Abstract
Aims
The aim of this study was to generate a biomarker-driven prognostic tool for patients with chronic HFrEF. Circulating levels of N-terminal pro B-type natriuretic peptide (NT-proBNP) and high-sensitivity cardiac troponin T (hs-cTnT) each have a marked positive relationship with adverse outcomes in heart failure with reduced ejection fraction (HFrEF). A risk model incorporating biomarkers and clinical variables has not been validated in contemporary heart failure (HF) trials.
Methods and results
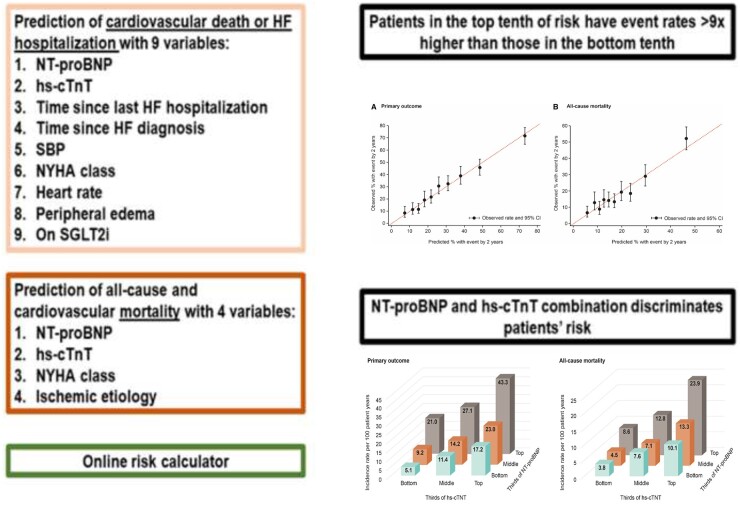
In EMPEROR-Reduced, 33 candidate variables were pre-selected. Multivariable Cox regression models were developed using stepwise selection for: (i) the primary composite outcome of HF hospitalization or cardiovascular death, (ii) all-cause death, and (iii) cardiovascular mortality. A total of 3730 patients were followed up for a median of 16 months, 823 (22%) patients had a primary outcome and 515 (14%) patients died, of whom 389 (10%) died from a cardiovascular cause. NT-proBNP and hs-cTnT were the dominant predictors of the primary outcome, and in addition, a shorter time since last HF hospitalization, longer time since HF diagnosis, lower systolic blood pressure, New York Heart Association (NYHA) Class III or IV, higher heart rate and peripheral oedema were key predictors (eight variables in total, all P < 0.001). The primary outcome risk score discriminated well (c-statistic = 0.73), with patients in the top 10th of risk having an event rate >9 times higher than those in the bottom 10th. Empagliflozin benefitted patients across risk levels for the primary outcome. NT-proBNP and hs-cTnT were also the dominant predictors of all-cause and cardiovascular mortality, followed by NYHA Class III or IV and ischaemic aetiology (four variables in total, all P < 0.001). The mortality risk model presented good event discrimination for all-cause and cardiovascular mortality (c-statistic = 0.69 for both). These simple models were externally validated in the BIOSTAT-CHF study, achieving similar c-statistics.
Conclusions
The combination of NT-proBNP and hs-cTnT with a small number of readily available clinical variables provides prognostic assessment for patients with HFrEF. This predictive tool kit can be easily implemented for routine clinical use.
Keywords: NT-proBNP, High-sensitivity cardiac troponin T, Heart failure, Risk score
Graphical Abstract
See page 4465 for the editorial comment for this article ‘Biomarker-driven prognostic model for risk prediction in heart failure: ready for Prime time?’, by D. Wussler and C. Mueller, https://doi.org/10.1093/eurheartj/ehab645.
Introduction
Patients with chronic heart failure with reduced ejection fraction (HFrEF) have a variable prognosis that may depend on patients’ characteristics, healthcare and social determinants, and treatments that modify the course of the disease.1
Prognostic models have been developed to capture the overall risk of each individual patient, thus allowing a better allocation of resources, therapies, and care organization strategies (e.g. nurse-led programmes or telemonitoring).2 A multitude of prognostic models has been developed in recent years. For example, the Seattle Heart Failure Model3 and the Meta-Analysis Global Group in Chronic Heart Failure (MAGGIC) score4 are tools that can be used to predict heart failure (HF) and survival outcomes in a clinical setting. However, these prognostic models were developed before the implementation of recent therapies that impact HFrEF outcomes [e.g. angiotensin receptor-neprilysin inhibitor and sodium-glucose cotransporter 2 inhibitors (SGLT2i)], and they do not incorporate biomarkers with strong prognostic value, e.g. N-terminal pro B-type natriuretic peptide (NT-proBNP) and high-sensitivity cardiac troponin T (hs-cTnT). More recent prognostic models have included natriuretic peptides but not hs-cTnT (e.g. the PREDICT-HF5 and BIOSTAT-CHF6), despite the importance of both biomarkers in predicting events,7 and they were developed before the advent of SGLT2i. Moreover, these models include a large number of clinical and laboratory variables as well as treatments, which necessarily limit their clinical implementation. For example, the PREDICT-HF score includes 30 variables for the prediction of all-cause mortality.5
Using data from the Empagliflozin Outcome Trial in Patients with Chronic Heart Failure with Reduced Ejection Fraction (EMPEROR-Reduced) trial, which assessed NT-proBNP and hs-cTnT in a contemporary setting, we developed simple and ready to implement biomarker-driven models for predicting the individual patient incidence of the composite of HF hospitalization or cardiovascular death, all-cause mortality, and cardiovascular mortality. These models can be incorporated into a biomarker-driven toolkit to facilitate appropriate individual patient management.
Methods
Study design
EMPEROR-Reduced: derivation cohort
The design and primary results of the EMPEROR-Reduced trial (NCT03057977) have been previously reported.8 , 9 In brief, 3730 patients with chronic HF and a left ventricular ejection fraction (LVEF) of 40% or less were randomized to receive placebo (n = 1867) or empagliflozin 10 mg daily (n = 1863). To prioritize the recruitment of higher-risk patients, either a hospitalization within 12 months of study enrolment or a meaningfully elevated NT-proBNP was required for study inclusion. The NT-proBNP inclusion criteria were ≥600, 1000, and 2500 pg/mL in patients with LVEF ≤30%, 31–35%, and 36–40%, respectively, and these thresholds were doubled in patients with atrial fibrillation. There was no eligibility criterion related to hs-cTnT.
The primary endpoint of EMPEROR-Reduced was time-to-first event in a composite of HF hospitalization or cardiovascular death. The mode of death and hospitalizations for HF were independently adjudicated by a blinded committee based on pre-specified criteria. The median follow-up time was 16 months.
Blood was collected for the measurement of NT-proBNP (expressed in pg/mL) and hs-cTnT (expressed in ng/L) (Roche Diagnostics, Risch-Rotkreuz, Switzerland) at baseline and measured in a central laboratory (using a Roche Elecsys® cobas analyzer). The institutional review board of each study site approved all study procedures and all patients provided informed consent.
BIOSTAT-CHF: validation cohort
From 2010 to 2012, BIOSTAT-CHF enrolled 2516 patients with worsening HF on lower than guideline-recommended doses of HF medication to investigate the factors influencing the up-titration of HF therapies. The design and first results of the study have been published.10 Briefly, patients were aged ≥18 years with signs and symptoms of worsening HF managed either in an outpatient setting or in an inpatient setting. The diagnosis of HF was confirmed either by an LVEF ≤40%, or B-type natriuretic peptide or NT-proBNP plasma levels >400 and/or >2000 pg/mL, respectively (without an entry cut-off point for hs-cTnT) and by treatment with oral or intravenous furosemide ≥40 mg/day or equivalent. Patients were treatment naive or were receiving <50% of target doses of angiotensin-converting enzyme inhibitors or angiotensin receptor blockers and beta-blockers. During a median follow-up of 21 months, hospitalizations for HF were identified by each treating physician, and cardiovascular mortality was centrally adjudicated by Dr Adriaan A. Voors.
Baseline blood measurements of NT-proBNP (expressed in pg/mL) and hs-cTnT (expressed in ng/L) (Roche Diagnostics) were measured in a central laboratory using a Roche Elecsys® cobas analyzer. Ethics board approval was obtained, and all participants signed written informed consent before entering the study.
Statistical analysis
For the primary composite outcome of HF hospitalization or cardiovascular death, and for all-cause mortality, multivariable Cox proportional hazard models were used to study the relation of patient variables at baseline to outcome incidence. First, we selected 33 candidate variables based on their potential clinical and prognostic importance; second, we used stepwise forward variable selection with P-value <0.001 as a criterion for inclusion (to keep the model parsimonious) with log-transformed NT-proBNP and hs-cTnT to achieve a good linear fit (the distribution of NT-proBNP and hs-cTnT is displayed in Supplementary material online, Figure S1); third, we evaluated model discrimination (using Harrell’s c-statistics) and calibration (by plotting the observed vs. predicted 2-year risk by deciles of risk). We assessed the form of associations between continuous variables and the risk of events using penalized splines with 4 degrees of freedom. For external validation, we applied the beta-coefficients and the baseline hazard derived from EMPEROR-Reduced to the BIOSTAT-CHF cohort. Missing data were rare, with all candidate covariates available in >90% of patients. We used single value imputation to impute missing data using the median for continuous variables or the mode for categorical variables. All analyses were performed using SAS, version 9.4 (SAS Institute, Cary, NC, USA).
Data sharing
Data will be made available on request in adherence with transparency conventions in medical research and through requests to the corresponding author. Following execution of pre-specified analyses a full EMPEROR-Reduced database will be made available in adherence with the transparency policy of the sponsor (available at https://trials.boehringer-ingelheim.com/transparency_policy.html).
Requests regarding the BIOSTAT-CHF data should be directed to Dr Adriaan A. Voors (a.a.voors@umcg.nl).
Results
EMPEROR-Reduced derivation models
Primary composite outcome of heart failure hospitalization or cardiovascular death
The primary composite outcome of HF hospitalization or cardiovascular death occurred in 823 (22%) of 3730 patients: 361 in the empagliflozin group and 462 in the placebo group [hazard ratio (HR) 0.75, 95% confidence interval (CI) 0.65–0.86; P < 0.001].
The 33 candidate predictor variables are listed in Supplementary material online, Table S1. After stepwise variable selection, the consequent prognostic model for the primary outcome included log-transformed NT-proBNP and hs-cTnT as the most powerful predictors (based on the χ 2 statistic for inclusion). These two biomarkers were followed by (i) shorter time since most recent HF hospitalization, (ii) longer time since HF diagnosis, (iii) lower systolic blood pressure (SBP), (iv) New York Heart Association (NYHA) functional Class III or IV, (v) peripheral oedema, and (vi) higher heart rate. Of note, the planned treatment (empagliflozin vs. placebo) remained highly predictive after adjustment for the 8 baseline predictors (Table 1).
Table 1.
The EMPEROR-Reduced risk model for the primary outcome (heart failure hospitalization or cardiovascular death)
| Hazard ratio (95% CI) | χ 2 statistic | Coefficient (SE)a | P-value | |
|---|---|---|---|---|
| Log NT-proBNPb (pg/mL) | 1.63 (1.50–1.77) | 131.0 | 0.49 (0.04) | <0.0001 |
| Log hs-cTnTb (ng/L) | 1.51 (1.37–1.67) | 65.1 | 0.41 (0.05) | <0.0001 |
| Time since most recent HHF (months) | ||||
| >6 | 1.00 (reference) | |||
| 3–6 | 1.55 (1.23–1.95) | 14.0 | 0.44 (0.12) | 0.0002 |
| <3 | 1.84 (1.55–2.19) | 47.7 | 0.61 (0.09) | <0.0001 |
| Time since HF diagnosis | ||||
| <1 year | 1.00 (reference) | |||
| 1–5 years | 1.46 (1.16–1.84) | 10.6 | 0.38 (0.12) | 0.0011 |
| ≥5 years | 1.79 (1.44–2.24) | 26.5 | 0.58 (0.11) | <0.0001 |
| SBP (per 10 mmHg lower) | 1.13 (1.08–1.18) | 26.9 | 0.12 (0.02) | <0.0001 |
| NYHA Class III/IV | 1.40 (1.21–1.63) | 20.2 | 0.33 (0.08) | <0.0001 |
| Heart rate (per 10 b.p.m. higher) | 1.11 (1.05–1.18) | 13.8 | 0.10 (0.03) | 0.0002 |
| Peripheral oedema | 1.32 (1.13–1.53) | 12.8 | 0.28 (0.08) | 0.0004 |
| Randomized to empagliflozin | 0.75 (0.65–0.86) | 17.3 | −0.29 (0.07) | <0.0001 |
An estimate of each individual’s 2-year risk can be calculated as follows: 1 − [0.9977^exp (0.49 × log NT-proBNP + 0.41 × log hs-cTnT + 0.44 × recent HHF1 + 0.61 × recent HHF2 + 0.38 × time since dignosis1 + 0.58 × time since dignosis2 − (SBP/10) × 0.12 + NYHA × 0.33 + peripheral oedema × 0.28 + (heart rate/10) × 0.10 + empagliflozin × −0.29)], where ‘recent HHF1’ and ‘recent HHF2’ are indicator variables for whether the most recent HHF was within 3–6 or <3 months, respectively; ‘time since dignosis1’ and ‘time since dignosis2’ are indicator variables for whether the time since most recent HF diagnosis between 1 and 5 years or ≥5 years, respectively. NYHA is an indicator variable for whether the patient’s NYHA class is III or IV. Peripheral oedema and empagliflozin are indicator variables for whether the patient has peripheral oedema or is to be treated with empagliflozin, respectively.
CI, confidence interval; HF, heart failure; HHF, hospitalization for heart failure; hs-cTnT, high-sensitivity cardiac troponin T; NT-proBNP, N-terminal pro B-type natriuretic peptide; NYHA, New York Heart Association; SBP, systolic blood pressure; SE, standard error.
Coefficient (SE) is the log hazard ratio and its standard error.
Per 1 unit higher on the log scale, equal to 2.7-fold levels of either NT-proBNP or troponin levels.
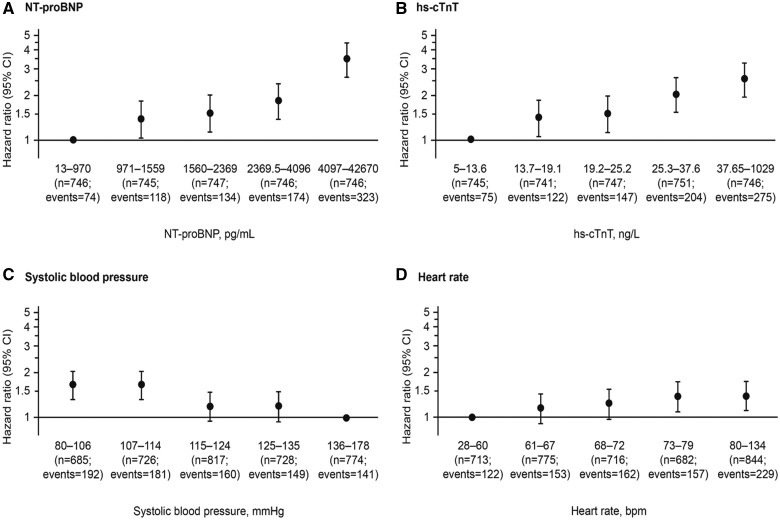
The strength and pattern of prognostic contributions for the four quantitative predictors are shown in Figure 1 and Supplementary material online, Figure S2. Each has been grouped into five ordered categories of approximately equal size. For NT-proBNP, we observed a strong monotonic risk gradient, with those in the top fifth (≥4097 pg/mL) having a HR of 3.52 (95% CI 2.69–4.59) as compared to those in the bottom fifth (≤970 pg/mL). For hs-cTnT, the risk gradient was slightly less marked, with the corresponding top fifth vs. bottom fifth (≥37.7 vs. <13.7 ng/L) having a HR of 2.54 (95% CI 1.94–3.32). The inverse association of SBP with risk is far less marked: when comparing the bottom fifth vs. top fifth (<107 vs. ≥136 mmHg), the HR was 1.66 (95% CI 1.33–2.06), but its contribution remained significant (P < 0.0001). The risk gradient was also less marked for heart rate, when comparing the bottom fifth vs. top fifth (<61 vs. ≥80 b.p.m.) with a HR of 1.42 (95% CI 1.13–1.77).
Figure 1.
(A) N-terminal pro B-type natriuretic peptide, (B) high-sensitivity cardiac troponin T, (C) systolic blood pressure, and (D) heart rate: their independent contributions according to fifths of their distributions to risk of the primary outcome (a composite of time-to-first heart failure hospitalization or cardiovascular death). CI, confidence interval; hs-cTnT, high-sensitivity cardiac troponin T; NT-proBNP, N-terminal pro B-type natriuretic peptide.
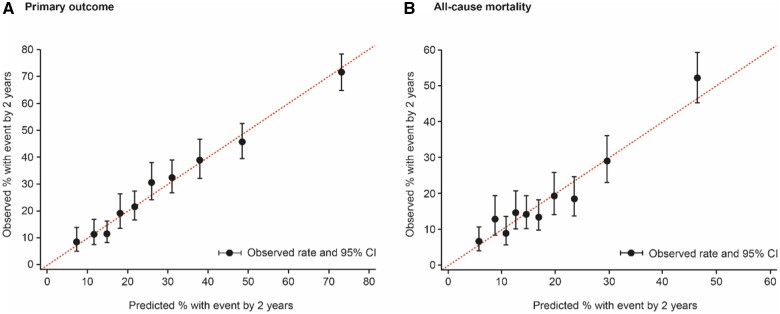
The goodness of fit and strength of prediction for this model—based on eight baseline predictors plus randomized treatment—are displayed in Figure 2A. A risk score (based on the coefficients in Table 1) has its distribution divided into 10 equal-sized groups. In each decile, there is good agreement between the observed and predicted patient risk, both expressed as the percentage having a primary event at 2 years. Comparing top and bottom deciles of risk, the observed event rates are 71.8% and 8.5%, respectively.
Figure 2.
Observed vs. predicted events by tenths of the risk score distribution. Hosmer–Lemeshow goodness-of-fit test P = 0.473 for the primary outcome (A) and P = 0.053 for all-cause mortality (B), indicating adequate calibration. CI, confidence interval.
The strength of prediction for the primary outcome is captured by the c-statistic of 0.729 (95% CI 0.711–0.746). We considered the option of extending the model to include other predictors that each achieved P-value <0.01 rather than the more stringent P-value <0.001; this would have added neutrophils, bilirubin, and potassium. The addition of these variables would have greatly increased the complexity of the model, without meaningful gain in terms of strength of prediction (c-statistic = 0.733). Of note, a more complex model (the PREDICT-HF risk score) included >20 baseline variables,5 but when applied to the EMPEROR-Reduced database, the PREDICT-HF score strength for prediction of the primary outcome was lower (c-statistic = 0.704).
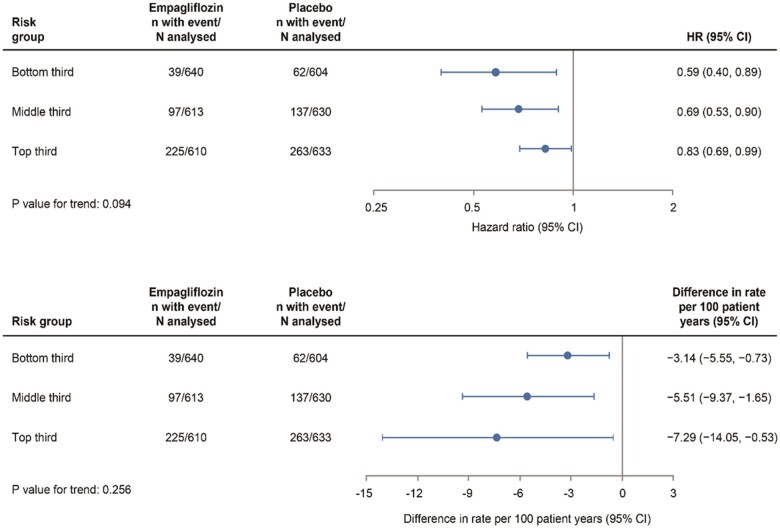
To evaluate the effect of empagliflozin at different levels of patient risk, we calculated a risk score using coefficients for the eight significant predictors in Table 1 (excluding the coefficient for randomized treatment), and we stratified patients into equal-sized thirds of risk, each containing over 1200 patients per risk tertile. Figure 3A shows the HR and 95% CIs for the primary outcome by tertiles of risk. All three showed a statistically significant relative reduction in risk of the primary outcome with empagliflozin, with a more marked relative reduction in low-risk patients (HR 0.59, 95% CI 0.40–0.89) and less marked relative reduction in high-risk patients (HR 0.83, 95% CI 0.69–0.99) (P for trend = 0.09). Figure 3B shows the absolute differences in risk for the same tertiles, expressed as the treatment difference in primary event rate per 100 patient-years. On an absolute scale, the reverse trend for the magnitude of the effect of empagliflozin was apparent (compared with the trend for relative risk reduction): rate difference −3.1 vs. −7.3 per 100 patient-years in low- and high-risk patients, respectively (P for trend = 0.26). Kaplan–Meier plots of the primary outcome over 24 months by risk tertiles and by treatment group demonstrated that empagliflozin reduced the cumulative incidence of a primary event at all levels of patient risk (Supplementary material online, Figure S3).
Figure 3.
Hazard ratios and rate differences for the primary outcome (empagliflozin vs. placebo) according to thirds of the risk score distribution. CI, confidence interval; HR, hazard ratio.
All-cause and cardiovascular mortality
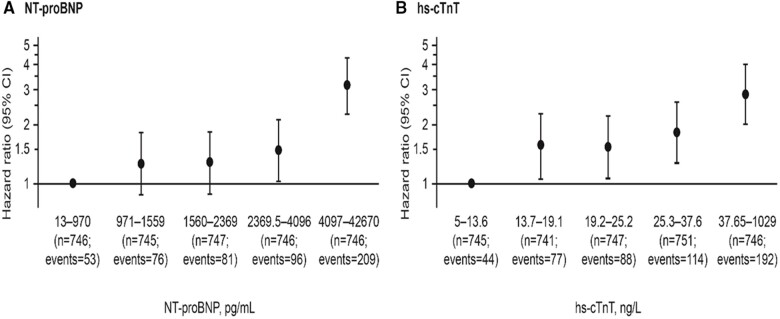
Death from any cause occurred in 515 (14%) of the 3730 patients: 249 in the empagliflozin group and 266 in the placebo group, with a HR of 0.92 (95% CI 0.77–1.10). A new risk model for mortality using the same set of candidate predictors yielded only four variables that achieved the inclusion criterion of P-value <0.001 (Table 2). NT-proBNP and hs-cTnT remained the most dominant risk factors for mortality, followed by NYHA functional Class III or IV and ischaemic (vs. non-ischaemic) HF. The consequent c-statistic = 0.687 (95% CI 0.661–0.713) and Figure 2B showed the model goodness-of-fit and strength of prediction. Figure 4 depicts how NT-proBNP and hs-cTnT are independently associated with mortality.
Table 2.
The EMPEROR-Reduced risk model for all-cause mortality
| Hazard ratio (95% CI) | χ 2 statistic | Coefficient (SE)a | P-value | |
|---|---|---|---|---|
| Log NT-proBNP (pg/mL)b | 1.61 (1.45–1.79) | 79.0 | 0.48 (0.05) | <0.0001 |
| Log hs-cTnT (ng/L)b | 1.58 (1.40–1.79) | 51.0 | 0.46 (0.06) | <0.0001 |
| NYHA Class III/IV (vs. Class II) | 1.40 (1.17–1.68) | 12.9 | 0.34 (0.09) | 0.0003 |
| Ischaemic (vs. non-ischaemic) heart failure | 1.38 (1.16–1.65) | 13.0 | 0.32 (0.09) | 0.0003 |
An estimate of each individual’s 2-year risk can be calculated as follows: 1 − [0.9991^exp(0.48 × log NT-proBNP + 0.46 × log hs-cTnT + 0.34 × NYHA + 0.32 × ischaemic)], where ‘NYHA’ is an indicator variable for whether the patient’s NYHA class is III or IV and ‘ischaemic’ is an indicator variable for whether their heart failure was of ischaemic aetiology.
CI, confidence interval; hs-cTnT, high-sensitivity cardiac troponin T; NT-proBNP, N-terminal pro B-type natriuretic peptide; NYHA, New York Heart Association; SE, standard error.
Coefficient (SE) is the log hazard ratio and its standard error.
Per 1 unit higher on the log scale, equal to 2.7-fold levels of either NT-proBNP or hs-cTnT levels.
Figure 4.
Mortality risks for N-terminal pro B-type natriuretic peptide (A) and high-sensitivity cardiac troponin T (B): their independent contributions according to fifths of their distribution. CI, confidence interval; hs-cTnT, high-sensitivity cardiac troponin T; NT-proBNP, N-terminal pro B-type natriuretic peptide.
A cardiovascular cause accounted for 76% of all deaths (n = 389): 187 in empagliflozin and 202 in placebo, with a HR of 0.92 (95% CI 0.75–1.12). Applying the all-cause mortality risk model to cardiovascular mortality yielded a statistically identical discrimination (c-statistic = 0.688, 95% CI 0.658–0.716).
We also explored a new risk model for cardiovascular death, but all gave results that were similar to the all-cause mortality model, with NT-proBNP and hs-cTnT being the most powerful predictors, followed by NYHA class and ischaemic aetiology. The c-statistic was 0.69 for both all-cause and cardiovascular mortality (Supplementary material online, Table S2).
Biomarker combination for risk prediction
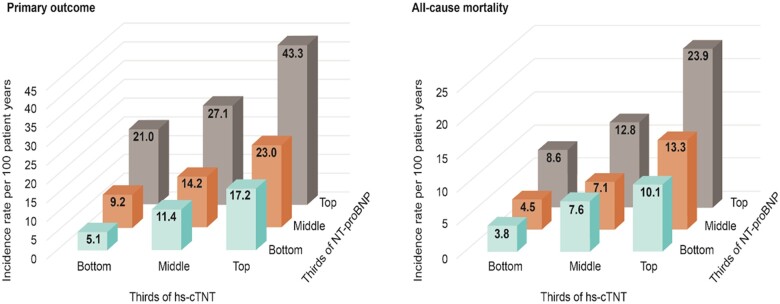
Given the dominant roles of NT-proBNP and hs-cTnT in influencing patient risks of the primary outcome and all-cause death, we explored how risk was affected by these biomarkers. In Supplementary material online, Table S3 and Figure 5, we simultaneously stratified both NT-proBNP and hs-cTnT into equal-sized thirds of their individual distribution and then showed how the event rates of the primary outcome and all-cause death varied according to these 9 patient groups. Patients with both the lowest NT-proBNP and lowest hs-cTnT had an event rate of 5.1 per 100 patient-years compared to 43.3 per 100 patient-years in those with both the highest NT-proBNP and the highest hs-cTnT, with a rate ratio of 8.5. For all-cause death, a similar pattern emerged, with rate ratio for the two extremes (highest-highest vs. lowest-lowest) of 6.2. The two biomarkers were positively correlated: patients with lowest-lowest and highest-highest biomarker values occurred in 619 and 619 patients, respectively, whereas highest-lowest and lowest-highest occurred in only 249 and 219 patients, respectively. The Pearson correlation for the values of log NT-proBNP and log hs-cTnT was r = 0.36.
Figure 5.
Incidence rates per 100 patient-years of (A) the primary outcome and (B) all-cause death for patients simultaneously grouped in thirds of N-terminal pro B-type natriuretic peptide and high-sensitivity cardiac troponin T. hs-cTnT, high-sensitivity cardiac troponin T; NT-proBNP, N-terminal pro B-type natriuretic peptide
BIOSTAT-CHF external validation
The models derived from EMPEROR-Reduced were applied to 1828 patients from BIOSTAT-CHF with an LVEF ≤40% in whom both NT-proBNP and hs-cTnT were available. In BIOSTAT-CHF, 599 (33%) patients experienced the composite of HF hospitalization or cardiovascular death and 432 (24%) died, of whom 329 (76%) died from a cardiovascular cause.
Application of the EMPEROR-Reduced risk score for all-cause mortality to the same outcome in BIOSTAT-CHF achieved a c-statistic of 0.712. The EMPEROR-Reduced risk score for all-cause mortality applied to cardiovascular mortality in BIOSTAT-CHF achieved a slightly better discrimination, with a c-statistic of 0.730.
The EMPEROR-Reduced model could not be used for validating the composite of HF hospitalization or cardiovascular death in BIOSTAT-CHF because time since HF diagnosis was not available in BIOSTAT-CHF, and the time since most recent HF hospitalization was recorded only as an event within the previous year (yes vs. no). To accommodate these limitations, we re-computed a modified EMPEROR-Reduced risk score to remove the time since HF diagnosis from the model and include prior HF hospitalization as a dichotomous variable based on whether the patient had or not been hospitalized for HF within the previous year. The c-statistics of this modified model were 0.710 in EMPEROR-Reduced and 0.691 in BIOSTAT-CHF.
When we assessed the observed vs. predicted risk of the composite of HF hospitalization or cardiovascular death and of all-cause death in the patients in BIOSTAT-CHF by fifths of their risk score distributions, there was a marked gradient in risk, but it was less steep than predicted by the EMPEROR-Reduced models (Supplementary material online, Figure S4).
The comparison of the baseline characteristics of the patients enrolled in EMPEROR-Reduced and BIOSTAT-CHF is displayed in Supplementary material online, Table S4. Most characteristics overlapped, except that BIOSTAT-CHF patients were more symptomatic (proportion in NYHA Class III/IV: 60.5 vs. 24.9), had higher heart rate (mean b.p.m.: 82 vs. 71), higher NT-proBNP levels (median 2549 vs. 1910 pg/mL), and higher hs-cTnT levels (median 29.6 vs. 22.0 pg/mL).
The summary of the findings is displayed in the Graphical Abstract, and the online risk calculator is provided in the Supplementary material online.
EMPEROR-Reduced risk model. CI, confidence interval; HF, heart failure; hs-cTnT, high-sensitivity cardiac troponin T; NT-proBNP, N-terminal pro B-type natriuretic peptide; NYHA, New York Heart Association; SBP, systolic blood pressure; SGLT2i, sodium-glucose cotransporter 2 inhibitor.
Discussion
The biomarker-driven models that we developed and validated showed good performance for event prediction in patients with HFrEF. Importantly, these models require only a handful of variables and can be easily implemented in clinical practice.
Prediction models that incorporate NT-proBNP have been developed for patients with HFrEF.5 However, models that incorporate both NT-proBNP and hs-cTnT have not been developed to date among contemporary HFrEF trials. The use of both biomarkers for event prediction was reported in patients with atrial fibrillation enrolled in the Apixaban for the Prevention of Stroke in Subjects With Atrial Fibrillation (ARISTOTLE) trial, where both NT-proBNP and hs-cTnT were the variables with the strongest association with cardiovascular death.11 Moreover, in a previous report from BIOSTAT-CHF, both hs-cTnT and NT-proBNP identified patients at the risk of cardiovascular death, whereas NT-proBNP (but not hs-cTnT) identified patients at the risk of death from non-cardiovascular causes.12
NT-proBNP is the biomarker with the strongest prognostic value in HF. Elevated levels of NT-proBNP reflect higher filling pressures and adverse cardiac remodelling and are strongly associated with severe symptoms and poor health status.13–16 Troponin is cardiac-specific and likely reflects the occurrence of ongoing loss of cardiomyocytes even in the absence of clinically apparent myocardial ischaemia and regardless of HF aetiology.17 Similar to NT-proBNP, recent data have linked hs-cTnT concentrations to cardiac remodelling, where the two biomarkers have a unique correlation, with NT-proBNP change preceding that of hs-cTnT.18 This leads to the hypothesis that increased myocardial stress (reflected in NT-proBNP concentrations) results in subsequent myocardial injury (reflected in hs-cTnT concentrations) ultimately culminating in adverse cardiac remodelling and worse prognosis.13 , 19 It is noteworthy that the lowest quintile for each biomarker identifies thresholds previously identified for risk stratification and cardiac remodelling for both NT-proBNP and hs-cTnT.18 Given the strong and independent prognostic value of NT-proBNP and hs-cTnT when evaluated individually, a great potential exists in combining both for refining risk prediction in HF.
In EMPEROR-Reduced, the utility of the biomarker combination of NT-proBNP and hs-cTnT was demonstrated by comparing the event rates of patients in the top third with those in the bottom third of the distributions of both biomarkers. Those with the highest values of both biomarkers had an 8.6-fold higher risk of a primary outcome event and a 6.2-fold higher risk of death than those with the lowest values of both biomarkers. In addition to elevated NT-proBNP and hs-cTnT, a recent HF hospitalization, longer HF duration, lower SBP, NYHA Class III or IV, and peripheral oedema were strongly associated with a subsequent occurrence of a primary outcome event. These clinical history and physical examination variables have each been previously shown to predict adverse outcomes in HF. Patients with a recent HF hospitalization are at higher risk of being re-hospitalized, a risk that inversely related to the time interval since the previous hospitalization, i.e. the shorter the time interval the higher the HF re-hospitalization risk.20 Patients with a long HF duration tend to be older, present with greater severity of signs and symptoms and comorbid conditions, independently of age.21 A lower blood pressure is associated with worse prognosis in patients with HFrEF, likely because it reflects poorer cardiac function.22 , 23
Signs of congestion (e.g. peripheral oedema) reflect HF decompensation and are strongly associated with HF events.24
Finally, treatment with empagliflozin (rather than placebo) is a strong primary outcome predictor and hence is included in our risk score. For future clinical use, this should be presented as ‘treatment with an SGLT2i’ since HFrEF trials showed similar benefits for both empagliflozin and dapagliflozin.25 We have deliberately not included other non-randomized treatments in our risk models since one cannot reliably distinguish between the actual treatment effect and the selection process that led to the introduction of each treatment to some patients and not to others. Thus, the application of our risk model is best suited to patients receiving appropriate background treatments for HFrEF, which include inhibitors of the renin-angiotensin system and neprilysin, beta-blockers, mineralocorticoid receptor antagonists, and, when indicated, cardiac devices.
Compared with the composite of HF hospitalization or cardiovascular death, an accurate prediction of all-cause mortality was achieved with only four variables, i.e. NT-proBNP, hs-cTnT, NYHA functional Class III or IV, and ischaemic aetiology. Patients with ischaemic heart disease have a higher risk of suffering further myocardial injurious events that accelerate the progression of cardiomyopathy.26 A prognostic model specific for cardiovascular mortality gave very similar findings to that for all-cause mortality, with NT-proBNP and hs-cTnT being (by far) the strongest predictors of cardiovascular mortality, with the former making a slightly greater contribution.
The baseline risk of a patient influences their response to empagliflozin, but this should be assessed both in relative and absolute terms. Patients in the bottom third of our novel risk score appear to experience a greater magnitude of benefit with empagliflozin on a relative scale with a 41% primary outcome event rate reduction compared with a 17% event rate reduction in the top third. However, an inverse pattern is apparent on an absolute scale where patients in the top third of the risk score experienced ∼2.5 times greater absolute event rate decrease with empagliflozin, when compared with patients at the bottom third of risk.
Importantly, these biomarker-driven models are simple to use because they rely only on a few readily available variables. Despite their simplicity, these models perform at least as well, if not better, than far more complex models with dozens of variables, many of which may not be easily ascertained from patient records.5 In contrast, NT-proBNP and hs-cTnT are available in most clinical settings, although the former is measured more frequently than the latter in HF patients. We compared our risk models with others available in the literature. For the composite of HF hospitalization or cardiovascular death, we applied the PREDICT-HF risk score (from the PARADIGM-HF trial), which gave a c-statistic of 0.704. For mortality, we applied the MAGGIC and the Seattle scores, which gave c-statistics of 0.641 and 0.629, respectively. Notably, when applied to EMPEROR-Reduced, each of these risk scores performed worse than the EMPEROR-Reduced scores and was vastly more complex to compute than the EMPEROR-Reduced risk scores. Our powerful and parsimonious models strongly support the routine assessment of hs-cTnT in the comprehensive assessment of patients with HFrEF.
One general problem is that prognostic models of any complexity are still used only in a tiny minority of patients with HF. For example, several prognostic models for mortality were evaluated in the European Society of Cardiology HF Long-Term Registry, and the authors concluded that the performance of prognostic scores is still limited and that physicians are reluctant to use them in daily practice.27 Thus, the need for contemporary, more precise, and simpler prognostic tools, as those here developed and validated.
Limitations
Some limitations should be highlighted. The primary outcome model could not be fully validated because information about HF duration was missing, and information regarding a prior HF hospitalization had not been fully recorded in BIOSTAT-CHF. Moreover, in BIOSTAT-CHF, the gradient in risk was less steep than predicted by the EMPEROR-Reduced models, probably because the distribution of log NT-proBNP and log hs-cTnT had a wider standard deviation in BIOSTAT-CHF than in EMPEROR-Reduced (1.26 vs. 0.90 for log NT-proBNP and 0.97 vs. 0.67 for log hs-cTnT). Consequently, Cox models would be expected to yield smaller HRs per log unit biomarker increase when applied to BIOSTAT-CHF than when applied to the EMPEROR-Reduced trial. Our risk models are deliberately parsimonious to facilitate their practical use. However, other variables not captured in EMPEROR-Reduced, such as frailty, social deprivation, or dementia, could have important prognostic impact. EMPEROR-Reduced had more exclusion criteria than BIOSTAT-CHF, but this did not cause a major impact on the external validity of our models. Still, prognostic models derived from a clinical trial database potentially have limited generalizability. Thus, we encourage further validation of our risk models in other populations of HFrEF patients.
Conclusion
The combination of NT-proBNP and hs-cTnT with a few readily available clinical variables provides a parsimonious prognostic assessment for individual patients with HFrEF. A predictive tool kit based on this novel model can be readily implemented for routine clinical use.
Supplementary material
Supplementary material is available at European Heart Journal online.
Funding
The EMPEROR-Reduced trial was funded by Boehringer Ingelheim and Eli Lilly (EMPEROR-Reduced ClinicalTrials.gov number, NCT03057977).
Conflict of interest: S.J.P. reports personal fees from Boehringer Ingelheim during the conduct of the study. J.P.F. reports consulting fees from Boehringer Ingelheim during the conduct of the study. J.G. reports personal fees from Boehringer Ingelheim during the conduct of the study. F.Z. reports personal fees from Boehringer Ingelheim during the conduct of the study; personal fees from Janssen, Novartis, Boston Scientific, Amgen, CVRx, AstraZeneca, Vifor Fresenius, Cardior, Cereno Pharmaceutical, Applied Therapeutics, Merck, Bayer, and Cellprothera outside the submitted work; and other support from CVCT and Cardiorenal, outside the submitted work. S.D.A. reports grants from Vifor; personal fees from Vifor, Bayer, Boehringer Ingelheim, Novartis, Servier, Impulse Dynamics, Cardiac Dimensions, and Thermo Fisher Scientific and grants and personal fees from Abbott Vascular, outside the submitted work. J.B. reports consultancy fees from Boehringer Ingelheim during the conduct of the study and consultancy fees from Abbott, Adrenomed, Amgen, Applied Therapeutics, Array, AstraZeneca, Bayer, BerlinCures, Boehringer Ingelheim, Cardior, CVRx, Foundry, G3 Pharma, Imbria, Impulse Dynamics, Innolife, Janssen, LivaNova, Luitpold, Medtronic, Merck, Novartis, NovoNordisk, Relypsa, Roche, Sanofi, Sequana Medical, V-Wave, and Vifor, outside the submitted work. G.F. reports receiving payment from Boehringer Ingelheim for being a trial committee member during the conduct of the study and from Medtronic, Vifor, Servier, and Novartis for being a trial committee member outside the submitted work. M.P. reports personal fees from Boehringer Ingelheim, during the conduct of the study; personal fees from AbbVie, Akcea, Amarin, AstraZeneca, Amgen, Boehringer Ingelheim, Cardiorentis, Daiichi Sankyo, Johnson & Johnson, Lilly, Novartis, Pfizer, Relypsa, Sanofi, Synthetic Biologics, Theravance, and NovoNordisk, outside the submitted work. M.B., N.D.G., and T.I. are employees of Boehringer Ingelheim International. J.L.J. is supported by the Hutter Family Professorship; is a Trustee of the American College of Cardiology; is a board member of Imbria Pharmaceuticals; has received grant support from Novartis Pharmaceuticals, Roche Diagnostics, Abbott, Singulex, and Prevencio; has received consulting income from Abbott, Janssen, Novartis, Pfizer, Merck, and Roche Diagnostics; and participates in clinical endpoint committees/data safety monitoring boards for Abbott, AbbVie, Amgen, Boehringer Ingelheim, Janssen, and Takeda.
Supplementary Material
Contributor Information
Stuart J Pocock, Department of Medical Statistics, London School of Hygiene and Tropical Medicine, London, UK.
João Pedro Ferreira, Université de Lorraine, Inserm, Centre d'Investigations Cliniques Plurithématique 1433, and Inserm U1116, CHRU, F-CRIN INI-CRCT (Cardiovascular and Renal Clinical Trialists), Nancy, France; Cardiovascular Research and Development Center, Department of Surgery and Physiology, Faculty of Medicine of the University of Porto, Porto, Portugal.
John Gregson, Department of Medical Statistics, London School of Hygiene and Tropical Medicine, London, UK.
Stefan D Anker, Department of Cardiology (CVK), Berlin Institute of Health Center for Regenerative Therapies (BCRT), German Centre for Cardiovascular Research (DZHK) partner site Berlin, Charité Universitätsmedizin Berlin, Berlin, Germany.
Javed Butler, Department of Medicine, University of Mississippi, Jackson.
Gerasimos Filippatos, Department of Cardiology, Attikon University Hospital, National and Kapodistrian University of Athens, School of Medicine, Athens, Greece.
Nicholas D Gollop, Boehringer Ingelheim International GmbH, Ingelheim, Germany.
Tomoko Iwata, Boehringer Ingelheim Pharma GmbH & Co. KG, Biberach, Germany.
Martina Brueckmann, Boehringer Ingelheim International GmbH, Ingelheim, Germany; Faculty of Medicine Mannheim, University of Heidelberg, Mannheim, Germany.
James L Januzzi, Jr, Massachusetts General Hospital, Boston, MA, USA; Harvard Medical School, Boston, MA, USA; Baim Institute for Clinical Research, Boston, MA, USA.
Adriaan A Voors, University of Groningen, Groningen, The Netherlands.
Faiez Zannad, Université de Lorraine, Inserm, Centre d'Investigations Cliniques Plurithématique 1433, and Inserm U1116, CHRU, F-CRIN INI-CRCT (Cardiovascular and Renal Clinical Trialists), Nancy, France.
Milton Packer, Baylor Heart and Vascular Institute, Baylor University Medical Center, Dallas, TX, USA; Imperial College London, London, UK.
References
- 1. Dokainish H, Teo K, Zhu J, Roy A, AlHabib KF, ElSayed A, Palileo-Villaneuva L, Lopez-Jaramillo P, Karaye K, Yusoff K, Orlandini A, Sliwa K, Mondo C, Lanas F, Prabhakaran D, Badr A, Elmaghawry M, Damasceno A, Tibazarwa K, Belley-Cote E, Balasubramanian K, Islam S, Yacoub MH, Huffman MD, Harkness K, Grinvalds A, McKelvie R, Bangdiwala SI, Yusuf S; INTER-CHF Investigators. Global mortality variations in patients with heart failure: results from the International Congestive Heart Failure (INTER-CHF) prospective cohort study. Lancet Glob Health 2017;5:e665–e672. [DOI] [PubMed] [Google Scholar]
- 2. Corrà U, Magini A, Paolillo S, Frigerio M. Comparison among different multiparametric scores for risk stratification in heart failure patients with reduced ejection fraction. Eur J Prev Cardiol 2020;27:12–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Levy WC, Mozaffarian D, Linker DT, Sutradhar SC, Anker SD, Cropp AB, Anand I, Maggioni A, Burton P, Sullivan MD, Pitt B, Poole-Wilson PA, Mann DL, Packer M. The Seattle Heart Failure Model: prediction of survival in heart failure. Circulation 2006;113: 1424–1433. [DOI] [PubMed] [Google Scholar]
- 4. Pocock SJ, Ariti CA, McMurray JJ, Maggioni A, Kober L, Squire IB, Swedberg K, Dobson J, Poppe KK, Whalley GA, Doughty RN; Meta-Analysis Global Group in Chronic Heart Failure. Predicting survival in heart failure: a risk score based on 39 372 patients from 30 studies. Eur Heart J 2013;34:1404–1413. [DOI] [PubMed] [Google Scholar]
- 5. Simpson J, Jhund PS, Lund LH, Padmanabhan S, Claggett BL, Shen L, Petrie MC, Abraham WT, Desai AS, Dickstein K, Køber L, Packer M, Rouleau JL, Mueller-Velten G, Solomon SD, Swedberg K, Zile MR, McMurray JJV. Prognostic models derived in PARADIGM-HF and validated in atmosphere and the Swedish Heart Failure Registry to predict mortality and morbidity in chronic heart failure. JAMA Cardiol 2020;5:432–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Voors AA, Ouwerkerk W, Zannad F, van Veldhuisen DJ, Samani NJ, Ponikowski P, Ng LL, Metra M, Ter Maaten JM, Lang CC, Hillege HL, van der Harst P, Filippatos G, Dickstein K, Cleland JG, Anker SD, Zwinderman AH. Development and validation of multivariable models to predict mortality and hospitalization in patients with heart failure. Eur J Heart Fail 2017;19:627–634. [DOI] [PubMed] [Google Scholar]
- 7. Rørth R, Jhund PS, Kristensen SL, Desai AS, Køber L, Rouleau JL, Solomon SD, Swedberg K, Zile MR, Packer M, McMurray JJV. The prognostic value of troponin T and N-terminal pro B-type natriuretic peptide, alone and in combination, in heart failure patients with and without diabetes. Eur J Heart Fail 2019;21:40–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Packer M, Anker SD, Butler J, Filippatos G, Pocock SJ, Carson P, Januzzi J, Verma S, Tsutsui H, Brueckmann M, Jamal W, Kimura K, Schnee J, Zeller C, Cotton D, Bocchi E, Bohm M, Choi DJ, Chopra V, Chuquiure E, Giannetti N, Janssens S, Zhang J, Gonzalez Juanatey JR, Kaul S, Brunner-La Rocca HP, Merkely B, Nicholls SJ, Perrone S, Pina I, Ponikowski P, Sattar N, Senni M, Seronde MF, Spinar J, Squire I, Taddei S, Wanner C, Zannad F; EMPEROR-Reduced Trial Investigators. Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med 2020;383:1413–1424. [DOI] [PubMed] [Google Scholar]
- 9. Packer M, Butler J, Filippatos GS, Jamal W, Salsali A, Schnee J, Kimura K, Zeller C, George J, Brueckmann M, Anker SD, Zannad F; EMPEROR-Reduced Trial Committees and Investigators. Evaluation of the effect of sodium-glucose co-transporter 2 inhibition with empagliflozin on morbidity and mortality of patients with chronic heart failure and a reduced ejection fraction: rationale for and design of the EMPEROR-Reduced trial. Eur J Heart Fail 2019;21:1270–1278. [DOI] [PubMed] [Google Scholar]
- 10. Voors AA, Anker SD, Cleland JG, Dickstein K, Filippatos G, van der Harst P, Hillege HL, Lang CC, Ter Maaten JM, Ng L, Ponikowski P, Samani NJ, van Veldhuisen DJ, Zannad F, Zwinderman AH, Metra M. A systems BIOlogy Study to TAilored Treatment in Chronic Heart Failure: rationale, design, and baseline characteristics of BIOSTAT-CHF. Eur J Heart Fail 2016;18:716–726. [DOI] [PubMed] [Google Scholar]
- 11. Sharma A, Hijazi Z, Andersson U, Al-Khatib SM, Lopes RD, Alexander JH, Held C, Hylek EM, Leonardi S, Hanna M, Ezekowitz JA, Siegbahn A, Granger CB, Wallentin L. Use of biomarkers to predict specific causes of death in patients with atrial fibrillation. Circulation 2018;138:1666–1676. [DOI] [PubMed] [Google Scholar]
- 12. Ferreira JP, Ouwerkerk W, Tromp J, Ng L, Dickstein K, Anker S, Filippatos G, Cleland JG, Metra M, van Veldhuisen DJ, Voors AA, Zannad F. Cardiovascular and non-cardiovascular death distinction: the utility of troponin beyond N-terminal pro-B-type natriuretic peptide. Findings from the BIOSTAT-CHF study. Eur J Heart Fail 2020;22:81–89. [DOI] [PubMed] [Google Scholar]
- 13. Januzzi JL Jr, Ahmad T, Mulder H, Coles A, Anstrom KJ, Adams KF, Ezekowitz JA, Fiuzat M, Houston-Miller N, Mark DB, Pina IL, Passmore G, Whellan DJ, Cooper LS, Leifer ES, Desvigne-Nickens P, Felker GM, O'Connor CM. Natriuretic peptide response and outcomes in chronic heart failure with reduced ejection fraction. J Am Coll Cardiol 2019;74:1205–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Januzzi JL Jr, Camacho A, Pina IL, Rocha R, Williamson KM, Maisel AS, Felker GM, Prescott MF, Butler J, Solomon SD; PROVE-HF Investigators. Reverse cardiac remodeling and outcome after initiation of sacubitril/valsartan. Circ Heart Fail 2020;13:e006946. [DOI] [PubMed] [Google Scholar]
- 15. Januzzi JL Jr, Prescott MF, Butler J, Felker GM, Maisel AS, McCague K, Camacho A, Pina IL, Rocha RA, Shah AM, Williamson KM, Solomon SD; PROVE-HF Investigators. Association of change in N-terminal pro-B-type natriuretic peptide following initiation of sacubitril-valsartan treatment with cardiac structure and function in patients with heart failure with reduced ejection fraction. JAMA 2019;322:1085–1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pina IL, Camacho A, Ibrahim NE, Felker GM, Butler J, Maisel AS, Prescott MF, Williamson KM, Claggett BL, Desai AS, Solomon SD, Januzzi JL; PROVE-HF Investigators. Improvement of health status following initiation of sacubitril/valsartan in heart failure and reduced ejection fraction. JACC Heart Fail 2021;9:42–51. [DOI] [PubMed] [Google Scholar]
- 17. Kociol RD, Pang PS, Gheorghiade M, Fonarow GC, O'Connor CM, Felker GM. Troponin elevation in heart failure: prevalence, mechanisms, and clinical implications. J Am Coll Cardiol 2010;56:1071–1078. [DOI] [PubMed] [Google Scholar]
- 18. Murphy SP, Prescott MF, Maisel AS, Butler J, Piña IL, Felker GM, Ward JH, Williamson KM, Camacho A, Kandanelly RR, Solomon SD, Januzzi JL. Association between angiotensin receptor-neprilysin inhibition, cardiovascular biomarkers, and cardiac remodeling in heart failure with reduced ejection fraction. Circ Heart Fail 2021;14:e008410. [DOI] [PubMed] [Google Scholar]
- 19. Daubert MA, Adams K, Yow E, Barnhart HX, Douglas PS, Rimmer S, Norris C, Cooper L, Leifer E, Desvigne-Nickens P, Anstrom K, Fiuzat M, Ezekowitz J, Mark DB, O’Connor CM, Januzzi J, Felker GM. NT-proBNP goal achievement is associated with significant reverse remodeling and improved clinical outcomes in HFrEF. JACC Heart Fail 2019;7:158–168. [DOI] [PubMed] [Google Scholar]
- 20. Bello NA, Claggett B, Desai AS, McMurray JJ, Granger CB, Yusuf S, Swedberg K, Pfeffer MA, Solomon SD. Influence of previous heart failure hospitalization on cardiovascular events in patients with reduced and preserved ejection fraction. Circ Heart Fail 2014;7:590–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Böhm M, Komajda M, Borer JS, Ford I, Maack C, Tavazzi L, Moyne A, Swedberg K; on behalf of the SHIFT Investigators. Duration of chronic heart failure affects outcomes with preserved effects of heart rate reduction with ivabradine: findings from SHIFT. Eur J Heart Fail 2018;20:373–381. [DOI] [PubMed] [Google Scholar]
- 22. Lee TT, Chen J, Cohen DJ, Tsao L. The association between blood pressure and mortality in patients with heart failure. Am Heart J 2006;151:76–83. [DOI] [PubMed] [Google Scholar]
- 23. Ferreira JP, Duarte K, Pfeffer MA, McMurray JJV, Pitt B, Dickstein K, Zannad F, Rossignol P; High-Risk Myocardial Infarction Database Initiative. Association between mean systolic and diastolic blood pressure throughout the follow-up and cardiovascular events in acute myocardial infarction patients with systolic dysfunction and/or heart failure: an analysis from the High-Risk Myocardial Infarction Database Initiative. Eur J Heart Fail 2018;20:323–331. [DOI] [PubMed] [Google Scholar]
- 24. Packer M, Anker SD, Butler J, Filippatos GS, Ferreira JP, Pocock S, Carson PE, Anand IS, Doehner W, Haass M, Komajda M, Miller AB, Pehrson S, Teerlink JR, Brueckmann M, Jamal W, Zeller C, Schnaidt S, Zannad F; For the EMPEROR-Reduced Trial Committees and Investigators. Effect of empagliflozin on the clinical stability of patients with heart failure and a reduced ejection fraction: the EMPEROR-Reduced trial. Circulation 2021;143:326–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zannad F, Ferreira JP, Pocock SJ, Anker SD, Butler J, Filippatos G, Brueckmann M, Ofstad AP, Pfarr E, Jamal W, Packer M. SGLT2 inhibitors in patients with heart failure with reduced ejection fraction: a meta-analysis of the EMPEROR-Reduced and DAPA-HF trials. Lancet 2020;396:819–829. [DOI] [PubMed] [Google Scholar]
- 26. Vedin O, Lam CSP, Koh AS, Benson L, Teng THK, Tay WT, Braun O, Savarese G, Dahlström U, Lund LH. Significance of ischemic heart disease in patients with heart failure and preserved, midrange, and reduced ejection fraction: a Nationwide Cohort Study. Circ Heart Fail 2017;10:e003875. [DOI] [PubMed] [Google Scholar]
- 27. Canepa M, Fonseca C, Chioncel O, Laroche C, Crespo-Leiro MG, Coats AJS, Mebazaa A, Piepoli MF, Tavazzi L, Maggioni AP; Term Registry Investigators. Performance of prognostic risk scores in chronic heart failure patients enrolled in the European Society of Cardiology Heart Failure Long-Term Registry. JACC Heart Fail 2018;6:452–462. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.