Abstract
Background
Up to 20 per cent of all operations for patients with colorectal cancer (CRC) are performed in octogenarians. Anastomotic leakage is a leading cause of morbidity and death after resection for CRC. The aim of this study was to assess the rate of anastomosis creation, the risk of anastomotic leakage and death in surgery for left-sided CRC in elderly patients.
Methods
This prospective cohort study compared patients less than 80 and 80 or more years with left-sided CRC resection performed between 2013 and 2019. Data were provided from a risk-adjusted surgical quality-assessment system with 219 participating centres in Germany. Outcome measures were the rate of anastomoses, anastomotic leakages, death at 30 days and 2-year overall survival (OS). Propensity score matching was used to control for selection bias and compare subgroups of patients of less than 80 and 80 or more years.
Results
Out of 18 959 patients, some 3169 (16.7 per cent) were octogenarians. Octogenarians were less likely to receive anastomoses (82.0 versus 92.9 per cent, P < 0.001; odds ratio 0.50 (95 per cent c.i. 0.44 to 0.58), P < 0.001). The rate of anastomotic leakages did not differ between age groups (8.6 versus 9.7 per cent, P = 0.084), but 30-day mortality rate after leakage was significantly higher in octogenarians (15.8 versus 3.5 per cent, P < 0.001). Overall, anastomotic leakage was the strongest predictor for death (odds ratio 4.95 (95 per cent c.i. 3.66 to 6.66), P < 0.001). In the subgroup with no leakage, octogenarians had a lower 2-year OS rate than younger patients (71 versus 87 per cent, P < 0.001), and in the population with anastomotic leakage, the 2-year OS was 80 per cent in younger and 43 per cent in elderly patients (P < 0.001). After propensity score matching, older age remained predictive for not receiving an anastomosis (odds ratio 0.54 (95 per cent c.i. 0.46 to 0.63), P < 0.001) and for death (odds ratio 2.60 (95 per cent c.i. 1.78 to 3.84), P < 0.001), but not for the occurrence of leakages (odds ratio 0.94 (95 per cent c.i. 0.76 to 1.15), P = 0.524).
Conclusion
Anastomotic leakage is not more common in octogenarians, but an age of 80 years or older is an independent factor for not receiving an anastomosis in surgery for left-sided CRC. The mortality rate in the case of leakage in octogenarians was reported to exceed 15 per cent.
The aim of this study was to assess the impact of age of 80 years or older on the creation of anastomoses, the risk of anastomotic leakage and death in surgery for left-sided colorectal cancer. The authors found that anastomotic leakage is not more common in octogenarians, but age of 80 years or older is an independent factor for not receiving an anastomosis in surgery for left-sided colorectal cancer. Death in the case of leakage is disproportionately high at 16 per cent in octogenarians, which should be addressed in shared decision making.
Introduction
Colorectal cancer (CRC) is predominantly a disease of the elderly and the age-specific incidence for CRC is highest in patients at least 80 years of age1,2. The group of elderly patients diagnosed with CRC is expected to increase as the population ages3. Octogenarians make up 4.0 per cent of the population today. In 2050, the proportion of octogenarians will rise to 8.6 per cent in the USA, 12.8 per cent in Germany and 15.6 per cent in Japan4. About 20 per cent of patients with CRC currently are octogenarians5. Octogenarians form a distinct group of patients due to their decreased functionality and increased prevalence and severity of co-morbidities. It has been shown previously that these patients are treated less aggressively by both surgery and radiochemotherapy, and also that prevention of complications is the key factor for long-term survival6,7. On the other hand, an increasing proportion of octogenarians has a good performance status and recent studies demonstrated comparable oncological outcomes7–9.
A reconstructive bowel anastomosis has a positive impact on the quality of life after colorectal resection10. However, anastomotic leakage is the major source of postoperative death11. The decision for or against an anastomosis is difficult, especially in elderly and co-morbid patients. However, the evidence to support decision making in octogenarians is limited and most studies involve only small numbers of patients9,12,13.
Estimates of the frequency of anastomotic leakages and death after leakage are particularly important for the elderly and clinicians in making a decision about the risks and benefits of anastomosis creation. The aim of the study was to evaluate the rate of anastomosis, the risk of anastomotic leakage and death after anastomosis in elderly patients with left-sided colon and rectum cancer resections. For this purpose, the Study, Documentation and Quality centre (StuDoQ)|ColorectalCancer, a German nationwide quality-assessment system for CRC surgery, was used as the data source for a large prospective cohort study.
Methods
Data source
StuDoQ is a German nationwide surgical patient outcome database with voluntary participation (https://www.dgav.de/english/studoq.html). StuDoQ was developed by the German Society of General and Visceral Surgery (DGAV) as an instrument for risk-adjusted quality assessment and as a framework for surgical research14–17. StuDoQ works with a common set of variables for various surgical procedures and organ-specific as well as technique-specific modules, including colon cancer, rectum cancer, pancreas surgery, sigmoid resections, HIPEC (hyperthermic intraperitoneal chemotherapy), liver surgery, metabolic and bariatric diseases, NOTES (natural orifice translumenal endoscopic surgery), thyroid surgery and robotic surgery14,16–21.
Data from participating centres are entered prospectively in pseudonymized form using a browser-based tool. The data-protection concept was approved by the Society for Technology, Methods and Infrastructure for Networked Medical Research19. The participating centres have each obtained approval from the local ethics committee as a basis for participation. All patients gave their written informed consent which included data collection, scientific analysis and publication of results.
Study design
The StuDoQ databases for colon carcinoma (StuDoQ|ColonCancer) and rectum carcinoma (StuDoQ|RectumCancer) vary slightly in certain organ-specific details, but share a broad number of common variables22,23. Both databases were merged for this analysis and are referred to as StuDoQ|ColorectalCancer. The complete data set includes more than 580 individual items per patient covering demographic data, preoperative risk factors, tumour-specific details, intraoperative and perioperative data, complications, oncological quality, 30-day morbidity and follow-up data. All data between the start of the database in January 2013 and December 2019 were used. The study was designed as a prospective cohort study and is reported adhering to the STROBE guidelines24.
Patient selection
The database of StuDoQ|ColorectalCancer included 40 029 patients from 219 participating centres (Fig. 1). Only valid cases (informed consent and 30 day morbidity completed), treated with elective surgery with resection for rectum or left sided colon cancer were used. Patients with local resection or palliative indication were excluded. Left-sided CRC was defined according to these ICD-10 codes: C18.5 Malignant neoplasm of splenic flexure, C18.6 Malignant neoplasm of descending colon, C18.7 Malignant neoplasm of sigmoid colon, C19 Malignant neoplasm of rectosigmoid junction, C20 Malignant neoplasm of rectum25. Since the aim of this study was to assess the creation of anastomoses and anastomotic complications, patients who received an abdominoperineal resection (Miles operation) without the technical possibility for an anastomosis were excluded.
Fig. 1.
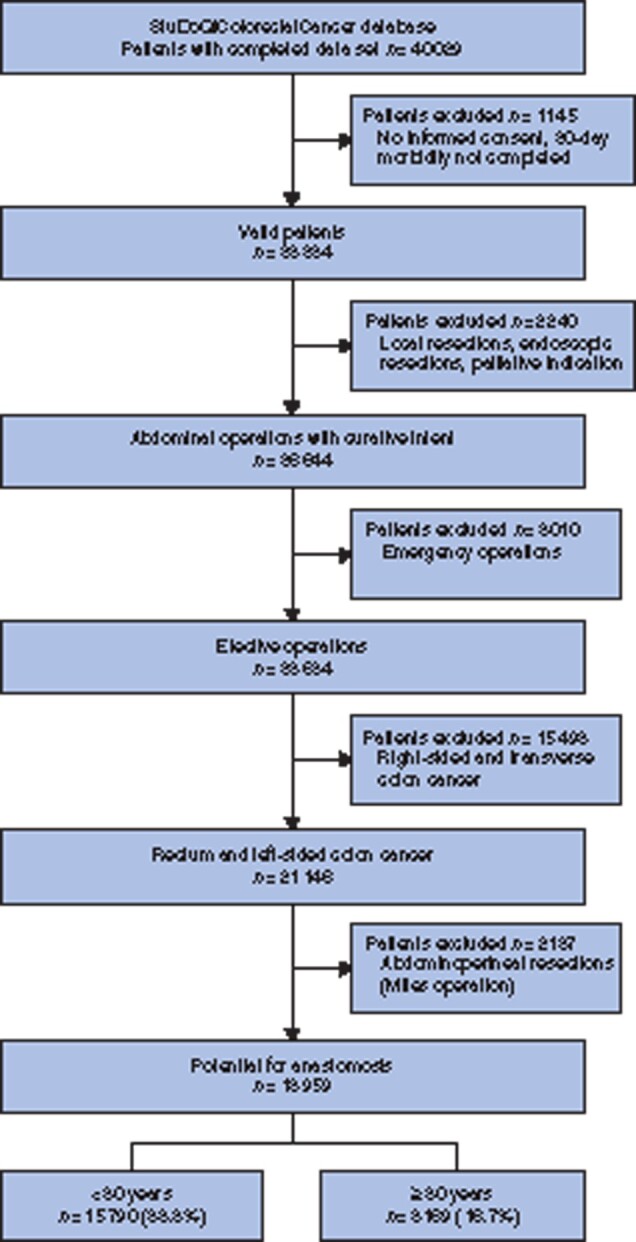
Study flow diagram
Preoperative characteristics and postoperative outcomes
The following variables were used to assess preoperative characteristics: alcohol abuse within 1 year before surgery according to ICD F10.1; anastomosis, defined as reconstructive bowel anastomosis; antiplatelet therapy within 5 days before surgery; ASA physical status classification system (ASA 1–2, ASA 3–5); BMI; cerebrovascular event, defined as history of cerebral stroke or transient ischaemic attack with or without neurological deficit; diabetes mellitus types 1 and 2; dialysis, defined as any form of renal replacement therapy; heart failure, defined as New York Heart Association (NYHA) functional classification for heart failure, NYHA stages 3 and 4; preoperative haemoglobin; arterial hypertension, defined as therapy with antihypertensive drugs; immunosuppressive therapy (excluding corticosteroids) until at least 3 months before surgery; Karnofsky performance score (KPS), which classifies cancer patients with regard to their functional impairment (self-sufficient, 70–100 per cent; requires assistance, 10–60 per cent)26,27; liver cirrhosis, defined as Child–Pugh scores A to C; peripheral artery disease with history of revascularization (interventional or surgical) or amputation; preoperative metastases in any organ; preoperative radiotherapy and/or chemotherapy; any type of protective stoma; severe chronic obstructive pulmonary disease (COPD) with bronchodilator therapy or dyspnoea; biological sex; steroid medication, defined as 7.5 mg or greater of prednisolone equivalent per day for more than 6 months to at least 3 months before surgery; location of the colorectal carcinoma (lower rectum less than 6 cm from the anal verge, middle/upper rectum 6–16 cm from the anal verge, left colon); unintentional weight loss of more than 10 per cent of body weight within 6 months before surgery.
Perioperative characteristics were reported with the following variables: surgical technique at the beginning of the operation (minimally invasive, open surgery, other); protective stoma (stoma that was created before or during the tumour operation); anastomotic technique (handsewn, stapled).
Postoperative outcomes were reported with the following variables: death within 30 days after surgery; anastomosis creation; anastomotic leakage during inpatient stay, grades A to C for rectum anastomoses, any leakage for colon anastomoses28; complications according to the Clavien–Dindo classification during inpatient stay (no complication, minor (Clavien–Dindo 1–3a), major (Clavien–Dindo 3b–4b), death)29; MTL30, a validated quality indicator composed of the three components M, (death at 30 days), T (transfer to another acute-care hospital within 30 days) and L (length of stay longer than 30 days), it is fulfilled if the patient is dead on postoperative day 30 or was transferred to another acute-care hospital or still requires inpatient treatment30–32; postoperative pneumonia defined as radiological or clinical evidence during inpatient stay; 30-day surgical-site infection (SSI) with surgical wound opening within 30 days after surgery; staging for CRC of the Union for International Cancer Control (UICC) (UICC 0 – 2, UICC 3, UICC 4); unplanned ventilation of more than 48 hours after surgery; postoperative inpatient stay in days.
Outcome measures
The patients were divided into two age groups of less than 80 and 80 or more years. The primary outcomes were: rate of reconstructive bowel anastomosis after colorectal resection; anastomotic leakages, defined as earlier; death in patients with anastomosis within 30 days after surgery; overall survival (OS), defined as 2-year survival after surgery.
Missing data
No methods to handle missing data were used, since missing data were infrequent and the distribution was even between the groups that were used for comparison.
Statistical analysis
Continuous variables were reported as mean(s.d.), and categorical variables as number (percent). Welch's unequal variances t-test was used to compare continuous variables between two independent groups. The χ2 test was used to compare categorical variables. The level of significance was 0.050 (two-sided) for each statistical test. Multivariable binary logistic regression analyses were used to investigate the preoperative co-variables on the outcome variables anastomosis creation, anastomotic leakage and death at 30 days, and results were presented as odds ratio (OR) or relative risk (RR) with 95 per cent confidence intervals. The results of regression analyses were displayed graphically as forest plots, which facilitates visual interpretation. All statistical analyses were conducted with R (R Foundation for Statistical Computing, Vienna, Austria, https://www.R-project.org/) version 3.6.333.
Kaplan–Meier estimates were calculated for 2-year OS with the last available contact date and the patient’s status (alive or dead) using the survival package for R34. All patients were included, including those who had surgery within the last 2 years of the study duration and were unable to complete the full 2-year follow-up. The log rank test was used for comparisons between groups. A considerable loss to follow-up was expected, since follow-up examinations were not mandatory due to legal restrictions in Germany. Loss to follow-up was assumed to be missing not at random (MNAR) and was not addressed with specific statistical measures35.
Propensity score matching (PSM) was used to control for selection bias in preoperative characteristics between the two age groups of less than 80 and 80 years or older. All preoperative co-variables were included, regardless of their significance in univariable or multivariable analyses36. In addition, postoperative UICC stage was included to compensate for differing tumour extent between the age groups. Patients were paired 1 : 1 with a nearest neighbour algorithm without replacement, using the MatchIt package for R37. A caliper width (maximum tolerated difference between matched subjects) of 0.1 times the standard deviation of the logit of the propensity score was used38. The quality of matching was evaluated with absolute standardized mean differences (SMD) using the cobalt package for R39. A threshold of 0.05 was used as an indicator of co-variable balance between matched groups40. SMD are graphically displayed as Love plot, a summary plot of co-variable balance before and after conditioning40.
Results
The final data set reviewed consisted of 18 959 patients (Fig. 1). The mean(s.d.) age of the study population was 67.8(11.9) years and 16.7 per cent were octogenarians (Table 1). Among data reviewed, some 21 of 33 variables had no missing data. Missing data were prevalent in less than 1.5 per cent for any of the remaining variables. In summary, octogenarians more frequently had cardiovascular co-morbidities, a higher ASA score, reduced KPS and a lower frequency of rectum cancer.
Table 1.
Preoperative characteristics
| <80 years | ≥80 years | Total | Missing | P | |
|---|---|---|---|---|---|
| All patients | 15 790 (83.3) | 3169 (16.7) | 18 959 | ||
| Age (years)* | 64.6(10.3) | 83.8(3.4) | 67.8(11.9) | 0 | <0.001 |
| Alcohol abuse | 699 (4.4) | 74 (2.3) | 773 (4.1) | 3 (0.0) | <0.001 |
| ASA 3–5 | 5424 (34.4) | 2139 (67.5) | 7563 (39.9) | 1 (0.0) | <0.001 |
| BMI (kg/m²)* | 26.8(5.0) | 25.8(4.3) | 26.7(4.9) | 2 (0.0) | <0.001 |
| Cerebrovascular event | 888 (5.6) | 343 (10.8) | 1231 (6.5) | 0 | <0.001 |
| Diabetes | 2748 (17.4) | 784 (24.7) | 3532 (18.6) | 0 | <0.001 |
| Dialysis | 80 (0.5) | 25 (0.8) | 105 (0.6) | 0 | 0.068 |
| Female sex | 5931 (37.6) | 1566 (49.4) | 7497 (39.5) | 0 | <0.001 |
| Immunosuppressive therapy | 159 (1.0) | 22 (0.7) | 181 (1.0) | 0 | 0.121 |
| Heart failure: NYHA 3–4 | 427 (2.7) | 325 (10.3) | 752 (4.0) | 0 | <0.001 |
| Hypertension | 8192 (51.9) | 2494 (78.7) | 10686 (56.4) | 0 | <0.001 |
| Karnofsky performance status | 0 | <0.001 | |||
| Self-sufficient (70–100%) | 14 972 (94.8) | 2409 (76.0) | 17 381 (91.7) | ||
| Requires assistance (10–60%) | 818 (5.2) | 760 (24.0) | 1578 (8.3) | ||
| Liver cirrhosis (Child A–C) | 305 (1.9) | 50 (1.6) | 355 (1.9) | 75 (0.4) | 0.209 |
| Peripheral artery disease | 376 (2.4) | 129 (4.1) | 505 (2.7) | 0 | <0.001 |
| Preoperative antiplatelet therapy | 2413 (15.3) | 913 (28.8) | 3326 (17.5) | 2 (0.0) | <0.001 |
| Preoperative haemoglobin (g/dl)* | 13.0(2.1) | 11.8(2.3) | 12.8(2.1) | 266 (1.4) | <0.001 |
| Preoperative metastases | 2135 (13.6) | 349 (11.2) | 2484 (13.2) | 181 (1.0) | <0.001 |
| Preoperative RCTx | 4030 (25.5) | 316 (10.0) | 4346 (22.9) | 0 | <0.001 |
| Severe COPD | 799 (5.1) | 213 (6.7) | 1012 (5.3) | 0 | <0.001 |
| Steroid medication | 191 (1.2) | 43 (1.4) | 234 (1.2) | 0 | 0.550 |
| Tumour location | 0 | <0.001 | |||
| Lower rectum | 1775 (11.2) | 243 (7.7) | 2018 (10.6) | ||
| Middle/upper rectum | 7448 (47.2) | 1328 (41.9) | 8776 (46.3) | ||
| Left colon | 6567 (41.6) | 1598 (50.4) | 8165 (43.1) | ||
| Weight loss | 1740 (11.0) | 473 (14.9) | 2213 (11.7) | 20 (0.1) | <0.001 |
Values in parentheses are percentages unless indicated otherwise; *values are mean(s.d.). NYHA, New York Heart Association; RCTx, radiotherapy or chemotherapy; COPD, chronic obstructive pulmonary disease.
There were some differences in the operative procedure (Table 2). Patients of 80 years of age and older were less likely to have minimally invasive resection in both colon and rectal cancer. In addition, the rate of protective stoma creation was lower in the elderly group with rectum cancer, while the rate was slightly higher in the group with colon cancer.
Table 2.
Perioperative characteristics for patients with rectum cancer and patients with colon cancer
| <80 years | ≥80 years | Total | Missing | P | |
|---|---|---|---|---|---|
| Rectum carcinoma | |||||
| All patients | 9223 (85.4) | 1571 (14.6) | 10794 | ||
| Operation technique | 86 (0.8) | <0.001 | |||
| Minimally invasive | 5538 (60.6) | 825 (52.6) | 6363 (59.4) | ||
| Open surgery | 3588 (39.3) | 738 (47.1) | 4326 (40.4) | ||
| Other | 15 (0.2) | 4 (0.3) | 19 (0.2) | ||
| Patients with anastomosis | 8335 (87.8) | 1154 (12.2) | 9489 | ||
| Protective stoma | 5978 (71.7) | 709 (61.5) | 6687 (70.5) | 5 (0.1) | <0.001 |
| Anastomotic technique | 0 | 0.512 | |||
| Handsewn | 1117 (13.4) | 146 (12.7) | 1263 (13.3) | ||
| Stapled | 7218 (86.6) | 1008 (87.3) | 8226 (86.7) | ||
| Colon carcinoma | |||||
| All patients | 6567 (80.4) | 1598 (19.6) | 8165 | ||
| Operation technique | 6 (0.1) | <0.001 | |||
| Minimally invasive | 3658 (55.7) | 752 (47.1) | 4410 (54.1) | ||
| Open surgery | 2901 (44.2) | 845 (52.9) | 3746 (45.9) | ||
| Other | 3 (0.0) | 0 | 3 (0.0) | ||
| Patients with anastomosis | 6246 (81.4) | 1426 (18.6) | 7672 | ||
| Protective stoma | 543 (9.0) | 135 (9.8) | 678 (9.1) | 262 (3.4) | 0.378 |
| Anastomotic technique | 0 | 0.014 | |||
| Handsewn | 1570 (25.1) | 404 (28.3) | 1974 (25.7) | ||
| Stapled | 4676 (74.9) | 1022 (71.7) | 5698 (74.3) |
Values in parentheses are percentages.
Patients 80 years and older had a significantly prolonged mean length of stay (15.8 versus 13.5 days) and a higher rate of complications as well as more severe complications (Table 3). While 30-day mortality rate in patients under 80 years of age was 1.1 per cent, it was almost five times higher in octogenarians. MTL30 was more than double in octogenarians (20.6 versus 9.5 per cent; P < 0.001).
Table 3.
Postoperative outcomes
| <80 years | ≥80 years | Total | Missing | P | |
|---|---|---|---|---|---|
| Death at 30 days (all patients) | 180 (1.1) | 165 (5.2) | 345 (1.8) | 0 | <0.001 |
| Death at 30 days (only patients with anastomotic leakage) | 50 (3.5) | 35 (15.8) | 85 (5.2) | 0 | <0.001 |
| 30-day SSI | 1049 (6.7) | 229 (7.2) | 1278 (6.8) | 39 (0.2) | 0.256 |
| Anastomotic leakage | 1416 (9.7) | 222 (8.6) | 1638 (9.5) | 113 (0.6) | 0.084 |
| Complications during inpatient stay | 0 | <0.001 | |||
| No complication | 10611 (67.2) | 1877 (59.2) | 12488 (65.9) | ||
| Minor (Clavien–Dindo 1–3a) | 3063 (19.4) | 727 (22.9) | 3790 (20.0) | ||
| Major (Clavien–Dindo 3b–4b) | 1930 (12.2) | 425 (13.4) | 2355 (12.4) | ||
| Death | 186 (1.2) | 140 (4.4) | 326 (1.7) | ||
| MTL30 | 1503 (9.5) | 653 (20.6) | 2156 (11.4) | 0 | <0.001 |
| Pneumonia | 431 (2.7) | 202 (6.4) | 633 (3.3) | 0 | <0.001 |
| Postoperative length of stay (days)* | 13.5(10.5) | 15.8(10.7) | 13.9(10.6) | 0 | <0.001 |
| UICC | 99 (0.5) | 0.004 | |||
| UICC 0 –2 | 9205 (58.6) | 1899 (60.1) | 11104 (58.9) | ||
| UICC 3 | 4328 (27.6) | 892 (28.2) | 5220 (27.7) | ||
| UICC 4 | 2169 (13.8) | 367 (11.6) | 2536 (13.4) | ||
| Unplanned ventilation (>48 hours) | 347 (2.2) | 125 (3.9) | 472 (2.5) | 0 | <0.001 |
Values in parentheses are percentages unless indicated otherwise; *values are mean(s.d.). SSI, surgical-site infection; MTL30, a validated quality indicator composed of the three components M (death at 30 days), T (transfer to another acute-care hospital within 30 days) and L (length of stay longer than 30 days); UICC, Union for International Cancer Control.
Creation of anastomoses and anastomotic leakages
Anastomoses were created in 91.1 per cent of all patients, with a significant trend towards resections without anastomosis (Hartmann procedure) in lower cancer locations (Table 4). This trend was greater in octogenarians, in whom 55 per cent received an anastomosis for low rectal cancer, compared with 83 per cent in younger patients.
Table 4.
Creation of anastomoses and anastomotic leakages for different tumour locations
| <80 years | ≥80 years | Total | Missing | P | |
|---|---|---|---|---|---|
| Creation of anastomoses | |||||
| Left colon (n = 8119) | 6246 (95.7) | 1426 (89.7) | 7672 (94.5) | 46 (0.6) | <0.001 |
| Middle/upper rectum (n = 8723) | 6878 (92.8) | 1022 (77.7) | 7900 (90.6) | 53 (0.6) | <0.001 |
| Lower rectum (n = 2004) | 1457 (82.6) | 132 (54.8) | 1589 (79.3) | 14 (0.7) | <0.001 |
| Total (n = 18 846) | 14 581 (92.9) | 2580 (82.0) | 17 161 (91.1) | 113 (0.6) | <0.001 |
| Anastomotic leakages | |||||
| Left colon (n = 7672) | 463 (7.4) | 108 (7.6) | 571 (7.4) | 0 | 0.878 |
| Middle/upper rectum (n = 7900) | 770 (11.2) | 102 (10.0) | 872 (11.0) | 0 | 0.270 |
| Lower rectum (n = 1589) | 183 (12.6) | 12 (9.1) | 195 (12.3) | 0 | 0.306 |
| Total (n = 17 161) | 1416 (9.7) | 222 (8.6) | 1638 (9.5) | 0 | 0.084 |
Values in parentheses are percentages.
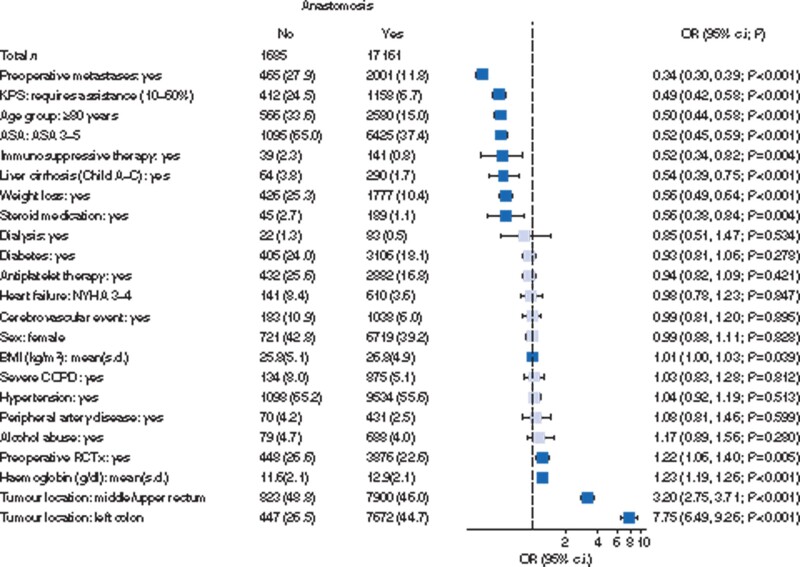
The regression analysis showing the clinical variables and their correlation with the creation of anastomoses is shown in Fig. 2. The odds against the creation of anastomoses correlated strongly with preoperative metastases (OR 0.34 (95 per cent c.i. 0.30 to 0.39), P < 0.001), physical performance status (OR 0.49 (95 per cent c.i. 0.42 to 0.58), P < 0.001), age 80 years or older (OR 0.50 (95 per cent c.i. 0.44 to 0.58), P < 0.001), ASA (OR 0.52 (95 per cent c.i. 0.45 to 0.59), P < 0.001) and weight loss (OR 0.56 (95 per cent c.i. 0.49 to 0.64), P < 0.001). The odds did not correlate with co-morbidities typically associated with cardiovascular disease such as antiplatelet therapy, hypertension or heart failure. Parameters such as liver disease, immunosuppression and steroid medication also influenced the decision to create an anastomosis negatively, but their prevalence was low (less than 6 per cent).
Fig. 2.
Forest plot of multivariable logistic regression analysis for factors influencing the creation of anastomoses.
Pale shade indicates non-significant odds ratios (ORs). The dashed line marks an OR of 1. Patient number (per cent) or mean(s.d.) for explanatory variables. KPS, Karnofsky performance score; NYHA, New York Heart Association; COPD, chronic obstructive pulmonary disease; RCTx, radiotherapy or chemotherapy.
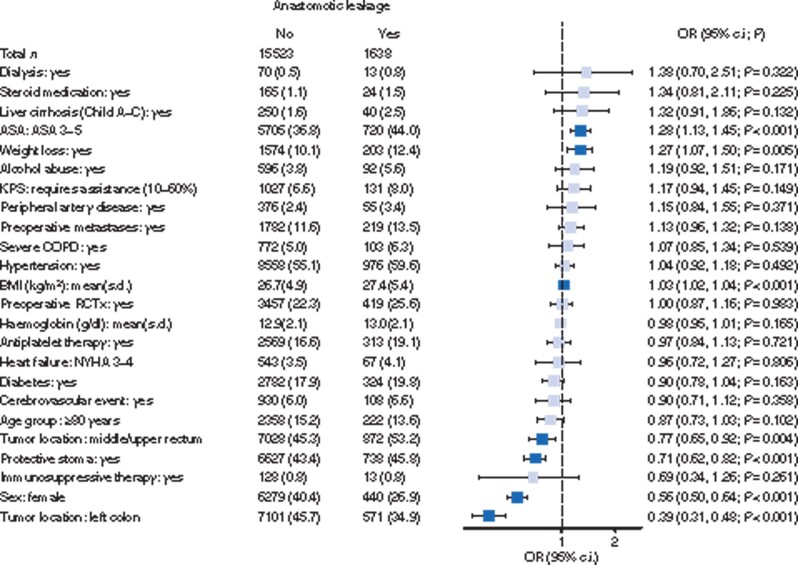
Higher ASA score and unintentional weight loss were the only strong predictors for anastomotic leakages in the regression analysis (Fig. 3). Higher tumour location, a defunctioning stoma and female gender were protective factors. Again, cardiovascular co-morbidity had no significant influence. No age-group differences were observed for the rate of anastomotic leakages, regardless of tumour location (Table 4).
Fig. 3.
Forest plot of multivariable logistic regression analysis for factors affecting anastomotic leakages.
Pale shade indicates non-significant odds ratios (ORs). The dashed line marks an OR of 1. Patient number (per cent) or mean(s.d.) for explanatory variables. KPS, Karnofsky performance score; COPD, chronic obstructive pulmonary disease; RCTx, radiotherapy or chemotherapy; NYHA, New York Heart Association.
Mortality rate in patients with anastomotic leakage
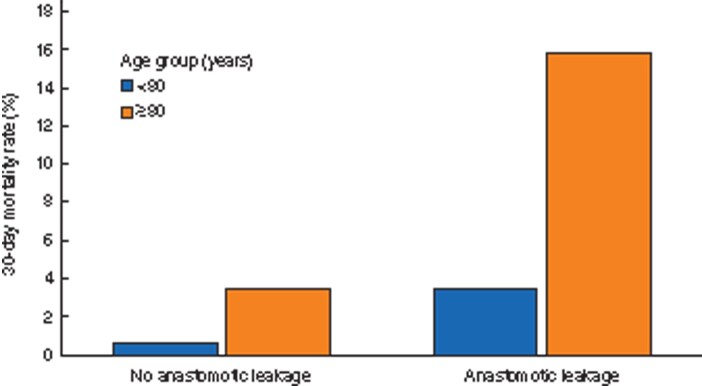
Overall, patients with anastomotic leakage had a significantly increased 30-day mortality rate compared with patients with unaffected anastomotic healing (5.2 versus 1.0 per cent, P < 0.001). A high mortality rate was observed in octogenarians with anastomotic leakage compared with that of younger patients (15.8 versus 3.5 per cent, P < 0.001, Table 3 and Fig. 4). For octogenarians, the RR of dying within 30 days after surgery was 5.58 (95 per cent c.i. 4.12 to 7.57, P < 0.001) for patients with anastomotic healing and 4.46 (95 per cent c.i. 2.97 to 6.72, P < 0.001) for patients with anastomotic leakage (Table 5).
Fig. 4.
30-day mortality rate in patients without and with anastomotic leakage
Table 5.
Relative mortality risk depending on the absence or occurrence of anastomotic leakage
| Death within 30 days of surgery |
Relative risk* | P | ||
|---|---|---|---|---|
| No | Yes | |||
| Patients without anastomotic leakage | ||||
| <80 years | 13 084 (99.4) | 81 (0.6) | 5.58 (4.12, 7.57) | <0.001 |
| ≥80 years | 2277 (96.6) | 81 (3.4) | ||
| Patients with anastomotic leakage | ||||
| <80 years | 1366 (96.5) | 50 (3.5) | 4.46 (2.97, 6.72) | <0.001 |
| ≥80 years | 187 (84.2) | 35 (15.8) | ||
Values in parentheses are percentages unless indicated otherwise; *values in parentheses are 95 per cent confidence intervals. Only patients with bowel anastomosis were included.
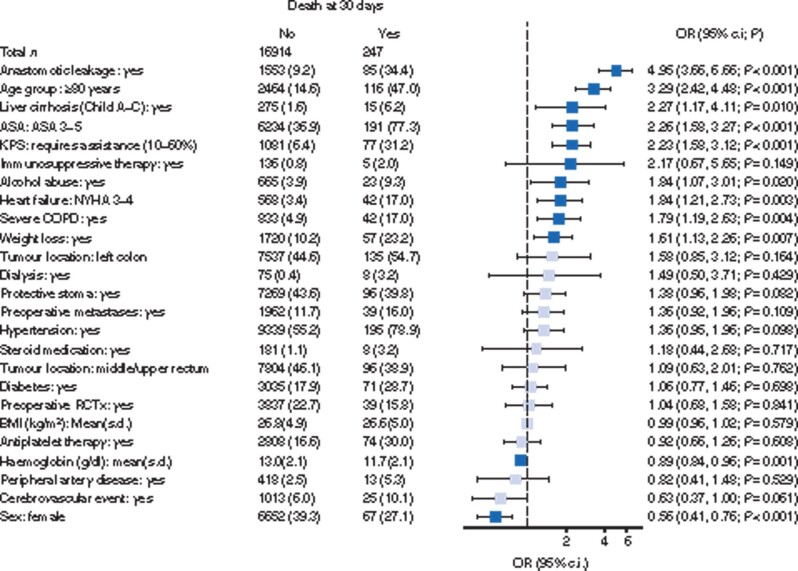
The strongest predictor for death at 30 days was anastomotic leakage, which occurred in 34 per cent of all patients who died (Fig. 5). Almost half of the patients (47 per cent) who died within 30 days were octogenarians and 31 per cent had a low physical performance score. ASA, heart failure, COPD and unintentional weight loss also had significant effects.
Fig. 5.
Forest plot of multivariable logistic regression analysis for factors influencing 30-day mortality rate in patients with anastomosis.
Pale shade indicates non-significant odds ratios (ORs). The dashed line marks an OR of 1. Patient number (per cent) or mean(s.d.) for explanatory variables. KPS, Karnofsky performance score; NYHA, New York Heart Association; COPD, chronic obstructive pulmonary disease; RCTx, radiotherapy or chemotherapy.
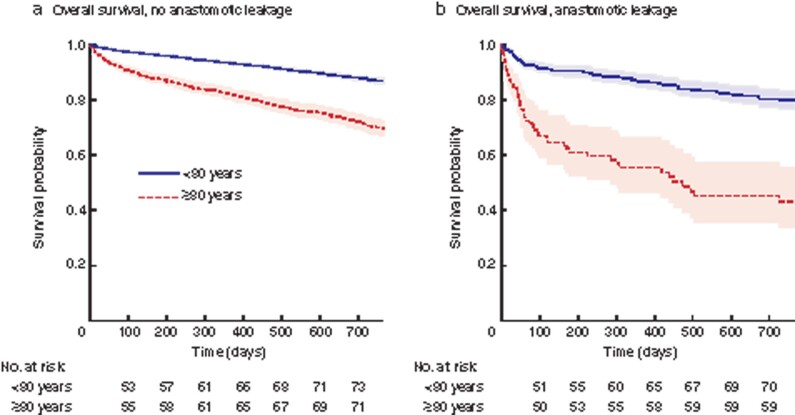
Kaplan–Meier estimates were calculated for OS. Octogenarians with anastomosis, but without leakage, showed a lower 2-year OS than younger patients (71 versus 87 per cent, Fig. 6). Anastomotic leakage reduced the 2-year survival probability to 80 per cent in younger patients and to 43 per cent in octogenarians. However, some 7257 (50.8 per cent) of the patients less than 80 years and 1557 (53.3 per cent) of the patients 80 years and older were censored after postoperative day 30.
Fig. 6.
Kaplan–Meier overall survival estimates
a Patients without anastomotic leakage (P < 0.001). b Patients with anastomotic leakage (P < 0.001). Shaded regions represent 95 per cent confidence intervals.
Propensity score matching
PSM was used for the age groups less than 80 and 80 years or older to adjust for these confounding co-variables. Some 2811 of 2990 (94.0 per cent) octogenarians could be matched. Unmatched cases were mainly patients with a low physical performance status (KPS 10–60 per cent; 166 of 179 unmatched patients). SMDs were less than 5 per cent for each of the 28 co-variables after PSM, which indicated balanced groups (Fig. S1).
After PSM, age 80 years or older remained an independent predictor in the regression analysis for not receiving an anastomosis (Table 6). The odds for anastomotic leakages did not differ between age groups. There was no significant difference in 30-day mortality rate between age groups when no anastomosis was created. However, octogenarians with anastomosis had significantly higher odds than younger patients of dying within 30 days after surgery.
Table 6.
Logistic regression analysis after propensity score matching: effect of age on the creation of anastomoses, anastomotic leakages and death
| <80 years | ≥80 years | Odds ratio* | P | |
|---|---|---|---|---|
| Anastomoses created | 2486 (88.4) | 2315 (82.4) | 0.54 (0.46, 0.63) | <0.001 |
| Anastomotic leakages | 229 (9.2) | 192 (8.3) | 0.94 (0.76, 1.15) | 0.524 |
| Death at 30 days in patients without anastomosis | 23 (7.1) | 35 (7.1) | 1.26 (0.70, 2.31) | 0.439 |
| Death at 30 days in patients with anastomosis | 43 (1.7) | 92 (4.0) | 2.60 (1.78, 3.84) | <0.001 |
Values in parentheses are percentages unless indicated otherwise; *values in parentheses are 95 per cent confidence intervals.
Discussion
The aim of this study was to evaluate the rate of anastomosis creation, anastomotic leakages and mortality for left-sided CRC in patients aged 80 years or older. Anastomoses were created at a significantly lower rate in octogenarians, particularly after rectum cancer resection. Although octogenarians were less likely to receive an anastomosis, the rate of anastomotic leakages did not differ between age groups. The relative risk of dying within 30 days after surgery was more than five times higher in octogenarians without anastomotic complications than in younger patients. In the case of anastomotic leakage, the relative mortality risk in octogenarians did not increase compared with that of younger patients, but the absolute mortality rate was 15.8 per cent. Also, less than 45 per cent of octogenarians were alive after 2 years, compared with 80 per cent of patients under 80 years of age.
Anastomotic leakage and age 80 years or older were the strongest predictors for death. The multivariable regression analysis after PSM confirmed these findings. Although preoperative risk factors were balanced between age groups, octogenarians continued to have lower odds of receiving an anastomosis but equal odds of anastomotic leakage. Age 80 years and older remained an independent risk factor for death in patients with anastomosis.
One of the strengths of the study is the large number of patients in a well defined surgical treatment group that reflects real world data. Since risk-adjusted quality assessment is the major objective of StuDoQ|ColorectalCancer, preoperative risk factors and outcome variables are defined precisely.
Another strength of the study is the selection of a relatively homogeneous patient group. Emergency surgery was excluded because it is known to have a higher morbidity rate5,41,42. In addition, elderly patients are more likely to undergo emergency surgery42. Together, this may bias the results significantly. Only resections for left-sided CRC were included, as anastomoses for left-sided resections are performed relatively homogeneously in the upper to lower rectum. These anastomoses have a leakage rate of about 10 per cent, whereas anastomoses for colon cancer in other locations have different leakage rates43,44. Coupled with the fact that older patients more often have right-sided CRC, this would create heterogeneity and may confound the results. Palliative and local resections were excluded for similar reasons.
One limitation of the study is the observational design. This makes it difficult to compare the outcome between age groups, as these groups differ in their risk profiles. However, this opens the view at clinical decision factors for or against the creation of an anastomosis, since this choice was probably made on the basis of the documented risk factors. PSM was used to balance the different risk profiles between age groups. This design made it possible to assess selectively the effect of age on the clinical outcome45,46. Some octogenarians could not be matched due to their low KPS, as no matching partner was found in younger patients. Nevertheless, 94 per cent of the patients 80 years and older could be matched, which resulted in two balanced and large groups that differed only in age but not in their co-morbidity.
Another limitation is the data collection after discharge from the hospital. Clinical assessment on postoperative day 30 is mandatory for all patients. Further follow-up examinations, however, are not mandatory due to legal restrictions in Germany. This limits the validity of the 2-year Kaplan–Meier estimates, as data collection is at the discretion of the respective clinic. Approximately 50 per cent of patients were lost to follow-up after day 30, which necessitates careful interpretation of the survival estimates.
To date, several studies addressed postoperative death and the impact of anastomoses after resection of CRC in elderly patients5,7,13,42,47. Overall, the heterogeneity of patients in these studies is high and most studies included relatively small patient numbers. Similar to the present analysis, some studies found that the patient’s numerical age is not a risk factor for development of anastomotic leakage, but that mortality rate increases considerably when leakage occurs. Other studies did not find a significant effect of age on survival. Most studies were unable to adjust their results for preoperative co-morbidity because of small patient numbers or lack of data.
A recent larger study analysing the effect of age on anastomotic leakages in CRC surgery used data from the Dutch ColoRectal Audit48. The authors found a protective effect of age on the rate of anastomotic leakages and a strong association of age with mortality rate after leakage. However, this study has some shortcomings. In particular, all CRC locations and emergency operations were included. This, along with different frequencies of cancer locations and emergency surgery in elderly patients, may affect the results significantly. These considerations may also explain why older age was found to have a protective effect on the rate of anastomotic leakages in this study.
In addition to the effect of anastomotic leakage on short-term outcome, another study also showed a long-lasting effect with much higher mortality rate at 1 year. While 1-year survival in patients younger than 65 years with anastomotic leakage was almost 90 per cent, it dropped to 33 per cent in octogenarians13.
The decision for or against an anastomosis has a major impact on the quality of life after colorectal surgery10. Octogenarians receive anastomoses at a significantly lower rate than younger patients, but they do not have a higher rate of anastomotic leakages. It stands to reason that those patients with anastomosis are selected based on preoperative risk factors, which may lead to selection of the strongest. However, after adjusting for the preoperative risk factors between age groups, higher age remained an independent prohibitive factor for the creation of anastomoses.
There are two points of interest regarding protective stoma creation. First, the creation of a stoma reduced the proportion of clinically apparent leakages. Second, the proportion of patients 80 years and older with rectum cancer and protective stoma was lower than that of younger patients. This can be explained by the lower rate of anastomosis formation in the group of the elderly, since these patients receive a Hartmann procedure more often than an anastomosis with a protective ostomy.
By far the strongest predictor of death was anastomotic leakage. Octogenarians do not have a higher risk of anastomotic leakage, but their risk of death is significantly increased after leakage.
The most difficult question in this context is whether the creation of an anastomosis is justified with knowledge of leakage and mortality rates. The risk of anastomotic leakage for rectum anastomoses is about 10 per cent, which is accepted and generally does not prevent the creation of an anastomosis43. The risk of death for younger patients with anastomotic leakage is around 3.5 per cent11. However, a risk of death after anastomotic leakage of more than 15 per cent in octogenarians probably deserves more discussion. In addition, key influencing factors can be addressed, such as a low ASA score or a reduced performance score.
In addition to preoperative co-morbidity, the decision for or against an anastomosis is influenced by various parameters, such as the tumour extent, a possible preoperative faecal incontinence or the individual estimation of the quality of life with or without a permanent stoma. These considerations speak in favour of detailed shared decision making, which specifically addresses the creation of anastomoses after bowel resection for CRC in very elderly patients.
Minimizing perioperative risk factors is a measure to reduce morbidity, especially in elderly and co-morbid patients. Obviously, age cannot be influenced, but cardiovascular co-morbidity, frailty and other factors can often be taken into account during surgery for benign disease. However, this is usually not possible in malignancies because of time constraints. Anastomotic leakages have the highest impact on mortality rate in patients with resections for left-sided colorectal carcinoma. The decision for or against an intestinal anastomosis is one of the few perioperative parameters that can be actively addressed by patients and surgeons in an upcoming CRC surgery. The creation of an anastomosis should be discussed in detail with elderly patients and is an important component of shared decision making.
Supplementary Material
Acknowledgements
The study was not preregistered in an independent registry. The data source for this study was the StuDoQ quality assessment system for colon cancer and rectum cancer of the German Society for General and Visceral Surgery (DGAV). A request for data access according to the publication guidelines (https://www.dgav.de/studoq/datenschutzkonzept-und-publikationsrichtlinien.html) can be made to the DGAV.
Disclosure. The authors declare no conflict of interest.
Supplementary material
Supplementary material is available at BJS Open online.
Contributor Information
Kai S Lehmann, Department of General, Visceral and Vascular Surgery, Charité—Universitätsmedizin Berlin, Berlin, Germany.
Carsten Klinger, Deutsche Gesellschaft für Allgemein- und Viszeralchirurgie E. V., Berlin, Germany.
Johannes Diers, Comprehensive Cancer Centre Mainfranken, University of Würzburg, Würzburg, Germany; Department of General, Visceral, Transplant, Vascular and Paediatric Surgery, University of Würzburg, Würzburg, Germany.
Heinz-Johannes Buhr, Deutsche Gesellschaft für Allgemein- und Viszeralchirurgie E. V., Berlin, Germany.
Christoph-Thomas Germer, Comprehensive Cancer Centre Mainfranken, University of Würzburg, Würzburg, Germany; Department of General, Visceral, Transplant, Vascular and Paediatric Surgery, University of Würzburg, Würzburg, Germany.
Armin Wiegering, Comprehensive Cancer Centre Mainfranken, University of Würzburg, Würzburg, Germany; Department of General, Visceral, Transplant, Vascular and Paediatric Surgery, University of Würzburg, Würzburg, Germany.
References
- 1. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin 2015;65:87–108. [DOI] [PubMed] [Google Scholar]
- 2. Surveillance, Epidemiology, and End Results (SEER) Program (www.seer.cancer.gov). SEERStat Database: SEER 21, SEER Research Data, 2000-2020. Accessed through SEERExplorer Application. 2020. https://seer.cancer.gov/ (accessed 21 August 2020).
- 3. Douaiher J, Ravipati A, Grams B, Chowdhury S, Alatise O, Are C. Colorectal cancer – global burden, trends, and geographical variations. J Surg Oncol 2017;115:619–630. [DOI] [PubMed] [Google Scholar]
- 4.United Nations, Department of Economic and Social Affairs, Population Division (2019). World Population Prospects 2019, Custom Data Acquired via Website. 2020. https://population.un.org/wpp/DataQuery/ (accessed 21 August 2020).
- 5. Pirrera B, Vaccari S, Cuicchi D, Lecce F, De Raffele E, Via BD et al. Impact of octogenarians on surgical outcome in colorectal cancer. Int J Surg 2016;35:28–33. [DOI] [PubMed] [Google Scholar]
- 6. Weerink LBM, Gant CM, van Leeuwen BL, de Bock GH, Kouwenhoven EA, Faneyte IF. Long-term survival in octogenarians after surgical treatment for colorectal cancer: prevention of postoperative complications is key. Ann Surg Oncol 2018;25:3874–3882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Surgery for colorectal cancer in elderly patients: a systematic review. Colorectal Cancer Collaborative Group. Lancet 2000;356:968–974. DOI:https://pubmed.ncbi.nlm.nih.gov/11041397/. [PubMed] [Google Scholar]
- 8. Mazzoni G, Tocchi A, Miccini M, Bettelli E, Cassini D, De Santis M et al. Surgical treatment of liver metastases from colorectal cancer in elderly patients. Int J Colorectal Dis 2007;22:77–83. [DOI] [PubMed] [Google Scholar]
- 9. Papamichael D, Audisio R, Horiot J-C, Glimelius B, Sastre J, Mitry E et al. ; SIOG. Treatment of the elderly colorectal cancer patient: SIOG expert recommendations. Ann Oncol 2009;20:5–16. [DOI] [PubMed] [Google Scholar]
- 10. Vermeulen J, Gosselink MP, Busschbach JJV, Lange JF. Avoiding or reversing Hartmann’s procedure provides improved quality of life after perforated diverticulitis. J Gastrointest Surg 2010;14:651–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jannasch O, Klinge T, Otto R, Chiapponi C, Udelnow A, Lippert H et al. Risk factors, short and long term outcome of anastomotic leaks in rectal cancer. Oncotarget 2015;6:36884–36893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Papamichael D, Audisio RA, Glimelius B, de Gramont A, Glynne-Jones R, Haller D et al. Treatment of colorectal cancer in older patients: International Society of Geriatric Oncology (SIOG) consensus recommendations 2013. Ann Oncol 2015;26:463–476. [DOI] [PubMed] [Google Scholar]
- 13. Duraes LC, Stocchi L, Dietz D, Kalady MF, Kessler H, Schroeder D et al. The disproportionate effect of perioperative complications on mortality within 1 year after colorectal cancer resection in octogenarians. Ann Surg Oncol 2016;23:4293–4301. [DOI] [PubMed] [Google Scholar]
- 14. Hardt J, Buhr H-J, Klinger C, Benz S, Ludwig K, Kalff J et al. Quality indicators for colon cancer surgery: evidence-based development of a set of indicators for the outcome quality. Chirurg 2018;89:17–25. [DOI] [PubMed] [Google Scholar]
- 15. Wellner UF, Keck T. Quality indicators in pancreatic surgery: lessons learned from the German DGAV StuDoQ|Pancreas Registry. Visc Med 2017;33:126–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Seyfried F, Buhr H-J, Klinger C, Huettel TP, Herbig B, Weiner S et al. Quality indicators for metabolic and bariatric surgery in Germany: evidence-based development of an indicator panel for the quality of results, indications and structure. Chirurg 2018;89:4–16. [DOI] [PubMed] [Google Scholar]
- 17. Wiegering A, Buhr H-J, Klinger C, Fürst A, Schiedeck T, Schwandner O et al. Quality indicators for surgery of rectal cancer: evidence-based development of a set of indicators for quality. Chirurg 2018;89:26–31. [DOI] [PubMed] [Google Scholar]
- 18. Lehmann KS, Ritz JP, Wibmer A, Gellert K, Zornig C, Burghardt J et al. The German Registry for Natural Orifice Translumenal Endoscopic Surgery: report of the first 551 patients. Ann Surg 2010;252:263–270. [DOI] [PubMed] [Google Scholar]
- 19. Wellner UF, Klinger C, Lehmann K, Buhr H, Neugebauer E, Keck T. The pancreatic surgery registry (StuDoQ|Pancreas) of the German Society for General and Visceral Surgery (DGAV) – presentation and systematic quality evaluation. Trials 2017;18:163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mintziras I, Keck T, Werner J, Fichtner-Feigl S, Wittel U, Senninger N et al. Implementation of Current ENETS Guidelines for surgery of small (≤2 cm) pancreatic neuroendocrine neoplasms in the German Surgical Community: an analysis of the prospective DGAV StuDoQ|Pancreas Registry. World J Surg 2019;43:175–182. [DOI] [PubMed] [Google Scholar]
- 21. Piso P, Nedelcut SD, Rau B, Königsrainer A, Glockzin G, Ströhlein MA et al. Morbidity and mortality following cytoreductive surgery and hyperthermic intraperitoneal chemotherapy: data from the DGAV StuDoQ Registry with 2149 consecutive patients. Ann Surg Oncol 2019;26:148–154. [DOI] [PubMed] [Google Scholar]
- 22. Jurowich C, Lichthardt S, Matthes N, Kastner C, Haubitz I, Prock A et al. Effects of anastomotic technique on early postoperative outcome in open right-sided hemicolectomy. BJS Open 2019;3:203–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lichthardt S, Wagner J, Löb S, Matthes N, Kastner C, Anger F et al. Pathological complete response due to a prolonged time interval between preoperative chemoradiation and surgery in locally advanced rectal cancer: analysis from the German StuDoQ|Rectalcarcinoma registry. BMC Cancer 2020;20:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP; STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: guidelines for reporting observational studies. Int J Surg 2014;12:1495–1499.25046131 [Google Scholar]
- 25. Classification of Diseases (ICD). 2021. https://www.who.int/standards/classifications/classification-of-diseases (accessed 28 April 2021).
- 26. Karnofsky DA, Abelmann WH, Craver LF, Burchenal JH. The use of the nitrogen mustards in the palliative treatment of carcinoma. With particular reference to bronchogenic carcinoma. Cancer 1948;1:634–656. [Google Scholar]
- 27. Péus D, Newcomb N, Hofer S. Appraisal of the Karnofsky Performance Status and proposal of a simple algorithmic system for its evaluation. BMC Med Inform Decis Mak 2013;13:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rahbari NN, Weitz J, Hohenberger W, Heald RJ, Moran B, Ulrich A et al. Definition and grading of anastomotic leakage following anterior resection of the rectum: a proposal by the International Study Group of Rectal Cancer. Surgery 2010;147:339–351. [DOI] [PubMed] [Google Scholar]
- 29. Dindo D, Demartines N, Clavien P-A. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004;240:205–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Matthes N, Diers J, Schlegel N, Hankir M, Haubitz I, Germer C-T et al. Validation of MTL30 as a quality indicator for colorectal surgery. PLoS One 2020;15:e0238473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wiegering A, Wellner U, Seyfried F, Hardt J, Klinger C, Buhr H et al. MTL30 as surrogate parameter for quality of surgically treated diseases: establishment based on the StuDoQ register of the German Society for General and Visceral Surgery. Chirurg 2017;88:977–982. [DOI] [PubMed] [Google Scholar]
- 32. Jurowich C, Lichthardt S, Kastner C, Haubitz I, Prock A, Filser J et al. Laparoscopic versus open right hemicolectomy in colon carcinoma: a propensity score analysis of the DGAV StuDoQ|ColonCancer registry. PLoS One 2019;14:e0218829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. R Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing, 2020.
- 34. Therneau TM, (until 2009), Lumley T (until 2009), Atkinson E, Crowson C. survival: Survival Analysis. 2020. https://CRAN.R-project.org/package=survival.
- 35. Kristman V, Manno M, Côté P. Loss to follow-up in cohort studies: how much is too much? Eur J Epidemiol 2003;19:751–760. [DOI] [PubMed] [Google Scholar]
- 36. Lonjon G, Porcher R, Ergina P, Fouet M, Boutron I. Potential pitfalls of reporting and bias in observational studies with propensity score analysis assessing a surgical procedure: a methodological systematic review. Ann Surg 2017;265:901–909. [DOI] [PubMed] [Google Scholar]
- 37. Ho D, Imai K, King G, Stuart E. Matching as nonparametric preprocessing for reducing model dependence in parametric causal inference. Polit Anal 2007;15:199–236. [Google Scholar]
- 38. Lunt M. Selecting an appropriate caliper can be essential for achieving good balance with propensity score matching. Am J Epidemiol 2014;179:226–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Greifer N. cobalt: Covariate Balance Tables and Plots. 2020. https://CRAN.R-project.org/package=cobalt (accessed 31 July 2020).
- 40. Zhang Z, Kim HJ, Lonjon G, Zhu Y; written on behalf of AME Big-Data Clinical Trial Collaborative Group. Balance diagnostics after propensity score matching. Ann Transl Med 2019;7:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Anderson JH, Hole D, McArdle CS. Elective versus emergency surgery for patients with colorectal cancer. Br J Surg 1992;79:706–709. [DOI] [PubMed] [Google Scholar]
- 42. Mulcahy HE, Patchett SE, Daly L, O'Donoghue DP. Prognosis of elderly patients with large bowel cancer. Br J Surg 1994;81:736–738. [DOI] [PubMed] [Google Scholar]
- 43. Diers J, Wagner J, Baum P, Lichthardt S, Kastner C, Matthes N et al. Nationwide in-hospital mortality rate following rectal resection for rectal cancer according to annual hospital volume in Germany. BJS Open 2020;4:310–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Krarup P-M, Jorgensen LN, Andreasen AH, Harling H; on behalf of the Danish Colorectal Cancer Group. A nationwide study on anastomotic leakage after colonic cancer surgery. Colorectal Dis 2012;14:e661–e667. [DOI] [PubMed] [Google Scholar]
- 45. Lin J, Gamalo-Siebers M, Tiwari R. Propensity score matched augmented controls in randomized clinical trials: a case study. Pharm Stat 2018;17:629–647. [DOI] [PubMed] [Google Scholar]
- 46. Kuss O, Blettner M, Börgermann J. Propensity score: an alternative method of analyzing treatment effects. Dtsch Arztebl Int 2016;113:597–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Tan K-K, Koh FH-X, Tan Y-Y, Liu JZ, Sim R. Long-term outcome following surgery for colorectal cancers in octogenarians: a single institution’s experience of 204 patients. J Gastrointest Surg 2012;16:1029–1036. [DOI] [PubMed] [Google Scholar]
- 48. Zaimi I, Sparreboom CL, Lingsma HF, Doornebosch PG, Menon AG, Kleinrensink G-J et al. ; on behalf of the Dutch ColoRectal Audit Group. The effect of age on anastomotic leakage in colorectal cancer surgery: a population-based study. J Surg Oncol 2018;118:113–120. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.