Abstract
Syringocystadenocarcinoma papilliferum (SCACP) is an extremely rare adnexal neoplasm of the sweat glands. It is believed to arise from the malignant transformation of syringocystadenoma papilliferum (SCAP). The majority of cases present on the head and neck and up to 17% of cases show metastatic progression. These tumors seldom occur in the anogenital area and, to date, only one case has been reported on the penis. Here, we report a rare case of SCACP in a 65-year-old man who presented with an erythematous, non-healing, ulcerated lesion on the penis.
Keywords: Syringocystadenocarcinoma papilliferum, Adnexal neoplasm, Anogenital area
Abbreviations: FDG-PET, fluorodeoxyglucose-positron emission tomography; SCACP, Syringocystadenocarcinoma papilliferum
Highlights
-
•
Syringocystadenocarcinoma papilliferum (SCACP) is an extremely rare adnexal neoplasm of the sweat glands.
-
•
The majority of SCACP cases present on the head and neck.
-
•
SCACP seldom occur in the anogenital area and, to date, only one case has been reported on the penis.
-
•
The present case is the second case of SCACP with involvement of the penis, the first involving the scrotum.
-
•
Clinicians should be aware of the possibility of SCACP in cases with chronic ulcerative nodular lesions in the genital area.
1. Introduction
Syringocystadenocarcinoma papilliferum (SCACP) is a rare malignant adnexal tumor that represents the malignant counterpart of syringocystadenoma papilliferum (SCAP).1 Mostly, it is believed to arise from SCAP, nevus sebaceous, or linear nevus verrucosus lesions.2 The majority of cases present on the head and neck and up to 17% of cases show metastatic progression.1 Only 52 cases of this malignancy have been reported to date, with limited data being available about its origin and etiology. Moreover, these tumors seldom occur in the anogenital area; to date, only one case has been reported to appear on the penis.2, 3, 4 Here, we report a case of SCACP in the anogenital area with penile involvement.
2. Case presentation
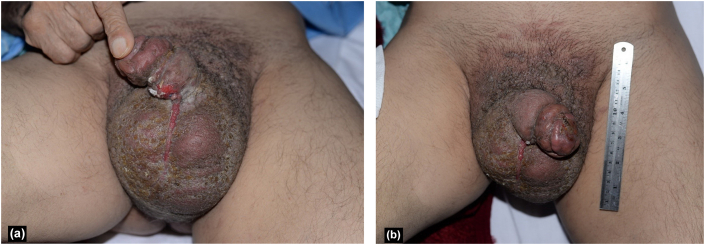
A 65-year-old Iranian man presented to the dermatology clinic with multiple papules, nodules, and exudative crust over the genitalia and an erythematous, non-healing, ulcerated lesion on the penis measuring 1 × 2 cm that had appeared two months earlier (Fig. 1). The patient complained of moderate irritating and obstructive urologic symptoms and had unintentionally lost approximately 10 kg of weight during the preceding 6 months. Severe lymphedema and subcutaneous edema were apparent in the scrotum. Skin biopsies were obtained from the skin ulcer and the adjacent papule. Sonography was performed, revealing irregularity in the bladder wall; the prostate volume was 45 ml, with bulging of 10 ml of the median lobe into the base of the bladder. No free fluid was detected in the abdominopelvic cavity. Testicular atrophy was prominent. The scrotal wall was thickened to about 19 mm with increased fat echogenicity in favor of soft tissue inflammation and edema. Bilateral complicated hydrocele was detected. Vascular flow was normal. Cystourethroscopy was performed under spinal anesthesia in the lithotomy position. There were some cotton-like, irregular, epithelial lesions in the distal part of the penile urethra, 2 cm proximal to the fossa navicularis; a cold cup biopsy was taken under vision. Other parts of the urethra had normal appearance and there was a prominent, enlarged prostate with kissing lobes. There was no visible abnormality in the bladder except for moderate trabeculation.
Fig. 1.
Multiple papules, nodules, and exudative crust over the genitalia and an erythematous, non-healing, ulcerated lesion on the penis along with severe scrotal edema (a, b).
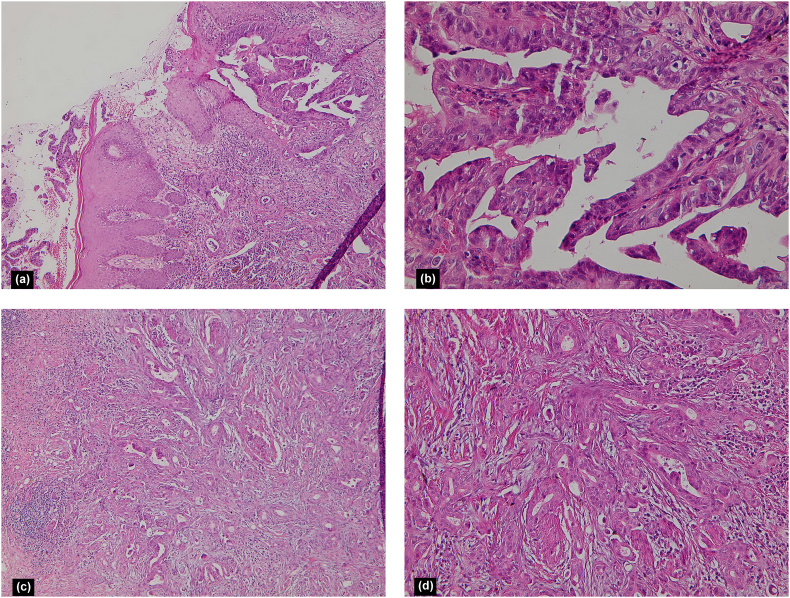
Histopathologic examination revealed hyperplastic epidermis and a crateriform lesion populated by papillary projections and lined by atypical epithelium, with the fibrovascular cores containing numerous plasma cells. Dermal invasion was seen, characterized by tubular structures, single cells, and small groups of cells that infiltrated the full thickness of the dermis and part of the subcutis. These structures were surrounded by desmoplastic stroma with lymphoplasmacytic infiltration and perineural invasion. Mild neutrophilic infiltration in dermal nests was identified along with isolated tumor cell necrosis. Overall, the manifestations were characterized as a malignant epithelial neoplasm with papillary and tubular structures in favor of SCACP (Fig. 2). Histologic evaluation of the urethral lesion was also consistent with adenocarcinoma. No distant metastasis was identified via a fluorodeoxyglucose (FDG)-positron emission tomography (PET) scan.
Fig. 2.
Hyperplastic epidermis and a crateriform lesion populated by papillary projections and lined by atypical epithelium, with the fibrovascular cores containing numerous plasma cells (a, H&E × 100; b, H&E × 400) (a, b). Dermal invasion was seen, characterized by tubular structures, single cells, and small groups of cells that infiltrated the full thickness of the dermis and part of the subcutis (c, H&E × 100; d, H&E × 200) (c, d).
3. Discussion
As an adnexal tumor, SCAP was first described by Stokes in 1917.1 Dissanayake and Salm, in 1980, were the first to discuss SCACP, which is considered as the malignant counterpart of SCAP.5 Since then, this is just the 53rd reported case of this malignancy, meaning that the clinical and pathological characteristics are not well defined.4 We performed a literature review of the Medline, EMBASE, and Cochrane databases to characterize the cases previously listed in the literature (Table 1). Our results show that SCACP predominantly involves the head and neck of patients over 60 years of age with no gender predilection, but can also occur on the chest, arm, anogenital region, and back (Table 1).
Table 1.
Reported cases of syringocystadenocarcinoma papilliferum.
| # | Reference | Clinical presentation | Sex/Age (year) | Location | Size (mm) | Duration | Diagnosis | Association | Follow-up | Treatment |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Dissanayake and Salm, 1980 | Exophytic tumor with copious secretion | F/74 | Scalp | 65 | 30 years | SCACP in situ | SCAP | NED (6.75 years) | Surgery |
| 2 | Dissanayake and Salm, 1980 | A lump | F/71 | Back | 30 | N/A | SCACP invasive | N/A | NED (7 years) | Surgery |
| 3 | Seco Navedo et al., 1982 | Tumor | F/50 | Scalp | 65 | Congenital | SCACP invasive | Nevus sebaceous | 3 Local lymph node, lymph node metastasis | Surgery + RTx + CTx (NED—2 years) |
| 4 | Numata et al., 1985 | Nodular, partially cystic and solid tumor | F/52 | Chest | 130 × 80 | 20 years | SCACP invasive | N/A | 1 Local lymph node, lymph node metastasis | Surgery NED (12 months) |
| 5 | Bondi and Urso, 1996 | Ulcerated and crusted lesion. | M/47 | Scalp | 25 | N/A | SCACP invasive | N/A | N/A | Surgery |
| 6 | Ishida-Yamamoto et al., 2001 | Enlarging nodule as a black exophytic tumor with a granular surface | M/61 | Perianal | 60 | 10 years | SCACP in situ | N/A | NED (11 months) | Surgery |
| 7 | Arai et al., 2003 | Enlarging tumor, bleed easily when pressed, surrounded by a bloody crust with macerated white papules on the surface | M/64 | Scalp | 35 | 2 years | SCACP in situ | SCAP | N/A | Surgery |
| 8 | Chi et al., 2004 | Two ulcerated verrucous plaques coated with yellow crust, painful, pruritic | M/60 | Auricle | 40 × 10 | Since childhood | SCACP invasive | SCAP | NED (72 months) | Surgery |
| 9 | Woestenborghs et al., 2006 | A bleeding raised tumor | F/81 | Scalp | 15 | N/A | SCACP in situ | SCAP | N/A | Surgery |
| 10 | Park et al., 2007 | Single erythematous dome-shaped and firm nodule surrounded by bloody crust | M/65 | Suprapubic region | 35 | 2 years | SCACP in situ | N/A | NED (24 months) | Surgery |
| 11 | Langner and Ott, 2009 | A nodule with partly cystic and partly solid appearance | M/83 | Perianal | 15 | N/A | SCACP in situ | SCAP | N/A | Surgery |
| 12 | Sroa et al., 2010 | Flesh-colored exophytic nodule with a peripheral crusted hyperpigmented border | M/77 | Calf | 25 | 9 years | SCACP invasive | N/A | NED (15 months) | Surgery |
| 13 | Kazakov et al., 2010 | Verrucous ulcerated nodule | F/56 | Neck | 20 | 10 years | SCACP in situ | SCAP | NED (9 months) | Surgery |
| 14 | Kazakov et al., 2010 | Clinical impression of a ruptured cyst or carcinoma | M/58 | Forehead | 25 | 25 years | SCACP invasive | SCAP | NED (4 years) | Surgery |
| 15 | Kazakov et al., 2010 | Ulcerated smelling neoplasm | F/46 | Scalp | 35 | N/A | SCACP invasive | SCAP | NED (6 years) | Surgery |
| 16 | Kazakov et al., 2010 | Ulcerated nodule | M/67 | Scalp | 25 | N/A | SCACP in situ | SCAP | NED (2 years) | Surgery |
| 17 | Kazakov et al., 2010 | Ulcerated tumor with Recent rapid growth. | F/60 | Scalp | 30 | >30 years | SCACP invasive | SCAP | N/A | Surgery |
| 18 | Kazakov et al., 2010 | Inflammatory plaque | M/81 | Scalp | 20 | N/A | SCACP invasive | SCAP | NED (21 months) | Surgery |
| 19 | Leeborg et al., 2010 | Erythematous to violaceous asymmetric papule with lobulated contours | F/86 | Neck | 45 | 4 months | SCACP invasive | Invasive squamous cell carcinoma | Local recurrence (18 months) | Surgery + RTx |
| 20 | Abrari and Mukherjee, 2011 | As described by the patient, a bead like, 1.0 cm axillary swelling, had been self-discovered and this lesion had excoriated and attained a size of 3.5 cm in 6 months. | M/62 | Axilla | 35 | 6 months | SCACP invasive | N/A | N/A | Surgery |
| 21 | Aydin et al., 2011 | Ulcerative nodular lesion | M/67 | Scalp | 40 | Since childhood | SCACP invasive | SCAP | NED (2 years) | Surgery |
| 22 | Hoekzema et al., 2011 | Exophytic nodule with a moist surface | F/83 | Arm | 30 | 7 years | SCACP invasive | SCAP nevus verrucosus | N/A | Surgery |
| 23 | Hoguet et al., 2012 | Erythematous, slightly elevated, centrally ulcerated and crusted nodule | M/86 | Eyelid | 4 | N/A | SCACP invasive | N/A | NED (3 months) | Surgery |
| 24 | Plant et al., 2012 | A non-healing ulcerated lesion | M/83 | Penis | 12 | N/A | SCACP in situ | N/A | N/A | Surgery |
| 25 | Bakhshi et al., 2012 | Hemispherical swelling was seen on the scalp which was firm in consistency with a granular surface and erosions and crustations | F/45 | Scalp | 60 × 30 | 12 months | N/A | SCAP | NED (12 months) | Surgery |
| 26 | Zhang et al., 2012 | Solitary tender erythematous ulcerated nodule within a background of red patch | F/75 | Arm | 15 | 12 months | SCACP invasive | SCAP | NED (6 months) | Surgery |
| 27 | Peterson et al., 2013 | Hairless flesh-colored exophytic tumor with serosanguinous exudate | M/65 | Scalp | 30 × 30 | 12 months | SCACP invasive | SCAP | NED | Surgery |
| 28 | Arslan et al., 2013 | Multinodular ulcerated lesions | M/66 | Scalp | N/A | 20 years | SCACP invasive | SCAP | 3 Local lymph node, lymph node metastasis | Surgery + RTx (NED—15 months) |
| 29 | Arslan et al., 2013 | Well-defined erythematous ulcerated nodule | F/66 | Scalp | 30 | >12 months | SCACP invasive | N/A | NED (2 years) | Surgery |
| 30 | Castillo et al., 2014 | Well-delimited oval dermal nontender nodule with a solid and cystic appearance. | F/32 | Scalp | 22 | N/A | SCACP in situ | N/A | Local recurrence (8 years) | Surgery |
| 31 | Paradiso et al., 2014 | A single painful erythematous, dome-shaped, and firm nodule surrounded by normal skin | M/88 | Shoulder | 15 × 15 | N/A | SCACP invasive | N/A | Died from other cause | N/A |
| 32 | Shan et al., 2014 | A pink ulcerated nodule without tenderness nor pruritis | M/93 | Popliteal fossa | 20 | >10 years | N/A | SCAP | NED | Surgery |
| 33 | Mohanty et al., 2014 | Exophytic lobulated mass which was tan-pink to red in color with soft to firm consistency and was non-tender. | F/80 | Scalp | 50 | 8 years | SCACP in situ | N/A | NED (5 years) | Surgery |
| 34 | Satter et al., 2014 | Focally ulcerated exophytic nodule associated with a few small satellite papules which bled with minor trauma | M/42 | Scalp | 45 × 40 | >1 month | SCACP invasive | SCAP and Nevus sebaceous | Lymph node metastasis | Surgery |
| 35 | Parekh et al., 2016 | A single well-demarcated red exophytic nodule with small foci of ulceration | M/74 | Scalp | 20 | Since childhood | SCACP invasive | SCAP, nevus sebaceous of Jadassohn, trichoblastoma | Lymph node metastasis | Surgery |
| 36 | Chen et al., 2016 | A hairless, rough, ill-defined erythematous erosive warty plaque on the right parietal scalp with serosanguinous exudate | F/60 | Scalp | 28 × 20 | 12 months | SCACP in situ | Nevus sebaceous | N/A | Surgery |
| 37 | Singh et al., 2017 | Well-defined erythematous plaque with overlying ulceration on the midback, 1 cm left of the spine. Adjoining skin showed a horseshoe-shaped oval area of lichenification | F/60 | Back | 15 × 10 | >10 years | SCACP in situ | SCACP in situ with macular amyloidosis | N/A | Surgery |
| 38 | Zhang et al., 2017 | Solitary red plaque | M/26 | Chest | 50 | 22 years | SCACP in situ | Invasive adenocarcinoma subcutis | Left axillary lymph node and bilateral lung metastases, DoD 2 months after diagnosis | Surgery + CTx |
| 39 | Zhang et al., 2017 | Solitary nodule with exudate | M/47 | Abdomen | 15 | 23 years | SCACP in situ | N/A | NED (9 years) | Surgery |
| 40 | Zhang et al., 2017 | Erythematous nodule | M/67 | Left Axilla | 20 | 6 years | SCACP in situ | Invasive adenocarcinoma subcutis | N/A | Surgery + left axilla lymphadenectomy |
| 41 | Zhang et al., 2017 | Flat verrucous neoplasm | M/64 | Scalp | 20 | 1 years | SCACP in situ | Invasive adenocarcinoma in dermis + mucinous metaplasia | Metastases to multiple distant lymph nodes and lung metastases, DoD 34 months after diagnosis | Surgery + RTx |
| 42 | Zhang et al., 2017 | Exophytic ulcerated nodule with bleeding | M/63 | Chest | 10 | 10 years | SCACP in situ | Invasive adenocarcinoma in dermis | NED (36 months) | Surgery |
| 43 | Zhang et al., 2017 | Exophytic pinkish nodule | M/74 | Chest | 20 | 6 years | SCACP in situ | Invasive adenocarcinoma subcutis | NED (30 months) | Surgery |
| 44 | Zhang et al., 2017 | Flat mass with granular surface | F/63 | Axilla | 50 | 3 months | SCACP in situ | Invasive adenocarcinoma + invasive squamous cell carcinoma | Widespread subcutaneous metastases, DoD 20 months after diagnosis | Surgery + right axilla lymphadenectomy |
| 45 | Zhang et al., 2017 | Keloid plaque | M/40 | Chest | 50 | 5 years | SCACP in situ | Invasive adenocarcinoma subcutis | NED (14 months) | Surgery + bilateral lymphadenectomy + CTx |
| 46 | Zhang et al., 2017 | Subcutaneous nodule | F/29 | Forehead | 15 | 2 years | SCACP in situ | Invasive squamous cell carcinoma | NED (10 months) | Surgery |
| 47 | Zhang et al., 2017 | Verrucous ulcerated mass | M/64 | Axilla | 22 | 10 years | SCACP in situ | Invasive adenocarcinoma subcutis | NED (3 months) | Surgery + right axilla lymphadenectomy + CTx |
| 48 | Muthusamy et al., 2017 | Ulcerated nodule with mild pain | F/78 | Scalp | 45 × 35 | 6 months | SCACP invasive | SCAP and Trichoblastoma | N/A | N/A |
| 49* | Yao et al., 2018 | Erythematous friable mass which bleed intermittently | F/73 | Scalp | 30 × 27 | Since birth | N/A | N/A | N/A | N/A |
| 50 | Agulló Pérez et al., 2018 | Pediculated erythematous-brownish papule which occasional bleeding caused by friction and exudative crust | M/40 | Chest | 10 | 1 year | SCACP invasive | N/A | NED (8 months) | Surgery |
| 51 | Pagano Boza et al., 2019 | Ulcerated, indurated, and erythematous nodule | M/63 | Eyelid | 50 × 70 | >6 years | SCACP invasive | SCAP | Local recurrence | Surgery |
| 52 | Alegría-Landa et al., 2019 | Eroded easily-bleeding nodule | F/90 | Scalp | N/A | 10 months | SCACP in situ with sarcomatoid appearance | N/A | Died from other cause 1 year after diagnosis | N/A |
| 53 | Present case | Non-healing erythematous ulcerated lesion, multiple papules and nodules, crustations and hyperpigmented patches. Scrotal Tense edema. | M/65 | Genitalia | 10× 20 | 2 months | SCACP invasive | Invasive Adenocarcinoma | Died from other cause 7 months after diagnosis | CTx |
Abbreviations: CTx, chemotherapy; DoD, died of disease; F, Female; M, Male; N/A, not available; NED, no evidence of disease; RTx, radiation therapy.
*Full text was not accessible online
These lesions clinically present as erythematous, skin-colored, brown or yellowish nodules, papules, and/or ulcerated lesions that may be associated with mild pain, pruritus, or easily induced bleedings (Table 1). In the present case, the initial differential diagnoses were angiosarcoma, Kaposi sarcoma, and squamous cell carcinoma; however, the histopathology findings were consistent with SCACP.
Currently, no treatment protocols for SCACP are available but surgery with wide margins is the most favorable option and Mohs surgery has been suggested.1,4 The application of chemotherapy and radiotherapy is controversial.4 Our therapeutic plan consisted of chemotherapy. Unfortunately, despite receiving five courses of chemotherapy, the patient died seven months after the diagnosis of SCACP.
There have been only eight cases reported with metastasis (Table 1); our patient did not show any signs of metastasis in the PET-FDG scan. On the other hand, there have been limited cases of SCACP associated with invasive adenocarcinoma (Table 1), as was the case for our patient.
To the best of our knowledge, the present case is the second case of SCACP with involvement of the penis, the first involving the scrotum, and one of the limited cases associated with invasive behavior of SCACP.
In conclusion, regarding the variable course of SCACP, in case of encountering chronic nodular and ulcerative lesions in the genital area, despite the rarity, clinicians should be aware of its possibility.
Ethics approval and consent to participate
Ethical approval was not required for this study in accordance with national guidelines.
Consent for publication
Written informed consent was obtained from the patient's family for publication of this case report and any accompanying images.
Declaration of competing interest
The authors have no conflicts of interest to declare.
Funding sources
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author contributions
MSD, FA and AAِ were involved in the diagnosis and management of the patients and have been responsible for the clinical part of the manuscript. AR reported the result of histopathological evaluation. FA and MB did literature review and drafted the manuscript. MSD, FA, AR and AA were responsible for final editing of the manuscript, and coordinated the study. All authors have read and approved the final manuscript.
Availability of data and material
Not applicable.
References
- 1.Pagano Boza C., Gonzalez-Barlatay J., Ugradar S., Pol M., Premoli E.J. Syringocystadenocarcinoma papilliferum with orbital invasion: a case report with literature review. Ther Adv Ophthalmol. 2019;11 doi: 10.1177/2515841419844087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hoekzema R., Leenarts M.F., Nijhuis E.W. Syringocystadenocarcinoma papilliferum in a linear nevus verrucosus. J Cutan Pathol. 2011;38(2):246–250. doi: 10.1111/j.1600-0560.2009.01419.x. [DOI] [PubMed] [Google Scholar]
- 3.Plant M.A., Sade S., Hong C., Ghazarian D.M. Syringocystadenocarcinoma papilliferum in situ of the penis. Eur J Dermatol. 2012;22(3):405–406. doi: 10.1684/ejd.2012.1723. [DOI] [PubMed] [Google Scholar]
- 4.Arslan H., Diyarbakrl M., Batur S., Demirkesen C. Syringocystadenocarcinoma papilliferum with squamous cell carcinoma differentiation and with locoregional metastasis. J Craniofac Surg. 2013;24(1):e38–e40. doi: 10.1097/SCS.0b013e31826cffc6. [DOI] [PubMed] [Google Scholar]
- 5.Dissanayake R., Salm R. Sweat‐gland carcinomas: prognosis related to histological type. Histopathology. 1980;4(4):445–466. doi: 10.1111/j.1365-2559.1980.tb02939.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.