Abstract
Conventional systemic and biologic agents are the mainstay of inflammatory bowel disease (IBD) management; however, many of these agents are associated with loss of clinical response, highlighting the need for effective, novel targeted therapies. Janus kinase (JAK) 1-3 and tyrosine kinase 2 (TYK2) mediate signal transduction events downstream of multiple cytokine receptors that regulate targeted gene transcription, including the interleukin-12, interleukin-23, and type I interferon receptors for TYK2. This review summarizes the role of TYK2 signaling in IBD pathogenesis, the differential selectivity of TYK2 inhibitors, and the potential clinical implications of TYK2 inhibition in IBD. A PubMed literature review was conducted to identify studies of JAK1-3 and TYK2 inhibitors in IBD and other immune-mediated inflammatory diseases. Key efficacy and safety information was extracted and summarized. Pan-JAK inhibitors provide inconsistent efficacy in patients with IBD and are associated with toxicities resulting from a lack of selectivity at therapeutic dosages. Selective inhibition of TYK2 signaling via an allosteric mechanism, with an agent that binds to the regulatory (pseudokinase) domain, may reduce potential toxicities typically associated with JAK1-3 inhibitors. Deucravacitinib, a novel, oral, selective TYK2 inhibitor, and brepocitinib and PF-06826647, TYK2 inhibitors that bind to the active site in the catalytic domain, are in development for IBD and other immune-mediated inflammatory diseases. Allosteric TYK2 inhibition is more selective than JAK1-3 inhibition and has the potential to limit toxicities typically associated with JAK1-3 inhibitors. Future studies will be important in establishing the role of selective, allosteric TYK2 inhibition in the management of IBD.
Keywords: Crohn disease, cytokine signaling, immune-mediated inflammatory disease, Janus kinase, ulcerative colitis
Introduction
Inflammatory bowel diseases (IBD), which include Crohn disease (CD) and ulcerative colitis (UC), are chronic, relapsing, systemic, immune-mediated inflammatory diseases (IMIDs) that cause damage to the gastrointestinal tract.1, 2 The IBD pathogenic process is triggered by genetic and environmental factors, resulting in a complex interplay of inflammatory events mediated by effectors of the innate and adaptive immune systems.3-9 Conventional systemic and biologic agents are the mainstay of symptom management in patients with IBD.10 However, many of these agents are associated with loss of clinical response in patients with CD or UC, highlighting the need for novel targeted therapies that provide sustained remission and mucosal healing.11, 12
An improved understanding of disease pathogenesis, especially the role of cytokines in the inflammatory cascade, has expanded the therapeutic armamentarium available for IBD. Monoclonal antibodies targeting single cytokines, such as tumor necrosis factor-alpha agents, are approved for the treatment of IBD.13 However, a substantial proportion of patients who receive monoclonal antibody therapy either do not respond or lose response over time.14 In addition, monoclonal antibodies require parenteral administration and may be associated with undesirable effects including immunogenicity.14 The Janus kinase–signal transducer and activator of transcription (JAK-STAT) superfamily of intracellular tyrosine kinases transduces downstream signals from multiple cytokines involved in the inflammatory cascade in IBD; as a result, targeting the JAK-STAT pathway can block multiple cytokine signals via the inhibition of a common signal transduction pathway.13-15 Several JAK inhibitors have been evaluated in IBD but have yielded conflicting efficacy results16 and are associated with various toxicities because of a lack of selectivity at therapeutic dosages.17, 18 Based on concerns about the long-term safety profile of pan-JAK inhibitors, including infections (especially herpes zoster reactivation) and laboratory parameter abnormalities,19, 20 selective, oral JAK inhibitors are currently in development for IBD. Selective inhibitors such as those targeting the JAK superfamily intracellular kinase tyrosine kinase 2 (TYK2) may be a viable approach to improving safety while maintaining efficacy in IMIDs such as IBD.
The aim of this review is to summarize the role of TYK2 signaling in IBD pathogenesis, to critically review the available evidence related to the differential selectivity of TYK2 inhibitors in development, and to discuss the potential clinical implications of TYK2 inhibition in IBD.
JAK-STAT and TYK2 Signaling in IBD
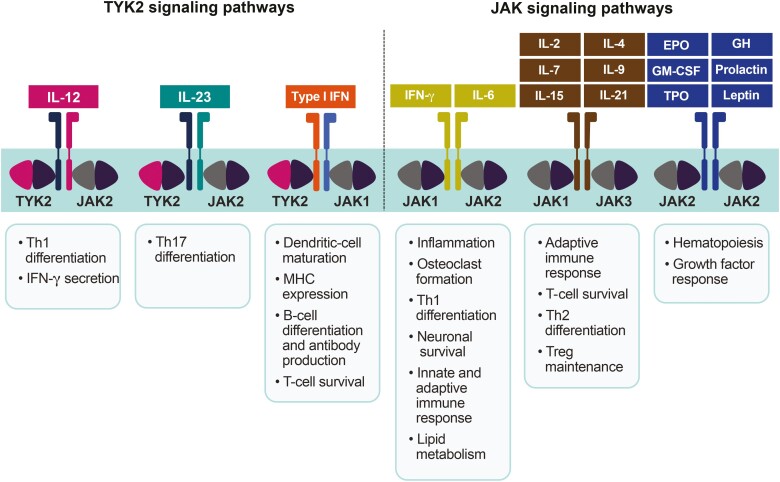
The JAK-STAT pathway plays a major role in intracellular cytokine signaling in IMIDs such as IBD, psoriasis (PsO), psoriatic arthritis (PsA), and lupus.21, 22 As described in detail elsewhere, JAK/TYK2 signaling is involved in innate immunity, adaptive immunity, and hematopoiesis, participating in cellular processes such as cell growth, survival, differentiation, and migration (Fig. 1).19-28
Figure 1.
Overview of the JAK/TYK2 signaling pathways.23-26 The JAK family consists of 4 TYKs of varying specificities, expression levels, and functions; JAK1 and JAK2 mediate signaling through gamma-chain26; JAK3 mediates signaling by 6 common gamma-chain cytokines, including IL-2, IL-4, IL-7, IL-9, IL-15, and IL-2125; and TYK2 plays a key role in mediating immune signaling by IL-10, IL-12, IL-22, IL-23, and type I and III IFNs.23, 25, 26 EPO indicates erythropoietin; GH, growth hormone; GM-CSF, granulocyte-macrophage colony-stimulating factor; MHC, major histocompatibility complex; TPO, thrombopoietin; Treg, regulatory T cell.
Chronic inflammation in CD is characterized by responses from helper T-cell subtypes 1 (Th1) and 17 (Th17) and inadequate regulatory T-cell activity. In contrast, chronic inflammation in UC is generally characterized by Th2 response (Fig. 2).3-9, 19 Many of the cytokines involved in these T-cell responses promote inflammation by signaling via JAK receptors.19 As an intracellular tyrosine kinase that is widely expressed in humans, TYK2 plays a key role in mediating signaling and functional responses downstream of the interleukin (IL)-12, IL-23, and type I interferon (IFN) receptors (Fig. 1).23-26 The IL-12 and IL-23 signaling pathways are involved in initiating and maintaining chronic inflammation in IBD.3-9 The cytokine signaling pathways and functional responses mediated by TYK2 are distinct from those driven by JAK1-3.23-26
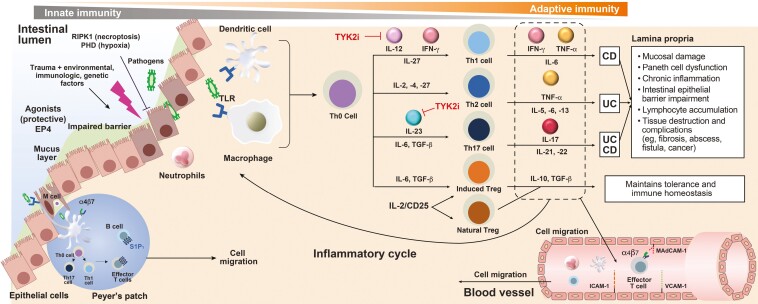
Figure 2.
IBD pathogenesis.3-9 Dysregulation of the mucosal immune response is a key trigger of IBD. The altered immune response is mediated through the activation of dendritic cells, which induces effector T cells, leading to an increase in the number of B cells and antibody production and an increase in the generation of proinflammatory signaling mediators such as IL-12, IL-23, IFN-gamma, IL-6, and TGF-β. TYK2 inhibitors disrupt dysregulated immune response by inhibiting IL-12, IL-23, and IFN-gamma inflammatory signaling. ILC indicates innate lymphoid cell; TGF, transforming growth factor; TLR, Toll-like receptor; TNF, tumor necrosis factor.
Research has shown that TYK2 heterodimerizes with JAK2 to regulate signal transduction pathways downstream of the IL-12 and IL-23 receptors (Fig. 1).23-26 Furthermore, IL-12 is involved in the development of Th1 cells and production of the proinflammatory cytokines tumor necrosis factor-α and IFN-γ, whereas IL-23 mediates the expansion and survival of Th17 cells; Th1 and Th17 cell effectors synergize to amplify inflammation.29-31 In addition, TYK2 heterodimerizes with JAK1 to regulate signal transduction pathways downstream of the receptors for the type I IFNs IFN-α and IFN-β, which are involved in various functions in the immune system including the regulation of dendritic cell maturation and activation, the expression of major histocompatibility complex and costimulation molecules, the moderation of B-cell differentiation and antibody production, and the regulation of T-cell survival.32, 33 The TYK2-mediated IL-12, IL-23, and type I IFN signaling activates STAT-dependent transcription and functional responses that perpetuate chronic inflammation.34, 35
Involvement of TYK2 in IL-12, IL-23, and type I IFN signaling
Previous studies in human cells, mice, and patients have shown that TYK2 is involved in IL-12, IL-23, and type I IFN signaling.36-38
Human Cells
An in vitro study of novel, small-molecule TYK2 and JAK1 inhibitors showed that TYK2 was required for IL-12 and IL-23 signaling but not for IFN-α signaling.36 In contrast, a TYK2-deficient human cell line did not respond to IFN-α, suggesting that TYK2 is essential for IFN-α signaling.39 Because the inhibition of IL-12 and IL-23 is efficacious in preclinical models of IBD, findings indicate that selective TYK2 inhibition may be a potential treatment for IBD.36
Murine Studies
In 1 study, the administration of IL-23 resulted in epidermal hyperplasia in TYK2-positive mice but not in TYK2-knockout mice.40 In addition, IL-23 induced dose-dependent IL-17 and IL-22 secretion in TYK2-positive lymphocytes but not in TYK2-knockout lymphocytes.40 Type I IFN signaling was reduced but not abrogated in TYK2-knockout mice.37 These results confirm that IL-23 signaling is mediated through TYK240 and the involvement of TYK2 in IFN-mediated signaling.37
Patients
In cells isolated from a patient with a mutation in the TYK2 locus, multiple cytokine signaling pathways including those involving IL-12, IL-23, and type I IFNs were impaired, and the introduction of an intact TYK2 gene into these cells successfully restored cytokine signaling.38 Findings substantiate the role of TYK2 in key cytokine signaling mechanisms involved in innate and acquired immunity.38
Role of TYK2 in IL-10 Family Signaling
Studies have also implicated TYK2 signaling in the response to various other cytokines including the IL-10 family.36 Research has shown that IL-10, which is produced by various immune cells, binds to its receptor and activates JAK1 and TYK2, triggering a diverse array of immunosuppressive and immunostimulatory effects.41, 42 Immunosuppressive effects include the inhibition of nuclear translocation of the nuclear factor kappa light chain enhancer of activated B cells, IFN-α– and IFN-γ–induced gene transcription, major histocompatibility complex class II expression by activated dendritic cells and macrophages, and T-cell activation and proliferation.19, 41, 42 Studies have shown that IL-10 exerts stimulatory effects on humoral immune responses such as promoting the differentiation, proliferation, and survival of B cells and the production of antibodies by B cells.41, 42 Contradictory effects of IL-10 have also been described for some cell types (eg, natural killer cells) depending on the cellular context.42 As an IL-10 family member produced in epithelial tissues in the skin and gastrointestinal tract, IL-22 activates JAK1 and TYK2 and is involved in maintaining epithelial integrity, primarily by promoting epithelial cell barrier function and by inducing antimicrobial peptide production. However, IL-22 also stimulates the epithelial production of chemokines, which may contribute to gastrointestinal inflammation and tissue damage.19, 36
TYK2 Inhibition in Ibd and other Imids
Currently, TYK2 inhibition is being evaluated as a therapeutic strategy in various IMIDS including IBD, PsO, PsA, and lupus. The mutation of TYK2 results in a near-complete loss of function and impairment of IL-12, IL-23, and type I IFN signaling but is not associated with immunodeficiency or an increased risk of infections or malignancies.43 Therefore, TYK2 inhibition has potential therapeutic value for the management of IMIDs such as IBD. Three TYK2 inhibitors are or were recently in clinical development for moderate to severe IBD (Table 1; Fig. 3).
Table 1.
Clinical Trials of Oral TYK2 Inhibitors in IBD
| Agent | MOA | Disease* | Clinical Trial | Patients, n | Study Design | Primary Endpoint | Projected Completion |
|---|---|---|---|---|---|---|---|
| Brepocitinib | Dual TYK2/JAK1 inhibitor, binds to the active site in the catalytic domain | UC | ClinicalTrials.gov identifier: NCT02958865 | 360 | Phase 2b, double-blind, randomized, placebo-controlled | Clinical remission at week 8 | May 2021 |
| CD | ClinicalTrials.gov identifier: NCT03395184 | 250 | Phase 2a, double-blind, randomized, placebo-controlled | Endoscopic improvement at week 12, safety up to week 68 | November 2022 | ||
| PF-06826647 | Dual TYK2/JAK2 inhibitor, binds to the active site in the catalytic domain | UC | ClinicalTrials.gov identifier: NCT04209556 | 202 | Phase 2b, double-blind, randomized, placebo-controlled | Endoscopic improvement at week 8, safety up to week 60 | Withdrawn |
| Deucravacitinib | TYK2 inhibitor, binds to the regulatory (pseudokinase) domain (allosteric inhibition) | UC | LATTICE-UC; ClinicalTrials.gov identifier: NCT03934216 | 120 | Phase 2, double-blind, randomized, placebo-controlled | Clinical remission at week 12 | July 2023 |
| CD | LATTICE-CD; ClinicalTrials.gov identifier: NCT03599622 | 240 | Phase 2, double-blind, randomized, placebo-controlled | Clinical remission at week 12, endoscopic response at week 12 | March 2024 |
*Patients were required to have moderate to severe disease.
MOA indicates mechanism of action.
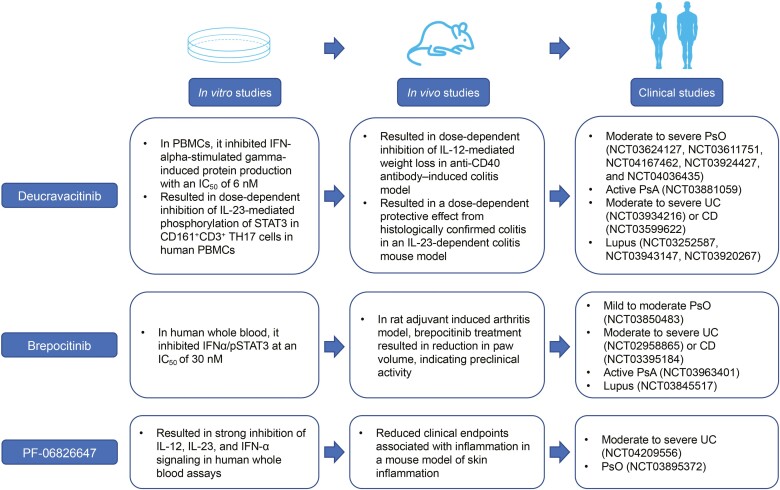
Figure 3.
Preclinical and clinical efficacy of TYK2 inhibitors. Deucravacitinib, brepocitinib, and PF-06826647 showed preclinical efficacy in in vitro studies of human PBMCs and human whole-blood assays and in vivo rodent models of colitis and autoimmune inflammatory diseases. They are currently being evaluated clinically in several autoimmune and inflammatory diseases.24, 44, 48 PBMCs indicate peripheral blood mononuclear cells; pSTAT, phosphorylated signal transducer and activator of transcription.
Brepocitinib
Brepocitinib is a dual oral TYK2/JAK1 inhibitor that binds to the active sites in the catalytic domains of TYK2 and JAK1 (Table 2).44, 45 The efficacy and safety of brepocitinib are being evaluated in PsO, PsA, IBD (UC and CD), and lupus. A phase 2a trial in patients with moderate to severe plaque PsO evaluated the efficacy and safety of oral brepocitinib in patients randomized to brepocitinib 30 mg once daily, 60 mg once daily, or placebo for a 4-week induction period followed by a predefined regimen of brepocitinib 10 mg once daily, 30 mg once daily, 100 mg once weekly, or placebo for an 8-week maintenance period (ClinicalTrials.gov identifier: NCT02969018).46 Findings indicated that a change from baseline in the Psoriasis Area and Severity Index (PASI) at week 12 (primary endpoint) was significantly higher in patients who received oral brepocitinib vs placebo (P < 0.05). Patients who remained on the 30 mg once-daily dose through week 12 had the highest PASI-75 response rates compared with placebo.46 Brepocitinib was generally well tolerated, and no herpes zoster infections were reported.21, 46 Biomarker analyses revealed that inflammatory gene and cellular pathway expression was reduced in patients treated using brepocitinib.47 After 2 weeks of treatment with brepocitinib, IL-17F and IL-12B levels were reduced in patients with moderate to severe plaque PsO. In addition, there was a significant reduction in markers of keratinocyte activation, epidermal thickness, and KRT16 and Ki-67 expression. Moreover, improvement in the clinical symptoms of chronic plaque PsO was associated with a reduction in the immune cell infiltrates CD3+/CD8+ T cells and CD11c dendritic cells.47 Oral brepocitinib development has been discontinued for PsO; however, a phase 2b trial will evaluate topical brepocitinib in patients with mild to moderate PsO (ClinicalTrials.gov identifier: NCT03850483). Two phase 2 trials will evaluate the efficacy and safety of oral brepocitinib in patients with moderate to severe UC (ClinicalTrials.gov identifier: NCT02958865) or CD (ClinicalTrials.gov identifier: NCT03395184). A phase 2 trial was completed in 2021 in active PsA (ClinicalTrials.gov identifier: NCT03963401) and is under way in lupus (ClinicalTrials.gov identifier: NCT03845517).
Table 2.
In Vitro Selectivity of Deucravacitinib and JAK Inhibitors for TYK2 and JAK1–3
| Inhibitor | Assay IC50 (nM) | ||||
|---|---|---|---|---|---|
| TYK2 Regulatory Domain | TYK2 Active Domain | JAK1 | JAK2 | JAK3 | |
| Tofacitinib (pan-JAK) | ND | 489 | 15 | 77 | 55 |
| Baricitinib (JAK1/2-selective) | ND | 61 | 4 | 7 | 787 |
| Filgotinib (JAK1-selective) | ND | 2600 | 363 | 2400 | >10,000 |
| Upadacitinib (JAK1-selective) | ND | 4690 | 47 | 120 | 2304 |
| Brepocitinib (dual JAK1/TYK2) | ND | 23 | 17 | 77 | 6494 |
| PF-06826647 (TYK2-selective) | ND | 17 | 383 | 74 | >10,000 |
| Deucravacitinib (TYK2-selective, allosteric) | 0.2 | >10,000 | >10,000 | >10,000 | >10,000 |
See Wrobleski et al.45
ND indicates not determined.
PF-06826647
PF-06826647 is a dual oral TYK2/JAK2 inhibitor that binds to the active sites in the catalytic domains of TYK2 and JAK2 (Table 2).24, 45 The binding of PF-06826647 to TYK2 elicits a potent inhibition of IL-12 and IL-23 signaling in human whole-blood assays.48 In a phase 1 study in patients with moderate to severe plaque PsO, treatment with once-daily PF-06826647 400 mg resulted in a significant improvement from baseline in the PASI score compared with placebo at 4 weeks of treatment. All treatment-emergent adverse events in patients treated using PF-06826647 were mild in severity.49 Significant decreases in IL-17A and IL-17F expression were observed at week 4 in the PF-06826647 group.49 A phase 2 trial evaluating the efficacy and safety of PF-06826647 in patients with moderate to severe PsO was completed in 2020 (ClinicalTrials.gov identifier: NCT03895372). A trial in UC planned to compare 4 doses of PF-06826647 vs placebo during an 8-week, double-blind induction period followed by a 52-week, open-label extension period. The primary endpoint was endoscopic response at week 8 and long-term safety at week 60, with study completion estimated to occur in October 2023; however, the study was withdrawn.
Deucravacitinib
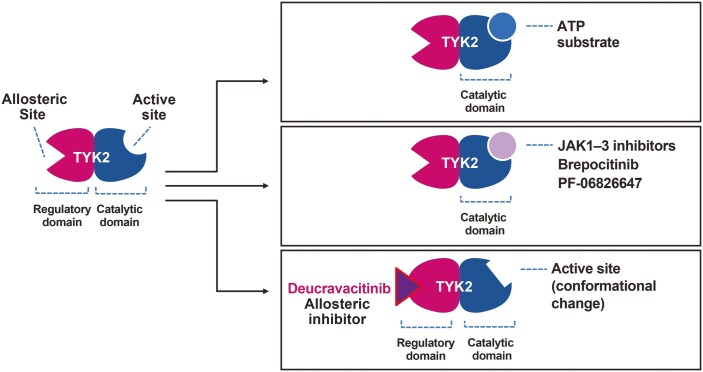
Deucravacitinib (formerly known as BMS-986165) is a novel, oral, selective TYK2 inhibitor with a mechanism of action distinct from that of JAK1-3 inhibitors.24, 45, 50, 51 Deucravacitinib inhibits TYK2 by binding to the regulatory (JH2 pseudokinase) domain rather than directly to the active site in the catalytic domain as JAK1-3 inhibitors do (Fig. 4).24 Binding allosterically locks the regulatory domain into an inhibitory interaction with the catalytic domain, which renders TYK2 inactive and thereby prevents receptor-mediated activation and downstream signal transduction.24
Figure 4.
Selective versus nonselective TYK2 inhibition. Deucravacitinib allosterically inhibits TYK2 through binding to the regulatory pseudokinase domain of TYK2, which inactivates TYK2 by locking the regulatory domain into an inhibitory interaction with the catalytic domain.24, 45 Brepocitinib, a dual TYK2/JAK1 inhibitor, and PF-06826647, a dual TYK2/JAK2 inhibitor, bind directly to the active site of the catalytic domain of TYK2.21, 44, 45 ATP indicates adenosine triphosphate.
Deucravacitinib is a highly selective TYK2 inhibitor and exerts only minimal or no activity against JAK1-3 (conversely, JAK1-3 inhibitors do not bind to the regulatory domain of TYK2). In cell-based assays, deucravacitinib has a greater than 100-fold selectivity for TYK2 over JAK1/JAK3 and a greater than 2000-fold selectivity for TYK2 over JAK2 (half-maximal inhibitory concentration [IC50] data; Table 2).24, 45 Deucravacitinib selectivity for TYK2 reduces the potential for toxicities such as those associated with JAK1-3 inhibitors.27
Moreover, the treatment of human peripheral blood mononuclear cells with deucravacitinib has resulted in the inhibition of IFN-α-stimulated gamma-induced protein production with an IC50 of 6 nM, which confirms that deucravacitinib suppresses TYK2-dependent functional cellular effects mediated by IFN stimulation.24 In addition, deucravacitinib has resulted in a dose-dependent inhibition of IL-23-mediated phosphorylation of STAT3 in CD161+ CD3+ TH17 cells in human peripheral blood mononuclear cells. The disruption of IL-23 signaling results in the inhibition of IL-17 production by CD4+ T cells with an IC50 of 2 nM.24
Orally administered deucravacitinib is currently being evaluated as a therapeutic option for several IMIDs: PsO, PsA, IBD (UC and CD), and lupus, including lupus nephritis.
PsO and PsA
In a phase 2 trial, the proportion of patients with moderate to severe PsO who achieved PASI 75 at week 12 (primary endpoint) was significantly higher with deucravacitinib 3 mg twice daily (69%), 6 mg twice daily (67%), and 12 mg once daily (75%) vs placebo (7%; P < 0.001).52 Deucravacitinib was generally well tolerated with no safety findings of concern, including no reported patients with herpes zoster infection or cardiovascular events.52 In addition, deucravacitinib treatment did not result in significant changes in hematologic parameters (hemoglobin levels, natural killer cells, lymphocytes, neutrophils, and platelets) or serum levels of lipids (high-density lipoprotein cholesterol and low-density lipoprotein cholesterol), liver enzymes, creatinine, or immunoglobulins.53 In contrast, JAK1-3 inhibitors have been associated with abnormalities in each of these parameters.52, 54, 55 Biomarker analysis indicated that deucravacitinib treatment inhibited expression of the IL-23/Th17 and type I IFN signaling pathways in patients with PsO.56
Several phase 3 trials are evaluating the efficacy and safety of deucravacitinib in patients with moderate to severe PsO (ClinicalTrials.gov identifiers: NCT03624127, NCT03611751, NCT04167462, NCT03924427, and NCT04036435). A phase 2 trial has evaluated the efficacy and safety of deucravacitinib in patients with active PsA (ClinicalTrials.gov identifier: NCT03881059).
IBD
Deucravacitinib prevented IL-12– and IL-23–dependent wasting and colitis in 2 murine models of IBD.24 In an anti-CD40 antibody–induced colitis model in severe combined immunodeficient mice, prophylactic treatment with deucravacitinib resulted in the dose-dependent inhibition of IL-12‒mediated weight loss.24 In another IL-23‒dependent colitis mouse model generated by the adoptive transfer of CD4+CD45RBhigh T cells to severe combined immunodeficient mice, deucravacitinib treatment resulted in a dose-dependent protective effect from histologically confirmed colitis.24 The safety and efficacy of deucravacitinib will be evaluated in two phase 2 clinical trials of patients with moderate to severe UC (LATTICE-UC; ClinicalTrials.gov identifier: NCT03934216) or CD (LATTICE-CD; ClinicalTrials.gov identifier: NCT03599622).
Lupus
The involvement of IFN-dependent inflammation in patients with lupus is well established. Deucravacitinib-treated whole blood from 31 patients with lupus resulted in the suppression of IFN-dependent gene expression.24 These preclinical results suggest that deucravacitinib has the potential to be an effective treatment in patients with lupus. The efficacy and safety of deucravacitinib will be evaluated in two phase 2 trials in patients with lupus (PAISLEY SLE; ClinicalTrials.gov identifier: NCT03252587; PAISLEY LN; ClinicalTrials.gov identifier: NCT03943147) and in a long-term safety and efficacy trial (ClinicalTrials.gov identifier: NCT03920267).
Conclusions
Novel oral therapies that are safe and effective are needed for the treatment of IBD. Studies have shown JAK1-3 inhibitors to have inconsistent efficacy in IBD and to be associated with toxicities. In addition, TYK2 plays a central role in the pathophysiology of IBD via the regulation of signaling and functional responses downstream of the IL-12, IL-23, and type I IFN receptors. Research has shown that TYK2 inhibition with an agent that prevents receptor-mediated activation by binding allosterically to the regulatory domain rather than to the active site in the catalytic domain results in selective inhibition, because the regulatory domain is present only in TYK2, whereas the active site in the catalytic domain is highly conserved across JAK superfamily members. Consequently, allosteric TYK2 inhibition is more selective than JAK1-3 inhibition and has the potential to limit toxicities associated with JAK1-3 inhibitors.
Several TYK2 inhibitors are currently being investigated in various IMID trials. Deucravacitinib, a novel, oral, selective TYK2 inhibitor that blocks IL-12, IL-23, and type I IFN signaling and functional responses, was shown to be efficacious and well tolerated in a phase 2 trial in patients with moderate to severe PsO. Clinical trials of deucravacitinib are ongoing in IBD (UC and CD), PsO, PsA, and lupus. Brepocitinib, a TYK2 inhibitor that targets the active site in the catalytic domain of TYK2, is also being evaluated in patients with IBD. Future studies will be important in establishing the role of selective, allosteric TYK2 inhibition in the management of IBD, including determining whether the selective inhibition of JAK-STAT signaling may overcome some of the challenges associated with less selective inhibition.
Acknowledgments
Professional medical writing from Ann Marie Fitzmaurice, PhD, and editorial assistance were provided by Peloton Advantage, LLC, an OPEN Health company, Parsippany, NJ, and were funded by Bristol Myers Squibb.
Funding
This publication was sponsored by Bristol Myers Squibb.
Conflicts of Interest
Silvio Danese has received consultancy fees from AbbVie, Allergan, Amgen, AstraZeneca, Athos Therapeutics, Biogen, Boehringer Ingelheim, Celgene, Celltrion, Eli Lilly, Enthera, Ferring, Gilead, Hospira, Inotrem, Janssen, Johnson & Johnson, MSD, Mundipharma, Mylan, Pfizer, Roche, Sandoz, Sublimity Therapeutics, Takeda, TiGenix, UCB, and Vifor and has received lecture fees from AbbVie, Amgen, Ferring, Gilead, Janssen, Mylan, Pfizer, and Takeda.
Laurent Peyrin-Biroulet has received personal fees from AbbVie, Janssen, Genentech, Ferring, Tillots, Pharmacosmos, Celltrion, Takeda, Boehringer Ingelheim, Pfizer, Index Pharmaceuticals, Sandoz, Celgene, Biogen, Samsung Bioepis, Alma, Sterna, Nestle, Enterome, Allergan, MSD, Roche, Arena, Gilead, Hikma, Amgen, BMS, Vifor, Norgine, Mylan, Lilly, Fresenius, Oppilan Pharma, Sublimity Therapeutics, Applied Molecular Transport, OSE Immunotherapeutics, and Enthera; has received grants from AbbVie, MSD, and Takeda; and has stock options with CTMA.
References
- 1. Faust AH, Halpern LF, Danoff-Burg S, et al. . Psychosocial factors contributing to inflammatory bowel disease activity and health-related quality of life. Gastroenterol Hepatol (N Y). 2012;8:173–181. [PMC free article] [PubMed] [Google Scholar]
- 2. Crohn’s & Colitis Foundation of America. The facts about inflammatory bowel diseases. Accessed May 12, 2021. https://www.crohnscolitisfoundation.org/sites/default/files/2019-02/Updated%20IBD%20Factbook.pdf. [Google Scholar]
- 3. de Souza HS, Fiocchi C. Immunopathogenesis of IBD: current state of the art. Nat Rev Gastroenterol Hepatol. 2016;13:13–27. [DOI] [PubMed] [Google Scholar]
- 4. Kim DH, Cheon JH. Pathogenesis of inflammatory bowel disease and recent advances in biologic therapies. Immune Netw. 2017;17:25–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ahluwalia B, Moraes L, Magnusson MK, et al. . Immunopathogenesis of inflammatory bowel disease and mechanisms of biological therapies. Scand J Gastroenterol. 2018;53:379–389. [DOI] [PubMed] [Google Scholar]
- 6. Peters CP, Mjösberg JM, Bernink JH, et al. . Innate lymphoid cells in inflammatory bowel diseases. Immunol Lett. 2016;172:124–131. [DOI] [PubMed] [Google Scholar]
- 7. Neurath MF. Cytokines in inflammatory bowel disease. Nat Rev Immunol. 2014;14:329–342. [DOI] [PubMed] [Google Scholar]
- 8. Allocca M, Furfaro F, Fiorino G, et al. . Can IL-23 be a good target for ulcerative colitis? Best Pract Res Clin Gastroenterol. 2018;32–33:95–102. [DOI] [PubMed] [Google Scholar]
- 9. Neurath MF. IL-23 in inflammatory bowel diseases and colon cancer. Cytokine Growth Factor Rev. 2019;45:1–8. [DOI] [PubMed] [Google Scholar]
- 10. Lichtenstein GR, Loftus EV, Isaacs KL, et al. . ACG clinical guideline: management of Crohn’s disease in adults. Am J Gastroenterol. 2018;113:481–517. [DOI] [PubMed] [Google Scholar]
- 11. Peyrin-Biroulet L, Lémann M. Review article: remission rates achievable by current therapies for inflammatory bowel disease. Aliment Pharmacol Ther. 2011;33:870–879. [DOI] [PubMed] [Google Scholar]
- 12. White JR, Phillips F, Monaghan T, et al. . Review article: novel oral-targeted therapies in inflammatory bowel disease. Aliment Pharmacol Ther. 2018;47:1610–1622. [DOI] [PubMed] [Google Scholar]
- 13. Danese S, Grisham M, Hodge J, et al. . JAK inhibition using tofacitinib for inflammatory bowel disease treatment: a hub for multiple inflammatory cytokines. Am J Physiol Gastrointest Liver Physiol. 2016;310:G155–G162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Olivera P, Danese S, Peyrin-Biroulet L. Next generation of small molecules in inflammatory bowel disease. Gut. 2017;66:199–209. [DOI] [PubMed] [Google Scholar]
- 15. D’Amico F, Fiorino G, Furfaro F, et al. . Janus kinase inhibitors for the treatment of inflammatory bowel diseases: developments from phase I and phase II clinical trials. Expert Opin Investig Drugs. 2018;27:595–599. [DOI] [PubMed] [Google Scholar]
- 16. Rogler G. JAK efficacy in Crohn’s disease. J Crohns Colitis. 2020;14:S746–S754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bonovas S, Lytras T, Nikolopoulos G, et al. . Editorial: tofacitinib and biologics for moderate-to-severe ulcerative colitis—what is best in class? Authors’ reply. Aliment Pharmacol Ther. 2018;47:540–541. [DOI] [PubMed] [Google Scholar]
- 18. Harigai M. Growing evidence of the safety of JAK inhibitors in patients with rheumatoid arthritis. Rheumatology (Oxford). 2019;58:i34–i42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pérez-Jeldres T, Tyler CJ, Boyer JD, et al. . Targeting cytokine signaling and lymphocyte traffic via small molecules in inflammatory bowel disease: JAK inhibitors and S1PR agonists. Front Pharmacol. 2019;10:212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Danese S, Argollo M, Le Berre C, et al. . JAK selectivity for inflammatory bowel disease treatment: does it clinically matter? Gut. 2019;68:1893–1899. [DOI] [PubMed] [Google Scholar]
- 21. Nogueira M, Puig L, Torres T. JAK inhibitors for treatment of psoriasis: focus on selective TYK2 inhibitors. Drugs. 2020;80:341–352. [DOI] [PubMed] [Google Scholar]
- 22. Salas A, Hernandez-Rocha C, Duijvestein M, et al. . JAK-STAT pathway targeting for the treatment of inflammatory bowel disease. Nat Rev Gastroenterol Hepatol. 2020;17:323–337. [DOI] [PubMed] [Google Scholar]
- 23. Baker KF, Isaacs JD. Novel therapies for immune-mediated inflammatory diseases: what can we learn from their use in rheumatoid arthritis, spondyloarthritis, systemic lupus erythematosus, psoriasis, Crohn’s disease and ulcerative colitis? Ann Rheum Dis. 2018;77:175–187. [DOI] [PubMed] [Google Scholar]
- 24. Burke JR, Cheng L, Gillooly KM, et al. . Autoimmune pathways in mice and humans are blocked by pharmacological stabilization of the TYK2 pseudokinase domain. Sci Transl Med. 2019;11:1–16. [DOI] [PubMed] [Google Scholar]
- 25. Jiang L, Li Z, Rui L. Leptin stimulates both JAK2-dependent and JAK2-independent signaling pathways. J Biol Chem. 2008;283:28066–28073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Xie J, LeBaron MJ, Nevalainen MT, et al. . Role of tyrosine kinase Jak2 in prolactin-induced differentiation and growth of mammary epithelial cells. J Biol Chem. 2002;277:14020–14030. [DOI] [PubMed] [Google Scholar]
- 27. Kvist-Hansen A, Hansen PR, Skov L. Systemic treatment of psoriasis with JAK inhibitors: a review. Dermatol Ther (Heidelb). 2020;10:29–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Balogh EA, Bashyam AM, Ghamrawi RI, et al. . Emerging systemic drugs in the treatment of plaque psoriasis. Expert Opin Emerg Drugs. 2020;25:89–100. [DOI] [PubMed] [Google Scholar]
- 29. Ghoreschi K, Laurence A, O’Shea JJ. Janus kinases in immune cell signaling. Immunol Rev. 2009;228:273–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tokarski JS, Zupa-Fernandez A, Tredup JA, et al. . Tyrosine kinase 2-mediated signal transduction in T lymphocytes is blocked by pharmacological stabilization of its pseudokinase domain. J Biol Chem. 2015;290:11061–11074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Alwan W, Nestle FO. Pathogenesis and treatment of psoriasis: exploiting pathophysiological pathways for precision medicine. Clin Exp Rheumatol. 2015;33:S2–S6. [PubMed] [Google Scholar]
- 32. Simmons DP, Wearsch PA, Canaday DH, et al. . Type I IFN drives a distinctive dendritic cell maturation phenotype that allows continued class II MHC synthesis and antigen processing. J Immunol. 2012;188:3116–3126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Eloranta ML, Rönnblom L. Cause and consequences of the activated type I interferon system in SLE. J Mol Med (Berl). 2016;94:1103–1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Schindler C, Levy DE, Decker T. JAK-STAT signaling: from interferons to cytokines. J Biol Chem. 2007;282:20059–20063. [DOI] [PubMed] [Google Scholar]
- 35. Clevenger CV. Roles and regulation of STAT family transcription factors in human breast cancer. Am J Pathol. 2004;165:1449–1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sohn SJ, Barrett K, Van Abbema A, et al. . A restricted role for TYK2 catalytic activity in human cytokine responses revealed by novel TYK2-selective inhibitors. J Immunol. 2013;191:2205–2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Karaghiosoff M, Neubauer H, Lassnig C, et al. . Partial impairment of cytokine responses in Tyk2-deficient mice. Immunity. 2000;13:549–560. [DOI] [PubMed] [Google Scholar]
- 38. Minegishi Y, Saito M, Morio T, et al. . Human tyrosine kinase 2 deficiency reveals its requisite roles in multiple cytokine signals involved in innate and acquired immunity. Immunity. 2006;25:745–755. [DOI] [PubMed] [Google Scholar]
- 39. Majoros A, Platanitis E, Kernbauer-Hölzl E, et al. . Canonical and non-canonical aspects of JAK-STAT signaling: lessons from interferons for cytokine responses. Front Immunol. 2017;8:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ishizaki M, Muromoto R, Akimoto T, et al. . Tyk2 is a therapeutic target for psoriasis-like skin inflammation. Int Immunol. 2014;26:257–267. [DOI] [PubMed] [Google Scholar]
- 41. Ouyang W, O’Garra A. IL-10 family cytokines IL-10 and IL-22: from basic science to clinical translation. Immunity. 2019;50:871–891. [DOI] [PubMed] [Google Scholar]
- 42. Saxena A, Khosraviani S, Noel S, et al. . Interleukin-10 paradox: a potent immunoregulatory cytokine that has been difficult to harness for immunotherapy. Cytokine. 2015;74:27–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Dendrou CA, Cortes A, Shipman L, et al. . Resolving TYK2 locus genotype-to-phenotype differences in autoimmunity. Sci Transl Med. 2016;8:363ra149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Fensome A, Ambler CM, Arnold E, et al. . Dual inhibition of TYK2 and JAK1 for the treatment of autoimmune diseases: discovery of ((S)-2,2-difluorocyclopropyl)((1 R,5 S)-3-(2-((1-methyl-1 H-pyrazol-4-yl)amino)pyrimidin-4-yl)-3,8-diazabicyclo[3.2.1]octan-8-yl)methanone (PF-06700841). J Med Chem. 2018;61:8597–8612. [DOI] [PubMed] [Google Scholar]
- 45. Wrobleski ST, Moslin R, Lin S, et al. . Highly selective inhibition of tyrosine kinase 2 (TYK2) for the treatment of autoimmune diseases: discovery of the allosteric inhibitor BMS-986165. J Med Chem. 2019;62:8973–8995. [DOI] [PubMed] [Google Scholar]
- 46. Forman S, Pariser DM, Poulin Y, et al. . A Phase 2A, randomised, double-blind, placebo-controlled study to evaluate efficacy and safety of PF-06700841 in patients with moderate-to-severe plaque psoriasis [abstract 86]. Exp Dermatol. 2018;27:37.28636759 [Google Scholar]
- 47. Page KM, Suarez-Farinas M, Suprun M, et al. . Molecular and cellular responses to the TYK2/JAK1 inhibitor PF-06700841 reveal reduction of skin inflammation in plaque psoriasis. J Invest Dermatol. 2020;140:1546–1555.e4. [DOI] [PubMed] [Google Scholar]
- 48. Gerstenberger BS, Ambler C, Arnold EP, et al. . Discovery of tyrosine kinase 2 (TYK2) inhibitor (PF-06826647) for the treatment of autoimmune diseases. J Med Chem. 2020;63:13561–13577. [DOI] [PubMed] [Google Scholar]
- 49. Tehlirian C, Peeva E, Kieras E, et al. . Safety, tolerability, efficacy, pharmacokinetics, and pharmacodynamics of the oral TYK2 inhibitor PF-06826647 in participants with plaque psoriasis: a phase 1, randomised, double-blind, placebo-controlled, parallel-group study. Lancet Rheumatol. 2021;3:e204–e213. [DOI] [PubMed] [Google Scholar]
- 50. Moslin R, Zhang Y, Wrobleski ST, et al. . Identification of N-methyl nicotinamide and N-methyl pyridazine-3-carboxamide pseudokinase domain ligands as highly selective allosteric inhibitors of tyrosine kinase 2 (TYK2). J Med Chem. 2019;62:8953–8972. [DOI] [PubMed] [Google Scholar]
- 51. Chang Y, Xu S, Ding K. Tyrosine kinase 2 (TYK2) allosteric inhibitors to treat autoimmune diseases. J Med Chem. 2019;62:8951–8952. [DOI] [PubMed] [Google Scholar]
- 52. Papp K, Gordon K, Thaçi D, et al. . Phase 2 trial of selective tyrosine kinase 2 inhibition in psoriasis. N Engl J Med. 2018;379:1313–1321. [DOI] [PubMed] [Google Scholar]
- 53. Gordon K, Papp K, Gooderham M, et al. . BMS-986165, an oral, selective tyrosine kinase 2 (TYK2) inhibitor: evaluation of changes in laboratory parameters in response to treatment in a Phase 2 trial in psoriasis patients. SKIN J Cutaneous Med. 2020;4:s28.
- 54. Winthrop KL. The emerging safety profile of JAK inhibitors in rheumatic disease. Nat Rev Rheumatol. 2017;13:234–243. [DOI] [PubMed] [Google Scholar]
- 55. Banfield C, Scaramozza M, Zhang W, et al. . The safety, tolerability, pharmacokinetics, and pharmacodynamics of a TYK2/JAK1 inhibitor (PF-06700841) in healthy subjects and patients with plaque psoriasis. J Clin Pharmacol. 2018;58:434–447. [DOI] [PubMed] [Google Scholar]
- 56. Krueger JG, Hu S, Banerjee S, et al. . A selective inhibitor of TYK2, BMS-986165, improves molecular, cellular and clinical biomarkers associated with efficacy in moderate-to-severe psoriasis. Presented at: Annual Congress of the European Academy of Dermatology and Venereology; September 12–16, 2018; Paris, France. [Google Scholar]