Abstract
Backgrounds
To date, the effects of SARS-CoV-2 vaccines on people living with HIV (PLWH) were mainly focused on messenger RNA (mRNA) and adenovirus vector-based vaccines, and little is known about the effects of inactivated virus-based vaccine. This study was designed to determine the effects of inactivated SARS-CoV-2 vaccines on PLWH.
Methods
Twenty-four HIV-positive individuals and 24 healthy donors (HD) were respectively recruited from Malipo Country People's Hospital and community in Kunming city. Enumeration of lymphocyte and CD4+CD45RO+ memory T cells were evaluated by flow cytometry. Competitive ELISA was used to measure the level of Anti-SARS-CoV-2 neutralization antibody. Spearman or Pearson correlation analysis was used to analyze the relationship between laboratory indicators and neutralization antibodies in PLWH. T-cell responses (Th1, Th2, Th17, Treg) and intracellular expression of cytokines (IL-2 and TNF-α) in CD4 or CD8 were induced by spike protein in SARS-CoV-2 (SARS-2-S) and further measured by intracellular staining.
Results
CD4, B cells, CD4+CD45RO+ memory T cells in peripheral blood of PLWH are dramatically decreased in comparison with HD. Importantly, PLWH display comparable neutralizing antibody positive rate to HD after inoculation with inactivated SARS-CoV-2 vaccine. However, PLWH showed weaker responses to vaccines exhibited by lower levels of neutralizing antibodies. Correlation analysis shows that this is possibly caused by low number of CD4 and B cells. Furthermore, SARS-2-S-induced Th2 and Th17 responses are also decreased in PLWH, while no influences on Treg and other cytokines (IL-2, TNF-α and IFN-γ) observed.
Conclusions
PLWH and HD have comparable neutralizing antibodies positive rates, but PLWH display weaker responses to inactivated SARS-CoV-2 vaccines in magnitude, which suggests that a booster dose or dose adjustment are required for HIV-infected individuals, especially for those with lower counts of CD4 T and B cells.
Keywords: COVID-19, SARS-CoV-2 vaccines, Neutralizing antibodies, HIV
1. Introduction
Recently, the pandemic of coronavirus disease 2019 (COVID-19) has posed a serious threat to the global public health and economic development [1]. To date, there are no effective preventive measures in treating COVID-19. Therefore, it is urgent to develop safe and effective COVID-19 vaccines. Increasing evidences have indicated that various anti-COVID-19 vaccines are effective in both clinical trials and real-world settings [2], [3], [4], [5], [6]. Nevertheless, most data on safety and effectiveness of vaccines are derived from general population, and little information is known about the immunogenicity of inactivated SARS-CoV-2 vaccines in HIV-positive individuals. Previous studies are mainly focused on mRNA and adenovirus vector-based vaccines, suggesting that mRNA vaccines, such as BNT162b2, could develop neutralizing antibodies to SARS-CoV-2 RBD and specific T-cell responses in persons living with HIV. In the study of adenovirus vector-based vaccines, it was found that ChAdOx1 nCoV-19 was safe and immunogenic for HIV-positive individuals, and vaccine-induced immune responses were dependent on types of vaccines and levels of CD4+T cells. However, it is still unknown that whether inactivated SARS-CoV-2 vaccines can elicit immunogenicity in people living with HIV (PLWH). This has caused panic among PLWH, including risks of serious illness and vaccine safety, which greatly restricts the progress of vaccination in China.
Herein, we aim to investigate the immunogenicity of inactivated SARS-CoV-2 vaccine in PLWH, further analyze the correlation between immunogenicity and specific T-cell responses. Our study will provide valuable insights into immunogenicity of anti-COVID-19 vaccines in people living with HIV.
2. Methods
2.1. Study design and participants
Twenty-four HIV-positive individuals who have received two injections of inactivated SARS-CoV-2 vaccines (CoronaVac, 3 μg/0.5 mL; or BBIBP-CorV, 4 μg/0.5 mL) were recruited from Malipo Country People’s Hospital, Yunnan province, China. Twenty-four healthy donors who were injected twice with inactivated SARS-CoV-2 vaccines are recruited from community in Kunming city, Yunnan province, China. Each participant was injected with 0.5 mL of vaccines each time. The key exclusion criteria include no history of exposure to COVID-19 and positive PCR tests for SARS-CoV-2 from pharyngeal or anal swab samples before enrolment. Written informed consent was obtained from each participant before screening for eligibility. The clinical trial protocol and informed consent forms were approved by Yunnan provincial traditional medicine hospital Ethics Committee (NO. K [2021]015), and all protocols were carried out according to the International Conference on Harmonization.
2.2. Lymphocyte Enumeration
BD Multitest™ 6-color TBNK reagent(BD Pharmingen)with optional BD Trucount™ tubes was used to identify and determine the percentages and absolute counts of T, B, as well as the CD4 and CD8 subpopulations of T cells in peripheral blood. Briefly, peripheral whole blood samples were collected from participants on about 40 days after the second injection of vaccines. The samples were collected in EDTA tubes, and stained with BD Multitest™ 6-Color TBNK Reagent. After staining, the samples were fixed and collected by FACS Canto instrument and analyzed by Flowjo.
2.3. Anti-SARS-CoV-2 neutralization antibodies measurement
The measurement of anti-SARS-CoV-2 neutralization antibodies were performed using a competitive ELISA kit following manufacturer’s instructions. Briefly, serum samples were collected from participants on about 40 days after the second injection of vaccines for the measurement of Anti-SARS-CoV-2 neutralization antibody. The microplates in the kit have been pre-coated with Human ACE2 Protein, and then 50 µL of serum samples, positive control and negative control were added to the wells followed by addition of HRP- SARS-CoV-2 Spike RBD. They were incubated at 37 °C for 1 h. After incubation, plate was washed three times and 50 µL of substrates were added to the well and incubated at 37 °C for 20 min. The reaction was terminated by the addition of stop solution and OD450 nm was measured. The neutralizing antibodies in samples will compete with ACE2 to bind to HRP-SARS-CoV-2 Spike RBD. The intensity of assay signal decreases proportionally due to the presence of anti-SARS-CoV-2 neutralizing antibody. Interpretation of results: percent inhibition= (1- OD 450 of sample/ OD 450 of negative control) × 100%. Positive: percent inhibition≧20% means anti-SARS-CoV-2 neutralizing antibody detected. Negative: percent inhibition < 20% means anti-SARS-CoV-2 neutralizing antibody not detected.
2.4. T Cell phenotyping
About 40 days after the second injection of vaccine, peripheral blood were collected for the measurement of memory T cells (CD3+CD4+CD45RO+ memory T cells), T-cell responses (Th1, Th2, Th17 and Treg) and intracellular cytokines expressions (IL-2 and TNF-α) in response to spike protein of SARS-CoV-2 (SARS-2-S). For measurement of memory T cells, 10 µL of blood samples were blocked with mouse-anti-human CD16/CD32 and were then incubated for 15 min at 4 °C with the following mAbs diluted in PBS with 0.2% BSA: FITC-conjugated-anti-human CD3 (HIT3a, Biolegend), PE-Cy7-conjugated anti-human CD4 (RPA-T4, Biolegend), and APC-conjugated anti-human CD45RO (UCHL1, Biolegend). The stained samples were then treated with BD FACS lysing solution to lyses erythrocytes under gentle hypotonic conditions. Finally, the cells were washed once with staining buffer, and then fixed, and harvested on FACS Canto™ II (BD Biosciences).
Intracellular staining of cytokines was performed according to the method previously described [7]. Briefly, peripheral blood mononuclear cells (PBMC) from COVID-19 vaccinated individuals were isolated by density gradient centrifugation, and the resulting PBMC (5 × 105) were restimulated for 24 h with SARS-CoV-2 S defined peptide pool (4 μg/ml) containing 100 peptides from the human SARS-CoV-2 virus (Mabtech AB) in the presence of Brefeldin A (10 μg/ml final, added in the last 8 h of incubation, Sigma-Aldrich). At the end of incubation, cells were collected and blocked with mouse-anti-human CD16/CD32, and then were stained with PE-Cy7-anti-human CD4 antibodies (RPA-T4, Biolegend) and APC-anti-human CD8 antibodies (SK1, Biolegend). After surface staining, cells were washed, fixed, permeabilized and intracellularly stained with PE anti-human IL-17A (BL168, Biolegend), PerCP/Cyanine5.5 anti-human IL-4 (MP4-25D2), Pacific Blue™ anti-human TNF-α (Mab11, Biolegend), FITC-anti-human IL-2 (MQ1-17H12, Biolegend), and APC/Cy7-anti-human IFN-γ(4S.B3, Biolegend). The above stimulations were not required for staining Foxp3 based on intracellular cytokines staining protocols. Samples were acquired on FACS Canto™ II (BD Biosciences) and analyzed using FlowJo software (Tree Star).
2.5. Statistical analysis
SPSS 20.0 software was used to perform the statistical analysis. Kolmogorov-Smirnov test was used to analyze whether the variables followed a normal distribution. If the variables were normally distributed, they were presented as mean ± SEM (standard error of the mean). Two independent samples tests were used for comparison between the two groups (PLWH versus HD). If they were not normally distributed, they were presented as Median (Q1-Q3) and non-parametric test was adopted. The enumeration data were described by frequency and percentage and compared by χ2 test. Pearson or Spearman correlation analysis was used to analyze the correlation between laboratory indicators and neutralizing antibodies. P < 0.05 was considered to be statistically significant.
3. Results
3.1. General information
Participants’ characteristics were summarized in Table.1 . In this study, we enrolled 48 participants, and all participants have received two injections of vaccines. Twenty-four healthy donors have negative HIV test results, while 24 participants living with HIV have positive HIV test results. 16 of 24 (64%) participants in HD were male, while 12 of 24 (50%) participants in HIV-positive group were male. There is no significant difference between HD and PLWH in age and sex (P > 0.05). None of these individuals had experienced serious adverse events, and the most commonly reported adverse reactions were pain at the site of vaccination.
Table 1.
Comparisons of demographic characteristics between the two groups.
| Variable | HD(n = 24) | PLWH(n = 24) | Z/χ2 | P |
|---|---|---|---|---|
| Age, [Median(Q3-Q1), years] | 37.00(26.25,47.25) | 44.00(39.00,48.75) | −1.796 | 0.072 |
| Gender | ||||
| Male, n(%) | 15(62.5%) | 12(50.0%) | 0.762 | 0.383 |
| Female, n(%) | 9(37.5%) | 12(50.0%) |
3.2. Lymphocyte Enumeration
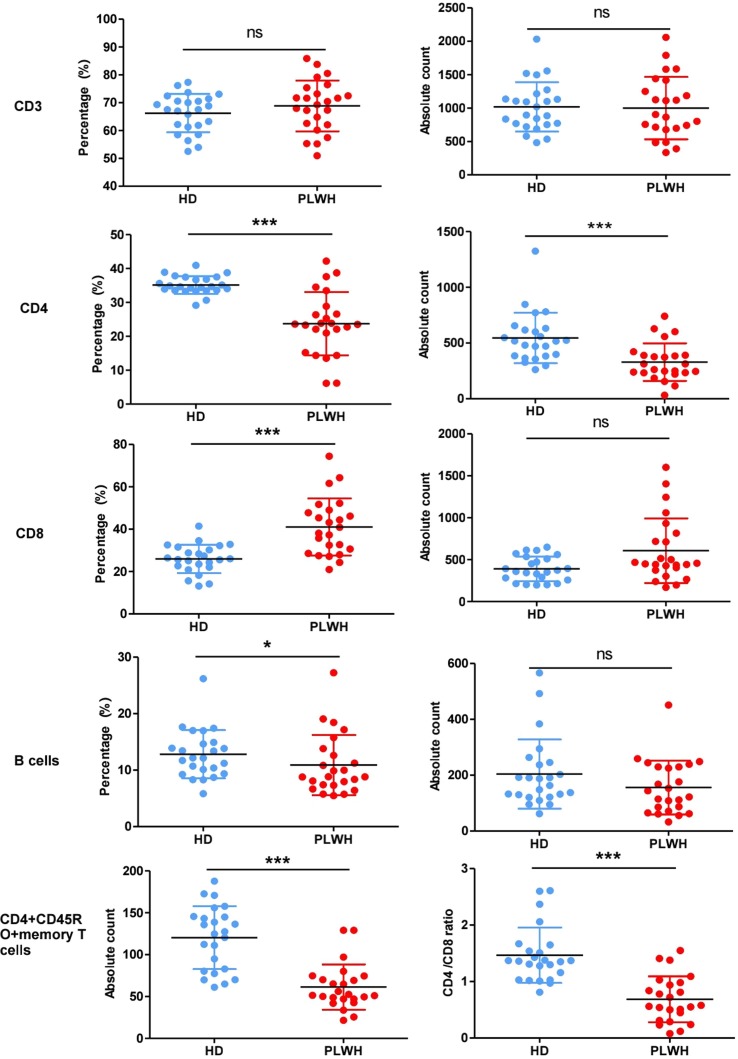
In order to induce specific antibody production, B cells require “help” from CD4+ T cells and have an important impact on antibody production. Thus, we analyzed the counts of lymphocytes using BD Multitest™ 6-color TBNK reagent. Surface marker staining showed that in comparison with HD, both percent and absolute counts of CD4 and CD4+CD45RO+ memory T cells in PLWH were dramatically decreased (P < 0.001), and the percent of B cells and ratio of CD4/CD8 were also obviously decreased, which were shown in Fig. 1 .
Fig.
1. Enumeration of lymphocytes in peripheral whole blood. Peripheral whole blood samples were collected from participants and then were stained with 6-Color TBNK Reagent or APC-conjugated anti-human CD45RO to determine the counts of lymphocytes using a flow cytometer. The expression of CD3 (A), CD4 (B), CD8 (C), B cells (D) and CD4 + CD45RO + memory T cells (E) were analyzed, and CD4/CD8 ratio (F) were calculated. N = 24, *P < 0.05, **P < 0.01.
3.3. Neutralizing antibodies and correlation analysis
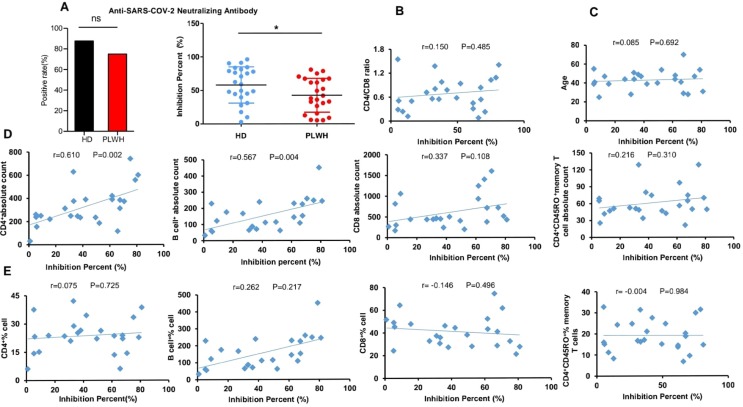
Neutralizing antibodies against SARS-CoV-2 Spike RBD were observed on about 40 days after participant's second injection (Fig. 2 ). Twenty-four (87.50%) participants in HD group are positive, and 19 (79.17%) participants in PLWH are positive. There is no significant difference between HD and PLWH in neutralizing antibody positive rates. However, we found that OD450 are higher in HD than in PLWH. Meanwhile, compared with HD, a significant decrease in the percentage inhibition was observed in PLWH group (P < 0.05), suggesting that PLWH’s immunogenicity to inactivated SARS-CoV-2 vaccine was smaller in magnitude. Therefore, a booster dose or dose adjustment might be needed for PLWH. Furthermore, the associations between neutralizing antibodies and levels of laboratory indicators were evaluated using Spearman or Pearson analysis. As showed in Fig. 2, a positive correlation was found between neutralizing antibodies and CD4 cells counts and B cells counts. Although the counts of CD4+CD45RO+ memory T cells and the ratio of CD4/CD8 were significantly decreased in PLWH, there were no significant associations between the counts of CD4+CD45RO+ memory T cells (P = 0.310), CD4 /CD8 ratio and neutralizing antibodies (P = 0.485).
Fig.
2. Neutralizing antibodies measurement and correlation analysis. Neutralizing antibodies were observed on 40 days after participants’ second injection using a competitive ELISA (A). Spearman or Pearson correlation analysis was used to analyze the correlation between laboratory indicators and neutralizing antibodies in PLWH (B-E). N = 24, *P < 0.05, **P < 0.01.
3.4. SARS-2-S-induced specific T cell response exhibited by intracellular expression of cytokines
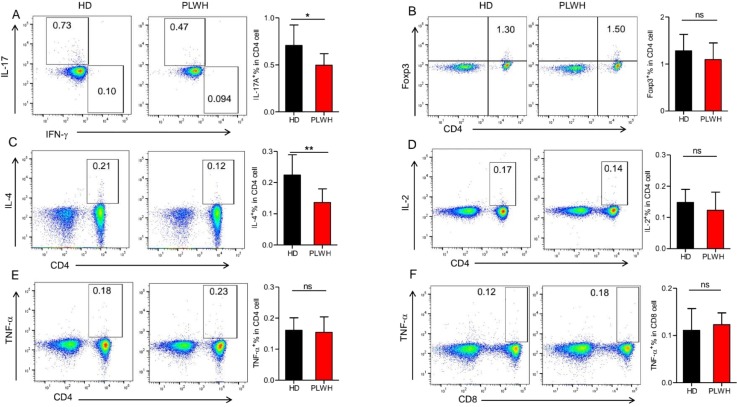
CD4+ T cells are crucial in orchestrating both the humoral and cellular immune responses, and have an important impact on antibody production. Effector CD4+ T cells could be divided into Th1, Th2, Th17, and Treg subsets according to their function. To further investigate whether the vaccine can induce different T-cell responses, we measured the expression of cytokines in CD4+ and CD8+T cells by using flow cytometry, as showed in Fig. 3 . PLWH exhibited significantly decreased expression of IL-17A and IL-4 in CD4+T cells than in HD. However, there were no significant differences in expression of IFN-γ, IL-2 and Foxp3 in CD4+T cells between the two groups, and no significant differences were found in expression of IL-2 and TNF-α in CD8+T cells.
Fig.
3. Specific T-cell responses to vaccination with inactivated SARS-CoV-2 vaccine. PBMC from participants were isolated by density gradient centrifugation, and the treated PBMC were restimulated for 24 h with SARS-CoV-2 S defined peptide pool in the presence of Brefeldin A (during the last 8 h). T cells responses (Th1, Th2, Th17 and Treg) and intracellular cytokines (IL-2 and TNF-α) expressions were observed by intracellular staining. Samples were acquired on a flow cytometer and analyzed using FlowJo software. The results were presented as mean ± SEM (standard error of the mean). N = 15, *P < 0.05, **P < 0.01.
4. Discussion
In HIV-infected individuals, cellular immunity is impaired due to the reduction or loss of CD4+ T lymphocytes. Previous reports have also revealed that immune responses to vaccines (eg, H1N1 2009 vaccine) in people living with HIV-1 are inferior to those in the general population [8]. Both neutralizing antibodies and T-cell responses were important in eliminating the virus and controlling disease development in patients with COVID-19 [9]. Thus, in the present study, we assessed the immunogenicity of inactivated SARS-CoV-2 vaccines in HIV positive individuals. It was found that inactivated SARS-CoV-2 vaccines exerted similar neutralizing antibodies positive rates on HIV positive individuals and HIV-negative controls. However, the magnitude of immunogenicity was smaller in HIV-positive individuals than HIV-negative controls. Correlation analysis further reveals that there is positive correlation between neutralizing antibodies and CD4 cells and B cells counts, which is consistent with the study that HIV-positive individuals with the lowest CD4 counts have a weaker response induced by SARS-CoV-2 mRNA vaccination. Neutralization antibodies are critical to eliminate SARS-CoV-2, which can reduce morbidity and mortality. Therefore, in order to achieve similar antiviral effects, a booster dose or dose adjustment might be needed when injecting HIV positive individuals with inactivated SARS-CoV-2 vaccines, particularly for those with lower CD4+T cells counts.
Aside from neutralizing antibodies, vaccine-induced T-cell responses are pivotal in directly attacking and killing virus-infected cells [10]. Thus, we determined the expression of cytokines in CD4+ and CD8+T cells. It was found that the expressions of Th2 and Th17 cytokines were lower in people living with HIV compared with HD, while other cytokines had no significant changes. Recent studies have demonstrated that Th17 proinflammatory responses might also contribute to an important component of vaccine-induced immune memory [11]. IL-4, mainly derived from Th2 cells, has crucial influence on production of immunoglobulin, particularly IgG1 [12] . However, it is still not completely clear that lack of adequate Th2 and Th17 is responsible for weaker T-cell responses in PLWH. More research will be needed to confirm our finding. This study still has several limitations. Firstly, the enrolled sample of participants with HIV-positive results is relatively small. This led to an inability to differentiate according to their CD4 counts. Secondly, in the current study, we only measured the immunogenicity of inactivated SARS-CoV-2 vaccine on about 40 days after the second injection, and long-term study, such as 6 months to 1 year follow-up study should be performed.
In summary, our study indicated that in HIV-infected individuals, inactivated SARS-CoV-2 vaccines could elicit comparable neutralizing antibodies positive rates to HIV-negative population, but HIV-infected individuals displayed weaker responses to inactivated SARS-CoV-2 vaccines in magnitude. Furthermore, the lower counts of CD4 cells and B cells may be responsible. This implies that a booster dose or dose adjustment is required for HIV-infected individuals, especially for those with lower CD4+T cells and B cells counts.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgements
We thank the Zheng lab, Kunming Institute of Zoology, Chinese Academy of Sciences, for their assistance in detecting specific T-cell responses to vaccination.
Funding
This work was financially supported by the National Natural Science Foundation of China (81960754), Yunnan Provincial Department of Education Teacher Science and Technology Project] under Grant Agreement (2020 J0290).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.intimp.2021.108383.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- 1.Sanders J.M., Monogue M.L., Jodlowski T.Z., Cutrell J.B. Pharmacologic Treatments for Coronavirus Disease 2019 (COVID-19): a Review. JAMA. 2020;323(18):1824–1836. doi: 10.1001/jama.2020.6019. [DOI] [PubMed] [Google Scholar]
- 2.Zhu F.C., Guan X.H., Li Y.H., Huang J.Y., Jiang T., Hou L.H., Li J.X., Yang B.F., Wang L., Wang W.J., Wu S.P., Wang Z., Wu X.H., Xu J.J., Zhang Z., Jia S.Y., Wang B.S., Hu Y., Liu J.J., Zhang J., Qian X.A., Li Q., Pan H.X., Jiang H.D., Deng P., Gou J.B., Wang X.W., Wang X.H., Chen W. Immunogenicity and safety of a recombinant adenovirus type-5-vectored COVID-19 vaccine in healthy adults aged 18 years or older: a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet. 2020;396(10249):479–488. doi: 10.1016/S0140-6736(20)31605-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang Y., Zeng G., Pan H., Li C., Hu Y., Chu K., Han W., Chen Z., Tang R., Yin W., Chen X., Hu Y., Liu X., Jiang C., Li J., Yang M., Song Y., Wang X., Gao Q., Zhu F. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine in healthy adults aged 18–59 years: a randomised, double-blind, placebo-controlled, phase 1/2 clinical trial. Lancet Infect. Dis. 2021;21(2):181–192. doi: 10.1016/S1473-3099(20)30843-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Paris C., Perrin S., Hamonic S., Bourget B., Roué C., Brassard O., Tadié E., Gicquel V., Bénézit F., Thibault V., Garlantézec R., Tattevin P. Effectiveness of mRNA-BNT162b2, mRNA-1273, and ChAdOx1 nCoV-19 vaccines against COVID-19 in healthcare workers: an observational study using surveillance data. Clin Microbiol Infect. 2021;27(11) doi: 10.1016/j.cmi.2021.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dagan N., Barda N., Kepten E., Miron O., Perchik S., Katz M.A., Hernán M.A., Lipsitch M., Reis B., Balicer R.D. BNT162b2 mRNA Covid-19 vaccine in a nationwide Mass vaccination setting. N Engl J Med. 2021;384(15):1412–1423. doi: 10.1056/NEJMoa2101765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xia S., Zhang Y., Wang Y., Wang H., Yang Y., Gao G.F., Tan W., Wu G., Xu M., Lou Z., Huang W., Xu W., Huang B., Wang H., Wang W., Zhang W., Li N., Xie Z., Ding L., You W., Zhao Y., Yang X., Liu Y., Wang Q., Huang L., Yang Y., Xu G., Luo B., Wang W., Liu P., Guo W., Yang X. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBIBP-CorV: a randomised, double-blind, placebo-controlled, phase 1/2 trial. Lancet Infect. Dis. 2021;21(1):39–51. doi: 10.1016/S1473-3099(20)30831-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhu F.C., Li Y.H., Guan X.H., Hou L.H., Wang W.J., Li J.X., Wu S.P., Wang B.S., Wang Z., Wang L., Jia S.Y., Jiang H.D., Wang L., Jiang T., Hu Y., Gou J.B., Xu S.B., Xu J.J., Wang X.W., Wang W., Chen W. Safety, tolerability, and immunogenicity of a recombinant adenovirus type-5 vectored COVID-19 vaccine: a dose-escalation, open-label, non-randomised, first-in-human trial. Lancet. 2020;395(10240):1845–1854. doi: 10.1016/S0140-6736(20)31208-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tebas P., Frank I., Lewis M., Quinn J., Zifchak L., Thomas A., Kenney T., Kappes R., Wagner W., Maffei K., Sullivan K. Poor immunogenicity of the H1N1 2009 vaccine in well controlled HIV-infected individuals. AIDS. 2010;24(14):2187–2192. doi: 10.1097/QAD.0b013e32833c6d5c. [DOI] [PubMed] [Google Scholar]
- 9.Tay M.Z., Poh C.M., Rénia L., MacAry P.A., Ng L.F.P. The trinity of COVID-19: immunity, inflammation and intervention. Nat. Rev. Immunol. 2020;20(6):363–374. doi: 10.1038/s41577-020-0311-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grifoni A., Weiskopf D., Ramirez S.I., Mateus J., Dan J.M., Moderbacher C.R., Rawlings S.A., Sutherland A., Premkumar L., Jadi R.S., Marrama D., de Silva A.M., Frazier A., Carlin A.F., Greenbaum J.A., Peters B., Krammer F., Smith D.M., Crotty S., Sette A. Targets of T Cell responses to SARS-CoV-2 coronavirus in Humans with COVID-19 disease And unexposed individuals. Cell. 2020;181(7):1489–1501.e15. doi: 10.1016/j.cell.2020.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Simon H.-U., Karaulov A., Bachmann M. Strategies to prevent SARS-CoV-2-mediated eosinophilic disease in association with COVID-19 vaccination and Infection. Int Arch Allergy Immunol. 2020;181(8):624–628. doi: 10.1159/000509368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Isakson P.C., Puré E., Vitetta E.S., Krammer P.H. T cell-derived B cell differentiation factor(s)effect on the isotype switch of murine B cells. J Exp Med. 1982;155(3):734–748. doi: 10.1084/jem.155.3.734. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.