Abstract
Introduction
Strengthening The Reporting Of Cohort Studies in Surgery (STROCSS) guidelines were developed in 2017 in order to improve the reporting quality of observational studies in surgery and updated in 2019. In order to maintain relevance and continue upholding good reporting quality among observational studies in surgery, we aimed to update STROCSS 2019 guidelines.
Methods
A STROCSS 2021 steering group was formed to come up with proposals to update STROCSS 2019 guidelines. An expert panel of researchers assessed these proposals and judged whether they should become part of STROCSS 2021 guidelines or not, through a Delphi consensus exercise.
Results
42 people (89%) completed the DELPHI survey and hence participated in the development of STROCSS 2021 guidelines. All items received a score between 7 and 9 by greater than 70% of the participants, indicating a high level of agreement among the DELPHI group members with the proposed changes to all the items.
Conclusion
We present updated STROCSS 2021 guidelines to ensure ongoing good reporting quality among observational studies in surgery.
Keywords: Cohort studies, Case-control studies, Cross-sectional studies, Reporting guideline, STROCSS
Highlights
-
•
In order to maintain relevance and continue upholding good reporting quality among observational studies in surgery, STROCSS 2019 guidelines were updated through a DELPHI consensus exercise.
-
•
42 people participated in the development of STROCSS 2021 guidelines and there was a high level of agreement among the DELPHI group members with the proposed changes to all the items.
-
•
Updated STROCSS 2021 guideline is presented.
1. Introduction
Observational studies often feature in the surgical literature [1]. However, poor reporting quality among observational studies in surgery has been highlighted [2]. In the absence of good reporting quality, readers are unable to meaningfully assess the research, rendering it less useful [3]. The existence of reporting guidelines and the mandatory implementation of these guidelines by journals have shown to improve the reporting quality among various types of studies [[4], [5], [6]].
Hence, Strengthening The Reporting Of Cohort Studies in Surgery (STROCSS) guidelines were developed in 2017 in order to improve the reporting quality of cohort studies in surgery. Despite the title, STROCSS guidelines aimed to improve the reporting quality of all observational studies in surgery, including case-control studies and cross-sectional studies, as well as cohort studies [7]. STROCSS 2017 guidelines were updated in 2019; since its inception, STROCSS guidelines have been cited over 1000 times illustrating their acceptance within the surgical research community [8]. We aimed to update STROCSS 2019 guidelines in order to maintain relevance and continue upholding good reporting quality among observational studies in surgery.
2. Methods
The DELPHI methodology used in the development of STROCSS 2017 and 2019 guidelines was used in the development of STROCSS 2021 guidelines [9].
2.1. Coming up with proposals to update STROCSS 2019 guidelines
A STROCSS 2021 steering group was formed; members collaborated over email, Google Docs and WhatsApp Messenger to come up with proposals to update STROCSS 2019 guidelines.
2.2. Delphi process
The proposals to update STROCSS 2019 guidelines were put to an expert panel of researchers; they were asked to assess the proposals and judge whether they should become part of STROCSS 2021 guidelines or not, through a Delphi consensus exercise.
The Delphi questionnaire was sent to all participants using Google Forms. The participants were required to indicate whether they disagreed or agreed with the proposed changes to the 17 items of the STROCSS 2019 guidelines, using a nine-point Likert scale, where 1 indicated “strongly disagree” and 9 indicated “strongly agree”. If greater than 70% of participants gave a score between 7 and 9 for a proposed change, this was deemed as consensus and the item was updated. If less than 70% of participants gave a score between 7 and 9 for a proposed change, the item was left unaltered.
2.3. Participants
Researchers who were involved in the development of STROCSS 2017 and 2019 guidelines were invited to participate again. In addition, members of the International Journal of Surgery (IJS) editorial board were invited; IJS has mandated authors submitting surgical research papers using observational methodology to comply with STROCSS guidelines and hence IJS is an ardent supporter of STROCSS guidelines. Participants were accomplished researchers, authors, journal reviewers, editorial board members and editors representing countries across North America, South America, Europe, Africa, Asia, and Australia.
3. Results
47 people agreed to participate in the development of STROCSS 2021 guidelines; 42 people (89%) completed the DELPHI survey and hence participated in the development of STROCSS 2021 guidelines. Table 1 shows a summary of the scores given by the Delphi participants to indicate agreement or disagreement with the proposed changes to each item of the STROCSS 2019 guidelines. All items received a score between 7 and 9 by greater than 70% of the participants, indicating consensus with the proposed changes to all the items. The revised STROCSS 2021 guidelines are shown in Table 2.
Table 1.
STROCSS 2021 Delphi participants' scores ranging between 1 (strongly disagree) and 9 (strongly agree). Items listed correspond to individual sections of STROCSS.
| Item | 1-3 (%) | 4-6 (%) | 7-9 (%) |
|---|---|---|---|
| 1 | 2.4 | 7.2 | 90.5 |
| 2a | 0.0 | 2.4 | 97.6 |
| 2b | 0.0 | 9.6 | 90.4 |
| 2c | 2.4 | 7.2 | 90.5 |
| 2d | 0.0 | 19.1 | 81.0 |
| 3 | 2.4 | 7.2 | 90.5 |
| 4a | 2.4 | 7.2 | 90.5 |
| 4b | 7.2 | 14.3 | 78.5 |
| 4c | 0.0 | 11.9 | 88.2 |
| 4d | 0.0 | 7.2 | 92.8 |
| 5a | 0.0 | 7.2 | 92.8 |
| 5b | 0.0 | 14.3 | 85.7 |
| 5c | 2.4 | 4.8 | 92.8 |
| 5d | 0.0 | 19.1 | 80.9 |
| 6a | 0.0 | 4.8 | 95.2 |
| 6b | 4.8 | 14.2 | 80.9 |
| 6c | 2.4 | 9.5 | 88.1 |
| 7a | 0.0 | 9.5 | 90.4 |
| 7b | 0.0 | 14.2 | 85.7 |
| 7c | 0.0 | 11.9 | 88.1 |
| 7d | 4.8 | 9.5 | 85.7 |
| 7e | 0.0 | 14.3 | 85.7 |
| 7f | 0.0 | 11.9 | 88.1 |
| 8 | 0.0 | 9.5 | 90.5 |
| 9 | 2.4 | 9.6 | 88.0 |
| 10a | 0.0 | 2.4 | 97.6 |
| 10b | 0.0 | 9.5 | 90.4 |
| 10c | 0.0 | 11.9 | 88.1 |
| 11a | 0.0 | 19.0 | 80.9 |
| 11b | 0.0 | 16.7 | 83.4 |
| 11c | 0.0 | 14.3 | 85.7 |
| 12 | 0.0 | 9.6 | 90.4 |
| 13 | 2.4 | 19.1 | 78.5 |
| 14 | 0.0 | 9.5 | 90.5 |
| 15 | 0.0 | 14.3 | 85.7 |
| 16 | 2.4 | 14.3 | 83.3 |
| 17a | 2.4 | 14.3 | 83.3 |
| 17b | 0.0 | 4.8 | 95.2 |
| 17c | 0.0 | 2.4 | 97.5 |
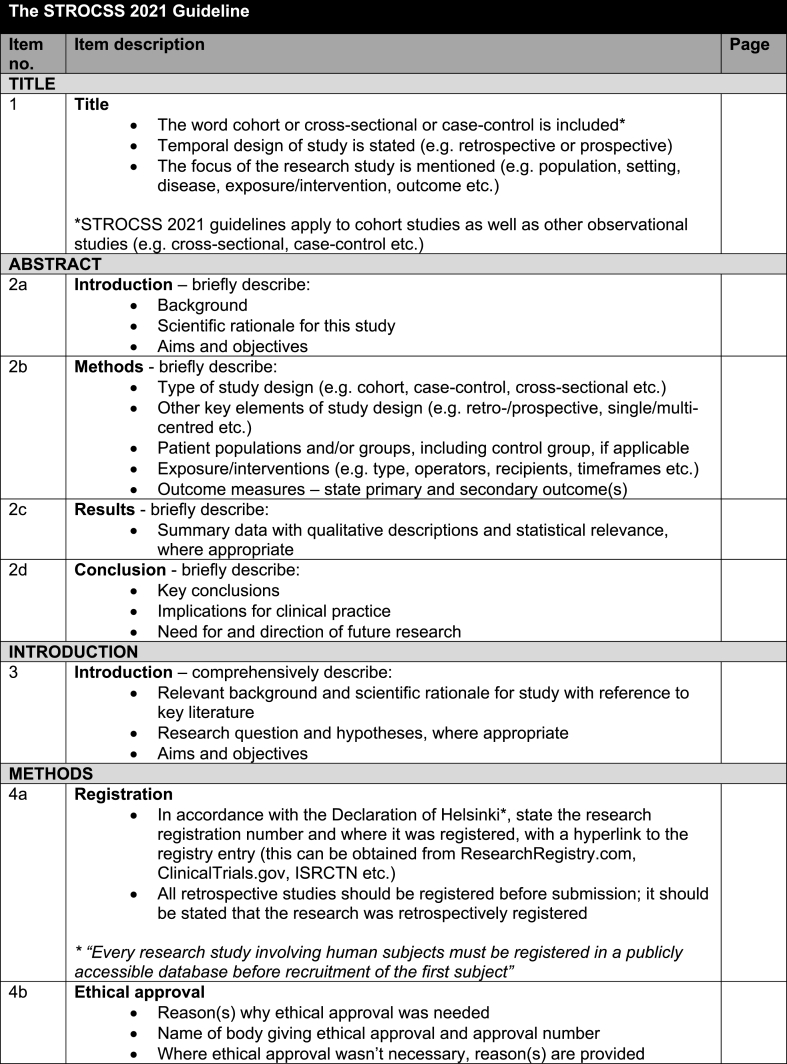
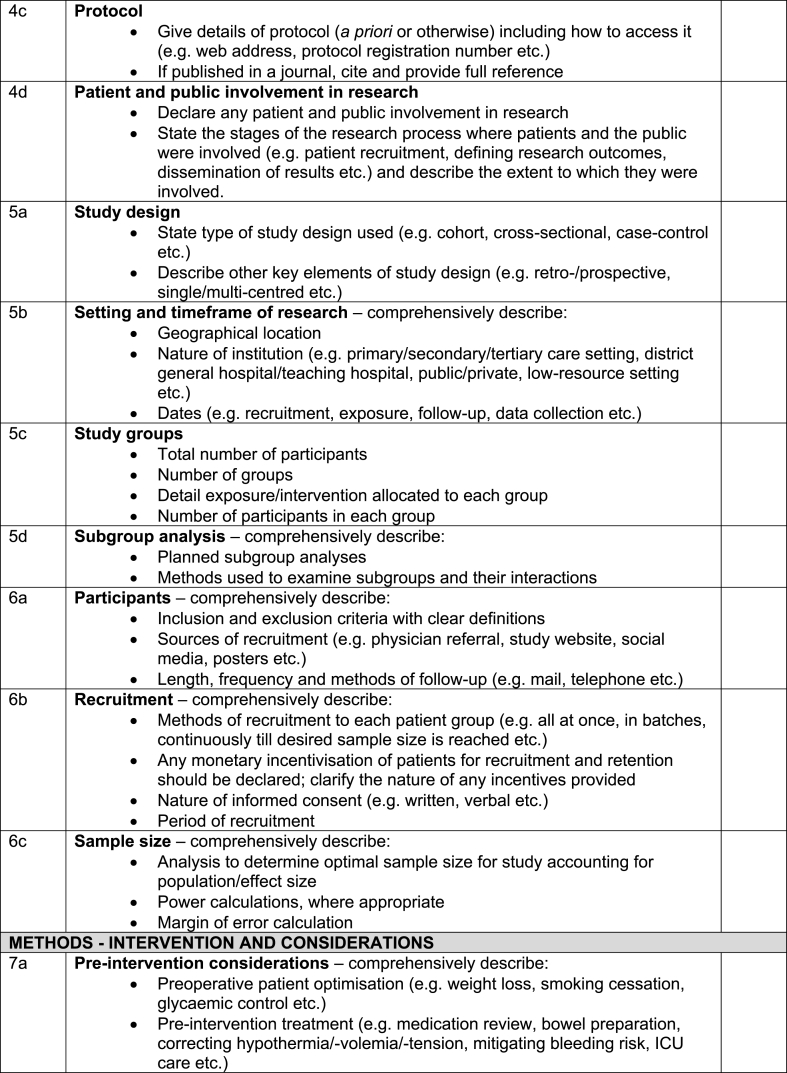
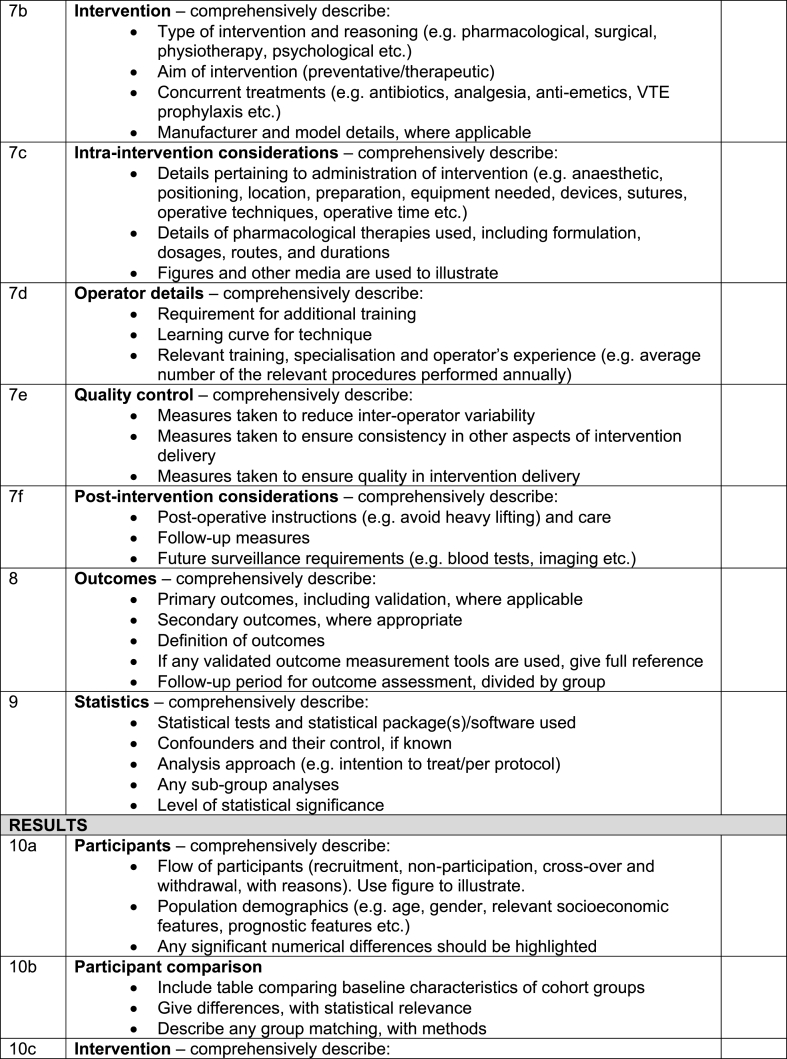
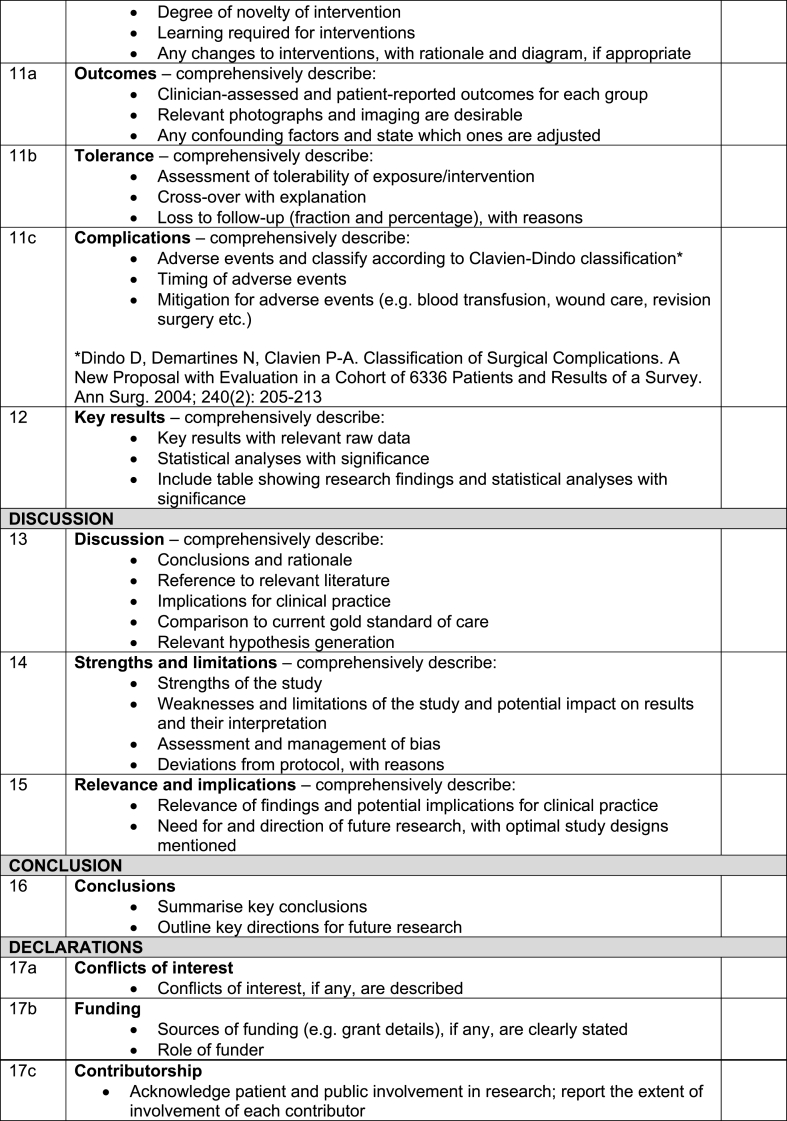
Table 2.
The full revised STROCSS 2021 checklist.
4. Discussion
Since the publication of STROCSS guidelines, it has been cited over 1000 times and thus enjoyed great acceptance within the surgical research community. We present the updated STROCSS 2021 guidelines to continue ensuring good reporting quality among observational studies in surgery; we encourage authors, reviewers, editors, and journals to adopt them.
Authors should cite STROCSS 2021 guidelines in their methods section; additionally, they should submit a completed STROCSS 2021 guidelines checklist alongside their manuscript for reviewers and editors to inspect and ensure compliance. STROCSS website (https://www.strocssguideline.com) has provided the STROCSS 2021 guidelines checklist in various formats to ensure accessibility.
5. Conclusion
We present updated STROCSS 2021 guidelines for authors, reviewers, editors, and journals to implement, with a view to ensuring good reporting quality among observational studies in surgery.
Sources of funding
None.
Ethical approval
Not applicable.
Research registration Unique Identifying number (UIN)
-
1.
Name of the registry: Not applicable
-
2.
Unique Identifying number or registration ID: Not applicable
-
3.
Hyperlink to your specific registration (must be publicly accessible and will be checked): Not applicable
Author contribution
RA: Concept and design, data interpretation and analysis, drafting, revision and approval of final manuscript. GM: Design, data collection, data interpretation and analysis, drafting, revision and approval of final manuscript.
Guarantor
Riaz Agha.
Declaration of competing interest
None declared - the authors have no financial, consultative, institutional, and other relationships that might lead to bias or conflict of interest.
Acknowledgements
Michelle Griffin, Stanford University, Palo Alto, United States.
Contributor Information
Ginimol Mathew, Email: ginimol.mathew.13@ucl.ac.uk.
STROCSS Group:
Joerg Albrecht, Prabudh Goel, Indraneil Mukherjee, Prathamesh Pai, Anil K. D'Cruz, Iain J. Nixon, Klappenbach Roberto, Syed Ather Enam, Somprakas Basu, Oliver J. Muensterer, Salvatore Giordano, Duilio Pagano, David Machado-Aranda, Patrick James Bradley, Mohammad Bashashati, Achilles Thoma, Raafat Y. Afifi, Maximilian Johnston, Ben Challacombe, James Chi-Yong Ngu, Mushtaq Chalkoo, Kandiah Raveendran, Jerome R. Hoffman, Boris Kirshtein, Wan Yee Lau, Mangesh A. Thorat, Diana Miguel, Andrew James Beamish, Gaurav Roy, Donagh Healy, M. Hammad Ather, Shahzad G. Raja, Zubing Mei, Todd G. Manning, Veeru Kasivisvanathan, Juan Gómez Rivas, Roberto Coppola, Burcin Ekser, Veena L. Karanth, Huseyin Kadioglu, Michele Valmasoni, and Ashraf Noureldin
References
- 1.Song J., Chung K. Observational studies: cohort and case-control studies. Plast. Reconstr. Surg. 2010;126(6):2234–2242. doi: 10.1097/PRS.0b013e3181f44abc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Agha R., Lee S., Jeong K., Fowler A., Orgill D. Reporting quality of observational studies in plastic surgery needs improvement. Ann. Plast. Surg. 2016;76(5):585–589. doi: 10.1097/SAP.0000000000000419. [DOI] [PubMed] [Google Scholar]
- 3.von Elm E., Altman D., Egger M., Pocock S., Gøtzsche P., Vandenbroucke J. Strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. PLoS Med. 2007;4(10):296. doi: 10.1371/journal.pmed.0040296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Agha R., Farwana R., Borrelli M., Tickunas T., Kusu-Orkar T., Millip M., Thavayogan R., Garner J., Orgill D. Impact of the SCARE guideline on the reporting of surgical case reports: a before and after study. Int. J. Surg. 2017;45:144–148. doi: 10.1016/j.ijsu.2017.07.099. [DOI] [PubMed] [Google Scholar]
- 5.Agha R., Borrelli M., Farwana R., Kusu-Orkar T., Millip M., Thavayogan R., Garner J., Darhouse N., Orgill D. Impact of the PROCESS guideline on the reporting of surgical case series: a before and after study. Int. J. Surg. 2017;45:92–97. doi: 10.1016/j.ijsu.2017.07.079. [DOI] [PubMed] [Google Scholar]
- 6.Agha R., Fowler A., Limb C., Whitehurst K., Coe R., Sagoo H., Jafree D., Chandrakumar C., Gundogan B. Impact of the mandatory implementation of reporting guidelines on reporting quality in a surgical journal: a before and after study. Int. J. Surg. 2016;30:169–172. doi: 10.1016/j.ijsu.2016.04.032. [DOI] [PubMed] [Google Scholar]
- 7.Agha R., Borrelli M., Vella-Baldacchino M., Thavayogan R., Orgill D., STROCSS Group The STROCSS statement: strengthening the reporting of cohort studies in surgery. Int. J. Surg. 2017;46:198–202. doi: 10.1016/j.ijsu.2017.08.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Agha R., Abdall-Razak A., Crossley E., Dowlut N., Iosifidis C., Mathew G., STROCSS Group, STROCSS 2019 Guideline: strengthening the reporting of cohort studies in surgery. Int. J. Surg. 2019;72:156–165. doi: 10.1016/j.ijsu.2019.11.002. [DOI] [PubMed] [Google Scholar]
- 9.Pill J. The Delphi method: substance, context, a critique and an annotated bibliography. Soc. Econ. Plann. Sci. 1971;5(1):57–71. [Google Scholar]