Abstract
NF-κB/Rel factors have been implicated in the regulation of liver cell death during development, after partial hepatectomy, and in hepatocytes in culture. Rat liver epithelial cells (RLEs) display many biochemical and ultrastructural characteristics of oval cells, which are multipotent cells that can differentiate into mature hepatocytes. While untransformed RLEs undergo growth arrest and apoptosis in response to transforming growth factor β1 (TGF-β1) treatment, oncogenic Ras- or Raf-transformed RLEs are insensitive to TGF-β1-mediated growth arrest. Here we have tested the hypothesis that Ras- or Raf-transformed RLEs have altered NF-κB regulation, leading to this resistance to TGF-β1. We show that classical NF-κB is aberrantly activated in Ras- or Raf-transformed RLEs, due to increased phosphorylation and degradation of IκB-α protein. Inhibition of NF-κB activity with a dominant negative form of IκB-α restored TGF-β1-mediated cell killing of transformed RLEs. IKK activity mediates this hyperphosphorylation of IκB-α protein. As judged by kinase assays and transfection of dominant negative IKK-1 and IKK-2 expression vectors, NF-κB activation by Ras appeared to be mediated by both IKK-1 and IKK-2, while Raf-induced NF-κB activation was mediated by IKK-2. NF-κB activation in the Ras-transformed cells was mediated by both the Raf and phosphatidylinositol 3-kinase pathways, while in the Raf-transformed cells, NF-κB induction was mediated by the mitogen-activated protein kinase cascade. Last, inhibition of either IKK-1 or IKK-2 reduced focus-forming activity in Ras-transformed RLEs. Overall, these studies elucidate a mechanism that contributes to the process of transformation of liver cells by oncogene Ras and Raf through the IκB kinase complex leading to constitutive activation of NF-κB.
NF-κB is a family of dimeric transcription factors with subunits that contain an approximately 300-amino-acid NH2-terminal stretch, termed the Rel homology domain, that shares homology with the v-Rel oncoprotein (3). Classical NF-κB is composed of a p50 (NF-κB1) and p65 (RelA) subunits (5, 75). NF-κB is ubiquitously expressed; however, in non-B cells it is sequestered in the cytoplasm with specific inhibitory proteins termed IκBs, of which IκB-α is the best characterized (4, 77). The mitogen-activated protein kinase (MAPK) kinase kinase NIK has been implicated in activation of NF-κB/Rel following cytokine stimulation (44). NIK, recruited to the receptors through interaction with TRAF adapter molecules, activates the IκB kinase complex, which consists of two IκB kinases, IKK-1 (IKK-α) and IKK-2 (IKK-β), and a 48-kDa essential component, alternatively termed IKK-associated protein 1 (IKKAP1), NF-κB essential modulator, or IKK-γ (51, 64). The protein serine kinases IKK-1 and IKK-2 contain a leucine zipper and a helix-loop-helix motif in the C-terminal region and a kinase domain at the N-terminal region (51). Activation of the IκB kinase complex is mediated via phosphorylation of either IKK-1 or IKK-2 (18, 52, 60, 80). IκB-α is then recruited in the IκB kinase complex, where it is phosphorylated by the functional IKK-1–IKK-2 heterodimer at serine residues at positions 32 and 36. This phosphorylation is followed by ubiquitination and rapid degradation through the proteasome pathway (11, 12, 17), allowing for migration of the released NF-κB to the nucleus (3).
Recently, NF-κB/Rel factors have been strongly implicated in the regulation of apoptosis, a key mechanism of normal and malignant cell growth control (29). Our laboratory demonstrated a direct role of NF-κB/Rel in cell survival of B-cell lymphomas, hepatocytes, and breast cancer cell lines (1, 2, 10, 68, 81). For example, inhibition of NF-κB activity in hepatocytes via direct microinjection of IκB-α protein led to apoptosis (10). Furthermore, inhibition of NF-κB/Rel activity by transforming growth factor β1 (TGF-β1) also induced apoptosis of normal hepatocyte cell lines, and conversely ectopic expression of the NF-κB subunit c-Rel could rescue these cells (1). These findings suggest that the observed embryonal lethality of RelA-deficient mice, resulting from massive liver degeneration due to hepatocyte apoptosis (8), is a direct effect due to a loss of a liver-specific antiapoptotic function of NF-κB during development. Furthermore, induction of NF-κB has also been shown to promote survival in cytokine-mediated cell death of epithelial, fibroblastic, and hematopoietic cells, which do not express NF-κB in a constitutive manner (9, 76, 78).
TGF-β1 belongs to a large superfamily of structurally related, regulatory cytokines, which include three mammalian isoforms of TGF-β (TGF-β1, -β2, and -β3), activins/inhibins, and bone morphogenic proteins (BMPs) (46). The proteins of this family mediate a variety of physiological processes including cell proliferation, embryogenesis, repair, remodeling, carcinogenesis, and programmed cell death (61). TGF-β1 has been suggested as signal for hepatic growth termination and apoptosis (22). TGF-β1 treatment induced cell death of primary cultures of rat hepatocytes and cooperated, in vivo, with the hepatomitogen cyproterone acetate in the induction of apoptosis of rat liver cells (54). Apoptotic hepatocytes in normal and preneoplastic liver showed immunostaining for TGF-β1 (55). Similarly, increased TGF-β1 expression was observed in rat hepatocytes undergoing apoptosis during allogeneic graft rejection (41). Furthermore, hepatic expression of mature TGF-β1 in transgenic mice resulted in multiple tissue lesions, including hepatic fibrosis and apoptotic cell death of hepatocytes (65). As discussed above, we found that TGF-β1 treatment of untransformed murine hepatocyte cell lines selectively downregulated classical NF-κB activity and caused cell death (2), indicating that TGF-β1-mediated signaling via NF-κB controls hepatocyte survival.
Rat liver epithelial cells (RLEs) display many biochemical and ultrastructural characteristics found in oval cells in vivo (31, 73, 74). Oval cells, which are believed to be derived from stem cells within the terminal biliary ductules, have been shown to be multipotent cells that can differentiate into mature hepatocytes (21). Normal RLEs undergo growth arrest and apoptosis following TGF-β1 treatment (50, 72). Transformation of RLEs by expression of Ha-ras or v-raf counteracts TGF-β1-induced growth arrest (33, 34, 36, 50), suggesting that disruption of TGF-β1 signaling contributes to oncogenic transformation of RLEs. Interestingly, oncogenic forms of Ha-Ras or of Raf-1 were observed to activate reporter gene expression controlled by multiple NF-κB sites (24), and in Ras- or Raf-transformed fibroblasts, NF-κB has been found to be constitutively active (25, 49). Thus, in this study we tested the hypothesis that v-ras and v-raf transformation of RLEs leads to aberrant NF-κB activity. We demonstrate that oncogenic Ras- or Raf-transformed RLEs aberrantly express elevated levels of functional NF-κB due to IκB-α hyperphosphorylation and reduced half-life, which renders them resistant to TGF-β1-induced growth arrest and apoptosis. Furthermore, we demonstrate for the first time a role of IKK-1 and IKK-2 in mediating the aberrant NF-κB activation in Ras- and Raf-mediated transformation of RLEs. Overall, these findings suggest that inhibition of NF-κB expression with TGF-β1 in combination with superrepressors of NF-κB might have clinical relevance in the treatment of hepatocellular carcinomas (HCCs).
MATERIALS AND METHODS
Cell culture and treatment conditions.
The F22-Ras and TH-Raf-transformed cell lines were isolated from v-Ha-ras- and v-raf-transformed RLE cells, respectively, as previously described (27). The T2 clone was derived as a single-cell clone from a tumor obtained by injection of the parental v-raf-transformed RLEs into a nude mouse (32). Wild-type (wt) transformed RLEs (50) were maintained in F-12 nutrient mixture (Ham) medium (Gibco/BRL, Gaithersburg, Md.) supplemented with penicillin (50 U/ml) and streptomycin (50 μg/ml) (all from Sigma Chemical Co., St. Louis, Mo.). For treatment, cells were incubated for the indicated periods of time with TGF-β1 (2 to 5 ng/ml; Austral Biological, San Ramon, Calif.) dissolved in 0.1% carrier bovine serum albumin (BSA) or, as a control, 0.1% BSA. Where indicated, cells were incubated for 1 h with 40 μM calpain inhibitor I (Boehringer Mannheim, Indianapolis, Ind.). To prevent protein synthesis, cells were treated with emetine (20 μg/ml; Sigma Chemical Co.) for the indicated periods of time. To block the phosphatidylinositol 3-kinase [PI(3)K] and MAPK pathways, the inhibitors wortmannin (Sigma) and PD98059 (Calbiochem, La Jolla, Calif.) were used as indicated.
Transfection conditions.
For transient transfection, RLEs were plated at various densities in P60, P35, or 96-mm wells. After removal from the medium of antibiotics and serum, cells were incubated for 3 h at 37°C with a solution of DNA and Lipofectamine reagent (Gibco/BRL) according to the manual's instructions. Cells were harvested after 24 to 48 h, and the resulting extracts were normalized for total protein content and β-galactosidase (β-Gal) expression as previously described (1). Equal amounts of lysates were incubated in duplicate in 2.5 μCi of [3H]acetyl coenzyme A (New England Nuclear, Boston, Mass.), 50 μM acetyl coenzyme A, and 1.6 mM chloramphenicol for 4 to 6 h, and the acetylated forms were extracted with ethyl acetate and assayed by liquid scintillation. Standard deviation was obtained using Student's t test. The upstream regulatory element (URE)-thymidine kinase (TK)-chloramphenicol acetyltransferase (CAT) plasmid (E8-CAT) and its double-mutant version (dmE8-CAT) have been described previously (20). The phosphorylation-defective mutants IKK-1 SS/AA and IKK-2 SS/AA and the dominant negative IKKAP1 (dnIKKAP1) have been described previously (52, 64). The human IκB-α clone, pMT2T-IκB-α, has been described elsewhere (11). The β-Gal-expressing vector pON407, in which the five putative NF-κB sites within the cytomegalovirus (CMV) promoter have been removed (13), was used to normalize transfection efficiency. The pSG5-V12 H-ras, pSG5-V12C40 H-ras, pSG5-V12S35 H-ras expression vectors have been described previously (62). The pUC19-LTR-ΔRaf and SR-αΔp85 constructs have been described elsewhere (39, 40).
EMSA.
The URE oligonucleotide (5′-AAGTCCGGGTTTTCCCCAACC-3′) was end labeled with Klenow enzyme and α-32P-labeled deoxynucleoside triphosphates and used in electrophoretic mobility shift assay (EMSA) as previously described (1). The Oct-1 oligonucleotide has the sequence 5′-TGTCGAATGCAAATCACTAGAA-3′. The core elements are indicated by underlining. Nuclear extracts were prepared from RLEs by the method of Strauss and Varshavsky as described elsewhere (20). Antibody against the p50 (SC114) or p65 (sc-109) subunit was purchased from Santa Cruz Biotechnology Inc. (Santa Cruz, Calif.); antibody against c-Rel (cross-reacting v-Rel antibody) was kindly provided by N. Rice (National Cancer Institute, Frederick, Md.).
Immunoblot analysis.
For isolation of cytoplasmic proteins, washed cells were resuspended in cold 10 mM Tris (pH 7.4)–10 mM NaCl–3 mM MgCl2–1 mM EDTA–0.2 mM phenylmethylsulfonyl fluoride–0.2 M dithiothreitol–leupeptin (10 μg/ml)–aprotinin (2 μg/ml)–pepstatin (0.7 μg/ml)–0.5% Nonidet P-40 and incubated 5 min on ice. Nuclei were separated by centrifugation. For two-dimensional gel electrophoresis, samples (20 to 40 μg) were separated according to isoelectric point by isoelectric focusing in the first dimension (56). Isoelectric focusing gels, or crude cytoplasmic extracts (20 to 40 μg) were then subjected to electrophoresis on a sodium dodecyl sulfate (SDS)–10% polyacrylamide gel, transferred to a polyvinylidene difluoride membrane (Millipore, Bedford, Mass.), and subjected to immunoblotting as previously described (1). Cytoplasmic extracts were immunoprecipitated by the protein A-Sepharose procedure as described previously (2). The antibodies preparation for IκB-β (SC-945), IκB-α (SC-371), IKK-1 (SC7182), and IKK-2 (SC7607) were purchased from Santa Cruz Biotechnology.
Kinase assay.
Kinase assay was done in kinase buffer C (20 mM HEPES [pH 7.7]), 2 mM MgCl2, 10 μM ATP, 3 μCi of [γ-32P]ATP, 10 mM β-glycerophosphate, 10 mM NaF, 10 mM p-nitrophenyl phosphate, 300 μM Na3 VO4, 1 mM benzamidine, 2 μM phenylmethylsulfonyl fluoride, aprotinin [10 μg/ml], leupeptin [1 μg/ml], pepstatin, [1 μg/ml], 1 mM dithiothreitol) at 30°C for 45 min in the presence of the indicated substrate. The kinase reaction was stopped by addition of 4× SDS-polyacrylamide gel electrophoresis (PAGE) sample buffer, subjected to SDS-PAGE analysis, and visualized by autoradiography.
Apoptosis assay.
For the nonradioactive cell proliferation assay (Promega, Madison, Wis.), cells were seeded at 20 × 104 in a 100-μl volume in 96-well dishes. Cells were incubated in triplicate for 4 h at 37°C in the presence of 3- (4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H- tetrazolium inner salt (MTS) solution (333 μg/ml) and 25 μM phenazine methosulfate according to the manufacturer's directions. The A490 was measured in an enzyme-linked immunosorbent assay plate reader.
Focus formation assay.
RLEs were transfected in 35-mm-diameter dishes by the Lipofectamine procedure as described above. Cells were plated at 104/ml in top plugs consisting of complete F-12 nutrient mixture (Ham) medium and 0.8% SeaPlaque agarose (FMC Bioproducts, Rockland, Maine). Plates were subsequently incubated for 14 days in a humidified incubator at 37°C. Plates were stained with 0.5 ml of 0.0005% crystal violet for 1 h, and colonies were counted using a dissecting microscope.
RESULTS
Effects of TGF-β1 treatment on growth and NF-κB activity in wt and Ras- or Raf-transformed RLEs.
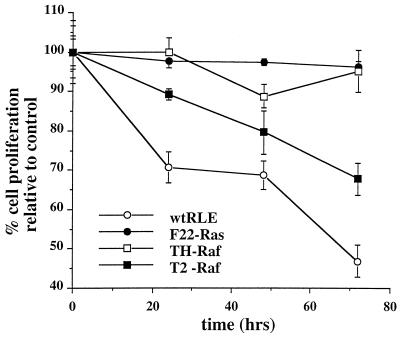
Several laboratories have shown that transformation of RLEs by expression of Ha-ras or v-raf counteracts the growth-inhibitory effects of TGF-β1 (33, 34, 36, 50). We first sought to confirm these observations in the Ha-ras-transformed F22 (F22-Ras) and v-raf-transformed F3611-T2 (T2-Raf) and F3611-TH (TH-Raf) lines. Cultures of normal and transformed RLEs were incubated in medium containing TGF-β1 (5 ng/ml) in 0.1% BSA carrier solution or in BSA carrier alone for 24, 48, and 72 h. Cell proliferation was monitored by conversion of MTS to its formazan derivative (Fig. 1). As seen previously, TGF-β1 treatment dramatically inhibited proliferation of wt RLEs. Furthermore, the effects of TGF-β1 on growth were paralleled by decreased cell viability as judged by trypan blue staining (data not shown). In contrast, TGF-β1 only modestly affected the proliferation of F22-Ras and TH-Raf cell lines, whereas the T2 line showed an intermediate sensitivity (Fig. 1). Thus, transformation with oncogenic ras and raf ablates growth arrest mediated by TGF-β1. Intriguingly, this resistance to TGF-β1 effects was not due to a defect in TGF-β1 signaling since the activity of the plasminogen activator inhibitor promoter was found to be induced approximately ninefold upon TGF-β1 treatment in the F22-Ras cells, and this induction was ablated by coexpression of a dominant negative variant of Smad3 (data not shown).
FIG. 1.
Oncogenic Ras- or Raf-transformed RLEs are resistant to TGF-β1-induced cell growth arrest. Cultures of wt and F22-Ras, TH-Raf, and T2-Raf RLEs were incubated in medium containing TGF-β1 (5 ng/ml) or BSA carrier solution for 24, 48, and 72 h. Cell proliferation was monitored by conversion of MTS to its formazan product. Means and standard deviations are representative of two independent experiments carried out in triplicate.
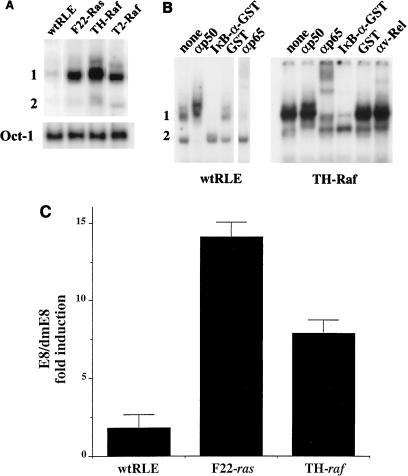
To test the hypothesis that constitutive expression of activated Ras or Raf leads to dysregulation of NF-κB activity, the levels of NF-κB binding were assessed in wt and transformed RLEs. EMSAs were performed using as probe the upstream NF-κB element (URE-κB) from the c-myc gene (20). Nuclear extracts from the normal RLEs displayed a very low level of two binding complexes (termed bands 1 and 2) (Fig. 2A). All three transformed lines exhibited similar two-band profiles. However, the levels of the upper complex (band 1) were greatly increased. The F22-Ras cells displayed a 6.9-fold increase of band 1, as assessed by densitometry normalized for levels of Oct-1 binding (bottom panel). Similarly, the TH-Raf and T2-Raf cells showed 9.6- and 3.7-fold induction of band 1, respectively. In contrast, only modest changes were seen in band 2 levels. Previously, we demonstrated that normal mouse hepatocyte lines constitutively expressed a similar two-band profile consisting of heterodimers of classical NF-κB (p50-p65) and p50 homodimers (2, 10). Supershift EMSA was performed with the extracts from the wt RLEs and F3611-TH cells (Fig. 2B). Of note, the autoradiogram for the wt RLEs represents an exposure for 2 weeks, versus 2 days for the TH-Raf cells. Addition of an antibody that preferentially recognized p50 in a homodimer complex ablated formation of band 2, while an antibody against the p65 subunit supershifted the upper complex (band 1). Addition of IκB–glutathione S-transferase (GST) fusion protein, which interacts with the p65 subunit in classical NF-κB preferentially compared to p50 in a homodimer form, selectively reduced formation of band 1, whereas addition of GST alone had little effect on binding. Furthermore, addition of an antibody against c-Rel to extracts from the TH-Raf cells had no effect on binding (Fig. 2B). Taken together, these results indicate that band 1 is a heterodimer of p50 and p65 or classical NF-κB and band 2 contains p50 homodimers. Similar results were obtained with extracts from F22-Ras cells (data not shown). Thus, ras and raf transformation lead to a dramatic increase in constitutive binding of classical NF-κB.
FIG. 2.
NF-κB is aberrantly expressed in Ras- or Raf-transformed RLEs. (A) Ras- and Raf-transformed cells display elevated levels of NF-κB binding. To measure the levels of NF-κB binding activity in wt and transformed RLEs, EMSA was performed using URE-κB motif from the c-myc gene as a probe (20) and nuclear extracts from exponentially growing wt and transformed (F-22, TH, and T2) RLEs. As control for equal loading, EMSA was also performed with an Oct-1 probe. (B) Transformed cells express classical NF-κB. For supershift analysis, following a 30-min incubation of nuclear extracts from the wt RLE or TH-Raf cells with the UREκB probe, 1 μl of antibody against either the p50 (SC114), p65 (sc-109), or c-Rel (cross-reacting v-Rel antibody, kindly provided by N. Rice) protein was added as indicated. The reaction mixture was incubated for an additional 1 h and subjected to EMSA. Alternatively, 100 ng of either IκB-α–GST or GST protein was added to the reaction mixture. (C) Transformed RLEs display elevated NF-κB activity. Wild-type and transformed RLEs in P60 dishes were transiently transfected by lipofection, in duplicate, with 6 μg of E8 or dmE8 reporter construct. The E8 vector has two copies of the URE-κB motif in front of the TK promoter driving the CAT reporter; the double-mutant vector has two copies of a mutant version of the URE with two G-to-C conversions, which is unresponsive to NF-κB transactivation (dmE8). The values for E8 CAT activity are represented as fold induction over dmE8 CAT activity, which was set at 1.0 for each cell line.
To test for transcriptional NF-κB activity, wt and transformed RLEs were transfected with a CAT reporter vector in which the TK heterologous promoter is driven by two copies of the URE (termed E8) (20). As control, we used a similar construct containing two copies of a mutant version of the URE with two G-to-C conversions, which is unresponsive to NF-κB transactivation (dmE8) (20). The basal activity of the E8 vector in exponentially growing F22-Ras and TH-Raf cells was much greater than in the normal RLEs (approximately 15- and 8-fold, respectively) (Fig. 2C). Thus, consistent with recent observations with NIH 3T3 fibroblasts (25, 49), oncogenic Ha-ras and v-raf transformation of RLEs significantly induces the functional activity of NF-κB.
Hyperphosphorylated IκB-α protein in F22-Ras and TH-Raf cells.
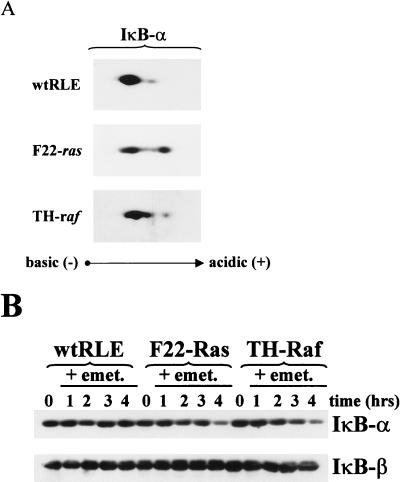
Based on the higher levels of NF-κB DNA binding activity in the F22-Ras, TH-Raf, and T2-Raf RLEs, we asked whether the basal levels of phosphorylation and rates of decay of IκB-α protein in the v-Ras or v-Raf transformation of RLEs compared to levels for wt RLEs. The phosphorylation levels of IκB-α protein were analyzed by two-dimensional gel electrophoresis. Exponentially growing wt, F22-Ras, and TH-Raf RLEs were treated for 1 h with a proteosome inhibitor to block IκB-α degradation, and cytoplasmic extracts were prepared. These were resolved on isoelectric focusing gels and then separated according to molecular weight by SDS-PAGE (10% gel) as described previously (2). Resulting immunoblots were analyzed using an IκB-α antibody preparation. Extracts from wild-type RLEs displayed predominantly the hypophosphorylated form of IκB-α, as judged by its migration toward a more basic pH (Fig. 3A). In addition, a minor spot positioned slightly to the acidic side of the major species (better seen with longer exposure) corresponding to a more phosphorylated form of IκB-α protein, was detected. Densitometric analysis revealed that the amount of the phosphorylated form represents only 11.2% of the total IκB-α protein. In contrast, extracts from F22-Ras cells yielded two distinct isoforms of the IκB-α protein of approximately equal intensity (Fig. 3A, middle panel). Densitometric analysis determined that the amount of phosphorylated IκB-α species is 39% of the total. Extracts from TH-Raf cells displayed three distinct complexes, two of which migrated toward the acidic domain presumably corresponding to more phosphorylated forms of IκB-α. Thus, oncogenic Ras or Raf transformation of RLEs leads to an IκB-α protein phosphorylation level higher than that of normal RLEs. Intriguingly, Raf-transformed RLEs extracts contain a different composition of phosphorylated species of IκB-α protein.
FIG. 3.
IκB-α protein is more phosphorylated and has a shorter half-life in F22-Ras and TH-Raf cells than in wt RLEs. (A) Phosphorylation state. Cytoplasmic extracts (40 μg) from exponentially growing wt, F22-Ras and TH-Raf RLEs, treated for 1 h with 40 μM calpain inhibitor I to inhibit IκB-α degradation, were resolved on isoelectric focusing gels, separated according to molecular weight by SDS-PAGE, and subjected to immunoblot analysis using an antibody preparation raised against the IκB-α product (SC-371). (B) Half-life of decay. Exponentially growing RLEs were treated with the protein synthesis inhibitor emetine (emet; 10 μg/ml) for 1 to 4 h. Cytoplasmic extracts (20 μg) were then subjected to immunoblot analysis for IκB-α as described above.
Because phosphorylation is a prerequisite for basal and induced degradation of IκB-α protein (11, 12, 17), we next analyzed the rate of IκB-α protein turnover. Exponentially growing cells were treated with the protein synthesis inhibitor emetine for a period of 1, 2, 3, or 4 h (Fig. 3B). Cytoplasmic extracts were then isolated and subjected to immunoblot analysis for IκB-α. In wt RLEs, no decay of IκB-α protein was detected; the inhibitor protein displayed a half-life longer than 4 h in these cells. In contrast, in the F22-Ras and TH-Raf cells, IκB-α half-lives of less than 3 h were measured. In either wt or transformed RLEs, IκB-β protein had a longer half-life and no change was detected over the 4-h time course (Fig. 3B). Thus, the presence of extremely low constitutive NF-κB levels in the wt RLEs is consistent with the low levels of phosphorylation and rate of turnover of the IκB-α protein seen in these cells. Furthermore, the higher turnover of IκB-α protein seen in the transformed RLEs appears due to increased phosphorylation.
Ras- and Raf-transformed cells maintain NF-κB DNA binding activity following TGF-β1 treatment.
Previously, we showed that TGF-β1 treatment of normal mouse hepatocytes resulted in a significant drop in the levels of NF-κB binding within 6 to 10 h (1). To test the hypothesis that persistent activation of NF-κB in the transformed but not wt RLEs correlates with protection from TGF-β1-induced apoptosis, we characterized changes of NF-κB binding activity following stimulation with TGF-β1. Cultures of wt and transformed RLEs were incubated for 24 h in the presence of TGF-β1 (5 ng/ml) or BSA carrier solution as a control, and the levels of NF-κB binding activity were monitored by EMSA. As expected, the constitutive level of NF-κB binding activity was significantly higher in nuclear extracts from the three transformed cell lines than in those from the normal RLEs (Fig. 4). In agreement with our previous observations in AML-12 and NMH cell lines (1), wt RLEs showed a TGF-β1-induced drop in NF-κB binding (Fig. 4). In contrast, no decrease in the levels of NF-κB binding activity were observed in the F22-Ras, TH-Raf, and T2 cells upon incubation with TGF-β1 for 24 h (Fig. 4), or even after 48 h in the case of the T2-raf cells (data not shown). Thus, Ras- and Raf-mediated transformation of RLEs leads to the maintenance of aberrant NF-κB expression in response to TGF-β1 treatment, suggesting that NF-κB activation may be essential for the transformed cells to escape from TGF-β1-induced apoptosis.
FIG. 4.
F22, TH, and T2 cells maintain NF-κB DNA binding activity following TGF-β1 treatment. Wild-type and transformed cells were treated for 24 h in the presence of TGF-β1 (5 ng/ml; T) or BSA carrier solution (B) as a control, and the levels of NF-κB binding activity to the URE-κB probe were monitored by EMSA analysis for Fig. 2B above.
NF-κB/Rel factors rescue wt RLEs from TGF-β1-mediated apoptosis.
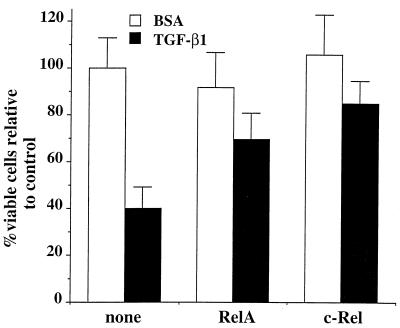
To determine whether ectopic expression of NF-κB/Rel family members could prevent TGF-β1-induced cell death of normal RLEs, cells were transiently cotransfected either with vectors directing expression of the RelA (p65) (11) or c-Rel (2) subunits, or pUC18 DNA as a control, and with a vector expressing β-Gal, to identify transfected cells within the population, as described previously (9). The basal CMV promoter-driven β-Gal expression vector pON407, in which the five NF-κB sites had been removed, was employed in the analysis, using a Lipofectamine technique of transfection, which yielded a 15 to 20% transfection efficiency (data not shown). RLEs were plated in triplicate at 70% confluence in 96-well plates and transfected with 75 ng of expression vector or control DNA and 25 ng of pON407 DNA. Six hours after transfection, TGF-β1 (5 ng/ml) or BSA carrier solution was added, and cells were incubated for an additional 48 h. Following staining with 5-bromo-4-chloro-3-indolyl-β-d-galactoside (X-Gal), the number of blue cells was determined. As expected, in the presence of the control pUC18 DNA, approximately 60% of the normal RLEs were killed following 48 h of TGF-β1 treatment (Fig. 5). In contrast, a significant protection from cell death was observed when either RelA or c-Rel subunits were expressed in combination with TGF-β1 treatment (approximately 30 and 15%, respectively). Thus, the expression of NF-κB/Rel factors prevents cell death by TGF-β1 of normal RLE cells.
FIG. 5.
NF-κB/Rel factors rescue wt RLEs from TGF-β1-mediated apoptosis. RLEs were plated at 70% confluence in 96-well plates and transiently transfected by lipofection. DNAs transfected included 25 ng of β-Gal-expressing vector pON407, in which the five putative NF-κB sites within the CMV promoter were removed in the presence of 75 ng of vector directing expression of either the RelA (p65) (11) or c-Rel (1) subunit or pUC18 DNA as control (none). Six hours after transfection, TGF-β1 (5 ng/ml) or BSA carrier solution was added, and cells were incubated for an additional 48 h. Cells were stained with X-Gal as described previously (9), and the viable (blue) cells were counted. Values are given relative to that for control cells (transfected with pUC18 DNA and treated with BSA), which was set at 100%. Means and standard deviations are representative of two independent experiments carried out in triplicate.
Inhibition of NF-κB activity enhances killing of Ras- and Raf-transformed RLEs by TGF-β1.
We next sought to determine whether specific inhibition of NF-κB was sufficient to promote TGF-β1-mediated apoptosis of Ras- and Raf-transformed RLEs. A 58% transfection efficiency of the F22-Ras and TH-Raf cell lines was measured using Lipofectamine (data not shown). Cells were transfected with the β-Gal-expressing vector pON407 in the absence or in the presence of the pMT2T-IκB-α construct (11), directing expression of the inhibitor IκB-α. Six hours after transfection, either TGF-β1 (5 ng/ml) or BSA carrier solution was added, and the cultures were incubated for an additional 48 h. In the absence of TGF-β1 treatment, IκB-α expression caused a significant decrease in viability of the F22-Ras and the TH-Raf cells (Fig. 6). Only approximately 25% of β-Gal-positive cells in both cell lines were still viable compared to cells transfected with plasmid pUC18 alone. This observation is in good agreement with previous findings demonstrating that inhibition of NF-κB in fibroblasts with activated Ras or Raf leads to a loss of cell viability (48). Upon 48 h of TGF-β1 treatment, viability of both the F22-Ras and the TH-Raf cells transfected with pUC18 DNA was unaffected, as expected, whereas transfection with the inhibitor IκB-α expression vector greatly enhanced cell death, with only 5 to 10% of cells remaining viable (Fig. 6). In fact, the extent of decrease in viability with the IκB-α-transfected F22-Ras and TH-Raf cells upon TGF-β1 treatment was even greater than that seen with the wt RLEs (Fig. 5). Furthermore, cotransfection with a RelA expression vector significantly rescued cell death caused by IκB-α, either alone or in combination with TGF-β1 (Fig. 6), suggesting a direct role of NF-κB in mediating cell survival. Overall, these data indicate that inhibition of NF-κB activity restores the response to TGF-β1-induced apoptosis in transformed RLEs.
FIG. 6.
Inhibition of NF-κB activity restores TGF-β1-mediated cell killing of oncogenic Ras- or Raf-transformed RLEs. F22-Ras (A) or TH-Raf (B) cells were lipofected with 25 ng of β-Gal-expressing vector pON407 in the presence of either 75 ng of pMT2T-IκB-α construct (11), directing expression of the inhibitor IκB-α, or pUC18 DNA, as indicated. Six hours after transfection, TGF-β1 (5 ng/ml) or BSA carrier solution was added, and cells were incubated for an additional 48 h. Alternatively, cells were cotransfected with 25 ng of pON407 plus 37.5 ng of vector expressing RelA in the absence or presence of 37.5 ng of pMT2T-IκB-α or pUC18 DNA. Cells were stained with X-Gal, and the viable (blue) cells were counted. Values are given relative to that for control cells, i.e., transfected with pUC18 DNA and treated with BSA, which was set at 100%. Means and standard deviations are representative of two independent experiments carried out in triplicate.
IKK activity mediates constitutive NF-κB activation in oncogenic Ras- or Raf-transformed RLEs.
It has been shown that distinct cytokine signaling pathways leading to NF-κB activation converge at the level of IKK-1 and IKK-2 that phosphorylate IκB-α protein (52). Thus, we explored the possibility that IKK-1 and IKK-2 mediate the IκB-α hyperphosphorylation seen in the Ras- or Raf-transformed RLEs, using NF-κB activity as the read-out. Cells were transiently transfected with the wt and double-mutant NF-κB reporter constructs in the absence or presence of vectors directing expression of two dnIKK forms of these kinases, IKK-1 SS/AA and IKK-2 SS/AA (52). The IKK-1 SS/AA and IKK-2 SS/AA genes were cloned into the pRC-βactin and pCMV-Neo vectors, respectively, and hence the parental vectors were used as control. As seen in Fig. 7A, the relative activity of the wt to mutant construct was much greater in exponentially growing F22-Ras- and TH-Raf-transformed RLEs than in wt RLEs even in the presence of the parental pRC-βactin and pCMV-Neo vectors. Ectopic expression of the IKK-1 SS/AA dominant negative construct reduced NF-κB activity by about 50% in the F22-Ras cells, whereas NF-κB transcriptional activity was unaffected in the TH-Raf cells (Fig. 7A). When we expressed the dominant negative form IKK-2 SS/AA, NF-κB activity was inhibited in both the Ras- and Raf-transformed RLEs (Fig. 7A).
FIG. 7.
IKK activity mediates constitutive NF-κB activation in oncogenic Ras- or Raf-transformed RLEs. (A) Inhibition of IKK activity. Cells were transiently transfected in P60 dishes with 2 μg of E8 or dmE8 NF-κB CAT construct in the absence or presence of 2 μg of vector directing expression dnIKK form IKK-1 SS/AA or IKK-2 SS/AA (51). As controls, the parental pRC-βactin and pCMV-Neo constructs, respectively, were similarly transfected. The values for E8 CAT activity are represented as fold induction over dmE8 CAT activity, which was set at 1.0 for each cell line. (B) Mutant version of IKKAP1 inhibits NF-κB activation in F22-Ras cells. Cells in P60 dishes were transiently transfected with 2 μg of E8 or dmE8 NF-κB CAT construct in the absence or presence of 2 μg of DNA of a vector directing expression of either full-length (FL) IKKAP1 or its C-terminus-deleted version ΔC IKKAP1 (51). In addition, 1 μg of simian virus 40–β-Gal expression vector was added to normalize for transfection efficiency. As control, the parental pCDNA3-EE construct was similarly transfected. The values are represented as percentage relative to the CAT activity of the E8 reporter in the presence of the parental vector, which was set at 100%. Means and standard deviations are representative of two independent experiments carried out in duplicate. (C) Kinase assays. (Top) Cells were transiently transfected with 5 μg of vector directing expression of wt IKK-1 or IKK-2 as described above. Following immunoprecipitation with antibodies against IKK-1 or IKK-2, extracts (10 μg) were subjected to kinase assays using either wt IκB-α–GST or the Ser32/36 double-mutant IκB-α–GST version, which cannot be phosphorylated. (Bottom) Equal aliquots of the immunoprecipitates from the lanes in the top panel, as indicated, were subjected to immunoblotting for IKK-1 and IKK-2 protein. (D) Extracts from exponentially growing cells (80 μg) were immunoprecipitated with antibodies against IKK-1 or IKK-2. Immunoprecipitated proteins were subjected to kinase assay using wt IκB-α–GST as substrate.
To verify that all of the NF-κB activation in F22-Ras cells occurs via the IκB kinase complex, we used a vector expressing a C-terminus-deleted dominant negative version of IKKAP1 (51). F22-Ras cells were transiently transfected with E8 or dmE8 NF-κB CAT constructs in the absence or presence of vectors directing expression of wt IKKAP1 or the dominant negative version (51). As a control, the parental pCDNA3-EE construct was similarly transfected. While expression of wt IKKAP1 had little effect on total reporter activity, as expected, expression of dnIKKAP1 reduced CAT activity of the wt E8 vector essentially to that of the double mutant (Fig. 7B). These findings suggest that Ras-induced activation of NF-κB is mediated entirely via the IκB kinase complex. Taken together, the results indicate that NF-κB activation by Ras is mediated by both IKK-1 and IKK-2, while Raf-induced NF-κB activation is mediated by IKK-2.
To confirm the involvement of the two IKK molecules, we carried out kinase assays using GST fusion proteins of either wt IκB-α or Ser32/36 double-mutant IκB-α, which cannot be phosphorylated, as a negative control. In the initial studies, RLEs were transiently transfected with vectors directing expression of wt IKK-1 or IKK-2. Extracts were prepared, immunoprecipitated with antibodies against IKK-1 or IKK-2, and subjected to kinase assays, as described previously (52). We were unable to detect IKK activity in normal RLEs ectopically expressing either IKK-1 or IKK-2 (Fig. 7C, top panel, lanes 1 to 4). In contrast, both immunoprecipitated IKK-1 and IKK-2 from the F22-Ras cell extracts were able to phosphorylate the wt but not the Ser32/36 IκB-α–GST protein (lanes 5 to 8). Only immunoprecipitated IKK-2 from the TH-Raf cells displayed kinase activity (lanes 9 to 12). The presence of the appropriate IKK protein in the immunoprecipitates was verified by immunoblotting (Fig. 7C, bottom panel). The activities of the endogenous IKK-1 and -2 kinases were similarly measured. Extracts from exponentially growing cells were immunoprecipitated with antibodies against either IKK-1 or IKK-2, and the immunoprecipitated proteins were subjected to kinase assay using the wt IκB-α–GST as substrate (Fig. 7D). Consistent with the findings in Fig. 7C, Ras-transformed cells contained activated IKK-1 and IKK-2, whereas only active IKK-2 was seen in Raf-transformed cells. Thus, Ras appears to function through both IKK-1 and IKK-2, whereas Raf appears to mediate its signals selectively through IKK-2.
Ras activates NF-κB through Raf- and PI(3)K-dependent pathways.
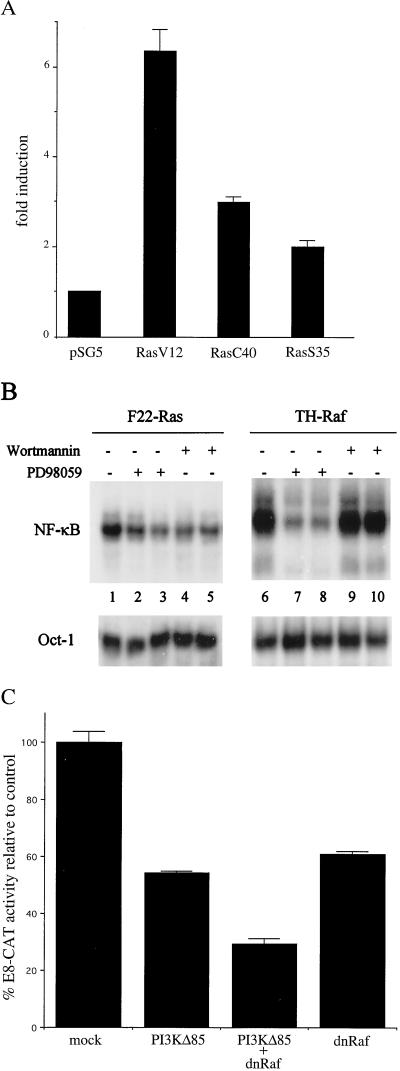
To further dissect the mechanism of oncogenic Ras-mediated NF-κB activation, we assessed the effects of several Ras effector mutants on NF-κB transcriptional activity in wt RLEs. V12 Ras interacts with all of the known Ras effectors, while the V12 C40 and V12 S35 interact only with Raf and PI(3)K, respectively (62). RLEs were transfected with constructs directing expression of the constitutively activated oncogenic V12 Ras or the partial loss of function V12 C40 or V12 S35 protein, along with the E8-CAT NF-κB reporter construct. As shown in Fig. 8A, V12 Ras induced E8-CAT activity about sixfold. Similarly, but with less potency, the V12 C40 and V12 S35 Ras proteins induced E8-CAT activity approximately three- and twofold, respectively. These results indicate that oncogenic Ras can activate NF-κB transcriptional activity through both the Raf and PI(3)K pathways.
FIG. 8.
Oncogenic Ras induces NF-κB translocation through both the PI(3)K and Raf pathways. (A) Wild-type RLE cells were transiently transfected in P100 dishes with 3 μg of wt E8 NF-κB CAT construct in the absence or presence of 1 μg of pSG5-V12 H-ras, pSG5-V12C40 H-ras, and pSG5-V12S35 H-ras expression vectors. As a control, the parental pSG5 vector was similarly transfected. The final amount of DNA was adjusted to 12 μg using pSG5 DNA. The values for E8 CAT activity are represented as fold induction over the E8 CAT activity in cells transfected with the pSG5 vector alone, which was set at 1. (B) F22-Ras and TH-Raf cells were treated for 3 h in the presence of 100 (lanes 2 and 3) or 150 (lanes 7 and 8) μM PD98059. Alternatively, F22-Ras and TH-Raf cells were treated for 3 h with 10 (lanes 4 and 5) or 100 (lanes 9 and 10) wortmannin. As a control, cells were treated with carrier dimethyl sulfoxide alone (lanes 1 and 6). Nuclear extracts were prepared, and the levels of binding activity to the URE (NF-κB) and Oct-1 probes were monitored by EMSA. (C) F22-Ras cells were transiently transfected in P100 dishes with 3 μg of wt E8 NF-κB CAT construct with 1 μg of SR-αΔp85 (PI3KD85), 1 μg of pUC19-LTR-ΔRaf (dnRaf), or both. As controls, the parental vectors were similarly transfected to bring the final amount of DNA to 12 μg. The values for E8 CAT activity are expressed as percentage of the E8 CAT activity in cells transfected with the parental vectors alone, which was set at 100.
We next asked whether these two pathways are utilized in Ras- and Raf-transformed cells using the specific inhibitors of MEK1 PD98059 and PI(3)K wortmannin. We treated F22-Ras and TH-Raf cells with increasing amounts of PD98059 or wortmannin and determined the effects on NF-κB binding activity by EMSA (Fig. 8B). Treatment with 100 or 150 μM PD98059 of F22-Ras (lanes 2 and 3) or TH-Raf (lane 7 and 8) cells resulted in a reduction in NF-κB binding activity of about 57 or 60% or 91 or 90%, respectively, relative to that of untreated cells, as determined by densitometric values normalized to the Oct-1 binding. When the F22-Ras cells were treated with either 10 or 100 nM wortmannin, we observed an approximately 30 to 40% reduction in NF-κB binding activity. In contrast, NF-κB binding levels in TH-Raf cells were unaffected by wortmannin treatment. These results suggest that activation of NF-κB is mediated by both the PI(3)K and Raf pathways in the Ras cells and only the Raf pathway in the TH-Raf cells.
To confirm that both pathways mediated NF-κB activity in the F22-Ras cells, cells were transfected with either a dominant negative mutant version of the p85 subunit of PI(3)K, PI(3)KΔ85 (40), or with a kinase mutant c-Raf (dnRaf) (39). As shown in Fig. 8C, ectopic expression of PI(3)KΔ85 reduced NF-κB transcriptional activity to approximately 55% relative to mock-transfected cells. In contrast, PI(3)KΔ85 only modestly affected NF-κB activity in the TH-Raf cells (data not shown). Ectopic expression of the dnRaf mutant inhibited NF-κB activity by approximately 40% in the F22-Ras cells. When the dnRaf was expressed in combination with PI(3)KΔ85, we observed further reduction of NF-κB transcriptional activity to approximately 29% of the basal level. Thus, we conclude that both the PI(3)K and Raf pathways mediate NF-κB translocation in the F22-Ras cells. In contrast, the induction of NF-κB activity in the TH-Raf cells appears mainly PI(3)K independent and MEK1 dependent, suggesting that in these cells only the Raf pathway mediates NF-κB activation.
Inhibition of IKKs activity renders transformed RLEs sensitive to TGF-β1-induced apoptosis.
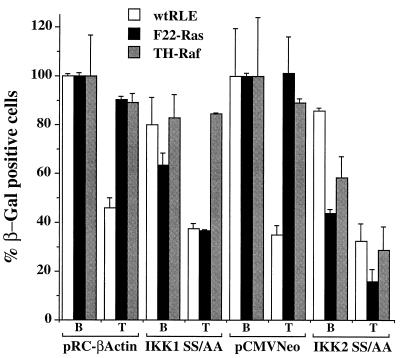
If activation of NF-κB activity through the IKKs is critical for enhanced cell survival, ectopic expression of dominant negative forms of IKKs should sensitize the transformed RLEs to TGF-β1-mediated cell killing. Wild-type, F22-Ras, and TH-Raf RLEs were transfected with a construct directing expression of IKK-1 SS/AA, IKK-2 SS/AA, or the appropriate parental protein, along with the β-Gal-expressing vector pON407, as described above. Following transfection, cells were treated with TGF-β1 (5 ng/ml) or BSA carrier solution and incubated for an additional 48 h, and viability was assessed by β-Gal staining. In cells that had been transfected with the parental, control vectors, TGF-β1 treatment had little effect on viability of either the F22-Ras or TH-Raf cells but led to cell death of approximately 50% of wt RLEs (Fig. 9). As seen above, ectopic expression of the IKK-1 SS/AA mutant alone (i.e., in BSA control-treated cells) killed approximately 35% of F22-Ras while not significantly affecting the viability of either wt or TH-Raf RLEs. This observation likely reflects the ability shown above of this dnIKK-1 form to repress NF-κB transcriptional activity in the F22-Ras RLEs but not in the TH-Raf RLEs (Fig. 7). Interestingly, transfection with IKK-1 SS/AA increased cell death mediated upon TGF-β1 treatment in F22-Ras cells (65% versus 38% viable cells), whereas it did not alter that of the TH-Raf cells (Fig. 9). Ectopic expression of the IKK-2 SS/AA mutant led to a significant cell killing of both Ras- and Raf-transformed RLEs in the absence of TGF-β1 treatment, consistent with the finding that IKK-2 mediates aberrant NF-κB activation in F22-Ras and TH-Raf cells. Furthermore, IKK-2 SS/AA increased the extent of cell death induced upon TGF-β1 treatment in the F22-Ras- and TH-Raf-transformed cells (Fig. 9), whereas it had little effect on wt RLEs. The viable cell numbers upon TGF-β1 treatment in the IKK-2 SS/AA-transfected F22-Ras and TH-Raf cell lines decreased from approximately 42 to 15% and from 58 to 25%, respectively (Fig. 9). Thus, inhibition of NF-κB activity via dnIKKs restores sensitivity to TGF-β1 cell killing of oncogenic Ras- and Raf-transformed RLEs.
FIG. 9.
Inhibition of IKK activity renders transformed RLEs sensitive to TGF-β1-induced apoptosis. Normal and transformed RLEs were lipofected with 25 ng of β-Gal-expressing vector pON407 in the presence of either 25 ng of IKK-1 SS/AA or IKK-2 SS/AA expression vector DNA. As controls, the respective parental pRC-βactin and pCMV-Neo constructs were similarly transfected. Six hours after transfection, TGF-β1 (5 ng/ml) or BSA carrier solution was added to the culture medium, and the cells were incubated for an additional 48 h. Viable cells and values were determined as described in the legend to Fig. 6.
Inhibition of IKK-1 or IKK-2 activity reduces oncogenic Ras focus-forming activity in RLEs.
To assess whether IKK-mediated activation of NF-κB is required for Ras-mediated transformation of RLEs, we tested whether inhibition of aberrant NF-κB expression via dnIKKs would affect Ras-enhanced ability of RLEs to form foci in soft agar. Following transfection of wt or F22-Ras RLEs with constructs directing expression of IKK-1SS/AA, IKK-2 SS/AA, or the appropriate parental control, cells were plated in soft agar and after 2 weeks foci were counted. As expected, wt RLEs did not yield any transformed foci (data not shown). F22-Ras cells transfected with the parental vector pRC-βActin or pCMV-Neo gave rise to about 300 to 350 CFU/cm2 (Fig. 10). Expression of either the IKK-1 SS/AA and IKK-2 SS/AA significantly reduced focus formation by approximately 60%, with IKK-2 slightly more effective. Given the transfection efficiency of approximately 60% observed with the F22-Ras RLEs, these findings indicate inhibition of IKK activity blocks focus-forming activity by Ras.
FIG. 10.
Inhibition of IKK-1 or IKK-2 activity reduces oncogenic Ras focus-forming activity in RLEs. F22-Ras cells were lipofected as described in the legend to Fig. 9. After 24 h, cells were plated in soft agar; after 2 weeks, the number of foci was scored. Means and standard deviation are representative of two independent experiments carried out in duplicate.
DISCUSSION
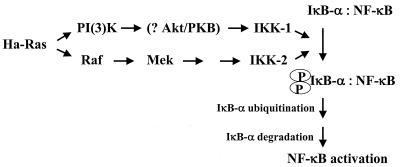
In this study we have shown that oncogenic Ras- and Raf-mediated transformation of RLEs leads to dysregulation of NF-κB expression via IKK signaling. In particular, transformation by Ras and Raf led to constitutively enhanced basal levels of NF-κB binding in RLEs that were maintained upon TGF-β1 treatment, promoting cell survival. These effects of Raf and Ras were mediated, in part, via signaling through IKK-2. Inhibition of IKK-2 activity downregulated NF-κB activity and restored TGF-β1 sensitivity; furthermore, this inhibition decreased the ability of the Ras-transformed cells to grow in soft agar. An additional component of Ras signaling appeared mediated by IKK-1. Intriguingly, Ras-mediated transformation induced NF-κB binding and transcriptional activities through both Raf and PI(3)K pathways, whereas oncogenic Raf appeared to activate NF-κB only through the MAPK pathway. Together, these findings suggest that MAPK and PI(3)K pathways signal via IKK-1 and IKK-2, respectively, as illustrated in Fig. 11. As expected, the constitutive IKK signaling in the transformed cells led to hyperphosphorylation and instability of IκB-α protein and to higher functional levels of NF-κB in the nucleus. Inhibition of NF-κB upon ectopic expression of IκB-α restored the sensitivity of Ras- or Raf-transformed RLEs to apoptosis upon TGF-β1 treatment, whereas RelA or c-Rel subunits rescued normal RLEs from TGF-β1 cell killing. Thus, aberrant activation of NF-κB binding plays a critical role in RLE survival and transformation. Overall, these findings elucidate a novel mechanism of oncogenic Ras- and Raf-mediated transformation of epithelial cells that involve IKK signaling, which leads to enhanced IκB-α phosphorylation and rate of degradation, resulting in dysregulated aberrant activation of NF-κB.
FIG. 11.
Schematic representation of Ha-Ras-mediated activation of NF-κB through IKK complex activation. Ras leads to activation of NF-κB via two pathways: PI(3)K and Raf, as discussed in the text. Akt/PKB, protein kinase B.
In murine liver tumors, Ras is activated due to mutation at codons 12, 13, and 61, with an overall frequency of 30% (45). Interestingly, a study by Kalkuhl et al. (37) reported a significant increase of Raf kinase in virtually all cases of murine 7,12-dimethylbenz(a)anthracene (DMBA)-induced tumors compared to that of normal liver. Intriguingly, Raf activation was also found in those tumors that were not harboring Ras mutations, suggesting the existence of additional coactivators of Raf, which are sensitive to carcinogens. Thus, it is tempting to speculate that carcinogen-mediated activation of the Ras-Raf pathway might lead to activation of NF-κB, which in turn results in a selective growth advantage of the transformed cells. Consistent with this hypothesis, we have recently shown that over 85% of DMBA-induced mammary tumors in Sprague-Dawley rats display elevated levels of NF-κB binding (68). This NF-κB activation was evident after only 3 weeks upon DMBA exposure, when tumors had not as yet developed, suggesting that NF-κB nuclear localization is an early event during mammary tumor formation (38). Consistently, expression of various NF-κB/Rel subunits was detected in specimens of primary human breast cancer (68). Thus, further analyses aimed at correlating Ras-Raf activation to NF-κB expression in carcinogen-induced carcinomas are needed.
Several investigators have been able to isolate and established long-term cultures of small, morphologically and functionally simple epithelial cells by enzymatic perfusion of fetal and adult rat liver and use of culture conditions that exclude hepatocytes (31). These RLEs share some phenotypic properties with both bile duct epithelial cells and hepatocytes but are more like some of the oval cell lines. The data summarizing the stem-like characteristic of the RLEs has recently been published (31). RLEs can be transformed in vitro with dominant oncogenes or chemical carcinogens, as well as spontaneously; when transplanted, they display a wide range of phenotypes, including well-differentiated HCC, cholangiomas, hepatoblastomas, and poorly differentiated or anaplastic tumors (66). While the undifferentiated RLEs have a very low level of NF-κB activity, all transformed RLEs examined so far have high NF-κB activity (S. S. Thorgeirrson, unpublished data). These results complement the data presented here and suggest that increased NF-κB activity is pivotal in the transformation process.
Many other tumors have recently been shown to display constitutive activation of NF-κB, including the human cutaneous T-cell lymphoma HuT-78 (30), Hodgkin's lymphomas (6), melanoma (67), pancreatic adenocarcinoma (79), and primary adult T-cell leukemias (53). Moreover, NF-κB induction has been found to inhibit tumor necrosis factor alpha (TNF-α)-induced cell death of breast, prostate, and bladder cancer cells (69, 70). Furthermore, the Tax transforming protein produced by human T-cell leukemia virus type 1 induces NF-κB activity through activation of both IKK-1 and IKK-2 (28).
Previously, Baldwin and coworkers demonstrated that NF-κB activity is essential for Ras-mediated transformation of rat and murine fibroblasts (25, 49). In these cases, however, no differences in the level of NF-κB DNA binding activity were noted despite the stronger induction of NF-κB transcriptional activity in Ras- or Raf-transformed NIH 3T3 cells than in wt cells. Instead, the authors demonstrated that the increased transcriptional activity in the Ras-transformed cells was due to activation of the transcriptional function of the RelA subunit through a translocation-independent mechanism. In contrast to these findings, here we show increased levels of binding in both the Raf- and Ras-transformed RLEs; furthermore, we can relate this increase to IKK activity. Inhibition of IKK-1 and IKK-2 activity leads to downregulation of NF-κB activity, to sensitization to TGF-β1-induced cell death, and to reduction of focus formation of the transformed RLEs. Thus, these results indicate an additional mechanism whereby Ras and Raf can signal activation of NF-κB. Furthermore, since the NF-κB activity of the Ras-transformed cells seemed somewhat higher than expected based solely on the binding levels, this component of the increase might be related to enhanced transcriptional activity of the p65 protein.
Inhibition of IKK-1 reduced NF-κB activity in F22-Ras but not TH-Raf cells, whereas downregulation of IKK-2 activity affected NF-κB expression in both cell types. These findings are consistent with a model whereby part of the Ras signaling occurs via Raf and IKK-2. An additional part of signaling by Ras appears to occur via another mechanism involving IKK-1, potentially mediated via PI(3)K (see below). Recently, mouse embryonic fibroblasts lacking the IKK-1 subunit displayed intact NF-κB activation in response to TNF-α and interleukin-1 (16, 35). However, IKK-1−/− mice died as neonates due to defects in skin and skeletal development (71). Thus, it appears that IKK-1 mediates NF-κB activation in early stages of embryonic development. Our results demonstrate that activation of IKK-1 upon Ras transformation counteracts the antiproliferative and proapoptotic effect of TGF-β1 through constitutive expression of NF-κB. Intriguingly, BMPs, which are members of the TGF-β superfamily of cytokines, have been implicated in the development of skeletal patterning in mammals and birds (19). Thus, it is tempting to speculate from our findings that specific BMPs might regulate NF-κB through IKK-1 during specific stages of bone development. Furthermore, we found that oncogenic Ras in part activates NF-κB through the PI(3)K pathway, raising the intriguing possibility that the activation of the Akt/protein kinase B serine-threonine kinase by PI(3)K might mediate NF-κB activation through IKK-1 phosphorylation, as described recently for TNF-α and platelet-derived growth factor signaling (58, 61).
Recently, IKK-2 has been implicated in the signaling of proinflammatory cytokines (48). Mouse embryonic fibroblasts isolated from IKK-2 knockout mice showed impaired activation of NF-κB in response to TNF-α or interleukin-1 treatment (42). Overall, it appears that IKK-2 is the key regulator of NF-κB activity in hepatocytes, since mice lacking the IKK-2 subunit died early in utero due to massive liver degeneration by apoptosis (42). This latter phenotype is similar to that of mice lacking the RelA subunit, which also died due to liver cell death (8). Our findings indicate that both oncogenic Ras and Raf can activate NF-κB through IKK-2, conferring a survival advantage to liver epithelial cells. Thus, IKK-2, which represents a ubiquitous mediator of NF-κB activation, might represent a candidate target for anticancer therapy.
TGF-β1 plays an important role in the pathophysiology of the liver (66). It inhibits the proliferation of normal hepatocytes in primary culture and after partial hepatectomy (26) and is implicated in the regulation of hepatic apoptosis both in vivo and in vitro (65). The level of TGF-β1 RNA and protein is very low in normal rodent and human livers. However, TGF-β1 is abundantly expressed during regenerative growth, inflammatory response to tissue injury, and hepatic fibrosis and in HCCs (7, 15, 23). Cancer cells can acquire resistance to the antiproliferative effect of TGF-β1 by a number of different mechanisms, including inability to activate the latent TGF-β1 complex, loss of expression or function of transmembrane serine-threonine receptor kinases (TβRI and TβRII), and disruption of postreceptor signal transduction pathways. Both TβRI and TβRII are required for the enactment of the biological effects of TGF-β1, and recent evidence suggests the existence of divergent signaling pathways for diverse TGF-β1 activities (47). That tumor cells commonly escape TGF-β1-mediated cell cycle control and the capacity of TGF-β1 to suppress the immune response, promote angiogenesis, and enhance extracellular matrix deposition implicate TGF-β1 as a key modulator of epithelial carcinogenesis (61). Recent work shows that the functions of TGF-β1 can vary from inhibition to stimulation of tumorigenesis, depending on the stage of neoplastic development and cooperation with different oncogenes and growth factors (14, 57, 59, 61). The upregulation of NF-κB in hepatitis B-associated liver cancer possibly due to X protein activation is of particular interest (43) and may, at least in part, explain the resistance of liver tumors to TGF-β1-induced apoptosis. Characterization of the mechanism(s) by which TGF-β1 modulates cancer development in general and liver cancer in particular may therefore provide opportunities for new therapeutic approaches.
ACKNOWLEDGMENTS
We gratefully acknowledge U. Siebenlist, E. Mocarski, N. Rice, J. Downward, W. Ogawa, and G. M. Cooper for kindly providing cloned DNAs or antibody reagents. We are indebted to P. Erhardt for insightful discussions. We thank D. Sloneker for assistance in preparation of the manuscript.
This work was supported by grants from the Charlotte Geyer Foundation (M.A.), ACS grant IRG-72-001-24 (M.A.), and NIH grants CA78616 (M.A.) and CA36355 (G.E.S.).
REFERENCES
- 1.Arsura M, FitzGerald M J, Fausto N, Sonenshein G E. NF-κB/Rel blocks transforming growth factor-β1-induced apoptosis of murine hepatocyte cell lines. Cell Growth Differ. 1997;8:1049–1059. [PubMed] [Google Scholar]
- 2.Arsura M, Wu M, Sonenshein G E. TGF-β1 inhibits NF-κB/Rel activity inducing apoptosis of B cells: transcriptional activation of IκB-α. Immunity. 1996;5:31–40. doi: 10.1016/s1074-7613(00)80307-6. [DOI] [PubMed] [Google Scholar]
- 3.Baeuerle P A, Henkel T. Function and activation of NF-κB in the immune system. Annu Rev Immunol. 1994;12:141–179. doi: 10.1146/annurev.iy.12.040194.001041. [DOI] [PubMed] [Google Scholar]
- 4.Baldwin A S., Jr The NF-κB and IκB proteins: new discoveries and insights. Annu Rev Immunol. 1996;14:649–681. doi: 10.1146/annurev.immunol.14.1.649. [DOI] [PubMed] [Google Scholar]
- 5.Ballard D, Dixon E, Peffer N, Bogard H, Doerre S, Stein B, Greene W. The 65-kDa subunit of human NF-κB functions as a potent transcriptional activator and a target for v-Rel-mediated repression. Proc Natl Acad Sci USA. 1992;85:1875–1880. doi: 10.1073/pnas.89.5.1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bargou R C, Emmerich F, Krappmann D, Mapara M Y, Arnold W, Royer H D, Grinstein E, Greiner A, Scheidereit C, Dorken B. Constitutive nuclear factor-kappaB-RelA activation is required for proliferation and survival of Hodgkin's disease tumor cells. J Clin Investig. 1997;100:2961–2969. doi: 10.1172/JCI119849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bedossa P, Peltier E, Terris B, Franco D, Poynard T. Transforming growth factor β1 (TGF-β1) and TGF-β1 receptors in normal, cirrhotic, and neoplastic human livers. Hepatology. 1995;21:760–766. [PubMed] [Google Scholar]
- 8.Beg A, Sha W, Bronson R, Ghosh S, Baltimore D. Embryonic lethality and liver degeneration in mice lacking the RelA component of NF-κB. Nature. 1995;376:167–170. doi: 10.1038/376167a0. [DOI] [PubMed] [Google Scholar]
- 9.Beg A, Baltimore D. An essential role for NF-κB in preventing TNF-α induced cell death. Science. 1996;274:782–784. doi: 10.1126/science.274.5288.782. [DOI] [PubMed] [Google Scholar]
- 10.Bellas R E, FitzGerald M J, Fausto N, Sonenshein G E. Inhibition of NF-κB activity induces apoptosis in murine hepatocytes. Am J Pathol. 1997;152:891–896. [PMC free article] [PubMed] [Google Scholar]
- 11.Brown K, Gerstenberger S, Carlson L, Franzoso G, Siebenlist U. Control of IκB-α proteolysis by site-specific signal-induced phosphorylation. Science. 1995;267:1485–1488. doi: 10.1126/science.7878466. [DOI] [PubMed] [Google Scholar]
- 12.Chen Z J, Parent L, Maniatis T. Site-specific phosphorylation of IκB-α by a novel ubiquitination-dependent protein kinase activity. Cell. 1996;84:853–862. doi: 10.1016/s0092-8674(00)81064-8. [DOI] [PubMed] [Google Scholar]
- 13.Cherrington J M, Mocarski E S. Human cytomegalovirus ie1 transactivates the alpha promoter-enhancer via an 18-base-pair repeat element. J Virol. 1989;63:1435–1440. doi: 10.1128/jvi.63.3.1435-1440.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cui W, Fowlis D J, Bryson S, Duffic E, Ireland H, Balman A, Akhurst R J. TGF-β I inhibits the formation of benign skin tumors but enhances progression to invasive spindle carcinomas in transgenic mice. Cell. 1996;86:531–542. doi: 10.1016/s0092-8674(00)80127-0. [DOI] [PubMed] [Google Scholar]
- 15.Czaja M J, Weiner F R, Flanders K C, Giambrone R, Wind L, Biempica L, Zern M A. In vitro and in vivo association of transforming growth factor β1 with hepatic fibrosis. J Cell Biol. 1989;108:2477–2482. doi: 10.1083/jcb.108.6.2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Delhase M, Hayakawa M, Chen Y, Karin M. Positive and negative regulation of IkappaB kinase activity through IKKbeta subunit phosphorylation. Science. 1999;284:309–313. doi: 10.1126/science.284.5412.309. [DOI] [PubMed] [Google Scholar]
- 17.DiDonato J, Mercurio F, Rosette C, Wu-Li J, Suyang H, Ghosh S, Karin M. Mapping of the inducible IκB phosphorylation sites that signal its ubiquitination and degradation. Mol Cell Biol. 1996;16:1295–1304. doi: 10.1128/mcb.16.4.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.DiDonato J, Hayakawa A, Rothwarf D M, Zandi E, Karin M. A cytokine-responsive IκB kinase that activates the transcription factor NF-κB. Nature. 1997;388:548–554. doi: 10.1038/41493. [DOI] [PubMed] [Google Scholar]
- 19.Duprez D, Bell E J, Richardson M K, Archer C W, Wolpert L, Brickell P M, Francis-West P H. Overexpression of BMP-2 and BMP-4 alters the size and shape of developing skeletal elements in the chick limb. Mech Dev. 1996;57:145–157. doi: 10.1016/0925-4773(96)00540-0. [DOI] [PubMed] [Google Scholar]
- 20.Duyao M P, Buckler A J, Sonenshein G E. Interaction of an NF-κB-like factor with a site upstream of the c-myc promoter. Proc Natl Acad Sci USA. 1990;87:4727–4731. doi: 10.1073/pnas.87.12.4727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Evarts R P, Nagy P, Nakatsukasa H, Marsden E, Thorgeirsson S S. In vivo differentiation of rat liver oval cells into hepatocytes. Cancer Res. 1989;49:1541–1547. [PubMed] [Google Scholar]
- 22.Fausto N. Growth factors in liver development, regeneration and carcinogenesis. Prog Growth Factor Res. 1991;3:219–234. doi: 10.1016/0955-2235(91)90008-r. [DOI] [PubMed] [Google Scholar]
- 23.Fausto N, Mead J E, Gruppuso P A, Braun L. TGF-β in liver development, regeneration, and carcinogenesis. Ann NY Acad Sci. 1990;593:231–242. doi: 10.1111/j.1749-6632.1990.tb16115.x. [DOI] [PubMed] [Google Scholar]
- 24.Finco T S, Baldwin A S., Jr κB site-dependent induction of gene expression by diverse inducers of nuclear factor κB requires raf-1. J Biol Chem. 1993;268:17676–17679. [PubMed] [Google Scholar]
- 25.Finco T S, Westwick J K, Norris J L, Beg A A, Der C J, Baldwin A S., Jr Oncogenic Ha-Ras-induced signalling activates NF-κB transcriptional activity, which is required for cellular transformation. J Biol Chem. 1997;272:24113–24116. doi: 10.1074/jbc.272.39.24113. [DOI] [PubMed] [Google Scholar]
- 26.Fynn T M, Reiss M. Resistance to inhibition of cell growth by transforming growth factor A and its role in oncogenesis. Crit Rev Oncog. 1993;4:493–540. [PubMed] [Google Scholar]
- 27.Garfield S, Huber B E, Nagy P, Cordingley M G, Thorgeirsson S S. Neoplastic transformation and lineage switching of rat liver epithelial cells by retrovirus-associated oncogenes. Mol Carcinog. 1988;1:189–195. doi: 10.1002/mc.2940010307. [DOI] [PubMed] [Google Scholar]
- 28.Geleziunas R, Ferrell S, Lin X, Mu Y, Cunningham E T, Jr, Grant M, Connelly M A, Hambor J E, Marcu K B, Greene W C. Human T-cell leukemia virus type 1 Tax induction of NF-κB involves activation of the IκB kinase alpha (IKKα) and IKKβ cellular kinases. Mol Cell Biol. 1998;18:5257–5265. doi: 10.1128/mcb.18.9.5157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gilmore T D, Koedood M, Piffat K A, White D W. Rel/NF-κB/IκB protein and cancer. Oncogene. 1996;13:1367–1378. [PubMed] [Google Scholar]
- 30.Giri D K, Aggarwal B B. Constitutive activation of NF-kappaB causes resistance to apoptosis in human cutaneous T cell lymphoma HuT-78 cells: autocrine role of tumor necrosis factor and reactive oxygen intermediates. J Biol Chem. 1998;273:14008–14014. doi: 10.1074/jbc.273.22.14008. [DOI] [PubMed] [Google Scholar]
- 31.Grisham J W, Thorgeirsson S S. Liver stem cells. In: Potten C S, editor. Stem cells. London, United Kingdom: Academic Press; 1997. pp. 233–282. [Google Scholar]
- 32.Hampton L L, Worland P J, Yu B, Thorgeirsson S S, Huggett A C. Expression of growth-related genes during tumor progression in v-raf-transformed rat liver epithelial cells. Cancer Res. 1990;50:7460–7467. [PubMed] [Google Scholar]
- 33.Houck K A, Michalopoulos G K, Strom S C. Introduction of a Ha-ras oncogene into rat liver epithelial cells and parenchymal hepatocytes confers resistance to the growth inhibitory effects of TGF-β. Oncogene. 1989;4:19–25. [PubMed] [Google Scholar]
- 34.Howe P H, Dobrowolski S F, Reddy K B, Stacey D W. Release from G1 growth arrest by transforming growth factor β1 requires cellular ras activity. J Biol Chem. 1993;268:21448–21452. [PubMed] [Google Scholar]
- 35.Hu Y, Baud V, Delhase M, Zhang P, Deerinck T, Ellisman M, Johnson R, Karin M. Abnormal morphogenesis but intact IKK activation in mice lacking the IKKalpha subunit of IkappaB kinase. Science. 1999;284:316–320. doi: 10.1126/science.284.5412.316. [DOI] [PubMed] [Google Scholar]
- 36.Huggett A C, Hampton L L, Ford C P, Wirth P J, Thorgeirsson S S. Altered responsiveness of rat liver epithelial cells to transforming growth factor beta 1 following their transformation with v-raf. Cancer Res. 1989;50:7468–7465. [PubMed] [Google Scholar]
- 37.Kalkuhl A, Troppmair J, Buchmann A, Stinchcombe S, Buenemann C L, Rapp U R, Kaestner K, Schwarz M. p21Ras downstream effectors are increased in activity or expression in mouse liver tumors but do not differ between ras-mutated and ras-wild-type lesions. Hepatology. 1998;27:1081–1088. doi: 10.1002/hep.510270425. [DOI] [PubMed] [Google Scholar]
- 38.Kim D W, Sovak M A, Zanieski G, Nonet G, Romieu-Mourez R, Lau A W, Hafer L J, Yaswen P, Stampfer M, Rogers A E, Russo J, Sonenshein G E. Activation of NF-κB/Rel occurs early during neoplastic transformation of mammary cells. Carcinogenesis. 2000;21:871–879. doi: 10.1093/carcin/21.5.871. [DOI] [PubMed] [Google Scholar]
- 39.Kolch W, Heidecker G, Kochs G, Hummel R, Vahidi H, Mischack H, Finkenzeller G, Marme D, Rapp U R. Protein kinase C alpha activates Raf-1 by direct phosphorylation. Nature. 1993;364:249–252. doi: 10.1038/364249a0. [DOI] [PubMed] [Google Scholar]
- 40.Kotani, K., K. Yonezawa, K. Hara, H. Hueda, Y. Kitamura, H. Sakaue, A. Ando, A. Chavanieu, B. Calas, and F. Grigorescu. Involvement of phosphoinositide 3-kinase in insulin- or IGF-1-induced membrane ruffling. EMBO J. 13:2313–2321. [DOI] [PMC free article] [PubMed]
- 41.Krams S M, Egawa H, Quinn M B, Villanueva J C, Garcia-Kennedy R, Martinez O M. Apoptosis as a mechanism of cell death in liver allograft rejection. Transplantation. 1995;59:621–625. [PubMed] [Google Scholar]
- 42.Li Q, Van Antwerp D, Mercurio F, Lee K F, Verma I M. Severe liver degeneration in mice lacking the IkappaB kinase 2 gene. Science. 1999;284:321–325. doi: 10.1126/science.284.5412.321. [DOI] [PubMed] [Google Scholar]
- 43.Lucito R, Schneider R J. Hepatitis B virus X protein activates transcription factor NF-κB without a requirement for protein kinase C. J Virol. 1992;66:983–991. doi: 10.1128/jvi.66.2.983-991.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Malinin N L, Boldin M P, Kovalenko A V, Wallach D. MAP3K-related kinase involved in NF-kappaB induction by TNF, CD95 and IL-1. Nature. 1997;385:540–544. doi: 10.1038/385540a0. [DOI] [PubMed] [Google Scholar]
- 45.Maronpot R R, Fox T, Malarkey D E, Goldsworthy T L. Mutations in the ras proto-oncogenes: clues to etiology and molecular pathogenesis of mouse liver tumors. Toxicology. 1995;101:125–156. doi: 10.1016/0300-483x(95)03112-s. [DOI] [PubMed] [Google Scholar]
- 46.Massague J. TGF-β signaling: receptors, transducers, and Mad proteins. Cell. 1996;85:947–950. doi: 10.1016/s0092-8674(00)81296-9. [DOI] [PubMed] [Google Scholar]
- 47.Massague J. The transforming growth factor-β family. Annu Rev Cell Biol. 1990;6:597–641. doi: 10.1146/annurev.cb.06.110190.003121. [DOI] [PubMed] [Google Scholar]
- 48.May M J, Ghosh S. IkappaB kinases: kinsmen with different crafts. Science. 1999;284:271–273. doi: 10.1126/science.284.5412.271. [DOI] [PubMed] [Google Scholar]
- 49.Mayo M W, Wang C-Y, Cogswell P C, Rogers-Graham K S, Lowe S W, Der C J, Baldwin A S., Jr Requirement of NF-κB activation to suppress p53-independent apoptosis induced by oncogenic Ras. Science. 1997;278:1812–1815. doi: 10.1126/science.278.5344.1812. [DOI] [PubMed] [Google Scholar]
- 50.McMahon J B, Richards W L, del Campo A A, Song M-K H, Thorgeirsson S S. Differential effects of transforming growth factor-β on proliferation of normal and malignant rat liver epithelial cells in culture. Cancer Res. 1986;46:4665–4671. [PubMed] [Google Scholar]
- 51.Mercurio F, Murray B W, Shevchenko A, Bennett B L, Young D B, Li J W, Pascual G, Motiwala A, Zhu H, Mann M, Manning A M. IκB kinase (IKK)-associated protein 1, a common component of the heterogeneous IKK complex. Mol Cell Biol. 1999;19:1526–1538. doi: 10.1128/mcb.19.2.1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mercurio F, Zhu H, Murray B W, Shevchenko A, Bennett B L, Li J W, Young D B, Barbosa M, Mann M, Manning A, Rao A. IKK-1 and IKK-2: cytokine-activated IκB kinases essential for NF-κB activation. Science. 1997;278:860–865. doi: 10.1126/science.278.5339.860. [DOI] [PubMed] [Google Scholar]
- 53.Mori N, Fujii M, Ikeda S, Yamada Y, Tomonaga M, Ballard D W, Yamamoto N. Constitutive activation of NF-kappaB in primary adult T-cell leukemia cells. Blood. 1999;93:2360–2368. [PubMed] [Google Scholar]
- 54.Oberhammer F A, Bursch W, Parzefall W, Breit P, Erber E, Stadler M, Schulte-Hermann R. Effect of transforming growth factor β on cell death of cultured rat hepatocytes. Cancer Res. 1991;51:2478–2485. [PubMed] [Google Scholar]
- 55.Oberhammer F A, Pavelka M, Sharma S, Tiefenbacher R, Purchio A F, Bursch W, Schulte-Hermann R. Induction of apoptosis in cultured hepatocytes and in regressing liver by transforming growth factor β1. Proc Natl Acad Sci USA. 1992;89:5408–5412. doi: 10.1073/pnas.89.12.5408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.O'Farrell P H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975;10:4007–4021. [PMC free article] [PubMed] [Google Scholar]
- 57.Oft M, Peli J, Rudaz C, Schwarz H, Beug H, Reichmann E. TGF-β1 and Ha-Ras collaborate in modulating the phenotypic plasticity and invasiveness of epithelial tumor cells. Genes Dev. 1996;10:2462–2477. doi: 10.1101/gad.10.19.2462. [DOI] [PubMed] [Google Scholar]
- 58.Ozes O N, Mayo L D, Gustin J A, Pfeffer S R, Pfeffer L M, Donner D B. NF-κB activation by tumour necrosis factor requires the Akt serine-threonine kinase. Nature. 1999;401:82–85. doi: 10.1038/43466. [DOI] [PubMed] [Google Scholar]
- 59.Pierce D F, Jr, Gorska A E, Chytil A, Meise K S, Page D L, Coffrey R J, Jr, Moses H L. Mammary tumor suppression by transforming growth factor β transgene expression. Proc Natl Acad Sci USA. 1995;92:4254–4258. doi: 10.1073/pnas.92.10.4254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Regnier C H, Song H Y, Gao X, Goeddel D V, Cao Z, Rothe M. Identification and characterization of an IκB kinase. Cell. 1997;90:373–383. doi: 10.1016/s0092-8674(00)80344-x. [DOI] [PubMed] [Google Scholar]
- 61.Roberts A B, Sporn M B. The transforming growth factor-betas. In: Sporn M B, Roberts A B, editors. Peptide growth factor and their receptors. Heidelberg, Germany: Springer-Verlag; 1990. pp. 419–472. [Google Scholar]
- 62.Rodriguez-Viciana P, Warne P H, Khwaya A, Marte B M, Pappin D, Das P, Waterfield M D, Ridley A, Downward J. Role of phosphoinositode 3-OH kinase in cell transformation and control of the actin cytoskeleton by Ras. Cell. 1997;89:457–467. doi: 10.1016/s0092-8674(00)80226-3. [DOI] [PubMed] [Google Scholar]
- 63.Romashkova J A, Makarov S S. NF-κB is a target of Akt in anti-apoptotic PDGF signalling. Nature. 1999;410:86–90. doi: 10.1038/43474. [DOI] [PubMed] [Google Scholar]
- 64.Rothwarf D M, Zandi E, Natoli G, Karin M. IKK-gamma is an essential regulatory subunit of the IkappaB kinase complex. Nature. 1998;395:297–300. doi: 10.1038/26261. [DOI] [PubMed] [Google Scholar]
- 65.Sanderson N, Factor V, Nagy P, Kopp J, Kondaiah P, Wakefield L, Roberts A B, Sporn M B, Thorgeirsson S S. Hepatic expression of mature transforming growth factor β1 in transgenic mice results in multiple tissue lesions. Proc Natl Acad Sci USA. 1995;92:2572–2576. doi: 10.1073/pnas.92.7.2572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Schulte-Hermann R, Grasl-Kraupp B, Bursch W. Apoptosis and hepatocarcinogenesis. In: Jirtle R L, editor. Liver regeneration and carcinogenesis: molecular and cellular mechanisms. San Diego, Calif: Academic Press; 1995. pp. 141–177. [Google Scholar]
- 67.Shattuck R L, Wood L D, Jaffe G J, Richmond A. MGSA/GRO transcription is differentially regulated in normal retinal pigment epithelial and melanoma cells. Mol Cell Biol. 1994;14:791–802. doi: 10.1128/mcb.14.1.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sovak M A, Bellas R E, Kim D W, Zanieski G J, Rogers A E, Traish A M, Sonenshein G E. Aberrant NF-κB/Rel expression in breast cancer: a role in protection from apoptosis. J Clin Investig. 1997;100:2952–2960. doi: 10.1172/JCI119848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sumitomo M, Tachibana M, Ozu C, Asakura H, Murai M, Hayakawa M, Nakamura H, Takayanagi A, Shimizu N. Induction of apoptosis of cytokine-producing bladder cancer cells by adenovirus-mediated IkappaBalpha overexpression. Hum Gene Ther. 1999;10:37–47. doi: 10.1089/10430349950019174. [DOI] [PubMed] [Google Scholar]
- 70.Sumitomo M, Tachibana M, Nakashima J, Murai M, Miyajima A, Kimura M, Hayakawa M, Nakamura H. An essential role for nuclear factor kappa B in preventing TNF-alpha-induced cell death in prostate cancer cells. J Urol. 1999;161:674–679. [PubMed] [Google Scholar]
- 71.Takeda K, Takeuchi O, Tsujimura T, Itami S, Adachi O, Kawai T, Sanjo H, Yoshikawa K, Terada N, Akira S. Limb and skin abnormalities in mice lacking IKKalpha. Science. 1999;284:313–316. doi: 10.1126/science.284.5412.313. [DOI] [PubMed] [Google Scholar]
- 72.Teramoto T, Kiss A, Thorgeirsson S S. Induction of p53 and Bax during TGF-beta 1 initiated apoptosis in rat liver epithelial cells. Biochem Biophys Res Commun. 1998;251:56–60. doi: 10.1006/bbrc.1998.9411. [DOI] [PubMed] [Google Scholar]
- 73.Thorgeirsson S S. Target cell populations in virus-associated hepatocarcinogenesis. Princess Takamatsu Symp. 1995;25:163–170. [PubMed] [Google Scholar]
- 74.Tsao M S, Smith J D, Nelson K G, Grisham J W. A diploid epithelial cell line from normal adult rat liver with phenotypic properties of “oval” cells. Exp Cell Res. 1984;154:38–52. doi: 10.1016/0014-4827(84)90666-9. [DOI] [PubMed] [Google Scholar]
- 75.Urban M B, Schreck R, Baeuerle P. NF-κB contacts DNA by a heterodimer of the p50 and p65 subunit. EMBO J. 1991;10:1817–1826. doi: 10.1002/j.1460-2075.1991.tb07707.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Van Antwerp D J, Seamus J M, Kafri T, Green D, Verma I M. Suppression of TNF-α-induced apoptosis by NF-κB. Science. 1996;274:787–789. doi: 10.1126/science.274.5288.787. [DOI] [PubMed] [Google Scholar]
- 77.Verma I M, Stevenson J K, Schwarz E M, Van Antwerp D, Miyamoto S. Rel/NF-κB/IκB family: intimate tales of association and dissociation. Genes Dev. 1995;9:2723–2735. doi: 10.1101/gad.9.22.2723. [DOI] [PubMed] [Google Scholar]
- 78.Wang C-Y, Mayo M W, Baldwin A S., Jr TNF- and cancer therapy-induced apoptosis: potentiation by inhibition of NF-κB. Science. 1996;274:784–787. doi: 10.1126/science.274.5288.784. [DOI] [PubMed] [Google Scholar]
- 79.Wang W, Abbruzzese J L, Evans D B, Larry L, Cleary K R, Chiao P J. The nuclear factor-kappa B RelA transcription factor is constitutively activated in human pancreatic adenocarcinoma cells. Clin Cancer Res. 1999;5:119–127. [PubMed] [Google Scholar]
- 80.Woronicz J D, Gao X, Cao Z, Rothe M, Goeddel D V. IkappaB kinase-beta: NF-kappaB activation and complex formation with IkappaB kinase-alpha and NIK. Science. 1997;278:866–869. doi: 10.1126/science.278.5339.866. [DOI] [PubMed] [Google Scholar]
- 81.Wu M, Lee H, Bellas R E, Schauer S L, Arsura M, Katz D, FitzGerald M J, Rothstein T L, Sherr D H, Sonenshein G E. Inhibition of NF-κB/Rel induces apoptosis of murine B cells. EMBO J. 1996;15:4682–4690. [PMC free article] [PubMed] [Google Scholar]