Abstract
Background
The ProMuscle in Practice intervention, comprising resistance exercise and an increased protein intake, was effective in improving muscle strength, lean body mass, and physical functioning in older adults aged 65 years and older (N = 168). However, a heterogeneous response to such interventions is common. Therefore, we explored the differences in responsiveness to the intervention in subgroups based on demographic characteristics and mobility-impairing disorders.
Method
Multiple regression analyses were performed to study mean changes between baseline and 12 weeks on the Short Physical Performance Battery, chair rise test, lean body mass, knee extension strength, leg press strength, and leg extension strength. The interaction term Treatment × Subgroup was included to study differences in effects between subgroups. Subgroups comprised age (≤75 vs >75 years), sex (men vs women), presence of frailty, presence of sarcopenia, and presence of osteoarthritis.
Results
A significant interaction effect including age was found on lean body mass (β = −0.8; 95% CI: −1.5, −0.2), favoring participants aged 75 years and younger. A significant interaction effect including sex was found on leg press strength (β = 15.5; 95% CI: 0.6, 30.3), favoring women. Participants with or without frailty, sarcopenia, or osteoarthritis responded equally to the intervention in terms of absolute effects.
Conclusions
Participants aged 75 years and younger and women benefited to a great extent from the intervention, as they improved significantly on nearly every outcome. Effects in participants with and without a mobility-impairing disorder were comparable, indicating that the intervention is suitable for both groups.
Keywords: Lifestyle intervention, Muscle mass, Muscle strength, Physical functioning, Responsiveness
Interindividual variability regarding health characteristics, such as functional status, mobility, and weight status, increases over the life course (1,2). This contributes to a heterogeneous older population, with the rate and consequences of the ageing process being different between individuals (3–6). One of those consequences is an impaired mobility, which is often the beginning of further functional decline (7–10). As the proportion of older adults is growing rapidly, it is important to delay or rather prevent the onset of functional decline (8,9,11).
An effective strategy in improving older adults’ physical functioning and mobility is the combination of resistance exercise (RE) and protein supplementation (PS) (12–14). Although a recent study showed that there are no nonresponders to RE among older adults (15), several meta-analyses reported that differential effect sizes and heterogeneity in responsiveness to RE and PS is common (13,14,16,17). On the one hand, the varying effects can be explained by heterogeneity in interventions, such as duration of the intervention, type of training sessions, and type, dose, and timing of PS. On the other hand, variation in effect sizes can be due to heterogeneity in study populations, including differences in age, sex, health status, and training status of the participants (13,14,16,17).
Several studies investigated the role of age and sex in the heterogeneous RE response (15,18,19). However, findings remain inconclusive, showing either no differences for age and sex groups (18), or sex-related differences favoring either men or women depending on the outcome studied (15,19). Interventions combining RE and PS also show inconsistent results in subgroups based on age or sex. A recent meta-analysis reported a higher increase in fat-free mass (FFM) in younger compared to older adults (≤45 vs >45 years) (20), whereas another meta-analysis found no significant differences among subgroups based on age but reported a higher increase in FFM in the older subgroup (≤65 vs >65 years) (21). A meta-analysis comparing intervention effects between men and women showed greater changes in lean body mass and leg strength in men compared to women (14), whereas 2 other meta-analyses reported no clear sex-based differences (20,21). However, the number of studies investigating the effects of RE and PS in women is limited, which complicates adequate comparison (14,20,21).
Varying effects in muscle health in response to RE and PS are also found in older adults suffering from mobility-impairing disorders, such as frailty, sarcopenia, and osteoarthritis. Although numerous interventions combining RE and an increased protein intake have been performed in healthy older adults aiming to prevent sarcopenia (12–14), limited interventions have been conducted specifically in sarcopenic older adults. Nevertheless, available literature showed that effects on muscle health in sarcopenic participants were rather positive (22). With regards to frail older adults, multiple interventions including RE and PS were conducted, resulting in improvements on lean body mass, leg strength, and physical mobility (23). To date, the preventive effects of RE and PS interventions have not been studied extensively in older adults suffering from osteoarthritis (24). However, aerobic, strengthening, and flexibility exercises are an important part of its treatment (25) and were found to reduce pain and increase physical functioning in osteoarthritis patients (26,27).
Thus, there is a discrepancy in literature related to the responsiveness of subgroups to interventions combining RE and PS. In our intervention, ProMuscle in Practice, we expect to find heterogeneous responses as well. Our 12-week intensive support intervention, including RE and an increased protein intake, was found to be effective in improving muscle strength, lean body mass, and physical functioning in community-dwelling older adults (28). However, variation was found in the effects, possibly indicating heterogeneous responses between subgroups. Therefore, we aimed to explore the differences in responsiveness to RE and PS between subgroups based on demographic characteristics and mobility-impairing disorders in the ProMuscle in Practice intervention.
Method
Study Design
The study design, intervention description, and main results of ProMuscle in Practice are described in detail elsewhere (28,29). In short, ProMuscle in Practice is a randomized controlled multicentre intervention, implemented in a phased manner at 5 Dutch municipalities (Apeldoorn, Epe, Ermelo/Putten, Harderwijk, and Ede) between 2016 and 2018.
Study Population
Researchers recruited and screened older adults aged 65 years and older, according to the following criteria: master the Dutch language, being prefrail or frail according to Fried criteria (9) or being nonfrail but experiencing difficulties in daily activities and being inactive (ie, not participating in RE >30 minutes a day on >2 days a week). The exclusion criteria (Supplementary Table S1) were checked by the older adults’ general practitioner (GP). In total, 168 older adults were randomized, stratified for sex and frailty state, to the intervention or control group. The study protocol was approved by the Wageningen University Medical Ethics Committee, and all subjects gave their written informed consent before the start of the study. The ProMuscle in Practice study is registered at the Dutch Trial Register (identifier NTR6038).
Description of the Intervention
The intervention consisted of a 12-week intensive support program, followed by a 12-week moderate support program. In the current study, we focused on the intensive intervention only. The intensive support program included twice weekly progressive RE, primarily focused on the leg muscles. Each session had a duration of 1 hour, was group-based (4–7 participants), and was supervised by physiotherapists according to ProMuscle in Practice manuals. Additionally, a dietitian advised participants to increase their protein intake to 25 g per main meal, via individual consultations (at baseline, Week 1, and Week 6) and by providing products, such as dairy foods and protein-rich cakes or desserts. The control group received usual health care and was asked to retain their habits regarding exercise and diet.
Measures
Outcome Measures
Outcomes were measured at baseline in Week 0 (T0) and after the 12-week intensive support intervention (T1). Data were collected by trained researchers and research assistants.
Physical functioning
The Short Physical Performance Battery (SPPB) was used to measure physical functioning (score 0–12, 12 representing best score). The test consists of 3 components: a standing balance, repeated chair rise, and gait speed test (30). In addition to total SPPB score, chair rise was included in the data analyses, as chair rise is highly correlated with lower body strength; the main training focus of the intervention (31).
Muscle mass
Lean body mass was measured through dual-energy x-ray absorptiometry (Lunar Prodigy Advance; GE Health Care, Madison, WI). Scans were performed in the morning. Participants were asked to consume a standardized breakfast and to defecate shortly before the scan. Bioelectrical impedance spectroscopy was used to assess hydration state (SFB7; ImpediMed Limited, Pinkenba, Queensland, Australia).
Muscle strength
Lower limb muscle strength was measured through 3-Repetition Maximum (3RM) tests on the leg press and leg extension machines. Before the measurement, a familiarization session was performed. The formula of Brzycki was used to recalculate 3RM to 1RM scores, reported in kilograms (32). Knee extension strength was measured using a hand-held dynamometer (MicroFET). Three repeated tests were performed alternating both legs. The highest measurement of the dominant leg was included for analyses, reported in Newton.
Subgroups
Subgroups were created based on demographic variables and mobility-impairing disorders, both assessed at baseline (T0). Demographic variables included age and sex. Age was divided in 2 groups based on median age (75 years). Mobility-impairing disorders included frailty, sarcopenia, and osteoarthritis and are described in detail below.
Frailty
Frailty was assessed according to the Fried frailty criteria (9). Participants in the categories “prefrail” and “frail” were combined due to low number of participants being frail (n = 7). Nonfrail participants were included in the study if they experienced difficulties in daily activities and being inactive (ie, not participating in RE >30 minutes a day on >2 days a week).
Sarcopenia
The revised sarcopenia definition of the European Working Group on Sarcopenia in Older People (EWGSOP2) was used (33). Low muscle strength (criterion 1) indicating probable sarcopenia was assessed with grip strength (<27 kg for men and <16 kg for women). Low muscle quantity (criterion 2) confirms the sarcopenia diagnosis and was assessed according to low appendicular muscle mass (<20 kg for men and <15 kg for women) on top of having low muscle strength. Severe sarcopenia is detected if low physical performance is present on top of low muscle strength and mass (criterion 3). Low physical performance was assessed according to gait speed (≤0.8 m/s for both men and women). Participants in the category “probable sarcopenia” were included in the analyses as being sarcopenic and remaining participants were classified as nonsarcopenic. Categories “sarcopenia diagnosis” and “severe sarcopenia” were too small to be analyzed separately (n = 10 and n = 6, respectively).
Osteoarthritis
Whether participants had osteoarthritis in the hips or knees was assessed via a questionnaire, including answer options “no,” “yes, not diagnosed by GP,” and “yes, diagnosed by GP.” The latter 2 categories were combined in the category indicating osteoarthritis.
Statistical Analyses
Baseline characteristics were analyzed using independent samples t tests or Mann–Whitney U tests for continuous data, and Pearson’s chi-squared test or Fisher exact probability tests for categorical data. Multiple regression analyses were performed to study the mean changes between T1 and T0 between the intervention and control group, in the total study population as well as in subgroups. Besides, differences in effects between subgroups were studied by including treatment × subgroup as interaction term. Subgroups comprised age, sex, frailty, sarcopenia, and osteoarthritis. Change scores were calculated by subtracting the effects at T0 from the effects at T1. Participants with complete measurements at T0 as well as T1 were included in the analyses, which were assessed per study outcome. Separate models were conducted for each outcome: SPPB total score, chair rise test, lean body mass, knee extension strength, leg press strength, and leg extension strength. We assessed the crude model as well as the adjusted model (adjusted for age, sex, educational level, and municipality). Estimated mean differences and 95% confidence intervals (CIs) are presented for the total study population, per subgroup and between subgroups (interaction effects). We additionally calculated “relative effects,” defined as changes relative to baseline in %, calculated as follows: (absolute effect T1 − T0/baseline measure) × 100%. Multiple regression analyses were also performed including relative effects as dependent variable. To assess the treatment effect in combinations of subgroups, 3-way interactions and related post hoc analyses were conducted. Three-way interactions were examined for the outcomes chair rise, lean body mass, and leg press strength, as chair rise is highly correlated with lower body strength, the main training focus of the intervention (31), and lean body mass and leg press strength showed significant 2-way interactions indicating further exploration of the pattern of treatment effects. Data were analyzed using SPSS version 23. Statistical significance was indicated with p value <.05.
Results
Baseline Characteristics
Table 1 presents the baseline characteristics for the intervention and control group separately. No significant differences between the 2 groups were observed at baseline. About 21% of the study population could be indicated with sarcopenia diagnosis, 52% as being prefrail or frail, and almost 48% suffered from osteoarthritis.
Table 1.
Baseline Characteristics of Participants of the ProMuscle in Practice Intervention
| Intervention Group (n = 82) | Control Group (n = 86) | |
|---|---|---|
| Age (y) | 74.7 ± 5.8 | 75.9 ± 6.5 |
| Sex (n female, %) | 51 (62%) | 51 (59%) |
| Bodyweight (kg) | 76.3 ± 14.4 | 75.6 ± 13.6 |
| Height (cm) | 167.6 ± 9.0 | 169.2 ± 9.3 |
| Education level (n, %)a | ||
| Low and intermediate | 56 (68%) | 46 (54%) |
| High | 26 (32%) | 40 (47%) |
| Ethnicity: native Dutch (n, %) | 79 (96%) | 81 (94%) |
| Care use (n, %) | 11 (13%) | 16 (19%) |
| Frailty status (n, %) | ||
| Nonfrail | 41 (50%) | 39 (45%) |
| Prefrail and frail | 41 (50%) | 47 (55%) |
| Sarcopenia (n, %)b | ||
| Probable | 17 (21%) | 19 (22%) |
| Diagnosis | 3 (4%) | 7 (8%) |
| Severe | 2 (2%) | 4 (5%) |
| Osteoarthritis (n, %) | 38 (46%) | 42 (49%) |
| SPPB total score (0–12)c | 10.1 ± 1.4 | 10.1 ± 2.0 |
| Standing balance (points) | 3.7 ± 0.6 | 3.6 ± 0.7 |
| 4-m gait speed (s) | 4.2 ± 0.9 | 4.2 ± 1.2 |
| Repeated chair rise (s) | 13.7 ± 3.4 | 13.1 ± 3.9 |
| Lean body mass (kg)d | 47.7 ± 9.1 | 48.0 ± 9.5 |
| Leg press strength (kg)e | 129.2 ± 41.1 | 122.8 ± 36.6 |
| Leg extension strength (kg)f | 66.8 ± 23.3 | 67.5 ± 22.9 |
| Knee extension strength (N)g | 309.9 ± 107.0 | 302.5 ± 96.1 |
Notes: SPPB = Short Physical Performance Battery. Data are presented as mean ± SD or n (%).
aEducation level: low: primary school or less; intermediate: secondary professional education or vocational school; and high: higher vocational education or university. Low and intermediate education level were combined due to low numbers in the low education category (n = 6). bN = 160. cN = 167. dN = 163; eN = 156. fN = 157. gN = 166.
Effects in Total Study Population and Per Subgroup
Table 2 presents the mean changes per study outcome after 12 weeks for the total study population and per subgroup. The model was adjusted for age, sex, educational level, and municipality. Adjustments affected the estimates of the interaction effect by more than 10% compared with the crude model (crude model; Supplementary Table S2). Further, 2-way interactions for Treatment × Subgroup are presented. In the total study population, significant mean changes were found on every study outcome. Effects in subgroups are described below and represent differences between the intervention and control group between T0 and T1.
Table 2.
Adjusted Mean Differences Per Study Outcome (mean Δ Int − Con) and Interaction Terms Treatment × Subgroup for Each Subgroup Separately
| SPPB (points) | Chair Rise (s) | Lean Body Mass (kg) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Treatment × Subgroup | Treatment × Subgroup | Treatment × Subgroup | |||||||
| N | Mean Δ Int − Con (95% CI) | β (95% CI) | N | Mean Δ Int − Con (95% CI) | β (95% CI) | N | Mean Δ Int – Con (95% CI) | β (95% CI) | |
| Total study population | 152 | 0.5 (0.1, 1.0)* | 148 | −1.6 (−2.6, −0.7)* | 150 | 0.5 (0.2, 0.9)* | |||
| Subgroup | |||||||||
| Age | |||||||||
| Age ≤ 75 | 84 | 0.7 (0.1, 1.3)* | −0.3 (−1.3, 0.6) | 84 | −2.0 (−3.2, −0.7)* | 0.8 (−1.2, 2.7) | 84 | 0.9 (0.5, 1.4)* | −0.8 (−1.5, −0.2)‡ |
| Age > 75 | 68 | 0.3 (−0.4, 1.0) | 64 | −1.2 (−2.7, 0.3) | 66 | 0.1 (−0.4, 0.6) | |||
| Sex | |||||||||
| Men | 63 | 0.3 (−0.4, 1.0) | 0.4 (−0.6, 1.3) | 62 | −1.0 (−2.5, 0.5) | −1.1 (−3.0, 0.9) | 65 | 0.8 (0.3, 1.3)* | −0.4 (−1.1, 0.3) |
| Women | 89 | 0.7 (0.1, 1.3)* | 86 | −2.1 (−3.3, −0.8)* | 85 | 0.4 (−0.1, 0.8) | |||
| Frailty state | |||||||||
| Prefrail and frail | 78 | 0.4 (−0.2, 1.1) | −0.1 (−1.1, 0.8) | 74 | −1.9 (−3.2, −0.5)* | −0.6 (−2.5, 1.4) | 76 | 0.5 (0.1, 1.0)* | 0.0 (−0.7, 0.7) |
| Nonfrail | 74 | 0.6 (−0.1, 1.3) | 74 | −1.3 (−2.7, 0.1) | 74 | 0.6 (0.1, 1.1)* | |||
| Sarcopenia | |||||||||
| Sarcopenia | 33 | 0.1 (−0.9, 1.1) | −0.6 (−1.7, 0.6) | 31 | −0.8 (−2.9, 1.3) | 1.1 (−1.2, 3.5) | 32 | 0.3 (−0.5, 1.0) | −0.3 (−1.2, 0.5) |
| No sarcopenia | 114 | 0.7 (0.1, 1.2)* | 112 | −1.9 (−3.0, −0.8)* | 113 | 0.6 (0.2, 1.0)* | |||
| Osteoarthritis | |||||||||
| Osteoarthritis | 72 | 0.7 (0.0, 1.4) | 0.3 (−0.7, 1.2) | 69 | −1.8 (−3.3, −0.4)* | −0.3 (−2.3, 1.6) | 69 | 0.5 (0.0, 1.0) | −0.1 (−0.8, 0.6) |
| No osteoarthritis | 80 | 0.4 (−0.3, 1.0) | 79 | −1.5 (−2.8, −0.2)* | 81 | 0.6 (0.1, 1.1)* | |||
| Knee Extension Strength (Newton) | Leg Press Strength (kg) | Leg Extension Strength (kg) | |||||||
| N | Mean Δ Int – Con (95% CI) | Treatment × Subgroup | N | Mean Δ Int − Con (95% CI) | Treatment × Subgroup | N | Mean Δ Int − Con (95% CI) | Treatment × Subgroup | |
| β (95% CI) | β (95% CI) | β (95% CI) | |||||||
| Total study population | 142 | 36.2 (17.8, 54.7)* | 136 | 16.0 (8.5, 23.6)* | 138 | 11.3 (7.6, 14.9)* | |||
| Subgroup | |||||||||
| Age | |||||||||
| Age ≤ 75 | 78 | 41.0 (16.0, 66.0)* | −10.3 (−47.1, 26.4) | 79 | 12.5 (2.6, 22.4)* | 8.4 (−6.7, 23.6) | 80 | 10.1 (5.3, 14.9)* | 2.7 (−4.7, 10.1) |
| Age > 75 | 64 | 30.6 (3.4, 57.8)* | 57 | 20.9 (9.4, 32.5)* | 58 | 12.8 (7.2, 18.4)* | |||
| Sex | |||||||||
| Men | 61 | 22.1 (−6.0, 50.2) | 24.3 (−12.3, 61.0) | 61 | 7.4 (−3.8, 18.6) | 15.5 (0.6, 30.3)‡ | 60 | 8.0 (2.4, 13.6)* | 5.7 (−1.6, 13.1) |
| Women | 81 | 46.5 (22.5, 70.5)* | 75 | 22.8 (12.9, 32.8)* | 78 | 13.7 (8.9, 18.5)* | |||
| Frailty state | |||||||||
| Prefrail and frail | 72 | 34.9 (9.1, 60.7)* | −3.2 (−40.8, 34.4) | 72 | 19.5 (9.2, 29.9)* | 7.7 (−7.7, 23.0) | 72 | 13.8 (8.8, 18.8)* | 4.8 (−2.6, 12.2) |
| Nonfrail | 70 | 38.2 (11.0, 65.3)* | 64 | 11.9 (0.6, 23.1)* | 66 | 9.0 (3.6, 14.4)* | |||
| Sarcopenia | |||||||||
| Sarcopenia | 30 | 23.5 (−16.9, 63.9) | −13.7 (−59.0, 31.6) | 29 | 23.0 (6.9, 39.1)* | 9.7 (−8.5, 27.8) | 29 | 15.4 (7.7, 23.1)* | 6.3 (−2.3, 15.0) |
| No sarcopenia | 108 | 37.2 (15.8, 58.5)* | 103 | 13.3 (4.7, 22.0)* | 104 | 9.1 (5.0, 13.1)* | |||
| Osteoarthritis | |||||||||
| Osteoarthritis | 67 | 45.1 (17.8, 72.4)* | 16.1 (−21.1, 53.3) | 60 | 16.7 (4.8, 28.5)* | 1.1 (−14.6, 16.8) | 63 | 10.3 (4.7, 15.9)* | −1.8 (−9.3, 5.8) |
| No osteoarthritis | 75 | 29.0 (3.9, 54.2)* | 76 | 15.6 (5.5, 25.7)* | 75 | 12.0 (7.0, 17.0)* | |||
Notes: Con = control group; Int = intervention group. Adjusted estimated mean differences between intervention and control group and 95% CIs are shown per subgroup; β-coefficients and 95% CIs are shown for the Treatment × Subgroup interaction. Adjusted for age, sex, educational level, and municipality. Treatment: control group is reference. Subgroup: reference groups are, respectively, men, age ≤ 75, nonfrail, no sarcopenia, and no osteoarthritis.
*Significant effect between intervention and control group (p < .05). ‡Significant interaction effect: Treatment × Subgroup (p < .05).
Age
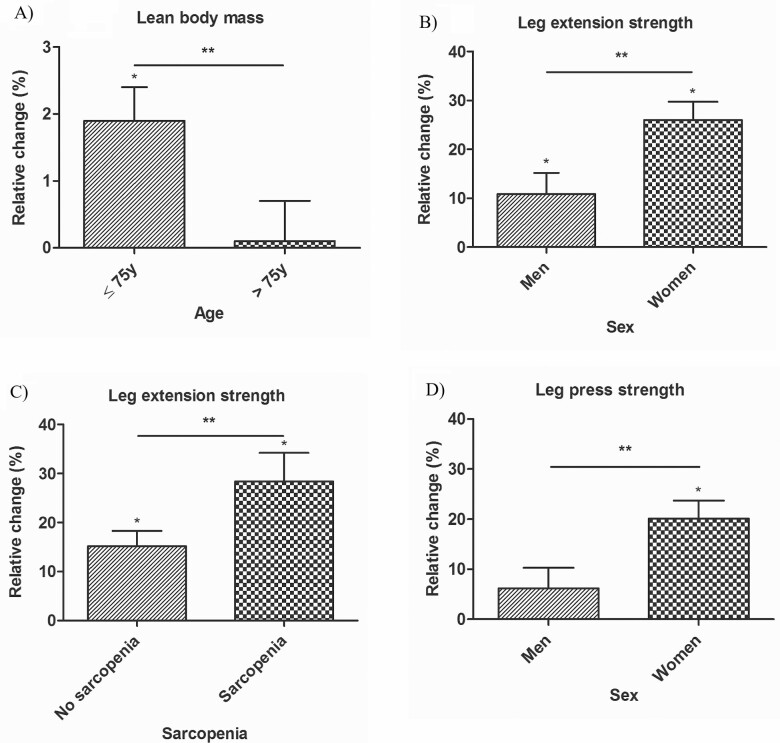
A significant positive effect on every study outcome was found for participants aged 75 years and younger (mean age: 71 ± 3). Participants older than 75 years (mean age: 81 ± 4) showed a significant increase on knee extension strength, leg press, and leg extension strength. The interaction effect Treatment × Age was found to be significant on lean body mass (β = −0.8; 95% CI: −1.5, −0.2). The increase in lean body mass for participants aged 75 years and younger was 0.9 kg (95% CI: 0.5, 1.4) and the increase in participants older than 75 years was 0.1 kg (95% CI: −0.4, 0.6). Relative changes and interaction effects were comparable to absolute changes and interaction effects (Supplementary Table S3); only the interaction Treatment × Age was found to be significant on lean body mass (β = −1.98; 95% CI: −3.3, −0.3), showing a higher increase in participants aged 75 years and younger compared to those aged older than 75 years (Figure 1A). No significant 3-way interaction effects including age were found.
Figure 1.
Significant 2-way interactions for relative changes (compared to baseline, in %) on (A) lean body mass: Treatment × Age, (B) leg extension strength: Treatment × Sex, (C) leg extension strength: Treatment × Sarcopenia, and (D) leg press strength: Treatment × Sex. Relative change is presented as adjusted estimated mean difference between intervention and control group in % and SE. Adjusted for age, sex, educational level, and municipality. *Significant effect in relative change compared to baseline (p < .05). **Significant interaction effect: Treatment × Subgroup (p < .05).
Sex
In women, a significant effect in the intervention compared to control group was found on every study outcome except for lean body mass. Men only showed a significant effect on lean body mass and leg extension strength. For leg press strength, the interaction effect Treatment × Sex was found to be significant (β = 15.5; 95% CI: 0.6, 30.3). The increase on leg press strength was 7.4 kg (95% CI: −3.8, 18.6) in men and 22.8 kg (95% CI: 12.9, 32.8) in women. Relative changes and interaction effects were comparable to absolute changes and interaction effects (Supplementary Table S3). However, when assessing relative changes, the interaction effect Treatment × Sex was significant for leg press strength (β = 13.9%; 95% CI: 3.1, 24.7), and leg extension strength (β = 15.2%; 95% CI: 4.0, 26.4), as presented in Figure 1, showing a higher relative change in women compared to men on both outcomes. Three-way interactions including sex were not significant. Only Treatment × Sex × Osteoarthritis for leg press strength was nearly significant (F = 3.047, p value = .051), showing the largest effects on leg press strength in women with osteoarthritis (mean effect: 26.5 kg, 95% CI: 12.8, 40.3).
Frailty
Nonfrail participants showed a significant increase on every study outcome, except for SPPB and chair rise. (Pre-)frail participants showed a significant improvement on every study outcome, except for SPPB. No significant 2- or 3-way interaction effects were found for frailty. Relative changes and interaction effects were comparable to absolute changes and interaction effects (Supplementary Table S3).
Sarcopenia
Nonsarcopenic participants showed significant improvements on every study outcome, whereas sarcopenic participants only showed significant effects on leg press and leg extension strength. No significant 2-way interaction effects were found. Relative changes and interaction effects were comparable to absolute changes and interaction effects, except for leg extension strength (Supplementary Table S3). The interaction effect Treatment × Sarcopenia was found to be significant for relative changes in leg extension strength (β = 13.2%; 95% CI: 0.4, 26.1), showing a larger increase in participants with sarcopenia compared to those without sarcopenia, as presented in Figure 1. The 3-way interaction Treatment × Osteoarthritis × Sarcopenia on leg press strength was found to be significant (F = 3.765, p value = .026). Exploring this interaction showed that the largest effects were found in sarcopenic participants without osteoarthritis (mean effect: 31.1 kg, 95% CI: 10.3, 52.0).
Osteoarthritis
Participants with osteoarthritis were found to have a significant increase on every study outcome, except for SPPB and lean body mass. Participants without osteoarthritis were found to have a significant increase on every study outcome, except for SPPB. No significant 2-way interaction effects were found for osteoarthritis. Relative changes and interaction effects were comparable to absolute changes and interaction effects (Supplementary Table S3). A nearly significant 3-way interaction on leg press strength was found for Treatment × Sex × Osteoarthritis (F = 3.047, p value = .051), and a significant 3-way interaction on leg press strength was found for Treatment × Osteoarthritis × Sarcopenia (F = 3.765, p value = .026), as described in previous paragraphs.
Discussion
The question “Who benefits most from ProMuscle in Practice?” can be answered after performing this in-depth analysis of the effects of the intervention. Participants aged 75 years and younger and women benefited to a great extent as they improved significantly on nearly every study outcome. Participants with and without a mobility-impairing disorder benefited equally from the intervention.
The randomized controlled trial was designed to study intervention effects in the total study population. As the current analyses were post hoc, the sample size was not calculated to detect differences between subgroups. Despite this, we were able to conduct in-depth analyses and report specific intervention effects in subgroups of older adults.
Age
Significant effects were found on every study outcome in participants aged 75 years and younger, whereas participants older than 75 years only showed significant effects on strength-related outcomes. Two-way interaction effects (Treatment × Age) were only significant for lean body mass, favoring participants aged 75 years and younger. In general, muscle mass is reported to decrease annually with 1%–2% after the age of 50 (34); however, our intervention led to a relative increase in lean body mass, being significantly higher in participants aged 75 years and younger (+1.9%) compared with participants aged older than 75 years (+0.1%). Two large meta-analyses of randomized controlled trials reported a trend toward higher changes in FFM in younger (±24 years) compared to older adults (±65 years) in response to RE and PS (16,20). This might be due to the blunted response of muscle protein synthesis to anabolic stimuli, such as exercise and food intake, that occurs with aging (35–37). Although other studies compared effects in younger and older adults, we were among the first to compare subgroups of older adults (≥65 years). Available meta-analyses reporting effects in FFM in older groups (>50 years and >65 years, respectively) (16,21) show results that are in line with the increases in lean body mass in our younger group (≤75 years). Hence, it is advised to start early with interventions such as ProMuscle in Practice, to prevent and counteract loss of muscle mass as early as possible.
Sex
In women, significant effects were found on every study outcome except lean body mass. On the contrary, in men, significant effects were only found on lean body mass and leg extension strength. Significant interaction effects including sex were found on leg press strength, favoring women. Additionally, the difference in relative change was found to be significant on leg press as well as leg extension strength, also indicating higher effects in women than in men. The sex differences cannot be attributed to compliance, as men and women adhered comparably to the intervention. Larger improvements in women might be due to their lower starting point compared with men, indicating more room for improvement. Mechanisms underlying these sex differences in leg strength changes in older adults are unclear to date and therefore need further investigation (38). Overall, similar studies show contradictory findings on changes in leg strength when comparing men and women. Retrospective analyses including RE studies in older adults also reported a higher relative change in 1RM in women compared with men (15); however, no statistically significant interaction with sex was found when assessing absolute effects (15,18). One meta-analysis reported a significant increase in leg strength in response to RE and PS in older men but not in older women. However, limited studies (N = 2) in older women were included (14). Another meta-analysis reported no sex differences in leg strength changes in older adults (39). Differences in results between these meta-analyses and the current study may be attributed to variations in population (older adults living in a nursing home or suffering from diseases vs community-dwelling older adults), length of the intervention (8–36 vs 12 weeks), type of RE intervention (RE or multicomponent exercise training vs RE), and type and amount of protein intake (supplementation of 3.0–40.8 g/d vs protein intake of 25 g per main meal) (14,39).
Only on lean body mass, significant effects were found in men and not in women. Comparable results were reported in a meta-analysis by Liao et al. (14). They indicated that sex may influence effects in muscle mass or muscle strength following RE and PS. The smaller changes in lean body mass in women might be due to older women’s decreased hypertrophy capacity in response to RE and their impaired ability to increase muscle protein synthesis after protein consumption (40). However, more research is needed to substantiate this. Conversely, 2 other meta-analyses found no difference in effects of RE and PS on changes in FFM between sexes (20,21). Again, the authors point to the fact that far less studies have been conducted in women (20). Altogether, more research is needed to examine sex-specific effects on lean body mass in response to RE and PS.
Mobility-Impairing Disorders
Because the study population included older adults with varying health status, tailoring the intervention to the (dis)abilities of the participants was essential (41). Results demonstrated no significant differences in 2-way interactions (regarding absolute effects) between treatment and, respectively, frailty, sarcopenia, and osteoarthritis. This indicates that both participants with and without mobility-impairing disorders can benefit from the intervention.
Frailty
Participants who were (pre-)frail improved significantly on every study outcome except for SPPB. Other studies also reported that exercise training combined with PS improved physical functioning, lean body mass, and leg strength in frail older adults (23,42,43).
Sarcopenia
Our findings indicate that participants with sarcopenia benefit to a great extent from the intervention in terms of leg strength, as they showed a significant increase on leg press and leg extension strength. Additionally, the 3-way interaction Treatment × Osteoarthritis × Sarcopenia for the outcome leg press strength was found to be significant, with the largest effects in sarcopenic participants without osteoarthritis. Moreover, when assessing relative changes to baseline, we found a significant difference in effects on leg extension strength between participants with and without sarcopenia. This is in line with results of a recent meta-regression analysis including interventions combining RE and PS, in which significant effects on leg muscle strength were found in older adults at high risk of sarcopenia and frailty (39). However, opposite to our results, 2 meta-analyses additionally reported improvements on physical functioning and muscle mass in sarcopenic older adults (21,39). The fact that we did not find comparable improvements in participants with sarcopenia may be due to several aspects. First, in this study, sarcopenia was defined as probable sarcopenia (based on low handgrip strength), which differed from the definition of sarcopenia in other studies. Second, the small subgroup of participants classified with probable sarcopenia (n = 36) likely decreased the power to detect differences between subgroups.
Osteoarthritis
Participants with osteoarthritis improved significantly on chair rise, knee extension, leg press, and leg extension strength. To date, few studies have investigated the effect of exercise training and PS in older adults with osteoarthritis. Nevertheless, a recent meta-analysis reported that exercise training and PS improved muscle mass, muscle strength, and functional outcomes in older adults with lower limb osteoarthritis (44). However, 5 of 6 interventions were postoperative, included participants who underwent total joint replacement, and most interventions consisted of 2 sessions/d for the duration of 2 weeks. Therefore, the populations and interventions included in the meta-analysis are not directly comparable to ProMuscle in Practice. Overall, interventions comprising RE and PS seem promising in improving muscle health in older adults, regardless of the nature of the intervention (preventive or postoperative) but more research is needed in both settings.
Outcome Reflections
SPPB score at baseline was relatively high (10.1 of 12 points). Besides, two-thirds of the participants had a SPPB score of 10 points or higher at baseline. This indicates that ceiling effects might have played a role in measuring change in physical performance (45). Therefore, a mix of outcome measures was included, consisting of chair rise performance, lean body mass, and strength-related measures, which were not subject to ceiling effects.
In addition to reporting “who benefits most” in terms of statistical significance, it is of importance to elaborate on the clinical meaningfulness of the results. Participants younger than 75 years and women improved significantly on nearly every outcome, including chair rise performance. Although the definition of a clinically meaningful change for chair rise performance is lacking to date, a definition for SPPB is available (46,47). Perera et al. (47) indicated that a 1.0 point change in SPPB could be considered a substantially meaningful change. In their study, a 1.0 point change represented a 12% increase in performance relative to baseline (SPPB: 8.3 ± 2.7). This corresponds with the percentage of change for chair rise performance in participants younger than 75 years and women in our study (17% and 16% mean change relative to baseline, respectively). Results for strength-related measurements are in line with this. Participants younger than 75 years showed mean changes ranging from 11% to 17% relative to baseline. Women showed mean changes ranging from 19% to 26% relative to baseline. Although definitions of clinically meaningful changes remain unclear for many outcomes, based on the aforementioned percentages, our results might be considered clinically meaningful.
Conclusion
Specific subgroups benefit to a greater extent from ProMuscle in Practice compared to others. In particular, older adults aged 75 years and younger and women exhibited larger increases on various outcomes compared to their counterparts. Additionally, participants with and without mobility-impairing disorders both benefited from ProMuscle in Practice, which suggests that the intervention is suitable for older adults regardless of having a mobility-impairing disorder. More insight is needed in underlying mechanisms to unravel the differences in responsiveness between subgroups.
Funding
This work was supported by the Dutch Ministry of Economic Affairs, Friesland Campina, and Innopastry (grant number TKI-AF-15206). Neither organization had any role in the design, analyses, or writing of this article.
Conflict of Interest
None declared.
Supplementary Material
Acknowledgments
We thank all participants, health care professionals, local organizations, municipalities, students, and research assistants involved in the ProMuscle in Practice study. The ProMuscle in Practice project is a public–private partnership. The public partners are responsible for the study design, data collection and analysis, decision to publish, and preparation of the manuscript. The private partners (FrieslandCampina, Innopastry, Nutrition and Healthcare Alliance, Zilveren Kruis) have contributed to the project through regular discussion, and financial and in-kind contributions.
Author Contributions
E.L.D., E.J.I.v.D., L.C.P.G.M.d.G., and A.H.-N. designed the ProMuscle in Practice study; B.G.D. and E.J.I.v.D. collected the data; B.G.D. performed the analyses and drafted the manuscript; and all authors provided suggestions to improve the manuscript.
References
- 1. Nelson EA, Dannefer D. Aged heterogeneity: fact or fiction? The fate of diversity in gerontological research. Gerontologist. 1992;32(1):17–23. doi: 10.1093/geront/32.1.17 [DOI] [PubMed] [Google Scholar]
- 2. Stone ME, Lin J, Dannefer D, Kelley-Moore JA. The continued eclipse of heterogeneity in gerontological research. J Gerontol B Psychol Sci Soc Sci. 2017;72(1):162–167. doi: 10.1093/geronb/gbv068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mitnitski A, Howlett SE, Rockwood K. Heterogeneity of human aging and its assessment. J Gerontol A Biol Sci Med Sci. 2017;72(7):877–884. doi: 10.1093/gerona/glw089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Carnes BA, Olshansky SJ. Heterogeneity and its biodemographic implications for longevity and mortality. Exp Gerontol. 2001;36(3):419–430. doi: 10.1016/s0531-5565(00)00254-0 [DOI] [PubMed] [Google Scholar]
- 5. Yashin AI, Arbeev KG, Arbeeva LS, et al. . How the effects of aging and stresses of life are integrated in mortality rates: insights for genetic studies of human health and longevity. Biogerontology. 2016;17(1):89–107. doi: 10.1007/s10522-015-9594-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Montero-Odasso M, Bergman H, Béland F, Sourial N, Fletcher JD, Dallaire L. Identifying mobility heterogeneity in very frail older adults. Are frail people all the same? Arch Gerontol Geriatr. 2009;49(2):272–277. doi: 10.1016/j.archger.2008.09.010 [DOI] [PubMed] [Google Scholar]
- 7. Rantakokko M, Mänty M, Rantanen T. Mobility decline in old age. Exerc Sport Sci Rev. 2013;41(1):19–25. doi: 10.1097/JES.0b013e3182556f1e [DOI] [PubMed] [Google Scholar]
- 8. Morley JE. Sarcopenia in the elderly. Fam Pract. 2012;29(1):i44–i48. doi: 10.1093/fampra/cmr063 [DOI] [PubMed] [Google Scholar]
- 9. Fried LP, Tangen CM, Walston J, et al. ; Cardiovascular Health Study Collaborative Research Group . Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56(3):M146–M156. doi: 10.1093/gerona/56.3.m146 [DOI] [PubMed] [Google Scholar]
- 10. Stuck AE, Walthert JM, Nikolaus T, Büla CJ, Hohmann C, Beck JC. Risk factors for functional status decline in community-living elderly people: a systematic literature review. Soc Sci Med. 1999;48(4):445–469. doi: 10.1016/s0277-9536(98)00370-0 [DOI] [PubMed] [Google Scholar]
- 11. StatLine. Prognose bevolking; geslacht en leeftijd, 2020–2060. 2019. https://opendata.cbs.nl/statline/#/CBS/nl/dataset/84646NED/table?ts=1604479523506. Accessed November 4, 2020.
- 12. Colonetti T, Grande AJ, Milton K, et al. . Effects of whey protein supplement in the elderly submitted to resistance training: systematic review and meta-analysis. Int J Food Sci Nutr. 2017;68(3):257–264. doi: 10.1080/09637486.2016.1232702 [DOI] [PubMed] [Google Scholar]
- 13. Finger D, Goltz FR, Umpierre D, Meyer E, Rosa LH, Schneider CD. Effects of protein supplementation in older adults undergoing resistance training: a systematic review and meta-analysis. Sports Med. 2015;45(2):245–255. doi: 10.1007/s40279-014-0269-4 [DOI] [PubMed] [Google Scholar]
- 14. Liao CD, Tsauo JY, Wu YT, et al. . Effects of protein supplementation combined with resistance exercise on body composition and physical function in older adults: a systematic review and meta-analysis. Am J Clin Nutr. 2017;106(4):1078–1091. doi: 10.3945/ajcn.116.143594 [DOI] [PubMed] [Google Scholar]
- 15. Churchward-Venne TA, Tieland M, Verdijk LB, et al. . There are no nonresponders to resistance-type exercise training in older men and women. J Am Med Dir Assoc. 2015;16(5):400–411. doi: 10.1016/j.jamda.2015.01.071 [DOI] [PubMed] [Google Scholar]
- 16. Cermak NM, Res PT, de Groot LC, Saris WH, van Loon LJ. Protein supplementation augments the adaptive response of skeletal muscle to resistance-type exercise training: a meta-analysis. Am J Clin Nutr. 2012;96:1454–1464. doi: 10.3945/ajcn.112.037556 [DOI] [PubMed] [Google Scholar]
- 17. Daly RM. Dietary protein, exercise and skeletal muscle: is there a synergistic effect in older adults and the elderly? In: Weaver C, Daly R, Bischoff-Ferrari H, ed. Nutritional Influences on Bone Health. Cham, Switzerland: Springer; 2016:63–75. doi: 10.1007/978-3-319-32417-3 [DOI] [Google Scholar]
- 18. Ahtiainen JP, Walker S, Peltonen H, et al. . Heterogeneity in resistance training-induced muscle strength and mass responses in men and women of different ages. Age (Dordr). 2016;38(1):10. doi: 10.1007/s11357-015-9870-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hubal MJ, Gordish-Dressman H, Thompson PD, et al. . Variability in muscle size and strength gain after unilateral resistance training. Med Sci Sports Exerc. 2005;37(6):964–972. doi:10.1249.01.mss.0000170469.90461.5f [PubMed] [Google Scholar]
- 20. Morton RW, Murphy KT, McKellar SR, et al. . A systematic review, meta-analysis and meta-regression of the effect of protein supplementation on resistance training-induced gains in muscle mass and strength in healthy adults. Br J Sports Med. 2018;52(6):376–384. doi: 10.1136/bjsports-2017-097608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hidayat K, Chen GC, Wang Y, et al. . Effects of milk proteins supplementation in older adults undergoing resistance training: a meta-analysis of randomized control trials. J Nutr Health Aging. 2018;22(2):237–245. doi: 10.1007/s12603-017-0899-y [DOI] [PubMed] [Google Scholar]
- 22. Anton S, Hida A, Mankowski R, et al. . Nutrition and exercise in sarcopenia. Curr Protein Pept Sci. 2017;17:1–19. doi: 10.2174/1389203717666161227144349 [DOI] [PubMed] [Google Scholar]
- 23. Liao CD, Lee PH, Hsiao DJ, et al. . Effects of protein supplementation combined with exercise intervention on frailty indices, body composition, and physical function in frail older adults. Nutrients. 2018;10(12):1–30. doi: 10.3390/nu10121916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. de Zwart AH, van der Leeden M, Roorda LD, et al. . Dietary protein intake and upper leg muscle strength in subjects with knee osteoarthritis: data from the osteoarthritis initiative. Rheumatol Int. 2019;39(2):277–284. doi: 10.1007/s00296-018-4223-x [DOI] [PubMed] [Google Scholar]
- 25. Hunter DJ, Bierma-Zeinstra S. Osteoarthritis. Lancet. 2019; 393(10182):1745–1759. doi: 10.1016/S0140-6736(19)30417-9 [DOI] [PubMed] [Google Scholar]
- 26. Fransen M, McConnell S, Hernandez-Molina G, Reichenbach S. Exercise for osteoarthritis of the hip. Annu Rev Gerontol Geriatr. 2014;36(1):155–168. doi: 10.1891/0198-8794.36.155 [DOI] [Google Scholar]
- 27. Fransen M, McConnell S, Harmer AR, Van der Esch M, Simic M, Bennell KL. Exercise for osteoarthritis of the knee: a Cochrane systematic review. Br J Sports Med. 2015;49(24):1554–1557. doi: 10.1136/bjsports-2015-095424 [DOI] [PubMed] [Google Scholar]
- 28. van Dongen EJI, Haveman-Nies A, Doets EL, Dorhout BG, de Groot LCPGM. Effectiveness of a diet and resistance exercise intervention on muscle health in older adults: ProMuscle in Practice. J Am Med Dir Assoc. 2020;21:1065–1072.e3. doi: 10.1016/j.jamda.2019.11.026 [DOI] [PubMed] [Google Scholar]
- 29. van Dongen EJI, Haveman-Nies A, Wezenbeek NLW, Dorhout BG, Doets EL, de Groot LCPGM. Effect, process, and economic evaluation of a combined resistance exercise and diet intervention (ProMuscle in Practice) for community-dwelling older adults: design and methods of a randomised controlled trial. BMC Public Health. 2018;18(1):877. doi: 10.1186/s12889-018-5788-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Guralnik JM, Simonsick EM, Ferrucci L, et al. . A Short Physical Performance Battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49(2):M85–M94. doi: 10.1093/geronj/49.2.m85 [DOI] [PubMed] [Google Scholar]
- 31. Jones CJ, Rikli RE, Beam WC. A 30-s chair-stand test as a measure of lower body strength in community-residing older adults. Res Q Exerc Sport. 1999;70(2):113–119. doi: 10.1080/02701367.1999.10608028 [DOI] [PubMed] [Google Scholar]
- 32. Brzychi M. Strength testing—predicting a one-rep max from reps-to-fatigue. J Phys Educ Recreat Danc. 1993;64(1):88–90. doi: 10.1080/07303084.1993.10606684 [DOI] [Google Scholar]
- 33. Cruz-Jentoft AJ, Bahat G, Bauer J, et al. . Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019;48( 1):16–31. doi: 10.1093/ageing/afy169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Abellan van Kan G. Epidemiology and consequences of sarcopenia. J Nutr Health Aging. 2009;13(8):708–712. doi: 10.1007/s12603-009-0201-z [DOI] [PubMed] [Google Scholar]
- 35. Fry CS, Rasmussen BB. Skeletal muscle protein balance and metabolism in the elderly. Curr Aging Sci. 2011;4(3):260–268. doi: 10.2174/1874609811104030260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Breen L, Phillips SM. Skeletal muscle protein metabolism in the elderly: interventions to counteract the “anabolic resistance” of ageing. Nutr Metab. 2011;8(1):68. doi: 10.1186/1743-7075-8-68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Burd NA, Gorissen SH, van Loon LJ. Anabolic resistance of muscle protein synthesis with aging. Exerc Sport Sci Rev. 2013;41(3):169–173. doi: 10.1097/JES.0b013e318292f3d5 [DOI] [PubMed] [Google Scholar]
- 38. Da Boit M, Sibson R, Meakin JR, et al. . Sex differences in the response to resistance exercise training in older people. Physiol Rep. 2016;4(12):1–8. doi: 10.14814/phy2.12834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. De Liao C, Chen HC, Huang SW, Liou TH. The role of muscle mass gain following protein supplementation plus exercise therapy in older adults with sarcopenia and frailty risks: a systematic review and meta-regression analysis of randomized trials. Nutrients. 2019;11(10):1–23. doi: 10.3390/nu11081713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Burd NA, Tang JE, Moore DR, Phillips SM. Exercise training and protein metabolism: influences of contraction, protein intake, and sex-based differences. J Appl Physiol (1985). 2009;106(5):1692–1701. doi: 10.1152/japplphysiol.91351.2008 [DOI] [PubMed] [Google Scholar]
- 41. van Dongen EJI, Doets EL, de Groot LCPGM, Dorhout BG, Haveman-Nies A. Process evaluation of a combined lifestyle intervention for community-dwelling older adults: ProMuscle in Practice. Gerontologist. 2020;60(8):1538–1554. doi: 10.1093/geront/gnaa027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Jadczak AD, Makwana N, Luscombe-Marsh N, Visvanathan R, Schultz TJ. Effectiveness of exercise interventions on physical function in community-dwelling frail older people: an umbrella review of systematic reviews. JBI Database System Rev Implement Rep. 2018;16(3):752–775. doi: 10.11124/JBISRIR-2017-003551 [DOI] [PubMed] [Google Scholar]
- 43. Tieland M, Dirks ML, van der Zwaluw N, et al. . Protein supplementation increases muscle mass gain during prolonged resistance-type exercise training in frail elderly people: a randomized, double-blind, placebo-controlled trial. J Am Med Dir Assoc. 2012;13(8):713–719. doi: 10.1016/j.jamda.2012.05.020 [DOI] [PubMed] [Google Scholar]
- 44. De Liao C, Wu YT, Tsauo JY, et al. . Effects of protein supplementation combined with exercise training on muscle mass and function in older adults with lower-extremity osteoarthritis: a systematic review and meta-analysis of randomized trials. Nutrients. 2020;12(8):1–19. doi: 10.3390/nu12082422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Guralnik JM, Ferrucci L, Pieper CF, et al. . Lower extremity function and subsequent disability: consistency across studies, predictive models, and value of gait speed alone compared with the Short Physical Performance Battery. J Gerontol A Biol Sci Med Sci. 2000;55(4):M221–M231. doi: 10.1093/gerona/55.4.m221 [DOI] [PubMed] [Google Scholar]
- 46. Guralnik J, Bandeen-Roche K, Bhasin SAR, et al. . Clinically meaningful change for physical performance: perspectives of the ICFSR task force. J Frailty Aging. 2020;9(1):9–13. doi: 10.14283/jfa.2019.33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Perera S, Mody SH, Woodman RC, Studenski SA. Meaningful change and responsiveness in common physical performance measures in older adults. J Am Geriatr Soc. 2006;54(5):743–749. doi: 10.1111/j.1532-5415.2006.00701.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.