Abstract
Epigenetic age acceleration is considered a measure of biological aging based on genome-wide patterns of DNA methylation. Although age acceleration has been associated with the incidence of diseases and death, less is known about how it is related to lifestyle behaviors. Among 2316 women, we evaluate associations between self-reported alcohol consumption and various metrics of epigenetic age acceleration. Recent average alcohol consumption was defined as the mean number of drinks consumed per week within the past year; lifetime average consumption was estimated as the mean number of drinks per year drinking. Whole-blood genome-wide DNA methylation was measured with HumanMethylation450 BeadChips and used to assess 4 epigenetic clocks (Hannum, Horvath, PhenoAge, and GrimAge) and their corresponding metrics of epigenetic age acceleration (Hannum AgeAccel, Horvath AgeAccel, PhenoAgeAccel, and GrimAgeAccel). Although alcohol consumption showed little association with most age acceleration metrics, both lifetime and recent average consumption measures were positively associated with GrimAgeAccel (lifetime, per additional 135 drinks/year: β = 0.30 years, 95% confidence interval [CI]: 0.11, 0.48, p = .002; recent, per additional 5 drinks/week: β = 0.19 years, 95% CI: 0.01, 0.37, p = .04). In a mutually adjusted model, only average lifetime alcohol consumption remained associated with GrimAgeAccel (lifetime, per additional 135 drinks/year: β = 0.27 years, 95% CI: 0.04, 0.50, p = .02; recent, per 5 additional drinks/week: β = 0.05 years, 95% CI: −0.16, 0.26, p = .64). Although alcohol use does not appear to be strongly associated with biological age measured by most epigenetic clocks, lifetime average consumption is associated with higher biological age assessed by the GrimAge epigenetic clock.
Keywords: Alcohol consumption, Biological age, DNA methylation, Epigenetic clock
Alcohol consumption has a broad range of health effects with evidence of both beneficial and deleterious consequences. For instance, although light to moderate alcohol use has been associated with lower risks of hypertension and frailty (1,2), alcohol consumption may also increase risks of breast cancer, comorbidity, and death (3,4). Molecular markers that track physiological changes and mortality risk, otherwise known as markers of “biological age,” may provide a unique perspective into the relationship between alcohol consumption and age-related health outcomes.
A variety of molecular markers are proposed to assess biological age (5,6); those based on genome-wide patterns of DNA methylation, or epigenetic clocks, are among the most studied (7). Epigenetic clocks use DNA methylation measured at different sets of cytosine-phosphate-guanine (CpG) dinucleotides located throughout the genome to estimate a person’s biological age (8). The difference between these biological age estimates and chronological age is termed “epigenetic age acceleration” and can have either positive or negative values (9). Age acceleration metrics based on the Hannum, Horvath, PhenoAge, and GrimAge epigenetic clocks have a varying association with physiological fitness, onset of disease, and death (10–14).
Associations between alcohol consumption and age acceleration metrics from earlier studies have been inconsistent and appear to be influenced by the interval for which alcohol use was assessed. For example, near-term measurements of alcohol intake, such as within the prior month, have demonstrated inverse associations with some metrics of age acceleration (13–15), whereas measures representing lifetime use have tended to show positive associations (16,17). Here, among a population of mostly low to moderate alcohol users, we use various metrics of epigenetic age acceleration to examine the collective health effects of both near-term and long-term measures of alcohol consumption.
Method
Study Population
The Sister Study is an ongoing, prospective cohort of 50 884 women recruited between 2003 and 2009 from the United States and Puerto Rico (18). Enrolled women completed a computer-assisted telephone interview that included information on demographics and lifestyle factors and a home visit where anthropomorphic measurements and whole-blood samples were collected. In July 2014, a case-cohort subsample of 2878 women was selected for genome-wide DNA methylation analysis; to limit confounding by ancestry, only non-Hispanic White women were eligible for selection. In total, 1336 women were randomly selected from the full Sister Study cohort. An additional 1542 women were selected because they developed incident breast cancer in the years after enrollment. Information about obtaining Sister Study data can be found at https://sisterstudy.niehs.nih.gov/English/coll-data.htm. Written informed consent was obtained at the time of the home visit. The study was approved by the Institutional Review Boards of the National Institute of Environmental Health Sciences and the Copernicus Group.
Alcohol Consumption Measurements
A woman’s history of alcohol consumption was obtained within 1 year of blood draw as part of the computer-assisted telephone interview. Women reported information including the age at which they started and stopped drinking alcohol. The frequency of alcohol consumption was reported as days per week, month, or year by decade of life. To assess the intensity of alcohol use, women were also asked about how many drinks they tended to consume on days in which they drank and separately about episodes of binge drinking. We focus our main analyses on recent average use, defined as the mean number of drinks consumed per week within the past 12 months, and lifetime average use, defined as the mean number of drinks consumed each year the woman reported drinking. Former drinkers were assigned zero values for average recent alcohol use and never drinkers were assigned zero values for both average recent and average lifetime alcohol use.
Methylation Processing and Age Acceleration Calculation
Details on the DNA methylation assessment have been reported (19). Methylation data preprocessing was completed using the ENmix R software package (20). Of the 2878 methylation samples, 102 were excluded due to poor quality (19). For the remaining 2776 samples, 4 epigenetic clocks (Hannum, Horvath, PhenoAge, and GrimAge) were calculated using an online calculator (https://dnamage.genetics.ucla.edu/home).
Age acceleration represents the difference between epigenetic age and chronological age (9). It is calculated by regressing epigenetic age on chronological age using normal linear regression models and calculating the residuals. The age acceleration residuals derived using the 4 epigenetic clocks are referred to as Hannum AgeAccel, Horvath AgeAccel, PhenoAgeAccel, and GrimAgeAccel. Two additional metrics were calculated by the online calculator which account for blood cell composition, whereby the underlying cell types were derived from the methylome of the sample and were not measured independently in peripheral blood mononuclear cells (9): Extrinsic epigenetic age acceleration (EEAA) is the Hannum AgeAccel metric upweighted by estimated naïve and exhausted CD 8+ T-cell and plasmablast abundances; intrinsic epigenetic age acceleration (IEAA) is constructed by regressing epigenetic age estimated by the Horvath epigenetic clock on chronological age and 6 white blood cell proportion estimates (CD8+ and CD4+ T cells, B cells, monocytes, natural killers, and granulocytes). Thus, IEAA represents a version of the Horvath AgeAccel metric that is adjusted for blood cell composition.
Statistical Analysis
In both descriptive and regression analyses, we accounted for the case-cohort sampling scheme by applying inverse probability of selection weights to the noncases (noncase weight assigned = 32.9; case weight assigned = 1), thereby standardizing the methylation subsample to approximate the full population of non-Hispanic White women enrolled in the Sister Study (Supplementary Table 1). We described the sample population characteristics using survey-weighted means and standard deviations (SDs) or survey-weighted proportions overall and stratified by median recent and lifetime alcohol use. We examined the weighted correlation of the average lifetime and recent alcohol consumption measures using Pearson’s correlation coefficient. In analyses of alcohol consumption and age acceleration, we used linear regression models to estimate β-coefficients and 95% confidence intervals (CIs) treating the alcohol measure (lifetime or recent average intakes, scaled per 1-SD increase in the unweighted population) as the independent variable and the age acceleration metric as the dependent variable.
We first tested associations with the age acceleration metrics using separate weighted linear regression models for recent and lifetime average alcohol consumption variables. Then, to determine whether the age acceleration associations were more sensitive to recent or lifetime alcohol use, we used a single, mutually adjusted model that included both alcohol consumption measurements together. We focused analyses on the 4 main measures of age acceleration (Hannum AgeAccel, Horvath AgeAccel, PhenoAgeAccel, and GrimAgeAccel). As a supplemental analysis, we examined average recent and lifetime alcohol consumption associations with the 8 components of the GrimAge epigenetic clock (ie, DNAm estimators of adrenomedullin, beta-2-microglobulin, cystatin C, growth differentiation factor 15, leptin, plasminogen activator inhibitor 1 [PAI-1], tissue inhibitor metalloproteinase 1, and smoking pack-years). We further explored age acceleration associations with current drinking status (never, former, and current), intensity of recent use (drinks per day on days drinking in the prior 12 months), and lifetime number of binges.
In our main analyses, we used model adjustment to account for potential confounding by education attainment (less than college, some college, advanced degree), body mass index (continuous, kg/m2), waist-to-hip ratio (continuous), smoking status (current, former, and never), and physical activity (continuous, metabolic equivalent tasks/week). To avoid the disproportional effect of outliers, we excluded participants if their age acceleration or alcohol intakes were greater than 4 standard deviations from the mean (age acceleration: n = 3; alcohol intake, n = 1) (21). To determine whether associations were driven by women with the highest alcohol use, we explored associations with the alcohol consumption measures as quintiles. We excluded women if they were missing information on alcohol consumption measures (n = 433) or confounders (n = 23). Our final analytic set included 2316 women. All analyses were conducted using Stata version 16 (College Station, TX).
Results
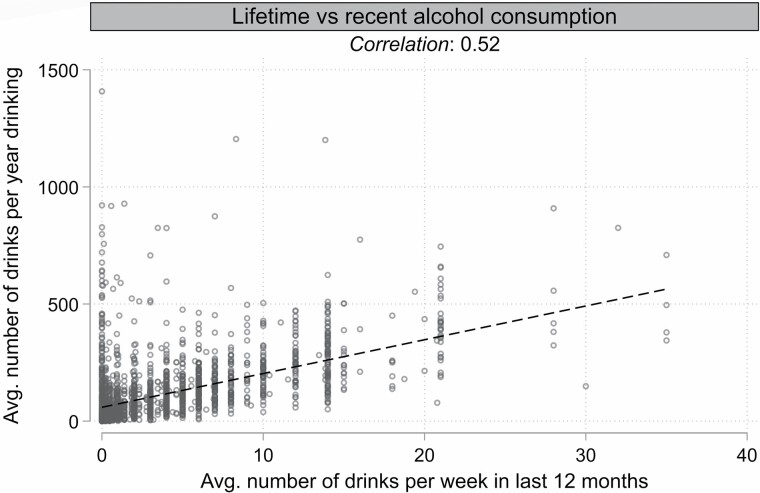
Of the women in the weighted sample, 97% reported ever drinking alcohol and 88% reported consuming alcohol within the past year (Table 1). In the last 12 months, the women reported a mean of 3.4 drinks per week (recent average consumption) and, for each year they reported drinking, a mean of 108 drinks per year (lifetime average consumption over a mean of 35 drinking-years). All women were cancer-free at blood draw and a majority of the women had more than high school education, were never smokers, and were postmenopausal at enrollment. Average recent and lifetime alcohol consumption were positively correlated (weighted ρ = 0.52, p < .001; Figure 1). Chronological age was positively correlated with all 4 epigenetic clocks (all weighted ρ > 0.85, all p < .001; Supplementary Figure 1) but, as expected by design, was not correlated with any of the age acceleration metrics (all weighted ρ < 0.05, all p > .05; Supplementary Figure 2). Hannum AgeAccel was highly correlated with its cell type-modified derivative, EEAA (ρ = 0.96, p < .001); similarly, Horvath AgeAccel was highly correlated with its cell type-modified derivative, IEAA (ρ = 0.96, p < .001). The age acceleration metrics were otherwise moderately to weakly correlated (ρ < 0.5; Supplementary Figure 3).
Table 1.
Weighted Participant Characteristics of the Sister Study Methylation Subsample (N = 2316)
| Average Recent Alcohol Use | Average Lifetime Alcohol Use | ||||
|---|---|---|---|---|---|
| Overall | < Median | > Median | < Median | > Median | |
| Age, years (SD) | 55.3 (9) | 55.4 (9) | 55.2 (9) | 55.6 (9) | 55.0 (9) |
| Body mass index, kg/m2 (SD) | 27.1 (6) | 27.9 (6) | 25.9 (5) | 27.7 (6) | 26.4 (5) |
| Waist-to-hip ratio (SD) | 0.80 (0.1) | 0.81 (0.1) | 0.80 (0.1) | 0.80 (0.1) | 0.81 (0.1) |
| Current physical activity, METs/week (SD) | 52.9 (32) | 52.4 (32) | 53.7 (33) | 53.8 (32) | 52.0 (33) |
| Education, % | |||||
| High school degree/GED | 15 | 17 | 13 | 15 | 16 |
| Attended college | 60 | 59 | 60 | 61 | 58 |
| Advanced degree | 25 | 24 | 26 | 24 | 26 |
| Smoking status, % | |||||
| Never | 50 | 55 | 43 | 59 | 40 |
| Former | 42 | 37 | 49 | 34 | 51 |
| Current | 8 | 8 | 7 | 7 | 9 |
| Menopause status, % | |||||
| Premenopausal | 36 | 35 | 37 | 34 | 38 |
| Postmenopausal | 64 | 65 | 63 | 66 | 62 |
| Ever drinkers, % | 97 | 94 | 100 | 93 | 100 |
| Current drinkers, % | 88 | 80 | 100 | 83 | 93 |
| Duration of alcohol use, years (SE)* | 35 (11) | 33 (11) | 37 (9) | 33 (11) | 36 (9) |
| Recent alcohol use, drinks/week (SD) | 3.4 (4.5) | 0.7 (0.7) | 7.3 (4.7) | 1.2 (1.6) | 5.7 (5.3) |
| Lifetime alcohol use, drinks/year drinking (SD) | 108 (124) | 68 (111) | 164 (121) | 29 (21) | 190 (134) |
Notes: METs = metabolic equivalent tasks; GED = general education development; SD = standard deviation. Characteristic proportions and means are weighted to correct for the case-cohort sampling of the methylation subsample.
*Among ever drinkers.
Figure 1.
Weighted correlation between lifetime and recent alcohol consumption. Scatterplot and linear fit line for self-reported lifetime and recent alcohol consumption. Although women who reported higher recent alcohol consumption were more likely to have higher lifetime estimates, the Pearson correlation coefficient was modest (ρ = 0.52).
In separate models accounting for confounders, both lifetime and recent alcohol consumption were positively associated with GrimAgeAccel (lifetime, per additional 135 drinks/year: β = 0.30 years, 95% CI: 0.11, 0.48, p = .002; recent, per additional 5 drinks/week: β = 0.19 years, 95% CI: 0.01, 0.37, p = .04; Table 2). However, in a mutually adjusted model that included both lifetime and recent measures of alcohol consumption, only lifetime alcohol consumption remained associated (lifetime, per additional 135 drinks/year: β = 0.27 years, 95% CI: 0.04, 0.50, p = .02; recent, per additional 5 drinks/week: β = 0.05 years, 95% CI: −0.16, 0.26, p = .64). Without adjustment for confounders, GrimAgeAccel showed stronger associations with alcohol use (Supplementary Table 2). Associations also appeared stronger among premenopausal women (Supplementary Table 3) and may be predominately driven by those reporting the highest alcohol use (Supplementary Table 4). The cell-type-modified age acceleration metrics (EEAA, IEAA) showed similar associations as the unmodified metrics (Hannum AgeAccel, Horvath AgeAccel; Supplementary Table 5). When the separate GrimAgeAccel components were tested with recent and lifetime consumption, in the mutually adjusted model, associations were strongest for lifetime use and the estimator for PAI-1 (β = 192, 95% CI: 51, 333, p = .008; Supplementary Table 6).
Table 2.
Survey-Weighted Alcohol Consumption Associations with the 4 Epigenetic Age Acceleration Metrics (N = 2316)
| Hannum AgeAccel | Horvath AgeAccel | PhenoAgeAccel | GrimAgeAccel | |||||
|---|---|---|---|---|---|---|---|---|
| β (95% CI) | p | β (95% CI) | p | β (95% CI) | p | β (95% CI) | p | |
| Separate alcohol consumption models | ||||||||
| Lifetime use | ||||||||
| Per additional 135 drinks/year | 0.24 (−0.07, 0.55) | .12 | −0.20 (−0.49, 0.08) | .16 | 0.13 (−0.25, 0.52) | .50 | 0.30 (0.11, 0.48) | .002 |
| Recent use | ||||||||
| Per additional 5 drinks/week | 0.23 (−0.08, 0.54) | .14 | −0.19 (−0.46, 0.08) | .16 | 0.02 (−0.34, 0.38) | .91 | 0.19 (0.01, 0.37) | .04 |
| Mutually adjusted alcohol consumption models | ||||||||
| Lifetime use | ||||||||
| Per additional 135 drinks/year | 0.17 (−0.18, 0.52) | .35 | −0.14 (−0.49, 0.20) | .41 | 0.17 (−0.30, 0.63) | .49 | 0.27 (0.04, 0.50) | .02 |
| Recent use | ||||||||
| Per additional 5 drinks/week | 0.15 (−0.21, 0.51) | .42 | −0.12 (−0.44, 0.21) | .48 | −0.06 (−0.50, 0.37) | .77 | 0.05 (−0.16, 0.26) | .64 |
Notes: METs = metabolic equivalent tasks; GED = general education development. All models adjust for education level (high school/GED, attended college, and advanced degree), body mass index (continuous, kg/m2), waist-to-hip ratio (continuous, ratio), smoking status (never, former, and current), and physical activity (continuous, METs/week).
Analyses of age acceleration with additional alcohol measures are reported in Supplementary Table 7. After accounting for average lifetime use, current drinking status was associated with greater Hannum AgeAccel (β = 1.25 years, 95% CI: 0.00, 2.49, p = .05) and former drinking status was associated with elevated GrimAgeAccel (compared to never drinkers, GrimAgeAccel: β = 1.05 years, 95% CI: 0.14, 1.7, p = .02; Supplementary Table 7). Associations with the intensity of recent alcohol use were similar to associations with average recent use, with one exception: greater intensity of recent use was associated with Hannum AgeAccel (per additional drink/day on days drinking: β = 0.26 years, 95% CI: 0.00, 0.51, p = .05). None of the age acceleration metrics was related to the number of lifetime binges.
Discussion
We find that alcohol consumption is most consistently associated with epigenetic age acceleration measured by the GrimAge epigenetic clock, especially among those reporting the highest alcohol use, and confirm the earlier finding by Zhao et al. (22) between alcohol consumption and the GrimAge component PAI-1. Although both recent and lifetime alcohol use measures showed positive associations with GrimAgeAccel, in a mutually adjusted model, only associations with lifetime use remained. We did not find that average alcohol use measures, either recent or lifetime, were meaningfully related to age acceleration based on the Hannum, Horvath, or PhenoAge epigenetic clocks. However, the Hannum clock showed associations with current drinking status and intensity of recent alcohol use. Although the possibility of residual confounding cannot be excluded, alcohol consumption appears to have modest associations with some methylation-based metrics of biological age.
Associations between alcohol consumption and metrics of age acceleration have been inconsistent across studies and may be related to the interval for which alcohol exposures were assessed (13–17,22,23). Specifically, self-reported drinks per week have been shown to have mixed associations with Hannum AgeAccel, Horvath AgeAccel, and PhenoAgeAccel, before and after accounting for confounders (13,15,23). Associations for recent alcohol use with GrimAgeAccel have also been inconsistent with reports of both inverse (14) and positive associations (22). While associations with self-reported lifetime alcohol use measures have not been examined in prior studies, clinical alcohol dependence was positively associated with Horvath AgeAccel and PhenoAgeAccel (16,17). Although GrimAgeAccel has not previously been examined with self-reported measures of long-term alcohol consumption, it was associated with average lifetime alcohol use in this study and has previously been reported to be a marker of fatty liver disease, a condition primarily caused by heavy, chronic alcohol consumption (14).
Methodological differences in the design of the epigenetic clocks may explain the varying associations with the history of alcohol consumption (13,14,24,25). The Hannum and Horvath epigenetic clocks were designed by selecting sets of CpGs where methylation is associated with chronological age (24,25). Prior studies suggest that these clocks have little association with lifestyle behaviors that are linked with mortality, such as smoking or physical inactivity (15,26). Conversely, the PhenoAge and GrimAge clocks use CpG site methylation to predict phenotypes associated with higher mortality risk (13,14). The PhenoAge epigenetic clock uses methylation to predict a mortality risk score based on nine clinical biomarkers and chronological age, whereas the GrimAge clock estimates mortality risk using a combination of chronological age, sex, and 8 separate DNA methylation estimators (7 estimators of plasma proteins and 1 for smoking pack-years). Thus, the 4 epigenetic clocks may be capturing different aspects of aging and mortality risk, with only the GrimAge epigenetic clock and the PAI-1 DNAm estimator, reflecting molecular processes affected by long-term measures of alcohol consumption.
Supplementary Material
Acknowledgments
J.K.K. performed the analysis and J.K.K. and J.A.T. drafted the manuscript. Z.X. provided additional data support. A.M.M.L., E.L.G., Z.X., A.J.W., and D.P.S. provided critical comments during manuscript revisions. All authors approved the final version of the manuscript. The funding sponsor had no role in the analysis, drafting of the manuscript, or the decision to publish. We would like to thank Drs Kaitlyn Lawrence and Katie O’Brien for providing the internal review of the manuscript.
Funding
This work was supported by the Intramural Research Program of the National Institutes of Health, National Institute of Environmental Health Sciences (Z01-ES044005, Z01-ES049033, Z01-ES049032).
Conflict of Interest
None declared.
References
- 1. Piano MR. Alcohol’s effects on the cardiovascular system. Alcohol Res. 2017;38:219–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Shah M, Paulson D, Nguyen V. Alcohol use and frailty risk among older adults over 12 years: the Health and Retirement Study. Clin Gerontol. 2018;41:315–325. doi: 10.1080/07317115.2017.1364681 [DOI] [PubMed] [Google Scholar]
- 3. White AJ, DeRoo LA, Weinberg CR, Sandler DP. Lifetime alcohol intake, binge drinking behaviors, and breast cancer risk. Am J Epidemiol. 2017;186:541–549. doi: 10.1093/aje/kwx118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Moore AA, Giuli L, Gould R, et al. Alcohol use, comorbidity, and mortality. J Am Geriatr Soc. 2006;54:757–762. doi: 10.1111/j.1532-5415.2006.00728.x [DOI] [PubMed] [Google Scholar]
- 5. Belsky DW, Moffitt TE, Cohen AA, et al. Eleven telomere, epigenetic clock, and biomarker-composite quantifications of biological aging: do they measure the same thing? Am J Epidemiol. 2018;187:1220–1230. doi:. doi: 10.1093/aje/kwx346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Li X, Ploner A, Wang Y, et al. Longitudinal trajectories, correlations and mortality associations of nine biological ages across 20-years follow-up. eLife. 2020;9:e51507. doi: 10.7554/eLife.51507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jylhävä J, Pedersen NL, Hägg S. Biological age predictors. EBioMedicine. 2017;21:29–36. doi: 10.1016/j.ebiom.2017.03.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Horvath S, Raj K. DNA methylation-based biomarkers and the epigenetic clock theory of ageing. Nat Rev Genet. 2018;19:371–384. doi: 10.1038/s41576-018-0004-3 [DOI] [PubMed] [Google Scholar]
- 9. Chen BH, Marioni RE, Colicino E, et al. DNA methylation-based measures of biological age: meta-analysis predicting time to death. Aging (Albany, NY). 2016;8:1844–1865. doi: 10.18632/aging.101020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kresovich JK, Xu Z, O’Brien KM, Weinberg CR, Sandler DP, Taylor JA. Methylation-based biological age and breast cancer risk. J Natl Cancer Inst. 2019;111:1051–1058. doi: 10.1093/jnci/djz020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kresovich JK, Xu Z, O’Brien KM, Weinberg CR, Sandler DP, Taylor JA. Epigenetic mortality predictors and incidence of breast cancer. Aging (Albany, NY). 2019;11:11975–11987. doi: 10.18632/aging.102523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Marioni RE, Shah S, McRae AF, et al. The epigenetic clock is correlated with physical and cognitive fitness in the Lothian Birth Cohort 1936. Int J Epidemiol. 2015;44:1388–1396 doi: 10.1093/ije/dyu277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Levine ME, Lu AT, Quach A, et al. An epigenetic biomarker of aging for lifespan and healthspan. Aging (Albany, NY). 2018;10:573–591. doi: 10.18632/aging.101414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lu AT, Quach A, Wilson JG, et al. DNA methylation GrimAge strongly predicts lifespan and healthspan. Aging (Albany, NY). 2019;11:303–327 doi: 10.18632/aging.101684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Quach A, Levine ME, Tanaka T, et al. Epigenetic clock analysis of diet, exercise, education, and lifestyle factors. Aging (Albany, NY). 2017;9:419–446. doi: 10.18632/aging.101168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rosen AD, Robertson KD, Hlady RA, et al. DNA methylation age is accelerated in alcohol dependence. Transl Psychiatry. 2018;8:182. doi: 10.1038/s41398-018-0233-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Luo A, Jung J, Longley M, et al. Epigenetic aging is accelerated in alcohol use disorder and regulated by genetic variation in APOL2. Neuropsychopharmacology. 2020;45:327–336. doi: 10.1038/s41386-019-0500-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sandler DP, Hodgson ME, Deming-Halverson SL, et al. The Sister Study cohort: baseline methods and participant characteristics. Environ Health Perspect. 2017;125:127003 doi: 10.1289/EHP1923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kresovich JK, O’Brien KM, Xu Z, Weinberg CR, Sandler DP, Taylor JA. Prediagnostic immune cell profiles and breast cancer. JAMA Netw Open. 2020;3:e1919536. doi: 10.1001/jamanetworkopen.2019.19536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Xu Z, Niu L, Li L, Taylor JA. ENmix: a novel background correction method for Illumina HumanMethylation450 BeadChip. Nucleic Acids Res. 2016;44:e20 doi: 10.1093/nar/gkv907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kresovich JK, Harmon QE, Xu Z, Nichols HB, Sandler DP, Taylor JA. Reproduction, DNA methylation and biological age. Hum Reprod. 2019;34:1965–1973. doi: 10.1093/humrep/dez149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zhao W, Ammous F, Ratliff S, et al. Education and lifestyle factors are associated with DNA methylation clocks in older African Americans. Int J Environ Res Public Health. 2019;16:1341. doi: 10.3390/ijerph16173141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Fiorito G, McCrory C, Robinson O, et al. ; BIOS Consortium; Lifepath Consortium . Socioeconomic position, lifestyle habits and biomarkers of epigenetic aging: a multi-cohort analysis. Aging (Albany, NY). 2019;11:2045–2070. doi: 10.18632/aging.101900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hannum G, Guinney J, Zhao L, et al. Genome-wide methylation profiles reveal quantitative views of human aging rates. Mol Cell. 2013;49:359–367. doi: 10.1016/j.molcel.2012.10.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Horvath S. DNA methylation age of human tissues and cell types. Genome Biol. 2013;14:R115. doi: 10.1186/gb-2013-14-10-r115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kresovich JK, Garval EL, Martinez Lopez AM, et al. Body composition and physical activity associations with multiple measures of epigenetic age acceleration. Am J Epidemiol 2021;190(6):984–993. doi: 10.1093/aje/kwaa251 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.