | –0.08 | –0.18 | –0.72 |
| Ear width | –0.06 | 0.41 | 0.58 | –0.16 | 0 | –0.29 |
| Length of the preocular scales | –0.26 | –0.48 | 0.21 | 0.33 | –0.43 | –0.17 |
| Preocular width | –0.25 | –0.38 | 0.51 | –0.26 | –0.25 | 0.03 |
| Length of the fourth supralabial flake | –0.29 | –0.68 | 0.23 | –0.09 | 0.05 | 0.04 |
| Length of the fourth lorilabial flake | –0.43 | –0.45 | 0.22 | –0.25 | –0.20 | 0.06 |
| Length between orbits | –0.62 | 0.33 | –0.05 | 0.45 | –0.35 | 0.12 |
| Length of the first finger of the forelimb, without the claw | –0.52 | 0.33 | –0.25 | –0.11 | –0.03 | –0.35 |
| Length of the claw of the fourth finger of the forelimb | –0.07 | 0.23 | –0.60 | 0.22 | –0.46 | 0.16 |
| Length of the fifth finger of the forelimb; without the claw | –0.31 | 0.18 | 0.14 | –0.22 | 0.06 | –0.61 |
| Humerus width | –0.54 | 0 | –0.12 | 0.47 | 0.37 | –0.03 |
| Distance from the insertion of the forelimb in the body toward the elbow | –0.72 | 0.18 | 0.21 | 0.14 | –0.13 | –0.27 |
| Thigh width | –0.66 | –0.52 | –0.07 | –0.13 | 0.37 | –0.06 |
| Length of the first finger of the hind limb, without the claw | –0.31 | 0.33 | –0.44 | –0.25 | –0.06 | –0.08 |
| Length of the claw of the fourth finger of the hind limb | –0.53 | 0.12 | –0.20 | –0.07 | –0.10 | –0.06 |
| Body width | –0.62 | –0.06 | 0.44 | 0.07 | 0.51 | 0.06 |
| Width of the base of the tail | –0.77 | –0.09 | –0.04 | 0.35 | 0.11 | –0.03 |
| Upper width of the pygal area | –0.22 | 0.64 | –0.20 | –0.05 | –0.03 | 0.09 |
| Length of the pygal area | –0.62 | 0.34 | –0.06 | –0.21 | 0.2 | 0.22 |
Fig. 8.

Plot of principal components scores to continuous characters for L. balagueri (black stars, n = 12), L. chiribaya (green circles, n = 10), L. insolitus (red triangles, n = 15), L. nazca (blue triangles, n = 7) y L. yarabamba sp. nov. (beige squares, n = 13). Eigenvectors, eigenvalues, and percent variation explained for the principal components are summarized in table 2.
Table 3.
Eigenvector, eigenvalues, and percentage of variance explained for the first five principal components from transformed data in the putative species of Liolaemus. Principal component axes loadings of meristic characters for L. balagueri (n = 12), L. chiribaya (n = 10), L. insolitus (n = 15), L. nazca (n = 7), and L. yarabamba sp. nov. (n = 13)
| Morphologial characteristics | PC1 | PC2 | PC3 | PC4 | PC5 | PC6 | PC7 |
| Percentage of variance | 25.97 | 10.8 | 8.45 | 6.86 | 5.4 | 4.6 | 4.47 |
| Eigenvalues | 8.57 | 3.56 | 2.79 | 2.26 | 1.78 | 1.52 | 1.47 |
| Number of scales around the interparietal scale | 0.03 | 0.25 | –0.10 | 0.19 | 0.1 | –0.12 | 0.78 |
| Supralabials number on the right side | 0.02 | 0.01 | –0.29 | –0.48 | 0.37 | 0.11 | 0.18 |
| Supralabials number on the left side | 0.27 | –0.25 | –0.60 | –0.28 | 0.09 | –0.17 | –0.04 |
| Infralabials number on the right side | 0.55 | –0.26 | –0.19 | 0.27 | –0.12 | 0.04 | –0.04 |
| Infralabials number on the left side | 0.44 | –0.17 | –0.22 | 0.2 | –0.50 | –0.29 | –0.01 |
| Number of scales around mental scale | 0.38 | 0.02 | –0.01 | –0.29 | –0.34 | –0.20 | 0.27 |
| Number of scales around the rostral scale | 0.42 | –0.46 | 0.51 | –0.24 | –0.04 | 0.02 | 0.21 |
| Number of lorilabials | –0.10 | 0.39 | 0.32 | –0.19 | –0.40 | 0.39 | 0.07 |
| Hellmich index | 0.27 | –0.55 | –0.44 | –0.34 | –0.04 | –0.05 | 0.24 |
| Subdigital lamellae of the first finger of the forelimb | 0.01 | 0.73 | –0.13 | 0.04 | –0.08 | –0.12 | 0.08 |
| Subdigital lamellae of the second finger of the forelimb | 0.12 | 0.56 | –0.48 | –0.14 | 0.34 | –0.27 | 0.07 |
| Subdigital lamellae of the third finger of the forelimb | –0.27 | 0.44 | –0.40 | –0.03 | –0.23 | –0.23 | –0.06 |
| Subdigital lamellae of the fourth finger of the forelimb | –0.74 | –0.04 | –0.27 | –0.19 | –0.11 | 0.16 | –0.22 |
| Subdigital lamellae of the fifth finger of the forelimb | –0.57 | 0.37 | 0.28 | 0.08 | 0.12 | 0.28 | 0.37 |
| Subdigital lamellae of the first toe of the hind limb | –0.44 | 0.11 | –0.26 | –0.08 | –0.09 | –0.14 | 0.11 |
| Subdigital lamellae of the second toe of the hind limb | –0.54 | 0.56 | 0.09 | –0.08 | –0.06 | –0.02 | 0.29 |
| Subdigital lamellae of the third toe of the hind limb | –0.51 | 0.19 | 0.07 | –0.18 | –0.27 | –0.31 | –0.19 |
| Subdigital lamellae of the fourth toe of the hind limb | –0.06 | 0.49 | 0.23 | –0.44 | –0.19 | –0.03 | –0.32 |
| Subdigital lamellae of the fifth toe of the hind limb | –0.24 | –0.14 | –0.36 | –0.08 | 0.23 | 0.28 | –0.03 |
| Number of dorsal scales between the occiput and the level of the anterior edge of the thigh | 0.55 | –0.24 | 0.03 | –0.50 | 0.02 | –0.28 | 0.13 |
| Precloacal number of pores | 0.23 | –0.07 | –0.43 | –0.32 | –0.15 | 0.52 | 0.08 |
| Number of scales between canthal and nasal | –0.58 | –0.12 | –0.41 | –0.23 | –0.08 | 0.1 | –0.05 |
| Number of scales around the nasal scale | –0.18 | –0.13 | –0.05 | –0.11 | –0.58 | 0.16 | 0.37 |
| Supraoculars number enlarged scale in the right side | 0.6 | 0.29 | 0.26 | –0.36 | 0.41 | –0.15 | 0.02 |
| Supraoculars number enlarged scale in the left side | 0.4 | 0 | 0.47 | –0.56 | 0.14 | 0.14 | –0.08 |
| Number of scales between canthal and nasal scales | 0.67 | 0.09 | –0.04 | –0.24 | –0.38 | 0.13 | –0.06 |
| Number of organs in the third lorilabial scale | –0.04 | 0.55 | –0.29 | –0.21 | 0.01 | 0.28 | –0.16 |
| Number of organs above the row of lorilabials scales and below the canthal and preocular scales | 0.63 | 0.34 | 0.14 | –0.12 | –0.12 | –0.33 | –0.09 |
| Gular number of scales | –0.89 | –0.26 | 0.2 | –0.12 | 0 | –0.11 | 0.06 |
| Number of scales around the middle body | –0.92 | –0.25 | 0.03 | –0.10 | 0.02 | –0.14 | 0.03 |
| Number of ventral scales | –0.92 | –0.24 | 0.09 | –0.11 | 0.03 | –0.16 | 0.03 |
| Number of auricular scales | –0.71 | –0.22 | 0.24 | –0.42 | –0.05 | –0.17 | 0.05 |
| Number of paravertebral spots in the right side | –0.87 | –0.11 | 0.01 | –0.10 | –0.01 | –0.19 | –0.08 |
Fig. 9.

Plot of principal components scores to meristic characters for L. balagueri (black stars, n = 12), L. chiribaya (green circles, n = 10), L. insolitus (red triangles, n = 15), L. nazca (blue triangles, n = 7) y L. yarabamba sp. nov. (beige squares, n = 13). Eigenvectors, eigenvalues, and percent variation explained for the principal components are summarized in table 3.
The first six components of the continuous characters explained 65.86% of the variation, and a screen plot test of the PCs indicated that the three first components contained nontrivial information. The first axis represents body size, loading for most variables negatively and accounting for 20.80% of the variation, with strong loadings for snout-vent length, width of the base of the tail, and humerus length. The second axis represents morphological variation, with strong loadings for width of the mental scale. The next axes account for the remaining variation, with correlation values below 0.70 for auditory meatus height. The first six components of the meristic characters explained 66.54% of the variation, and a screen plot test of the PCs indicated that only those components contain relevant information. The seven axes represent morphological variation, especially in the number of scales around midbody, the number of ventral scales, the number of gular scales and the number of paravertebral spots. The seven axes account for the remaining variation, albeit with values below 0.70 for number of scales around of interparietal, subdigital lamellae of the 4th finger of the forelimb and 1st finger of the hind limb, and number of auricular scales.
The position of species based on their scores of the two morphological principal component axes is illustrated in the figures 8 and 9. The spatial distribution of the continuous characters indicates that morphological variation is sufficient to virtually separate the five Liolaemus species. These species can also be distinguished by their position analyzing meristic characters only. In both analyses, Liolaemus yarabamba sp. nov. can be differentiated from other phylogenetically related species by its morphological variation and body size.
To further clarify the position of the five Liolaemus species in the morphospace of both continuous and meristic characters, a DFA was carried out where the group membership was determined a priori for a PCA. The result obtained through the DFA for the five operational taxonomic units was not significant for continuous (Wilk’s Lambda = 0.90, F = 0.59, P = 0.67) and meristic morphological characters (Wilk’s Lambda = 0.84, F = 0.93, P = 0.47); however, the jackknife satisfactory classification was developed at 100% rate. These results show that L. yarabamba sp. nov., can be reliably distinguished from other species inside the L. reichei clade by a combination of morphological characters.
Phylogenetic analysis: The objective of the phylogenetic analysis performed here was not to resolve the relationships of the L. montanus species group, which is beyond the goal of this article, but to detect the phylogenetic relationships of L. yarabamba sp. nov., with the species with which it was confused (L. insolitus), with the recently described species L. balagueri, L. chiribaya and L. nazca, and the rest of the L. reichei clade proposed by Abdala et al. (2020).
The morphological phylogenetic hypothesis indicates that L. yarabamba sp. nov. belongs to the L. montanus species group and within it to the L. reichei clade sensu Abdala et al. (2020), together with L. audituvelatus, L. balagueri, L. chiribaya, L. insolitus, L. nazca, L. poconchilensis, L. reichei, L. torresi, and eight unnamed populations. This clade is supported by 13 synapomorphies, of which three are continuous characters (lowest number of scales from rostral to occiput; low number of scales around midbody and lowest ratio of tail length /SVL), and six are discrete characters (ventral scales of the body equal to or slightly larger than the dorsal; sides of the body with color not conspicuous, with little or absence of ventral sexual dichromatism; absence of white line in the temporal region; diameter of the eye larger than the distance between the margin anterior of the eye and the rostral scale, isognathic profile, inhabitating predominantly sandy substrates). The L. reichei clade is divided into two large subclades, one with species and populations unnamed from Chile (L. audituvelatus, L. poconchilensis, L. reichei and L. torresi) and the other with species and populations from central and southern Peru (L. balagueri, L. chiribaya, L. insolitus and L. nazca); the subclade corresponding to Peru, where the new species belongs, is supported by 19 synapomorphies, among which stands out the ratio of auditory meatus height/head height, number of pygals, number of lorilabials contacting the subocular, number of supraoculars, dorsal surface of head (rugouse), scales on external edge of forelimbs (subimbricate), scales of dorsal hind limbs (subimbricate), with notch in edge of scales of gular fold, scales of pygal región (subimbricate), with dark line through the eye; white posterior edge of paravertebral spots in both sex (present), black dots scattered on dorsal region of hind limbs in males (absent), dark line through the eye in females (present). Liolaemus yarabamba sp. nov. clustered in morphological analyses as the closest related to populations of unnamed Liolaemus from southern Peru, with particular morphological characteristics forming the clade: (L. yarabamba sp. nov. (L. aff. insolitus5 (L. aff. insolitus7 + L. aff. insolitus4))). Liolaemus yarabamba sp. nov. is supported by 21 autopomorphies in the tree, of which 12 are continuous characters and nine are discrete.
The molecular analysis is consistent with the results presented by Aguilar et al. (2017), in which the L. reichei clade is paraphyletic (Fig. 10). The L. reichei clade is divided into two subclades, one with the group (L. yarabamba sp. nov. + L. insolitus) that supports the inference of its sister relationship, and on the other hand, a group that includes the rest of the species and populations of the L. reichei clade analyzed. The three DNA (Cyt-b) obtained for L. yarabamba (intraspecific distance = 0%) fall within the same clade, supporting the identification of the new species (Fig. 10). The nearest neighbor (BS = 99) is L. insolitus, a coastal species. The mean distance between L. yarabamba and L. reichei clade (11.4%) is similar the overall divergence within the L. reichei clade (11.1%) (Table 4).
Fig. 10.
Phylogenetic analysis trees showing the specific classification of L. yarabamba sp. nov. (red), and its position among L. montanus species group lineages. (A) Total evidence phylogenetic analysis; (B) Molecular phylogenetic analysis using Maximum likelihood, the percentage of replicate trees in which the associated taxa clustered together in the bootstrap test (1000 replicates) are shown above the branches; (C) Morphology phylogenetic analysis. Morphology and total evidence analyses under parsimony criteria; the values correspond to the support measure (symmetric resampling).
Table 4.
Mean genetic distances based on cyt-b sequences using Kimura 2-parameter method for distinct phylogenetic arrangement of Liolaemus species, with special reference to Liolaemus yarabamba sp. nov. N, number of specimens used in the dataset
| Group | N | Mean (minimum — maximum) |
| L. reichei clade (described + undescribed species) | 40 | 11.1% (4.7—14.4) |
| L. yarabamba sp. nov. vs. L. reichei clade | 40 | 11.4% (4.7—14.4) |
| L. yarabamba sp. nov. vs. peruvian group | 25 | 11% |
| L. yarabamba sp. nov. vs. L. insolitus | 7 | 4.8% (4.7—4.9) |
Total evidence analysis of the phylogenetic relationships also recovers the L. reichei clade as monophyletic (Abdala et al. 2019) which, as in the morphological analyses, is divided into two subclades, one with the species from Chile and the other with those from Peru. Liolaemus yarabamba sp. nov. is recovered as the sister species of L. insolitus, this relationship is basal to a clade that includes L. chiribaya and six unnamed populations.
DISCUSSION
Our understanding of the L. montanus species group is still incipient and has many gaps. Considerable progress has been made recently in phylogenetic (Aguilar et al. 2013; Aguilar-Puntriano et al. 2018 2019; Abdala et al. 2020) and taxonomic studies (Laurent 1998; Gutiérrez et al. 2018; Aguilar-Puntriano et al. 2017; Chaparro et al. 2020; Villegas-Paredes et al. 2020); however, other disciplines such as ecology, physiology, biogeography, morphology, and conservation remain little explored. Although several Peruvian species of L. montanus group were described during the 19th century (Dumeril and Bibron 1837; Cope 1875; Steindachner 1891) and the beginning of the 20th century (Boulenger 1901; Boulenger 1902), there was a period of stagnation for 80 years before formal descriptions were made of L. ortizi (Laurent 1982) and L. insolitus (Cei and Péfaur 1982). Until the pioneering work of Raymond Laurent (1990), little was known on the taxonomy, systematics, and phylogenetics of the Liolaemus species in Peru. The revision of specimens housed in the major museums of Peru during the last four years and field trips to underexplored regions of the country have led to the description of six new species (Gutiérrez et al. 2018; Aguilar-Puntriano et al. 2019; Chaparro et al. 2020; Villegas-Paredes et al. 2020). It is remarkable that, of the last five species described, three belong to the same group (L. reichei clade). This advance is congruent with the phylogenetic hypothesis of the L. montanus group by Abdala et al. (2020), which proposes 38 taxonomically innominate populations; this study presents and revalidates the L. reichei clade composed of L. insolitus and L. poconchilensis species.
Liolaemus yarabamba sp. nov. for ca. 30 years has been considered to be the highest altitude populations of L. insolitus, based on the proposal by Cei and Péfaur (1982). However, field and laboratory observations by some of us revealed a combination of morphological characters that deserved further evaluations, including this population as a different terminal in the total evidence phylogeny proposed by Abdala et al. (2020), allowed to differentiate morphological, molecular, and geographical distribution of the population of L. yarabamba sp. nov., which is phylogenetically close to L. insolitus.
The morphological analysis shows that L. yarabamba sp. nov. has 21 autopomorphies. It is possible to highlight as a remarkable character the presence of celestial scale on the body and tail in females. This evolutionary novelty has not been formally reported in any species of the L. montanus species group, and we found this state in a few species, such as L. silvanae from the L. lineomaculatus, L. cyanogaster, L. lemniscatus, and L. platei group inside subgenus Liolaemus sensu stricto. Another original characteristic that emerged from this study is the evident keel in the ventral scales of thigh that was found also in the type material of L. chiribaya (Fig. 11). This discovery is relevant, because these species have this kind of scales that have been reported in the genus Stenocercus and Kentropyx. Undoubtedly, this morphological state gives remarkable validity to the taxonomic status of L. chiribaya, beyond the evidence presented by Aguilar-Puntriano et al. (2019).
Fig. 11.
Ventral scales of thigh of L. chiribaya paratypes exhibiting keeled scales. (A) MUSM 31547, (B) MUSM 31387.
The phylogenetic relationships in the L. reichei clade have yet to be studied in depth, considering the taxonomic status of the unnamed populations reported by Abdala et al. (2020) and Aguilar-Puntriano et al. (2017). However, the results obtained in this study give us an approximation that opens the possibility for future work in the field of taxonomy, systematics, and phylogenetic relationships. The unnamed populations are mostly lizards that are endemic to different desert areas, with altitudes ranging from 1,000 to 3,000 m asl. Liolaemus chiribaya is found at the highest altitude (3,005 m asl) and L. yarabamba the second highest (near 2,935 m asl); these are in contrast with other species that inhabit coastal deserts, close to sea level (e.g., L. insolitus, L. balagueri). This wide range of altitudes within the L. reichei clade, as well as their latitudinal distribution (from northern of Ica Department in Peru, to the Atacama Region in Chile), includes many xerophytic habitats.
The L. reichei clade contains two groups, sensu Abdala et al. (2020): Peruvian and Chilean groups; separated in distribution near the border between both countries. According to the biogeographical regionalization proposed by Morrone (2014), two provinces of the South American Transition Zone, the Desert and Atacaman provinces, correspond to the distribution of these two groups. This reinforces the phylogenetic proposal of L. reichei clade, where we propose that the Peruvian group is endemic to the Desert province and the Chilean group is endemic to the Atacaman province.
The main threat to L. yarabamba sp. nov. is habitat loss, mostly resulting from increases in large-scale mining activities and urban expansion, close to the geographical distribution area. Mining has a direct effect on local habitat degradation through the removal of native vegetation and soil, promoting changes at the landscape level, such as the opening and secondary accesses of roads or urbanization (Sonter et al. 2014). The batholith of Arequipa or “La Caldera” is of strong economic interest, and has historically hosted several mines (García 1968; Waszkis 1993) and is actually an important open-pit of copper and molybdenum mining complex (CerroVerde Mine) in the Yarabamba and Uchumayo Districts, overlapping with the habitat of L. yarabamba sp. nov. Also, in the surroundings of Chapi locality, informal non-metallic mining occurs, such as brickmaking, in which they take out “clay” or clay soil for the elaboration of bricks for construction, modifying the habitat of this species. The impact of mining on the distribution range of the fauna in Yarabamba, and throughout the whole La Caldera batholith range, is still unknown. Despite this, considering estimated area of occupancy is less than 500 km2, that it is known from less than five locations, and that mining and urban expansion activities in the area are accelerating, the species is experiencing major threats, which are leading the increase of the loss of suitable habitat for this species and declines in their populations; we therefore conclude that the category of Endangered EN [B1ab(iii)+ 2ab (iii)] is adequate, according to IUCN criteria and subcriteria (IUCN 2020), for the conservation status of L. yarabamba sp. nov.
CONCLUSIONS
Based on morphological characters and molecular data (Cyt-b), Liolaemus yarabamba sp nov. constitutes an independent lineage within the L. montanus species group from the southwestern Andes of Peru. The description of this new species not only clarifies the taxonomy and phylogeny of the L. montanus species group, but also increases the knowledge of the diversity of reptiles in the Andes of southern Peru. In addition, this study identifies the endemism and conservation status of the new species.
Key to species of the clade
1a. With tympanic membrane covered with scales, more than 73 scales around the body ............................... L. audituvelatus
1b. Tympanic membrane without scales, less than 73 scales around the body ............................... 2
2a. Dorsal body scales with evident keel and imbricate, green scales on the sides of the body ............................... L. nazca
2b. Dorsal body scales without keel, juxtaposed or subimbricated, with or without green scales on the sides of the body ............................... 3
3a. With the presence of light blue scales on the back, and sides of the body ............................... 4
3b. Without light blue scales on the back, and sides of the body ............................... 7
4a. Body with light blue scales crowded, or forming spots ............................... 5
4b. Body with light blue scales unevenly distributed ............................... 6
5a. With keel scales on the thigh, less than 34 scales on the neck, less than 77 ventral scales, sides of the belly immaculate or slightly tinged with orange, light blue scales crowded, or forming irregular spots, not strictly related to paravertebral, and, or lateral spots ............................... L. chiribaya
5b. Without keel scales on the thigh, more than 34 scales on the neck, more than 77 ventral scales, deep orange tinted sides of the belly, light blue scales forming regular spots and related to paravertebral, and, or, lateral spots ............................... L. poconchilensis
6a. With large number of light blue scales on the body, less than 30 scales on the neck, scales on the back of the body with a slight keel, no red nuances on the back of the body, belly without deep orange sides ............................... L. yarabamba sp. nov.
6b. With few light blue scales on the body, more than 30 scales on the neck, scales on the back of the body smooth, with reddish shades on the back of the body, belly with sides in deep orange ............................... L. insolitus
7a. With evident ocelli on the back of the body, with green spots, and scales on the sides of the body, variegated throat, and orange belly, less than 80 ventral scales ............................... L. balagueri
7b. Without obvious ocelli on the back of the body, no spots, and green scales on the sides of the body, immaculate throat, white belly, more than 80 ventral scales ............................... 8
8a. With more than 60 scales around the body, and 70 dorsal scales............................... L. torresi
8b. Fewer than 60 scales around the body, and less than 70 dorsal scales ............................... 9
9a. With variegated belly, yellow spots on the sides of the body, more than eight supralabials, more than seven lorilabials, distinguishable parietal, and interparietal scales, not fragmented and a maximum SVL of 50 mm ............................... L. reichei
9b. Immaculate belly, no yellow spots on body sides, less than eight supralabials, less than seven lorilabials; small, fragmented, parietal and interparietal scales and a maximum SVL of 57 mm ............................... L. stolzmanni
Supplementary materials
Specimens examined.
GenBank codes for sequences of Liolaemus lizards and the outgroup used in this study.
Acknowledgments
This work and the new species name were registered with ZooBank under urn:lsid:zoobank.org:pub:68FF41D3-7488-4354-9515-62D42D44DD26. We are grateful to the staff of Museo de Historia Natural, Universidad Nacional de San Agustin de Arequipa (MUSA), Museo de Biodiversidad del Perú (MUBI), Sonia Kretzschmar and Esteban Lavilla of Fundación Miguel Lillo (FML) and César Aguilar Puntriano (Museo de Historia Natural, Universidad Nacional Mayor de San Marcos) for allowing the review of specimens from the museum collections under their care. We thank to Jackie Farfán, Maria Gracia Lozada, Jefferson Bedregal, Moises Mamani and Luis Arapa for their support in the field work. We are grateful to Steffany Cárdenas Ninasivincha for your support in laboratory molecular analysis. AJQ, RCG and ELT thank Horacio Zeballos for encouraging the study of this lizard species. WHM thanks to “Convenio de Dempeño Regional UTA1795” and “UTA1799”. The first author would like to thank the Universidad Nacional de San Agustin de Arequipa for financial support through the contract number TP 01-2019-UNSA.
Footnotes
Authors’ contributions: AJQ wrote the draft manuscript and other authors performed the critical revision of the manuscript; AJQ and JJM designed the study, and identified the study material; AJQ, LHV, RCG, CSA described the new species; JC and WHM designed and supervised the molecular phylogenetic analyses; AJAK performed the statistical analysis; CSA, PVF, JCC performed total evidence and morphological analysis; ELT, ALR supervised the study.
Competing interests: The authors declare that they have no conflict of interests.
Availability of data and materials: Type and non-type specimens are deposited and available for review at the Museo de Historia Natural Universidad Nacional de San Agustín de Arequipa and Museo de Biodiversidad del Perú. The sequences will be available on GenBank. The manuscript will be incorporated into ZooBank.
Consent for publication: The authors give their consent to publish.
Ethics approval consent to participate: Not applicable.
References
- Abdala CS. 2007. Phylogeny of the L. boulengeri group (Iguania: Liolaemidae, Liolaemus) based on morphological and molecular charaters. Zootaxa 1538:1–84. doi:10.11646/zootaxa.1538.1.1.
- Abdala CS, Acosta JC, Cabrera MR, Villavicencio HJ, Marinero J. 2009. A new Andean Liolaemus of the L. montanus series (Squamata: Iguania: Liolaemidae) from western Argentina. South Am J Herpetol 4:91–102.
- Abdala CS, Aguilar-Kirigin AJ, Semhan RV, Bulacios-Arroyo AL, Valdes J, Paz MM, Gutiérrez-Poblete R, Valladeres-Faundez P, Langstroth R, Aparicio J. 2019. Description and phylogeny of a new species of Liolaemus (Iguania: Liolaemidae) endemic to the south of the Plurinational State of Bolivia. PLoS ONE 14(12):e0225815. doi:10.1371/journal.pone.0225815. [DOI] [PMC free article] [PubMed]
- Abdala CS, Juarez VI. 2013. Taxonomía y filogenia de un grupo de lagartos amenazados: el grupo de Liolaemus anomalus (Iguania: Liolaemidae). Cuad Herpetol 27:109–153.
- Abdala CS, Paz MM, Semhan RV. 2013. Nuevo Liolaemus (Iguania: Liolaemidae) fronterizo de Argentina y Chile que exhibe un novedoso caracter morfológico. Rev Biol Trop 61:1563–1584. [PubMed]
- Abdala CS, Quinteros AS. 2008. Una nueva especie de Liolaemus (Iguanidae: Liolemini) endémica de la sierra de Fiambalá, Catamarca, Argentina. Cuadernos de Herpetología 22:35–47.
- Abdala CS, Quinteros AS. 2014. Los últimos 30 años de estudios de la familia de las lagartijas más diversa de Argentina: Actualización taxonómica y sistemática de Liolaemidae. Cuadernos de Herpetología 28:55–82.
- Abdala CS, Quinteros AS, Espinoza RE. 2008. Two new species of Liolaemus (Iguania: Liolaemidae) from the Puna of northwestern Argentina. Herpetologica 64:458–471. doi:10.1655/08-022R1.1.
- Abdala CS, Quinteros AS, Semhan RV, Bulacios-Arroyo AL, Schulte J, Paz MM, Ruiz-Monachesi MR, Laspiur A, Aguilar-Kirigin AJ, Gutiérrez Poblete R, Valladares Faundez P, Valdés J, Portelli S, Santa-Cruz R, Aparacio J, García N, Langstroth R. 2020. Unravelling interspecific relationships among hihland lizards: first phylogenetic hypothesis sing total evidence of the Liolaemus montanus group (Iguania: Liolaemidae). Zool Linn Soc-Lond 189(1):349–377. doi:10.1093/zoolinnean/zlz114.
- Abdala V, Abdala CS, Tulli MJ. 2006. Three traditional muscular characters in the phylogeny of Liolaemus (Squamata: Tropiduridae) a reappraisal. Zootaxa 1205:55–68.
- Aguilar C, Wood P, Cusi J, Guzmán A, Huari F, Lundberg M, Mortensen E, Ramirez C, Robles D, Suarez J, Ticona A, Vargas V, Venegas P. 2013. Integrative taxonomy and preliminary asessment of species limits in the Liolaemus walkeri complex (Squamata, Liolaemidae) with descriptions of three new species from Peru. ZooKeys 364:47–91. doi:10.3897/zookeys.364.6109. [DOI] [PMC free article] [PubMed]
- Aguilar-Kirigin A, Abdala CS. 2016. Primer registro de Liolaemus puritamensis Núñez & Fox, 1989 para el sur de Bolivia (Reptilia, Squamata, Liolaemidae). Cuadernos de Herpetología 30:45–47.
- Aguilar-Kirigin AJ, Abdala CS, Aparicio J, Langstroth R. 2016. Primer registro de Liolaemus pleopholis Laurent, 1998 para Bolivia (Reptilia, Squamata, Liolaemidae). Cuadernos de Herpetología 30:89–92.
- Aguilar-Puntriano C, Avila LJ, De la Riva I, Johnson L, Morando M, Troncoso-Palacios J, Wood PL Jr, Sites JW Jr. 2018. The shadow of the past: convergence of young and old South American desert lizards as measured by head shape traits. Ecology and Evolution 8:11399–11409. doi:10.1002/ece3.4548. [DOI] [PMC free article] [PubMed]
- Aguilar-Puntriano C, Ramírez C, Castillo E, Mendoza, A, Vargas V, Sites JW Jr. 2019. Three New Lizard Species of the Liolaemus montanus Group from Peru. Diversity 11(9):161. doi:10.3390/d11090161.
- Aguilar-Puntriano C, Wood PL Jr, Belk M, Duff MH, Sites JW Jr. 2017. Different roads lead to Rome: Integrative taxonomic approaches lead to the discovery of two new lizard lineages in the Liolaemus montanus group (Squamata: Liolaemidae). Biological Journal Linnaean Society 120:448–467. doi:10.1111/bij.12890.
- Avila L, Morando M, Sites JW Jr. 2006. Congeneric phylogeography: hypothesizing species limits and evolutionary processes in Patagonian lizards of the Liolaemus boulengeri group (Squamata: Liolaemini). Biol Linn Soc 89:241–275. doi:10.1111/j.1095-8312.2006.00666.x.
- Boulenger GA. 1901. Further descriptions of new reptiles collected by Mr. P. O. Simons in Peru and Bolivia. Ann Mag Nat Hist 7(42):546–549. doi:10.1080/00222930108678513.
- Boulenger GA. 1902. Descriptions of new batrachians and reptiles from the Andes of Peru and Bolivia. Ann Mag Nat Hist 10(59):394–402. doi:10.1080/00222930208678691.
- Breitman MF, Bonino MF, Sites JW Jr, Avila LJ, Morando M. 2015. Morphological variation, niche divergence, and phylogeography of lizards of the Liolaemus lineomaculatus section (Liolaemini) from southern Patagonia. Herpetol Monogr 29:65–88. doi:10.1655/HERPMONOGRAPHS-D-14-00003.
- Breitman MF, Morando M, Avila LJ. 2013. Past and present taxonomy of the Liolaemus lineomaculatus section (Liolaemidae): is the morphological arrangement hypothesis valid? Zoo J Linn Soc-Lond 168:612–668. doi:10.1111/zoj.12037.
- Carrillo N, Icochea J. 1995. Lista taxonómica preliminar de los Reptiles vivientes del Perú. Publicaciones del Museo de Historia Natural Universidad Nacional Mayor de San Marcos 49:1–27.
- Cei JM. 1978. A new species of Liolaemus (Sauria: Iguanidae) from the Andean Mountains of the southern Mendoza volcanic region of Argentina. Occasional Papers of The Museum of Natural History University of Kansas (No. 76) 1978:1–6.
- Cei JM, Péfaur JE. 1982. Una especie nueva de Liolaemus (Iguanidae: Squamata): su sistemática, ecología y distribución. In: Actas 8vo Congreso Latinoamericano de Zoología, Mérida, Venezuela, Octubre1980, pp. 573–586.
- Chaparro JC, Quiroz, AJ, Mamani L, Gutiérrez RC, Condori P, De la Riva I, Herrera-Juárez G, Cerdeña J, Arapa LP, and Abdala CS. 2020. An endemic new species of Andean lizard of the genus Liolaemus from southern Peru (Iguania: Liolaemidae) and its phylogenetic position. Amphibian & Reptile Conservation 14(2):47–63. Available at http://amphibian-reptile-conservation. org/pdfs/Volume/Vol_14_no_2/ARC_14_2_[General_ Section]_47-63_e238.pdf. Accessed 28 May 2020.
- Cope ED. 1875. Report on the Reptiles brought by Professor James Orton from the middle and upper Amazon and western Peru. Journal of the Academy of Natural Sciences of Philadelphia N.S. 8(2):159–183.
- Corl A, Davis A, Kuchta S, Comendant T, Sinervo B. 2010. Alternative mating strategies and the evolution of sexual size dimorphisn in the side-blotched lizard, Uta stansburiana: a population-level comparative analysis. Evolution 64:79–96. doi:10.1111/j.1558-5646.2009.00791.x. [DOI] [PubMed]
- De Queiroz K. 1998. The general concept of species, species criteria, and the process of speciation: a conceptual unification and terminological recommendations. pp. 57–75. In: Howard, D. & Berlocher, S.H. (Eds.), Endless Forms: Species and Speciation. Oxford University Press, New York, New York, U.S.A.
- De Queiroz K. 2007. Species concepts and species delimitation. Syst Biol 56:879–886. doi:10.1080/10635150701701083.
- Demangel MD, Sepúlveda C, Jara M, Pincheira-Donoso D, Núñez H. 2015. Liolaemus omorfi, una nueva especie de lagarto de los andes del norte de Chile (Sauria, Liolaemidae). Boletín del Museo Nacional de Historia Natural, Chile 64:139–155.
- Duméril AMC, Bibron G. 1837. Erpétologie Générale ou Histoire Naturelle Complete des Reptiles. Vol. 4. Libr. Encyclopédique Roret, Paris, 570 pp. doi:10.5962/bhl.title.45973.
- Etheridge R. 1995. Redescription of Ctenoblepharys adspersa Tschudi, 1845, and the taxonomy of Liolaeminae (Reptilia, Squamata, Tropiduridae). Am Mus Novit 3142:1–34.
- Etheridge R. 2000. A review of lizards of the Liolaemus wiegmannii Group (Squamata, Iguania, Tropiduridae). And a history of morphological change in the sand-dwelling species. Herpetol Monogr 14:293–352. doi:10.2307/1467049.
- García W. 1968. Geología de los Cuadrangulo de Mollendo y La Joya. Servicio de Geología y Minería, Boletín 19, 103 pp. https://es.calameo.com/books/0008201299ceeee304f7d.
- Goloboff PA. 1993. Estimating character weights during tree search. Cladistics 9:83–91. doi:10.1111/j.1096-0031.1993.tb00209.x. [DOI] [PubMed]
- Goloboff P, Farris J, Nixon K. 2003. TNT: Tree analysis using new technology, v. 1.0. Available at http://www.zmuc.dk/public/phylogeny/TNT/.
- Goloboff PA, Mattoni CI, Quinteros AS. 2006. Continuous characters analyzed as such. Cladistics 22:589–601. doi:10.1111/j.1096-0031.2006.00122.x. [DOI] [PubMed]
- Gutiérrez R, Chaparro JC, Vásquez M, Quiroz A, Langsthroth R. 2013. Taxonomy, Diversity, and Abundance of the Genera Liolaemus and Proctoporus (Reptilia: Squamata) in the Area of Influence of the PERU LNG Pipeline in Monitoring biodiversity. Alonso, Dallmeier y Servat Eds. Smithsonian Press, USA, pp. 400–412.
- Gutiérrez R, Chaparro JC, Vásquez MY, Quiroz AJ, Aguilar-Kirigin AJ, Abdala CS. 2018. Una nueva especie de Liolaemus (Iguania: Liolaemidae) de Perú, y notas sobre el grupo L. montanus. Cuadernos de Herpetología 32:81–99. doi:10.31017/CdH.2018.
- Irschick DJ, Losos JB. 1996. Morphology, ecology, and behavior of the Twig anole, Anolis angusticeps. In: Powell R, Henderson RW, editors. Contributions to West Indian herpetology: a tribute to Albert Schwartz. Contributions to herpetology. Society for the Study of Amphibians and Reptiles, pp. 291–301.
- IUCN. 2020. The IUCN Red List of Threatened Species. Version 2020-1. Available at https://www.iucnredlist.org. Accessed 28 May 2020.
- Kaiser H, Crother BJ, Kelly CMR, Luiselli L, O’Shea M, Ota H, Passos P, Schleip WD, Wüster W. 2013. Best practices: in the 21st century, taxonomic decisions in herpetology are acceptable only when supported by a body of evidence and published via peer-review. Herpetological Review 44:8–23.
- Kumar S, Stecher G, Li M, Knyaz C, Tamura K. 2018. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol Biol Evol 35(6):1547–1549. doi:10.1093/molbev/msy096. [DOI] [PMC free article] [PubMed]
- Langstroth R. 2011. On the species identities of a complex Liolaemus fauna from the Atiplano and Atacama Desert: insights on Liolaemus stozmanni, L. reichei, L. jamesi pachecoi, and L. poconchilenis (Squamata: Liolaemidae). Zootaxa 2809:20–32. doi:10.11646/zootaxa.2809.1.2.
- Laurent RF. 1982. Las especies y “variedades” de Liolaemus descritas por J. Koslowsky (Sauria Iguanidae). Neotropica (La Plata) 28:87–96.
- Laurent RF. 1985. Segunda contribución al conocimiento de la estructura taxonómica del Género Liolaemus Wiegmann Iguanidae. Cuadernos de herpetología 1:1–37.
- Laurent RF. 1990. Una especie apartada del género Liolaemus Wiegmann (Iguanidae, Lacertilia). Acta Zoológica Lilloana 39:79–84.
- Laurent RF. 1998. New forms of Lizards of the subgenus Eulaemus of the genus Liolaemus (Reptilia: Squamata: Tropiduridae) fron Perú and Northern Chile. Acta Zoológica Lilloana 44:1–26.
- Losos JB. 1990. Ecomorphology, performance capability, and scalling of West Indian Anolis lizards: and evolutionary analysis. Ecol Monogr 60:369–388. doi:10.2307/1943062.
- Lobo F, Espinoza RE. 1999. Two new cryptic species of Liolaemus (Iguania: Tropiduridae) from northwestern Argentina: resolution of the purported reproductive bimodality of Liolaemus alticolor. Copeia 1999:122–140. doi:10.2307/1447393.
- Lovett GM, Weathers KC, Sobczak WV. 2000. Nitrogen saturation and retention in forested watersheds of the Catskill Mountains, New York. Ecol Appl 10:73–84. doi:10.1890/1051-0761(2000)010 [0073:NSARIF]2.0.CO;2.
- Manly BFL. 2000. Multivariate statistical methods. Florida: Chapman and Hall/CRC, Boca Raton.
- McCune B, Grace JB. 2002. Analysis of ecological communities. Oregon: MjM Software Design. doi:10.1016/S0022-0981(03)00091-1.
- McGarigal K, Cushman S, Stafford S. 2000. Multivariate statistics for wildlife and ecology research. 1st ed. New York: Springer Science & Business Media, Inc. doi:10.1007/978-1-4612-1288-1.
- Morrone JJ. 2014. Biogeographical regionalisation of the Neotropical region. Zootaxa 3782 (1):1–110. doi:10.11646/zootaxa.3782.1.1. [DOI] [PubMed]
- Olave M, Avila LJ, Sites JW Jr, Morando M. 2015. Model-based approach to test hard polytomies in the Eulaemus clade of the most diverse South American lizard genus Liolaemus (Liolaemini, Squamata). Zool J Linn Soc-Lond 174(1):169–184. doi:10.1111/zoj.12231.
- Peres-Neto PR, Jackson DA. 2001. The importance of scaling of multivariate analysis in ecological studies. Ecoscience 8:522–526. doi:10.1080/11956860.2001.11682683.
- Quinn GP, Keough MJ. 2002. Experimental design and data analysis for biologists. 1st ed. Cambridge University Press, New York, USA.
- Quinteros AS. 2013. A morphology-based phylogeny of the Liolaemus alticolor-bibronii group (Iguania: Liolaemidae). Zootaxa 3670:1–32. doi:10.11646/zootaxa.3670.1.1. [PubMed]
- Quinteros AS, Abdala CS. 2011. A new species of the Liolaemus montanus section (Iguania: Liolaemidae) from Northwestern Argentina. Zootaxa 2789:35–48. doi:10.11646/zootaxa.2789.1.2.
- Quinteros AS, Abdala CS, Lobo FJ. 2008. Redescription of Liolaemus dorbignyi Koslowsky, 1898 and description of a new species of Liolaemus (Iguania: Liolaemidae). Zootaxa 1717:51–67. doi:10.11646/zootaxa.1717.1.5.
- Quinteros AS, Ruiz-Monachesi MR, Abdala CS. 2020. Solving the Liolaemus bibronii puzzle, an integrative taxonomy approach: redescription of L. bibronii and description of three new species (Iguania: Liolaemidae). Zool J Linn Soc-Lond 189(1):315–348. doi:10.1093/zoolinnean/zlz113.
- Ruiz de Gamboa M, Correa M, Marambio-Alfaro Y, Riveros-Riffo, Ortiz J. 2018. Molecular evidence for conspecificity of two desert Liolaemus lizards (Iguania: Liolaemidae). Zootaxa 4438(2):283–298. doi:10.11646/zootaxa.4438.2.4. [DOI] [PubMed]
- Schulte JA II, Macey JR, Espinoza RE, Larson A. 2000. Phylogenetic relationships in the iguanid lizard genus Liolaemus: multiple origins of viviparous reproduction and evidence for recurring Andean vicariance and dispersal. Biol J Linn Soc 69:75–102. doi:10.1006/bijl.1999.0346.
- Sonter LJ, Barrett DJ, Soares-Filho BS. 2014. Offsetting the impacts of mining to achieve no-net-loss to native vegetation and its biodiversity. Conserv Biol 28(4):1068–1076. doi:10.1111/cobi.12260. [DOI] [PubMed]
- Sokal RR, Rohlf FJ. 1998. Biometry. The principle and practice of statistics in biological research. 3rd ed. New York: FreemanWH and Company.
- Statsoft INC (Statistica software). 2004. Version 7.0. Available from: http://www.statsoft.com. Accessed 28 May 2020.
- Steindachner F. 1891. Über einige neue und seltene Reptilien-und Amphibienarten. Sitzungsb. Ber Kais Akad Wiss, Wien, Abt. 1 100(6):289–313.
- Troncoso-Palacios J, Escobar-Gimpel V. 2020. On the taxonomy of the desert lizard Liolaemus stolzmanni (Steindachner, 1891) A third point of view (Squamata: Liolaemidae). Zootaxa 4763(1):138–144. doi:10.11646/zootaxa.4763.1.12. [DOI] [PubMed]
- Troncoso-Palacios J, Esquerré D, Urra FA, Díaz HA, Castro-Pastene C, Ruiz MS. The true identity of the new world iguanid lizard Liolaemus chillanensis Müller and Hellmich 1932 (Iguania: Liolaemidae) and description of a new species in the Liolaemus elongatus group. Zool Stud 57:22. doi:10.6620/ZS.2018.57-22. [DOI] [PMC free article] [PubMed]
- Valladares P, Etheridge R, Abdala CS. 2018. Resurrection and redescription of Liolaemus reichei, proposal of a Neotype to stabilization of the taxonomy. Revista Mexicana de Biodiversidad 89:393–401. doi:10.22201/ib.20078706e.2018.2.2086.
- Van den Brink PJ, Van den Brink NW, Ter Braak CJF. 2003. Multivariate analysis of ecotoxicological data using ordination: demonstration of utility on the basis of various examples. Australas J Ecotoxicol 9:141–156.
- Verrastro L, Veronese L, Bujes C, Dias-Filho MM. 2003. A new species of Liolaemus from Southern Brazil (Iguania: Tropiduridae). Herpetologica 59(1):105–118.
- Villegas-Paredes L, Huamaní-Valderrama L, Luque-Fernández C, Gutiérrez-Poblete RC, Quiróz AJ, Abdala CS. 2020. Una nueva especie de Liolaemus (Iguania: Liolaemidae) perteneciente al grupo L. montanus en las lomas costeras del sur de Perú. Revista de Biología Tropical 68(1):69–86. doi:10.15517/rbt.v68i1.34861.
- Waszkis H. 1993. Mining in the Americas: Stories and History. Woodhead Publishing, Cambridge, UK, 304 pp.
- Zar JH. 2010. Biostatistical analysis. 5th ed. New Jersey: Prentice Hall, Inc.
- Zeballos H, López E, Villegas L, Jiménez P, Gutiérrez R. 2002. Distribución de los reptiles de Arequipa, sur del Perú. Dilloniana 4:27–34.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Specimens examined.
GenBank codes for sequences of Liolaemus lizards and the outgroup used in this study.