Abstract
Background
Dietary recommendations may underestimate the protein older adults need for optimal bone health. This study sought to determine associations of protein intake with bone mineral density (BMD) and fracture among community-dwelling White and Black older adults.
Method
Protein as a percentage of total energy intake (TEI) was assessed with a Food Frequency Questionnaire in 2160 older adults (73.5 ± 2.8 years; 51.5% women; 35.8% Black) in the Health, Aging, and Body Composition prospective cohort. Hip, femoral neck, and whole body BMD was assessed by dual-energy x-ray absorptiometry at baseline and 4 years, and lumbar trabecular, cortical, and integral BMD was assessed by computed tomography at baseline and 5 years. Fragility fractures over 5 years were adjudicated from self-report data collected every 6 months. Associations with tertiles of protein intake were assessed using analysis of covariance for BMD and multivariate Cox regression for fracture, adjusting for confounders.
Results
Participants in the upper protein tertile (≥15% TEI) had 1.8%–6.0% higher mean hip and lumbar spine BMD compared to the lower protein tertile (<13% TEI; p < .05). Protein intake did not affect change in BMD at any site over the follow-up period. Participants in the upper protein tertile had a reduced risk of clinical vertebral fracture over 5 years of follow-up (hazard ratio: 0.36 [95% confidence interval: 0.14, 0.97] vs lower protein tertile, p = .04).
Conclusions
Older adults with higher protein intake (≥15% TEI) had higher BMD at the hip, whole body, and lumbar spine, and a lower risk of vertebral fracture.
Keywords: Computed tomography (CT), Dual-energy x-ray absorptiometry (DXA), Food Frequency Questionnaire, Nutrition, Osteoporosis
The Acceptable Macronutrient Distribution Range (10%–35% of total energy intake [TEI]) and Recommended Dietary Allowance (RDA; 0.8 g/kg body wt/d) for protein were derived predominately from urinary nitrogen balance studies in young adults (1) and may underestimate the protein needs for older adults to optimally preserve bone, lean mass, and physical function (2–13). Lower dietary protein intake may exacerbate age-related decreases in bone mineral density (BMD) that lead to osteoporosis and fractures, which are associated with significant mortality and morbidity (14,15). Systematic reviews and meta-analyses have found positive associations between protein intake and BMD at the hip, whole body, and lumbar spine (16,17), with protein intake above the RDA accounting for 2%–4% of BMD variance (16). Another meta-analysis found moderate evidence that higher protein intake may protect against lumbar spine BMD loss in older adults (18). Two meta-analyses found higher protein intake reduced hip fracture risk by 11%–16% (17,19), while others found no significant associations (16,18), possibly due to insufficient events (18). Based on synthesis of the recent literature, the expert consensus endorsed by the International Osteoporosis Foundation and by the European Society for Clinical and Economical Aspects of Osteoporosis, Osteoarthritis, and Musculoskeletal Diseases concluded that higher protein intake above the current RDA is associated with higher BMD, slower bone loss, and reduced hip fracture risk, as long as dietary calcium intake is adequate (20). However, further evidence is needed, especially on the effects of higher protein intake on fracture incidence and the synergistic effects of protein and calcium intake on bone health (18). Vitamin D intake also contributes, along with dietary protein and calcium, to maintenance of muscle and bone health (21), and interactions between protein and calcium–vitamin D on BMD have been observed (18). Concurrent use of osteoporosis medications (eg, bisphosphonates) will affect bone remodeling, and this has not been controlled for in many cohort studies investigating dietary protein effects on bone health (22–26). Despite the existing evidence, additional data are needed to understand and quantify the impact of dietary protein intake on measures of older adult bone health (BMD and fractures), and to explore if the associations differ by gender, race, and calcium intake, while also controlling for vitamin D supplement and osteoporosis medication use. Dietary intake, areal and volumetric BMD, and fracture data collected in the Health, Aging, and Body Composition (Health ABC) Study, a large cohort of older White and Black men and women, offer an opportunity to address these gaps.
The objective of this study was to examine the association of total dietary protein intake, as well as animal and vegetable protein intake, with BMD and fracture incidence over 5 years of follow-up among older, community-dwelling White and Black men and women. The primary hypothesis was that higher protein intake would be associated with higher baseline BMD. Higher protein intake was also hypothesized to attenuate BMD loss and fracture risk over 5 years of follow-up.
Method
Study Design
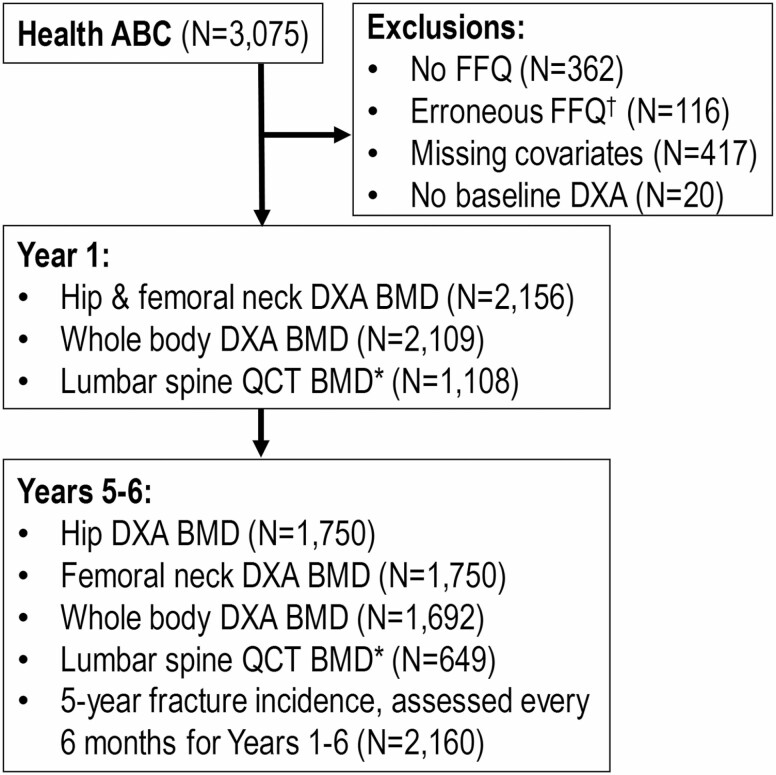
Associations between protein intake and baseline BMD, BMD change, and fracture incidence were examined in community-dwelling older adults enrolled in the Health ABC Study, a prospective cohort study investigating age-related body composition and functional changes. The Health ABC Study enrolled 3075 White and Black men and women aged 70–79 recruited from the Pittsburgh, PA and Memphis, TN metropolitan areas between April 1997 and June 1998. Participants were eligible if they reported no difficulty walking one fourth of a mile, climbing 10 steps, or performing basic activities of daily living; were free of life-threatening illness; planned to remain in the geographic area for at least 3 years; and were not enrolled in lifestyle intervention trials. All participants provided written informed consent, and all protocols were approved by the institutional review boards at the University of Pittsburgh (Pittsburgh, PA) and University of Tennessee Health Science Center (Memphis, TN). For this analysis, participants were excluded if they lacked a Food Frequency Questionnaire (FFQ; n = 362), had serious errors (<3 or >17 solid foods consumed per day) on the FFQ (n = 57) or reported implausible energy intakes (<500 kcal/d or >3500 kcal/d in women and <800 kcal/d or >4000 kcal/d in men; n = 59), were missing pertinent covariates (n = 417), or were missing baseline BMD (n = 20). The final study sample for this analysis includes 2160 men and women who had baseline total dietary protein intake from a FFQ and baseline BMD from dual-energy x-ray absorptiometry (DXA) scans. Figure 1 shows a consort diagram of the selection of the participant samples for each outcome measure.
Figure 1.
Consort diagram of Health ABC participant selection with sample sizes for each outcome.Notes: BMD = bone mineral density; DXA = dual-energy x-ray absorptiometry; FFQ = Food Frequency Questionnaire; Health ABC = Health, Aging, and Body Composition Study; QCT = quantitative computed tomography. †Erroneous FFQ exclusions include those with serious errors on the FFQ (<3 or >17 solid foods/d) and those who reported implausible energy intakes (<500 kcal/d or >3500 kcal/d [women] or <800 kcal/d or >4000 kcal/d [men]). *QCT assessments only done at the Pittsburgh site.
Dietary Intake
Participants completed a 108-item, interviewer-administered modified version of the FFQ in Year 2 (1 year from baseline) (27). This FFQ food list was specifically developed for the Health ABC Study based on dietary recall data from the third National Health and Nutrition Examination Survey (NHANES) for non-Hispanic White and Black adults (ages 65+) living in the Northeast or the South. The FFQ asked participants about their “usual eating habits over the past year or so.” Wood blocks, food models, standard kitchen measures, and flash cards were used by trained interviewers to assist participants in estimating food portion sizes. Energy intake and macronutrient and micronutrient content were calculated from the FFQ by Block Dietary Data Systems (Berkeley, CA). Total protein intake, as well as the source of protein (eg, animal or vegetable), was computed. For the current study, protein intake as a percentage of TEI was stratified by tertile for total protein intake (<13%, 13%–15%, ≥15%), animal protein intake (<7%, 7%–9%, ≥9%), and vegetable protein intake (<6%, 6%–7%, ≥7%). Validation of the original, NHANES II-based FFQ against dietary food records yielded correlations >0.7 for energy and 17 selected nutrients including protein (27). Although the Health ABC FFQ has not been independently validated, the validity of the Women’s Health Initiative (WHI) FFQ, which was similarly modified to reflect regional and racial variations in food types among older women, has previously been examined (28). Protein intake according to the WHI FFQ was moderately correlated with food diary methods (Pearson correlation coefficient, r = .51).
Bone Mineral Density
Total hip, femoral neck, and whole body areal BMD (g/cm2) was assessed from hip and whole body DXA scans (Hologic 4500A, software v.9.03; Bedford, MA) acquired in Years 1 and 5. Dual-energy x-ray absorptiometry quality assurance measurements using daily and cross-calibration phantoms were performed to ensure scanner reliability, and identical patient scan protocols were used at both study sites. Quantitative computed tomography (QCT) scans (9800 Advantage; General Electric, Milwaukee, WI) of the L3 vertebra were acquired in University of Pittsburgh participants only in Years 1 and 6 (n = 1108). Participants were scanned with a bone calibration phantom (Image Analysis, Columbia, KY) positioned under their lower back. Quantitative computed tomography data were analyzed with a standardized protocol at the University of California, San Francisco, to measure volumetric trabecular, cortical, and integral BMD (mg/cm3) of the L3 vertebra (29). The “change in BMD” reported in this paper refers to the 4-year change in DXA-derived BMD and 5-year change in CT-derived BMD.
Fractures
Incident fragility fractures (defined as spontaneous or with modest trauma, such as fall from standing height) over the 5-year period (Years 1–6) were assessed by self-report at annual clinic visits and at intermediate 6-month phone interviews. Participants were also asked to notify the study team as soon as possible after any fracture. Follow-up was >95% complete. All fractures were verified by medical documentation, including radiology reports (except rib, toe, and finger fractures). Fractures were excluded if they were due to excessive trauma (eg, motor vehicle crash), stress, a pathologic condition (eg, cancer), or other/unknown causes.
Covariates
Demographic characteristics (age, gender, race, education, and study site), smoking status, alcohol consumption, physical activity, history of osteoporosis diagnosis, and falls in the prior 12 months were collected via an interviewer-administered questionnaire at baseline. Smoking status and alcohol consumption were categorized as current, former, or never. Education was categorized as less than high school, high school graduate, or postsecondary. Physical activity was based on the reported time spent walking for exercise or in other walking (eg, transportation) over the prior 7 days, and categorized as 0, 1–149, or ≥150 min. Participant height was measured in millimeters using a Harpenden stadiometer (Holtain Ltd, Crosswell, UK) and weight was measured in kilograms with a calibrated balance-beam scale. Body mass index was computed by dividing weight by the square of height in meters. Participants brought their medications from the prior week to the clinic; osteoporosis medications (bisphosphonates, calcitonin, raloxifene, and fluoride), calcium and vitamin D supplement use, and estrogen therapy were coded using the Iowa Drug Information System ingredient codes (30). Cognitive function was assessed using the Modified Mini-Mental State Examination (31). An expanded version of the Short Physical Performance Battery (SPPB), known as the Health ABC Physical Performance Battery (PPB), was conducted to minimize ceiling effects of the SPPB: 5 repeated chair stands, a 6-m walk for usual gait speed, and balance tests consisting of 30-second full tandem, semi-tandem, and single leg stands and a narrow walk test as previously described (score range 0–4) (32).
Statistical Analysis
Baseline characteristics of participants were compared across tertiles of total protein intake as a percentage of TEI using analysis of variance for continuous variables and chi-squared tests for categorical variables. Baseline BMD and change in BMD were compared across tertiles of total protein intake, animal protein intake, and vegetable protein intake (calculated as % TEI) with analysis of covariance, followed by tests for linear trends across tertiles of protein intake using the median value in each protein category as a continuous variable in the linear regression models. Base models were adjusted for age, gender, race, study site, and energy intake. Full models were additionally adjusted for smoking status, alcohol consumption, education, physical activity, BMI, osteoporosis diagnosis, osteoporosis medications, dietary calcium intake, calcium and vitamin D supplement use, estrogen therapy, and cognitive function. Time to first fragility fracture over a 5-year period was assessed by protein tertile using multivariate Cox regression; participants were censored at the earliest date of fracture, death, loss to follow-up, or end of the 5-year period. Subgroup analyses were conducted for each type of fragility fracture (clinical vertebral fracture, hip fracture, and non-hip, non-spine fracture). Models were adjusted using the same covariates in the BMD analyses, as well as the Health ABC PPB and falls in the prior 12 months recorded at baseline. Gender, race, and calcium intake (defined as ≥800 mg dietary intake or use of a calcium supplement, or <800 mg dietary intake and no use of calcium supplement) by protein interactions were tested in the fully adjusted models. In cases where the gender, race, or calcium by protein interactions were significant, models were stratified by male versus females, White versus Black, or adequate versus inadequate calcium intake. Osteoporosis medication by protein interactions were not tested since <5% of the cohort was taking these medications. Statistical analyses were performed using SAS software (v9.4; SAS Institute, Cary, NC) with α = .05 indicating statistical significance.
Results
The mean age of the study population was 73.5 years; 51.5% were women and 35.8% were Black. The mean ± SD daily protein intake was 0.9 ± 0.4 g/kg body weight/d, accounting for 14 ± 3% TEI daily. Descriptive characteristics of the study population at baseline by total protein % TEI tertile are shown in Table 1. Total daily protein intake was grouped by the following tertiles: lower (<13% TEI; mean ± SD, 0.8 ± 0.3 g/kg body weight/d), middle (13%–15% TEI; 0.9 ± 0.3 g/kg body weight/d), and upper (≥15% TEI; 1.1 ± 0.4 g/kg body weight/d). Participants in the upper protein tertile were more likely to be female, White, nonsmokers, nonsedentary, have lower energy intake with lower fat and carbohydrate % TEIs, have higher dietary calcium and vitamin D intakes, be on calcium, vitamin D, or estrogen supplementation, and have higher cognitive function (Table 1). Osteoporosis diagnosis and medication use did not vary across the protein tertiles.
Table 1.
Baseline Characteristics by Tertile of Dietary Protein Intake: The Health, Aging, and Body Composition Study
| Dietary Protein Tertile, % of Total Energy Intake | p Value | ||||
|---|---|---|---|---|---|
| Overall | <13% | 13%–15% | ≥15% | ||
| N | 2160 | 718 | 703 | 739 | |
| Age (y) | 73.5 ± 2.8 | 73.5 ± 2.9 | 73.4 ± 2.8 | 73.7 ± 2.9 | .31 |
| Female gender, n (%) | 1113 (51.5) | 335 (47.0) | 371 (52.2) | 407 (55.3) | .006 |
| Black race, n (%) | 773 (35.8) | 305 (42.8) | 236 (33.2) | 232 (31.5) | <.001 |
| Study site, Pittsburgh, n (%) | 1130 (52.3) | 392 (55.0) | 363 (51.1) | 375 (51.0) | .22 |
| Smoking history, n (%) | <.001 | ||||
| Never | 970 (44.9) | 268 (37.6) | 333 (46.8) | 369 (50.1) | |
| Current | 192 (8.9) | 104 (14.6) | 44 (6.2) | 44 (6.0) | |
| Former | 998 (46.2) | 341 (47.8) | 334 (47.0) | 323 (43.9) | |
| Alcohol use, n (%) | .09 | ||||
| Never | 587 (27.2) | 171 (24.0) | 204 (28.7) | 212 (28.8) | |
| Current | 1118 (51.8) | 394 (55.3) | 347 (48.8) | 377 (51.2) | |
| Former | 455 (21.1) | 148 (20.8) | 160 (22.5) | 147 (20.0) | |
| Education completed, n (%) | .17 | ||||
| Less than high school | 466 (21.6) | 166 (23.3) | 154 (21.7) | 146 (19.8) | |
| High school | 719 (33.3) | 250 (35.1) | 222 (31.2) | 247 (33.6) | |
| Postsecondary education | 975 (45.1) | 297 (41.7) | 335 (47.1) | 343 (46.6) | |
| Minutes of walking/wk, n (%) | .009 | ||||
| 0 (sedentary) | 870 (40.3) | 313 (43.9) | 285 (40.1) | 272 (37.0) | |
| 1–149 | 672 (31.1) | 209 (29.3) | 240 (33.8) | 223 (30.3) | |
| ≥150 | 618 (28.6) | 191 (26.8) | 186 (26.2) | 241 (32.7) | |
| BMI (kg/m2) | 27.2 ± 4.6 | 27.1 ± 4.7 | 27.0 ± 4.4 | 27.5 ± 4.8 | .07 |
| Dietary intake | |||||
| Total energy (kcal/d) | 1838 ± 626 | 1933 ± 656 | 1825 ± 589 | 1758 ± 619 | <.001 |
| Fat (% energy) | 33 ± 7 | 34 ± 7 | 34 ± 7 | 32 ± 8 | <.001 |
| Carbohydrate (% energy) | 54 ± 8 | 55 ± 8 | 53 ± 8 | 52 ± 8 | <.001 |
| Protein (% energy) | 14 ± 3 | 12 ± 1 | 14 ± 1 | 18 ± 2 | <.001 |
| Protein (g/kg body weight/d) | 0.9 ± 0.4 | 0.8 ± 0.3 | 0.9 ± 0.3 | 1.1 ± 0.4 | <.001 |
| Osteoporosis diagnosis, n (%) | 226 (10.5) | 65 (9.1) | 78 (11.0) | 83 (11.3) | .35 |
| Osteoporosis medications, n (%) | 100 (4.6) | 24 (3.4) | 37 (5.2) | 39 (5.3) | .15 |
| Dietary calcium intake (mg) | 773 ± 360 | 685 ± 293 | 779 ± 343 | 854 ± 413 | <.001 |
| Dietary vitamin D intake (IU) | 208 ± 133 | 182 ± 116 | 209 ± 128 | 232 ± 147 | .001 |
| Calcium supplements, n (%) | 424 (19.6) | 107 (15.0) | 140 (19.7) | 177 (24.0) | <.001 |
| Vitamin D supplements, n (%) | 194 (9.0) | 47 (6.6) | 70 (9.8) | 77 (10.5) | .02 |
| Oral estrogen, n (%) | 258 (11.9) | 63 (8.8) | 91 (12.8) | 104 (14.1) | .006 |
| Fractured bone after age 45, n (%) | 493 (22.8) | 170 (23.8) | 158 (22.2) | 165 (22.4) | .73 |
| Preexisting vertebral fracture, n (%) | 72 (3.3) | 25 (3.5) | 25 (3.5) | 22 (3.0) | .81 |
| Cognitive function, Modified Mini-Mental State Examination (3MS) | 91.0 ± 7.5 | 90.4 ± 8.0 | 91.2 ± 8.0 | 91.5 ± 8.2 | .02 |
| Physical Performance Battery Score (range: 0–4) | 2.2 ± 0.5 | 2.2 ± 0.5 | 2.3 ± 0.5 | 2.3 ± 0.5 | .97 |
| Fall in the prior 12 mo, n (%) | 463 (21.4) | 144 (20.2) | 158 (22.2) | 161 (21.9) | .61 |
Notes: BMI = body mass index. Data presented as means ± SD for the overall population unless otherwise noted as n (%). Statistical comparisons across protein tertiles were assessed with analysis of variance for continuous variables or chi-squared tests for categorical variables.
The mean ± SD baseline BMD was 0.89 ± 0.17 g/cm2 at the total hip, 0.74 ± 0.14 g/cm2 at the femoral neck, and 1.09 ± 0.14 g/cm2 for the whole body. At the L3 vertebra, mean baseline BMD was 119.1 ± 41.0 mg/cm3 (trabecular), 288.9 ± 56.4 mg/cm3 (cortical), and 246.1 ± 52.4 mg/cm3 (integral). Baseline total hip, L3 trabecular, L3 cortical, and L3 integral BMD was significantly higher in participants in the upper protein tertile (≥15% TEI) compared to the lower (<13% TEI) protein tertiles in the base and fully adjusted models (all p < .05; Table 2). Positive linear trends were found for total hip, whole body, L3 trabecular, L3 cortical, and L3 integral baseline BMD measures across the protein tertiles in the base and full models (all p < .05; Table 2), with a trend for femoral neck (p = .06) baseline BMD. Compared to participants in the lower protein tertile, mean baseline BMD in participants in the upper protein tertile was 1.8% higher for the total hip, and 6.0% (trabecular), 2.7% (cortical), and 3.3% (integral) higher for the L3 vertebra. Baseline BMD did not differ between the lower and middle protein tertiles (all p > .05). There was a significant interaction between gender and total protein intake on baseline femoral neck BMD (full model p = .01); thus, separate models were examined for males versus females. In males, the effect of total protein intake on baseline femoral neck BMD was similar to the unstratified results (Table 2), with a positive linear association between femoral neck BMD and protein intake (p = .04; Supplementary Table A1). However, baseline femoral neck BMD did not differ between the protein tertiles for females. There were no significant interactions between protein intake and race on baseline BMD at any site. There was a significant interaction between calcium intake and total protein intake on baseline whole body BMD (full model p = .003); thus, separate models were examined for participants with adequate versus inadequate calcium intake. With inadequate calcium intake (<800 mg dietary intake and no supplement use), the effect of total protein intake on baseline whole body BMD was similar to the unstratified results (Table 2), with participants in the upper protein tertile having significantly higher baseline whole body BMD compared to participants in the lower and middle protein tertiles (linear trend p < .001; group comparison p < .001 and .009, respectively; Supplementary Table A2). However, baseline whole body BMD did not differ between the protein tertiles for participants with adequate calcium intake (≥800 mg dietary intake or supplement use).
Table 2.
Baseline Bone Mineral Density by Tertile of Total Dietary Protein Intake: The Health, Aging, and Body Composition Study
| Dietary Protein Tertile, % of Total Energy Intake | ||||
|---|---|---|---|---|
| <13% | 13%–15% | ≥15% | Linear Trend p Value | |
| Total hip (g/cm2) | ||||
| Base modela | 0.890 ± 0.005 a | 0.891 ± 0.005 a | 0.918 ± 0.005 b | <.001 |
| Full modelb | 0.896 ± 0.010 a | 0.895 ± 0.010 a | 0.912 ± 0.010 b | .02 |
| Femoral neck (g/cm2) | ||||
| Base modela | 0.748 ± 0.005 a | 0.748 ± 0.005 a | 0.770 ± 0.005 b | <.001 |
| Full modelb | 0.753 ± 0.009 a,b | 0.751 ± 0.009 a | 0.764 ± 0.009 b | .06 |
| Whole body (g/cm2) | ||||
| Base modela | 1.091 ± 0.004 a | 1.093 ± 0.004 a | 1.112 ± 0.004 b | <.001 |
| Full modelb | 1.108 ± 0.008 a,b | 1.107 ± 0.008 a | 1.119 ± 0.008 b | .05 |
| L3 trabecular vertebra (mg/cm3) | ||||
| Base modela | 118.3 ± 2.1 a | 120.6 ± 2.0 a | 127.2 ± 1.9 b | <.001 |
| Full modelb | 123.8 ± 4.2 a | 126.1 ± 4.2 a,b | 131.2 ± 4.2 b | .01 |
| L3 cortical vertebra (mg/cm3) | ||||
| Base modela | 288.3 ± 2.9 a | 289.7 ± 2.8 a | 299.8 ± 2.6 b | .002 |
| Full modelb | 297.6 ± 5.8 a | 298.6 ± 5.8 a,b | 305.5 ± 5.8 b | .03 |
| L3 integral vertebra (mg/cm3) | ||||
| Base modela | 245.4 ± 2.7 a | 247.1 ± 2.6 a | 256.9 ± 2.4 b | <.001 |
| Full modelb | 253.2 ± 5.4 a | 254.8 ± 5.4 a,b | 261.6 ± 5.3 b | .02 |
Notes: Data are presented as least square means ± SE. Different letters (a, b) indicate statistically significant differences between protein tertiles.
aBase model adjusted for age, gender, race, study site, and energy intake (all measured at baseline). bFull model adjusted for age, gender, race, study site, smoking status, alcohol consumption, education, physical activity, body mass index, energy intake, osteoporosis medications, dietary calcium intake, calcium and vitamin D supplement use, estrogen therapy, cognitive function, and osteoporosis diagnosis (all measured at baseline).
Positive linear trends were found for whole body, L3 trabecular, and L3 integral baseline BMD measures across the animal protein tertiles in the base and full models (all p < .05; Table 3). In the fully adjusted models, baseline L3 trabecular BMD was significantly higher in participants in the upper animal protein tertile (≥9% TEI) compared to the middle (7%–9% TEI) animal protein tertile (p = .03; Table 3). Baseline BMD did not differ between the lower and middle animal protein tertiles or between tertiles of vegetable protein intake (all p > .05).
Table 3.
Baseline Bone Mineral Density by Tertile of Animal and Vegetable Dietary Protein Intake: The Health, Aging, and Body Composition Study
| Animal Dietary Protein Tertile, % of Total Energy Intake | Linear Trend p Value | Vegetable Dietary Protein Tertile, % of Total Energy Intake | Linear Trend p Value | |||||
|---|---|---|---|---|---|---|---|---|
| <7% | 7%–9% | ≥9% | Animal | <6% | 6%–7% | ≥7% | Vegetable | |
| Total hip (g/cm2) | ||||||||
| Base modela | 0.892 ± 0.005 a | 0.890 ± 0.005 a | 0.916 ± 0.005 b | <.001 | 0.894 ± 0.005 a | 0.906 ± 0.005 a | 0.899 ± 0.005 a | .52 |
| Full modelb | 0.898 ± 0.010 a | 0.897 ± 0.010 a | 0.909 ± 0.010 a | .10 | 0.898 ± 0.010 a | 0.902 ± 0.010 a | 0.903 ± 0.010 a | .52 |
| Femoral neck (g/cm2) | ||||||||
| Base modela | 0.747 ± 0.005 a | 0.749 ± 0.005 a | 0.769 ± 0.005 b | <.001 | 0.749 ± 0.005 a | 0.760 ± 0.005 a | 0.756 ± 0.005 a | .35 |
| Full modelb | 0.752 ± 0.009 a | 0.754 ± 0.009 a | 0.763 ± 0.009 a | .08 | 0.752 ± 0.009 a | 0.757 ± 0.009 a | 0.759 ± 0.009 a | .33 |
| Whole body (g/cm2) | ||||||||
| Base modela | 1.091 ± 0.004 a | 1.093 ± 0.004 a | 1.112 ± 0.004 b | <.001 | 1.093 ± 0.004 a | 1.101 ± 0.004 a | 1.102 ± 0.004 a | .16 |
| Full modelb | 1.107 ± 0.009 a | 1.108 ± 0.008 a | 1.119 ± 0.009 a | .04 | 1.110 ± 0.008 a | 1.111 ± 0.008 a | 1.113 ± 0.009 a | .66 |
| L3 trabecular vertebra (mg/cm3) | ||||||||
| Base modela | 119.4 ± 2.1 a | 119.8 ± 2.0 a | 127.0 ± 1.9 b | .003 | 120.3 ± 2.0 a | 124.3 ± 2.0 a | 121.5 ± 2.0 a | .71 |
| Full modelb | 125.2 ± 4.3 a,b | 125.2 ± 4.2 a | 131.0 ± 4.2 b | .03 | 125.8 ± 4.2 a | 129.2 ± 4.2 a | 126.5 ± 4.3 a | .84 |
| L3 cortical vertebra (mg/cm3) | ||||||||
| Base modela | 288.6 ± 2.9 a | 289.8 ± 2.8 a | 299.6 ± 2.6 b | .002 | 289.9 ± 2.7 a | 295.4 ± 2.8 a | 292.8 ± 2.8 a | .48 |
| Full modelb | 298.2 ± 5.9 a | 298.7 ± 5.7 a | 305.3 ± 5.8 a | .06 | 298.9 ± 5.7 a | 302.5 ± 5.8 a | 300.8 ± 5.9 a | .65 |
| L3 integral vertebra (mg/cm3) | ||||||||
| Base modela | 245.7 ± 2.7 a | 247.3 ± 2.6 a | 256.6 ± 2.4 b | .001 | 247.3 ± 2.5 a | 252.6 ± 2.6 a | 249.6 ± 2.6 a | .56 |
| Full modelb | 253.8 ± 5.5 a | 254.9 ± 5.3 a | 261.2 ± 5.4 a | .04 | 254.9 ± 5.3 a | 258.5 ± 5.4 a | 256.5 ± 5.4 a | .70 |
Notes: Data are presented as least square means ± SE. Different letters (a, b) indicate statistically significant differences between protein tertiles.
aBase model adjusted for age, gender, race, study site, and energy intake (all measured at baseline). bFull model adjusted for age, gender, race, study site, smoking status, alcohol consumption, education, physical activity, body mass index, energy intake, osteoporosis medications, dietary calcium intake, calcium and vitamin D supplement use, estrogen therapy, cognitive function, and osteoporosis diagnosis (all measured at baseline).
There were no significant interactions between gender and either animal or vegetable protein intake for baseline BMD. There was a significant interaction between race and animal protein intake on baseline L3 cortical and integral BMD (full model, both p = .02; Supplementary Table A3). White participants in the upper animal protein tertile had significantly higher baseline L3 cortical and integral BMD compared to participants in the lower animal protein tertile (linear trend p = .01; group comparisons p < .01). However, a linear trend with animal protein tertile and these L3 BMD measures was not observed in Black participants (p > .05).
There was a significant interaction between calcium intake and animal protein intake on baseline hip and whole body BMD (full model p = .008 and <.001, respectively; Supplementary Table A4). With inadequate calcium intake, participants in the upper animal protein tertile had significantly higher baseline hip and whole body BMD compared to participants in the lower and middle protein tertiles (linear trend p = .003; all group comparisons p ≤.01). However, baseline hip and whole body BMD did not differ between the animal protein tertiles for participants with adequate calcium intake (all p > .05). There was also a significant interaction between calcium intake and vegetable protein intake on baseline hip and femoral neck BMD (full model p < .001 and p = .009, respectively). With inadequate calcium intake, participants in the upper vegetable protein tertile had significantly higher baseline hip BMD compared to participants in the middle tertile (p = .01), but all other tertile group comparisons and linear trend tests were not statistically significant. With adequate calcium intake, participants in the middle vegetable protein tertile had significantly higher baseline hip BMD compared to the lower and upper protein tertiles (both p < .03), and significantly higher baseline femoral neck BMD compared to the lower protein tertile (p = .02). All other tertile group comparisons and linear trend tests for participants with adequate calcium intake were not statistically significant.
Mean ± SD changes in BMD were −0.01 ± 0.04 g/cm2 (total hip), −0.01 ± 0.04 g/cm2 (femoral neck), −0.02 ± 0.04 g/cm2 (whole body), −4.91 ± 20.31 mg/cm3 (L3 trabecular), and 7.58 ± 21.13 mg/cm3 (L3 integral); follow-up data on L3 cortical BMD was not available in the Health ABC data set. Change in total hip, femoral neck, whole body, L3 trabecular, and L3 integral BMD was not associated with total protein intake (all p for trend >.05; Supplementary Table A5). Change in BMD at any site did not differ between tertiles of animal or vegetable protein intake (data not shown). There were no significant interactions between protein intake and gender, race, or calcium intake on change in BMD at any site.
Over the 5-year period, 6.3% of the study population (N = 136 participants) had at least one fragility fracture; these first fractures included 31 clinical vertebral fractures, 20 hip fractures, and 85 non-hip, non-spine fractures. Total protein intake was not associated with 5-year overall fragility fracture risk (Table 4), although reduced risk of clinical vertebral fracture was observed among participants in the upper protein tertile compared to those in the lower protein tertile (hazard ratio: 0.36 [95% confidence interval (CI): 0.14, 0.97], p = .04). Clinical vertebral fracture incidence over the 5-year period was 2.13% (95% CI: 1.26, 3.56) in the lower protein tertile, 1.37% (95% CI: 0.71, 2.61) in the middle protein tertile, and 1.13% (95% CI: 0.56, 2.24) in the upper protein tertile. There were no significant interactions between protein intake and gender, race, or calcium intake on the incidence of vertebral fracture or any other fracture types over the 5-year period.
Table 4.
Five-Year Fracture Incidence by Tertile of Total Dietary Protein Intake: The Health, Aging, and Body Composition (Health ABC) Study
| Dietary Protein Tertile, % of Total Energy Intake | |||
|---|---|---|---|
| <13% (reference) | <13%–15% | ≥15% | |
| Fragility fracture (cases, n) | 49 | 42 | 45 |
| Base modela | 1.00 | 0.81 (0.54, 1.23) | 0.76 (0.50, 1.15) |
| Full modelb | 1.00 | 0.76 (0.50, 1.15) | 0.71 (0.45, 1.11) |
| Clinical vertebral fracture (cases, n) | 14 | 9 | 8 |
| Base modela | 1.00 | 0.64 (0.27, 1.49) | 0.52 (0.21, 1.26) |
| Full modelb | 1.00 | 0.49 (0.20, 1.19) | 0.36 (0.14, 0.97) |
| Hip fracture (cases, n) | 5 | 7 | 8 |
| Base modela | 1.00 | 1.60 (0.50, 5.13) | 1.61 (0.51, 5.04) |
| Full modelb | 1.00 | 1.51 (0.45, 5.06) | 1.82 (0.54, 6.11) |
| Non-hip, non-spine fracture (cases, n) | 30 | 26 | 29 |
| Base modela | 1.00 | 0.78 (0.46, 1.33) | 0.74 (0.44, 1.25) |
| Full modelb | 1.00 | 0.76 (0.44, 1.30) | 0.72 (0.41, 1.27) |
Data are presented as hazard ratios (95% confidence intervals).
aBase model adjusted for age, gender, race, study site, and energy intake (all measured at baseline). bFull model adjusted for age, gender, race, study site, smoking status, alcohol consumption, education, physical activity, body mass index, energy intake, osteoporosis medications, dietary calcium intake, calcium and vitamin D supplement use, estrogen therapy, cognitive function, osteoporosis diagnosis, Health ABC Physical Performance Battery, and falls in the prior 12 mo (all measured at baseline).
Discussion
Higher protein intake (≥15% TEI) was associated with higher baseline BMD of the hip and lumbar spine in older men and women aged in their 70s when compared to lower protein intake (<13% TEI), especially in those with inadequate calcium intake. While there was no association with change in BMD, higher protein intake was associated with lower vertebral fracture risk. These results suggest that the lower end of the 10%–35% Acceptable Macronutrient Distribution Range and 0.8 g/kg body weight/d RDA for protein may be suboptimal for BMD preservation in older adults (1).
The 1.8%–6.0% higher mean baseline BMD observed in the upper protein tertile (≥15% TEI; mean: 1.1 g/kg body weight/d) in Health ABC aligns with the 2%–4% increase in BMD attributed to protein intake above the RDA in a meta-analysis of cross-sectional studies (16). Further supporting our findings, two thirds of these cross-sectional surveys reported a positive association between protein intake and at least one BMD site, with the remainder finding no significant association. Recent cross-sectional surveys of large cohorts of older men and postmenopausal women have also associated higher protein intake with higher BMD at the hip (22,24), whole body (24), and spine (24). Other cohort studies found no gender-by-protein interactions on BMD (23), generally aligning with our study which only found a gender-by-protein interaction on femoral neck baseline BMD.
Higher protein intake may preserve bone mass by increasing calcium absorption and circulating insulin-like growth factor (7,33–35). As protein works synergistically with calcium, adequate calcium intake is recommended to accompany higher protein intake to optimize bone health (20,36). However, we found higher protein intake to be associated with higher BMD at the hip and whole body even in participants with inadequate calcium intake. Other meta-analyses have found limited or insufficient evidence to support a synergistic effect of protein with calcium on BMD loss or fracture (18). It is possible that calcium intake may be more important than protein intake in the association with BMD, and may explain the attenuated effect of protein intake on BMD in participants with adequate calcium intake.
In Health ABC, positive linear associations between animal protein intake and whole body and lumbar spine BMD at baseline were found (p < .05), and positive trends were observed at the hip (p = .10) and femoral neck (p = .08). This agrees with findings in older men and postmenopausal women (ages 50+) in the Canadian Multicentre Osteoporosis Study, where positive associations were found between dairy protein intake and spine BMD (men only) and hip BMD (both sexes), and nondairy animal protein intake and spine BMD (women only) (37). Higher animal protein intake was positively associated with hip, femoral neck, whole body, and spine BMD in women in the Rancho Bernardo cohort study (ages 55+) (25), and hip BMD in the Osteoporotic Fractures in Men (MrOS) cohort (ages 65+) (22). While vegetable protein intake did not affect BMD in the Health ABC or the MrOS cohorts (22), vegetable protein intake has been negatively associated with BMD at several sites in other older adult cohorts (25,37). However, a meta-analysis of randomized controlled trials comparing equivalent amounts of protein intake by source (animal vs vegetable) found no effect on BMD (38). As individuals with higher total protein intake tend to consume a higher ratio of animal to vegetable protein (26), the positive effects on BMD with increased animal protein intake observed in our study may stem from a higher total protein intake, rather than the specific protein source.
Protein intake did not affect changes in BMD at any skeletal site in Health ABC. Other meta-analyses have found moderate evidence that lower protein intake caused more lumbar BMD loss (16,18), but did not affect BMD change at other sites (eg, hip, femoral neck, whole body) (18). Increased clinical vertebral fracture risk over 5 years of follow-up was observed in the Health ABC participants with lower protein intake, which could be mediated by lumbar BMD losses. Protein intake did not decrease hip fracture risk in our cohort, as others have reported (17,19,20,22); however, the <1% incidence of hip fracture in our cohort limited the study’s power to detect associations with protein intake.
Strengths of this study include a large sample of community-dwelling older White and Black men and women; the use of DXA and QCT to obtain BMD measures; adjudicated fracture outcome data; a long follow-up (5 years); and adjustment for potential confounders. The use of QCT allowed for assessment of BMD specific to the bone compartments (eg, trabecular, cortical, integral). However, the QCT data were only collected at the Pittsburgh site. We explored the effect of protein intake on cross-sectional (baseline BMD) and longitudinal measures (BMD change and fracture) of bone health. We assessed these effects based on total protein intake, and by protein source (animal vs vegetable). Food Frequency Questionnaires are limited in their ability to precisely assess absolute and relative nutrient intakes, which may bias our results (27,39). However, these limitations are common to any self-report of diet, including dietary records (40). The FFQ was administered at a single time point (at 1-year follow-up) and reflects participant eating habits over the past year (from baseline to 1-year follow-up), but does not account for changes in diet that may have occurred over the entire follow-up period; however, energy, protein, and calcium intake from FFQs repeated 1 year later in ~400 participants were strongly correlated (intraclass correlation coefficients: 81%–88%), suggesting that dietary intake among the Health ABC participants did not change substantially from year to year. Longitudinal changes were assessed at 5 years (as opposed to ≥10 years) to reduce confounding due to diet changes, and to coincide with the collection of DXA and QCT follow-up scans. We only adjusted for osteoporosis medications and calcium and vitamin D supplement use at baseline; however, the use of osteoporosis medications and calcium and vitamin D supplements only increased by ~5% over the follow-up period and did not differ by protein tertile. However, the relatively low fracture rates and the 5-year length of follow-up may have limited the ability to detect significant differences in this study population. While the main findings in this older adult population (ages 70–79) align with those of other cohorts (ages 50+), they may not be generalizable to younger individuals. Although analyses were adjusted for many potential confounders, there may be unmeasured confounders that relate to diet or bone health. Finally, the observational nature of this study does not allow for evaluation of a causal association between dietary protein intake and changes in BMD and fracture risk.
In conclusion, greater baseline BMD at the hip, whole body, and lumbar spine was observed in community-dwelling older White and Black men and women with higher total protein intake. Protein intake did not affect changes in BMD at any site. However, participants with higher total protein intake (≥15% TEI) had lower risk of clinical vertebral fracture over the 5-year follow-up period. A better understanding of the association between dietary protein and BMD may lead to improved dietary recommendations to preserve bone health in over 40 million older Americans, particularly the 5 million who consume less protein than recommended (41), and the 54 million with low bone mass who are at higher risk for fracture (42).
Funding
This work was supported by the National Institute on Aging grant numbers N01-AG-6-2101, N01-AG-6-2103, N01-AG-6-2106, R01-AG-028050, K25-AG-058804, R03-AG045492, and P30-AG-021332, and the National Institute of Nursing Research grant number R01-NR012459.
Conflict of Interest
None declared.
Supplementary Material
Acknowledgments
A.A.W., J.A.T., and D.K.H. designed research; all authors conducted research; J.A.C., D.C.B., F.A.T., and S.B.K. provided essential data collected in the Health ABC Study; A.A.W., J.A.T., and D.K.H. analyzed data and performed statistical analysis; A.A.W. wrote the paper; all authors contributed to data interpretation, content of the paper, and revisions; A.A.W. and D.K.H. had primary responsibility for final content, and take responsibility for the integrity of the data analysis. All authors read and approved the final manuscript.
References
- 1. Trumbo P, Schlicker S, Yates AA, Poos M; Food and Nutrition Board of the Institute of Medicine, The National Academies . Dietary reference intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein and amino acids. J Am Diet Assoc. 2002;102:1621–1630. doi: 10.1016/s0002-8223(02)90346-9 [DOI] [PubMed] [Google Scholar]
- 2. Bauer J, Biolo G, Cederholm T, et al. . Evidence-based recommendations for optimal dietary protein intake in older people: a position paper from the PROT-AGE Study Group. J Am Med Dir Assoc. 2013;14:542–559. doi: 10.1016/j.jamda.2013.05.021 [DOI] [PubMed] [Google Scholar]
- 3. Campbell WW, Trappe TA, Wolfe RR, Evans WJ. The recommended dietary allowance for protein may not be adequate for older people to maintain skeletal muscle. J Gerontol A Biol Sci Med Sci. 2001;56:M373–M380. doi: 10.1093/gerona/56.6.m373 [DOI] [PubMed] [Google Scholar]
- 4. Wolfe RR, Miller SL, Miller KB. Optimal protein intake in the elderly. Clin Nutr. 2008;27:675–684. doi: 10.1016/j.clnu.2008.06.008 [DOI] [PubMed] [Google Scholar]
- 5. Baum JI, Kim IY, Wolfe RR. Protein consumption and the elderly: what is the optimal level of intake? Nutrients. 2016;8:359–367. doi: 10.3390/nu8060359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Volpi E, Campbell WW, Dwyer JT, et al. . Is the optimal level of protein intake for older adults greater than the recommended dietary allowance? J Gerontol A Biol Sci Med Sci. 2013;68:677–681. doi: 10.1093/gerona/gls229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gaffney-Stomberg E, Insogna KL, Rodriguez NR, Kerstetter JE. Increasing dietary protein requirements in elderly people for optimal muscle and bone health. J Am Geriatr Soc. 2009;57:1073–1079. doi: 10.1111/j.1532-5415.2009.02285.x [DOI] [PubMed] [Google Scholar]
- 8. Chernoff R. Protein and older adults. J Am Coll Nutr. 2004;23(suppl. 6):627S–630S. doi: 10.1080/07315724.2004.10719434 [DOI] [PubMed] [Google Scholar]
- 9. Evans WJ. Protein nutrition, exercise and aging. J Am Coll Nutr. 2004;23(suppl. 6):601S–609S. doi: 10.1080/07315724.2004.10719430 [DOI] [PubMed] [Google Scholar]
- 10. Paddon-Jones D, Short KR, Campbell WW, Volpi E, Wolfe RR. Role of dietary protein in the sarcopenia of aging. Am J Clin Nutr. 2008;87:1562S–1566S. doi: 10.1093/ajcn/87.5.1562S [DOI] [PubMed] [Google Scholar]
- 11. Beasley JM, Shikany JM, Thomson CA. The role of dietary protein intake in the prevention of sarcopenia of aging. Nutr Clin Pract. 2013;28:684–690. doi: 10.1177/0884533613507607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wolfe RR. The role of dietary protein in optimizing muscle mass, function and health outcomes in older individuals. Br J Nutr. 2012;108(suppl. 2):S88–S93. doi: 10.1017/S0007114512002590 [DOI] [PubMed] [Google Scholar]
- 13. Courtney-Martin G, Ball RO, Pencharz PB, Elango R. Protein requirements during aging. Nutrients. 2016;8:492–503. doi:10.3390/nu8080492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cockerill W, Lunt M, Silman AJ, et al. . Health-related quality of life and radiographic vertebral fracture. Osteoporos Int. 2004;15:113–119. doi: 10.1007/s00198-003-1547-4 [DOI] [PubMed] [Google Scholar]
- 15. Center JR, Nguyen TV, Schneider D, Sambrook PN, Eisman JA. Mortality after all major types of osteoporotic fracture in men and women: an observational study. Lancet. 1999;353:878–882. doi: 10.1016/S0140-6736(98)09075-8 [DOI] [PubMed] [Google Scholar]
- 16. Darling AL, Millward DJ, Torgerson DJ, Hewitt CE, Lanham-New SA. Dietary protein and bone health: a systematic review and meta-analysis. Am J Clin Nutr. 2009;90:1674–1692. doi: 10.3945/ajcn.2009.27799 [DOI] [PubMed] [Google Scholar]
- 17. Wallace TC, Frankenfeld CL. Dietary protein intake above the current RDA and bone health: a systematic review and meta-analysis. J Am Coll Nutr. 2017;36:481–496. doi: 10.1080/07315724.2017.1322924 [DOI] [PubMed] [Google Scholar]
- 18. Shams-White MM, Chung M, Du M, et al. . Dietary protein and bone health: a systematic review and meta-analysis from the National Osteoporosis Foundation. Am J Clin Nutr. 2017;105:1528–1543. doi: 10.3945/ajcn.116.145110 [DOI] [PubMed] [Google Scholar]
- 19. Wu AM, Sun XL, Lv QB, et al. . The relationship between dietary protein consumption and risk of fracture: a subgroup and dose-response meta-analysis of prospective cohort studies. Sci Rep. 2015;5:9151. doi: 10.1038/srep09151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rizzoli R, Biver E, Bonjour JP, et al. . Benefits and safety of dietary protein for bone health—an expert consensus paper endorsed by the European Society for Clinical and Economical Aspects of Osteoporosis, Osteoarthritis, and Musculoskeletal Diseases and by the International Osteoporosis Foundation. Osteoporos Int. 2018;29:1933–1948. doi: 10.1007/s00198-018-4534-5 [DOI] [PubMed] [Google Scholar]
- 21. Rizzoli R, Stevenson JC, Bauer JM, et al. ; ESCEO Task Force . The role of dietary protein and vitamin D in maintaining musculoskeletal health in postmenopausal women: a consensus statement from the European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis (ESCEO). Maturitas. 2014;79:122–132. doi: 10.1016/j.maturitas.2014.07.005 [DOI] [PubMed] [Google Scholar]
- 22. Langsetmo L, Shikany JM, Cawthon PM, et al. ; Osteoporotic Fractures in Men (MrOS) Research Group . The association between protein intake by source and osteoporotic fracture in older men: a prospective cohort study. J Bone Miner Res. 2017;32:592–600. doi: 10.1002/jbmr.3058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hannan MT, Tucker KL, Dawson-Hughes B, Cupples LA, Felson DT, Kiel DP. Effect of dietary protein on bone loss in elderly men and women: the Framingham Osteoporosis Study. J Bone Miner Res. 2000;15:2504–2512. doi: 10.1359/jbmr.2000.15.12.2504 [DOI] [PubMed] [Google Scholar]
- 24. Beasley JM, LaCroix AZ, Larson JC, et al. . Biomarker-calibrated protein intake and bone health in the Women’s Health Initiative clinical trials and observational study. Am J Clin Nutr. 2014;99:934–940. doi: 10.3945/ajcn.113.076786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Promislow JH, Goodman-Gruen D, Slymen DJ, Barrett-Connor E. Protein consumption and bone mineral density in the elderly: the Rancho Bernardo Study. Am J Epidemiol. 2002;155:636–644. doi: 10.1093/aje/155.7.636 [DOI] [PubMed] [Google Scholar]
- 26. Sellmeyer DE, Stone KL, Sebastian A, Cummings SR. A high ratio of dietary animal to vegetable protein increases the rate of bone loss and the risk of fracture in postmenopausal women. Study of Osteoporotic Fractures Research Group. Am J Clin Nutr. 2001;73:118–122. doi: 10.1093/ajcn/73.1.118 [DOI] [PubMed] [Google Scholar]
- 27. Block G, Hartman AM, Dresser CM, Carroll MD, Gannon J, Gardner L. A data-based approach to diet questionnaire design and testing. Am J Epidemiol. 1986;124:453–469. doi: 10.1093/oxfordjournals.aje.a114416 [DOI] [PubMed] [Google Scholar]
- 28. Patterson RE, Kristal AR, Tinker LF, Carter RA, Bolton MP, Agurs-Collins T. Measurement characteristics of the Women’s Health Initiative Food Frequency Questionnaire. Ann Epidemiol. 1999;9:178–187. doi: 10.1016/s1047-2797(98)00055-6 [DOI] [PubMed] [Google Scholar]
- 29. Mackey DC, Eby JG, Harris F, et al. ; Health, Aging, and Body Composition Study Group . Prediction of clinical non-spine fractures in older black and white men and women with volumetric BMD of the spine and areal BMD of the hip: the Health, Aging, and Body Composition Study*. J Bone Miner Res. 2007;22:1862–1868. doi: 10.1359/jbmr.070807 [DOI] [PubMed] [Google Scholar]
- 30. Pahor M, Chrischilles EA, Guralnik JM, Brown SL, Wallace RB, Carbonin P. Drug data coding and analysis in epidemiologic studies. Eur J Epidemiol. 1994;10:405–411. doi: 10.1007/BF01719664 [DOI] [PubMed] [Google Scholar]
- 31. Teng EL, Chui HC. The Modified Mini-Mental State (3MS) examination. J Clin Psychiatry. 1987;48:314–318. [PubMed] [Google Scholar]
- 32. Simonsick EM, Newman AB, Nevitt MC, et al. ; Health ABC Study Group . Measuring higher level physical function in well-functioning older adults: expanding familiar approaches in the Health ABC study. J Gerontol A Biol Sci Med Sci. 2001;56:M644–M649. doi: 10.1093/gerona/56.10.m644 [DOI] [PubMed] [Google Scholar]
- 33. Shapses SA, Sukumar D. Bone metabolism in obesity and weight loss. Annu Rev Nutr. 2012;32:287–309. doi: 10.1146/annurev.nutr.012809.104655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kerstetter JE, O’Brien KO, Caseria DM, Wall DE, Insogna KL. The impact of dietary protein on calcium absorption and kinetic measures of bone turnover in women. J Clin Endocrinol Metab. 2005;90:26–31. doi: 10.1210/jc.2004-0179 [DOI] [PubMed] [Google Scholar]
- 35. Dawson-Hughes B. Interaction of dietary calcium and protein in bone health in humans. J Nutr. 2003;133:852S–854S. doi: 10.1093/jn/133.3.852S [DOI] [PubMed] [Google Scholar]
- 36. Kerstetter JE, Kenny AM, Insogna KL. Dietary protein and skeletal health: a review of recent human research. Curr Opin Lipidol. 2011;22:16–20. doi: 10.1097/MOL.0b013e3283419441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Langsetmo L, Barr SI, Berger C, et al. ; CaMos Research Group . Associations of protein intake and protein source with bone mineral density and fracture risk: a population-based cohort study. J Nutr Health Aging. 2015;19:861–868. doi: 10.1007/s12603-015-0544-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Shams-White MM, Chung M, Fu Z, et al. . Animal versus plant protein and adult bone health: a systematic review and meta-analysis from the National Osteoporosis Foundation. PLoS ONE 2018;13:e0192459. doi: 10.1371/journal.pone.0192459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Willett WC, Browne ML, Bain C, et al. . Relative weight and risk of breast cancer among premenopausal women. Am J Epidemiol. 1985;122:731–740. doi: 10.1093/oxfordjournals.aje.a114156 [DOI] [PubMed] [Google Scholar]
- 40. Kim WW, Mertz W, Judd JT, Marshall MW, Kelsay JL, Prather ES. Effect of making duplicate food collections on nutrient intakes calculated from diet records. Am J Clin Nutr. 1984;40(suppl. 6):1333–1337. doi: 10.1093/ajcn/40.6.1333 [DOI] [PubMed] [Google Scholar]
- 41. Berner LA, Becker G, Wise M, Doi J. Characterization of dietary protein among older adults in the United States: amount, animal sources, and meal patterns. J Acad Nutr Diet. 2013;113:809–815. doi: 10.1016/j.jand.2013.01.014 [DOI] [PubMed] [Google Scholar]
- 42. National Osteoporosis Foundation. 54 Million Americans Affected by Osteoporosis and Low Bone Mass. 2014. https://www.nof.org/news/54-million-americans-affected-by-osteoporosis-and-low-bone-mass/. Accessed January 8, 2020. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.