Abstract
Coronary microvascular disease (CMD), characterized by impaired coronary flow reserve (CFR), is a common finding in patients with stable angina. Impaired CFR, in the absence of obstructive coronary artery disease, is also present in up to 75% of patients with heart failure with preserved ejection fraction (HFpEF). Heart failure with preserved ejection fraction is a heterogeneous syndrome comprising distinct endotypes and it has been hypothesized that CMD lies at the centre of the pathogenesis of one such entity: the CMD–HFpEF endotype. This article provides a contemporary review of the pathophysiology underlying CMD, with a focus on the mechanistic link between CMD and HFpEF. We discuss the central role played by subendocardial ischaemia and impaired lusitropy in the development of CMD–HFpEF, as well as the clinical and research implications of the CMD–HFpEF mechanistic link. Future prospective follow-up studies detailing outcomes in patients with CMD and HFpEF are much needed to enhance our understanding of the pathological processes driving these conditions, which may lead to the development of physiology-stratified therapy to improve the quality of life and prognosis in these patients.
Keywords: Coronary microvascular disease, Coronary flow reserve, Subendocardial ischaemia, Lusitropy, Heart failure with preserved ejection fraction
Graphical Abstract
Introduction
Up to half of patients with angina and non-obstructive coronary artery disease (NOCAD) have evidence of coronary microvascular disease (CMD).1 Coronary microvascular disease is defined as an abnormality in the microcirculation leading to an inadequate vasodilatory response, or a pathological vasoconstrictive response, to physiological or pharmacological stress.2 Coronary microvascular disease can be due to endothelial and/or vascular smooth muscle (VSM) (endothelium-independent) dysfunction. Contemporary invasive tests used to diagnose CMD are based on assessing the ability of the microvasculature to augment blood flow in response to a vasodilator stimulus, i.e. the flow reserve.3–5 Endothelium-independent CMD is defined as a coronary flow reserve (CFR) of <2.5 in response to adenosine, whereas endothelium-dependent CMD is defined as acetylcholine flow reserve of <1.5 in response to acetylcholine.4 , 6 In the context of NOCAD, diminished CFR and/or acetylcholine flow reserve is associated with inducible ischaemia, impaired quality of life, and increased risk of adverse cardiovascular outcomes.7–11 There is emerging evidence linking coronary microvascular dysfunction to heart failure with preserved ejection fraction (HFpEF),12–14 with up to 75% of patients with HFpEF suffering from impaired CFR despite the absence of obstructive coronary artery disease.15 Heart failure with preserved ejection fraction is a heterogeneous syndrome characterized by exercise intolerance. The contemporary cognizance is that HFpEF, like NOCAD, is an umbrella term comprising distinct endotypes, each with disparate underlying pathophysiology, therapeutic targets, and prognosis. Coronary microvascular disease-related HFpEF (CMD–HFpEF) represents one distinct endotype (Figure 1). In this review article, we will discuss the mechanistic role played by CMD in the pathogenesis of CMD–HFpEF, including abnormalities in cardiac–coronary coupling, which underlie the intimate relationship between myocardial perfusion and diastolic function.
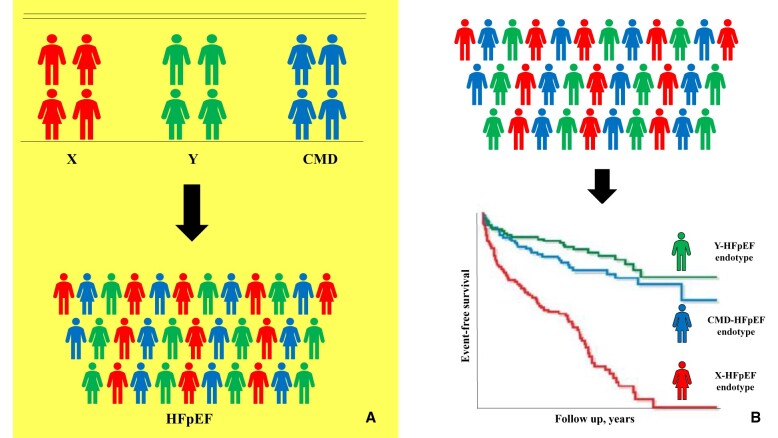
Figure 1.
This figure illustrates that heart failure with preserved ejection fraction is a heterogeneous syndrome comprising distinct endotypes, each with disparate underlying pathophysiology, therapeutic options, and outcomes. Patients can be characterized clinically according to factors such as pathophysiology (A), or they can be characterized using artificial intelligence-derived clusters that groups patients according to their clinical characteristics and clinical outcomes (B). Note: (B) is illustrative and not based on real data. CMD, coronary microvascular disease; HFpEF, heart failure with preserved ejection fraction.
Cardiac–coronary coupling
Myocardial perfusion is dependent on CFR and the dynamic interaction between myocardium and microvasculature. As a result of the phasic compression and decompression of intramyocardial vessels, coronary flow is intimately linked to myocardial relaxation and contraction; this process is called cardiac–coronary coupling, which can be readily characterized by coronary wave intensity analysis.16 Wave intensity analysis defines the nature (accelerating or decelerating flow) and origin (aortic, designated as forward, or microcirculatory, designated as backward) of energy fluxes that govern coronary blood flow (CBF). The backward expansion wave (BEW) is the main driver of flow in the healthy heart and it is secondary to decompression of the microvasculature in early diastole. Therefore, it is directly related to the degree of ventricular relaxation (lusitropy). The other major accelerating wave is the forward compression wave, which corresponds to the rise in aortic pressure after the aortic valve opens in early systole. The major decelerating wave is the backward compression wave (BCW), which arises during isovolumetric contraction. The relative balance of these wave energies is encapsulated in perfusion efficiency, which is the proportion of accelerating energy in relation to total energy flux; perfusion efficiency increases with exercise and pharmacologically induced microvascular dilation in health.17 In contrast, perfusion efficiency decreases with both exercise and pharmacologically induced microvascular dilation in CMD,3 primarily driven by attenuation of the accelerating BEW and accentuation of the decelerating BCW during exercise. Attenuation of the BEW during exercise can result from impaired lusitropy and a diastolic dysfunction phenotype that manifests during higher workloads, with resultant subendocardial ischaemia. Alternatively, subendocardial ischaemia during exercise may precipitate ischaemia-induced diastolic dysfunction, with diminished lusitropy subsequently impairing coronary perfusion further and resulting in an ischaemic cascade. As coronary flow is intimately linked with myocardial relaxation (and contraction), it can be challenging to ascertain causality between ischaemia and diastolic dysfunction.
Impaired ventricular relaxation has an adverse impact on coronary flow. In a coronary Doppler study of patients with unobstructed coronary arteries and either normal left ventricular (LV) function or a cardiomyopathic process, early diastolic coronary flow at rest and CFR were attenuated in those with abnormal LV relaxation. Diastolic coronary flow was further reduced with an increase in heart rate in patients with impaired LV relaxation at rest.18
These concepts underpin the intimate relationship between coronary flow and ventricular relaxation state (Figure 2). Alteration of this relationship lies at the forefront of the CMD–HFpEF pathophysiology. This will be discussed in more detail later in the review article.
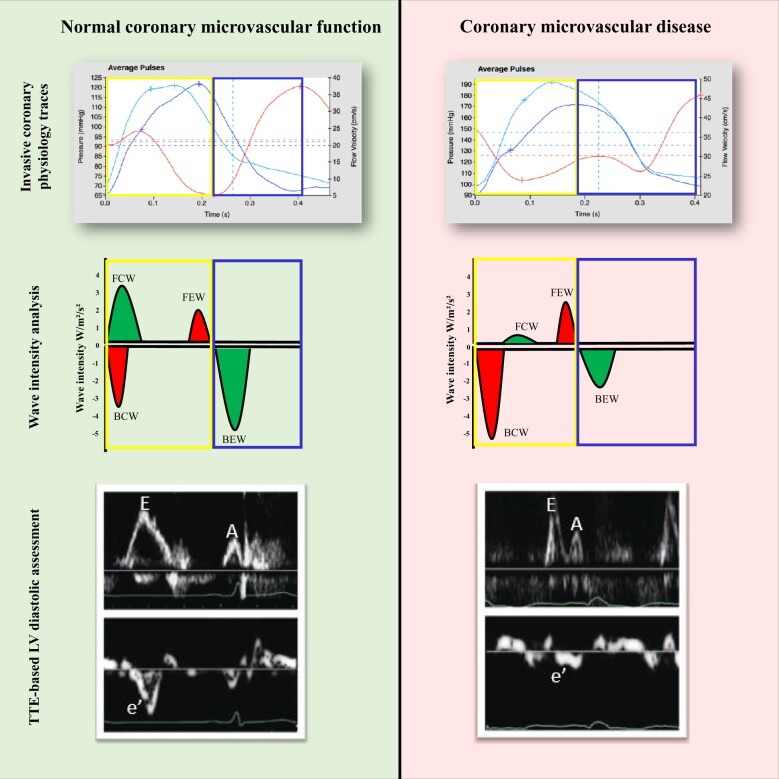
Figure 2.
The left panel represents a normal control: the backward expansion wave becomes augmented on exertion, indicating enhanced lusitropy and myocardial perfusion. The right panel represents a patient with coronary microvascular disease: The backward compression wave indicates deceleration of flow and is augmented during exertion in these patients, whereas the backward expansion wave is attenuated. Note that diastole is defined electrically and all haemodynamic traces are gated to the R wave. The traces of aortic pressure, coronary pressure, and flow velocity are ensemble-averaged waveforms in a single calibrated wave. The wave intensity values (W/m2/s2) are for illustration purposes only and do not represent real data. The transthoracic echocardiogram-derived Doppler traces demonstrate normal left ventricular diastolic function in a control patient and impaired left ventricular diastolic function in a patient with coronary microvascular disease. BCW, backward compression wave; BEW, backward expansion wave; FCW, forward compression wave; FEW, forward expansion wave; LV, left ventricular; TTE, transthoracic echocardiogram.
Pathophysiology of coronary microvascular disease
Traditionally, CMD has been attributed to a combination of microvascular architectural changes (such as microvascular obstruction and rarefaction), endothelial dysfunction, and/or VSM dysfunction. Endothelial or VSM (endothelium-independent) dysfunction may lead to an attenuated vasodilatory or a pathological vasoconstrictive response to stimuli, leading to a blunted augmentation of, or reduction of, CBF in response to stress.19 This can cause a supply–demand mismatch, leading to myocardial ischaemia. The cellular mechanisms regulating microvascular tone are summarized in Figure 3. However, recent animal models and clinical physiology evaluations suggest that coronary microvascular dysfunction may be a heterogeneous condition comprising distinct entities that form part of a disease spectrum. Based on physiology assessment in the catheter laboratory, we have described the presence of two distinct CMD endotypes, termed ‘structural CMD’ and ‘functional CMD’.3 Both endotypes display impaired augmentation of CBF in response to intravenous adenosine (CFR < 2.5). However, whilst patients with structural CMD have an elevated minimal microvascular resistance (MR) (which translates to reduced maximal CBF), patients with functional CMD have a normal minimal MR but an attenuated vasodilatory reserve as they have reduced tone at rest.3 , 4 The endotypes have a similar core phenotype, with both groups demonstrating high prevalence of inducible ischaemia and inefficient cardiac–coronary coupling during physical exercise, but their pathogenesis differs at the microvascular level.3 , 4 Vascular tone is mediated by nitric oxide (NO), which is synthesized by NO synthase (NOS). Patients with functional CMD have heightened resting CBF and NOS activity, reflecting a near-maximal vasodilatory state at rest (reduced resting microvascular tone), leading to an attenuated vasodilatory capacity in response to physiological stress.4 Endothelial NOS (eNOS) is thought to maintain hyperaemic CBF in response to hypoxia and shear stress, whilst neuronal NOS (nNOS) is thought to maintain CBF at rest (at least in the healthy heart).20 , 21 The elevated resting CBF in patients with functional CMD could be due to up-regulation of nNOS either as an appropriate response to an increased myocardial oxygen demand at rest or due to disordered autoregulation. Conversely, patients with structural CMD have normal resting CBF3 , 4 but an impaired ability to augment CBF in response to physiological stress and diminished peripheral endothelium-dependent dilatation, precipitating exercise-induced hypertension. The attenuated reduction in afterload with exercise interrupts the usual synergistic response of the coronary and peripheral circulations and predisposes to ischaemia in patients with structural CMD.3 However, it remains unclear whether patients with structural CMD have an impaired ability to augment their CBF as a result of irreversible architectural changes, such as microvascular hypertrophy and fibrosis, limiting their ability to vasodilate, or whether this reflects a reversible disequilibrium of the pathways that mediate vasomotor tone, such as eNOS dysfunction.
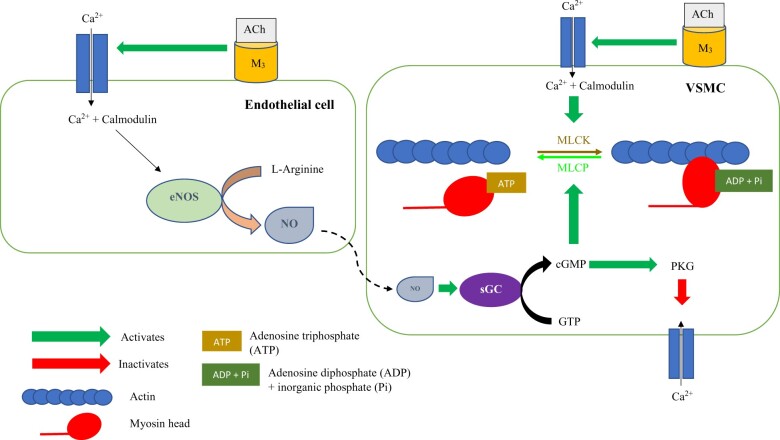
Figure 3.
Acetylcholine has dual effects on coronary microvasculature. It binds to the muscarinic 3 receptor on endothelial cells and leads to an influx of intracellular calcium via the L-type calcium channels. Intracellular calcium binds to the protein calmodulin, and the calcium–calmodulin complex activates the endothelial nitric oxide synthase enzyme, which catalyzes the conversion of L-Arginine into nitric oxide. Nitric oxide then diffuses into the neighbouring vascular smooth muscle cell and activates soluble Guanylate Cyclase enzyme to catalyze the conversion of Guanosine Triphosphate into cyclic Guanosine Monophosphate. Cyclic Guanosine Monophosphate activates the protein kinase G, which, via a series of intracellular events, inactivates the calcium channels on the vascular smooth muscle cell. This reduces the intracellular influx of calcium into the vascular smooth muscle cell, therefore leading to vasodilation. Acetylcholine also binds to the muscarinic 3 receptor on the surface of vascular smooth muscle cells and, in the presence of endothelial dysfunction, leads to unopposed vasoconstriction. Calcium enters vascular smooth muscle cells via the L-type calcium channels and binds to the protein calmodulin. The calcium–calmodulin complex activates myosin light chain kinase, which phosphorylates myosin light chains. Myosin light chains are found on the myosin heads and myosin light chain phosphorylation leads to cross-bridge formation between the myosin heads and the actin filaments, leading to vascular smooth muscle contraction. Myosin light chain phosphatase dephosphorylates myosin light chain and promotes unbinding of the myosin-actin filaments, therefore leading to vasodilation. Cyclic Guanosine Monophosphate promotes myosin light chain phosphatase activity. The myosin head detaches from the actin binding site after adenosine triphosphate attaches to the myosin head. This adenosine triphosphate is then hydrolyzed to adenosine diphosphate and inorganic phosphate by the myosin head; this adenosine diphosphate and inorganic phosphate is then released by the myosin head after the power stroke. At this point, the myosin head is ready for the next adenosine triphosphate to allow detachment from the myosin head. ACh, acetylcholine; ADP, adenosine diphosphate; ATP, adenosine triphosphate; Ca2+, calcium; cGMP, cyclic Guanosine Monophosphate; eNOS, endothelial nitric oxide synthase; GTP, Guanosine Triphosphate; M3, muscarinic 3; MLCs, myosin light chains; MLCK, myosin light chain kinase; MLCP, Myosin light chain phosphatase; NO, nitric oxide; Pi, inorganic phosphate; PKG, protein kinase G; sGC, soluble Guanylate Cyclase; VSMC, vascular smooth muscle cell.
Similar pathobiological endotypes have been described by other groups. A bimodal distribution of impaired CFR has been reported in patients with type 2 diabetes mellitus (T2DM) depending on the duration of diabetes.22 In the early stages of diabetes (<10-year duration), CFR was diminished due to elevated resting CBF whereas in the latter stages of the disease (>10-year duration), this was mainly due to a reduction in maximal CBF (secondary to heightened hyperaemic MR). The elevated resting flow in the early stages of T2DM may represent impaired coronary microvascular autoregulation or an appropriate adaptive response to altered myocardial energy metabolism. Furthermore, it is conceivable that the increased resting CBF in the early stages of T2DM may lead to shear stress-induced architectural changes in the coronary microvasculature, contributing to heightened MR during hyperaemia, leading to an attenuated maximal CBF in the later stages of the disease.22 Animal studies have corroborated the findings of elevated resting CBF being associated with coronary microvascular dysfunction and myocardial ischaemia. In a swine model, animals with CMD were found to have heightened resting CBF, with a correspondingly high basal myocardial oxygen consumption (MVO2). The former meant that, despite maintaining their hyperaemic CBF, there was reduced CFR.23 As elevated MVO2 could not be matched by augmenting blood flow (i.e. myocardial oxygen delivery), there was a reduction in lactate consumption indicating anaerobic metabolism, and therefore, ischaemia. The authors of this study suggested that the basal oxygen demand was elevated either due to a myocardial substrate shift towards fatty acid oxidation leading to a reduced phosphate:oxygen ratio and an increased oxygen consumption for adenosine triphosphate (ATP) production or that it was due to mitochondrial uncoupling leading to a reduction in the phosphate:oxygen ratio, thereby increasing oxygen consumption at any given level of cardiac work.23 Interestingly, in a cohort of 74 women with angina, unobstructed coronary arteries and impaired CFR, low basal CBF (measured indirectly as basal average peak velocity) was associated with higher LV end-diastolic pressure and impaired diastolic strain. There was no difference in cardiovascular risk factors or LV structure between women with low and high basal CBF. The authors concluded that low basal CBF is associated with worse myocardial performance and may eventually lead to heart failure.24 This cohort of patients demonstrated similar physiological properties as patients with structural CMD. These mechanistic and clinical studies suggest that coronary microvascular dysfunction may lie on a spectrum, with normal function at one end and CMD–HFpEF at the other.
Finally, whilst the NO pathway is central to the development of CMD, dysfunction of the endothelin-1 (ET-1) pathway has also been implicated.25 , 26 Endothelin-1 is a highly potent coronary arteriolar vasoconstrictor; this effect is mediated by activation of the G-protein coupled endothelin A receptors on VSM cells. A specific genetic allele, which is associated with higher serum ET-1 levels, impaired myocardial perfusion on cardiac magnetic resonance imaging and reduced exercise tolerance has been identified in patients with angina and CMD.27 This supports the role of ET-1 dysregulation in the pathogenesis of CMD, as well as the possibility of precision medicine using genetics to target the ET-1 pathway in patients with CMD.28
Pathophysiological links between coronary microvascular disease and heart failure with preserved ejection fraction
Impaired lusitropy
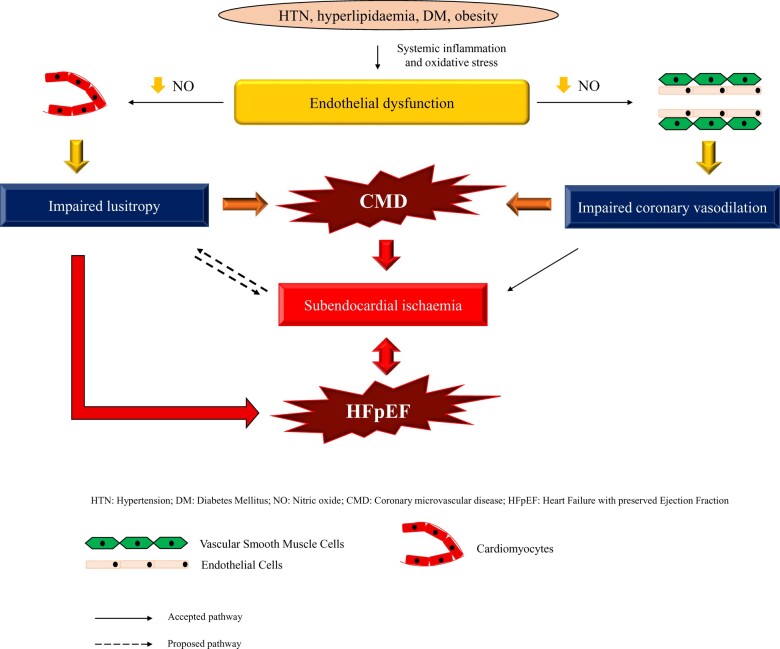
Whilst the significance of impaired NO pathways is well established in the development of CMD, eNOS dysfunction is also thought to play a central role in the pathogenesis of HFpEF.29 A systemic inflammatory state, induced by cardiovascular risk factors, leads to increased endothelial production of reactive oxygen species.30 Reactive oxygen species, such as superoxide ions, react with NO to form peroxynitrite. Peroxynitrite oxidizes the eNOS cofactor tetrahydrobiopterin (BH4), which results in eNOS uncoupling and reduced NO production and bioavailability, which, in turn, leads to reduced cyclic guanosine monophosphate (cGMP) and protein kinase G (PKG) activity. Protein kinase G is involved in titin phosphorylation; titin is a cytoskeletal protein that acts as a bidirectional spring and is responsible for early diastolic recoil and late diastolic distensibility of cardiomyocytes. Therefore, titin hypophosphorylation makes cardiomyocytes less compliant.31 The impaired NO–cGMP–PKG axis leads to heightened diastolic stiffness through hypophosphorylation of titin in cardiomyocytes, leading to impaired lusitropy and LV diastolic reserve (Graphical Abstract).
The mechanistic link between coronary microvascular disease and heart failure with preserved ejection fraction.
Furthermore, an up-regulation of inducible nitric oxide synthase (iNOS) has also been implicated in the pathogenesis of HFpEF.32 Pharmacological eNOS inhibition, in a murine model of HFpEF, enhanced iNOS activity. This led to dysregulation of protein quality control, resulting in accumulation of misfolded proteins, which ultimately resulted in cardiomyocyte dysfunction and impaired lusitropy. Importantly, pharmacological inhibition of iNOS improved lusitropy and exercise intolerance in this model of HFpEF.32
Subendocardial ischaemia
Mechanistic studies have consistently demonstrated that patients with HFpEF have an inability to adequately augment their myocardial blood flow (MBF) and oxygen delivery during stress. Patients with HFpEF and NOCAD demonstrate a higher LV external work, MVO2 and MBF at rest but blunted rise in these indices during dobutamine-induced stress, compared to control subjects.33 Although myocardial oxygen extraction is enhanced, the rise in MBF (supply) is inadequate to meet the change in LV external work (demand) in patients with HFpEF. The myocardial oxygen supply:demand mismatch is associated with limitations in LV systolic and diastolic reserve, alongside the inability to adequately increase cardiac output during exercise, which can lead to sufficient ischaemia to cause elevation in high-sensitivity troponin T levels.34 Patients with HFpEF have reduced phosphocreatine:ATP ratio during exercise,35 which may explain the impaired LV diastolic reserve as ATP is required during diastole for the detachment of the myosin head from actin and for the extrusion of intracellular calcium ions. Reduced ATP production, due to inadequate myocardial perfusion, will likely lead to incomplete diastolic relaxation due to diastolic cross-bridge cycling. Whilst these data suggest that subendocardial ischaemia, due to attenuated augmentation of MBF during stress, may be pathogenetic in HFpEF, it is not clear whether this precedes the changes in myocardial reserve or is caused by the latter. A vicious cycle has been proposed, whereby attenuated oxygen delivery impairs myocyte relaxation, which promotes heightened myocardial tension and increased oxygen consumption demands.36 , 37
In summary, cardiovascular risk factors, such as hypertension, diabetes mellitus, and hyperlipidaemia, lead to a systemic inflammatory state, which leads to endothelial dysfunction through eNOS uncoupling. Coronary endothelial dysfunction, clinically diagnosed as an inability to augment CBF by ≥50% in response to acetylcholine, leads to impaired lusitropy through the NO–cGMP–PKG pathway. Impaired lusitropy may potentiate subendocardial ischaemia, both leading to impaired myocardial reserve and HFpEF. Coronary microvascular disease, which may represent a later stage in the coronary microvascular dysfunction spectrum than endothelial dysfunction, leads to subendocardial ischaemia through the impaired ability of the VSM to relax in response to appropriate stimuli. This may potentiate impaired lusitropy and lead to impaired myocardial reserve and HFpEF (Graphical abstract).
Clinical links between coronary microvascular disease and heart failure with preserved ejection fraction
Cardiovascular risk factors, such as hypertension, diabetes mellitus, hyperlipidaemia, and obesity, lead to a cascade of pro-inflammatory events that eventually lead to eNOS uncoupling and endothelial dysfunction. Hypertension has been implicated in the development of CMD.38 In addition to causing eNOS dysfunction, it leads to enhanced endothelin production resulting in inappropriate coronary endothelial vasoconstriction.39 Hypertension is also associated with architectural changes, such as capillary rarefaction, medial hypertrophy, and fibrosis of arteriolar vessels. These result in an inability of the coronary vasculature to augment its blood flow. Insulin resistance and hyperglycaemia, both cardinal features of diabetes mellitus, also alter coronary vascular tone regulation. Coronary microvascular disease is prevalent in patients with T2DM22 and studies have shown that impaired myocardial flow reserve is strongly associated with the degree of albuminuria in these patients.40 Coronary microvascular disease and albuminuria may, therefore, share common mechanisms related to the pathogenesis of diabetic micro-vasculopathy.
Women may be susceptible to coronary vasomotor disorders due to gender-specific risk factors, such as systemic inflammation and endocrine changes. During adulthood, men tend to have a higher inflammatory predisposition than women; however, this predilection swaps genders after menopause. Oestradiol is generally protective against inflammation and reduced oestrogen levels post-menopause are associated with altered vascular function, heightened systemic inflammation41 and up-regulation of the renin–angiotensin–aldosterone and sympathetic nervous systems. These have all been implicated in the pathogenesis of CMD and HFpEF and serve as the reasons for why women may be biologically more likely to develop CMD–HFpEF.
Research and clinical implications
Research implications
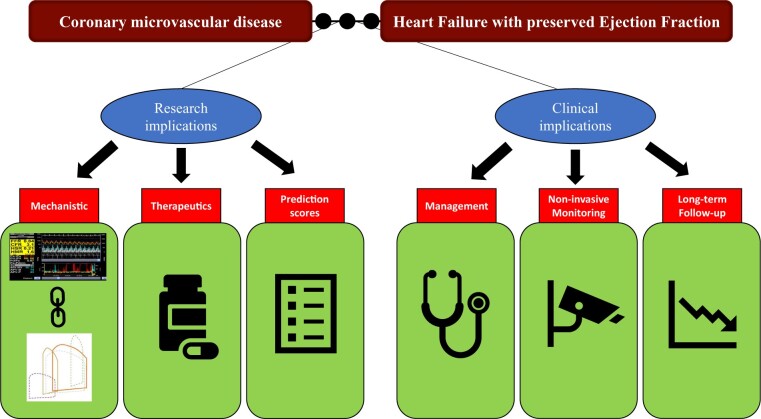
Impaired flow reserve, the hallmark of CMD, likely leads to impairment of myocardial reserve, which eventually results in HFpEF. Impaired CFR, as measured by the diminished response to adenosine and acetylcholine, has been closely linked to exertional pulmonary arterial wedge pressure in patients with HFpEF.42 Future research should prospectively assess the dynamic relationship between intracoronary physiology and LV haemodynamics during exercise; this may help untangle the ‘cause vs. effect’ dilemma that currently plagues our understanding of this intimate relationship. Along with human studies, animal HFpEF models (for instance, using Dahl salt-sensitive rats) may also be useful to understand the longitudinal pathophysiological link between CMD and HFpEF. Once a mechanistic link has been confirmed, the next step would be development of pharmacotherapy targeting specific abnormalities within the pathway. Although the recent ‘one size fits all’ therapeutic studies targeting the NO–cGMP–PKG pathway have yielded disappointing results, it is conceivable that better disease characterization and endotype-specific therapeutic targets may yield positive outcomes. Informatics and machine-learning techniques may help to identify different endotypes based on statistically clustered clinical and biological characteristics.
Furthermore, a better understanding of the mechanistic link between CMD and HFpEF may provide further therapeutic targets for the CMD–HFpEF endotype, which target the subendocardial ischaemia pathway. Finally, development of risk prediction scores, using invasive and non-invasive parameters of coronary microvascular function, may allow clinicians to predict the likelihood of patients developing CMD–HFpEF.
Clinical implications
Once the diagnosis of CMD has been confirmed, aggressive pharmacotherapy should be commenced with the primary aim of ameliorating microvascular dysfunction and the secondary aim of preventing, or delaying, the onset of CMD–HFpEF. Small studies have demonstrated improvement of CFR with angiotensin converting enzyme inhibitors and statins.43 Longitudinal studies are needed to assess whether this treatment strategy prevents the development of HFpEF in patients with CMD. To this effect, routine non-invasive monitoring of systemic microvascular function, via peripheral endothelial assessment or non-invasive coronary flow velocity reserve measurement, may permit focused titration of prognostic and anti-ischaemic therapy in patients with CMD.
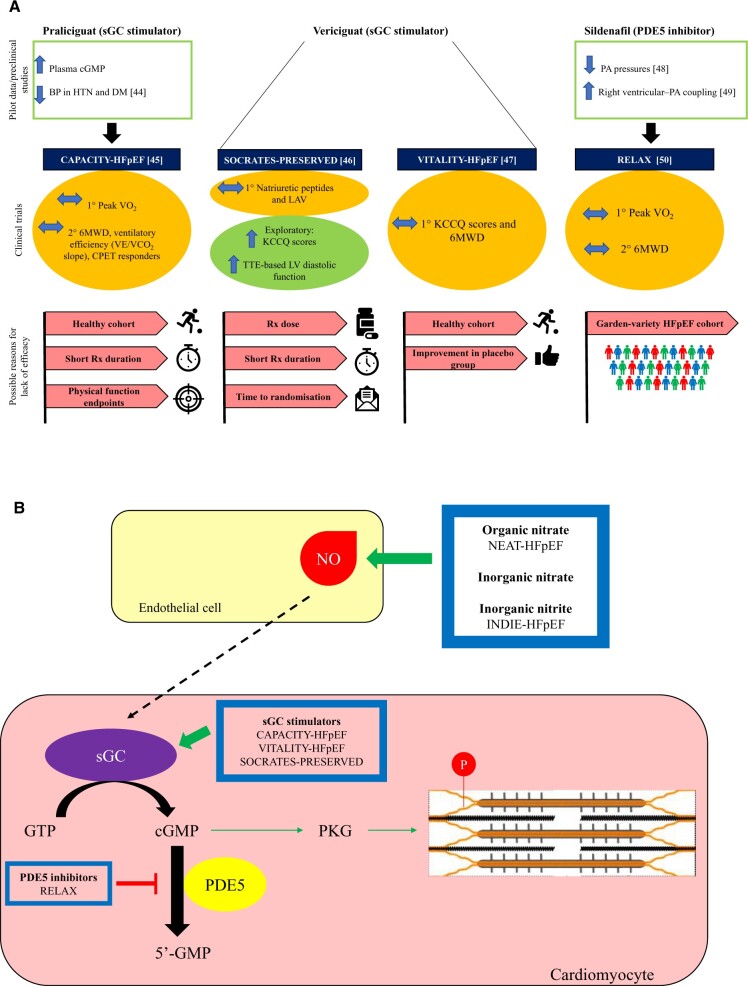
After the disappointing results of renin–angiotensin–aldosterone system inhibitors and beta-blockers in patients with HFpEF, the scientific community turned to agents that could modulate the NO–cGMP–PKG pathway. However, despite initial positive outcomes from pre-clinical and pilot studies, none of these drugs have demonstrated favourable outcomes in large trials. The study results and the potential reasons behind the neutral outcomes are illustrated in Figure 4.44–50
Figure 4.
Results of therapeutic trials targeting the nitric oxide–cyclic Guanosine Monophosphate–protein kinase G pathway. (A) Summarizes the pilot data (or pre-clinical data), clinical trial data and the potential reasons for neutral outcomes, whilst (B) highlights the specific intracellular pathways that each of the novel agents act on. None of these trials met their primary endpoints. However, all of these trials are plagued by specific and generic trial design limitations. The treatment arm of CAPACITY-HFpEF and SOCRATES-PRESERVED trials lasted for only 12 weeks, which may not have been long enough to lead to sustained improvements in the study endpoints. The patient cohort recruited in the CAPACITY-HFpEF and VITALITY-HFpEF trials may represent a ‘healthier’ cohort that is not representative of the ‘real-world’ patient cohort. In the CAPACITY-HFpEF study, only 20% of patients had elevated filling pressures and a majority of patients had New York Heart Association II symptoms. Attenuation of cyclic Guanosine Monophosphate levels in patients with HFpEF is due to the loss of upstream nitric oxide rather than an excessive breakdown of cyclic Guanosine Monophosphate.31 It is, therefore, not unexpected that attempting to augment cyclic Guanosine Monophosphate by inhibiting its breakdown did not lead to clinically meaningful improvements in patients’ haemodynamics or exercise capacity in the RELAX trial. Furthermore, whilst the agents used in these trials target the nitric oxide–cyclic Guanosine Monophosphate–protein kinase G pathway, none of the trials directly studied physiological endpoints of impaired vascular function, such as peripheral or coronary endothelial function. Additionally, the physical functioning endpoints (6-min walk distance and change in peak VO2) may not be able to discriminate among effective therapies because patients with HFpEF usually suffer from multiple comorbidities and their impaired physical functioning may be multifactorial in nature. BP, blood pressure; cGMP, cyclic Guanosine Monophosphate; DM, diabetes mellitus; GTP, Guanosine Triphosphate; HFpEF, heart failure with preserved ejection fraction; HTN, hypertension; KCCQ: Kansas City Cardiomyopathy Questionnaire; LAV, left atrial volume; NO, nitric oxide; PA, pulmonary artery; PKG, protein kinase G; sGC, soluble Guanylate Cyclase; 6MWD, 6-min walk distance.
It is now increasingly recognized that HFpEF is a heterogeneous condition comprising distinct endotypes, which have vastly disparate underlying pathophysiology. Therefore, a ‘one size fits all’ approach is unlikely to yield positive therapeutic outcomes. Coronary microvascular disease–HFpEF is a distinct HFpEF endotype, with impaired CFR and ensuing subendocardial ischaemia and impaired lusitropy being at the centre of its pathogenesis. None of the aforementioned therapeutic trials reported patients’ flow reserve (in response to adenosine or acetylcholine) or stipulated an impaired flow reserve in their inclusion criteria. Therefore, it is conceivable that a large percentage of patients recruited in these studies did not have the CMD–HFpEF endotype. Furthermore, although the agents used in these trials theoretically potentiate the NO–cGMP–PKG pathway, none of them ameliorate the subendocardial ischaemia that is fundamental to the development of CMD–HFpEF. Future therapeutic studies should trial endotype-specific agents to target the underlying pathological pathways.
Finally, long-term follow-up of patients with CMD–HFpEF is required to track the natural trajectory of this disease process.
Figure 5 summarizes the future research and clinical implications of the CMD–HFpEF mechanistic link.
Figure 5.
Clinical and research implications of the coronary microvascular disease–heart failure with preserved ejection fraction mechanistic link.
Conclusion
Coronary microvascular disease–HFpEF may be a distinct HFpEF endotype with coronary microvascular dysfunction being the underlying mechanism leading to impaired myocardial reserve. We have scrutinized the role of subendocardial ischaemia and impaired lusitropy in the development of CMD–HFpEF and have explored the challenges in demonstrating causality between CMD and HFpEF. We have also discussed the role of gender in CMD–HFpEF and have explored the limitations of the contemporary HFpEF therapeutic trials targeting the NO–cGMP–PKG pathway. Further longitudinal and mechanistic studies are required to verify the proposed mechanistic link between CMD and HFpEF. A better understanding of the common pathophysiology may pave the way for the development of therapeutic agents that can improve these patients’ quality of life and cardiovascular outcomes.
Funding
The authors’ work is supported by grants from the Medical Research Council (MR/T029390/1), British Heart Foundation (FS/16/49/32320, CH/1999001/11735, and RE/18/2/34213), the UK National Institute for Health Research (through the Biomedical Research Centre award to King’s College London and Guy’s and St Thomas’ Hospital), and the Fondation Leducq.
Conflict of interest: none declared.
Contributor Information
Aish Sinha, British Heart Foundation Centre of Excellence and National Institute for Health Research Biomedical Research Centre at the School of Cardiovascular Medicine and Sciences, King’s College London, St. Thomas' Hospital, Westminster bridge road, London SE1 7EH, UK.
Haseeb Rahman, British Heart Foundation Centre of Excellence and National Institute for Health Research Biomedical Research Centre at the School of Cardiovascular Medicine and Sciences, King’s College London, St. Thomas' Hospital, Westminster bridge road, London SE1 7EH, UK.
Andrew Webb, British Heart Foundation Centre of Excellence and National Institute for Health Research Biomedical Research Centre at the School of Cardiovascular Medicine and Sciences, King’s College London, St. Thomas' Hospital, Westminster bridge road, London SE1 7EH, UK.
Ajay M Shah, British Heart Foundation Centre of Excellence and National Institute for Health Research Biomedical Research Centre at the School of Cardiovascular Medicine and Sciences, King’s College London, St. Thomas' Hospital, Westminster bridge road, London SE1 7EH, UK.
Divaka Perera, British Heart Foundation Centre of Excellence and National Institute for Health Research Biomedical Research Centre at the School of Cardiovascular Medicine and Sciences, King’s College London, St. Thomas' Hospital, Westminster bridge road, London SE1 7EH, UK.
References
- 1. Sara J, Widmer R, Matsuzawa Y, Lennon R, Lerman L, Lerman A. Prevalence of coronary microvascular dysfunction among patients with chest pain and nonobstructive coronary artery disease. JACC Cardiovasc Interv 2015;8:1445–1453. [DOI] [PubMed] [Google Scholar]
- 2. Cannon R, Epstein S. “Microvascular angina” as a cause of chest pain with angiographically normal coronary arteries. Am J Cardiol 1988;61:1338–1343. [DOI] [PubMed] [Google Scholar]
- 3. Rahman H, Ryan M, Lumley M, Modi B, McConkey H, Ellis H, Scannell C, Clapp B, Marber M, Webb A, Chiribiri A, Perera D. Coronary microvascular dysfunction is associated with myocardial ischemia and abnormal coronary perfusion during exercise. Circulation 2019;140:1805–1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rahman H, Demir OM, Khan F, Ryan M, Ellis H, Mills MT, Chiribiri A, Webb A, Perera D. Physiological stratification of patients with angina due to coronary microvascular dysfunction. J Am Coll Cardiol 2020;75:2538–2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bairey Merz CN, Pepine CJ, Walsh MN, Fleg JL. Ischemia and no obstructive coronary artery disease (INOCA): developing evidence-based therapies and research agenda for the next decade. Circulation 2017;135:1075–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rahman H, Demir OM, Ryan M, McConkey H, Scannell C, Ellis H, Webb A, Chiribiri A, Perera D. Optimal use of vasodilators for diagnosis of microvascular angina in the cardiac catheterization laboratory. Circ Cardiovasc Interv 2020;13:e009019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gdowski MA, Murthy VL, Doering M, Monroy-Gonzalez AG, Slart R, Brown DL. Association of isolated coronary microvascular dysfunction with mortality and major adverse cardiac events: a systematic review and meta-analysis of aggregate data. J Am Heart Assoc 2020;9:e014954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Suwaidi J, Hamasaki S, Higano S, Nishimura R, Holmes D Jr, Lerman A. Long-term follow-up of patients with mild coronary artery disease and endothelial dysfunction. Circulation 2000;101:948–954. [DOI] [PubMed] [Google Scholar]
- 9. Tavella R, Cutri N, Tucker G, Adams R, Spertus J, Beltrame J. Natural history of patients with insignificant coronary artery disease. Eur Heart J Qual Care Clin Outcomes 2016;2:117–124. [DOI] [PubMed] [Google Scholar]
- 10. Jespersen L, Hvelplund A, Abildstrom SZ, Pedersen F, Galatius S, Madsen JK, Jorgensen E, Kelbaek H, Prescott E. Stable angina pectoris with no obstructive coronary artery disease is associated with increased risks of major adverse cardiovascular events. Eur Heart J 2012;33:734–744. [DOI] [PubMed] [Google Scholar]
- 11. AlBadri A, Bairey Merz CN, Johnson BD, Wei J, Mehta PK, Cook-Wiens G, Reis SE, Kelsey SF, Bittner V, Sopko G, Shaw LJ, Pepine CJ, Ahmed B. Impact of abnormal coronary reactivity on long-term clinical outcomes in women. J Am Coll Cardiol 2019;73:684–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Taqueti VR, Solomon SD, Shah AM, Desai AS, Groarke JD, Osborne MT, Hainer J, Bibbo CF, Dorbala S, Blankstein R, Di Carli MF. Coronary microvascular dysfunction and future risk of heart failure with preserved ejection fraction. Eur Heart J 2018;39:840–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yang JH, Obokata M, Reddy YN, Redfield MM, Lerman A, Borlaug BA. Endothelium‐dependent and independent coronary microvascular dysfunction in patients with heart failure with preserved ejection fraction. Eur J Heart Fail 2020;22:432–441. [DOI] [PubMed] [Google Scholar]
- 14. Mahfouz RA, Gouda M, Abdelhamid M. Relation of microvascular dysfunction and exercise tolerance in patients with heart failure with preserved ejection fraction. Echocardiography 2020;37:1192–1198. [DOI] [PubMed] [Google Scholar]
- 15. Shah SJ, Lam CSP, Svedlund S, Saraste A, Hage C, Tan R-S, Beussink-Nelson L, Ljung Faxén U, Fermer ML, Broberg MA, Gan L-M, Lund LH. Prevalence and correlates of coronary microvascular dysfunction in heart failure with preserved ejection fraction: PROMIS-HFpEF. Eur Heart J 2018;39:3439–3450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Perera D. Cardiac-coronary coupling. J Am Coll Cardiol 2016;68:1661–1663. [DOI] [PubMed] [Google Scholar]
- 17. Lumley M, Williams R, Asrress KN, Arri S, Briceno N, Ellis H, Rajani R, Siebes M, Piek JJ, Clapp B, Redwood SR, Marber MS, Chambers JB, Perera D. Coronary physiology during exercise and vasodilation in the healthy heart and in severe aortic stenosis. J Am Coll Cardiol 2016;68:688–697. [DOI] [PubMed] [Google Scholar]
- 18. Masuyama T, Uematsu M, Doi Y, Yamamoto K, Mano T, Naito J, Kondo H, Nagano R, Hori M, Kamada T. Abnormal coronary flow dynamics at rest and during tachycardia associated with impaired left ventricular relaxation in humans: implication for tachycardia-induced myocardial ischemia. J Am Coll Cardiol 1994;24:1625–1632. [DOI] [PubMed] [Google Scholar]
- 19. Taqueti V, Carli M. Coronary microvascular disease pathogenic mechanisms and therapeutic options: JACC state-of-the-art review. J Am Coll Cardiol 2018;72:2625–2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Seddon M, Melikian N, Dworakowski R, Shabeeh H, Jiang B, Byrne J, Casadei B, Chowienczyk P, Shah A. Effects of neuronal nitric oxide synthase (nNOS) on human coronary diameter and blood flow in vivo. Circulation 2009;119:2656–2662. [DOI] [PubMed] [Google Scholar]
- 21. Shabeeh H, Melikian N, Dworakowski R, Casadei B, Chowienczyk P, Shah A. Differential role of endothelial versus neuronal nitric oxide synthase in the regulation of coronary blood flow during pacing-induced increases in cardiac workload. Am J Physiol Heart Circ Physiol 2013;304:H1277–H1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sezer M, Kocaaga M, Aslanger E, Atici A, Demirkiran A, Bugra Z, Umman S, Umman B. Bimodal pattern of coronary microvascular involvement in diabetes mellitus. J Am Heart Assoc 2016;5:e003995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. van de Wouw J, Sorop O, van Drie RWA, van Duin RWB, Nguyen ITN, Joles JA, Verhaar MC, Merkus D, Duncker DJ. Perturbations in myocardial perfusion and oxygen balance in swine with multiple risk factors: a novel model of ischemia and no obstructive coronary artery disease. Basic Res Cardiol 2020;115:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Suppogu N, Wei J, Nelson MD, Cook-Wiens G, Cheng S, Shufelt CL, Thomson LEJ, Tamarappoo B, Berman DS, Samuels B, Azarbal B, Anderson RD, Petersen JW, Handberg EM, Pepine CJ, Merz CNB. Resting coronary velocity and myocardial performance in women with impaired coronary flow reserve: results from the Women's Ischemia Syndrome Evaluation-Coronary Vascular Dysfunction (WISE-CVD) study. Int J Cardiol 2020;309:19–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lerman A, Holmes D Jr, Bell M, Garratt K, Nishimura R, Burnett J Jr. Endothelin in coronary endothelial dysfunction and early atherosclerosis in humans. Circulation 1995;92:2426–2431. [DOI] [PubMed] [Google Scholar]
- 26. Ford TJ, Rocchiccioli P, Good R, McEntegart M, Eteiba H, Watkins S, Shaukat A, Lindsay M, Robertson K, Hood S, Yii E, Sidik N, Harvey A, Montezano AC, Beattie E, Haddow L, Oldroyd KG, Touyz RM, Berry C. Systemic microvascular dysfunction in microvascular and vasospastic angina. Eur Heart J 2018;39:4086–4097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ford TJ, Corcoran D, Padmanabhan S, Aman A, Rocchiccioli P, Good R, McEntegart M, Maguire JJ, Watkins S, Eteiba H, Shaukat A, Lindsay M, Robertson K, Hood S, McGeoch R, McDade R, Yii E, Sattar N, Hsu LY, Arai AE, Oldroyd KG, Touyz RM, Davenport AP, Berry C. Genetic dysregulation of endothelin-1 is implicated in coronary microvascular dysfunction. Eur Heart J 2020;41:3239–3252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Morrow AJ, Ford TJ, Mangion K, Kotecha T, Rakhit R, Galasko G, Hoole S, Davenport A, Kharbanda R, Ferreira VM, Shanmuganathan M, Chiribiri A, Perera D, Rahman H, Arnold JR, Greenwood JP, Fisher M, Husmeier D, Hill NA, Luo X, Williams N, Miller L, Dempster J, Macfarlane PW, Welsh P, Sattar N, Whittaker A, Connachie AM, Padmanabhan S, Berry C. Rationale and design of the Medical Research Council's Precision Medicine with Zibotentan in Microvascular Angina (PRIZE) trial. Am Heart J 2020;229:70–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Paulus WJ, Tschöpe C. A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol 2013;62:263–271. [DOI] [PubMed] [Google Scholar]
- 30. Yamamoto E, Hirata Y, Tokitsu T, Kusaka H, Sakamoto K, Yamamuro M, Kaikita K, Watanabe H, Hokimoto S, Sugiyama S, Maruyama T, Ogawa H. The pivotal role of eNOS uncoupling in vascular endothelial dysfunction in patients with heart failure with preserved ejection fraction. Int J Cardiol 2015;190:335–337. [DOI] [PubMed] [Google Scholar]
- 31. van Heerebeek L, Hamdani N, Falcão-Pires I, Leite-Moreira AF, Begieneman MP, Bronzwaer JG, van der Velden J, Stienen GJ, Laarman GJ, Somsen A, Verheugt FW, Niessen HW, Paulus WJ. Low myocardial protein kinase G activity in heart failure with preserved ejection fraction. Circulation 2012;126:830–839. [DOI] [PubMed] [Google Scholar]
- 32. Schiattarella GG, Altamirano F, Tong D, French KM, Villalobos E, Kim SY, Luo X, Jiang N, May HI, Wang ZV, Hill TM, Mammen PPA, Huang J, Lee DI, Hahn VS, Sharma K, Kass DA, Lavandero S, Gillette TG, Hill JA. Nitrosative stress drives heart failure with preserved ejection fraction. Nature 2019;568:351–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. AbouEzzeddine OF, Kemp BJ, Borlaug BA, Mullan BP, Behfar A, Pislaru SV, Fudim M, Redfield MM, Chareonthaitawee P. Myocardial energetics in heart failure with preserved ejection fraction. Circ Heart Fail 2019;12:e006240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Obokata M, Reddy YNV, Melenovsky V, Kane GC, Olson TP, Jarolim P, Borlaug BA. Myocardial injury and cardiac reserve in patients with heart failure and preserved ejection fraction. J Am Coll Cardiol 2018;72:29–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Phan TT, Abozguia K, Nallur Shivu G, Mahadevan G, Ahmed I, Williams L, Dwivedi G, Patel K, Steendijk P, Ashrafian H, Henning A, Frenneaux M. Heart failure with preserved ejection fraction is characterized by dynamic impairment of active relaxation and contraction of the left ventricle on exercise and associated with myocardial energy deficiency. J Am Coll Cardiol 2009;54:402–409. [DOI] [PubMed] [Google Scholar]
- 36. Crea F, Merz CNB, Beltrame JF, Kaski JC, Ogawa H, Ong P, Sechtem U, Shimokawa H, Camici PG; Coronary Vasomotion Disorders International Study Group (COVADIS). The parallel tales of microvascular angina and heart failure with preserved ejection fraction: a paradigm shift. Eur Heart J 2017;38:473–477. [DOI] [PubMed] [Google Scholar]
- 37. Tschöpe C, Post H. Latent ischaemia as a trigger for a circulus vitiosus of inflammation, fibrosis, and stiffness in HFPEF. Eur J Heart Fail 2015;17:1210–1212. [DOI] [PubMed] [Google Scholar]
- 38. Rizzoni D, Palombo C, Porteri E, Muiesan ML, Kozàkovà M, La Canna G, Nardi M, Guelfi D, Salvetti M, Morizzo C, Vittone F, Rosei EA. Relationships between coronary flow vasodilator capacity and small artery remodelling in hypertensive patients. J Hypertens 2003;21:625–631. [DOI] [PubMed] [Google Scholar]
- 39. Kaski JC, Cox ID, Crook JR, Salomone OA, Fredericks S, Hann C, Holt D. Differential plasma endothelin levels in subgroups of patients with angina and angiographically normal coronary arteries. Coronary Artery Disease Research Group. Am Heart J 1998;136:412–417. [DOI] [PubMed] [Google Scholar]
- 40. Potier L, Chequer R, Roussel R, Mohammedi K, Sismail S, Hartemann A, Amouyal C, Marre M, Le Guludec D, Hyafil F. Relationship between cardiac microvascular dysfunction measured with 82Rubidium-PET and albuminuria in patients with diabetes mellitus. Cardiovasc Diabetol 2018;17:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Klein SL, Flanagan KL. Sex differences in immune responses. Nat Rev Immunol 2016;16:626–638. [DOI] [PubMed] [Google Scholar]
- 42. Ahmad A, Corban MT, Toya T, Verbrugge FH, Sara JD, Lerman LO, Borlaug BA, Lerman A. Coronary microvascular dysfunction is associated with exertional haemodynamic abnormalities in patients with heart failure with preserved ejection fraction. Eur J Heart Fail 2021;23:765–772. [DOI] [PubMed] [Google Scholar]
- 43. Yong J, Tian J, Yang X, Xing H, He Y, Song X. Effects of oral drugs on coronary microvascular function in patients without significant stenosis of epicardial coronary arteries: a systematic review and meta-analysis of coronary flow reserve. Front Cardiovasc Med 2020;7:580419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hanrahan JP, Seferovic JP, Wakefield JD, Wilson PJ, Chickering JG, Jung J, Carlson KE, Zimmer DP, Frelinger AL 3rd, Michelson AD, Morrow L, Hall M, Currie MG, Milne GT, Profy AT. An exploratory, randomised, placebo-controlled, 14 day trial of the soluble guanylate cyclase stimulator praliciguat in participants with type 2 diabetes and hypertension. Diabetologia 2020;63:733–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Udelson JE, Lewis GD, Shah SJ, Zile MR, Redfield MM, Burnett J Jr, Parker J, Seferovic JP, Wilson P, Mittleman RS, Profy AT, Konstam MA. Effect of praliciguat on peak rate of oxygen consumption in patients with heart failure with preserved ejection fraction: the CAPACITY HFpEF randomized clinical trial. JAMA 2020;324:1522–1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Pieske B, Maggioni AP, Lam CSP, Pieske-Kraigher E, Filippatos G, Butler J, Ponikowski P, Shah SJ, Solomon SD, Scalise AV, Mueller K, Roessig L, Gheorghiade M. Vericiguat in patients with worsening chronic heart failure and preserved ejection fraction: results of the SOluble guanylate Cyclase stimulatoR in heArT failurE patientS with PRESERVED EF (SOCRATES-PRESERVED) study. Eur Heart J 2017;38:1119–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Armstrong PW, Lam CSP, Anstrom KJ, Ezekowitz J, Hernandez AF, O'Connor CM, Pieske B, Ponikowski P, Shah SJ, Solomon SD, Voors AA, She L, Vlajnic V, Carvalho F, Bamber L, Blaustein RO, Roessig L, Butler J; VITALITY-HFpEF Study Group. Effect of vericiguat vs placebo on quality of life in patients with heart failure and preserved ejection fraction: the VITALITY-HFpEF randomized clinical trial. JAMA 2020;324:1512–1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Galiè N, Ghofrani HA, Torbicki A, Barst RJ, Rubin LJ, Badesch D, Fleming T, Parpia T, Burgess G, Branzi A, Grimminger F, Kurzyna M, Simonneau G; Sildenafil Use in Pulmonary Arterial Hypertension (SUPER) Study Group. Sildenafil citrate therapy for pulmonary arterial hypertension. N Engl J Med 2005;353:2148–2157. [DOI] [PubMed] [Google Scholar]
- 49. Guazzi M, Vicenzi M, Arena R, Guazzi MD. Pulmonary hypertension in heart failure with preserved ejection fraction: a target of phosphodiesterase-5 inhibition in a 1-year study. Circulation 2011;124:164–174. [DOI] [PubMed] [Google Scholar]
- 50. Redfield MM, Chen HH, Borlaug BA, Semigran MJ, Lee KL, Lewis G, LeWinter MM, Rouleau JL, Bull DA, Mann DL, Deswal A, Stevenson LW, Givertz MM, Ofili EO, O'Connor CM, Felker GM, Goldsmith SR, Bart BA, McNulty SE, Ibarra JC, Lin G, Oh JK, Patel MR, Kim RJ, Tracy RP, Velazquez EJ, Anstrom KJ, Hernandez AF, Mascette AM, Braunwald E; RELAX Trial. Effect of phosphodiesterase-5 inhibition on exercise capacity and clinical status in heart failure with preserved ejection fraction: a randomized clinical trial. JAMA 2013;309:1268–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]